An Assessment of the Functional Properties of Black Amaranth Flour During Fermentation with Probiotic Lactic Acid Bacteria
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Amaranth Flour
2.2. Chemicals and Reagents
2.3. Physico-Chemical Analysis of Amaranth Seeds
2.4. Starter Culture of Lactic Acid Bacteria Reactivation and Fermentation
2.5. pH and Total Titratable Acidity (TTA) Measurement
2.6. Antioxidant Activity (AA)
2.7. Total Flavonoid Content (TFC)
2.8. Total Polyphenolic Content (TPC)
2.9. Reverse-Phase High-Performance Liquid Chromatography (HPLC) Analysis of Phenolic Compounds
2.10. Protein Content
2.11. Statistical Analysis
3. Results and Discussion
3.1. Proximal Composition of Black Amaranth Seeds
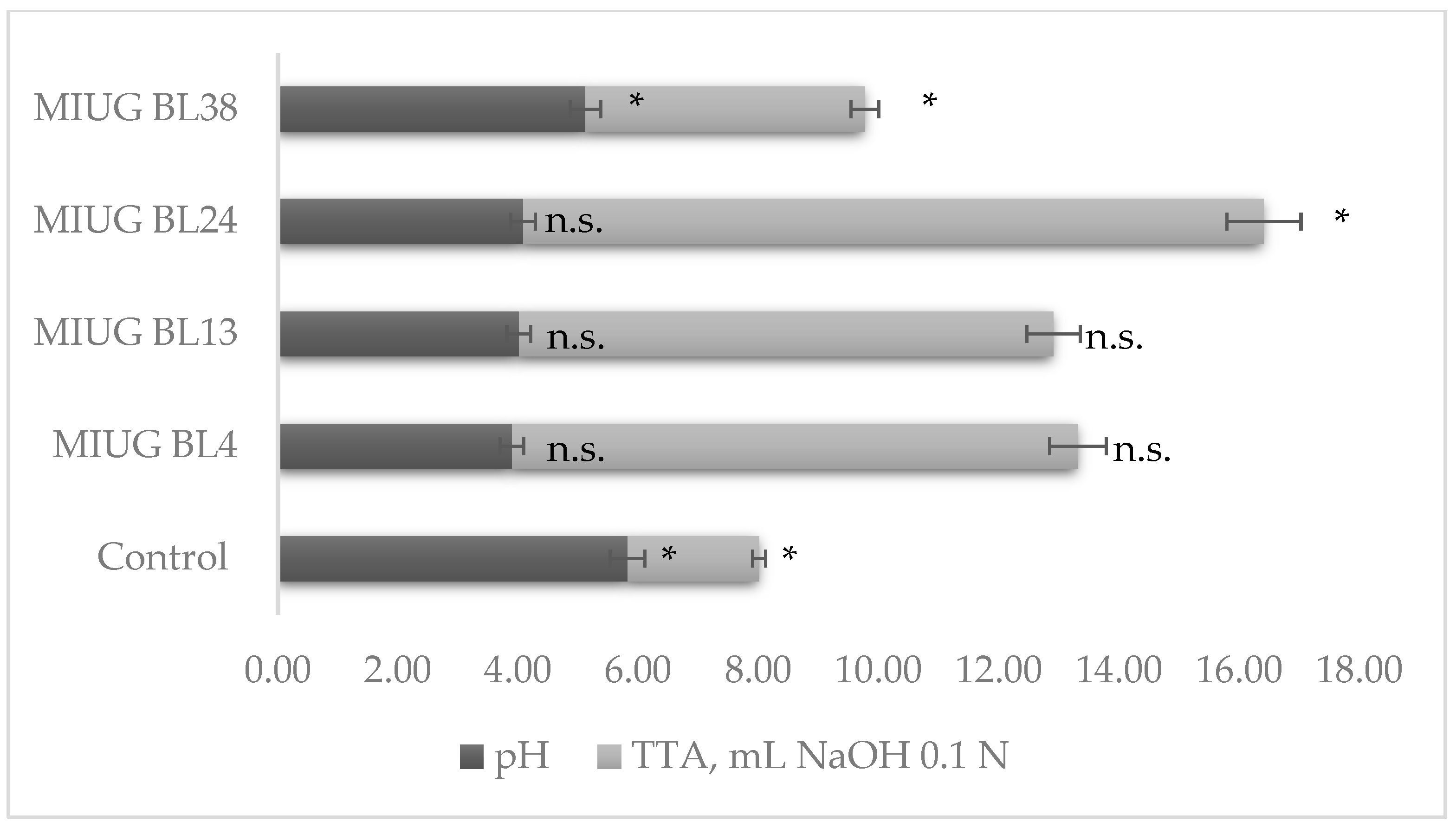
3.2. pH and Total Titratable Acidity (TTA)
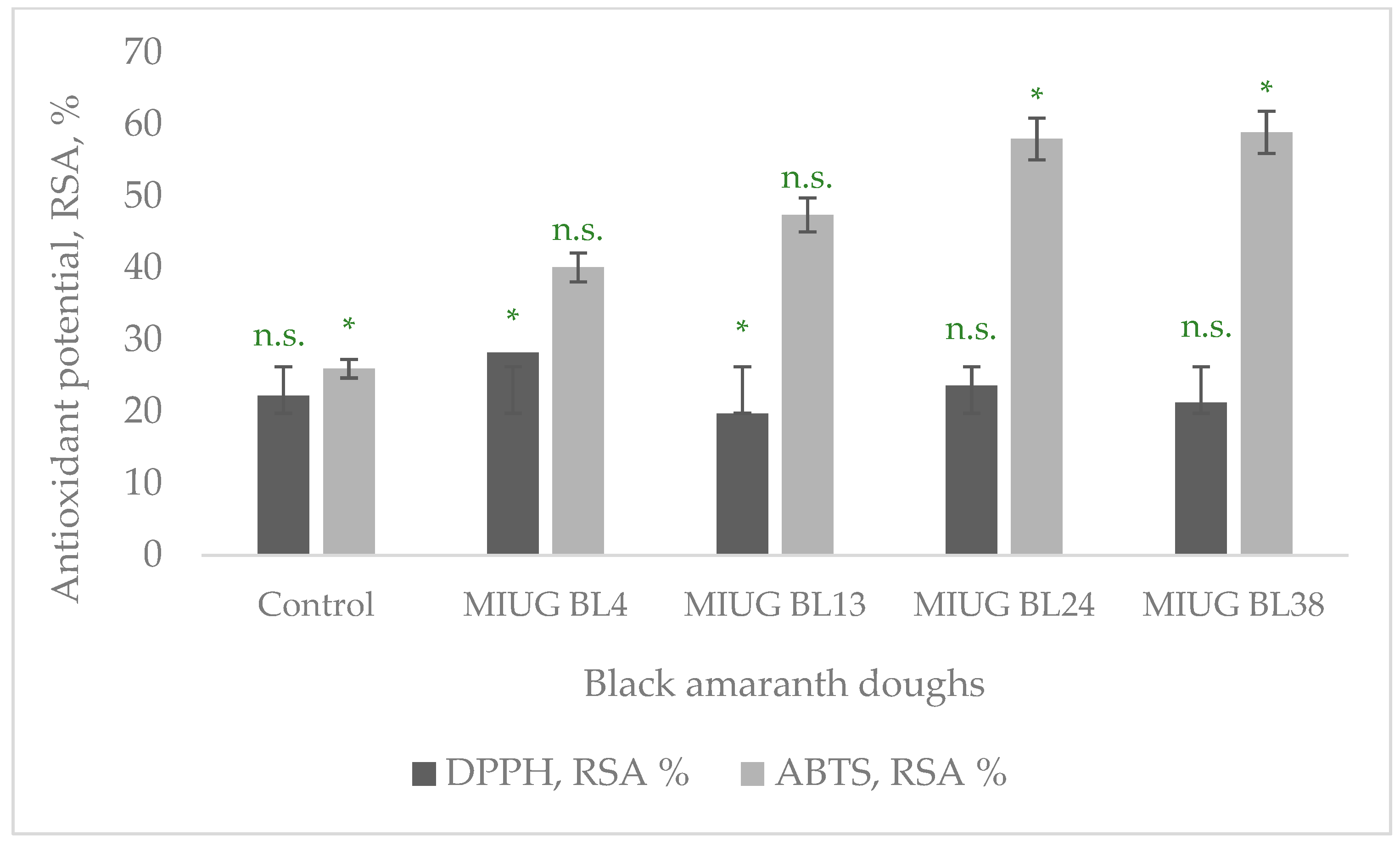
3.3. Antioxidant Activity (AA) of the BA Doughs
3.4. Bioactive and Protein Composition Characterisation of Black Amaranth Doughs
3.4.1. Total Polyphenol Content (TPC) of the BA Doughs
3.4.2. Total Flavonoid Content (TFC) of the BA Doughs
3.4.3. Protein Content (PC) of the BA Doughs
3.5. RP-HPLC Characterisation of BA Fermented Dough
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aiking, H. Future Protein Supply. Trends Food Sci. Technol. 2011, 22, 112–120. [Google Scholar] [CrossRef]
- Boland, M.J.; Rae, A.N.; Vereijken, J.M.; Meuwissen, M.P.M.; Fischer, A.R.H.; Van Boekel, M.A.J.S.; Rutherfurd, S.M.; Gruppen, H.; Moughan, P.J.; Hendriks, W.H. The Future Supply of Animal-Derived Protein for Human Consumption. Trends Food Sci. Technol. 2013, 29, 62–73. [Google Scholar] [CrossRef]
- Nandan, A.; Koirala, P.; Dutt Tripathi, A.; Vikranta, U.; Shah, K.; Gupta, A.J.; Agarwal, A.; Nirmal, N. Nutritional and Functional Perspectives of Pseudocereals. Food Chem. 2024, 448, 139072. [Google Scholar] [CrossRef] [PubMed]
- Schönlechner, R.; Bender, D. Chapter 20—Pseudocereals. In ICC Handbook of 21st Century Cereal Science and Technology; Shewry, P.R., Koksel, H., Taylor, J.R.N., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 191–198. ISBN 978-0-323-95295-8. [Google Scholar]
- Fletcher, R.J. Pseudocereals, Overview. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; p. B9780081005965000391. ISBN 978-0-08-100596-5. [Google Scholar]
- Zhu, F. Amaranth Proteins and Peptides: Biological Properties and Food Uses. Food Res. Int. 2023, 164, 112405. [Google Scholar] [CrossRef] [PubMed]
- Upasana; Yadav, L. Pseudocereals: A Novel Path towards Healthy Eating. In Pseudocereals; IntechOpen: London, UK, 2022; ISBN 978-1-80355-181-4. [Google Scholar]
- Pirzadah, T.B.; Malik, B. Pseudocereals as Super Foods of 21st Century: Recent Technological Interventions. J. Agric. Food Res. 2020, 2, 100052. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive Value of Pseudocereals and Their Increasing Use as Functional Gluten-Free Ingredients. Trends Food Sci. Technol. 2010, 21, 106–113. [Google Scholar] [CrossRef]
- Singh, A.; Punia, D. Characterization and Nutritive Values of Amaranth Seeds. Curr. J. Appl. Sci. Technol. 2020, 39, 27–33. [Google Scholar] [CrossRef]
- Santiago, P.; Anaberta, C.-M.; Tenbergen, K.; Velez-Jimenez, E. Functional Attributes of Amaranth. Austin J. Nutr. Food Sci. 2014, 2, 6. [Google Scholar]
- Adhikary, D.; Khatri-Chhetri, U.; Slaski, J.; Adhikary, D.; Khatri-Chhetri, U.; Slaski, J. Amaranth: An Ancient and High-Quality Wholesome Crop. In Nutritional Value of Amaranth; IntechOpen: London, UK, 2020; ISBN 978-1-83880-084-0. [Google Scholar]
- Emmanuel, O.C.; Babalola, O.O. Amaranth Production and Consumption in South Africa: The Challenges of Sustainability for Food and Nutrition Security. Int. J. Agric. Sustain. 2022, 20, 449–460. [Google Scholar] [CrossRef]
- Calabrò, S.; Oteri, M.; Vastolo, A.; Cutrignelli, M.I.; Todaro, M.; Chiofalo, B.; Gresta, F. Amaranthus Grain as a New Ingredient in Diets for Dairy Cows: Productive, Qualitative, and in Vitro Fermentation Traits. J. Sci. Food Agric. 2022, 102, 4121–4130. [Google Scholar] [CrossRef] [PubMed]
- Mukuwapasi, B.; Mavengahama, S.; Gerrano, A.S. Grain Amaranth: A Versatile Untapped Climate-Smart Crop for Enhancing Food and Nutritional Security. Discov. Agric. 2024, 2, 44. [Google Scholar] [CrossRef]
- Sánchez-López, F.; Robles-Olvera, V.J.; Hidalgo-Morales, M.; Tsopmo, A. Characterization of Amaranthus hypochondriacus Seed Protein Fractions, and Their Antioxidant Activity after Hydrolysis with Lactic Acid Bacteria. J. Cereal Sci. 2020, 95, 103075. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Bioactive Protein Hydrolysates Obtained from Amaranth by Fermentation with Lactic Acid Bacteria and Bacillus Species. Heliyon 2023, 9, e13491. [Google Scholar] [CrossRef] [PubMed]
- Sterr, Y.; Weiss, A.; Schmidt, H. Evaluation of Lactic Acid Bacteria for Sourdough Fermentation of Amaranth. Int. J. Food Microbiol. 2009, 136, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Makovicky, P.; Makovicky, P.; Caja, F.; Rimarova, K.; Samasca, G.; Vannucci, L. Celiac Disease and Gluten-Free Diet: Past, Present, and Future. Gastroenterol. Hepatol. Bed Bench 2020, 13, 1–7. [Google Scholar] [PubMed]
- Hamzehpour, R.; Dastgerdi, A.A. The Effects of Quinoa and Amaranth Flour on the Qualitative Characteristics of Gluten-Free Cakes. Int. J. Food Sci. 2023, 2023, 6042636. [Google Scholar] [CrossRef] [PubMed]
- Sattar, M.; Saeed, F.; Afzaal, M.; Rasheed, A.; Asif, A.; Sharif, S.; Hussain, M.; Rehman, H.; Raza, M.; Munir, H.; et al. An Overview of the Nutritional and Therapeutic Properties of Amaranth. Int. J. Food Prop. 2024, 27, 263–272. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation Transforms the Phenolic Profiles and Bioactivities of Plant-Based Foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef] [PubMed]
- Abdelshafy, A.M.; Rashwan, A.K.; Osman, A.I. Potential Food Applications and Biological Activities of Fermented Quinoa: A Review. Trends Food Sci. Technol. 2024, 144, 104339. [Google Scholar] [CrossRef]
- Cotârleţ, M.; Vasile, A.M.; Gaspar-Pintiliescu, A.; Oancea, A.; Bahrim, G.E. Tribiotication Strategy for the Functionalization of Bovine Colostrum through the Biochemical Activities of Artisanal and Selected Starter Cultures. CyTA 2020, 18, 274–280. [Google Scholar] [CrossRef]
- Castro-Alba, V.; Lazarte, C.E.; Perez-Rea, D.; Carlsson, N.; Almgren, A.; Bergenståhl, B.; Granfeldt, Y. Fermentation of Pseudocereals Quinoa, Canihua, and Amaranth to Improve Mineral Accessibility through Degradation of Phytate. J. Sci. Food Agric. 2019, 99, 5239–5248. [Google Scholar] [CrossRef] [PubMed]
- Popoola, O.O. Phenolic Compounds Composition and in Vitro Antioxidant Activity of Nigerian Amaranthus viridis Seed as Affected by Autoclaving and Germination. Meas. Food 2022, 6, 100028. [Google Scholar] [CrossRef]
- Oncică, F.-G.; Stoica, F.; Constantin, O.E.; Turturică, M.; Aprodu, I.; Ratu, R.N.; Andronoiu, D.G.; Stănciuc, N.; Râpeanu, G. Development of Value-Added Muffins Using Carrot Pomace Powder as a Natural Pigment. Ann. Univ. Dunarea Jos Galati Fascicle VI 2024, 48, 192–210. [Google Scholar] [CrossRef]
- Cotârleț, M.; Pihurov, M.; Păcularu-Burada, B.; Vasile, A.M.; Bahrim, G.E.; Grigore-Gurgu, L. Selection of New Lactobacilli Strains with Potentially Probiotic Properties. Ann. Univ. Dunarea Jos Galati Fascicle VI 2023, 47, 27–50. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, Y.; Zhao, L.; Wang, Y.; Rao, L.; Liao, X. Pressure-Resistant Acclimation of Lactic Acid Bacteria from a Natural Fermentation Product Using High Pressure. Innov. Food Sci. Emerg. Technol. 2021, 69, 102660. [Google Scholar] [CrossRef]
- Păcularu-Burada, B.; Grigore-Gurgu, L.; Cotârleț, M.; Vasile, A.M.; Nistor, O.V.; Cîrciumaru, A.; Râpeanu, G.; Bahrim, G.E.; Nicoleta, S. Freeze-Dried Biotics Based on Lactiplantibacillus plantarum and Lactiplantibacillus paraplantarum with Enhanced Selected Biological Activity. LWT 2024, 203, 116339. [Google Scholar] [CrossRef]
- Nionelli, L.; Wang, Y.; Pontonio, E.; Immonen, M.; Rizzello, C.G.; Maina, H.N.; Katina, K.; Coda, R. Antifungal Effect of Bioprocessed Surplus Bread as Ingredient for Bread-Making: Identification of Active Compounds and Impact on Shelf-Life. Food Control 2020, 118, 107437. [Google Scholar] [CrossRef]
- Mërtiri, I.; Coman, G.; Cotârleț, M.; Turturică, M.; Balan, N.; Râpeanu, G.; Stănciuc, N.; Mihalcea, L. Phytochemical Profile Screening and Selected Bioactivity of Myrtus Communis Berries Extracts Obtained from Ultrasound-Assisted and Supercritical Fluid Extraction. Separations 2025, 12, 8. [Google Scholar] [CrossRef]
- Gavril (Rațu), R.N.; Constantin, O.E.; Enachi, E.; Stoica, F.; Lipșa, F.D.; Stănciuc, N.; Aprodu, I.; Râpeanu, G. Optimization of the Parameters Influencing the Antioxidant Activity and Concentration of Carotenoids Extracted from Pumpkin Peel Using a Central Composite Design. Plants 2024, 13, 1447. [Google Scholar] [CrossRef] [PubMed]
- Bayoï, J.R.; Râpeanu, G.; Stănciuc, N.; Cotârleț, M.; Constantin, O.E.; Etoa, F.-X. In Vitro Antioxidant Potential and Antimicrobial Activity of Some Cameroonian Plant Extracts. Ann. Univ. Dunarea Jos Galati Fascicle VI 2021, 45, 96–116. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Różewicz, M. Nutritional Value and Potential Uses of Amaranth Seeds and the Outlook to Increase the Area under the Amaranth Crop in Poland. Pol. J. Agron. 2021, 46, 40–48. [Google Scholar] [CrossRef]
- Muchekeza, J.T.; Jombo, T.Z.; Magogo, C.; Mugari, A.; Manjeru, P.; Manhokwe, S. Proximate, Physico-Chemical, Functional and Sensory Properties of Quinoa and Amaranth Flour as Potential Binders in Beef Sausages. Food Chem. 2021, 365, 130619. [Google Scholar] [CrossRef] [PubMed]
- Anuradha; Kumari, M.; Zinta, G.; Chauhan, R.; Kumar, A.; Singh, S.; Singh, S. Genetic Resources and Breeding Approaches for Improvement of Amaranth (Amaranthus spp.) and Quinoa (Chenopodium quinoa). Front. Nutr. 2023, 10, 1129723. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Bao, J.; Wang, Z.; Du, S.; Gao, C.; Nan, D.; Yan, X.; Ge, G. Evaluation of the Fermentation Performance and Functional Properties of Bacterial Communities of Amaranth Silage Supplemented with Limosilactobacillus fermentum and Latilactobacillus graminis. Chem. Biol. Technol. Agric. 2023, 10, 103. [Google Scholar] [CrossRef]
- Matejčeková, Z.; Liptáková, D.; Valík, Ľ. Evaluation of The Potential of Amaranth Flour for Lactic Acid Fermentation. J. Pharm. Nutr. Sci. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Xie, C.; Coda, R.; Chamlagain, B.; Edelmann, M.; Varmanen, P.; Piironen, V.; Katina, K. Fermentation of Cereal, Pseudo-Cereal and Legume Materials with Propionibacterium freudenreichii and Levilactobacillus brevis for Vitamin B12 Fortification. LWT 2021, 137, 110431. [Google Scholar] [CrossRef]
- Neelam, Y.; Shruti, S.; Alka, S.; Sudha, T. Effect Of Fermentation On The Anti Nutritional Factors, Antiradical Activity And In Vitro Protein Digestibility Of Cicer arietinum L. Int. J. Curr. Res. Rev. 2012, 4, 85–93. [Google Scholar]
- Kia, P.S.; Sadeghi, A.; Kashaninejad, M.; Zarali, M.; Khomeiri, M. Application of Controlled Fermented Amaranth Supplemented with Purslane (Portulaca oleracea) Powder to Improve Technological Functionalities of Wheat Bread. Appl. Food Res. 2024, 4, 100395. [Google Scholar] [CrossRef]
- Araujo-León, J.A.; Sánchez-del Pino, I.; Ortiz-Andrade, R.; Hidalgo-Figueroa, S.; Carrera-Lanestosa, A.; Brito-Argáez, L.G.; González-Sánchez, A.; Giácoman-Vallejos, G.; Hernández-Abreu, O.; Peraza-Sánchez, S.R.; et al. HPLC-Based Metabolomic Analysis and Characterization of Amaranthus cruentus Leaf and Inflorescence Extracts for Their Antidiabetic and Antihypertensive Potential. Molecules 2024, 29, 2003. [Google Scholar] [CrossRef] [PubMed]
- Yeşil, S.; Levent, H. The Influence of Fermented Buckwheat, Quinoa and Amaranth Flour on Gluten-Free Bread Quality. LWT 2022, 160, 113301. [Google Scholar] [CrossRef]
- Vento, M.; Della Croce, C.M.; Bellani, L.; Tassi, E.L.; Echeverria, M.C.; Giorgetti, L. Effect of Sprouting, Fermentation and Cooking on Antioxidant Content and Total Antioxidant Activity in Quinoa and Amaranth. Int. J. Mol. Sci. 2024, 25, 10972. [Google Scholar] [CrossRef] [PubMed]
- Gobbetti, M.; De Angelis, M.; Di Cagno, R.; Polo, A.; Rizzello, C.G. The Sourdough Fermentation Is the Powerful Process to Exploit the Potential of Legumes, Pseudo-Cereals and Milling by-Products in Baking Industry. Crit. Rev. Food Sci. Nutr. 2020, 60, 2158–2173. [Google Scholar] [CrossRef] [PubMed]
- Vasyliev, G.S.; Vorobyova, V.I.; Linyucheva, O.V. Evaluation of Reducing Ability and Antioxidant Activity of Fruit Pomace Extracts by Spectrophotometric and Electrochemical Methods. J. Anal. Methods Chem. 2020, 2020, 8869436. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, W.; Pan, X.; Lao, F.; Liao, X.; Shi, Y.; Wu, J. Improvement of Antioxidant Properties of Jujube Puree by Biotransformation of Polyphenols via Streptococcus thermophilus Fermentation. Food Chem. X 2022, 13, 100214. [Google Scholar] [CrossRef] [PubMed]
- Đorđević, T.M.; Šiler-Marinković, S.S.; Dimitrijević-Branković, S.I. Effect of Fermentation on Antioxidant Properties of Some Cereals and Pseudo Cereals. Food Chem. 2010, 119, 957–963. [Google Scholar] [CrossRef]
- Hansen, H.; Rasmussen, C.; Knudsen, K.; Hansen, Å. Effect of Genotype and Harvest Year on Content and Composition of Dietary Fibre in Rye (Secale cereale L) Grain. J. Sci. Food Agric. 2002, 83, 76–85. [Google Scholar] [CrossRef]
- Kariluoto, S.; Aittamaa, M.; Korhola, M.; Salovaara, H.; Vahteristo, L.; Piironen, V. Effects of Yeasts and Bacteria on the Levels of Folates in Rye Sourdoughs. Int. J. Food Microbiol. 2006, 106, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Zahra, M.; Abrahamse, H.; George, B.P. Flavonoids: Antioxidant Powerhouses and Their Role in Nanomedicine. Antioxidants 2024, 13, 922. [Google Scholar] [CrossRef] [PubMed]
- Kamal, H.; Mudgil, P.; Bhaskar, B.; Fisayo, A.F.; Gan, C.-Y.; Maqsood, S. Amaranth Proteins as Potential Source of Bioactive Peptides with Enhanced Inhibition of Enzymatic Markers Linked with Hypertension and Diabetes. J. Cereal Sci. 2021, 101, 103308. [Google Scholar] [CrossRef]
- Suárez, S.; Aphalo, P.; Rinaldi, G.; Añón, M.C.; Quiroga, A. Effect of Amaranth Proteins on the RAS System. In Vitro, in Vivo and Ex Vivo Assays. Food Chem. 2020, 308, 125601. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Shams, R.; Dash, K.K.; Dar, A.H. A Comprehensive Review of Pseudo-Cereals: Nutritional Profile, Phytochemicals Constituents and Potential Health Promoting Benefits. Appl. Food Res. 2023, 3, 100351. [Google Scholar] [CrossRef]
| Probiotic Strains | Isolation Source | Accession No. |
|---|---|---|
| Lc. paracasei MIUG BL4 | Borsch | MT626061.1 |
| Lc. paracasei MIUG BL13 | Corn flour | MT611827.1 |
| Lp. pentosus MIUG BL24 | Whey | OK325938.1 |
| Lc. rhamnosus MIUG BL38 | Chickpea | MT463821.1 |
| Physico-Chemical Characteristics | Results |
|---|---|
| Ash, % | 3.50 ± 0.14 |
| Moisture, % | 11.05 ± 0.21 |
| Fats, %, | 5.25 ± 0.13 |
| Saturated fatty acids, % | 0.80 ± 0.14 |
| Proteins, % | 18.85 ± 0.35 |
| Carbohydrates, % | 61.09 ± 0.90 |
| Sugars, % | 3.50 ± 0.35 |
| Samples Fermented with LAB Strains | |||||
|---|---|---|---|---|---|
| Control | MIUG BL4 | MIUG BL13 | MIUG BL24 | MIUG BL38 | |
| TPC, mg GAE/g DM | 2.62 ± 0.18 | 2.24 ± 0.22 * | 2.02 ± 0.22 ** | 2.00 ± 0.09 ** | 2.33 ± 0.20 * |
| TFC, mg CE/g DM | 2.00 ± 0.21 | 2.20 ± 0.16 | 2.14 ± 0.02 | 2.12 ± 0.09 | 1.97 ± 0.19 |
| PC, mg/g DM | 1.23 ± 0.03 | 0.75 ± 0.07 **** | 1.01 ± 0.03 *** | 1.05 ± 0.08 ** | 1.18 ± 0.06 * |
| Identified Compounds, ng/µL | Control | Lc. paracasei MIUG BL4 | Lc. paracasei MIUG BL13 | Lp. pentosus MIUG BL24 | Lc. rhamnosus MIUG BL38 |
|---|---|---|---|---|---|
| Phenolic acids | |||||
| Gallic acid | 161.21 ± 0.82 | 19.15 ± 0.25 **** | 27.67 ± 7.98 **** | n.d. | n.d. |
| Protocatechuic acid | 13.34 ± 1.47 | 12.57 ± 0.70 n.s. | 15.33 ± 0.33 n.s. | 16.25 ± 0.59 * | 19.61 ± 0.54 ** |
| 4-Hydroxybenzoic acid | 3.73 ± 0.09 | n.d. | n.d. | n.d. | 2.96 ± 0.02 ** |
| p-Coumaric acid | 9.49 ± 0.48 | n.d. | n.d. | n.d. | n.d. |
| Flavonoids | |||||
| Epigallocatechin | 7789.88 ± 17.07 | 3941.11 ± 11.61 **** | 3566.02 ± 11.24 **** | 4983.16 ± 7.29 **** | 6942.47 ± 5.63 *** |
| Epicatechin | 880.02 ± 15.33 | 415.13 ± 13.11 *** | 274.92 ± 7.43 **** | 337.72 ± 2.53 **** | 242.50 ± 9.14 **** |
| Catechin | n.d. | 217.33 ± 4.85 | 237.20 ± 2.74 | 226.48 ± 2.78 | 434.81 ± 5.63 ** |
| Epigallocatechin | TFC | TPC | PC | DPPH | ABTS | |
|---|---|---|---|---|---|---|
| Epigallocatechin | 1.000 | |||||
| TFC | −0.920 * | 1.000 | ||||
| TPC | 0.822 n.s. | −0.638 n.s. | 1.000 | |||
| PC | 0.822 n.s. | −0.916 * | 0.466 n.s. | 1.000 | ||
| DPPH | −0.266 n.s. | 0.560 n.s. | 0.037 n.s. | −0.741 n.s. | 1.000 | |
| ABTS | −0.265 n.s. | 0.006 n.s. | −0.692 n.s. | −0.020 n.s. | −0.195 n.s. | 1.000 |
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
|---|---|---|---|---|---|
| Regression | 1 | 11,626,204.0 | 11,626,204.0 | 16.45 | 0.027 |
| TFC | 1 | 11,626,204.0 | 11,626,204.0 | 16.45 | 0.027 |
| Error | 3 | 2,120,221.0 | 706,740.0 | ||
| Total | 4 | 13,746,425.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souare, M.L.; Diallo, A.O.S.; Balan, N.; Vasile, M.A.; Traore, L.; Bahrim, G.E.; Cotârleț, M.; Dumitru, C.N. An Assessment of the Functional Properties of Black Amaranth Flour During Fermentation with Probiotic Lactic Acid Bacteria. Fermentation 2025, 11, 414. https://doi.org/10.3390/fermentation11070414
Souare ML, Diallo AOS, Balan N, Vasile MA, Traore L, Bahrim GE, Cotârleț M, Dumitru CN. An Assessment of the Functional Properties of Black Amaranth Flour During Fermentation with Probiotic Lactic Acid Bacteria. Fermentation. 2025; 11(7):414. https://doi.org/10.3390/fermentation11070414
Chicago/Turabian StyleSouare, Mamadou Lamarana, Alpha Oumar Sily Diallo, Nicoleta Balan, Mihaela Aida Vasile, Lounceny Traore, Gabriela Elena Bahrim, Mihaela Cotârleț, and Caterina Nela Dumitru. 2025. "An Assessment of the Functional Properties of Black Amaranth Flour During Fermentation with Probiotic Lactic Acid Bacteria" Fermentation 11, no. 7: 414. https://doi.org/10.3390/fermentation11070414
APA StyleSouare, M. L., Diallo, A. O. S., Balan, N., Vasile, M. A., Traore, L., Bahrim, G. E., Cotârleț, M., & Dumitru, C. N. (2025). An Assessment of the Functional Properties of Black Amaranth Flour During Fermentation with Probiotic Lactic Acid Bacteria. Fermentation, 11(7), 414. https://doi.org/10.3390/fermentation11070414









