Abstract
Allium genus vegetables have significant functional effects on health. In recent years, consumer demand for black forms of these vegetables, produced from fresh Alliums through spontaneous fermentation at high temperature and humidity, has increased, while their functional effects have yet to be fully elucidated. The aim of this study was to compare the antioxidant capacity and total phenol content of various Allium vegetables (yellow onion, red onion, white onion, and garlic) in both their raw and black (fermented) forms. For the production of black onions and black garlic, unpeeled raw forms of these vegetables were kept at 75 °C and 90% humidity for 9 days. Afterward, fresh and fermented samples were lyophilized, dried, and evaluated for total phenol content by the Folin–Ciocalteu method and for antioxidant activity by the ABTS and DPPH methods. The total phenol content increased significantly in all samples after fermentation (p < 0.05), with the highest increases observed in garlic (216%), while the increases in onion species ranged between 44.6% and 118.3%. The increase in antioxidant capacity was also significant in all samples (p < 0.05) and was higher in garlic than in onions. Changes in antioxidant capacity and total phenol content indicate that fermentation improves the nutritional quality of these vegetables.
1. Introduction
Onion (Allium cepa) and garlic (Allium sativum) are the most widely produced and consumed species of the Allium genus, and they have an important place in world cuisine with their sensory characteristics and functional effects [1]. Before the discovery and widespread use of pharmaceuticals, onions and garlic have historically played an important role in traditional medicine. Charaka, the pioneer of Ayurvedic medicine; Ibn Sina, known as Avicenna by Western societies; Hippocrates, considered the father of medicine; and many other famous physicians and scientists defined onion and garlic as medicinal plants by distinguishing them from traditional foods [2]. Due to the increasing tendency towards traditional/complementary medicine practices, vegetables in this group can be consumed in different industrial forms besides their known raw forms. Among the current consumption forms, black onion and black garlic preparations attract the most attention [3].
Black onion and black garlic are produced by fermenting raw onion and garlic at high temperatures (70–75 °C) and humidity (90%) using endophytic bacteria or with the addition of exogenous starter cultures [4]. Studies investigating the endophytic bacteria responsible for the fermentation of Allium vegetables under these conditions—without exogenous microorganisms—have identified various endophytes, particularly heat-resistant Bacillus strains such as B. subtilis, B. methylotrophicus, and B. amyloliquefaciens [5]. The heat resistance of Allium endophytes is further supported by the identification of four other dominant strains from black garlic, namely Thermus, Corynebacterium, Streptococcus, and Brevundimonas, even after processing in a heating oven at 80 °C for 12 days [6].
During fermentation, heat treatment and decreasing pH values lead to the hydrolysis of polysaccharides into oligosaccharides and monosaccharides (glucose and fructose), resulting in increased levels of reduced carbohydrates. This process also diminishes the characteristic odor and taste of these vegetables, enhancing their sensory palatability. In recent years, consumers seeking the functional benefits of onion and garlic (antimicrobial, antibacterial, antiparasitic, antifungal, antiviral, and anti-inflammatory/immunomodulatory) have increasingly turned to black onion and garlic, which offer more appealing sensory properties [7].
Although the positive effects of onion and garlic against many diseases (cardiovascular diseases, diabetes, cancer, etc.) have been proven in literature, many processes applied from pre-harvest to consumption can cause changes in the functional activity of these vegetables [8,9,10]. In this study, the aim was to investigate the antioxidant capacity and total phenol content of yellow onion, white onion, red onion, and garlic in both their raw and fermented forms.
2. Materials and Methods
2.1. Chemicals
Folin–Ciocalteu reagent, 2,20-azino-bis-(3-ethylbenzothiazoline-6-sulphonate) diammonium salts (ABTS), 2,2-diphenyl-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethyl-2-carboxylic acid (Trolox), hydrochloric acid (HCl), sodium carbonate (Na2CO3), ferric chloride (FeCl3), and gallic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). All chemicals used in this study were of analytical grade.
2.2. Plant Materials and Processing of Samples
The onion and garlic samples used in the study were obtained from a market selling local products in the Bolu province (Türkiye). Care was taken to ensure that the onions and garlic were not sprouted or crushed. One part each of the onion and garlic samples was randomly selected to prepare raw onion and garlic samples, and the other part was selected for the fermented samples. Black garlic and onion were produced at 75 °C and approximately 90% relative humidity, without the addition of starter culture, in 9 days. The aim was to obtain similar dry matter and moisture contents of raw and fermented samples in the analyses to be carried out, and therefore, it was decided to dry all samples using the lyophilization method. Accordingly, raw and fermented onion and garlic samples were first peeled and diced with a ceramic knife and kept in covered petri dishes at −80 °C for one day. Then, the samples were transferred to the lyophilizer with the same petri dishes, lyophilized for 36 h, and powdered in a porcelain mortar before extract preparation.
2.3. Preparation of Methanolic Extracts from Fresh and Fermented Allium Species
For the determination of total phenol and antioxidant activity, 1 ± 0.001 g of the lyophilized and powdered samples were weighed and mixed with 10 mL of 60% methanol. Then, it was centrifuged at 3000 rpm for 30 min. After centrifugation, the supernatant was taken, 10 mL of methanol was added, and the process was repeated. As a result, 1 g of the sample was extracted with 20 mL of 60% methanol, and all samples were stored at −20 °C until the analysis was performed.
2.4. Determination of Total Phenolic Content
The total phenolic content of each extract was determined in triplicate by the Folin–Ciocalteu procedures according to the method of Lu et al. (2011) [11], with minor changes. In brief, 750 μL of the Folin–Ciocalteu reagent diluted at a volume ratio of 1:10 was mixed with 100 μL of 60% methanolic onion and garlic extracts and incubated for 10 min at room temperature. Then, 750 μL of 2% sodium carbonate solution was added and mixed with a vortex device. After 45 min in the dark at room temperature, the absorbance at 765 nm was measured in a spectrophotometer. Gallic acid (0–50 mg/L), widely preferred in the determination of total phenolic compounds, was used as the analysis standard and performed in 3 replicates. The amounts of gallic acid corresponding to the absorbance values of the samples were calculated with the calibration curve specific to the gallic acid standard, and the results were expressed as gallic acid equivalents.
2.5. Determination of Total Antioxidant Capacity
2.5.1. DPPH Free Radical Scavenging Activity
A 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay was performed using the method of Sharma et al. (2015) [12] but with some modifications. Firstly, 24 mg of DPPH was dissolved in 100 mL of 60% methanol to prepare a stock DPPH solution, and then the stock solution was diluted (1:10) with 60% methanol and used for analysis. For the analysis, 50 μL of the sample extracts were taken, and 1950 μL of DPPH solution was added, mixed with a vortex device, and kept at room temperature for 30 min. Trolox (0–500 μmol/L) was used as the analysis standard, and the analyses were performed in triplicate. Absorbance values of samples (As) and controls (Ac) were read at 517 nm in a UV-VIS spectrophotometer. The amount of Trolox corresponding to the absorbance value of the samples was calculated with the calibration curve specific to the Trolox standard, and the results were expressed as μmol Trolox equivalent/g dry material.
2.5.2. ABTS Radical Cation Scavenging Activity
The 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonate) assay was performed using the method of Toledano-Medina et al. (2016) [13] but with some modifications. Firstly, 0.1920 g of ABTS for the 7 mM ABTS solution was mixed with 0.0331 g of K2S2O8 (2.25 mM) dissolved in distilled water at a different location, and this solution was kept in the dark at room temperature for approximately 12–16 h. This stock solution was diluted with methanol, and the absorbance at a wavelength of 734 nm was adjusted to approximately 0.7. After adjusting the absorbance, 980 μL of ABTS solution was taken, 20 μL of sample extract was added, and the absorbance after 6 min was read at a 734 nm wavelength. Trolox (0–500 μmol/L) was used as the analysis standard, and the analyses were performed in triplicate. The amount of Trolox corresponding to the absorbance value of the samples was calculated with the calibration curve specific to the Trolox standard, and the results were expressed as μmol Trolox equivalent/g dry material.
The radical scavenging activity for both the DPPH and ABTS techniques was expressed as a percentage of inhibition, which can be calculated using the equation below:
Ac represents the absorbance of the control, and As represents the absorbance of the sample.
2.6. Determination of the pH
The pHs of the samples were measured using the potentiometric method in a 1:5 mixture of homogenate and deionized water, with a laboratory pH meter probe (Thermo Scientific-Orion Star A211, Waltham, MA, USA) at room temperature. Measurements were taken in three independent replications.
2.7. Statistical Analysis
The SPSS Statistics 24.0 package program was used for the statistical analysis of the research data. In order to determine the statistical analysis method, the normality of the distribution of the data was first examined by looking at the kurtosis and skewness values, and it was accepted that the values with kurtosis and skewness values between ±1.5 were normally distributed. Since the kurtosis and skewness values of all parameters were between ±1.5, statistical analyses were performed with parametric tests. The paired t-test analyzed within-group changes, and the one-way Anova test analyzed between-group differences. The Tukey test, one of the post hoc tests, was used to determine the source of the difference between the groups. All analyses evaluated the results at a 95% confidence interval and a p < 0.05 significance level.
3. Results
3.1. Physical Changes and pH
Onion and garlic samples were fermented at 75 °C and high humidity for 9–12 days without adding exogenous microorganisms, and their colors turned brown/black. Photographs of onion and garlic samples after fermentation are shown in the figure below (Figure 1).
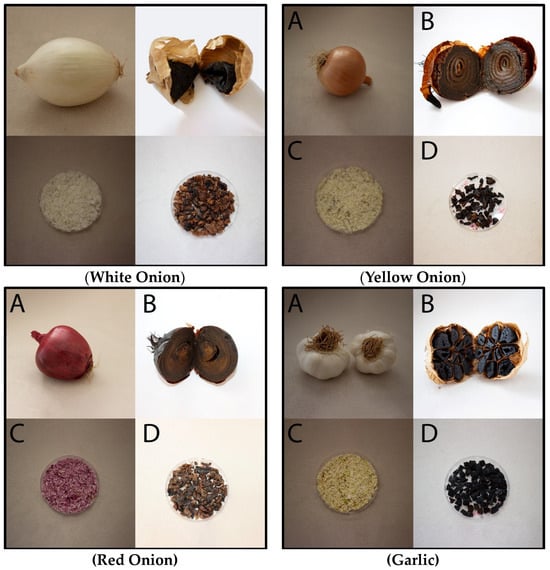
Figure 1.
Fermentation-induced color changes in Allium vegetables (A: raw, B: fermented, C: raw-lyophilized, and D: fermented-lyophilized).
The weights of onions and garlic decreased due to the fermentation process. The mean weights of white onion, red onion, yellow onion, and garlic before fermentation were 117.3 ± 1.15, 186.7 ± 21.6, 208 ± 36.5, and 52.4 ± 4.55 g, respectively, and the mean weights after fermentation were 38.3 ± 1.39, 66.1 ± 7.65, 79.83 ± 11.34, and 11.50 ± 0.61 g, respectively. While the weight losses due to fermentation in onion samples varied between 61.6% and 67.3%, proportionally, the highest weight loss was realized in white onion, and the lowest weight loss was realized in yellow onion. The weight loss in garlic was higher than the weight loss in onion species and was determined to be 78%. The difference between pre-fermentation weight and post-fermentation weight was statistically significant (p < 0.001) for all onion and garlic species.
The mean pH values of white onion, red onion, yellow onion, and garlic before fermentation were 5.04 ± 0.03, 4.85 ± 0.04, 4.91 ± 0.06, and 5.26 ± 0.05, respectively, while the mean pH values after fermentation were 3.87 ± 0.03, 3.80 ± 0.02, 3.83 ± 0.03, and 4.13 ± 0.23, respectively. The difference between the pre-fermentation pH value and post-fermentation pH value was statistically significant (p < 0.001) in all onion and garlic species (Table 1).

Table 1.
Intra- and intergroup differences in total phenolic content, antioxidant activity, and pH of garlic and onions.
3.2. Changes in Total Phenolic Substance Content
The total phenolic matter contents of onion and garlic samples before and after fermentation were expressed as mg gallic acid equivalent (GAE)/g dry material (dm), as shown in Table 1. According to the results obtained, the highest mean phenolic content was found in red onion (7.13 ± 0.48 mg GAE/dm) before fermentation, followed by garlic (5.27 ± 0.18 mg GAE/dm), yellow onion (3.44 ± 0.17 mg GAE/dm), and white onion (2.85 ± 0.12 mg GAE/dm). After fermentation, the highest mean phenolic content was found in garlic (16.66 ± 0.48 mg GAE/dm), followed by red onion (10.46 ± 0.6 mg GAE/dm), yellow onion (7.51 ± 0.56 mg GAE/dm), and white onion (4.12 ± 0.23 mg GAE/dm). The difference in total phenol content between the groups before and after fermentation was statistically significant (p < 0.001).
The increases in the total phenolic contents of onion species varied between 44.6% and 118.3%, with the highest increase in yellow onion (p = 0.004) and the lowest increase in white onion (p = 0.024). The increase in total phenol content of garlic (216%) was higher than that of the onion species (p < 0.001). The level of increase in total phenol content before and after fermentation in all samples was statistically significant.
3.3. Changes in Antioxidant Activity Values
3.3.1. DPPH
The antioxidant activity values of onion and garlic samples determined by the DPPH method before and after fermentation were expressed as μmol Trolox/dm, and the values are shown in Table 1. According to the results obtained, the highest mean antioxidant activity value before fermentation was observed in red onion (11.2 ± 0.5 μmol Trolox/dm), followed by garlic (10.1 ± 0.3 μmol Trolox/dm), yellow onion (6.74 ± 0.12 μmol Trolox/dm), and white onion (5.50 ± 0.21 μmol Trolox/dm). After fermentation, the highest mean antioxidant activity value was found in garlic (35.64 ± 1.13 μmol Trolox/dm), followed by red onion (18.9 ± 0.81 μmol Trolox/dm), white onion (11.74 ± 0.58 μmol Trolox/dm), and yellow onion (11.22 ± 0.44 μmol Trolox/dm). The difference in antioxidant activity values between the groups before and after fermentation was statistically significant (p < 0.001).
The increase in the antioxidant activity value of onion species varied between 66.47% and 113%; the highest increase was observed in white onion (p = 0.003), and the lowest increase was observed in yellow onion (p = 0.004). The increase in the antioxidant activity value of garlic (251.9%) was higher than that of the onion species (p < 0.001). The level of increase in antioxidant activity before and after fermentation was observed to be statistically significant in all samples.
3.3.2. ABTS
The antioxidant activity values of onion and garlic samples determined by the ABTS method before and after fermentation were expressed as µmol Trolox/dm, as shown in Table 1. Based on the results obtained, the highest mean antioxidant activity value before fermentation was observed in red onion (24.61 ± 0.7 µmol Trolox/dm), followed by garlic (19.7 ± 0.49 µmol Trolox/dm), yellow onion (8.79 ± 0.29 µmol Trolox/dm), and white onion (7.97 ± 0.23 µmol Trolox/dm). After fermentation, the highest mean antioxidant activity value was found in garlic (51.35 ± 1.94 µmol Trolox/dm), followed by red onion (31.9 ± 1.09 µmol Trolox/dm), yellow onion (19.29 ± 0.72 µmol Trolox/dm), and white onion (16.26 ± 0.66 µmol Trolox/dm). The difference in the antioxidant activity values of the groups before and after fermentation was statistically significant (p < 0.001).
The increase in the antioxidant activity values of the onion species varied between 29.6% and 119.45%, with the highest increase in yellow onion (p < 0.001) and the lowest increase in red onion (p < 0.001). The increase in the antioxidant activity value of garlic (160.6%) was higher than that of the onion species (p < 0.001). It was observed that the level of increase in antioxidant activity before and after fermentation in all samples was statistically significant.
4. Discussion
The fermentation of onions and garlic under high temperature and high humidity conditions caused the color to change to brown or black, increased the total phenol content and antioxidant activity, and decreased the pH value.
The high temperature during the fermentation process causes Maillard reactions to develop in onions and garlic. Melanoidin and other brown polymer compounds produced due to Maillard reactions give the food a characteristic dark brown/black color. The decrease in pH value caused by the black onion and garlic production process is also associated with Maillard reaction products. When the –NH2 groups of amino acids or proteins are closed by the carbonyl groups of carbohydrates, an amino–sugar complex is formed during the processing period while the –COOH groups remain, decreasing pH [14]. In this study, it was found that the fermentation of onion and garlic at high temperatures and high humidity caused the color to change to brown/black and the pH value and weight to decrease, and the findings are consistent with literature data [15,16,17]. On the other hand, the decrease in pH value contributes to reducing toxicological risks in black garlic and black onion. This is because lactic acid bacteria, as well as many yeast and mold species, can grow well at pH levels below 4.2, while many microorganisms that cause food poisoning lose their viability at this pH. Applying high temperatures during the fermentation process and the resulting decreases in water content and pH value prolong the storage life of black onions and garlic [16].
There are a limited number of studies in literature investigating the change in total phenol content during the black onion production process. In the only onion-related study in literature [18], shallots were transformed into black onions at a temperature range of 65–70 °C for 7–28 days at 90% humidity, and the changes in the contents of seven different flavonoids (quercetin, isorhamnetin, quarsetin-3-O-glucoside, luteolin, quercetin diglycoside, quarsetin-4-O-glucoside, and isorhamnetin-4′-O-glucoside) were examined chromatographically. As a result, it was found that all flavonoids decreased with the black onion transformation process, and it was stated that the decrease may be due to the reaction of flavonoids with other quinones or proteins or due to the oxidation to semi-quinoid intermediates. Although there is no study investigating the changes in total phenol content with the production process of black onions, there are studies evaluating the total phenol contents of different-colored onions. In some of these studies, it was reported that the total phenol content of yellow onion was higher than that in red onion [19] and white onion [20], while in some studies, the total phenol content of red onion was higher than that of yellow onion, white onion [11,12], and garlic [21,22]. In this study, it was determined that the total phenol content of unfermented red onion was higher than the total phenol contents of unfermented yellow onion, white onion, and garlic, and the findings obtained are consistent with the results in literature [11,12].
In literature, there are a sufficient number of studies evaluating the total phenol content in the black garlic production process. The majority of these studies show parallel results where the total phenol content increases with the black garlic production process [15,16], and the changes in total phenol content show a wide range. Changes in total phenol content can vary, depending on the methods of analysis, the type of solvent used in extraction, fermentation conditions, and duration [23,24,25].
Apart from these factors, it has been reported that freezing (at −16 °C for 30 h) [26], steaming (4–6 min) [27], and fermentation by separating into cloves [13] before converting garlic into black garlic can also increase the total phenol content. According to the findings obtained from this study, it was determined that the total phenol content of garlic increased approximately three times as a result of fermentation, and the rate of increase was similar to some studies in literature [13,16,17,23].
It was observed that total phenol content increased with the fermentation process in all onion species, as well as garlic. Considering the changes in total phenol content, it is understood that fermentation at high temperatures and humidity increases the biological quality of these vegetables. The fact that the increase in the total phenol content of garlic was higher than that of the onion species suggests that garlic is more suitable for the fermentation process. Various explanations have been proposed for the increase in total phenol content of black garlic during the production process, and it is argued that the fermentation process breaks the glycosylated and esterified bound forms of phenolic compounds, and thus, an increase in free forms occurs. The latter is suggested to be due to increased complex polyphenol levels due to Maillard reactions in the fermentation process [16,28]. In this study, the increase observed in the total phenol content in the production process of black onion and black garlic is thought to be due to the increased free forms of phenolic compounds and complex polyphenol levels due to Maillard reactions.
In literature, only one study was found in which the changes in antioxidant capacity were examined with the black onion production process in the time period of the research. In this study, it was concluded that the oxygen radical scavenging activity (ORAC) decreased in fermented shallots, while there was no significant change in the antioxidant activity values determined using the DPPH and ABTS methods [18]. It was stated that the differences in the results obtained from the three antioxidant activity assays (ORAC, ABTS, and DPPH) may be due to the chemical principles on which the analysis techniques are based. Although no other study investigated the changes in antioxidant capacity during the black onion production process, studies evaluated the antioxidant capacities of onions of different colors. In some of these studies, it was reported that the antioxidant capacity of red onion was higher than that of garlic [21], yellow onion [29], and white onion [11,12], while it could also be concluded that the antioxidant capacity of garlic was higher than that of red onion [30]. In addition, there may be differences in the antioxidant capacity ranking of Allium species, depending on the analysis methods [22]. This study found that the antioxidant capacity of unfermented red onion was higher than that of unfermented yellow onion, white onion, and garlic, and the findings obtained were compatible with the results of the literature mentioned above.
There are a sufficient number of studies in literature evaluating the changes in antioxidant capacity during the black garlic production process. The majority of these studies reported parallel results showing that the antioxidant capacity increased with the black garlic production process [15,23,24]. Changes in the antioxidant capacity during the black garlic transformation process depended on the analysis methods [31], the type of solvent used in the extraction [24,25], fermentation conditions, and duration [13,14,15,17,23,26]. In addition, it was reported that freezing (30 h at −16 °C) [26] and steaming (4–6 min) [27] before converting garlic into black garlic can provide a further increase in antioxidant capacity, while no significant difference in antioxidant capacity was observed in whole and clove-separated fermented garlic [13].
As a result, it is understood that the anti-oxidative properties of onion and garlic are strengthened in the production process of black onion and garlic. Similar to the total phenol content, the increase in antioxidant activity levels was higher in garlic than in the onion species. Considering the increase in antioxidant activity levels, it is thought that fermented forms of onion and garlic in high-temperature and high-humidity environments may be more effective against oxidative stress. Studies in literature suggest that the increase in antioxidant capacity observed with the black garlic production process may be due to increased polyphenol content [13,15,16,32,33]; conversion of unstable compounds in raw forms to stable soluble compounds with high antioxidant power [13,23,34]; increased levels of compounds, such as S-allyl cysteine, an alliin derivative [16,17,34,35]; and anti-oxidative compounds formed due to Maillard reactions (melanoidins, 5-hydroxymethyl furfuraldehyde) [16,36,37,38]. In this study, the findings regarding the increase in total phenol content and antioxidant capacity confirmed the hypothesis that the increase in phenol content may play a role in the increased antioxidant capacity. In addition, the proven antioxidant activity of melanoidins isolated from black garlic [36,38], evidence that glucose/fructose–cysteine- and glucose/fructose–tyrosine-mediated Maillard reaction products have greater antioxidant activities than other reducing sugar–amino acid-mediated Maillard reaction products [39], and the fact that the Maillard reactions that take place in the process of obtaining black onion and garlic are mainly mediated by cysteine and tyrosine amino acids confirm the hypothesis that Maillard reactions may play a role in the high antioxidant capacity of black onion and garlic, compared to their normal forms.
5. Conclusions
The fermentation of garlic and onions at high temperatures and humidity, without the addition of exogenous microorganisms, significantly increased the total phenol content and antioxidant capacity in all samples. Given the negative impact of alternative heat treatments on the nutritional composition and functional properties of these vegetables, it is evident that fermented onions and garlic offer a healthier form of consumption. Additionally, the fermentation process can extend the shelf life of these vegetables by reducing both pH levels and water content.
Author Contributions
Conceptualization, T.G.Ü. and F.P.Ç.; methodology, T.G.Ü. and F.P.Ç.; formal analysis, T.G.Ü. and F.P.Ç.; investigation, T.G.Ü.; resources, T.G.Ü. and F.P.Ç.; data curation, T.G.Ü. and F.P.Ç.; writing—original draft preparation, T.G.Ü. and F.P.Ç.; writing—review and editing, T.G.Ü. and F.P.Ç.; visualization, T.G.Ü.; supervision, F.P.Ç.; project administration, T.G.Ü. and F.P.Ç.; funding acquisition, T.G.Ü. and F.P.Ç. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Acknowledgments
This study is based on a doctoral thesis completed at Ankara University, Türkiye.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Corzo-Martínez, M.; Corzo, N.; Villamiel, M. Biological properties of onions and garlic. Trends Food Sci. Technol. 2007, 18, 609–625. [Google Scholar] [CrossRef]
- Ekşi, G.; Özkan, A.M.G.; Koyuncu, M. Garlic and onions: An eastern tale. J. Ethnopharmacol. 2020, 253, 112675. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Tung, Y.C.; Pan, M.H.; Su, N.W.; Lai, Y.J.; Cheng, K.C. Black garlic: A critical review of its production, bioactivity, and application. JFDA 2017, 25, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Kang, D. Physicochemical properties, biological activity, health benefits, and general limitations of aged black garlic: A review. Molecules 2017, 22, 919. [Google Scholar] [CrossRef]
- Qiu, Z.; Lu, X.; Li, N.; Zhang, M.; Qiao, X. Characterization of garlic endophytes isolated from the black garlic processing. MicrobiologyOpen 2018, 7, e00547. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, N.; Lu, X.; Zheng, Z.; Zhang, M.; Qiao, X. Characterization of microbial community structure and metabolic potential using Illumina MiSeq platform during the black garlic processing. Food Res. Int. 2018, 106, 428–438. [Google Scholar] [CrossRef]
- Yang, P.; Song, H.; Wang, L.; Jing, H. Characterization of key aroma-active compounds in black garlic by sensory-directed flavor analysis. J. Agric. Food Chem. 2019, 67, 7926–7934. [Google Scholar] [CrossRef]
- Cavagnaro, P.F.; Camargo, A.; Galmarini, C.R.; Simon, P.W. Effect of cooking on garlic (Allium sativum L.) antiplatelet activity and thiosulfinates content. J. Agric. Food Chem. 2007, 55, 1280–1288. [Google Scholar] [CrossRef]
- Cavagnaro, P.F.; Sance, M.M.; Galmarini, C.R. Effect of heating on onion (Allium cepa L.) antiplatelet activity and pungency sensory perception. Food Sci. Technol. Int. 2007, 13, 447–453. [Google Scholar] [CrossRef]
- Hansen, E.A.; Folts, J.D.; Goldman, I.L. Steam-cooking rapidly destroys and reverses onion-induced antiplatelet activity. Nutr. J. 2012, 11, 76. [Google Scholar] [CrossRef]
- Lu, X.; Wang, J.; Al-Qadiri, H.M.; Ross, C.F.; Powers, J.R.; Tang, J.; Rasco, B.A. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 2011, 129, 637–644. [Google Scholar] [CrossRef]
- Sharma, K.; Ko, E.Y.; Assefa, A.D.; Ha, S.; Nile, S.H.; Lee, E.T.; Park, S.W. Temperature-dependent studies on the total phenolics, flavonoids, antioxidant activities, and sugar content in six onion varieties. JFDA 2015, 23, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Toledano-Medina, M.A.; Pérez-Aparicio, J.; Moreno-Rojas, R.; Merinas-Amo, T. Evolution of some physicochemical and antioxidant properties of black garlic whole bulbs and peeled cloves. Food Chem. 2016, 199, 135–139. [Google Scholar] [CrossRef]
- Zhang, Z.; Lei, M.; Liu, R.; Gao, Y.; Xu, M.; Zhang, M. Evaluation of alliin, saccharide contents and antioxidant activities of black garlic during thermal processing. J. Food Biochem. 2015, 39, 39–47. [Google Scholar] [CrossRef]
- Chang, T.C.; Jang, H.D. Optimization of aging time for improved antioxidant activity and bacteriostatic capacity of fresh and black garlic. Appl. Sci. 2021, 11, 2377. [Google Scholar] [CrossRef]
- Najman, K.; Sadowska, A.; Hallmann, E. Influence of thermal processing on the bioactive, antioxidant, and physicochemical properties of conventional and organic agriculture black garlic (Allium sativum L.). Appl. Sci. 2020, 10, 8638. [Google Scholar] [CrossRef]
- Toledano Medina, M.Á.; Merinas-Amo, T.; Fernández-Bedmar, Z.; Font, R.; Del Río-Celestino, M.; Pérez-Aparicio, J.; Moreno-Rojas, R. Physicochemical characterization and biological activities of black and white garlic: In vivo and in vitro assays. Foods 2019, 8, 220. [Google Scholar] [CrossRef]
- Moreno-Ortega, A.; Pereira-Caro, G.; Ordóñez, J.L.; Muñoz-Redondo, J.M.; Moreno-Rojas, R.; Pérez-Aparicio, J.; Moreno-Rojas, J.M. Changes in the antioxidant activity and metabolite profile of three onion varieties during the elaboration of ‘black onion’. Food Chem. 2020, 311, 125958. [Google Scholar] [CrossRef]
- Cheng, A.; Chen, X.; Jin, Q.; Wang, W.; Shi, J.; Liu, Y. Comparison of phenolic content and antioxidant capacity of red and yellow onions. Czech J. Food Sci. 2013, 31, 501–508. [Google Scholar] [CrossRef]
- Soto, V.C.; Gonzalez, R.E.; Sance, M.M.; Galmarini, C.R. Organosulfur and phenolic content of garlic (Allium sativum L.) and onion (Allium cepa L.) and its relationship with antioxidant activity. VII Int. Symp. Edible Alliaceae 2015, 1143, 277–290. [Google Scholar] [CrossRef]
- Bouhenni, H.; Doukani, K.; Hanganu, D.; Olah, N.K.; Şekeroğlu, N.; Gezici, S. Analysis of bioactive compounds and antioxidant activities of cultivated garlic (Allium sativum L.) and red onion (Allium cepa L.) in Algeria. JAEFS 2021, 5, 550–560. [Google Scholar] [CrossRef]
- Gorinstein, S.; Leontowicz, H.; Leontowicz, M.; Namiesnik, J.; Najman, K.; Drzewiecki, J.; Trakhtenberg, S. Comparison of the main bioactive compounds and antioxidant activities in garlic and white and red onions after treatment protocols. J. Agric. Food Chem. 2008, 56, 4418–4426. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.S.; Cha, H.S.; Lee, Y.S. Physicochemical and antioxidant properties of black garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Lee, H.J.; Yoon, D.K.; Ji, D.S.; Kim, J.H.; Lee, C.H. Antioxidant and antimicrobial activities of fresh garlic and aged garlic by-products extracted with different solvents. Food Sci. Biotechnol. 2018, 27, 219–225. [Google Scholar] [CrossRef]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition analysis and antioxidant properties of black garlic extract. JFDA 2017, 25, 340–349. [Google Scholar] [CrossRef]
- Kandemirli, F.; İçli, N.; Bakır, T.K.; Nazlı, B.; Aydın, S. The investigation of the effect of freezing pretreatment on properties of black garlic produced from Kastamonu garlic. Food Health 2020, 6, 1–8. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; Supapvanich, S.; Kaewthong, P.; Takeungwongtrakul, S. Impact of steaming pretreatment process on characteristics and antioxidant activities of black garlic (Allium sativum L.). JFST 2021, 58, 1869–1876. [Google Scholar] [CrossRef]
- Kim, J.S.; Kang, O.J.; Gweon, O.C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods. 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Saplonţai-Pop, A.; Moţ, A.; Moldovan, M.; Oprean, R.; Silaghi-Dumitrescu, R.; Orășan, O.; Ionescu, C. Testing antiplatelet and antioxidant activity of the extract of seven varieties of Allium cepa L. Open Life Sci. 2015, 10, 89–98. [Google Scholar] [CrossRef]
- Benkeblia, N. Free-radical scavenging capacity and antioxidant properties of some selected onions (Allium cepa L.) and garlic (Allium sativum L.) extracts. Braz. Arch. Biol. Technol. 2005, 48, 753–759. [Google Scholar] [CrossRef]
- Kim, J.H.; Nam, S.H.; Rico, C.W.; Kang, M.Y. A comparative study on the antioxidative and anti-allergic activities of fresh and aged black garlic extracts. IJFST 2012, 47, 1176–1182. [Google Scholar] [CrossRef]
- Lee, Y.M.; Gweon, O.C.; Seo, Y.J.; Im, J.; Kang, M.J.; Kim, M.J.; Kim, J.I. Antioxidant effect of garlic and aged black garlic in animal model of type 2 diabetes mellitus. Nutr. Res. Pract. 2009, 3, 156–161. [Google Scholar] [CrossRef]
- Sato, E.; Kohno, M.; Hamano, H.; Niwano, Y. Increased anti-oxidative potency of garlic by spontaneous short-term fermentation. Plant Foods Hum. Nutr. 2006, 61, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Casas, L.; Lage-Yusty, M.; López-Hernández, J. Changes in the aromatic profile, sugars, and bioactive compounds when purple garlic is transformed into black garlic. J. Agric. Food Chem. 2017, 65, 10804–10811. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.E.; Cho, S.Y.; Won, Y.D.; Lee, S.H.; Park, H.J. A comparative study of the different analytical methods for analysis of S-allyl cysteine in black garlic by HPLC. LWT Food Sci. Technol. 2012, 46, 532–535. [Google Scholar] [CrossRef]
- Kim, J.S. Antioxidant activity of various soluble melanoidins isolated from black garlic after different thermal processing steps. Prev. Nutr. Food Sci. 2020, 25, 301. [Google Scholar] [CrossRef]
- Nakagawa, K.; Maeda, H.; Yamaya, Y.; Tonosaki, Y. Maillard reaction intermediates and related phytochemicals in black garlic determined by EPR and HPLC analyses. Molecules 2020, 25, 4578. [Google Scholar] [CrossRef]
- Wu, J.; Jin, Y.; Zhang, M. Evaluation on the physicochemical and digestive properties of melanoidin from black garlic and their antioxidant activities in vitro. Food Chem. 2021, 340, 127934. [Google Scholar] [CrossRef]
- Hwang, I.G.; Kim, H.Y.; Woo, K.S.; Lee, J.; Jeong, H.S. Biological activities of Maillard reaction products (MRPs) in a sugar–amino acid model system. Food Chem. 2011, 126, 221–227. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).