Adding Ethanol to the Batch and Continuous Transplantation Co-Culture of Maize Straw Fermented by Rumen Fluid for the Production of Caproic Acid
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate and Rumen Fluid
2.2. Sequential Batch Culture
2.3. Sampling and Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Effects of Ethanol on Continuous Subcultured Culture of Maize Straw with Rumen Liquid
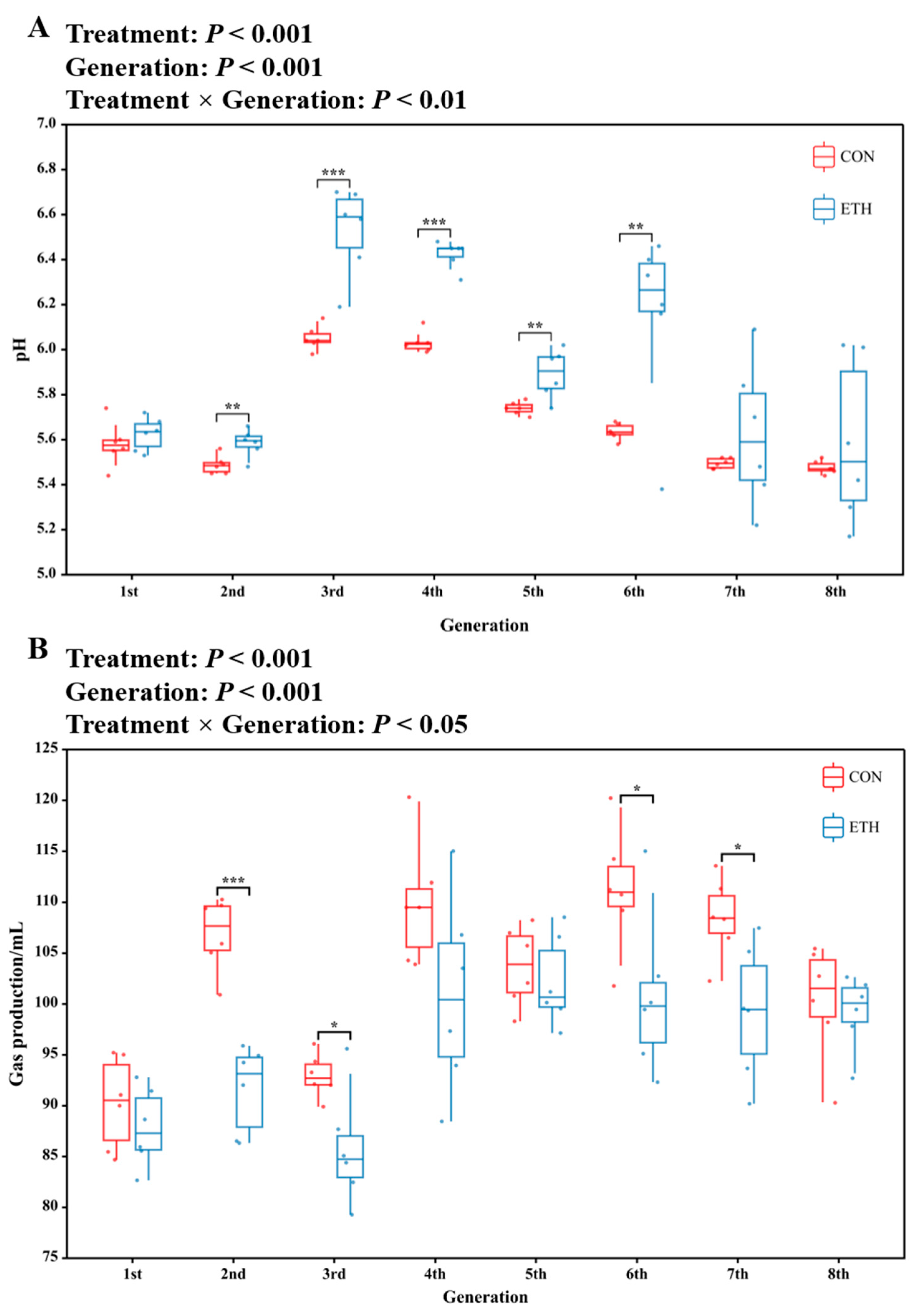
3.1.1. pH and Gas Production
3.1.2. Ethanol Promotes the Production of Caproic Acid and Chain Extension
3.2. Microbial Community Analysis
3.2.1. Alpha and Beta Diversity Analysis
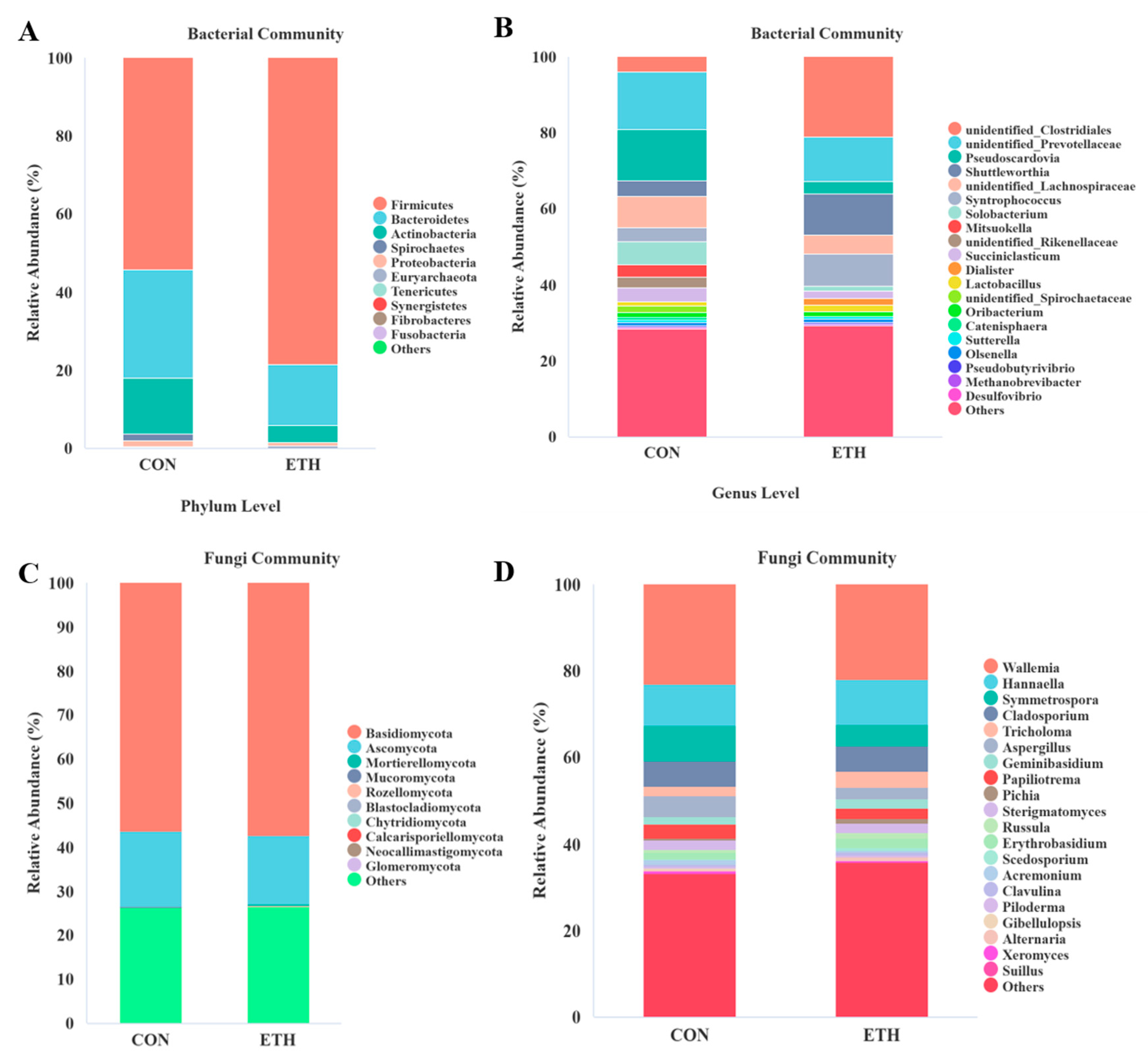
3.2.2. Community Structure of Bacteria and Fungi
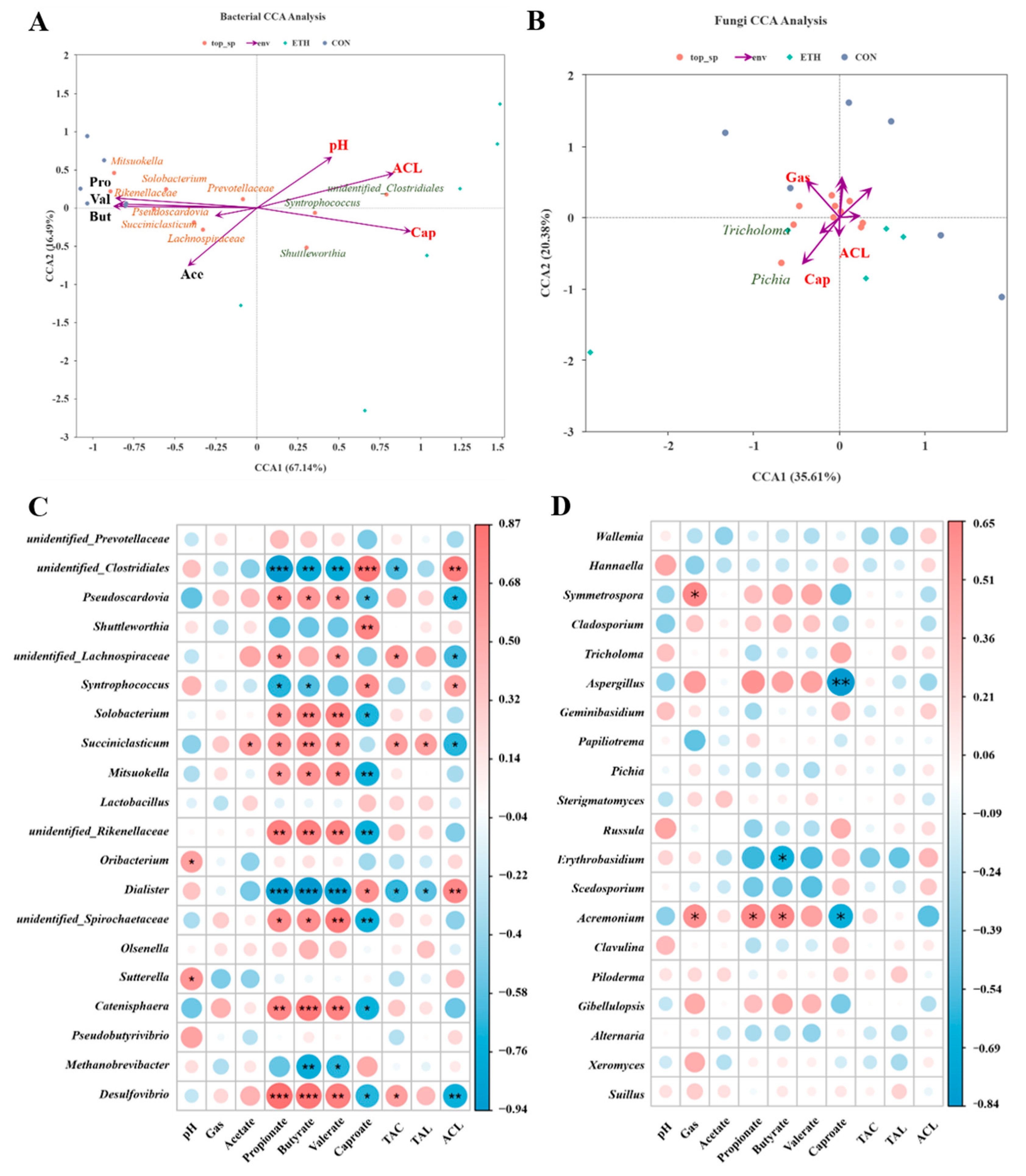
3.2.3. Correlation Between Microorganisms and Fatty Acid Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Phylum | Treatments | p-Value | |
|---|---|---|---|
| CON 1 | ETH 2 | ||
| Bacterial | |||
| Firmicutes | 54.11 ± 2.42 | 78.58 ± 5.01 | 0.001 |
| Bacteroidetes | 27.89 ± 1.99 | 15.58 ± 3.21 | 0.009 |
| Actinobacteria | 14.35 ± 1.40 | 4.31 ± 2.27 | 0.004 |
| Spirochaetes | 1.72 ± 0.26 | 0.08 ± 0.03 | 0.001 |
| Proteobacteria | 1.39 ± 0.18 | 0.82 ± 0.20 | 0.061 |
| Fungi | |||
| Basidiomycota | 56.39 ± 1.56 | 57.34 ± 1.47 | 0.673 |
| Ascomycota | 16.97 ± 1.02 | 15.59 ± 1.51 | 0.454 |
| Phylum | Genus | Treatments | p-Value | |
|---|---|---|---|---|
| CON 1 | ETH 2 | |||
| Bacterial | ||||
| Firmicutes | unidentified_Clostridiales | 3.91 ± 0.58 | 20.97 ± 3.75 | 0.006 |
| Shuttleworthia | 4.09 ± 0.62 | 10.7 ± 1.89 | 0.016 | |
| unidentified_Lachnospiraceae | 8.27 ± 1.21 | 5.00 ± 2.29 | 0.237 | |
| Syntrophococcus | 3.74 ± 0.50 | 8.42 ± 1.75 | 0.043 | |
| Solobacterium | 5.98 ± 0.60 | 1.44 ± 0.29 | <0.001 | |
| Mitsuokella | 3.26 ± 0.79 | 0.03 ± 0.01 | 0.010 | |
| Succiniclasticum | 3.60 ± 0.33 | 1.84 ± 0.60 | 0.027 | |
| Dialister | 0.02 ± 0.00 | 1.77 ± 0.69 | 0.053 | |
| Lactobacillus | 1.20 ± 0.23 | 1.75 ± 0.36 | 0.226 | |
| Oribacterium | 1.36 ± 0.19 | 1.16 ± 0.25 | 0.547 | |
| Bacteroidetes | unidentified_Prevotellaceae | 15.29 ± 1.34 | 11.69 ± 2.5 | 0.233 |
| unidentified_Rikenellaceae | 2.88 ± 0.80 | 0.02 ± 0.01 | 0.016 | |
| Actinobacteria | Pseudoscardovia | 13.36 ± 1.42 | 3.41 ± 2.24 | 0.004 |
| Spirochaetes | unidentified_Spirochaetaceae | 1.69 ± 0.26 | 0.08 ± 0.03 | 0.001 |
| Fungi | ||||
| Basidiomycota | Wallemia | 23.00 ± 4.00 | 22.00 ± 2.22 | 0.841 |
| Hannaella | 9.63 ± 0.95 | 10.42 ± 1.73 | 0.682 | |
| Symmetrospora | 8.24 ± 1.27 | 4.93 ± 0.22 | 0.044 | |
| Tricholoma | 2.11 ± 0.85 | 3.78 ± 1.2 | 0.274 | |
| Geminibasidium | 1.75 ± 0.82 | 2.08 ± 0.56 | 0.762 | |
| Papiliotrema | 3.11 ± 0.34 | 2.52 ± 0.71 | 0.452 | |
| Sterigmatomyces | 2.18 ± 0.37 | 2.04 ± 0.43 | 0.810 | |
| Erythrobasidium | 1.48 ± 0.41 | 2.18 ± 0.20 | 0.184 | |
| Russula | 0.64 ± 0.29 | 1.37 ± 0.57 | 0.259 | |
| Ascomycota | Cladosporium | 5.84 ± 0.46 | 5.82 ± 1.32 | 0.984 |
| Aspergillus | 4.83 ± 0.23 | 2.58 ± 0.49 | 0.002 | |
| Acremonium | 1.13 ± 0.14 | 0.47 ± 0.13 | 0.008 | |
| Pichia | 0.51 ± 0.15 | 1.03 ± 0.64 | 0.403 | |
References
- Langsdorf, A.; Volkmar, M.; Holtmann, D.; Ulber, R. Material Utilization of Green Waste: A Review on Potential Valorization Methods. Bioresour. Bioprocess. 2021, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.Z.; Liu, Y.P.; Li, X.J.; Wang, K.S.; Yuan, H.R. Improving Biodegradability and Biogas Production of Corn Stover through Sodium Hydroxide Solid State Pretreatment. Energy Fuels 2008, 22, 2761–2766. [Google Scholar] [CrossRef]
- Ajayi-Banji, A.A.; Rahman, S.; Cihacek, L.; Nahar, N. Comparison of the Reactor Performance of Alkaline-Pretreated Corn Stover Co-Digested with Dairy Manure Under Solid-State. Waste Biomass Valor. 2020, 11, 5211–5222. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, X.; Yuan, W.; Wang, Y.; Zhou, W.; Wang, G.; Liu, Y. Fed-Batch Enzymatic Hydrolysis of Alkaline Organosolv-Pretreated Corn Stover Facilitating High Concentrations and Yields of Fermentable Sugars for Microbial Lipid Production. Biotechnol. Biofuels 2020, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Hernández, J.E.; Loera, O.; Méndez-Hernández, E.M.; Herrera, E.; Arce-Cervantes, O.; Soto-Cruz, N.Ó. Fungal Pretreatment of Corn Stover by Fomes Sp. EUM1: Simultaneous Production of Readily Hydrolysable Biomass and Useful Biocatalysts. Waste Biomass Valor. 2019, 10, 2637–2650. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Cai, D.; Zhao, G. Co-Generation of Ethanol and l-Lactic Acid from Corn Stalk under a Hybrid Process. Biotechnol. Biofuels 2018, 11, 331. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-J.; Park, G.W.; Kang, J.; Kim, Y.-C.; Chang, H.N. Volatile Fatty Acid Production from Lignocellulosic Biomass by Lime Pretreatment and Its Applications to Industrial Biotechnology. Biotechnol. Bioprocess Eng. 2013, 18, 1163–1168. [Google Scholar] [CrossRef]
- Tu, W.; Zhang, D.; Wang, H. Polyhydroxyalkanoates (PHA) Production from Fermented Thermal-Hydrolyzed Sludge by Mixed Microbial Cultures: The Link between Phosphorus and PHA Yields. Waste Manag. 2019, 96, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Loh, K.-C.; Kuroki, A.; Dai, Y.; Tong, Y.W. Microbial Biodiesel Production from Industrial Organic Wastes by Oleaginous Microorganisms: Current Status and Prospects. J. Hazard. Mater. 2021, 402, 123543. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.J.; Candry, P.; Basadre, T.; Khor, W.C.; Roume, H.; Hernandez-Sanabria, E.; Coma, M.; Rabaey, K. Electrolytic Extraction Drives Volatile Fatty Acid Chain Elongation through Lactic Acid and Replaces Chemical pH Control in Thin Stillage Fermentation. Biotechnol. Biofuels 2015, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Weimer, P.J.; Nerdahl, M.; Brandl, D.J. Production of Medium-Chain Volatile Fatty Acids by Mixed Ruminal Microorganisms Is Enhanced by Ethanol in Co-Culture with Clostridium Kluyveri. Bioresour. Technol. 2015, 175, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Bayané, A.; Guiot, S.R. Animal Digestive Strategies versus Anaerobic Digestion Bioprocesses for Biogas Production from Lignocellulosic Biomass. Rev. Environ. Sci. Biotechnol. 2011, 10, 43–62. [Google Scholar] [CrossRef]
- Noel, S.J.; Attwood, G.T.; Rakonjac, J.; Moon, C.D.; Waghorn, G.C.; Janssen, P.H. Seasonal Changes in the Digesta-Adherent Rumen Bacterial Communities of Dairy Cattle Grazing Pasture. PLoS ONE 2017, 12, e0173819. [Google Scholar] [CrossRef] [PubMed]
- Wei, S. The Application of Biotechnology on the Enhancing of Biogas Production from Lignocellulosic Waste. Appl. Microbiol. Biotechnol. 2016, 100, 9821–9836. [Google Scholar] [CrossRef] [PubMed]
- Nerdahl, M.A.; Weimer, P.J. Redox Mediators Modify End Product Distribution in Biomass Fermentations by Mixed Ruminal Microbes In Vitro. AMB Expr. 2015, 5, 44. [Google Scholar] [CrossRef]
- Cavalcante, W.d.A.; Leitão, R.C.; Gehring, T.A.; Angenent, L.T.; Santaella, S.T. Anaerobic Fermentation for N-Caproic Acid Production: A Review. Process Biochem. 2017, 54, 106–119. [Google Scholar] [CrossRef]
- Shi, X.; Wu, L.; Wei, W.; Ni, B.-J. Insights into the Microbiomes for Medium-Chain Carboxylic Acids Production from Biowastes through Chain Elongation. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3787–3812. [Google Scholar] [CrossRef]
- Spirito, C.M.; Richter, H.; Rabaey, K.; Stams, A.J.; Angenent, L.T. Chain Elongation in Anaerobic Reactor Microbiomes to Recover Resources from Waste. Curr. Opin. Biotechnol. 2014, 27, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Yu, Z.; Wu, W.; Fu, P.; Xia, C.; Shiung Lam, S.; Boer Emilia, D.; Wang, Q.; Gao, M. Effects of Ethanol Addition on Caproic Acid Production and Rumen Microorganism Community Structure from Straw Fermentation. Fuel 2022, 327, 125142. [Google Scholar] [CrossRef]
- Lin, M.; Dai, X.; Weimer, P.J. Shifts in Fermentation End Products and Bacterial Community Composition in Long-Term, Sequentially Transferred in Vitro Ruminal Enrichment Cultures Fed Switchgrass with and without Ethanol as a Co-Substrate. Bioresour. Technol. 2019, 285, 121324. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Feng, L.; Cheng, Z.; Wang, K. Effect of Ethanol or Lactic Acid on Volatile Fatty Acid Profile and Microbial Community in Short-Term Sequentially Transfers by Ruminal Fermented with Wheat Straw In Vitro. Process Biochem. 2021, 102, 369–375. [Google Scholar] [CrossRef]
- Menke, K.H.; Raab, L.; Salewski, A.; Steingass, H.; Fritz, D.; Schneider, W. The Estimation of the Digestibility and Metabolizable Energy Content of Ruminant Feedingstuffs from the Gas Production When They Are Incubated with Rumen Liquor In Vitro. J. Agric. Sci. 1979, 93, 217–222. [Google Scholar] [CrossRef]
- Lin, M.; Schaefer, D.M.; Zhao, G.Q.; Meng, Q.X. Effects of Nitrate Adaptation by Rumen Inocula Donors and Substrate Fiber Proportion on In Vitro Nitrate Disappearance, Methanogenesis, and Rumen Fermentation Acid. Animal 2013, 7, 1099–1105. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cheng, Z.; Meng, Z.; Tan, D.; Datsomor, O.; Zhan, K.; Lin, M.; Zhao, G. Effects of Supplementation of Sodium Acetate on Rumen Fermentation and Microbiota in Postpartum Dairy Cows. Front. Microbiol. 2022, 13, 1053503. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree Visualization by One Table (tvBOT): A Web Application for Visualizing, Modifying and Annotating Phylogenetic Trees. Nucleic Acids Res. 2023, 51, W587–W592. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-S.; Strik, D.P.B.T.B.; Buisman, C.J.N.; Kroeze, C. Production of Caproic Acid from Mixed Organic Waste: An Environmental Life Cycle Perspective. Environ. Sci. Technol. 2017, 51, 7159–7168. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Bao, X.; Guo, W.; Wang, B.; Li, Y.; Luo, H.; Wang, H.; Ren, N. Medium Chain Carboxylic Acids Production from Waste Biomass: Current Advances and Perspectives. Biotechnol. Adv. 2019, 37, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Liu, Z.; Varrone, C.; Zhou, A.; He, Z.; Li, H.; Zhang, J.; Liu, W.; Yue, X. Elucidating the Microbial Ecological Mechanisms on the Electro-Fermentation of Caproate Production from Acetate via Ethanol-Driven Chain Elongation. Environ. Res. 2022, 203, 111875. [Google Scholar] [CrossRef] [PubMed]
- Suo, M.; Liu, L.; Fan, H.; Li, N.; Pan, H.; Hrynsphan, D.; Tatsiana, S.; Robles-Iglesias, R.; Wang, Z.; Chen, J. Advancements in Chain Elongation Technology: Transforming Lactic Acid into Caproic Acid for Sustainable Biochemical Production. Bioresour. Technol. 2025, 425, 132312. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ma, H.; Gao, M.; Qian, D.; Wang, Q.; Liu, L. Production of Caproate from Lactate by Chain Elongation under Electro-Fermentation: Dual Role of Exogenous Ethanol Electron Donor. Bioresour. Technol. 2025, 424, 132272. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Cheng, Z.; Zong, Y.; Shen, Y.; Jama, S.M.; Lin, M. Enrichment of Rumen Solid-Phase Bacteria for Production of Volatile Fatty Acids by Long-Term Subculturing In Vitro. Fermentation 2025, 11, 173. [Google Scholar] [CrossRef]
- Mickdam, E.; Khiaosa-ard, R.; Metzler-Zebeli, B.U.; Klevenhusen, F.; Chizzola, R.; Zebeli, Q. Rumen Microbial Abundance and Fermentation Profile during Severe Subacute Ruminal Acidosis and Its Modulation by Plant Derived Alkaloids In Vitro. Anaerobe 2016, 39, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zhu, S.; Xue, M.-Y.; Chen, H.; Xu, J.; Song, M.; Tang, Y.; Liu, X.; Tao, Y.; Zhang, T.; et al. Single-Cell Transcriptomics across 2,534 Microbial Species Reveals Functional Heterogeneity in the Rumen Microbiome. Nat. Microbiol. 2024, 9, 1884–1898. [Google Scholar] [CrossRef] [PubMed]
- Mizrahi, I.; Wallace, R.J.; Moraïs, S. The Rumen Microbiome: Balancing Food Security and Environmental Impacts. Nat. Rev. Microbiol. 2021, 19, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, S.N.; Thakur, D. Actinobacteria. In Beneficial Microbes in Agro-Ecology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 443–476. ISBN 978-0-12-823414-3. [Google Scholar]
- Li, J.; Rui, J.; Pei, Z.; Sun, X.; Zhang, S.; Yan, Z.; Wang, Y.; Liu, X.; Zheng, T.; Li, X. Straw- and Slurry-Associated Prokaryotic Communities Differ during Co-Fermentation of Straw and Swine Manure. Appl. Microbiol. Biotechnol. 2014, 98, 4771–4780. [Google Scholar] [CrossRef] [PubMed]
- Chwialkowska, J.; Duber, A.; Zagrodnik, R.; Walkiewicz, F.; Łężyk, M.; Oleskowicz-Popiel, P. Caproic Acid Production from Acid Whey via Open Culture Fermentation—Evaluation of the Role of Electron Donors and Downstream Processing. Bioresour. Technol. 2019, 279, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Mandra Harahap, B.; Ahring, B.K. Caproic Acid Production from Short-Chained Carboxylic Acids Produced by Arrested Anaerobic Digestion of Green Waste: Development and Optimization of a Mixed Caproic Acid Producing Culture. Bioresour. Technol. 2024, 414, 131573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, H.; Wu, P.; Li, J.; Zhang, J. Clostridium Kluyveri Enhances Caproate Production by Synergistically Cooperating with Acetogens in Mixed Microbial Community of Electro-Fermentation System. Bioresour. Technol. 2023, 369, 128436. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, Y. Syntrophococcus. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 1–5. ISBN 978-1-118-96060-8. [Google Scholar]


| Items | Treatments | p-Value | |
|---|---|---|---|
| CON | ETH | ||
| Bacterial 1 | |||
| Ace | 320.79 ± 7.17 | 242.43 ± 8.17 | <0.001 |
| Chao1 | 322.50 ± 7.94 | 249.25 ± 18.11 | 0.004 |
| Shannon | 5.49 ± 0.08 | 4.57 ± 0.08 | <0.001 |
| Simpson | 0.9564 ± 0.003 | 0.9141 ± 0.007 | <0.001 |
| Fungi 2 | |||
| Ace | 986.72 ± 42.64 | 1111.47 ± 47.04 | 0.081 |
| Chao1 | 986.28 ± 42.30 | 1121.72 ± 52.29 | 0.072 |
| Shannon | 6.60 ± 0.12 | 7.13 ± 0.19 | 0.035 |
| Simpson | 0.9686 ± 0.003 | 0.9739 ± 0.004 | 0.314 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Meng, Z.; Shen, Y.; Liu, W.; Liu, L.; Zhao, G.; Wang, L.; Lin, M. Adding Ethanol to the Batch and Continuous Transplantation Co-Culture of Maize Straw Fermented by Rumen Fluid for the Production of Caproic Acid. Fermentation 2025, 11, 413. https://doi.org/10.3390/fermentation11070413
Cheng Z, Meng Z, Shen Y, Liu W, Liu L, Zhao G, Wang L, Lin M. Adding Ethanol to the Batch and Continuous Transplantation Co-Culture of Maize Straw Fermented by Rumen Fluid for the Production of Caproic Acid. Fermentation. 2025; 11(7):413. https://doi.org/10.3390/fermentation11070413
Chicago/Turabian StyleCheng, Zhiqiang, Zitong Meng, Yue Shen, Wengboyang Liu, Li Liu, Guoqi Zhao, Lin Wang, and Miao Lin. 2025. "Adding Ethanol to the Batch and Continuous Transplantation Co-Culture of Maize Straw Fermented by Rumen Fluid for the Production of Caproic Acid" Fermentation 11, no. 7: 413. https://doi.org/10.3390/fermentation11070413
APA StyleCheng, Z., Meng, Z., Shen, Y., Liu, W., Liu, L., Zhao, G., Wang, L., & Lin, M. (2025). Adding Ethanol to the Batch and Continuous Transplantation Co-Culture of Maize Straw Fermented by Rumen Fluid for the Production of Caproic Acid. Fermentation, 11(7), 413. https://doi.org/10.3390/fermentation11070413






