Valorization of Spent Coffee Grounds as a Substrate for Fungal Laccase Production and Biosorbents for Textile Dye Decolorization
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Spent Coffee Grounds (SCG)
2.3. Laccase Production
2.4. Determination of Laccase Activity
2.5. Full-Factorial Design
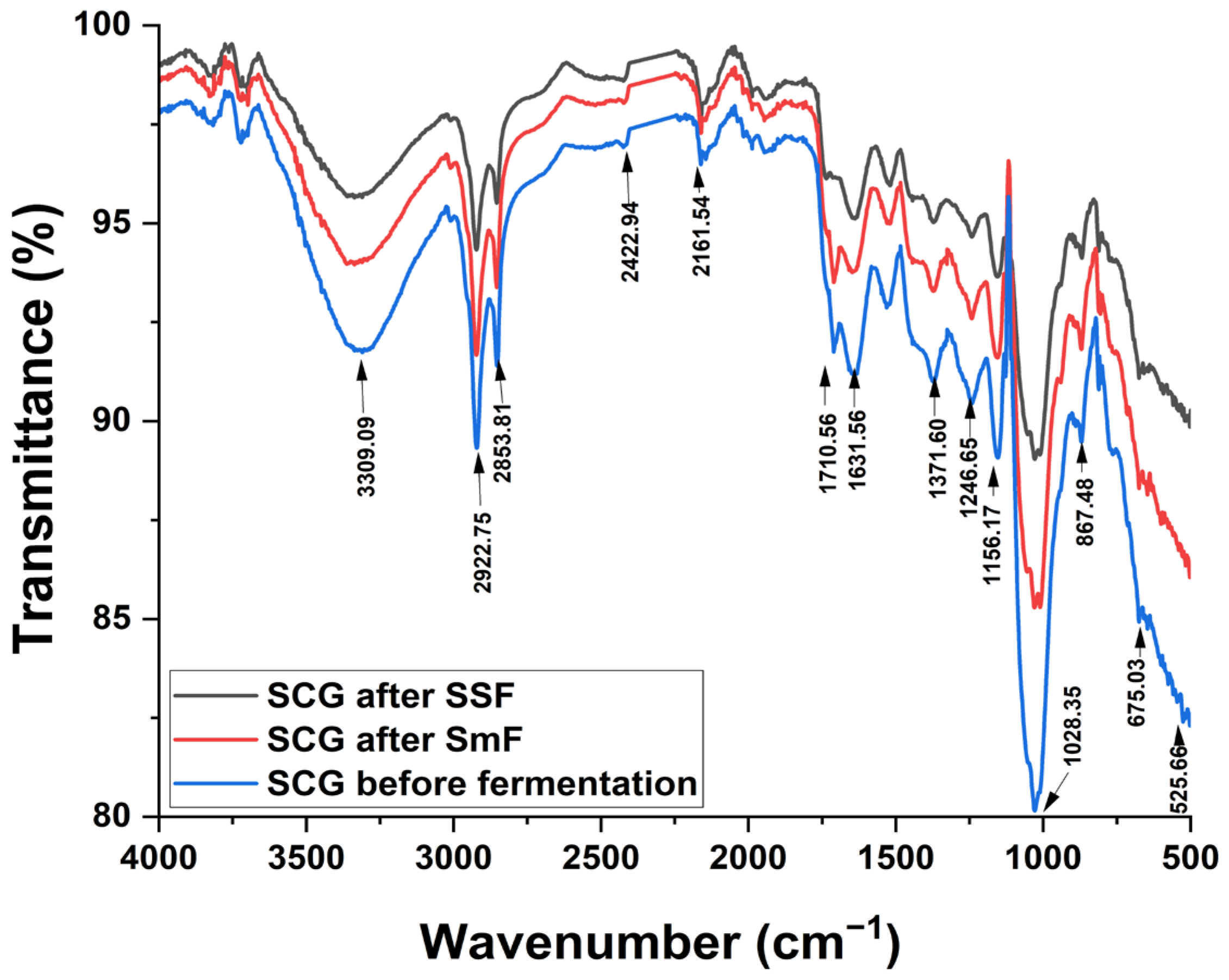
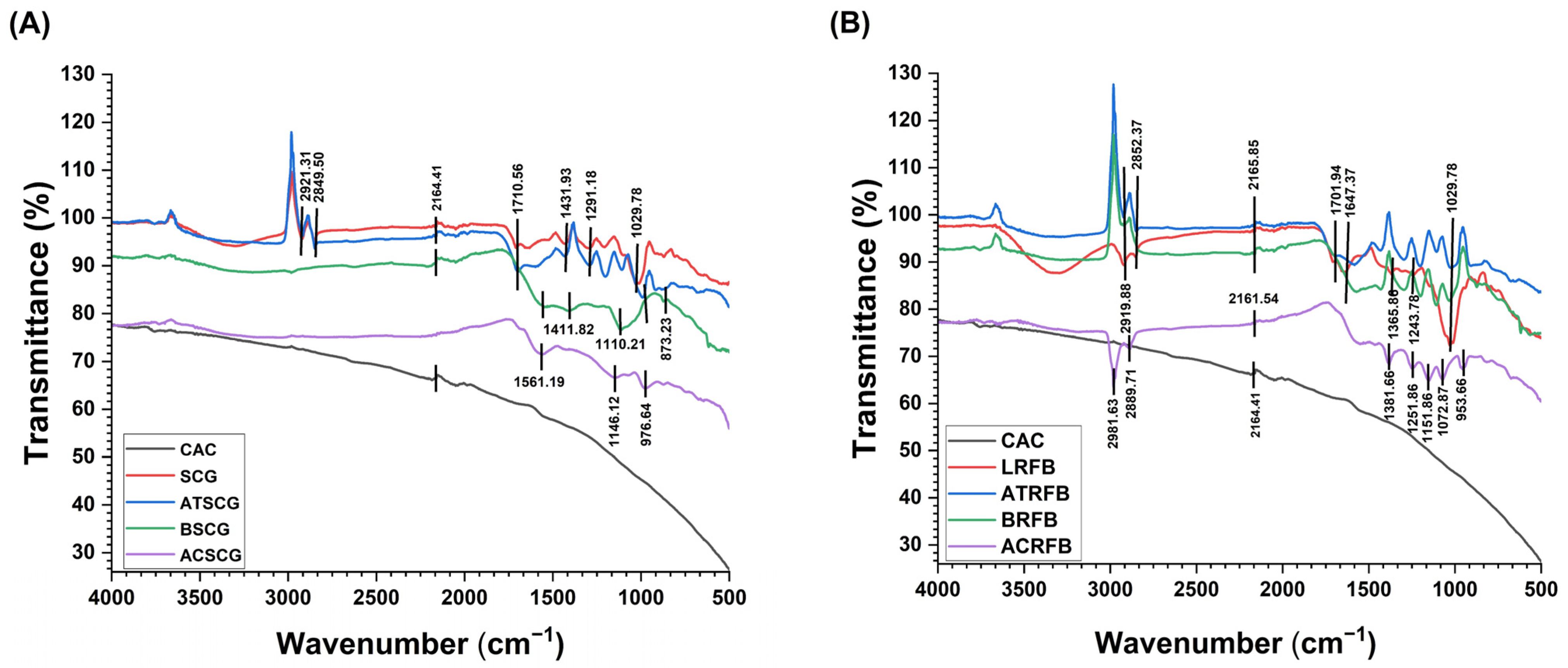
2.6. Identification of Structural Changes in SCG
2.7. Determination of Protein Concentration and Phenolic Compound Content in SCG
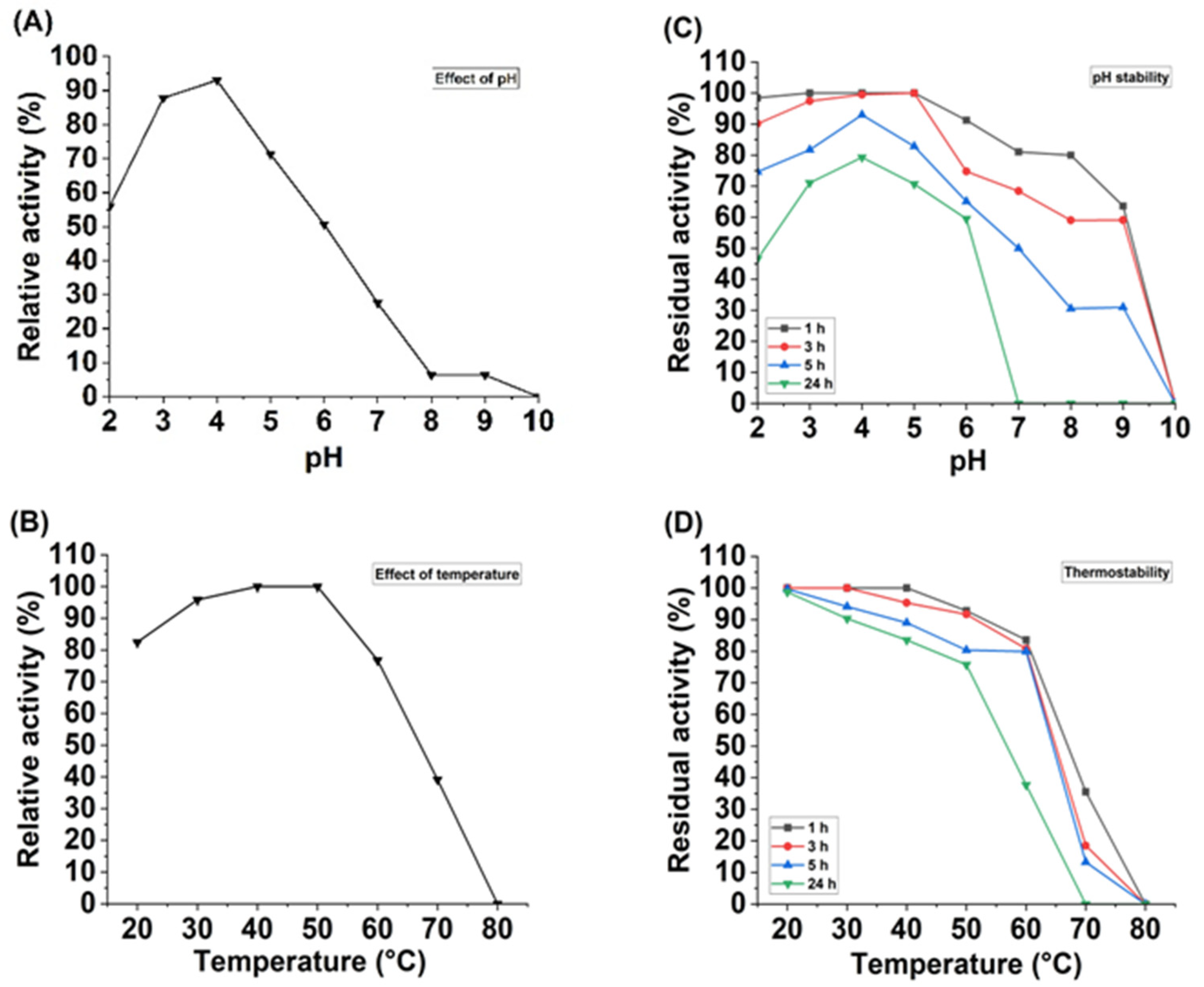
2.8. Effect of pH and Temperature on Laccase Activity and Stability
2.9. Preparation of Biosorbents
- Activated carbons: Activated carbon from SCG (ACSCG) and activated carbon from RFB (RFBAC) were prepared following the methodology of Prabawati et al. [18] with modifications. Initially, both SCG and RFB were separately impregnated with phosphoric acid (H3PO4) at a concentration of 85% in a 1:1 (w/v) ratio. The impregnated samples were dried in a forced-air circulating oven (Marconi, model MA035, Brazil) at 105 °C ± 0.2 for 24 h. After drying, the materials were carbonized in a muffle furnace at 400 °C ± 0.2 for 2 h.
- Acid treatment: The same methodology of impregnation with H3PO4 and subsequent drying was used, as described above. The residual acid was removed by repeated washes with distilled water, followed by drying at 65 °C ± 0.2 until complete dehydration. Acid-treated SCG (ATSCG) and acid-treated RFB (ATRFB) were then obtained.
- Biochars: The biochars of SCG (BSCG) and RFB (BRFB) were prepared by carbonizing the samples at 400 °C ± 0.2 for 2 h.
- Lyophilization: Lyophilized RFB (LRFB) was obtained after freezing at −40 °C for 24 h and lyophilization for 72 h in an Advantage Plus EL-85 lyophilizer (SP Scientific, Warminster, PA, USA). The lyophilized material was stored in desiccators and later kept in hermetically sealed containers until further use.
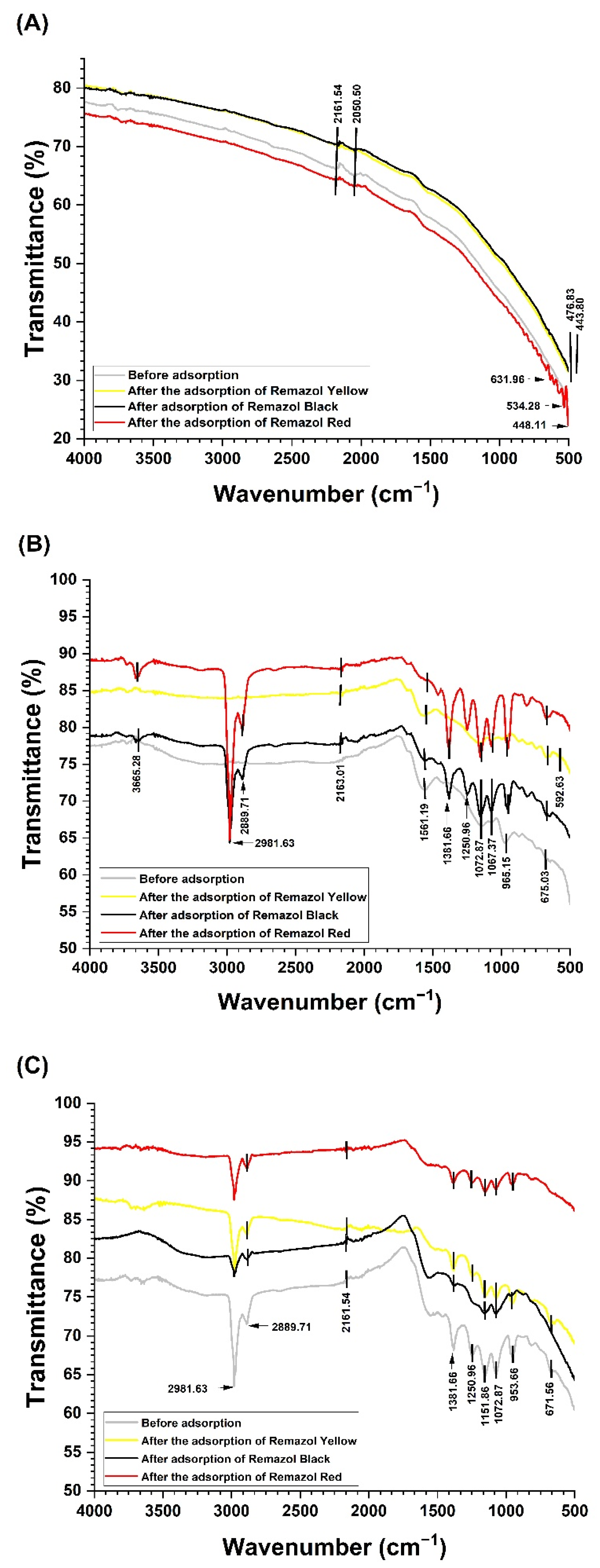
2.10. Adsorption Assay
3. Results and Discussion
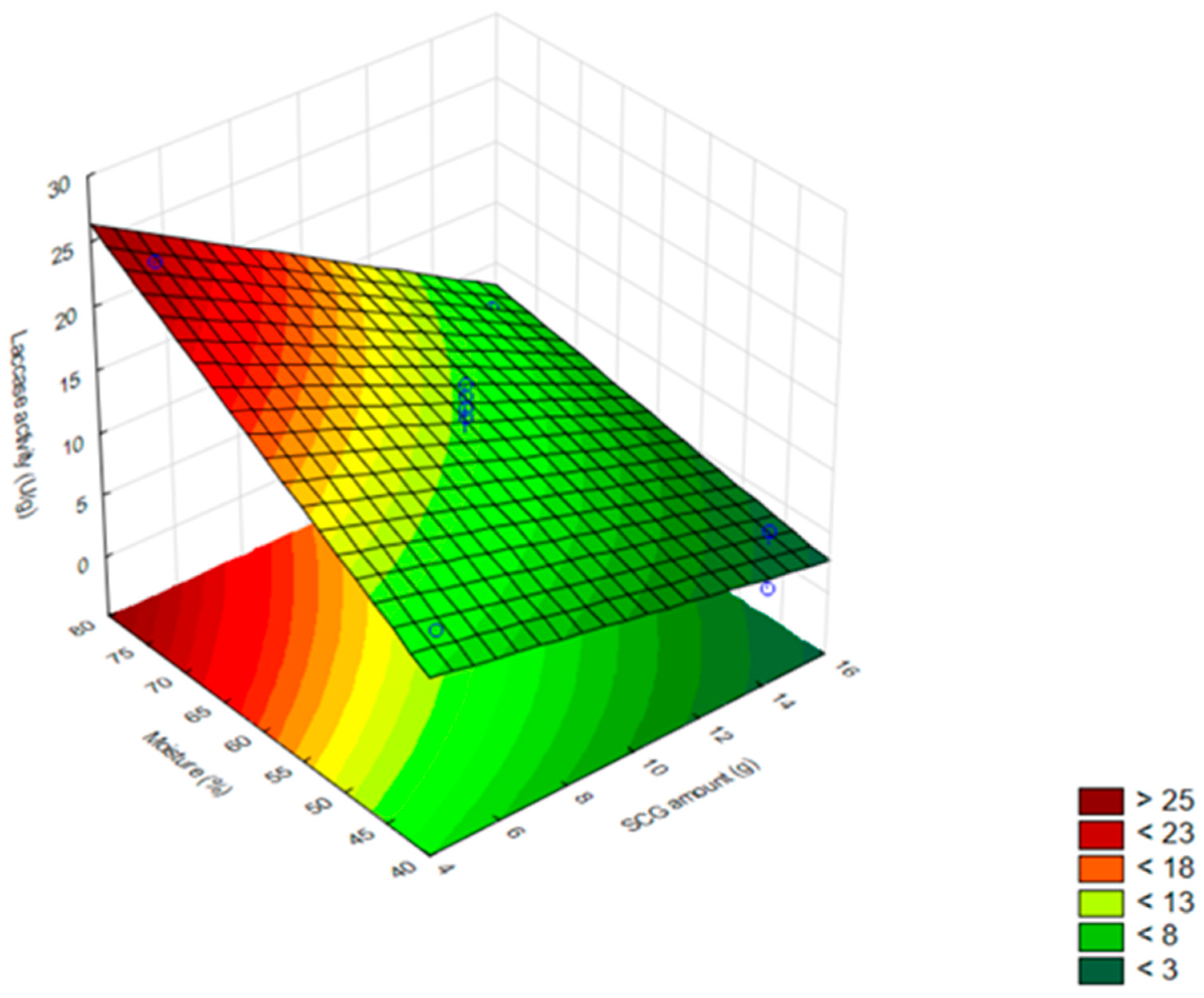
3.1. Production of Fungal Laccase Using SCG
3.2. Production of Laccase by L. crinitus in SSF
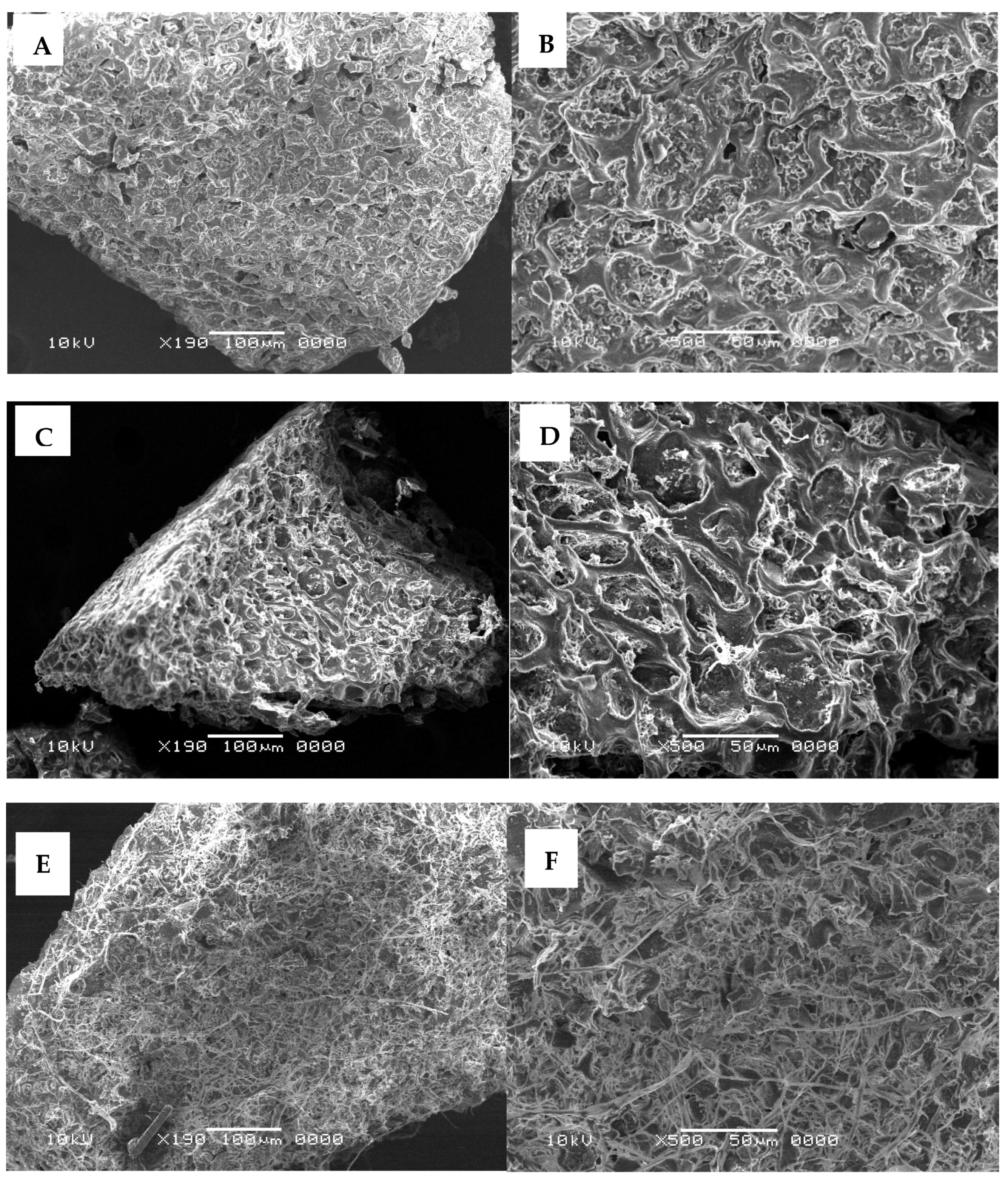
3.3. Identification of Structural Changes in SCG After Fermentation
3.4. Total Protein and Phenolic Compound Content of SCG
3.5. Effect of pH and Temperature on Laccase Activity and Stability
3.6. Characterization of the Biosorbents
3.7. Adsorption Efficiency of Dyes Remazol by Biosorbents
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ogbu, C.C.; Okey, S. Agro-Industrial Waste Management: The Circular and Bioeconomic Perspective. In Agricultural Waste—New Insights; Ahmad, F., Sultan, M., Eds.; IntechOpen: London, UK, 2023; Available online: https://www.intechopen.com/chapters/85597 (accessed on 23 August 2024).
- Senthilkumar, K.; Naveen, K.M.; Chitra, D.V.; Saravanan, K.; Easwaramoorthi, S. Agro-Industrial Waste Valorization to Energy and Value Added Products for Environmental Sustainability. In Biomass Valorization to Bioenergy; Energy, Environment, and Sustainability; Praveen, K.R., Bharathiraja, B., Kataki, R., Moholkar, V.S., Eds.; Springer: Singapore, 2020; pp. 1–9. Available online: http://link.springer.com/10.1007/978-981-15-0410-5_1 (accessed on 16 August 2024).
- Voltolini, G.B.; Carvalho, G.R.; Andrade, V.T.; Ferreira, A.D.; Raposo, F.V.; Carvalho, J.P.F.; Vilela, D.J.M.; da Silva, C.A.; Costa, J.D.O.; Abreu, G.B.; et al. Agronomic Performance of Irrigated and Rainfed Arabica Coffee Cultivars in the Cerrado Mineiro Region. Agronomy 2025, 15, 222. [Google Scholar] [CrossRef]
- Bairwan, R.D.; Khalil, H.P.S.A.; Nuryawan, A.; Ahmad, M.I.; Kassim, M.H.M.; Ahmad, A. Enhancing biopolymer materials with coffee waste-derived reinforcements. Polym. Eng. Sci. 2025, 65, 455–477. [Google Scholar] [CrossRef]
- Afriliana, A.; Hidayat, E.; Mitoma, Y.; Masuda, T.; Harada, H. Studies on Composting Spent Coffee Grounds by Aspergillus sp. and Penicillium sp.; in Aerobic Static Batch Temperature Control. J. Agric. Chem. Environ. 2021, 10, 91–112. [Google Scholar]
- Ravindran, R.; Williams, G.A.; Jaiswal, A.K. Spent Coffee Waste as a Potential Media Component for Xylanase Production and Potential Application in Juice Enrichment. Foods 2019, 8, 585. [Google Scholar] [CrossRef]
- Cardoso, B.K.; Linde, G.A.; Colauto, N.B.; Do Valle, J.S. Panus strigellus laccase decolorizes anthraquinone, azo, and triphenylmethane dyes. Biocatal. Agric. Biotechnol. 2018, 16, 558–563. [Google Scholar] [CrossRef]
- Latif, W.; Ciniglia, C.; Iovinella, M.; Shafiq, M.; Papa, S. Role of White Rot Fungi in Industrial Wastewater Treatment: A Review. Appl. Sci. 2023, 13, 8318. [Google Scholar] [CrossRef]
- Khan, M.F. Recent Advances in Microbial Enzyme Applications for Sustainable Textile Processing and Waste Management. Science 2025, 7, 46. [Google Scholar] [CrossRef]
- Bhoyar, S.S.; Chaudhari, A.U.; Desai, M.A.; Latpate, R.V.; Sartale, S.D.; Kodam, K.M. Wheat bran as an efficient agro-process waste for enhanced yellow laccase production by Lentinus tigrinus SSB_W2 and its application in anthraquinone dye degradation. 3 Biotech 2024, 14, 33. [Google Scholar] [CrossRef]
- Sukarta, I.N.; Suyasa, I.W.B.; Mahardika, I.G.; Suprihatin, I.E.; Sastrawidana, I.D.K. Innovation of Remazol yellow FG dye adsorption using biochar from coffee fruit shell waste. J. Ecol. Eng. 2024, 26, 273–285. [Google Scholar] [CrossRef]
- Tiwari, H.; Tripathi, P.; Sonwani, R.K.; Singh, R.S. A synergistic approach combining Adsorption and Biodegradation for effective treatment of Acid Blue 113 dye by Klebsiella grimontii entrapped Graphene Oxide-Calcium Alginate Hydrogel Beads. Bioresour. Technol. 2023, 387, 129614. [Google Scholar] [CrossRef]
- Chamoli, S.; Singh, A.; Kapoor, R.K.; Singh, S.; Singh, R.K.; Saini, J.K. Purification and characterization of laccase from Ganoderma lucidum and its application in decolorization of malachite green dye. Bioresour. Technol. Rep. 2023, 21, 101368. [Google Scholar]
- Ranimol, G.; Venugopal, T.; Gopalakrishnan, S.; Sunkar, S. Production of laccase from Trichoderma harzianum and its application in dye decolourisation. Biocatal. Agric. Biotechnol. 2018, 16, 400–404. [Google Scholar]
- Alnsour, L.; Issa, R.; Awwad, S.; Albals, D.; Al-Momani, I. Quantification of Total Phenols and Antioxidants in Coffee Samples of Different Origins and Evaluation of the Effect of Degree of Roasting on Their Levels. Molecules 2022, 27, 1591. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Park, B.H. Phenolic compound extraction from spent coffee grounds for antioxidant recovery. Korean J. Chem. Eng. 2019, 36, 186–190. [Google Scholar] [CrossRef]
- Gutiérrez-Antón, M.; Santiago-Hernández, A.; Rodríguez-Mendoza, J.; Cano-Ramírez, C.; Bustos-Jaimes, I.; Aguilar-Osorio, G.; Campos, J.E.; Hidalgo-Lara, M.E. Improvement of Laccase Production by Thielavia terrestris Co3Bag1. Enhancing the Bio-Catalytic Performance of the Native Thermophilic TtLacA via Immobilization in Copper Alginate Gel Beads. J. Fungi 2023, 9, 308. [Google Scholar] [CrossRef]
- Prabawati, S.Y.; Widiakongko, P.D.; Taqwim, M.A. Activated Charcoal from Coffee Dregs Waste as an Alternative Biosorbent of Cu(II) and Ag(I). Indones. J. Chem. 2023, 23, 1120. [Google Scholar] [CrossRef]
- Tigrine, Z.; Benhabiles, O.; Merabti, L.; Chekir, N.; Mellal, M.; Aoudj, S.; Abdeslam, N.A.; Tassalit, D.; Lebouachera, S.E.I.; Drouiche, N. Sustainable Activated Carbon from Agricultural Waste: A Study on Adsorption Efficiency for Humic Acid and Methyl Orange Dyes. Sustainability 2024, 16, 9308. [Google Scholar] [CrossRef]
- Wang, F.; Xu, L.; Zhao, L.; Ding, Z.; Ma, H.; Terry, N. Fungal Laccase Production from Lignocellulosic Agricultural Wastes by Solid-State Fermentation: A Review. Microorganisms 2019, 7, 665. [Google Scholar] [CrossRef]
- Hasan, S.; Anwar, Z.; Khalid, W.; Afzal, F.; Zafar, M.; Ali, U.; Refai, M.Y.; Afifi, M.; Al-Farga, A.; Aljobair, M.O. Laccase Production from Local Biomass Using Solid State Fermentation. Fermentation 2023, 9, 179. [Google Scholar] [CrossRef]
- Marín, M.; Artola, A.; Sánchez, A. Optimization of Down-Stream for Cellulases Produced Under Solid-State Fermentation of Coffee Husk. Waste Biomass Valor. 2019, 10, 2761–2772. [Google Scholar] [CrossRef]
- Filipe, D.; Fernandes, H.; Castro, C.; Peres, H.; Oliva-Teles, A.; Belo, I.; Salgado, J.M. Improved lignocellulolytic enzyme production and antioxidant extraction using solid-state fermentation of olive pomace mixed with winery waste. Biofuels Bioprod. Bioref. 2020, 14, 78–91. [Google Scholar] [CrossRef]
- Leite, P.; Sousa, D.; Fernandes, H.; Ferreira, M.; Costa, A.R.; Filipe, D.; Gonçalves, M.; Peres, H.; Belo, I.; Salgado, J.M. Recent advances in production of lignocellulolytic enzymes by solid-state fermentation of agro-industrial wastes. Curr. Opin. Green Sustain. Chem. 2021, 27, 100407. [Google Scholar] [CrossRef]
- Mollea, C.; Bosco, F. Solid-State Fermentation of Brewery Spent Grains to Enhance Biomolecule Extraction. Separations 2025, 12, 58. [Google Scholar] [CrossRef]
- Singh, J.; Mandal, A.; Nandabalan, Y.K. Synergistic effect on lignolytic enzyme production through co-culturing of white rot fungi during solid state fermentation of agricultural residues. Bull. Natl. Res. Cent. 2025, 49, 3. [Google Scholar] [CrossRef]
- An, Q.; Li, C.S.; Yuan, Y.N.; Dou, X.Y.; Wang, Y.H.; Guo, S.; Chen, Z.; Pen, A.; Zhang, T.; Yang, Q.; et al. Utilization of agroindustrial wastes for the production of laccase by Pleurotus eryngii Han 1787 and Lentinus edodes Han 1788. BioResources 2022, 18, 570–583. [Google Scholar] [CrossRef]
- Xu, L.; Sun, K.; Wang, F.; Zhao, L.; Hu, J.; Ma, H.; Dingc, Z. Laccase production by Trametes versicolor in solid-state fermentation using tea residues as substrate and its application in dye decolorization. J. Environ. Manag. 2020, 270, 110904. [Google Scholar] [CrossRef]
- Akpinar, M.; Urek, R.O. Decolorization and degradation potential of enhanced lignocellulolytic enzymes production by Pleurotus eryngii using cherry waste from industry. Biotech App Biochem. 2020, 67, 760–773. [Google Scholar] [CrossRef]
- Tišma, M.; Jurić, A.; Bucić-Kojić, A.; Panjičko, M.; Planinić, M. Biovalorization of brewers’ spent grain for the production of laccase and polyphenols: Biovalorization of brewers’ spent grain. J. Inst. Brew. 2018, 124, 182–186. [Google Scholar] [CrossRef]
- Ergun, S.O.; Urek, R.O. Production of ligninolytic enzymes by solid state fermentation using Pleurotus ostreatus. Ann. Agrar. Sci. 2017, 15, 273–277. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Ávila, S.; Hornung, P.S.; Júnior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar]
- Hao, L.; Wang, P.; Valiyaveettil, S. Successive extraction of As(V), Cu(II) and P(V) ions from water using spent coffee powder as renewable bioadsorbents. Sci. Rep. 2017, 7, 42881. [Google Scholar] [CrossRef] [PubMed]
- Okur, I.; Soyler, B.; Sezer, P.; Oztop, M.H.; Alpas, H. Improving the Recovery of Phenolic Compounds from Spent Coffee Grounds (SCG) by Environmentally Friendly Extraction Techniques. Molecules 2021, 26, 613. [Google Scholar] [CrossRef] [PubMed]
- Atabani, A.E.; Shobana, S.; Mohammed, M.N.; Uğuz, G.; Kumar, G.; Arvindnarayan, S.; Aslam, M.; Al-Muhtaseb, A.H. Integrated valorization of waste cooking oil and spent coffee grounds for biodiesel production: Blending with higher alcohols, FT–IR, TGA, DSC and NMR characterizations. Fuel 2019, 244, 419–430. [Google Scholar] [CrossRef]
- Akcay, C.; Ceylan, F.; Arslan, R. Production of oyster mushroom (Pleurotus ostreatus) from some waste lignocellulosic materials and FTIR characterization of structural changes. Sci. Rep. 2023, 13, 12897. [Google Scholar] [CrossRef]
- Lun, L.W.; Gunny, A.A.N.; Kasim, F.H.; Arbain, D. Fourier Transform Infrared Spectroscopy (FTIR) Analysis of Paddy Straw Pulp Treated Using Deep Eutectic Solvent. In Proceedings of the International Conference on Advanced Material Engineering and Technology 2016, Kaohsiung City, Taiwan, 8–9 December 2016; AIP Publishing: Melville, NY, USA, 2017. Available online: https://pubs.aip.org/aip/acp/article/583564 (accessed on 21 August 2024).
- Mulana, F.; Ismail, T.A.; Hafdiansyah, M.F. Activation and characterization of waste coffee grounds as bio-sorbent. IOP Conf. Ser. Mater. Sci. Eng. 2018, 334, 012029. [Google Scholar]
- Roychand, R.; Kilmartin-Lynch, S.; Saberian, M.; Li, J.; Zhang, G.; Li, C.Q. Transforming spent coffee grounds into a valuable resource for the enhancement of concrete strength. J. Clean. Prod. 2023, 419, 138205. [Google Scholar] [CrossRef]
- Ganash, M.; Abdelghany, T.M.; Abboud, M.A.; Alawlaqi, M.; Qanash, H.; Amin, B.H. Lignocellulolytic Activity of Pleurotus ostreatus under Solid State Fermentation Using Silage, Stover, and Cobs of Maize. BioResources 2021, 16, 3797–3807. [Google Scholar] [CrossRef]
- Andrade, C.; Perestrelo, R.; Câmara, J.S. Bioactive Compounds and Antioxidant Activity from Spent Coffee Grounds as a Powerful Approach for Its Valorization. Molecules 2022, 27, 7504. [Google Scholar] [CrossRef]
- Abbasi-Parizad, P.; De Nisi, P.; Scaglia, B.; Scarafoni, A.; Pilu, S.; Adani, F. Recovery of phenolic compounds from agro-industrial by-products: Evaluating antiradical activities and immunomodulatory properties. Food Bioprod. Process. 2021, 127, 338–348. [Google Scholar] [CrossRef]
- Ozuna, C.; Mulík, S.; Valdez-Rodríguez, B.; Abraham-Juárez, M.D.R.; Fernández-López, C.L. The effect of organic farming on total phenols, total flavonoids, brown compounds and antioxidant activity of spent coffee grounds from Mexico. Biol. Agric. Hortic. 2020, 36, 107–118. [Google Scholar] [CrossRef]
- Nagai, M.; Sato, T.; Watanabe, H.; Saito, K.; Kawata, M.; Enei, H. Purification and characterization of an extracellular laccase from the edible mushroom Lentinula edodes, and decolorization of chemically different dyes. Appl. Microbiol. Biotechnol. 2002, 60, 327–335. [Google Scholar] [PubMed]
- Pacheco, S.M.V.; Soares, C.H.L. Immobilization and Characterization of Laccase and Its Use in the Biodegradation of Paper Mill Effluent. Química Nova 2014, 37, 209–214. Available online: https://quimicanova.sbq.org.br/audiencia_pdf.asp?aid2=3&nomeArquivo=v37n2a03.pdf (accessed on 17 August 2024). [CrossRef]
- González-González, P.; Gómez-Manzo, S.; Tomasini, A.; Pérez, J.L.M.Y.; Nieto, E.G.; Anaya-Hernández, A.; Ortiz, E.O.; Rodriguez, R.A.C.; Marcial-Quino, J.; Montiel-González, A.M. Laccase Production from Agrocybe pediades: Purification and Functional Characterization of a Consistent Laccase Isoenzyme in Liquid Culture. Microorganisms 2023, 11, 568. [Google Scholar] [CrossRef] [PubMed]
- Cen, Q.; Wu, X.; Cao, L.; Lu, Y.; Lu, X.; Chen, J.; Fu, G.; Liu, Y.; Ruan, R. Green production of a yellow laccase by Coriolopsis gallica for phenolic pollutants removal. AMB Expr. 2022, 12, 96. [Google Scholar] [CrossRef]
- Umar, A.; Ahmed, S. Optimization, purification and characterization of laccase from Ganoderma leucocontextum along with its phylogenetic relationship. Sci. Rep. 2022, 12, 2416. [Google Scholar] [CrossRef]
- Si, J.; Wu, Y.; Ma, H.F.; Cao, Y.J.; Sun, Y.F.; Cui, B.K. Selection of a pH-and temperature-stable laccase from Ganoderma australe and its application for bioremediation of textile dyes. J. Environ. Manag. 2021, 299, 113619. [Google Scholar] [CrossRef]
- Campbell, R.; Xiao, B.; Mangwandi, C. Production of activated carbon from spent coffee grounds (SCG) for removal of hexavalent chromium from synthetic wastewater solutions. J. Environ. Manag. 2024, 366, 121682. [Google Scholar] [CrossRef]
- Loulidi, I.; Jabri, M.; Amar, A.; Kali, A.; Alrashdi, A.A.; Hadey, C.; Ouchabi, M.; Abdullah, P.S.; Lgaz, H.; Cho, Y.; et al. Comparative Study on Adsorption of Crystal Violet and Chromium (VI) by Activated Carbon Derived from Spent Coffee Grounds. Appl. Sci. 2023, 13, 985. [Google Scholar] [CrossRef]
- Aouay, F.; Attia, A.; Dammak, L.; Amar, B.R.; Deratani, A. Activated Carbon Prepared from Waste Coffee Grounds: Characterization and Adsorption Properties of Dyes. Materials 2024, 17, 3078. [Google Scholar] [CrossRef]
- Chouchane, H.; Najjari, A.; Neifar, M.; Cherif, H.; Askri, R.; Naili, F.; Ouzari, H.I.; Cherif, A. Unravelling the characteristics of a heteropolysaccharide–protein from an Haloarchaeal strain with flocculation effectiveness in heavy metals and dyes removal. Environ. Technol. 2020, 41, 2180–2195. [Google Scholar] [CrossRef]
- Zuluaga, R.; Hoyos, C.G.; Velásquez-Cock, J.; Vélez-Acosta, L.; Valencia, P.I.; Torres, R.J.A.; Rojo, P.G. Exploring Spent Coffee Grounds: Comprehensive Morphological Analysis and Chemical Characterization for Potential Uses. Molecules 2024, 29, 5866. [Google Scholar] [CrossRef] [PubMed]
- Ismanto, A.; Sukmono, Y.; Hadibarata, T.; Yeow, P.K.; Indrayanti, E.; Ismunarti, D.H.; Handoyo, G. Removal of Remazol brilliant blue r and Remazol brilliant violet 5r dyes from aqueous solution by adsorption using coffee residue. Environ. Qual. Mgmt. 2024, 33, 47–57. [Google Scholar] [CrossRef]
- Kim, S.; Won, S.W.; Cho, C.W.; Yun, Y.S. Valorization of Escherichia coli waste biomass as a biosorbent for removing reactive dyes from aqueous solutions. Desalination Water Treat. 2016, 57, 20084–20090. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Rosine, G.M.L.; Kaur, S.; Hegde, K.; Brar, S.K.; Drogui, P.; Verma, M. Novel biomaterials from citric acid fermentation as biosorbents for removal of metals from waste chromated copper arsenate wood leachates. Int. Biodeterior. Biodegrad. 2017, 119, 147–154. [Google Scholar] [CrossRef]
- Lucaci, A.R.; Bulgariu, D.; Ahmad, I.; Lisă, G.; Mocanu, A.M.; Bulgariu, L. Potential Use of Biochar from Various Waste Biomass as Biosorbent in Co(II) Removal Processes. Water 2019, 11, 1565. [Google Scholar] [CrossRef]
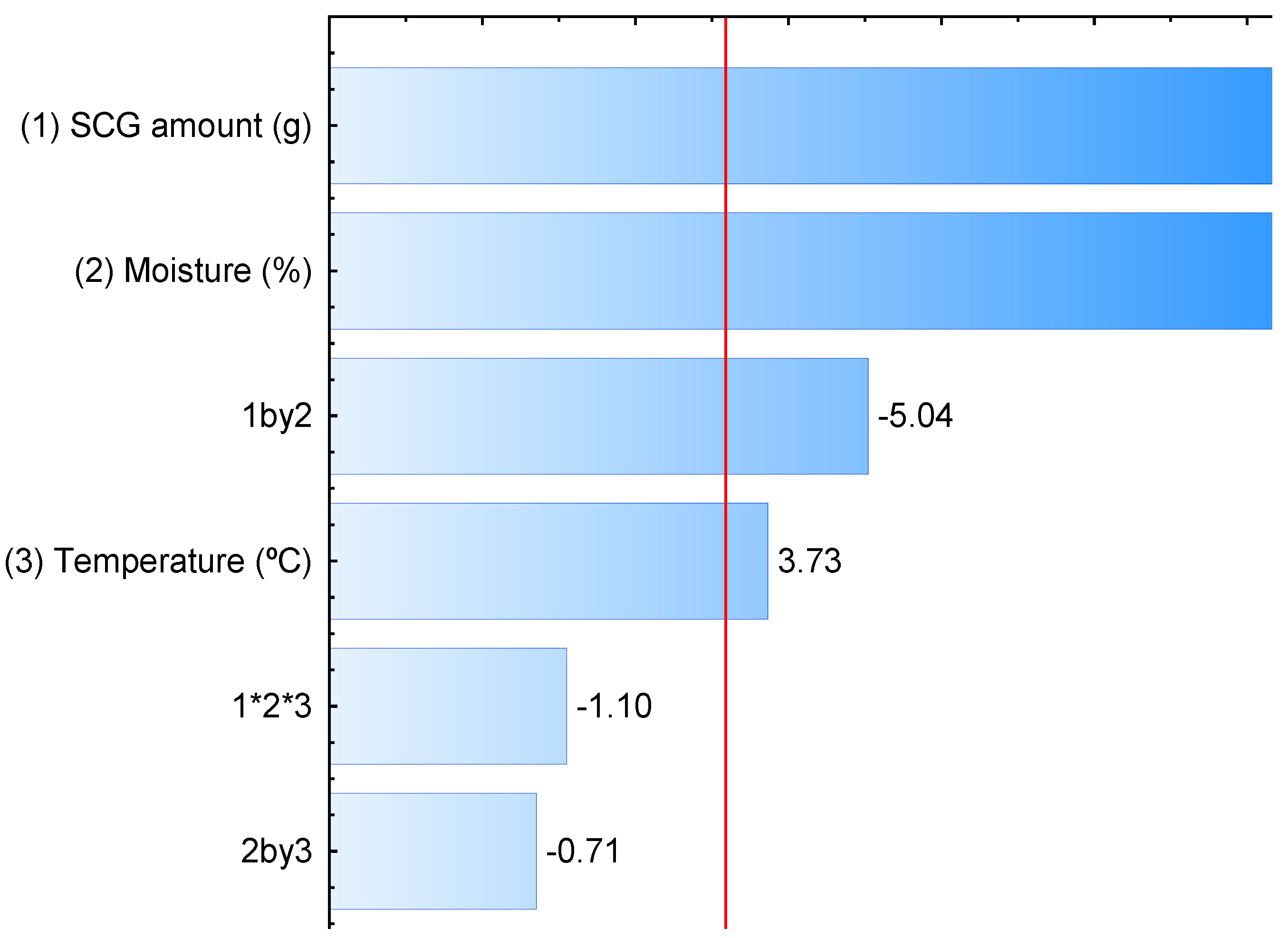
| Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| SCG amount (g) | 5 | 10 | 15 |
| Moisture (%) | 40 | 60 | 80 |
| Temperature (°C) | 26 | 28 | 30 |
| Fungi | Agro-Industrial Wastes | Fermentation Time (Days) | Laccase Activity (U/g) | References |
|---|---|---|---|---|
| L. crinitus UCP 1206 | Spent coffee grounds | 15 | 14.62 | Present study |
| Phanerochaete chrysosporium MUCL 19343 | Brewery spent grain | 7 | 0.008 | [25] |
| Trametes versicolor + P. chrysosporium | Cotton stalk Wheat straw Paddy straw | 15 | 8.10 6.75 5.55 | [26] |
| L. edodes Han 1788 | Cottonseed shell | 14 | 5.65 | [27] |
| Leaf of corncob | 11 | 4.46 | ||
| Corncob | 13 | 2.31 | ||
| T. versicolor | Tea residues | 7 | 6.40 | [28] |
| P. eryngii | Cherry waste | 15 | 0.04 | [29] |
| T. versicolor | Brewery spent grain | 7 | 0.78 | [30] |
| Pleurotus ostreatus | Potato peel waste | 17 | 1.60 | [31] |
| Assays | SCG Amount (g) | Moisture (%) | Temperature (°C) | Laccase Activity (U/g) |
|---|---|---|---|---|
| 1 | 5 | 40 | 26 | 7.38 |
| 2 | 15 | 40 | 26 | 0.16 |
| 3 | 5 | 80 | 26 | 20.64 |
| 4 | 15 | 80 | 26 | 7.05 |
| 5 | 5 | 40 | 30 | 9.88 |
| 6 | 15 | 40 | 30 | 4.83 |
| 7 | 5 | 80 | 30 | 23.78 |
| 8 | 15 | 80 | 30 | 8.80 |
| 9 | 10 | 60 | 28 | 14.41 |
| 10 | 10 | 60 | 28 | 12.73 |
| 11 | 10 | 60 | 28 | 15.35 |
| 12 | 10 | 60 | 28 | 13.44 |
| Biosorbents | Dye Adsorption (mg/L) | ||
|---|---|---|---|
| Remazol Yellow | Remazol Black | Remazol Red | |
| CAC | 98.17 ± 0.01 | 99.27 ± 0.01 | 96.94 ± 0.03 |
| SCG | 25.08 ± 0.01 | 19.24 ± 0.02 | 4.95 ± 0.00 |
| ACSCG | 98.78 ± 0.00 | 99.13 ± 0.01 | 98.34 ± 0.01 |
| ATSCG | 98.22 ± 0.00 | 43.48 ± 0.02 | 78.83 ± 0.00 |
| BSCG | 40.39 ± 0.04 | 49.68 ± 0.06 | 27.91 ± 0.02 |
| LRFB | 26.92 ± 0.02 | 14.34 ± 0.04 | 16.34 ± 0.03 |
| ACRFB | 99.02 ± 0.00 | 62.18 ± 0.05 | 68.01 ± 0.00 |
| ATRFB | 14.70 ± 0.00 | 0.00 ± 0.04 | 14.50± 0.00 |
| BRFB | 48.35 ± 0.05 | 62.18 ± 0.06 | 0.00 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
França, E.d.S.; de Souza, A.F.; Rodríguez, D.M.; de Paula, N.Z.; Neves, A.G.D.; Cardoso, K.B.B.; Campos-Takaki, G.M.d.; de Lima, M.A.B.; Porto, A.L.F. Valorization of Spent Coffee Grounds as a Substrate for Fungal Laccase Production and Biosorbents for Textile Dye Decolorization. Fermentation 2025, 11, 396. https://doi.org/10.3390/fermentation11070396
França EdS, de Souza AF, Rodríguez DM, de Paula NZ, Neves AGD, Cardoso KBB, Campos-Takaki GMd, de Lima MAB, Porto ALF. Valorization of Spent Coffee Grounds as a Substrate for Fungal Laccase Production and Biosorbents for Textile Dye Decolorization. Fermentation. 2025; 11(7):396. https://doi.org/10.3390/fermentation11070396
Chicago/Turabian StyleFrança, Eduardo da Silva, Adriana Ferreira de Souza, Dayana Montero Rodríguez, Nazareth Zimiani de Paula, Anna Gabrielly Duarte Neves, Kethylen Barbara Barbosa Cardoso, Galba Maria de Campos-Takaki, Marcos Antonio Barbosa de Lima, and Ana Lucia Figueiredo Porto. 2025. "Valorization of Spent Coffee Grounds as a Substrate for Fungal Laccase Production and Biosorbents for Textile Dye Decolorization" Fermentation 11, no. 7: 396. https://doi.org/10.3390/fermentation11070396
APA StyleFrança, E. d. S., de Souza, A. F., Rodríguez, D. M., de Paula, N. Z., Neves, A. G. D., Cardoso, K. B. B., Campos-Takaki, G. M. d., de Lima, M. A. B., & Porto, A. L. F. (2025). Valorization of Spent Coffee Grounds as a Substrate for Fungal Laccase Production and Biosorbents for Textile Dye Decolorization. Fermentation, 11(7), 396. https://doi.org/10.3390/fermentation11070396








