Pigments from Microorganisms: A Sustainable Alternative for Synthetic Food Coloring
Abstract
1. Introduction
2. Importance of Microbial Pigments
3. Classification and Some Important Food-Grade Pigments
3.1. Lycopene
3.2. β-Carotene
3.3. Canthaxanthin
3.4. Astaxanthin
3.5. Phycocyanin
3.6. Prodigiosin
3.7. Riboflavin
3.8. Violacein
3.9. Melanin
| Pigment | Source Organism(s) | Major Industrial Producer (Biotechnological Source) | Mol. Formula and Solubility | Application | Stability Profile (pH/Temp. Light) | Refs. |
|---|---|---|---|---|---|---|
| Astaxanthin (orange-pink-red) | Serratia marcescens, Halobacterium salinarium, Agrobacterium aurantiacum, Paracoccus carotinifaciens, Pseudoalteromonas rubra | Haematococcus pluvialis [GRAS GRN 294/580], Xanthophyllomyces dendrorhous [No GRAS], and engineered E. coli (introducing genes from H. pluvialis, like β-carotenoid hydroxylase and ketolase) [no GRAS] | C40H52O4 596.8 g/mol lipid soluble | Animal and fish food, food colorants, anti-aging, and memory improvement | pH 5–8, stable up to 70 °C, moderately light-sensitive | [102,103,104,105,106,107,108,109,110] |
| Phycocyanin (blue) | Pseudomonas aeruginosa, Aphanizomenon flos-aquae, Spirulina sp. | Spirulina platensis [GRAS GRN 424] | C33H38N4O6 water-soluble | Ice creams and sweets | pH 6.5–7.5, unstable > 45 °C, very light-sensitive | [111,112,113,114,115,116,117] |
| Prodigiosin (red) | Pseudoalteromonas rubra, Serratia marcescens | Recombinant E. coli (Sau3A fragments of S. marcescens DNA were introduced into E. coli K-12), Pseudomonas putida KT2440 | C20H25N3O 323.4 g/mol water-insoluble | Colorants in yogurt, milk, and carbonated beverages | pH 4–6, degrades > 60 °C, light-sensitive | [70,118,119,120,121,122] |
| Riboflavin (yellow) | Debaryomyces subglobosus, Ashbya gossypii, Clostridium acetobutylicum | Ashbya gossypii, Bacillus subtilis [GRAS under 21 CFR 184.1695] | C17H20N4O6 376.4 g/mol water-soluble | Food industries and dietary supplements | pH 5–7, stable up to 100 °C, extremely light-sensitive (photo-labile) | [78,123,124,125,126,127] |
| β-carotene (yellow) | Rhodotorula gracilis, Rhodotorula rubra, Blakeslea trispora | Yarrowia lipolytica [GRAS GRN632 and BSL1 status] | C40H56 536.9 g/mol water-insoluble | Vitamin A sources, food industries, and boosts immunity | pH 4–8, degrades > 60 °C, highly light-sensitive | [128,129,130,131,132] |
| Violacein (purple) | Chromobacterium violaceum, Janthinobacterium lividum, Pseudoalteromonas spp., Pseudoalteromonas tunicata | E. coli strain (B8/pTRPH1-pVio-VioE), Citrobacter freundii, Corynebacterium glutamicum, and Yarrowia lipolytica | C20H13N3O3 343.3 g/mol | Cosmetics, textiles, medicine, and food industries | pH 5–7, stable < 60 °C, light-sensitive | [84,124,133,134] |
| Melanin (black) | Burkholderia cenocepacia, Rubrivivax benzoatilyticus JA2 | Gliocephalotrichum simplex, MEL1 mutant of Aspergillus nidulans, Streptomyces kathirae strain SC-1 | C18H10N2O4 318.3 g/mol | Cosmetic creams, food industries, and anti-HIV activity | pH 2–10, highly heat-stable, light-stable | [135,136,137,138,139] |
| Lycopene (red) | Fusarium sp., Blakeslea trispora | Blakeslea trispora [no GRAS but approved in EU (2006/721/EC)] | C40H56 536.9 g/mol | Meat colorants | pH 4–7, degrades > 60 °C, highly light-sensitive | [140,141,142] |
| Canthaxanthin (orange-pink) | Halobacterium sp., Bradyrhizobium sp., Fusarium Sporotrichioides | Haematococcus pluvialis [GRAS GRN 294], genetically engineered Mucor circinelloides, and Saccharomyces cerevisiae [no GRAS] | C40H52O2 564.8 g/mol | Food colorant, salmon food and poultry feed | pH 4–9, stable up to 80 °C, relatively light-stable | [143,144,145] |
4. Economic Evaluation of Microbial Pigments
5. Industrial Considerations in the Use of Bacterial and Fungal Pigment Producers
6. Advances in Large-Scale Production and Genetic Engineering of Microbial Pigments
6.1. Industrial Production of Microbial Pigments
6.2. Genetically Engineered Microorganisms for Pigment Production
| Pigment | Microorganism | Genetic Engineering Strategy | Yield Before Modification | Reported Yield After Modification | Refs. |
|---|---|---|---|---|---|
| Astaxanthin | E. coli | Optimization of the astaxanthin biosynthetic pathway with multivariate modular methods | Not reported | Not reported | [198] |
| β-carotene | E. coli | Overexpression of dxs (1-deoxy-D-xylulose-5-phosphate synthase) and idi (isopentenyl diphosphate isomerase), CrtE (GGPP synthase), CrtB (phytoene synthase), and CrtI (phytoene desaturase) | ~20 mg/L | ~450 mg/L | [199] |
| Violacein | E. coli | Violacein biosynthesis genes’ heterologous expression | Not reported | Not reported | [200] |
| Astaxanthin | Y. lipolytica | Combination of HpCrtZ and HpCrtW from H. pluvialis | Not reported | 3.3 g/L or 41.3 mg/g DCW under fed-batch fer mentation conditions | [201] |
| β-carotene | Y. lipolytica | Optimized promoter gene pairs based on Golden Gate DNA assembly | 17 mg/L | 408 mg/L | [131,193] |
| Violacein | Y. lipolytica | Golden Gate Assembly method | Not reported | 70.04 mg/L | [202] |
| Astaxanthin | S. cerevisiae | Recombination of CrtW and CrtZ genes in vitro and in vivo | Not reported | 4.7 mg/g DCW | [203] |
| β-carotene | S. cerevisiae | Integration of CrtI, CrtE, and CrtYB (bifunctional phytoene synthase and lycopene cyclase) and additional copies of tHMG1 from X. dendrorhous using CRISPR-Cas9 | Not reported | 5.9 mg/g dry weight | [204] |
| Lycopene | S. cerevisiae | Assembly of IDI, CrtE, and CrtB | Not reported | 41.8 mg/g DCW | [205,206] |
| β-carotene | S. cerevisiae | Lipase expression and introduction of the β-carotene synthetic pathway from X. dendrorhous | Not reported | 772.8 mg/L | [207,208] |
6.3. Issues and Implications of Different Pigment-Producing Microorganisms
7. Applications in the Food Industry
7.1. Pigment-Producing Microorganisms and Applications in the Food Industry
7.2. Food Preservatives
7.3. Pharmacological Activities
7.4. Alternative Applications
8. Emerging Application: Microbial Pigment-Mediated Nanoparticle Synthesis
9. Challenges and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Mejia, E.G.; Zhang, Q.; Penta, K.; Eroglu, A.; Lila, M.A. The Colors of Health: Chemistry, Bioactivity, and Market Demand for Colorful Foods and Natural Food Sources of Colorants. Annu. Rev. Food Sci. Technol. 2020, 11, 145–182. [Google Scholar] [CrossRef]
- Poorniammal, R.; Prabhu, S.; Dufossé, L.; Kannan, J. Safety Evaluation of Fungal Pigments for Food Applications. J. Fungi 2021, 7, 692. [Google Scholar] [CrossRef]
- Barciela, P.; Perez-Vazquez, A.; Prieto, M.A. Azo Dyes in the Food Industry: Features, Classification, Toxicity, Alternatives, and Regulation. Food Chem. Toxicol. 2023, 178, 113935. [Google Scholar] [CrossRef] [PubMed]
- Okafor, S.N.; Obonga, W.; Ezeokonkwo, M.A.; Nurudeen, J.; Orovwigho, U.; Ahiabuike, J. Assessment of the Health Implications of Synthetic and Natural Food Colourants—A Critical Review. Pharm. Biosci. J. 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Stachová, I.; Lhotská, I.; Solich, P.; Šatínský, D. Determination of Green, Blue and Yellow Artificial Food Colorants and Their Abuse in Herb-Coloured Green Easter Beers on Tap. Food Addit. Contam. Part A 2016, 33, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Yang, H.-H.; Muhammad, S.; Khan, Z.I.; Yu, H.; Luqman, M.; Tofail, M.; Hussain, M.I.; Awan, M.U.F. Cross Talk between Synthetic Food Colors (Azo Dyes), Oral Flora, and Cardiovascular Disorders. Appl. Sci. 2022, 12, 7084. [Google Scholar] [CrossRef]
- Trasande, L.; Shaffer, R.M.; Sathyanarayana, S. Food Additives and Child Health. Pediatrics 2018, 142, e20181410. [Google Scholar] [CrossRef]
- Gupta, R.; Ranjan, S.; Yadav, A.; Verma, B.; Malhotra, K.; Madan, M.; Chopra, O.; Jain, S.; Gupta, S.; Joshi, A.; et al. Toxic Effects of Food Colorants Erythrosine and Tartrazine on Zebrafish Embryo Development. Curr. Res. Nutr. Food Sci. 2019, 7, 876–885. [Google Scholar] [CrossRef]
- Mpountoukas, P.; Pantazaki, A.; Kostareli, E.; Christodoulou, P.; Kareli, D.; Poliliou, S.; Mourelatos, C.; Lambropoulou, V.; Lialiaris, T. Cytogenetic Evaluation and DNA Interaction Studies of the Food Colorants Amaranth, Erythrosine and Tartrazine. Food Chem. Toxicol. 2010, 48, 2934–2944. [Google Scholar] [CrossRef]
- Kiki, M.J. Biopigments of Microbial Origin and Their Application in the Cosmetic Industry. Cosmetics 2023, 10, 47. [Google Scholar] [CrossRef]
- Sharma, N.; Shekhar, P.; Kumar, V.; Kaur, H.; Jayasena, V. Microbial Pigments: Sources, Current Status, Future Challenges in Cosmetics and Therapeutic Applications. J. Basic. Microbiol. 2024, 64, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Nwoba, E.G.; Ogbonna, C.N.; Ishika, T.; Vadiveloo, A. Microalgal Pigments: A Source of Natural Food Colors. In Microalgae Biotechnology for Food, Health and High Value Products; Alam, M.A., Xu, J.-L., Wang, Z., Eds.; Springer: Singapore, 2020; pp. 81–123. ISBN 9789811501685. [Google Scholar]
- Rana, B.; Bhattacharyya, M.; Patni, B.; Arya, M.; Joshi, G.K. The Realm of Microbial Pigments in the Food Color Market. Front. Sustain. Food Syst. 2021, 5, 603892. [Google Scholar] [CrossRef]
- Pailliè-Jiménez, M.E.; Stincone, P.; Brandelli, A. Natural Pigments of Microbial Origin. Front. Sustain. Food Syst. 2020, 4, 590439. [Google Scholar] [CrossRef]
- Jacobsen, I.H.; Ledesma-Amaro, R.; Martinez, J.L. Recombinant β-Carotene Production by Yarrowia lipolytica—Assessing the Potential of Micro-Scale Fermentation Analysis in Cell Factory Design and Bioreaction Optimization. Front. Bioeng. Biotechnol. 2020, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, C.; Prasastha, V.R.; Venkatachalam, M.; Dufossé, L. Natural Substrates and Culture Conditions to Produce Pigments from Potential Microbes in Submerged Fermentation. Fermentation 2022, 8, 460. [Google Scholar] [CrossRef]
- Hu, J.; Meng, W.; Su, Y.; Qian, C.; Fu, W. Emerging Technologies for Advancing Microalgal Photosynthesis and Metabolism toward Sustainable Production. Front. Mar. Sci. 2023, 10, 1260709. [Google Scholar] [CrossRef]
- Morocho-Jácome, A.L.; Ruscinc, N.; Martinez, R.M.; De Carvalho, J.C.M.; Santos De Almeida, T.; Rosado, C.; Costa, J.G.; Velasco, M.V.R.; Baby, A.R. (Bio)Technological Aspects of Microalgae Pigments for Cosmetics. Appl. Microbiol. Biotechnol. 2020, 104, 9513–9522. [Google Scholar] [CrossRef]
- Barone, G.D.; Ferizović, D.; Biundo, A.; Lindblad, P. Hints at the Applicability of Microalgae and Cyanobacteria for the Biodegradation of Plastics. Sustainability 2020, 12, 10449. [Google Scholar] [CrossRef]
- Venil, C.K.; Velmurugan, P.; Dufossé, L.; Renuka Devi, P.; Veera Ravi, A. Fungal Pigments: Potential Coloring Compounds for Wide Ranging Applications in Textile Dyeing. J. Fungi 2020, 6, 68. [Google Scholar] [CrossRef]
- Afroz Toma, M.; Rahman, M.H.; Rahman, M.S.; Arif, M.; Nazir, K.H.M.N.H.; Dufossé, L. Fungal Pigments: Carotenoids, Riboflavin, and Polyketides with Diverse Applications. J. Fungi 2023, 9, 454. [Google Scholar] [CrossRef]
- Lin, L.; Xu, J. Fungal Pigments and Their Roles Associated with Human Health. J. Fungi 2020, 6, 280. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Bajpai, S.; Mishra, A.; Kohli, I.; Varma, A.; Fouillaud, M.; Dufossé, L.; Joshi, N.C. Bacterial Pigments and Their Multifaceted Roles in Contemporary Biotechnology and Pharmacological Applications. Microorganisms 2023, 11, 614. [Google Scholar] [CrossRef]
- Huang, X.; Gan, L.; He, Z.; Jiang, G.; He, T. Bacterial Pigments as a Promising Alternative to Synthetic Colorants: From Fundamentals to Applications. J. Microbiol. Biotechnol. 2024, 34, 2153–2165. [Google Scholar] [CrossRef]
- Aman Mohammadi, M.; Ahangari, H.; Mousazadeh, S.; Hosseini, S.M.; Dufossé, L. Microbial Pigments as an Alternative to Synthetic Dyes and Food Additives: A Brief Review of Recent Studies. Bioprocess Biosyst. Eng. 2022, 45, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Anshi; Kapil, S.; Goswami, L.; Sharma, V. Unveiling the Intricacies of Microbial Pigments as Sustainable Alternatives to Synthetic Colorants: Recent Trends and Advancements. Micro 2024, 4, 621–640. [Google Scholar] [CrossRef]
- Sen, T.; Barrow, C.J.; Deshmukh, S.K. Microbial Pigments in the Food Industry—Challenges and the Way Forward. Front. Nutr. 2019, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Maharshi, A.; Gohil, N. Microbial Pigments: A Potential Substitute of Synthetic Colorants in the Food and Healthcare Sectors. In Microbial Products for Health and Nutrition; Kothari, V., Ray, S., Kumar, P., Eds.; Springer Nature Singapore: Singapore, 2024; pp. 191–220. ISBN 978-981-9742-34-9. [Google Scholar]
- Barreto, J.V.D.O.; Casanova, L.M.; Junior, A.N.; Reis-Mansur, M.C.P.P.; Vermelho, A.B. Microbial Pigments: Major Groups and Industrial Applications. Microorganisms 2023, 11, 2920. [Google Scholar] [CrossRef]
- Kumar Roy Choudhury, A. Eco—Friendly Dyes and Dyeing. Adv. Mat. Technol. Environ. 2018, 2, 145–176. [Google Scholar]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye Removal from Water and Wastewater Using Various Physical, Chemical, and Biological Processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef]
- Rane, A.; Joshi, S.J. Biodecolorization and Biodegradation of Dyes: A Review. Open Biotechnol. J. 2021, 15, 97–108. [Google Scholar] [CrossRef]
- Popli, S.; Patel, U.D. Destruction of Azo Dyes by Anaerobic-Aerobic Sequential Biological Treatment: A Review. Int. J. Environ. Sci. Technol. 2015, 12, 405–420. [Google Scholar] [CrossRef]
- Alzain, H.; Kalimugogo, V.; Hussein, K. A Review of Environmental Impact of Azo Dyes. Int. J. Res. Rev. 2023, 10, 64–689. [Google Scholar] [CrossRef]
- Chatragadda, R.; Dufossé, L. Ecological and Biotechnological Aspects of Pigmented Microbes: A Way Forward in Development of Food and Pharmaceutical Grade Pigments. Microorganisms 2021, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Numan, M.; Bashir, S.; Mumtaz, R.; Tayyab, S.; Rehman, N.U.; Khan, A.L.; Shinwari, Z.K.; Al-Harrasi, A. Therapeutic Applications of Bacterial Pigments: A Review of Current Status and Future Opportunities. 3 Biotech 2018, 8, 207. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, X.; Gao, S.; Li, D.; Zhou, J. Production of Natural Pigments Using Microorganisms. J. Agric. Food Chem. 2023, 71, 9243–9254. [Google Scholar] [CrossRef] [PubMed]
- Aldaghi, S.A.; Ubais, R.; Schmitt, I.; Wendisch, V.F.; Costamagna, M.; Perucca, M. Life Cycle Assessment of Bacterial, Algal, and Synthetic Approaches for Astaxanthin Production at a Laboratory Scale: Comparative Environmental Analysis and Sensitivity of Energy Sources. Processes 2023, 11, 2911. [Google Scholar] [CrossRef]
- Delgove, M.A.F.; Laurent, A.; Woodley, J.M.; De Wildeman, S.M.A.; Bernaerts, K.V.; van der Meer, Y. A Prospective Life Cycle Assessment (LCA) of Monomer Synthesis: Comparison of Biocatalytic and Oxidative Chemistry. ChemSusChem 2019, 12, 1349–1360. [Google Scholar] [CrossRef]
- Renita, A.A.; Gajaria, T.K.; Sathish, S.; Kumar, J.A.; Lakshmi, D.S.; Kujawa, J.; Kujawski, W. Progress and Prospective of the Industrial Development and Applications of Eco-Friendly Colorants: An Insight into Environmental Impact and Sustainability Issues. Foods 2023, 12, 1521. [Google Scholar] [CrossRef]
- Díez, B.H.; Torres, C.A.V.; Gaudêncio, S.P. Actinomycete-Derived Pigments: A Path Toward Sustainable Industrial Colorants. Mar. Drugs 2025, 23, 39. [Google Scholar] [CrossRef]
- Durán, N.; Teixeira, M.F.S.; De Conti, R.; Esposito, E. Ecological-Friendly Pigments From Fungi. Crit. Rev. Food Sci. Nutr. 2002, 42, 53–66. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Classification of Dye and Pigments. In Dyes and Pigments; Gürses, A., Açıkyıldız, M., Güneş, K., Gürses, M.S., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 31–45. ISBN 978-3-319-33890-3. [Google Scholar]
- Li, M.; Xia, Q.; Zhang, H.; Zhang, R.; Yang, J. Metabolic Engineering of Different Microbial Hosts for Lycopene Production. J. Agric. Food Chem. 2020, 68, 14104–14122. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, R.; Yoshimura, T.; Hemmi, H. Reconstruction of the “Archaeal” Mevalonate Pathway from the Methanogenic Archaeon Methanosarcina Mazei in Escherichia Coli Cells. Appl. Environ. Microbiol. 2020, 86, e02889–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Guo, L. Biosynthesis of β-Carotene in Engineered E. Coli Using the MEP and MVA Pathways. Microb. Cell Fact. 2014, 13, 160. [Google Scholar] [CrossRef]
- Mehta, B.J.; Cerdá-Olmedo, E. Mutants of Carotene Production in Blakeslea Trispora. Appl. Microbiol. Biotechnol. 1995, 42, 836–838. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, J.; Yang, Q.; Yang, J. Metabolic Engineering Escherichia coli for the Production of Lycopene. Molecules 2020, 25, 3136. [Google Scholar] [CrossRef]
- López-Nieto, M.J.; Costa, J.; Peiro, E.; Méndez, E.; Rodríguez-Sáiz, M.; De La Fuente, J.L.; Cabri, W.; Barredo, J.L. Biotechnological Lycopene Production by Mated Fermentation of Blakeslea Trispora. Appl. Microbiol. Biotechnol. 2004, 66, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Miao, L.; Li, Q.; Dai, G.; Lu, F.; Liu, T.; Zhang, X.; Ma, Y. Production of Lycopene by Metabolically-Engineered Escherichia coli. Biotechnol. Lett. 2014, 36, 1515–1522. [Google Scholar] [CrossRef]
- Kong, K.-W.; Khoo, H.-E.; Prasad, K.N.; Ismail, A.; Tan, C.-P.; Rajab, N.F. Revealing the Power of the Natural Red Pigment Lycopene. Molecules 2010, 15, 959–987. [Google Scholar] [CrossRef]
- Mannazzu, I.; Landolfo, S.; Da Silva, T.L.; Buzzini, P. Red Yeasts and Carotenoid Production: Outlining a Future for Non-Conventional Yeasts of Biotechnological Interest. World J. Microbiol. Biotechnol. 2015, 31, 1665–1673. [Google Scholar] [CrossRef]
- Paniagua-Michel, J.; Olmos-Soto, J.; Ruiz, M.A. Pathways of Carotenoid Biosynthesis in Bacteria and Microalgae. In Microbial Carotenoids from Bacteria and Microalgae; Barredo, J.-L., Ed.; Humana Press: Totowa, NJ, USA, 2012; Volume 892, pp. 1–12. ISBN 978-1-61779-878-8. [Google Scholar]
- Gupta, I.; Adin, S.N.; Panda, B.P.; Mujeeb, M. β-Carotene—Production Methods, Biosynthesis from Phaffia Rhodozyma, Factors Affecting Its Production during Fermentation, Pharmacological Properties: A Review. Biotechnol. Appl. Biochem. 2022, 69, 2517–2529. [Google Scholar] [CrossRef]
- Berman, J.; Zorrilla-López, U.; Farré, G.; Zhu, C.; Sandmann, G.; Twyman, R.M.; Capell, T.; Christou, P. Nutritionally Important Carotenoids as Consumer Products. Phytochem. Rev. 2015, 14, 727–743. [Google Scholar] [CrossRef]
- Chen, M.; Li, M.; Ye, L.; Yu, H. Construction of Canthaxanthin-Producing Yeast by Combining Spatiotemporal Regulation and Pleiotropic Drug Resistance Engineering. ACS Synth. Biol. 2022, 11, 325–333. [Google Scholar] [CrossRef]
- Steiger, S.; Sandmann, G. Cloning of Two Carotenoid Ketolase Genes from Nostoc Punctiforme for the Heterologous Production of Canthaxanthin and Astaxanthin. Biotechnol. Lett. 2004, 26, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Papp, T.; Csernetics, Á.; Nagy, G.; Bencsik, O.; Iturriaga, E.A.; Eslava, A.P.; Vágvölgyi, C. Canthaxanthin Production with Modified Mucor circinelloides Strains. Appl. Microbiol. Biotechnol. 2013, 97, 4937–4950. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Rimbach, G. Canthaxanthin: From Molecule to Function. Mol. Nutr. Food Res. 2017, 61, 1600469. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Wiegertjes, G. Properties of Carotenoids in Fish Fitness: A Review. Mar. Drugs 2020, 18, 568. [Google Scholar] [CrossRef]
- Goswami, G.; Chaudhuri, S.; Dutta, D. The Present Perspective of Astaxanthin with Reference to Biosynthesis and Pharmacological Importance. World J. Microbiol. Biotechnol. 2010, 26, 1925–1939. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Domínguez-Bocanegra, A.R.; Ponce-Noyola, T.; Torres-Muñoz, J.A. Astaxanthin Production by Phaffia rhodozyma and Haematococcus pluvialis: A Comparative Study. Appl. Microbiol. Biotechnol. 2007, 75, 783–791. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, H.; Li, S.; Xue, Y.; Chen, Y.; Adu-Frimpong, M.; Xu, Y.; Yu, J.; Xu, X.; Smyth, H.D.C.; et al. Preparation of Astaxanthin-loaded Composite Micelles with Coaxial Electrospray Technology for Enhanced Oral Bioavailability and Improved Antioxidation Capability. J. Sci. Food Agric. 2024, 104, 1408–1419. [Google Scholar] [CrossRef]
- Martínez-Álvarez, Ó.; Calvo, M.M.; Gómez-Estaca, J. Recent Advances in Astaxanthin Micro/Nanoencapsulation to Improve Its Stability and Functionality as a Food Ingredient. Mar. Drugs 2020, 18, 406. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, R.; Zhang, G.; Liu, Z.; Jiang, H.; Mao, X. Heterologous Expression of the Plant-Derived Astaxanthin Biosynthesis Pathway in Yarrowia Lipolytica for Glycosylated Astaxanthin Production. J. Agric. Food Chem. 2023, 71, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.C.D.; Bicas, J.L. Natural Blue Pigments and Bikaverin. Microbiol. Res. 2021, 244, 126653. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, I.; Caycedo-Soler, F.; Harris, D.; Yochelis, S.; Huelga, S.F.; Plenio, M.B.; Adir, N.; Keren, N.; Paltiel, Y. Regulating the Energy Flow in a Cyanobacterial Light-Harvesting Antenna Complex. J. Phys. Chem. B 2017, 121, 1240–1247. [Google Scholar] [CrossRef]
- Zhong, Y.; Sun, S.; Dai, T.; Zhang, H.; Wu, J.; Gong, E.S. Phycocyanin-Chitosan Complex Stabilized Emulsion: Preparation, Characteristics, Digestibility, and Stability. Int. J. Biol. Macromol. 2024, 260, 129253. [Google Scholar] [CrossRef]
- Setiyono, E.; Adhiwibawa, M.A.S.; Indrawati, R.; Prihastyanti, M.N.U.; Shioi, Y.; Brotosudarmo, T.H.P. An Indonesian Marine Bacterium, Pseudoalteromonas rubra, Produces Antimicrobial Prodiginine Pigments. ACS Omega 2020, 5, 4626–4635. [Google Scholar] [CrossRef]
- Han, R.; Xiang, R.; Li, J.; Wang, F.; Wang, C. High-level Production of Microbial Prodigiosin: A Review. J. Basic. Microbiol. 2021, 61, 506–523. [Google Scholar] [CrossRef]
- Kuo, Y.-Y.; Li, S.-Y. Solid-State Fermentation of Food Waste by Serratia marcescens NCHU05 for Prodigiosin Production. J. Taiwan. Inst. Chem. Eng. 2024, 160, 105260. [Google Scholar] [CrossRef]
- Williamson, N.R.; Simonsen, H.T.; Ahmed, R.A.A.; Goldet, G.; Slater, H.; Woodley, L.; Leeper, F.J.; Salmond, G.P.C. Biosynthesis of the Red Antibiotic, Prodigiosin, in Serratia: Identification of a Novel 2-methyl-3-n-amyl-pyrrole (MAP) Assembly Pathway, Definition of the Terminal Condensing Enzyme, and Implications for Undecylprodigiosin Biosynthesis in Streptomyces. Mol. Microbiol. 2005, 56, 971–989. [Google Scholar] [CrossRef]
- Lapenda, J.C.L.; Alves, V.P.; Adam, M.L.; Rodrigues, M.D.; Nascimento, S.C. Cytotoxic Effect of Prodigiosin, Natural Red Pigment, Isolated from Serratia marcescens UFPEDA 398. Indian J. Microbiol. 2020, 60, 182–195. [Google Scholar] [CrossRef]
- Wang, Z.; Li, B.; Zhou, L.; Yu, S.; Su, Z.; Song, J.; Sun, Q.; Sha, O.; Wang, X.; Jiang, W.; et al. Prodigiosin Inhibits Wnt/β-Catenin Signaling and Exerts Anticancer Activity in Breast Cancer Cells. Proc. Natl. Acad. Sci. USA 2016, 113, 13150–13155. [Google Scholar] [CrossRef]
- Li, D.; Liu, J.; Wang, X.; Kong, D.; Du, W.; Li, H.; Hse, C.-Y.; Shupe, T.; Zhou, D.; Zhao, K. Biological Potential and Mechanism of Prodigiosin from Serratia marcescens Subsp. Lawsoniana in Human Choriocarcinoma and Prostate Cancer Cell Lines. Int. J. Mol. Sci. 2018, 19, 3465. [Google Scholar] [CrossRef]
- Lapenda, J.C.; Silva, P.A.; Vicalvi, M.C.; Sena, K.X.F.R.; Nascimento, S.C. Antimicrobial Activity of Prodigiosin Isolated from Serratia marcescens UFPEDA 398. World J. Microbiol. Biotechnol. 2015, 31, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Stahmann, K.-P.; Revuelta, J.L.; Seulberger, H. Three Biotechnical Processes Using Ashbya gossypii, Candida famata, or Bacillus subtilis Compete with Chemical Riboflavin Production. Appl. Microbiol. Biotechnol. 2000, 53, 509–516. [Google Scholar] [CrossRef]
- Macheroux, P.; Kappes, B.; Ealick, S.E. Flavogenomics—A Genomic and Structural View of Flavin-dependent Proteins. FEBS J. 2011, 278, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Rhee, H.-W.; Choi, S.J.; Yoo, S.H.; Jang, Y.O.; Park, H.H.; Pinto, R.M.; Cameselle, J.C.; Sandoval, F.J.; Roje, S.; Han, K.; et al. A Bifunctional Molecule as an Artificial Flavin Mononucleotide Cyclase and a Chemosensor for Selective Fluorescent Detection of Flavins. J. Am. Chem. Soc. 2009, 131, 10107–10112. [Google Scholar] [CrossRef] [PubMed]
- Powers, H.J. Riboflavin (Vitamin B-2) and Health. Am. J. Clin. Nutr. 2003, 77, 1352–1360. [Google Scholar] [CrossRef]
- Choi, S.Y.; Yoon, K.; Lee, J.I.; Mitchell, R.J. Violacein: Properties and Production of a Versatile Bacterial Pigment. BioMed Res. Int. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Balibar, C.J.; Walsh, C.T. In Vitro Biosynthesis of Violacein from l-Tryptophan by the Enzymes VioA−E from Chromobacterium Violaceum. Biochemistry 2006, 45, 15444–15457. [Google Scholar] [CrossRef]
- Ahmed, A.; Ahmad, A.; Li, R.; AL-Ansi, W.; Fatima, M.; Mushtaq, B.S.; Basharat, S.; Li, Y.; Bai, Z. Recent Advances in Synthetic, Industrial and Biological Applications of Violacein and Its Heterologous Production. J. Microbiol. Biotechnol. 2021, 31, 1465–1480. [Google Scholar] [CrossRef]
- Park, H.; Park, S.; Yang, Y.-H.; Choi, K.-Y. Microbial Synthesis of Violacein Pigment and Its Potential Applications. Crit. Rev. Biotechnol. 2021, 41, 879–901. [Google Scholar] [CrossRef] [PubMed]
- Yada, S.; Wang, Y.; Zou, Y.; Nagasaki, K.; Hosokawa, K.; Osaka, I.; Arakawa, R.; Enomoto, K. Isolation and Characterization of Two Groups of Novel Marine Bacteria Producing Violacein. Mar. Biotechnol. 2008, 10, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Aranda, S.; Montes-Borrego, M.; Landa, B.B. Purple-Pigmented Violacein-Producing Duganella Spp. Inhabit the Rhizosphere of Wild and Cultivated Olives in Southern Spain. Microb. Ecol. 2011, 62, 446–459. [Google Scholar] [CrossRef]
- Chang, P.-K.; Cary, J.W.; Lebar, M.D. Biosynthesis of Conidial and Sclerotial Pigments in Aspergillus Species. Appl. Microbiol. Biotechnol. 2020, 104, 2277–2286. [Google Scholar] [CrossRef]
- Rzepka, Z.; Buszman, E.; Beberok, A.; Wrześniok, D. From Tyrosine to Melanin: Signaling Pathways and Factors Regulating Melanogenesis. Postep. Hig. Med. Dosw. 2016, 70, 695–708. [Google Scholar] [CrossRef]
- Eisenman, H.C.; Casadevall, A. Synthesis and Assembly of Fungal Melanin. Appl. Microbiol. Biotechnol. 2012, 93, 931–940. [Google Scholar] [CrossRef]
- Glagoleva, A.Y.; Shoeva, O.Y.; Khlestkina, E.K. Melanin Pigment in Plants: Current Knowledge and Future Perspectives. Front. Plant Sci. 2020, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, C.; Vitiello, G.; Adinolfi, B.; Silvestri, B.; Armanetti, P.; Manini, P.; Pezzella, A.; d’Ischia, M.; Luciani, G.; Menichetti, L. Melanin and Melanin-Like Hybrid Materials in Regenerative Medicine. Nanomaterials 2020, 10, 1518. [Google Scholar] [CrossRef]
- Song, W.; Yang, H.; Liu, S.; Yu, H.; Li, D.; Li, P.; Xing, R. Melanin: Insights into Structure, Analysis, and Biological Activities for Future Development. J. Mater. Chem. B 2023, 11, 7528–7543. [Google Scholar] [CrossRef]
- Keith, K.E.; Killip, L.; He, P.; Moran, G.R.; Valvano, M.A. Burkholderia cenocepacia C5424 Produces a Pigment with Antioxidant Properties Using a Homogentisate Intermediate. J. Bacteriol. 2007, 189, 9057–9065. [Google Scholar] [CrossRef]
- Ahmad, S.; Mohammed, M.; Mekala, L.P.; Chintalapati, S.; Chintalapati, V.R. Tryptophan, a Non-Canonical Melanin Precursor: New L-Tryptophan Based Melanin Production by Rubrivivax benzoatilyticus JA2. Sci. Rep. 2020, 10, 8925. [Google Scholar] [CrossRef] [PubMed]
- El-Sayyad, G.S.; Mosallam, F.M.; El-Sayed, S.S.; El-Batal, A.I. Facile Biosynthesis of Tellurium Dioxide Nanoparticles by Streptomyces cyaneus Melanin Pigment and Gamma Radiation for Repressing Some Aspergillus Pathogens and Bacterial Wound Cultures. J. Clust. Sci. 2020, 31, 147–159. [Google Scholar] [CrossRef]
- Valiante, V.; Baldin, C.; Hortschansky, P.; Jain, R.; Thywißen, A.; Straßburger, M.; Shelest, E.; Heinekamp, T.; Brakhage, A.A. The Aspergillus Fumigatus Conidial Melanin Production Is Regulated by the Bifunctional bHLH DevR and MADS-box RlmA Transcription Factors. Mol. Microbiol. 2016, 102, 321–335. [Google Scholar] [CrossRef]
- Gómez, B.L.; Nosanchuk, J.D. Melanin and Fungi. Curr. Opin. Infect. Dis. 2003, 16, 91–96. [Google Scholar] [CrossRef]
- Wang, Z.; Tschirhart, T.; Schultzhaus, Z.; Kelly, E.E.; Chen, A.; Oh, E.; Nag, O.; Glaser, E.R.; Kim, E.; Lloyd, P.F.; et al. Melanin Produced by the Fast-Growing Marine Bacterium Vibrio natriegens through Heterologous Biosynthesis: Characterization and Application. Appl. Environ. Microbiol. 2020, 86, e02749-19. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Xu, W.; Li, L.; Fan, B.; Peng, X.; Qu, B.; Wang, L.; Li, T.; Li, S.; Zhang, R. Ultrasmall Endogenous Biopolymer Nanoparticles for Magnetic Resonance/Photoacoustic Dual-Modal Imaging-Guided Photothermal Therapy. Nanoscale 2018, 10, 10584–10595. [Google Scholar] [CrossRef]
- Guo, K.; Jiao, Z.; Zhao, X.; Hu, Y.; Zhao, N.; Xu, F.-J. Melanin-Based Immunoregulatory Nanohybrids Enhance Antitumor Immune Responses in Breast Cancer Mouse Model. ACS Nano 2023, 17, 10792–10805. [Google Scholar] [CrossRef]
- Pogorzelska, E.; Godziszewska, J.; Brodowska, M.; Wierzbicka, A. Antioxidant Potential of Haematococcus pluvialis Extract Rich in Astaxanthin on Colour and Oxidative Stability of Raw Ground Pork Meat during Refrigerated Storage. Meat Sci. 2018, 135, 54–61. [Google Scholar] [CrossRef]
- Asker, D. Isolation and Characterization of a Novel, Highly Selective Astaxanthin-Producing Marine Bacterium. J. Agric. Food Chem. 2017, 65, 9101–9109. [Google Scholar] [CrossRef]
- Dufossé, L. Current and Potential Natural Pigments from Microorganisms (Bacteria, Yeasts, Fungi, Microalgae). In Handbook on Natural Pigments in Food and Beverages; Schweiggert, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 337–354. ISBN 978-0-08-100371-8. [Google Scholar]
- Capelli, B.; Cysewki, G.R. Natural Astaxanthin the World’s Best Kept Health Secret; Cyanotech Corporation: Kailua-Kona, HI, USA, 2012; p. 202. [Google Scholar]
- Schmidt, I.; Schewe, H.; Gassel, S.; Jin, C.; Buckingham, J.; Hümbelin, M.; Sandmann, G.; Schrader, J. Biotechnological Production of Astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl. Microbiol. Biotechnol. 2011, 89, 555–571. [Google Scholar] [CrossRef]
- Zhou, P.; Ye, L.; Xie, W.; Lv, X.; Yu, H. Highly Efficient Biosynthesis of Astaxanthin in Saccharomyces cerevisiae by Integration and Tuning of Algal crtZ and Bkt. Appl. Microbiol. Biotechnol. 2015, 99, 8419–8428. [Google Scholar] [CrossRef] [PubMed]
- Le-Feuvre, R.; Moraga-Suazo, P.; Gonzalez, J.; Martin, S.S.; Henríquez, V.; Donoso, A.; Agurto-Muñoz, C. Biotechnology Applied to Haematococcus pluvialis Fotow: Challenges and Prospects for the Enhancement of Astaxanthin Accumulation. J. Appl. Phycol. 2020, 32, 3831–3852. [Google Scholar] [CrossRef]
- Lemuth, K.; Steuer, K.; Albermann, C. Engineering of a Plasmid-Free Escherichia coli Strain for Improved in Vivo Biosynthesis of Astaxanthin. Microb. Cell Factories 2011, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; McClements, D.J.; Cao, Y.; Xiao, H. Chemical and Physical Stability of Astaxanthin-Enriched Emulsion-Based Delivery Systems. Food Biophys. 2016, 11, 302–310. [Google Scholar] [CrossRef]
- Eriksen, N.T. Production of Phycocyanin—A Pigment with Applications in Biology, Biotechnology, Foods and Medicine. Appl. Microbiol. Biotechnol. 2008, 80, 1–14. [Google Scholar] [CrossRef]
- Dufossé; Chatragadda, R.; Nambali Valsalan, V.; Ramalingam, K.; Chidambaram Kulandaisamy, V.; Laurent, D. Multifaceted Applications of Microbial Pigments: Current Knowledge, Challenges and Future Directions for Public Health Implications. Microorganisms 2019, 7, 186. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Aguilar-Hernandez, I.; Cardenas-Chavez, D.L.; Ornelas-Soto, N.; Romero-Ogawa, M.A.; Parra-Saldivar, R. Extraction and Purification of High-value Metabolites from Microalgae: Essential Lipids, Astaxanthin and Phycobiliproteins. Microb. Biotechnol. 2015, 8, 190–209. [Google Scholar] [CrossRef] [PubMed]
- Oddities and Curiosities in the Algal World. In Algae; CRC Press: Boca Raton, FL, USA, 2014; pp. 325–342. ISBN 978-0-429-10718-4.
- Abdelaziz, A.A.; Kamer, A.M.A.; Al-Monofy, K.B.; Al-Madboly, L.A. Pseudomonas aeruginosa’s Greenish-Blue Pigment Pyocyanin: Its Production and Biological Activities. Microb. Cell Factories 2023, 22, 110. [Google Scholar] [CrossRef]
- Julianti, E.; Susanti, S.; Singgih, M.; Mulyani, L.N. Optimization of Extraction Method and Characterization of Phycocyanin Pigment from Spirulina platensis. J. Math. Fundam Sci. 2019, 51, 168–176. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Figueira, F.D.S.; Silveira, J.T.D.; Morais, M.G.D.; Costa, J.A.V.; Kalil, S.J. Improvement of Thermal Stability of C-Phycocyanin by Nanofiber and Preservative Agents: C-Phycocyanin Stability: Preservative and Nanofiber. J. Food Process. Preserv. 2016, 40, 1264–1269. [Google Scholar] [CrossRef]
- Namazkar, S.; Ahmad, W.A. Spray-Dried Prodigiosin from Serratia marcescens As A Colorant. Biosci. Biotechnol. Res. Asia 2013, 10, 69–76. [Google Scholar] [CrossRef]
- Dauenhauer, S.A.; Hull, R.A.; Williams, R.P. Cloning and Expression in Escherichia coli of Serratia Marcescens Genes Encoding Prodigiosin Biosynthesis. J. Bacteriol. 1984, 158, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Domröse, A.; Klein, A.S.; Hage-Hülsmann, J.; Thies, S.; Svensson, V.; Classen, T.; Pietruszka, J.; Jaeger, K.-E.; Drepper, T.; Loeschcke, A. Efficient Recombinant Production of Prodigiosin in Pseudomonas putida. Front. Microbiol. 2015, 6, 972. [Google Scholar] [CrossRef]
- Pan, X.; Tang, M.; You, J.; Liu, F.; Sun, C.; Osire, T.; Fu, W.; Yi, G.; Yang, T.; Yang, S.-T.; et al. Regulator RcsB Controls Prodigiosin Synthesis and Various Cellular Processes in Serratia marcescens JNB5-1. Appl. Environ. Microbiol. 2021, 87, e02052–20. [Google Scholar] [CrossRef]
- Dos Santos, R.A.; Rodríguez, D.M.; Da Silva, L.A.R.; De Almeida, S.M.; De Campos-Takaki, G.M.; De Lima, M.A.B. Enhanced Production of Prodigiosin by Serratia marcescens UCP 1549 Using Agrosubstrates in Solid-State Fermentation. Arch. Microbiol. 2021, 203, 4091–4100. [Google Scholar] [CrossRef]
- Hong, M.Y.; Seeram, N.P.; Zhang, Y.; Heber, D. Anticancer Effects of Chinese Red Yeast Rice versus Monacolin K Alone on Colon Cancer Cells. J. Nutr. Biochem. 2008, 19, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Dufossé, L. Microbial Pigments from Bacteria, Yeasts, Fungi, and Microalgae for the Food and Feed Industries. In Natural and Artificial Flavoring Agents and Food Dyes; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 7, pp. 113–132. ISBN 978-0-12-811518-3. [Google Scholar]
- Averianova, L.A.; Balabanova, L.A.; Son, O.M.; Podvolotskaya, A.B.; Tekutyeva, L.A. Production of Vitamin B2 (Riboflavin) by Microorganisms: An Overview. Front. Bioeng. Biotechnol. 2020, 8, 570828. [Google Scholar] [CrossRef]
- Schwechheimer, S.K.; Park, E.Y.; Revuelta, J.L.; Becker, J.; Wittmann, C. Biotechnology of Riboflavin. Appl. Microbiol. Biotechnol. 2016, 100, 2107–2119. [Google Scholar] [CrossRef]
- Hustad, S.; Eussen, S.; Midttun, Ø.; Ulvik, A.; Van De Kant, P.M.; Mørkrid, L.; Gislefoss, R.; Ueland, P.M. Kinetic Modeling of Storage Effects on Biomarkers Related to B Vitamin Status and One-Carbon Metabolism. Clin. Chem. 2012, 58, 402–410. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural Colorants: Food Colorants from Natural Sources. Annu. Rev. Food Sci. Technol. 2017, 8, 261–280. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kurcz, A.; Gientka, I.; Kieliszek, M. Rhodotorula Glutinis—Potential Source of Lipids, Carotenoids, and Enzymes for Use in Industries. Appl. Microbiol. Biotechnol. 2016, 100, 6103–6117. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, A.; Hruszkewycz, D.P.; Dela Sena, C.; Narayanasamy, S.; Riedl, K.M.; Kopec, R.E.; Schwartz, S.J.; Curley, R.W.; Harrison, E.H. Naturally Occurring Eccentric Cleavage Products of Provitamin A β-Carotene Function as Antagonists of Retinoic Acid Receptors. J. Biol. Chem. 2012, 287, 15886–15895. [Google Scholar] [CrossRef] [PubMed]
- Larroude, M.; Celinska, E.; Back, A.; Thomas, S.; Nicaud, J.; Ledesma-Amaro, R. A Synthetic Biology Approach to Transform Yarrowia lipolytica into a Competitive Biotechnological Producer of Β-carotene. Biotechnol. Bioeng. 2018, 115, 464–472. [Google Scholar] [CrossRef]
- Aman, R.; Schieber, A.; Carle, R. Effects of Heating and Illumination on Trans. − Cis Isomerization and Degradation of β-Carotene and Lutein in Isolated Spinach Chloroplasts. J. Agric. Food Chem. 2005, 53, 9512–9518. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fang, M.-Y.; Li, G.; Zhang, C.; Xing, X.-H. Enhanced Production of Crude Violacein from Glucose in Escherichia coli by Overexpression of Rate-Limiting Key Enzyme(S) Involved in Violacein Biosynthesis. Appl. Biochem. Biotechnol. 2018, 186, 909–916. [Google Scholar] [CrossRef]
- Rabasco Alvarez, A.M.; González Rodríguez, M.L. Lipids in Pharmaceutical and Cosmetic Preparations. Grasas Aceites 2000, 51, 74–96. [Google Scholar] [CrossRef]
- Surwase, S.N.; Jadhav, S.B.; Phugare, S.S.; Jadhav, J.P. Optimization of Melanin Production by Brevundimonas Sp. SGJ Using Response Surface Methodology. 3 Biotech 2013, 3, 187–194. [Google Scholar] [CrossRef]
- Jalmi, P.; Bodke, P.; Wahidullah, S.; Raghukumar, S. The Fungus Gliocephalotrichum simplex as a Source of Abundant, Extracellular Melanin for Biotechnological Applications. World J. Microbiol. Biotechnol. 2012, 28, 505–512. [Google Scholar] [CrossRef]
- Campanhol, B.S.; Ribeiro, B.D.; Casellato, F.; Medina, K.J.D.; Sponchiado, S.R.P. Improvement of DOPA-Melanin Production by Aspergillus nidulans Using Eco-Friendly and Inexpensive Substrates. J. Fungi 2023, 9, 714. [Google Scholar] [CrossRef]
- Guo, J.; Rao, Z.; Yang, T.; Man, Z.; Xu, M.; Zhang, X. High-Level Production of Melanin by a Novel Isolate of Streptomyces kathirae. FEMS Microbiol. Lett. 2014, 357, 85–91. [Google Scholar] [CrossRef]
- Mavridi-Printezi, A.; Guernelli, M.; Menichetti, A.; Montalti, M. Bio-Applications of Multifunctional Melanin Nanoparticles: From Nanomedicine to Nanocosmetics. Nanomaterials 2020, 10, 2276. [Google Scholar] [CrossRef]
- Giovannucci, E.; Rimm, E.B.; Liu, Y.; Stampfer, M.J.; Willett, W. A Prospective Study of Tomato Products, Lycopene, and Prostate Cancer Risk. J. Natl. Cancer Inst. 2002, 94, 391–398. [Google Scholar] [CrossRef]
- Papaioannou, E.H.; Liakopoulou-Kyriakides, M.; Karabelas, A.J. Natural Origin Lycopene and Its “Green” Downstream Processing. Crit. Rev. Food Sci. Nutr. 2016, 56, 686–709. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.F.; Favaro-Trindade, C.S.; Rocha, G.A.; Thomazini, M. Microencapsulation of Lycopene by Gelatin-Pectin Complex Coacervation. J. Food Process. Preserv. 2012, 36, 185–190. [Google Scholar] [CrossRef]
- Hamidi, M.; Hejazi, M.S.; Nazemyieh, H.; Hejazi, M.A.; Naziri, D. Halorubrum Sp. TBZ112, an Extremely Halophilic Carotenoid- Producing Archaeon Isolated from Urmia Lake. Pharm. Sci. 2017, 23, 150–158. [Google Scholar] [CrossRef]
- Naz, T.; Yang, J.; Nosheen, S.; Sun, C.; Nazir, Y.; Mohamed, H.; Fazili, A.B.A.; Ullah, S.; Li, S.; Yang, W.; et al. Genetic Modification of Mucor circinelloides for Canthaxanthin Production by Heterologous Expression of β-Carotene Ketolase Gene. Front. Nutr. 2021, 8, 756218. [Google Scholar] [CrossRef]
- Promdonkoy, P.; Watcharawipas, A.; Bubphasawan, S.; Sansatchanon, K.; Suwanakitti, N.; Kocharin, K.; Runguphan, W. Metabolic Engineering of Saccharomyces cerevisiae for Production of Canthaxanthin, Zeaxanthin, and Astaxanthin. J. Fungi 2024, 10, 433. [Google Scholar] [CrossRef]
- Neza, E.; Centini, M. Microbiologically Contaminated and Over-Preserved Cosmetic Products According Rapex 2008–2014. Cosmetics 2016, 3, 3. [Google Scholar] [CrossRef]
- Durán, N.; Menck, C.F.M. Chromobacterium violaceum: A Review of Pharmacological and Industiral Perspectives. Crit. Rev. Microbiol. 2001, 27, 201–222. [Google Scholar] [CrossRef]
- Tuli, H.S.; Chaudhary, P.; Beniwal, V.; Sharma, A.K. Microbial Pigments as Natural Color Sources: Current Trends and Future Perspectives. J. Food Sci. Technol. 2015, 52, 4669–4678. [Google Scholar] [CrossRef]
- Mummaleti, G.; Udo, T.; Mohan, A.; Kong, F. Synthesis, Characterization and Application of Microbial Pigments in Foods as Natural Colors. Crit. Rev. Food Sci. Nutr. 2024, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Venil, C.K.; Zakaria, Z.A.; Ahmad, W.A. Bacterial Pigments and Their Applications. Process Biochem. 2013, 48, 1065–1079. [Google Scholar] [CrossRef]
- Raita, S.; Feldmane, L.; Kusnere, Z.; Spalvins, K.; Kuzmika, I.; Berzina, I.; Mika, T. Microbial Carotenoids Production: Strains, Conditions, and Yield Affecting Factors. Environ. Clim. Technol. 2023, 27, 1027–1048. [Google Scholar] [CrossRef]
- Grand View Research. Beta-Carotene Market Size, Share & Trends Analysis Report by Type (Natural, Synthetic), by End-Use (Food & Beverages, Pharmaceutical, Dietary Supplements), by Region (North America, Asia Pacific), And Segment Forecasts, 2024–2030; Grand View Research: San Francisco, CA, USA, 2024. [Google Scholar]
- Fact.MR. Synthetic Beta-Carotene Market Outlook (2024–2034); Fact.MR: Rockville, MD, USA, 2024. [Google Scholar]
- Cortez, R.; Luna-Vital, D.A.; Margulis, D.; Gonzalez De Mejia, E. Natural Pigments: Stabilization Methods of Anthocyanins for Food Applications. Comp. Rev. Food Sci. Food Safe 2017, 16, 180–198. [Google Scholar] [CrossRef]
- Jardine, A.; Sayed, S. Valorisation of Chitinous Biomass for Antimicrobial Applications. Pure Appl. Chem. 2018, 90, 293–304. [Google Scholar] [CrossRef]
- Nawaz, A.; Chaudhary, R.; Shah, Z.; Dufossé, L.; Fouillaud, M.; Mukhtar, H.; Ul Haq, I. An Overview on Industrial and Medical Applications of Bio-Pigments Synthesized by Marine Bacteria. Microorganisms 2020, 9, 11. [Google Scholar] [CrossRef]
- Lin, L.; Xu, J. Production of Fungal Pigments: Molecular Processes and Their Applications. J. Fungi 2022, 9, 44. [Google Scholar] [CrossRef]
- Huang, T.; Wang, M.; Shi, K.; Chen, G.; Tian, X.; Wu, Z. Metabolism and Secretion of Yellow Pigment under High Glucose Stress with Monascus ruber. AMB Expr. 2017, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yang, S.; Wang, C.; Shi, K.; Zhao, X.; Wu, Z. Investigation of the Mycelial Morphology of Monascus and the Expression of Pigment Biosynthetic Genes in High-Salt-Stress Fermentation. Appl. Microbiol. Biotechnol. 2020, 104, 2469–2479. [Google Scholar] [CrossRef]
- Garcia-Cortes, A.; Garcia-Vásquez, J.A.; Aranguren, Y.; Ramirez-Castrillon, M. Pigment Production Improvement in Rhodotorula mucilaginosa AJB01 Using Design of Experiments. Microorganisms 2021, 9, 387. [Google Scholar] [CrossRef]
- Carvalho, J.C.D.; Oishi, B.O.; Pandey, A.; Soccol, C.R. Biopigments from Monascus: Strains Selection, Citrinin Production and Color Stability. Braz. Arch. Biol. Technol. 2005, 48, 885–894. [Google Scholar] [CrossRef]
- Li, Y.-P.; Pan, Y.-F.; Zou, L.-H.; Xu, Y.; Huang, Z.-B.; He, Q.-H. Lower Citrinin Production by Gene Disruption of ctnB Involved in Citrinin Biosynthesis in Monascus aurantiacus Li AS3.4384. J. Agric. Food Chem. 2013, 61, 7397–7402. [Google Scholar] [CrossRef] [PubMed]
- Hölker, U.; Höfer, M.; Lenz, J. Biotechnological Advantages of Laboratory-Scale Solid-State Fermentation with Fungi. Appl. Microbiol. Biotechnol. 2004, 64, 175–186. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Hosseini, S.M.; Assarehzadegan, M.; Khosravi-Darani, K.; Hosseini, H. Safety Assays and Nutritional Values of Mycoprotein Produced by Fusarium venenatum IR372C from Date Waste as Substrate. J. Sci. Food Agric. 2020, 100, 4433–4441. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Du, X.-J.; Li, P.; Sun, C.-C.; Wang, S. Investigation of Citrinin and Pigment Biosynthesis Mechanisms in Monascus purpureus by Transcriptomic Analysis. Front. Microbiol. 2018, 9, 1374. [Google Scholar] [CrossRef]
- Liu, W.; An, C.; Shu, X.; Meng, X.; Yao, Y.; Zhang, J.; Chen, F.; Xiang, H.; Yang, S.; Gao, X.; et al. A Dual-Plasmid CRISPR/Cas System for Mycotoxin Elimination in Polykaryotic Industrial Fungi. ACS Synth. Biol. 2020, 9, 2087–2095. [Google Scholar] [CrossRef]
- Silva, T.R.E.; Silva, L.C.F.; De Queiroz, A.C.; Alexandre Moreira, M.S.; De Carvalho Fraga, C.A.; De Menezes, G.C.A.; Rosa, L.H.; Bicas, J.; De Oliveira, V.M.; Duarte, A.W.F. Pigments from Antarctic Bacteria and Their Biotechnological Applications. Crit. Rev. Biotechnol. 2021, 41, 809–826. [Google Scholar] [CrossRef]
- Cerda, A.; Artola, A.; Barrena, R.; Font, X.; Gea, T.; Sánchez, A. Innovative Production of Bioproducts From Organic Waste Through Solid-State Fermentation. Front. Sustain. Food Syst. 2019, 3, 63. [Google Scholar] [CrossRef]
- Chmelová, D.; Legerská, B.; Kunstová, J.; Ondrejovič, M.; Miertuš, S. The Production of Laccases by White-Rot Fungi under Solid-State Fermentation Conditions. World J. Microbiol. Biotechnol. 2022, 38, 21. [Google Scholar] [CrossRef]
- Egbune, E.O.; Ezedom, T.; Orororo, O.C.; Egbune, O.U.; Avwioroko, O.J.; Aganbi, E.; Anigboro, A.A.; Tonukari, N.J. Solid-State Fermentation of Cassava (Manihot Esculenta Crantz): A Review. World J. Microbiol. Biotechnol. 2023, 39, 259. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, G.; Sage, V.; Xu, J.; Sun, G.; He, J.; Sun, Y. Optimization of Dark Fermentation for Biohydrogen Production Using a Hybrid Artificial Neural Network (ANN) and Response Surface Methodology (RSM) Approach. Environ. Prog. Sustain. Energy 2021, 40, e13485. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Shum-Chéong-Sing, A.; Dufossé, L.; Fouillaud, M. Statistical Optimization of the Physico-Chemical Parameters for Pigment Production in Submerged Fermentation of Talaromyces Albobiverticillius 30548. Microorganisms 2020, 8, 711. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Shekh, A.; Jakhu, S.; Sharma, Y.; Kapoor, R.; Sharma, T.R. Bioengineering of Microalgae: Recent Advances, Perspectives, and Regulatory Challenges for Industrial Application. Front. Bioeng. Biotechnol. 2020, 8, 914. [Google Scholar] [CrossRef]
- Nigam, P.S.; Luke, J.S. Food Additives: Production of Microbial Pigments and Their Antioxidant Properties. Curr. Opin. Food Sci. 2016, 7, 93–100. [Google Scholar] [CrossRef]
- Uyar, B.; Ali, M.D.; Uyar, G.E.O. Design Parameters Comparison of Bubble Column, Airlift and Stirred Tank Photobioreactors for Microalgae Production. Bioprocess. Biosyst. Eng. 2024, 47, 195–209. [Google Scholar] [CrossRef]
- Yang, J.; Lu, C.; Stasny, B.; Henley, J.; Guinto, W.; Gonzalez, C.; Gleason, J.; Fung, M.; Collopy, B.; Benjamino, M.; et al. Fed-batch Bioreactor Process Scale-up from 3-L to 2,500-L Scale for Monoclonal Antibody Production from Cell Culture. Biotechnol. Bioeng. 2007, 98, 141–154. [Google Scholar] [CrossRef]
- Hajjaj, H.; Blanc, P.; Groussac, E.; Uribelarrea, J.-L.; Goma, G.; Loubiere, P. Kinetic Analysis of Red Pigment and Citrinin Production by Monascus ruber as a Function of Organic Acid Accumulation. Enzym. Microb. Technol. 2000, 27, 619–625. [Google Scholar] [CrossRef]
- De Carvalho, J.C.; Cardoso, L.C.; Ghiggi, V.; Woiciechowski, A.L.; De Souza Vandenberghe, L.P.; Soccol, C.R. Microbial Pigments. In Biotransformation of Waste Biomass into High Value Biochemicals; Brar, S.K., Dhillon, G.S., Soccol, C.R., Eds.; Springer: New York, NY, USA, 2014; pp. 73–97. ISBN 978-1-4614-8004-4. [Google Scholar]
- Ghosh, A.; Goyal, A.; Jain, R.K. Study of Methanol-Induced Phenotypic Changes in a Novel Strain of Acinetobacter lwoffii. Arch. Microbiol. 2007, 188, 533–539. [Google Scholar] [CrossRef]
- Robledo, J.A.; Murillo, A.M.; Rouzaud, F. Physiological Role and Potential Clinical Interest of Mycobacterial Pigments. IUBMB Life 2011, 63, 71–78. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The Shikimate Pathway and Aromatic Amino Acid Biosynthesis in Plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Zhang, X.; Heinonen, S.; Levänen, E. Applications of Supercritical Carbon Dioxide in Materials Processing and Synthesis. RSC Adv. 2014, 4, 61137–61152. [Google Scholar] [CrossRef]
- Fabrowska, J.; Messyasz, B.; Szyling, J.; Walkowiak, J.; Łęska, B. Isolation of Chlorophylls and Carotenoids from Freshwater Algae Using Different Extraction Methods. Phycol. Res. 2018, 66, 52–57. [Google Scholar] [CrossRef]
- Venil, C.K.; Dufossé, L.; Renuka Devi, P. Bacterial Pigments: Sustainable Compounds with Market Potential for Pharma and Food Industry. Front. Sustain. Food Syst. 2020, 4, 100. [Google Scholar] [CrossRef]
- Da Silva, A.F.; Moreira, A.F.; Miguel, S.P.; Coutinho, P. Recent Advances in Microalgae Encapsulation Techniques for Biomedical Applications. Adv. Colloid Interface Sci. 2024, 333, 103297. [Google Scholar] [CrossRef] [PubMed]
- Dinu, V.; Kilic, A.; Wang, Q.; Ayed, C.; Fadel, A.; Harding, S.E.; Yakubov, G.E.; Fisk, I.D. Policy, Toxicology and Physicochemical Considerations on the Inhalation of High Concentrations of Food Flavour. Npj Sci. Food 2020, 4, 15. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, J.; Perčulija, V.; Li, Y.; Lu, Q.; Chen, J.; Ouyang, S. Structural Insights into Target DNA Recognition and Cleavage by the CRISPR-Cas12c1 System. Nucleic Acids Res. 2022, 50, 11820–11833. [Google Scholar] [CrossRef]
- Heider, S.A.E.; Wolf, N.; Hofemeier, A.; Peters-Wendisch, P.; Wendisch, V.F. Optimization of the IPP Precursor Supply for the Production of Lycopene, Decaprenoxanthin and Astaxanthin by Corynebacterium glutamicum. Front. Bioeng. Biotechnol. 2014, 2, 28. [Google Scholar] [CrossRef]
- Shu, C.; Zhang, S.; Wu, S.; Liu, S.; Xu, J.; Zhao, J.; Li, B. Microorganism-Mediated Production of Anthocyanins: Current Progress and Future Prospects. Food Res. Int. 2025, 201, 115550. [Google Scholar] [CrossRef]
- Yan, Y.; Chemler, J.; Huang, L.; Martens, S.; Koffas, M.A.G. Metabolic Engineering of Anthocyanin Biosynthesis in Escherichia coli. Appl. Environ. Microbiol. 2005, 71, 3617–3623. [Google Scholar] [CrossRef]
- Zha, J.; Zang, Y.; Mattozzi, M.; Plassmeier, J.; Gupta, M.; Wu, X.; Clarkson, S.; Koffas, M.A.G. Metabolic Engineering of Corynebacterium Glutamicum for Anthocyanin Production. Microb. Cell Fact. 2018, 17, 143. [Google Scholar] [CrossRef]
- Jones, J.A.; Vernacchio, V.R.; Collins, S.M.; Shirke, A.N.; Xiu, Y.; Englaender, J.A.; Cress, B.F.; McCutcheon, C.C.; Linhardt, R.J.; Gross, R.A.; et al. Complete Biosynthesis of Anthocyanins Using E. coli Polycultures. mBio 2017, 8, e00621-17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-K.; Wang, D.-N.; Chen, J.; Liu, Z.-J.; Wei, L.-J.; Hua, Q. Metabolic Engineering of β-Carotene Biosynthesis in Yarrowia lipolytica. Biotechnol. Lett. 2020, 42, 945–956. [Google Scholar] [CrossRef]
- Ahmad, A.; Jamil, A.; Munawar, N. GMOs or Non-GMOs? The CRISPR Conundrum. Front. Plant Sci. 2023, 14, 1232938. [Google Scholar] [CrossRef] [PubMed]
- Ravi, R.; Janodia, M.D. Factors Affecting Technology Transfer and Commercialization of University Research in India: A Cross-Sectional Study. J. Knowl. Econ. 2022, 13, 787–803. [Google Scholar] [CrossRef]
- Flammini, S.; Arcese, G.; Lucchetti, M.C.; Mortara, L. Business Model Configuration and Dynamics for Technology Commercialization in Mature Markets. Br. Food J. 2017, 119, 2340–2358. [Google Scholar] [CrossRef]
- Mendoza-Moheno, J.; Cruz-Coria, E.; González-Cruz, T.F. Socio-Technical Innovation in Community-Based Tourism Organizations: A Proposal for Local Development. Technol. Forecast. Social. Change 2021, 171, 120949. [Google Scholar] [CrossRef]
- Zhou, P.; Xie, W.; Yao, Z.; Zhu, Y.; Ye, L.; Yu, H. Development of a Temperature-responsive Yeast Cell Factory Using Engineered Gal4 as a Protein Switch. Biotechnol. Bioeng. 2018, 115, 1321–1330. [Google Scholar] [CrossRef]
- Kim, S.-W.; Kim, J.-B.; Jung, W.-H.; Kim, J.-H.; Jung, J.-K. Over-Production of β-Carotene from Metabolically Engineered Escherichia Coli. Biotechnol. Lett. 2006, 28, 897–904. [Google Scholar] [CrossRef]
- Xu, X.; Chu, X.; Du, B.; Huang, C.; Xie, C.; Zhang, Z.; Jiang, L. Functional Characterization of a Novel Violacein Biosynthesis Operon from Janthinobacterium Sp. B9–8. Appl. Microbiol. Biotechnol. 2022, 106, 2903–2916. [Google Scholar] [CrossRef]
- Zhu, H.-Z.; Jiang, S.; Wu, J.-J.; Zhou, X.-R.; Liu, P.-Y.; Huang, F.-H.; Wan, X. Production of High Levels of 3 S,3′ S -Astaxanthin in Yarrowia lipolytica via Iterative Metabolic Engineering. J. Agric. Food Chem. 2022, 70, 2673–2683. [Google Scholar] [CrossRef]
- Tong, Y.; Zhou, J.; Zhang, L.; Xu, P. A Golden-Gate Based Cloning Toolkit to Build Violacein Pathway Libraries in Yarrowia lipolytica. ACS Synth. Biol. 2021, 10, 115–124. [Google Scholar] [CrossRef]
- Qi, D.-D.; Jin, J.; Liu, D.; Jia, B.; Yuan, Y.-J. In Vitro and in Vivo Recombination of Heterologous Modules for Improving Biosynthesis of Astaxanthin in Yeast. Microb. Cell Fact. 2020, 19, 103. [Google Scholar] [CrossRef]
- Verwaal, R.; Wang, J.; Meijnen, J.-P.; Visser, H.; Sandmann, G.; Van Den Berg, J.A.; Van Ooyen, A.J.J. High-Level Production of Beta-Carotene in Saccharomyces cerevisiae by Successive Transformation with Carotenogenic Genes from Xanthophyllomyces dendrorhous. Appl. Environ. Microbiol. 2007, 73, 4342–4350. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Ma, T.; Liu, M.; Qu, J.; Liu, Z.; Zhang, H.; Shi, B.; Fu, S.; Ma, J.; Lai, L.T.F.; et al. Modular Enzyme Assembly for Enhanced Cascade Biocatalysis and Metabolic Flux. Nat. Commun. 2019, 10, 4248. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Park, S.-H.; Kim, S.; Kim, S.-W.; Hahn, J.-S. Efficient Production of Lycopene in Saccharomyces cerevisiae by Enzyme Engineering and Increasing Membrane Flexibility and NAPDH Production. Appl. Microbiol. Biotechnol. 2019, 103, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Fathi, Z.; Tramontin, L.R.R.; Ebrahimipour, G.; Borodina, I.; Darvishi, F. Metabolic Engineering of Saccharomyces cerevisiae for Production of β-Carotene from Hydrophobic Substrates. FEMS Yeast Res. 2021, 21, foaa068. [Google Scholar] [CrossRef]
- Sun, L.; Atkinson, C.A.; Lee, Y.; Jin, Y. High-level Β-carotene Production from Xylose by Engineered Saccharomyces cerevisiae without Overexpression of a Truncated HMG1 (t HMG1). Biotechnol. Bioeng. 2020, 117, 3522–3532. [Google Scholar] [CrossRef]
- Ferreira, R.L.; Rezende, G.S.; Damas, M.S.F.; Oliveira-Silva, M.; Pitondo-Silva, A.; Brito, M.C.A.; Leonardecz, E.; Góes, F.R.D.; Campanini, E.B.; Malavazi, I.; et al. Characterization of KPC-Producing Serratia marcescens in an Intensive Care Unit of a Brazilian Tertiary Hospital. Front. Microbiol. 2020, 11, 956. [Google Scholar] [CrossRef]
- Khayyat, A.N.; Hegazy, W.A.H.; Shaldam, M.A.; Mosbah, R.; Almalki, A.J.; Ibrahim, T.S.; Khayat, M.T.; Khafagy, E.-S.; Soliman, W.E.; Abbas, H.A. Xylitol Inhibits Growth and Blocks Virulence in Serratia marcescens. Microorganisms 2021, 9, 1083. [Google Scholar] [CrossRef]
- Tavares-Carreon, F.; De Anda-Mora, K.; Rojas-Barrera, I.C.; Andrade, A. Serratia marcescens Antibiotic Resistance Mechanisms of an Opportunistic Pathogen: A Literature Review. PeerJ 2023, 11, e14399. [Google Scholar] [CrossRef]
- Hamilton, J.J.; Marlow, V.L.; Owen, R.A.; Costa, M.D.A.A.; Guo, M.; Buchanan, G.; Chandra, G.; Trost, M.; Coulthurst, S.J.; Palmer, T.; et al. A Holin and an Endopeptidase Are Essential for Chitinolytic Protein Secretion in Serratia marcescens. J. Cell Biol. 2014, 207, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Mudaliar, S.B.; Bharath Prasad, A.S. A Biomedical Perspective of Pyocyanin from Pseudomonas aeruginosa: Its Applications and Challenges. World J. Microbiol. Biotechnol. 2024, 40, 90. [Google Scholar] [CrossRef] [PubMed]
- Lau, G.W.; Ran, H.; Kong, F.; Hassett, D.J.; Mavrodi, D. Pseudomonas aeruginosa Pyocyanin Is Critical for Lung Infection in Mice. Infect. Immun. 2004, 72, 4275–4278. [Google Scholar] [CrossRef]
- Ran, H.; Hassett, D.J.; Lau, G.W. Human Targets of Pseudomonas aeruginosa Pyocyanin. Proc. Natl. Acad. Sci. USA 2003, 100, 14315–14320. [Google Scholar] [CrossRef]
- Venkatramanan, M.; Nalini, E. Regulation of Virulence in Chromobacterium violaceum and Strategies to Combat It. Front. Microbiol. 2024, 15, 1303595. [Google Scholar] [CrossRef]
- Oh, W.T.; Giri, S.S.; Yun, S.; Kim, H.J.; Kim, S.G.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J.; Jun, J.W.; et al. Janthinobacterium lividum as An Emerging Pathogenic Bacterium Affecting Rainbow Trout (Oncorhynchus Mykiss) Fisheries in Korea. Pathogens 2019, 8, 146. [Google Scholar] [CrossRef] [PubMed]
- Narsing Rao, M.P.; Xiao, M.; Li, W.-J. Fungal and Bacterial Pigments: Secondary Metabolites with Wide Applications. Front. Microbiol. 2017, 8, 1113. [Google Scholar] [CrossRef]
- Gong, P.; Shi, R.; Tang, J.; Wang, J.; Luo, Q.; Zhang, J.; Ruan, X.; Wang, C.; Chen, W. Effect of Exogenous and Endogenous Ectoine on Monascus Development, Metabolism, and Pigment Stability. Foods 2023, 12, 3217. [Google Scholar] [CrossRef]
- Wang, J.-J.; Lee, C.-L.; Pan, T.-M. Modified Mutation Method for Screening Low Citrinin-Producing Strains of Monascus purpureus on Rice Culture. J. Agric. Food Chem. 2004, 52, 6977–6982. [Google Scholar] [CrossRef]
- Reverberi, M.; Ricelli, A.; Zjalic, S.; Fabbri, A.A.; Fanelli, C. Natural Functions of Mycotoxins and Control of Their Biosynthesis in Fungi. Appl. Microbiol. Biotechnol. 2010, 87, 899–911. [Google Scholar] [CrossRef]
- Nikitin, D.A.; Ivanova, E.A.; Semenov, M.V.; Zhelezova, A.D.; Ksenofontova, N.A.; Tkhakakhova, A.K.; Kholodov, V.A. Diversity, Ecological Characteristics and Identification of Some Problematic Phytopathogenic Fusarium in Soil: A Review. Diversity 2023, 15, 49. [Google Scholar] [CrossRef]
- Zain, M.; Yasmin, S.; Hafeez, F. Isolation and Characterization of Plant Growth Promoting Antagonistic Bacteria from Cotton and Sugarcane Plants for Suppression of Phytopathogenic Fusarium Species. Iran. J. Biotechnol. 2019, 17, 61–70. [Google Scholar] [CrossRef]
- Gong, Z.; Zhang, S.; Liu, J. Recent Advances in Chitin Biosynthesis Associated with the Morphology and Secondary Metabolite Synthesis of Filamentous Fungi in Submerged Fermentation. J. Fungi 2023, 9, 205. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, Z.; Liu, Y.; Hua, X.; Gao, C.; Liu, J. Morphological Engineering of Filamentous Fungi: Research Progress and Perspectives. J. Microbiol. Biotechnol. 2024, 34, 1197–1205. [Google Scholar] [CrossRef]
- Méndez, A.; Pérez, C.; Montañéz, J.C.; Martínez, G.; Aguilar, C.N. Red Pigment Production by Penicillium purpurogenum GH2 Is Influenced by pH and Temperature. J. Zhejiang Univ. Sci. B 2011, 12, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. High-Value Products from Microalgae—Their Development and Commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Dębowski, M.; Świca, I.; Kazimierowicz, J.; Zieliński, M. Large Scale Microalgae Biofuel Technology—Development Perspectives in Light of the Barriers and Limitations. Energies 2022, 16, 81. [Google Scholar] [CrossRef]
- Uduman, N.; Qi, Y.; Danquah, M.K.; Forde, G.M.; Hoadley, A. Dewatering of Microalgal Cultures: A Major Bottleneck to Algae-Based Fuels. J. Renew. Sustain. Energy 2010, 2, 012701. [Google Scholar] [CrossRef]
- De Souza, M.P.; Hoeltz, M.; Gressler, P.D.; Benitez, L.B.; Schneider, R.C.S. Potential of Microalgal Bioproducts: General Perspectives and Main Challenges. Waste Biomass Valorization 2019, 10, 2139–2156. [Google Scholar] [CrossRef]
- Diaconu, M.; Soreanu, G.; Balan, C.D.; Buciscanu, I.I.; Maier, V.; Cretescu, I. Study of Spirulina platensis (Arthrospira) Development under the Heavy Metals Influence, as a Potential Promoter of Wastewater Remediation. Water 2023, 15, 3962. [Google Scholar] [CrossRef]
- Spicer, A.; Molnar, A. Gene Editing of Microalgae: Scientific Progress and Regulatory Challenges in Europe. Biology 2018, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Dufossé, L. Back to Nature, Microbial Production of Pigments and Colorants for Food Use. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2022; Volume 102, pp. 93–122. ISBN 978-0-323-99084-4. [Google Scholar]
- Patakova, P. Monascus Secondary Metabolites: Production and Biological Activity. J. Ind. Microbiol. Biotechnol. 2013, 40, 169–181. [Google Scholar] [CrossRef]
- Vendruscolo, F.; Bühler, R.M.M.; De Carvalho, J.C.; De Oliveira, D.; Moritz, D.E.; Schmidell, W.; Ninow, J.L. Monascus: A Reality on the Production and Application of Microbial Pigments. Appl. Biochem. Biotechnol. 2016, 178, 211–223. [Google Scholar] [CrossRef]
- Zhang, Y.; Navarro, E.; Cánovas-Márquez, J.T.; Almagro, L.; Chen, H.; Chen, Y.Q.; Zhang, H.; Torres-Martínez, S.; Chen, W.; Garre, V. A New Regulatory Mechanism Controlling Carotenogenesis in the Fungus Mucor circinelloides as a Target to Generate β-Carotene over-Producing Strains by Genetic Engineering. Microb. Cell Fact. 2016, 15, 99. [Google Scholar] [CrossRef]
- Mak, S.; Nodwell, J.R. Actinorhodin Is a Redox-active Antibiotic with a Complex Mode of Action against Gram-positive Cells. Mol. Microbiol. 2017, 106, 597–613. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.; Pedrolli, D.B.; Teixeira, M.F.S.; De Carvalho Santos-Ebinuma, V. Water-Soluble Fluorescent Red Colorant Production by Talaromyces amestolkiae. Appl. Microbiol. Biotechnol. 2019, 103, 6529–6541. [Google Scholar] [CrossRef]
- Arivizhivendhan, K.V.; Mahesh, M.; Boopathy, R.; Swarnalatha, S.; Regina Mary, R.; Sekaran, G. Antioxidant and Antimicrobial Activity of Bioactive Prodigiosin Produces from Serratia marcescens Using Agricultural Waste as a Substrate. J. Food Sci. Technol. 2018, 55, 2661–2670. [Google Scholar] [CrossRef] [PubMed]
- Eghbaljoo, H.; Alizadeh Sani, M.; Sani, I.K.; Maragheh, S.M.; Sain, D.K.; Jawhar, Z.H.; Pirsa, S.; Kadi, A.; Dadkhodayi, R.; Zhang, F.; et al. Development of Smart Packaging Halochromic Films Embedded with Anthocyanin Pigments; Recent Advances. Crit. Rev. Food Sci. Nutr. 2025, 65, 770–786. [Google Scholar] [CrossRef]
- Nupur, L.N.U.; Vats, A.; Dhanda, S.K.; Raghava, G.P.S.; Pinnaka, A.K.; Kumar, A. ProCarDB: A Database of Bacterial Carotenoids. BMC Microbiol. 2016, 16, 96. [Google Scholar] [CrossRef]
- Arivuselvam, R.; Dera, A.A.; Parween Ali, S.; Alraey, Y.; Saif, A.; Hani, U.; Arumugam Ramakrishnan, S.; Azeeze, M.S.T.A.; Rajeshkumar, R.; Susil, A.; et al. Isolation, Identification, and Antibacterial Properties of Prodigiosin, a Bioactive Product Produced by a New Serratia marcescens JSSCPM1 Strain: Exploring the Biosynthetic Gene Clusters of Serratia Species for Biological Applications. Antibiotics 2023, 12, 1466. [Google Scholar] [CrossRef]
- Darshan, N.; Manonmani, H.K. Prodigiosin Inhibits Motility and Activates Bacterial Cell Death Revealing Molecular Biomarkers of Programmed Cell Death. AMB Expr. 2016, 6, 50. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, F.; Tosin, I.; Giachini, A.J.; Schmidell, W.; Ninow, J.L. Antimicrobial Activity of M. onascus Pigments Produced in Submerged Fermentation: Antimicrobial Activity of Monascus Pigments. J. Food Process. Preserv. 2014, 38, 1860–1865. [Google Scholar] [CrossRef]
- Zhao, G.-P.; Li, Y.-Q.; Yang, J.; Cui, K.-Y. Antibacterial Characteristics of Orange Pigment Extracted from Monascus pigments against Escherichia coli. Czech J. Food Sci. 2016, 34, 197–203. [Google Scholar] [CrossRef]
- Lu, W.; Shi, Y.; Wang, R.; Su, D.; Tang, M.; Liu, Y.; Li, Z. Antioxidant Activity and Healthy Benefits of Natural Pigments in Fruits: A Review. Int. J. Mol. Sci. 2021, 22, 4945. [Google Scholar] [CrossRef] [PubMed]
- Igielska-Kalwat, J.; Gościańska, J.; Nowak, I. Carotenoids as Natural Antioxidants. Postep. Hig. Med. Dosw. 2015, 69, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Brotosudarmo, T.H.P.; Limantara, L.; Setiyono, E.; Heriyanto. Structures of Astaxanthin and Their Consequences for Therapeutic Application. Int. J. Food Sci. 2020, 2020, 2156582. [Google Scholar] [CrossRef]
- Li, C.; Cheng, P.; Li, Z.; Xu, Y.; Sun, Y.; Qin, D.; Yu, G. Transcriptomic and Metabolomic Analyses Provide Insights into the Enhancement of Torulene and Torularhodin Production in Rhodotorula glutinis ZHK under Moderate Salt Conditions. J. Agric. Food Chem. 2021, 69, 11523–11533. [Google Scholar] [CrossRef]
- Hamilton, A.J.; Holdom, M.D. Antioxidant Systems in the Pathogenic Fungi of Man and Their Role in Virulence. Med. Mycol. 1999, 37, 375–389. [Google Scholar] [CrossRef]
- Solano, F. Photoprotection and Skin Pigmentation: Melanin-Related Molecules and Some Other New Agents Obtained from Natural Sources. Molecules 2020, 25, 1537. [Google Scholar] [CrossRef]
- Oh, J.-J.; Kim, J.Y.; Son, S.H.; Jung, W.-J.; Kim, D.H.; Seo, J.-W.; Kim, G.-H. Fungal Melanin as a Biocompatible Broad-Spectrum Sunscreen with High Antioxidant Activity. RSC Adv. 2021, 11, 19682–19689. [Google Scholar] [CrossRef]
- Panathur, N.; Gokhale, N.; Dalimba, U.; Koushik, P.V.; Yogeeswari, P.; Sriram, D. New Indole–Isoxazolone Derivatives: Synthesis, Characterisation and in Vitro SIRT1 Inhibition Studies. Bioorganic Med. Chem. Lett. 2015, 25, 2768–2772. [Google Scholar] [CrossRef]
- Venegas, F.A.; Köllisch, G.; Mark, K.; Diederich, W.E.; Kaufmann, A.; Bauer, S.; Chavarría, M.; Araya, J.J.; García-Piñeres, A.J. The Bacterial Product Violacein Exerts an Immunostimulatory Effect Via TLR8. Sci. Rep. 2019, 9, 13661. [Google Scholar] [CrossRef]
- Zhang, W.; Hua, H.; Guo, Y.; Cheng, Y.; Pi, F.; Yao, W.; Xie, Y.; Qian, H. Torularhodin from Sporidiobolus pararoseus Attenuates d-Galactose/AlCl3 Induced Cognitive Impairment, Oxidative Stress, and Neuroinflammation via the Nrf2/NF-κB Pathway. J. Agric. Food Chem. 2020, 68, 6604–6614. [Google Scholar] [CrossRef] [PubMed]
- Corsi, F.; Deidda Tarquini, G.; Urbani, M.; Bejarano, I.; Traversa, E.; Ghibelli, L. The Impressive Anti-Inflammatory Activity of Cerium Oxide Nanoparticles: More than Redox? Nanomaterials 2023, 13, 2803. [Google Scholar] [CrossRef] [PubMed]
- Regourd, J.; Al-Sheikh Ali, A.; Thompson, A. Synthesis and Anti-Cancer Activity of C-Ring-Functionalized Prodigiosin Analogues. J. Med. Chem. 2007, 50, 1528–1536. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Y.; Liu, J.; Wei, D. Antimetastatic Effect of Prodigiosin through Inhibition of Tumor Invasion. Biochem. Pharmacol. 2005, 69, 407–414. [Google Scholar] [CrossRef]
- Zheng, Y.; Xin, Y.; Shi, X.; Guo, Y. Cytotoxicity of Monascus Pigments and Their Derivatives to Human Cancer Cells. J. Agric. Food Chem. 2010, 58, 9523–9528. [Google Scholar] [CrossRef] [PubMed]
- Reyes, F.G.R.; Valim, M.F.C.F.A.; Vercesi, A.E. Effect of Organic Synthetic Food Colours on Mitochondrial Respiration. Food Addit. Contam. 1996, 13, 5–11. [Google Scholar] [CrossRef]
- Di Mascio, P.; Kaiser, S.; Sies, H. Lycopene as the Most Efficient Biological Carotenoid Singlet Oxygen Quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Jesisca; Hsieh, C.; Lee, D.-J.; Chang, C.-H.; Chang, J.-S. Production, Extraction and Stabilization of Lutein from Microalga Chlorella sorokiniana MB-1. Bioresour. Technol. 2016, 200, 500–505. [Google Scholar] [CrossRef]
- Ventura Pinto, A.; Lisboa De Castro, S. The Trypanocidal Activity of Naphthoquinones: A Review. Molecules 2009, 14, 4570–4590. [Google Scholar] [CrossRef]
- Fuhrman, B.; Elis, A.; Aviram, M. Hypocholesterolemic Effect of Lycopene and β-Carotene Is Related to Suppression of Cholesterol Synthesis and Augmentation of LDL Receptor Activity in Macrophages. Biochem. Biophys. Res. Commun. 1997, 233, 658–662. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.W.; Yang, J.E.; Choi, Y.J. Isolation and Characterization of a Yellow Xanthophyll Pigment-Producing Marine Bacterium, Erythrobacter Sp. SDW2 Strain, in Coastal Seawater. Mar. Drugs 2022, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Aruldass, C.A.; Dufossé, L.; Ahmad, W.A. Current Perspective of Yellowish-Orange Pigments from Microorganisms- a Review. J. Clean. Prod. 2018, 180, 168–182. [Google Scholar] [CrossRef]
- Sikkandar, S.; Murugan, K.; Al-Sohaibani, S.; Rayappan, F.; Nair, A.; Tilton, F. Halophilic Bacteria-A Potent Source of Carotenoids with Antioxidant and Anticancer Potentials. J. Pure Appl. Microbiol. 2013, 7, 2825–2830. [Google Scholar]
- Suresh, R.; Uma, G.; Gokulakrishnan, R. Antibacterial Property of Halobacterial Carotenoids against Human Bacterial Pathogens. J. Sci. Ind. Res. 2016, 75, 253–257. [Google Scholar]
- Rezaeeyan, Z.; Safarpour, A.; Amoozegar, M.A.; Babavalian, H.; Tebyanian; Shakeri, F. High Carotenoid Production by a Halotolerant Bacterium, Kocuria Sp. Strain QWT-12 and Anticancer Activity of Its Carotenoid. EXCLI J. 2017, 16, 840–851. [Google Scholar] [CrossRef]
- Megha, S.; Varalakshmi Kilingar, N. Yellow Pigment from a Novel Bacteria, Micrococcus terreus, Activates Caspases and Leads to Apoptosis of Cervical and Liver Cancer Cell Lines. J. Appl. Pharm. Sci. 2021, 11, 77–84. [Google Scholar] [CrossRef]
- Hsu, L.-C.; Hsu, Y.-W.; Liang, Y.-H.; Kuo, Y.-H.; Pan, T.-M. Anti-Tumor and Anti-Inflammatory Properties of Ankaflavin and Monaphilone A from Monascus purpureus NTU 568. J. Agric. Food Chem. 2011, 59, 1124–1130. [Google Scholar] [CrossRef]
- Malik, K.; Tokkas, J.; Goyal, S. Microbial Pigments: A Review. Int. J. Microb. Resour. Technol. 2012, 1, 361–365. [Google Scholar]
- Tsubokura, A.; Yoneda, H.; Mizuta, H. Paracoccus Carotinifaciens Sp. Nov., a New Aerobic Gram-Negative Astaxanthin-Producing Bacterium. Int. J. Syst. Evol. Microbiol. 1999, 49, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Correa-Llantén, D.N.; Amenábar, M.J.; Blamey, J.M. Antioxidant Capacity of Novel Pigments from an Antarctic Bacterium. J. Microbiol. 2012, 50, 374–379. [Google Scholar] [CrossRef]
- Ramírez, J.; Nuñez, M.L.; Valdivia, R. Increased Astaxanthin Production by a Phaffia rhodozyma Mutant Grown on Date Juice from Yucca fillifera. J. Ind. Microbiol. Biotechnol. 2000, 24, 187–190. [Google Scholar] [CrossRef]
- Sonani, R.R.; Singh, N.K.; Kumar, J.; Thakar, D.; Madamwar, D. Concurrent Purification and Antioxidant Activity of Phycobiliproteins from Lyngbya Sp. A09DM: An Antioxidant and Anti-Aging Potential of Phycoerythrin in Caenorhabditis elegans. Process Biochem. 2014, 49, 1757–1766. [Google Scholar] [CrossRef]
- Melvin, M.S.; Tomlinson, J.T.; Saluta, G.R.; Kucera, G.L.; Lindquist, N.; Manderville, R.A. Double-Strand DNA Cleavage by Copper·Prodigiosin. J. Am. Chem. Soc. 2000, 122, 6333–6334. [Google Scholar] [CrossRef]
- Wang, Y.; Nakajima, A.; Hosokawa, K.; Soliev, A.B.; Osaka, I.; Arakawa, R.; Enomoto, K. Cytotoxic Prodigiosin Family Pigments from Pseudoalteromonas Sp. 1020R Isolated from the Pacific Coast of Japan. Biosci. Biotechnol. Biochem. 2012, 76, 1229–1232. [Google Scholar] [CrossRef]
- Konzen, M.; De Marco, D.; Cordova, C.A.S.; Vieira, T.O.; Antônio, R.V.; Creczynski-Pasa, T.B. Antioxidant Properties of Violacein: Possible Relation on Its Biological Function. Bioorganic Med. Chem. 2006, 14, 8307–8313. [Google Scholar] [CrossRef]
- Antonisamy, P.; Ignacimuthu, S. Immunomodulatory, Analgesic and Antipyretic Effects of Violacein Isolated from Chromobacterium violaceum. Phytomedicine 2010, 17, 300–304. [Google Scholar] [CrossRef]
- Baron, S.S.; Rowe, J.J. Antibiotic Action of Pyocyanin. Antimicrob. Agents Chemother. 1981, 20, 814–820. [Google Scholar] [CrossRef]
- Wahyudi, A.T.; Nursari, R.; Purwaningtyas, W.E.; Cahlia, U.; Priyanto, J.A. Crude Extract of Blue-Green Pigment Derived from the Marine Bacterium Pseudomonas aeruginosa P1.S9 Has Antibacterial, Antioxidant and Cytotoxic Activities. OnLine J. Biol. Sci. 2022, 22, 118–125. [Google Scholar] [CrossRef]
- Asmae, Z.; Jaouad, A.; Jamal, G.; Khalid, S.; El Hassouni, M. Antioxidant and Antimicrobial Activities of Melanin Produced by a Pseudomonas balearica Strain. J. Biotechnol. Lett. 2014, 5, 87–94. [Google Scholar]
- Elsayed, Y.; Refaat, J.; R Abdelmohsen, U.; A Fouad, M. The Genus Rhodococcus as a Source of Novel Bioactive Substances: A Review. J. Pharmacogn. Phytochem. 2017, 6, 83–92. [Google Scholar]
- Xie, Z.-T.; Mi, B.-Q.; Lu, Y.-J.; Chen, M.-T.; Ye, Z.-W. Research Progress on Carotenoid Production by Rhodosporidium toruloides. Appl. Microbiol. Biotechnol. 2024, 108, 7. [Google Scholar] [CrossRef]
- Vinarov, A.; Robucheva, Z.; Sidorenko, T.; Dirina, E. Microbial Biosynthesis and Making of Pigment Melanin. Commun. Agric. Appl. Biol. Sci. 2003, 68, 325–326. [Google Scholar]
- Kavitha, R.; Aiswariya, S.; Ratnavali, C.M.G. Anticancer Activity of Red Pigment from Serratia marcescens in Human Cervix Carcinoma. Int. J. PharmTech Res. 2010, 2, 784–787. [Google Scholar]
- Clauditz, A.; Resch, A.; Wieland, K.-P.; Peschel, A.; Götz, F. Staphyloxanthin Plays a Role in the Fitness of Staphylococcus aureus and Its Ability to Cope with Oxidative Stress. Infect. Immun. 2006, 74, 4950–4953. [Google Scholar] [CrossRef]
- Balagurunathan, R.; Selvameenal, L.; Radhakrishnan, M. Antibiotic Pigment from Desert Soil Actinomycetes; Biological Activity, Purification and Chemical Screening. Indian. J. Pharm. Sci. 2009, 71, 499–504. [Google Scholar] [CrossRef]
- Darshan, N.; Manonmani, H.K. Prodigiosin and Its Potential Applications. J. Food Sci. Technol. 2015, 52, 5393–5407. [Google Scholar] [CrossRef]
- Sibero, M.T.; Bachtiarini, T.U.; Trianto, A.; Lupita, A.H.; Sari, D.P.; Igarashi, Y.; Harunari, E.; Sharma, A.R.; Radjasa, O.K.; Sabdono, A. Characterization of a Yellow Pigmented Coral-Associated Bacterium Exhibiting Anti-Bacterial Activity Against Multidrug Resistant (MDR) Organism. Egypt. J. Aquat. Res. 2019, 45, 81–87. [Google Scholar] [CrossRef]
- Rekha, R.; Nimsi, K.A.; Manjusha, K.; Sirajudheen, T.K. Marine Yeast Rhodotorula paludigena VA 242 a Pigment Enhancing Feed Additive for the Ornamental Fish Koi Carp. Aquac. Fish. 2024, 9, 66–70. [Google Scholar] [CrossRef]
- Vaidya, A.A.; O’Callahan, D.; Donaldson, L.; West, M.; Campion, S.; Singh, T. A Closed-Loop Circularity in Wood Sugar as a Renewable Carbon Source for Fungal Pigment Production and Application of Pigments in Wood Colouration. Bioresour. Technol. Rep. 2023, 24, 101648. [Google Scholar] [CrossRef]
- Shiva Krishna, P.; Sudha, S.; Reddy, K.A.; Al-Dhabaan, F.A.; Meher; Prakasham, R.S.; Singara Charya, M.A. Studies on Wound Healing Potential of Red Pigment Isolated from Marine Bacterium Vibrio Sp. Saudi J. Biol. Sci. 2019, 26, 723–729. [Google Scholar] [CrossRef]
- Suwannarach, N.; Kumla, J.; Nishizaki, Y.; Sugimoto, N.; Meerak, J.; Matsui, K.; Lumyong, S. Optimization and Characterization of Red Pigment Production from an Endophytic Fungus, Nigrospora aurantiaca CMU-ZY2045, and Its Potential Source of Natural Dye for Use in Textile Dyeing. Appl. Microbiol. Biotechnol. 2019, 103, 6973–6987. [Google Scholar] [CrossRef]
- Velmurugan, P.; Kamala-Kannan, S.; Balachandar, V.; Lakshmanaperumalsamy, P.; Chae, J.-C.; Oh, B.-T. Natural Pigment Extraction from Five Filamentous Fungi for Industrial Applications and Dyeing of Leather. Carbohydr. Polym. 2010, 79, 262–268. [Google Scholar] [CrossRef]
- Ben Tahar, I.; Kus-Liśkiewicz, M.; Lara, Y.; Javaux, E.; Fickers, P. Characterization of a Nontoxic Pyomelanin Pigment Produced by the Yeast Yarrowia lipolytica. Biotechnol. Prog. 2020, 36, 2912. [Google Scholar] [CrossRef]
- Metwally, R.A.; El Sikaily, A.; El-Sersy, N.A.; Ghozlan, H.A.; Sabry, S.A. Antimicrobial Activity of Textile Fabrics Dyed with Prodigiosin Pigment Extracted from Marine Serratia rubidaea RAM_Alex Bacteria. Egypt. J. Aquat. Res. 2021, 47, 301–305. [Google Scholar] [CrossRef]
- Rocha Balbino, T.; Sánchez-Muñoz, S.; Díaz-Ruíz, E.; Moura Rocha, T.; Mier-Alba, E.; Custódio Inácio, S.; Jose Castro-Alonso, M.; De Carvalho Santos-Ebinuma, V.; Fernando Brandão Pereira, J.; César Santos, J.; et al. Lignocellulosic Biorefineries as a Platform for the Production of High-Value Yeast Derived Pigments—A Review. Bioresour. Technol. 2023, 386, 129549. [Google Scholar] [CrossRef]
- Dulf, F.V.; Vodnar, D.C.; Dulf, E.-H. Solid-State Fermentation with Zygomycetes Fungi as a Tool for Biofortification of Apple Pomace with γ-Linolenic Acid, Carotenoid Pigments and Phenolic Antioxidants. Food Res. Int. 2023, 173, 113448. [Google Scholar] [CrossRef]
- Umesh, M.; Suresh, S.; Santosh, A.S.; Prasad, S.; Chinnathambi, A.; Al Obaid, S.; Jhanani, G.K.; Shanmugam, S. Valorization of Pineapple Peel Waste for Fungal Pigment Production Using Talaromyces albobiverticillius: Insights into Antibacterial, Antioxidant and Textile Dyeing Properties. Environ. Res. 2023, 229, 115973. [Google Scholar] [CrossRef]
- Koli, S.H.; Patil, S.V.; Mohite, B.V.; Otari, S.V. Microbial Pigment-Mediated Synthesis of Metal Nanoparticles. J. Clust. Sci. 2024, 35, 1625–1642. [Google Scholar] [CrossRef]
- El-Baz, A.F.; El-Batal, A.I.; Abomosalam, F.M.; Tayel, A.A.; Shetaia, Y.M.; Yang, S. Extracellular biosynthesis of anti-Candida silver nanoparticles using Monascus purpureus. J. Basic Microbiol. 2016, 56, 531–540. [Google Scholar] [CrossRef]
- Lian, Q.; Yao, L.; Uddin Ahmad, Z.; Gang, D.D.; Konggidinata, M.I.; Gallo, A.A.; Zappi, M.E. Enhanced Pb(II) Adsorption onto Functionalized Ordered Mesoporous Carbon (OMC) from Aqueous Solutions: The Important Role of Surface Property and Adsorption Mechanism. Environ. Sci. Pollut. Res. 2020, 27, 23616–23630. [Google Scholar] [CrossRef]
- Fu, H.; Liu, H.; Mao, J.; Chu, W.; Li, Q.; Alvarez, P.J.J.; Qu, X.; Zhu, D. Photochemistry of Dissolved Black Carbon Released from Biochar: Reactive Oxygen Species Generation and Phototransformation. Environ. Sci. Technol. 2016, 50, 1218–1226. [Google Scholar] [CrossRef]
- Chen, G.; Wu, Z. Production and Biological Activities of Yellow Pigments from Monascus Fungi. World J. Microbiol. Biotechnol. 2016, 32, 136. [Google Scholar] [CrossRef]
- Lin, G.; Zhao, C.; Liao, W.; Yang, J.; Zheng, Y. Eco-Friendly Green Synthesis of Rubropunctatin Functionalized Silver Nanoparticles and Evaluation of Antibacterial Activity. Nanomaterials 2022, 12, 4052. [Google Scholar] [CrossRef]
- Bhatnagar, S.; Kobori, T.; Ganesh, D.; Ogawa, K.; Aoyagi, H. Biosynthesis of Silver Nanoparticles Mediated by Extracellular Pigment from Talaromyces purpurogenus and Their Biomedical Applications. Nanomaterials 2019, 9, 1042. [Google Scholar] [CrossRef]
- Chavan, A.; Pawar, J.; Kakde, U. Fungal Pigment and Its Potential in Nanoparticle Synthesis, Characterization and Its Application. Adv. Biores. 2023, 14, 349–355. [Google Scholar] [CrossRef]
- Rahul, S.; Chandrashekhar, P.; Hemant, B.; Bipinchandra, S.; Mouray, E.; Grellier, P.; Satish, P. In Vitro Antiparasitic Activity of Microbial Pigments and Their Combination with Phytosynthesized Metal Nanoparticles. Parasitol. Int. 2015, 64, 353–356. [Google Scholar] [CrossRef]
- Manikprabhu, D.; Lingappa, K. Antibacterial Activity of Silver Nanoparticles against Methicillin-Resistant Staphylococcus aureus Synthesized Using Model Streptomyces Sp. Pigment by Photo-Irradiation Method. J. Pharm. Res. 2013, 6, 255–260. [Google Scholar] [CrossRef]
- Salunkhe, N.S.; Koli, S.H.; Mohite, B.V.; Patil, V.S.; Patil, S.V. Xanthomonadin Mediated Synthesis of Biocidal and Photo-Protective Silver Nanoparticles (XP-AgNPs). Results Chem. 2022, 4, 100663. [Google Scholar] [CrossRef]
- Baraka, A.; Dickson, S.; Gobara, M.; El-Sayyad, G.S.; Zorainy, M.; Awaad, M.I.; Hatem, H.; Kotb, M.M.; Tawfic, A.F. Synthesis of Silver Nanoparticles Using Natural Pigments Extracted from Alfalfa Leaves and Its Use for Antimicrobial Activity. Chem. Pap. 2017, 71, 2271–2281. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, J.; Guo, X.; Li, W.; Xing, Y.; Wang, Y. Monascus Pigment-mediated Green Synthesis of Silver Nanoparticles: Catalytic, Antioxidant, and Antibacterial Activity. Appl. Organomet. Chem. 2021, 35, e6120. [Google Scholar] [CrossRef]
- Poorniammal, R.; Prabhu, S. Exploitation of Thermomyces Fungal Pigment for the Synthesis of Silver Nanoparticles for Textile Application. Emergent Life Sci. Res. 2023, 09, 40–48. [Google Scholar] [CrossRef]
- Nair, V.; Sambre, D.; Joshi, S.; Bankar, A.; Ravi Kumar, A.; Zinjarde, S. Yeast-Derived Melanin Mediated Synthesis of Gold Nanoparticles. J. Bionanosci. 2013, 7, 159–168. [Google Scholar] [CrossRef]
- El-Sayyad, G.S.; Mosallam, F.M.; El-Batal, A.I. One-Pot Green Synthesis of Magnesium Oxide Nanoparticles Using Penicillium Chrysogenum Melanin Pigment and Gamma Rays with Antimicrobial Activity against Multidrug-Resistant Microbes. Adv. Powder Technol. 2018, 29, 2616–2625. [Google Scholar] [CrossRef]
- Sowani, H.; Mohite, P.; Damale, S.; Kulkarni, M.; Zinjarde, S. Carotenoid Stabilized Gold and Silver Nanoparticles Derived from the Actinomycete Gordonia Amicalis HS-11 as Effective Free Radical Scavengers. Enzym. Microb. Technol. 2016, 95, 164–173. [Google Scholar] [CrossRef]
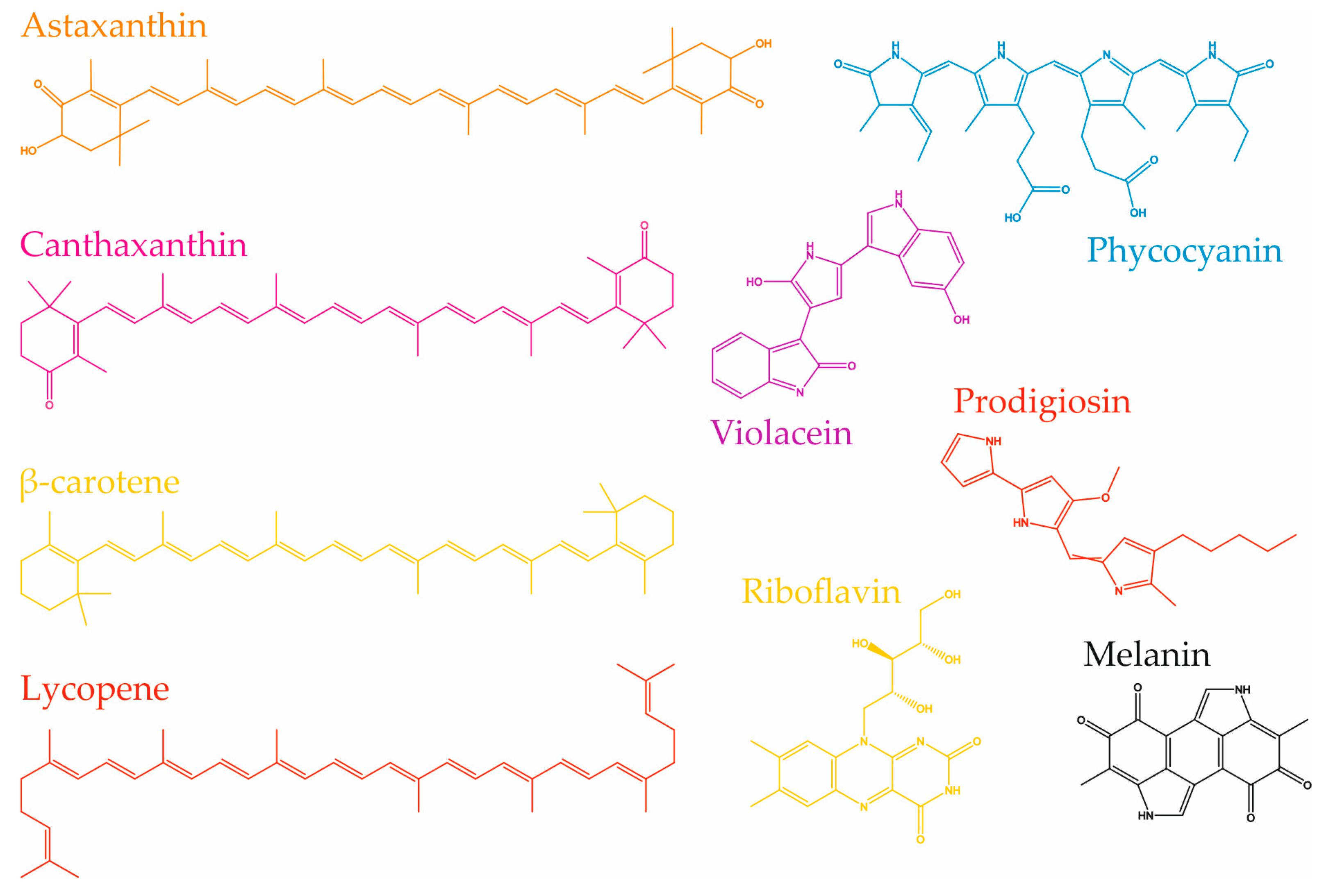
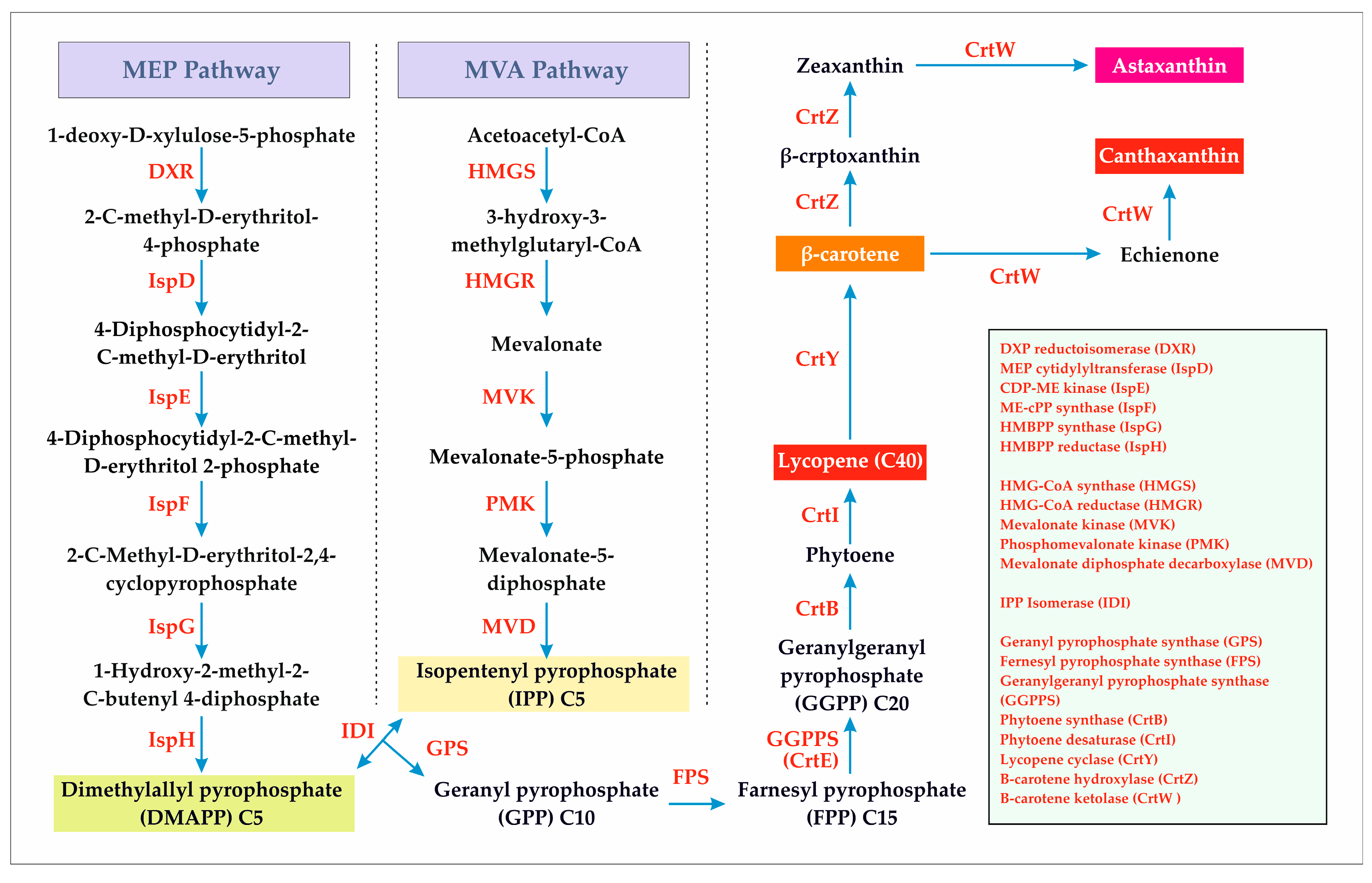
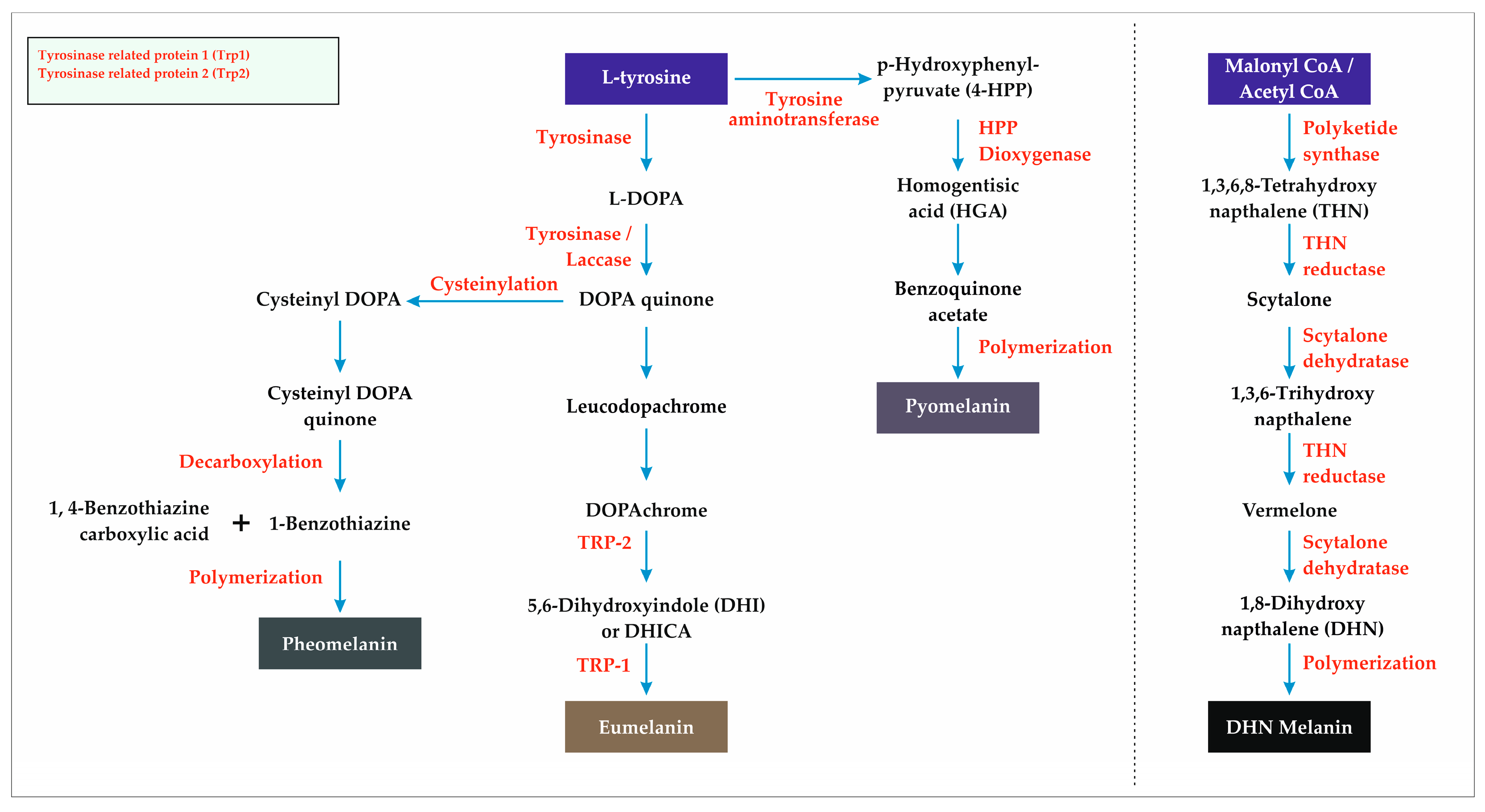
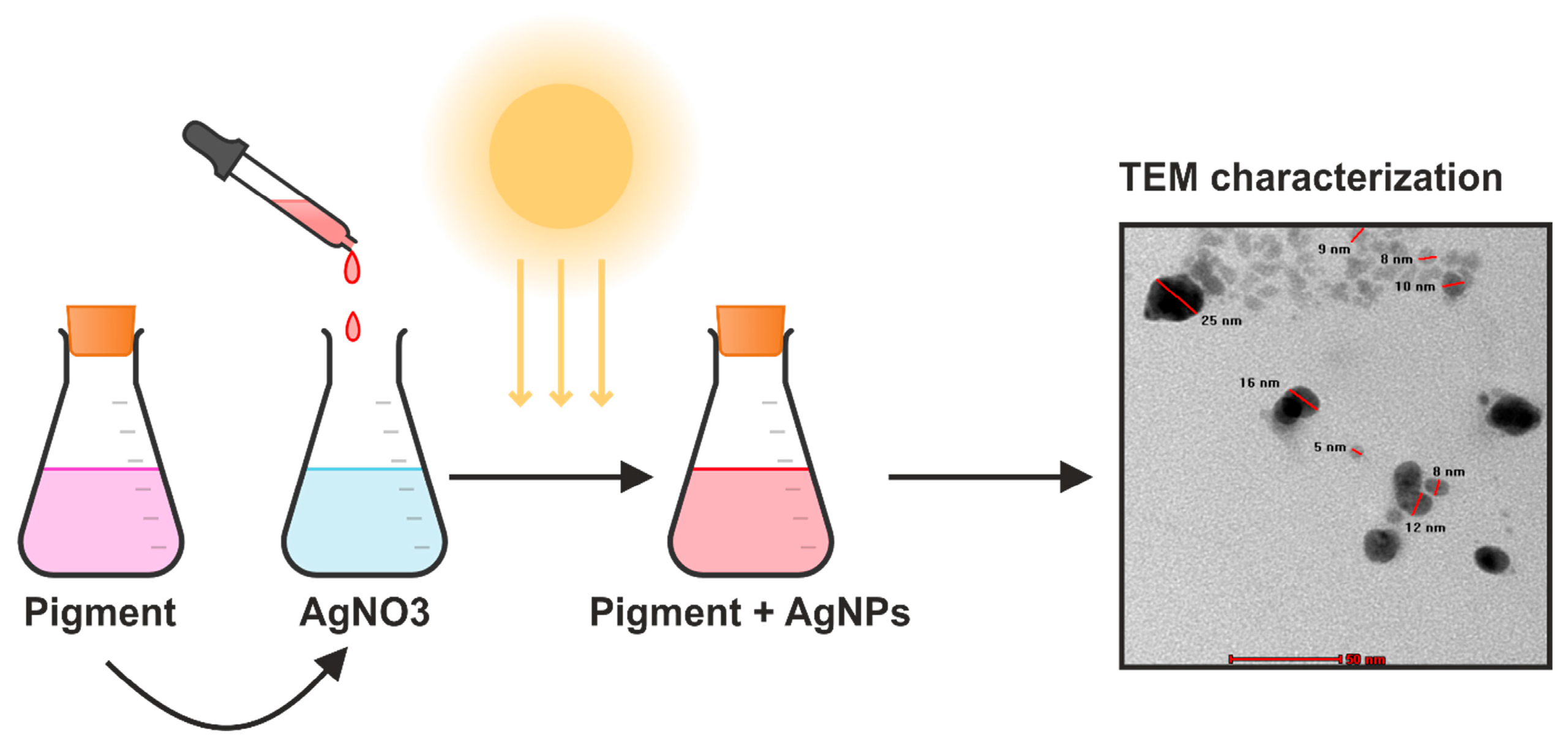
| Synthetic Food Colorants | Microbial Food Colorants | |
|---|---|---|
| Advantages | High stability against oxygen, light, and heat (>100 °C) High pH stability (stable across pH 2–9) High tinctorial strength Availability of all shades of color Synthetic food colorants are less expensive than natural food colorants Widely approved by FDA/EFSA, e.g., Tartrazine E102, Allura Red E129 | Biodegradable and environmentally friendly Generally regarded as safe and non-toxic Exhibit bioactivities such as anticancer, antimicrobial, and antioxidant activities, among others Can be produced using agro-industrial waste as a substrate (support the circular economy) |
| Disadvantages | Often linked to adverse health effects (carcinogenicity, hyperactivity in children, allergies) Environmental persistence and non-biodegradability | Relatively low stability towards oxygen, light, and heat (degrade above 60–80 °C) Low pH stability (stable between pH 4 and 8) Relatively low color saturation Not all shades of color are available Higher production costs and regulatory constraints Regulatory approval and large-scale standardization remain challenges Limited approvals by FDA/EFSA (e.g., β-carotene, Monascus pigment) |
| Pigment Name | Color | Industry Name | Producing Microorganism |
|---|---|---|---|
| Phycocyanin | Blue | Fermentalg | Arthrospira sp. (formerly Spirulina sp.) and Galdieria sulphuraria |
| Lycopene | Red | DSM Nutritional Products | Blakeslea trispora |
| β-Carotene | Yellow-orange | DSM Nutritional Products | Blakeslea trispora |
| β-Carotene | Yellow-orange | Henkel-Cognis Australia | Dunaliella salina |
| Astaxanthin | Pink-red | JX Nippon Oil & Energy | Paracoccus carotinifaciens |
| Astaxanthin | Pink-red | AstaReal | Haematococcus pluvialis |
| Anthraquinones | Red | Natural Red | Penicillium oxalicum and many others |
| Microorganisms | Pigment | Color | Notable Functional Benefit | Ref. |
|---|---|---|---|---|
| Agrobacterium aurantiacum | Astaxanthin | Pink-red | Antioxidant, anticancer, anti-inflammatory | [260] |
| Ashbya gossypi | Riboflavin | Yellow | Anticancer, antioxidant | [81] |
| Blakeslea trispora | B-carotene, Lycopene | Cream/red | Anticancer, antioxidant | [261] |
| Chlorococcum, Chlorella | Lutein | Yellow | Antioxidant | [262] |
| Cordyceps unilateralis | Naphtoquinone | Deep blood-red | Anticancer, antibacterial, trypanocidal | [263] |
| Dunaliella salina | B-Carotene | Red, orange | Suppression of cholesterol synthesis, antioxidant, anticancer | [264] |
| Erythrobacter sp. SDW2 | Xanthophyll | Yellow | Antioxidant activity and potential for foods, cosmetics, and pharmaceuticals | [265] |
| Flavobacterium sp., Paracoccus zeaxanthinifaciens | Zeaxanthin | Yellow | Antioxidant, photoprotectant | [266] |
| Fusarium sporotrichioides | Lycopene | Red | Anticancer, antioxidant | [261] |
| Haloferax volcanii | Carotenoids | Yellow | Anticancer activity (human liver carcinoma cell HepG2) | [267] |
| Halomonas sp. | Carotenoid | Yellow | Antimicrobial activity | [268] |
| Kocuria marina, Meiothermus | Carotenoids | Yellow | Antioxidant activity | [269] |
| Kocuria sp. QWT-12 | Carotenoids | Yellow | Anticancer (breast cancer cell lines MCF-7) | [269] |
| Micrococcus terreus | -------- | Yellow | Anticancer activity against cervical and liver cancer | [270] |
| Monascus purpureus | Monascin Ankaflavin | Red-yellow | Antitumor, anti-inflammatory | [271] |
| Pacilomyces farinosus | Anthraquinone | Red | Antifungal, virucidal | [272] |
| Paracoccus carotinifaciens | Astaxanthin | Pink-red | Antioxidant, anticancer, anti-inflammatory | [273] |
| Pedobacter | Carotenoids | Yellow | Antioxidant activity | [274] |
| Phaffia rhodozyma | Astaxanthin | Pink-red | Anti-inflammatory, antioxidant, anticancer | [275] |
| Porphyridium cruentum | Phycoerythrin | Red | Antioxidant, antitumor, immunoregulatory | [276] |
| Pseudoalteromonas rubra | Prodigiosin | Red | Immunosuppressant, anticancer | [277] |
| Pseudoalteromonas sp. | Prodigiosin | Red | Cytotoxicity against U937 leukemia cells | [278] |
| Pseudoalteromonas | Violoacein | Purple | Antioxidant activity | [279,280] |
| Pseudomonas aeruginosa | Phyocyanin | Blue-green | Ciliary dysmotility, cytotoxicity | [281] |
| Pseudomonas Aeruginosa P1.S9 | -------- | Blue-green | Cytotoxic activities, antibacterial, antioxidant | [282] |
| Pseudomonas balearica | Melanin | Black | Antimicrobial activity against phytopathogenic strains | [283] |
| Rhodococcus maris | -------- | Yellow | The risk of breast cancer was shown to be reduced | [284] |
| Rhodosporidium toruloides | Carotenoid | Yellow–orange-red | Antioxidant activity | [285] |
| Saccharomyces neoformans var. nigricans | Melanin | Black melanin | Antimicrobial, antibiofilm, antioxidant | [286] |
| Pseudomoalteromonas rubra | Prodigiosin | Red | Anticancer activity against human cervix carcinoma | [287] |
| Staphylococcus aureus | Staphyloxanthin Zeaxanthin | Golden yellow | Antioxidant | [288] |
| Streptomyces hygroscopicus | -------- | Yellow | Antibacterial activity against MRSA, VRSA, and ESBL cultures | [289] |
| Streptoverticillium rubrireticuli | Prodigiosin | Red | Antibacterial, antimalarial, antineoplastic | [290] |
| Vibrio owensii TNKJ.CR.24-7 (MH488980.1) | B Carotene | Yellow | Antibacterial activity | [291] |
| Xanthophyllomyces dendrorhous | Astaxanthin | Pink-red | Antioxidant, anticancer, anti-inflammatory | [275] |
| Microorganism | Pigment | NPs | Biomedical Applications | Ref. |
|---|---|---|---|---|
| Talaromyces purpurogenus | ----- | AgNPs | Antimicrobial and anticancer | [308] |
| Talaromyces australis | ----- | AgNPs | Antibacterial | [309] |
| Serratia nematodophila (NMCC 76), C. violaceum (KM226331) | ----- | AgNPs, AuNPs | Antiparasitic, antiplasmodial | [310] |
| Streptomyces coelicolor | Actinorhodin | AgNPs | Antibacterial | [311] |
| Xanthomonas sp. | Xanthomonadin | AgNPs | Photoprotecting, antioxidant | [312] |
| Monascus purpureus NRRL 1992 | ----- | AgNPs | Antibacterial, antifungal | [313] |
| Monascus ruber | ----- | AgNPs | Antibacterial, antioxidant, catalytic degradation of toxic dyes | [314] |
| Thermomyces sp. | Yellow | AgNPs | Textile application | [315] |
| Yarrowia lipolytica NCYC789 | Melanin | AuNPs | Antibacterial | [316] |
| Penicillium chrysogenum | Melanin | MgONPs | Antibacterial, antifungal | [317] |
| Gordonia amicalis HS-11 | Carotenoids | AuNPs | Free radical scavenging activity | [318] |
| Streptomycetes coelicolor | Actinorhodin | AgNPs | Enhancement of antibacterial activity | [311] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavan, A.; Pawar, J.; Kakde, U.; Venkatachalam, M.; Fouillaud, M.; Dufossé, L.; Deshmukh, S.K. Pigments from Microorganisms: A Sustainable Alternative for Synthetic Food Coloring. Fermentation 2025, 11, 395. https://doi.org/10.3390/fermentation11070395
Chavan A, Pawar J, Kakde U, Venkatachalam M, Fouillaud M, Dufossé L, Deshmukh SK. Pigments from Microorganisms: A Sustainable Alternative for Synthetic Food Coloring. Fermentation. 2025; 11(7):395. https://doi.org/10.3390/fermentation11070395
Chicago/Turabian StyleChavan, Akshay, Jaya Pawar, Umesh Kakde, Mekala Venkatachalam, Mireille Fouillaud, Laurent Dufossé, and Sunil Kumar Deshmukh. 2025. "Pigments from Microorganisms: A Sustainable Alternative for Synthetic Food Coloring" Fermentation 11, no. 7: 395. https://doi.org/10.3390/fermentation11070395
APA StyleChavan, A., Pawar, J., Kakde, U., Venkatachalam, M., Fouillaud, M., Dufossé, L., & Deshmukh, S. K. (2025). Pigments from Microorganisms: A Sustainable Alternative for Synthetic Food Coloring. Fermentation, 11(7), 395. https://doi.org/10.3390/fermentation11070395











