In Vitro Fermentation Characteristics of Pelagic Sargassum for Inclusion in Integral Diets for Ruminants
Abstract
1. Introduction
2. Materials and Methods
2.1. Location
2.2. Animal Management
2.3. Sample Preparation
2.4. In Vitro Trial and Experimental Design
2.5. In Vitro Gas Production
2.6. Fermentation Parameters
2.7. Chemical Analysis
2.8. Statistical Analysis
3. Results
3.1. Chemical Composition
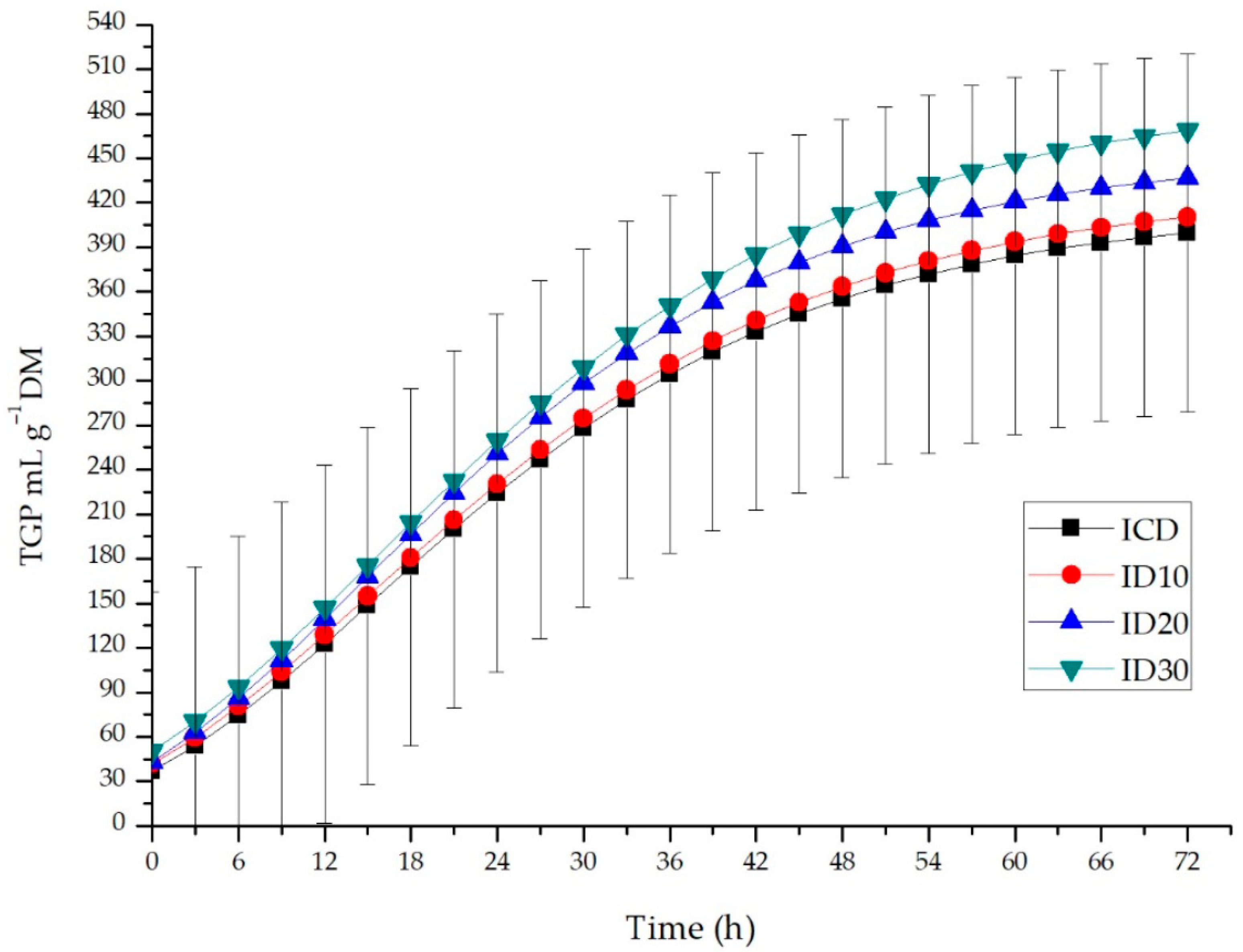
3.2. Total Gas Production and Characteristics of In Vitro Fermentation
3.3. Protozoan Population
4. Discussion
4.1. Total Gas Production and Characteristics of In Vitro Fermentation
4.2. Protozoa Population
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evans, F.D.; Critchley, A.T. Seaweeds for animal production use. J. Appl. Phycol. 2014, 26, 891–899. [Google Scholar] [CrossRef]
- Costa, M.; Cardoso, C.; Afonso, C.; Bandarra, N.M.; Prates, J.A.M. Current knowledge and future perspectives of the use of seaweeds for livestock production and meat quality: A systematic review. J. Anim. Physiol. Anim. Nutr. 2021, 105, 1075–1102. [Google Scholar] [CrossRef] [PubMed]
- Bikker, P.; van Krimpen, M.M.; van Wikselaar, P.; Houweling-Tan, B.; Scaccia, N.; van Hal, J.W.; Huijgen, W.J.J.; Cone, J.W.; López-Contreras, A.M. Biorefinery of the green seaweed Ulva lactuca to produce animal feed, chemicals and biofuels. J. Appl. Phycol. 2016, 28, 3511–3525. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, M.; Graham, C.; Vera, E.; Escalante-Mancera, E.; Álvarez-Filip, L.; van Tussenbroek, B.I. Temporal changes in the composition and biomass of beached pelagic Sargassum species in the Mexican Caribbean. Aquatic Botany 2020, 167, 103275. [Google Scholar] [CrossRef]
- Espinosa, L.A.; Li Ng, J.J. The risk of Sargassum to the economy and tourism of Quintana Roo and Mexico. BBVA Res. 2020, 20, 1–35. [Google Scholar]
- Lapointe, B.E.; Burkholder, J.M.; Van Alstyne, K.L. Harmful Macroalgal Blooms in a Changing World: Causes, Impacts, and Management. Harmful Algal Bloom. 2018, 515–560. [Google Scholar] [CrossRef]
- Resiere, D.; Mehdaoui, H.; Florentin, J.; Gueye, P.; Lebrun, T.; Blateau, A.; Viguier, J.; Valentino, R.; Brouste, Y.; Kallel, H.; et al. Sargassum seaweed health menace in the Caribbean: Clinical characteristics of a population exposed to hydrogen sulfide during the 2018 massive stranding. Clin. Toxicol. 2020, 59, 215–223. [Google Scholar] [CrossRef]
- Chávez, V.; Uribe-Martínez, A.; Cuevas, E.; Rodríguez-Martínez, R.E.; van Tussenbroek, B.I.; Francisco, V.; Estévez, M.; Celis, L.B.; Monroy-Velázquez, L.V.; Leal-Bautista, R.; et al. Massive influx of pelagic Sargassum spp. on the coasts of the Mexican Caribbean 2014–2020: Challenges and opportunities. Water 2020, 12, 2908. [Google Scholar] [CrossRef]
- Liranzo-Gómez, R.E.; García-Cortés, D.; Jáuregui-Haza, U. Adaptation and Sustainable Management of Massive Influx of Sargassum in the Caribbean. Procedia Environ. Sci. Eng. Manag. 2021, 8, 543–553. [Google Scholar]
- Rodríguez-Martínez, R.E.; Torres-Conde, E.G.; Jordán-Dahlgren, E. Pelagic Sargassum cleanup cost in Mexico. Ocean Coast. Manag. 2023, 237, 106542. [Google Scholar] [CrossRef]
- Cabrita, A.R.J.; Maia, M.R.G.; Oliveira, H.M.; Sousa-Pinto, I.; Almeida, A.A.; Pinto, E.; Fonseca, A.J.M. Tracing seaweeds as mineral sources for farm-animals. J. Appl. Phycol. 2016, 28, 3135–3150. [Google Scholar] [CrossRef]
- Belanche, A.; Ramos-Morales, E.; Newbold, C.J. In vitro screening of natural feed additives from crustaceans, diatoms, seaweeds and plant extracts to manipulate rumen fermentation. J. Sci. Food Agric. 2016, 96, 3069–3078. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.; Tomkins, N.; Magnusson, M.; Midgley, D.J.; de Nys, R.; Rosewarne, C.P. In Vitro Response of Rumen Microbiota to the Antimethanogenic Red Macroalga Asparagopsis taxiformis. Microb. Ecol. 2018, 75, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Lee, S.J.; Lee, Y.J.; Kim, H.S.; Eom, J.S.; Kim, S.C.; Kim, E.T.; Lee, S.S. New challenges for efficient usage of Sargassum fusiforme for ruminant production. Sci. Rep. 2020, 10, 19655. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Zhang, W.; Smid, S.D. Phlorotannins: A review on biosynthesis, chemistry and bioactivity. Food Biosci. 2021, 39, 100832. [Google Scholar] [CrossRef]
- Machado, L.; Magnusson, M.; Paul, N.A.; Kinley, R.; de Nys, R.; Tomkins, N. Dose-response effects of Asparagopsis taxiformis and Oedogonium sp. on in vitro fermentation and methane production. J. Appl. Phycol. 2015, 28, 1443–1452. [Google Scholar] [CrossRef]
- Vijay, K.; Balasundari, S.; Jeyashakila, R.; Velayathum, P.; Masilan, K. Proximate and mineral composition of brown seaweed from Gulf of Mannar. Int. J. Fish. Aquat. Stud. 2017, 5, 106–112. [Google Scholar]
- Choi, Y.Y.; Shin, N.H.; Lee, S.J.; Lee, Y.J.; Kim, H.S.; Eom, J.S.; Lee, S.S.; Kim, E.T.; Lee, S.S. In vitro five brown algae extracts for efficiency of ruminal fermentation and methane yield. J. Appl. Phycol. 2021, 33, 1253–1262. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, S.J.; Kim, H.S.; Eom, J.S.; Jo, S.U.; Guan, L.L.; Seo, J.; Kim, H.; Lee, S.S.; Lee, S.S. Effects of seaweed extracts on in vitro rumen fermentation characteristics, methane production, and microbial abundance. Sci. Rep. 2021, 11, 24092. [Google Scholar] [CrossRef]
- Maia, M.R.G.; Fonseca, A.J.M.; Cortez, P.P.; Cabrita, A.R.J. In vitro evaluation of macroalgae as unconventional ingredients in ruminant animal feeds. Algal Res. 2019, 40, 101481. [Google Scholar] [CrossRef]
- Roque, B.M.; Brooke, C.G.; Ladau, J.; Polley, T.; Marsh, L.J.; Najafi, N.; Pandey, P.; Singh, L.; Kinley, R.; Salwen, J.K.; et al. Effect of the macroalgae Asparagopsis taxiformis on methane production and rumen microbiome assemblage. Anim. Microbiome 2019, 1, 3. [Google Scholar] [CrossRef]
- Belanche, A.; Jones, E.; Parveen, I.; Newbold, C.J. A metagenomics approach to evaluate the impact of dietary supplementation with Ascophyllum nodosum or Laminaria digitata on rumen function in Rusitec fermenters. Front. Microbiol. 2016, 7, 299. [Google Scholar] [CrossRef] [PubMed]
- Milledge, J.J.; Maneein, S.; López, E.A.; Bartlett, D. Sargassum inundations in Turks and Caicos: Methane potential and proximate, ultimate, lipid, amino acid, metal and metalloid analyses. Energies 2020, 13, 1523. [Google Scholar] [CrossRef]
- Davis, D.; Simister, R.; Campbell, S.; Marston, M.; Bose, S.; McQueen-Mason, S.J.; Gomez, L.D.; Gallimore, W.A.; Tonon, T. Biomass composition of the golden tide pelagic seaweeds Sargassum fluitans and S. natans (morphotypes I and VIII) to inform valorisation pathways. Sci. Total Environ. 2021, 762, 143134. [Google Scholar] [CrossRef]
- Canul-Ku, L.A.; Sanginés-García, J.R.; Urquizo, E.A.; Canul-Solís, J.R.; Valdivieso-Pérez, I.A.; Vargas-Bello-Pérez, E.; Molina-Botero, I.; Arango, J.; Piñeiro-Vázquez, Á.T. Effect of Pelagic Sargassum on In Vitro Dry Matter and Organic Matter Degradation, Gas Production, and Protozoa Population. Animals 2023, 13, 1858. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, Y.R.; Oh, S.; Paik, M.J.; Je, J.G.; Heo, J.H.; Lee, T.K.; Fu, X.; Xu, J.; Gao, X.; et al. Arsenic removal from the popular edible seaweed Sargassum fusiforme by sequential processing involving hot water, citric acid, and fermentation. Chemosphere 2022, 292, 133409. [Google Scholar] [CrossRef]
- Rjiba-Ktita, S.; Chermiti, A.; Bodas, R.; France, J.; López, S. Aquatic plants and macroalgae as potential feed ingredients in ruminant diets. J. Appl. Phycol. 2017, 29, 449–458. [Google Scholar] [CrossRef]
- INEGI (Instituto Nacional de Estadística y Geografía). Standard for Quality Assurance of Statistical and Geographic Information of the National Institute of Statistics and Geography; INEGI: Aguascalientes, Mexico, 2014. [Google Scholar]
- García, E. Modifications to the Köppen Climate Classification System, 5th ed.; Autonomous University of Mexico: Mexico City, Mexico, 2004. [Google Scholar]
- NOM-062-Z00 1999; Technical Specifications for the Production, Care, and Use of Laboratory Animals. Norma Oficial Mexicana: Mexico City, Mexico, 2001.
- NRC. Nutrient Requirements of Small ruminants: Sheep, Goats, Cervids, and New World Camelids; National Academies Press: Washington, DC, USA, 2007.
- Ramos-Morales, E.; Arco-Pérez, A.; Martín-García, A.I.; Yáñez-Ruiz, D.R.; Frutos, P.; Hervás, G. Use of stomach tubing as an alternative to rumen cannulation to study ruminal fermentation and microbiota in sheep and goats. Anim. Feed Sci. Technol. 2014, 198, 57–66. [Google Scholar] [CrossRef]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Theodorou, M.K.; Williams, B.A.; Dhanoa, M.S.; McAllan, A.B.; France, J. A simple gas production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed Sci. Technol. 1994, 48, 185–197. [Google Scholar] [CrossRef]
- Beuvink, J.M.; Kogut, J. Modeling gas production kinetics of grass silages incubated with buffered ruminal fluid. J. Anim. Sci. 1993, 71, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Machado, L.; Magnusson, M.; Paul, N.A.; De Nys, R.; Tomkins, N. Effects of marine and freshwater macroalgae on in vitro total gas and methane production. PLoS ONE 2014, 9, e85289. [Google Scholar] [CrossRef] [PubMed]
- Ogimoto, K.; Imai, S. Atlas of Rumen Microbiology; Japan Scientific Societies: Tokyo, Japan, 1981. [Google Scholar]
- AOAC. Association of Official Analytical Chemists, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- SAS. Statistical Analysis Systems (SAS OnLine Doc. Version 8); Statistical Analysis Systems Institute: Cary, NC, USA, 1999. [Google Scholar]
- Munde, V.K.; Das, A.; Singh, P.; Verma, A.K.; Muwel, N.; Mishra, A.; Deb, R.; Raje, K. Influence of Kappaphycus Alvarezii and Gracilaria Salicornia Supplementation on in Vitro Fermentation Pattern, Total Gas and Methane Production of Mixed Substrates. Res. Sq. 2021, 1, 1–19. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Carro, M.D.; Roleda, M.Y.; Weisbjerg, M.R.; Lind, V.; Novoa-Garrido, M. In vitro ruminal fermentation and methane production of different seaweed species. Anim. Feed Sci. Technol. 2017, 228, 1–12. [Google Scholar] [CrossRef]
- de la Moneda, A.; Carro, M.D.; Weisbjerg, M.R.; Roleda, M.Y.; Lind, V.; Novoa-Garrido, M.; Molina-Alcaide, E. Variability and potential of seaweeds as ingredients of ruminant diets: An in vitro study. Animals 2019, 9, 851. [Google Scholar] [CrossRef]
- Zheng, Y.; Xue, S.; Zhao, Y.; Li, S. Effect of cassava residue substituting for crushed maize on in vitro ruminal fermentation characteristics of dairy cows at mid-lactation. Animals 2020, 10, 893. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Lee, S.J.; Lee, Y.J.; Kim, H.S.; Eom, J.S.; Jo, S.U.; Lee, S.S. In vitro and in situ evaluation of Undaria pinnatifida as a feed ingredient for ruminants. J. Appl. Phycol. 2019, 32, 729–739. [Google Scholar] [CrossRef]
- Choi, Y.Y.; Lee, S.J.; Kim, H.S.; Eom, J.S.; Kim, D.H.; Lee, S.S. The potential nutritive value of Sargassum fulvellum as a feed ingredient for ruminants. Algal Res. 2020, 45, 101761. [Google Scholar] [CrossRef]
- Widiawati, Y.; Hikmawan, D. Enteric methane mitigation by using seaweed Eucheuma cottonii. IOP Conf. Ser. Earth Environ. Sci. 2021, 788, 012152. [Google Scholar] [CrossRef]
- Maia, M.R.G.; Fonseca, A.J.M.; Oliveira, H.M.; Mendonça, C.; Cabrita, A.R.J. The potential role of seaweeds in the natural manipulation of rumen fermentation and methane production. Sci. Rep. 2016, 6, 32321. [Google Scholar] [CrossRef] [PubMed]
- Özkan Gülzari, S.; Lind, V.; Aasen, I.M.; Steinshamn, H. Effect of supplementing sheep diets with macroalgae species on in vivo nutrient digestibility, rumen fermentation and blood amino acid profile. Animal 2019, 13, 2792–2801. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, Y.; Wang, X.; Wei, Y.; Pan, M.; Zhao, G. Effects of Phlorotannins from Sargassum on In Vitro Rumen Fermentation, Microbiota and Fatty Acid Profile. Animals 2023, 13, 2854. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Jo, Y.H.; Ghassemi Nejad, J.; Lee, J.C.; Lee, H.G. Evaluation of nutritional value of Ulva sp. and Sargassum horneri as potential eco-friendly ruminant feed. Algal Res. 2022, 65, 102706. [Google Scholar] [CrossRef]
- Gaillard, C.; Bhatti, H.S.; Novoa-Garrido, M.; Lind, V.; Roleda, M.Y.; Weisbjerg, M.R. Amino acid profiles of nine seaweed species and their in situ degradability in dairy cows. Anim. Feed Sci. Technol. 2018, 241, 210–222. [Google Scholar] [CrossRef]
- Saldarriaga-Hernandez, S.; Melchor-Martínez, E.M.; Carrillo-Nieves, D.; Parra-Saldívar, R.; Iqbal, H.M.N. Seasonal characterization and quantification of biomolecules from sargassum collected from Mexican Caribbean coast—A preliminary study as a step forward to blue economy. J. Environ. Manag. 2021, 298, 113507. [Google Scholar] [CrossRef]
- Liu, L.; Heinrich, M.; Myers, S.; Dworjanyn, S.A. Towards a better understanding of medicinal uses of the brown seaweed Sargassum in Traditional Chinese Medicine: A phytochemical and pharmacological review. J. Ethnopharmacol. 2012, 142, 591–619. [Google Scholar] [CrossRef]
- Carrillo-Domínguez, S.; Rodríguez-Martínez, R.E.; Díaz-Martínez, M.; Magaña-Gallegos, E.; Cuchillo-Hilario, M. Potential application of pelagic Sargassum spp. in animal feeding. J. Appl. Phycol. 2023, 35, 433–444. [Google Scholar] [CrossRef]
- Vázquez-Delfín, E.; Freile-Pelegrín, Y.; Salazar-Garibay, A.; Serviere-Zaragoza, E.; Méndez-Rodríguez, L.C.; Robledo, D. Species composition and chemical characterization of Sargassum influx at six different locations along the Mexican Caribbean coast. Sci. Total Environ. 2021, 795, 148852. [Google Scholar] [CrossRef]
- Williams, A.G.; Withers, S.; Sutherland, A.D. The potential of bacteria isolated from ruminal contents of seaweed-eating North Ronaldsay sheep to hydrolyse seaweed components and produce methane by anaerobic digestion in vitro. Microb. Biotechnol. 2013, 6, 45–52. [Google Scholar] [CrossRef]
- Dai, X.; Kalscheur, K.F.; Huhtanen, P.; Faciola, A.P. Efectos de los protozoos ruminales en las emisiones de metano en rumiantes: Un metanálisis. Rev. Cienc. Láct. 2022, 105, 7482–7491. [Google Scholar] [CrossRef]
- Prayitno, C.H.; Hidayat, N. In Vitro Rumen Methanogenesis Inhibition Ability Of Brown Seaweed From Nusakambangan Coast, Cilacap, Indonesia. NVEO-Nat. Volatiles Essent. Oils J. 2021, 8, 2081–2089. [Google Scholar]
- Ford, L.; Stratakos, A.C.; Theodoridou, K.; Dick, J.T.A.; Sheldrake, G.N.; Linton, M.; Corcionivoschi, N.; Walsh, P.J. Polyphenols from Brown Seaweeds as a Potential Antimicrobial Agent in Animal Feeds. ACS Omega 2020, 5, 9093–9103. [Google Scholar] [CrossRef]
- Abbott, D.W.; Aasen, I.M.; Beauchemin, K.A.; Grondahl, F.; Gruninger, R.; Hayes, M.; Huws, S.; Kenny, D.A.; Krizsan, S.J.; Kirwan, S.F.; et al. Seaweed and seaweed bioactives for mitigation of enteric methane: Challenges and opportunities. Animals 2020, 10, 2432. [Google Scholar] [CrossRef] [PubMed]
- Antaya, N.T.; Ghelichkhan, M.; Pereira, A.B.D.; Soder, K.J.; Brito, A.F. Production, milk iodine, and nutrient utilization in Jersey cows supplemented with the brown seaweed Ascophyllum nodosum (kelp meal) during the grazing season. J. Dairy Sci. 2019, 102, 8040–8058. [Google Scholar] [CrossRef]
- Kinley, R.D.; Martinez-Fernandez, G.; Matthews, M.K.; de Nys, R.; Magnusson, M.; Tomkins, N.W. Mitigating the carbon footprint and improving productivity of ruminant livestock agriculture using a red seaweed. J. Clean. Prod. 2020, 259, 120836. [Google Scholar] [CrossRef]
- Roque, B.M.; Venegas, M.; Kinley, R.D.; De Nys, R.; Duarte, T.L.; Yang, X.; Kebreab, E. Red seaweed (Asparagopsis taxiformis) supplementation reduces enteric methane by over 80 percent in beef steers. PLoS ONE 2021, 16, e0247820. [Google Scholar] [CrossRef]
- Pradhan, B.; Nayak, R.; Bhuyan, P.P.; Patra, S.; Behera, C.; Sahoo, S.; Ki, J.S.; Quarta, A.; Ragusa, A.; Jena, M. Algal phlorotannins as novel antibacterial agents with reference to the antioxidant modulation: Current advances and future directions. Mar. Drugs 2022, 20, 403. [Google Scholar] [CrossRef]
- Bolkenov, B.; Duarte, T.; Yang, L.; Yang, F.; Roque, B.; Kebreab, E.; Yang, X. Effects of red macroalgae Asparagopsis taxiformis supplementation on the shelf life of fresh whole muscle beef. Transl. Anim. Sci. 2021, 5, 1–9. [Google Scholar] [CrossRef]
- Brooke, C.G.; Roque, B.M.; Shaw, C.; Najafi, N.; Gonzalez, M.; Pfefferlen, A.; De Anda, V.; Ginsburg, D.W.; Harden, M.C.; Nuzhdin, S.V.; et al. Methane Reduction Potential of Two Pacific Coast Macroalgae During in vitro Ruminant Fermentation. Front. Mar. Sci. 2020, 7, 1–7. [Google Scholar] [CrossRef]
- Demarco, M.; Oliveira de Moraes, J.; Matos, Â.P.; Derner, R.B.; de Farias Neves, F.; Tribuzi, G. Digestibility, bioaccessibility and bioactivity of compounds from algae. Trends Food Sci. Technol. 2022, 121, 114–128. [Google Scholar] [CrossRef]
- Cheong, K.L.; Zhang, Y.; Li, Z.; Li, T.; Ou, Y.; Shen, J.; Zhong, S.; Tan, K. Role of Polysaccharides from Marine Seaweed as Feed Additives for Methane Mitigation in Ruminants: A Critical Review. Polymers 2023, 15, 3153. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Van Truong, H.B.; Tran, N.H.V.; Quach, T.M.T.; Nguyen, T.N.; Bui, M.L.; Yuguchi, Y.; Thanh, T.T.T. Structure, conformation in aqueous solution and antimicrobial activity of ulvan extracted from green seaweed Ulva reticulata. Nat. Prod. Res. 2018, 32, 2291–2296. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; de Almeida, R.R.; Pedrosa, R.; et al. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, Y.; Chen, Z.; Li, D.; Liu, W.; Huang, L.; Zou, C.; Cao, M.J.; Liu, G.M.; Wang, Y. Antibacterial activity of sulfated galactans from Eucheuma serra and Gracilari verrucosa against diarrheagenic Escherichia coli via the disruption of the cell membrane structure. Mar. Drugs 2020, 18, 397. [Google Scholar] [CrossRef]
| Components | Sargassum | Treatments (Inclusion Level of Sargassum) | |||||
|---|---|---|---|---|---|---|---|
| ICD | ID10 | ID20 | ID30 | SEM | p Value | ||
| Ingredients and levels of inclusion (%) | |||||||
| CT-115 grass | 64.2 | 47.95 | 31.7 | 15.46 | |||
| Corn | 22.27 | 26.67 | 31.07 | 35.47 | |||
| Soybean | 3.54 | 5.38 | 7.23 | 9.07 | |||
| Molasses | 10 | 10 | 10 | 10 | |||
| Sargassum | 0 | 10 | 20 | 30 | |||
| Chemical composition (%) | |||||||
| DM | 94.11 | 94.05 a | 93.52 a | 92.62 a | 91.42 a | 0.440 | 0.0834 |
| OM | 58.89 | 90.09 d | 88.48 c | 86.62 b | 83.93 a | 0.170 | 0.0005 |
| CP | 6.73 | 12.00 a | 11.99 a | 12.05 a | 12.00 a | 0.070 | 0.7134 |
| aNDF | 45.04 | 52.04 c | 42.97 b | 37.06 a | 32.35 a | 0.840 | 0.0016 |
| ADF | 30.93 | 31.94 c | 26.19 b | 23.03 b | 19.02 a | 0.510 | 0.0013 |
| Lignina (sa) | 5.32 | 2.06 a | 2.24 a | 2.47 a | 2.49 a | 0.300 | 0.8144 |
| Time (h) | Treatments (Inclusion Level of Sargassum) | SEM | p Value | |||
|---|---|---|---|---|---|---|
| ICD | ID10 | ID20 | ID30 | |||
| Total gas production (mL g−1 DM) | ||||||
| 24 | 255.27 c | 278.19 b | 288.81 b | 311.10 a | 4.844 | ˂0.0001 |
| 48 | 342.00 b | 345.21 b | 375.96 a | 389.92 a | 6.382 | 0.0004 |
| 72 | 416.33 c | 428.83 bc | 453.62 b | 493.42 a | 5.985 | ˂0.0001 |
| DM degradability (g kg−1) | ||||||
| 0 | 375.68 b | 385.96 b | 471.29 a | 484.26 a | 6.282 | <0.0001 |
| 24 | 493.39 c | 519.75 b | 544.44 b | 592.57 a | 6.066 | <0.0001 |
| 48 | 509.07 c | 559.83 b | 587.54 ab | 613.92 a | 7.544 | <0.0001 |
| 72 | 523.34 c | 571.97 b | 594.25 b | 636.23 a | 7.484 | <0.0001 |
| OM degradability (g kg−1) | ||||||
| 0 | 349.29 b | 344.90 b | 428.17 a | 425.86 a | 6.074 | <0.0001 |
| 24 | 468.04 c | 492.43 bc | 511.82 b | 553.68 a | 6.419 | <0.0001 |
| 48 | 486.77 c | 536.07 b | 556.98 ab | 581.03 a | 8.390 | <0.0001 |
| 72 | 505.55 c | 551.96 b | 568.30 b | 604.97 a | 7.659 | <0.0001 |
| Time (h) | Treatments (Inclusion Level of Sargassum) | SEM | p Value | |||
|---|---|---|---|---|---|---|
| ICD | ID10 | ID20 | ID30 | |||
| pH | ||||||
| 24 | 6.83 | 6.88 | 6.93 | 6.93 | 0.027 | 0.0532 |
| 48 | 6.83 a | 6.89 a | 6.82 a | 6.69 b | 0.026 | 0.0010 |
| 72 | 6.77 | 6.83 | 6.87 | 6.80 | 0.033 | 0.2142 |
| Total protozoa (×105 cel/mL) log10 | ||||||
| 24 | 7.32 a | 7.03 ab | 6.99 ab | 6.75 b | 0.109 | 0.0247 |
| 48 | 6.71 | 6.59 | 6.83 | 6.64 | 0.120 | 0.5401 |
| 72 | 6.39 | 6.24 | 6.03 | 5.99 | 0.135 | 0.1789 |
| Total Entodinium (×105 cel/mL) log10 | ||||||
| 24 | 4.23 | 4.14 | 4.02 | 3.93 | 0.076 | 0.0834 |
| 48 | 3.74 | 3.69 | 3.81 | 3.70 | 0.086 | 0.7334 |
| 72 | 3.57 | 3.42 | 3.21 | 3.09 | 0.144 | 0.1462 |
| Total Holotrichs (×105 cel/mL) log10 | ||||||
| 24 | 3.09 | 2.89 | 2.97 | 2.82 | 0.076 | 0.1289 |
| 48 | 2.97 | 2.89 | 3.01 | 2.94 | 0.102 | 0.8630 |
| 72 | 2.82 | 2.82 | 2.82 | 2.89 | 0.037 | 0.4262 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canul-Ku, L.A.; Casanova-Lugo, F.; Aguilar-Urquizo, E.; Valdivieso-Pérez, I.; Arcos-Álvarez, D.; Canul-Solís, J.; Castillo-Sánchez, L.; Chay-Canul, A.; Dzib-Castillo, B.; Piñeiro-Vázquez, A. In Vitro Fermentation Characteristics of Pelagic Sargassum for Inclusion in Integral Diets for Ruminants. Fermentation 2025, 11, 390. https://doi.org/10.3390/fermentation11070390
Canul-Ku LA, Casanova-Lugo F, Aguilar-Urquizo E, Valdivieso-Pérez I, Arcos-Álvarez D, Canul-Solís J, Castillo-Sánchez L, Chay-Canul A, Dzib-Castillo B, Piñeiro-Vázquez A. In Vitro Fermentation Characteristics of Pelagic Sargassum for Inclusion in Integral Diets for Ruminants. Fermentation. 2025; 11(7):390. https://doi.org/10.3390/fermentation11070390
Chicago/Turabian StyleCanul-Ku, Luis Alberto, Fernando Casanova-Lugo, Edgar Aguilar-Urquizo, Ingrid Valdivieso-Pérez, Darwin Arcos-Álvarez, Jorge Canul-Solís, Luis Castillo-Sánchez, Alfonso Chay-Canul, Benito Dzib-Castillo, and Angel Piñeiro-Vázquez. 2025. "In Vitro Fermentation Characteristics of Pelagic Sargassum for Inclusion in Integral Diets for Ruminants" Fermentation 11, no. 7: 390. https://doi.org/10.3390/fermentation11070390
APA StyleCanul-Ku, L. A., Casanova-Lugo, F., Aguilar-Urquizo, E., Valdivieso-Pérez, I., Arcos-Álvarez, D., Canul-Solís, J., Castillo-Sánchez, L., Chay-Canul, A., Dzib-Castillo, B., & Piñeiro-Vázquez, A. (2025). In Vitro Fermentation Characteristics of Pelagic Sargassum for Inclusion in Integral Diets for Ruminants. Fermentation, 11(7), 390. https://doi.org/10.3390/fermentation11070390





