Incorporation of Agglomerated Spirulina platensis Powder in Yogurt: A Strategy for Enhancing Nutritional Quality and Bioactive Compounds
Abstract
1. Introduction
2. Materials and Methods
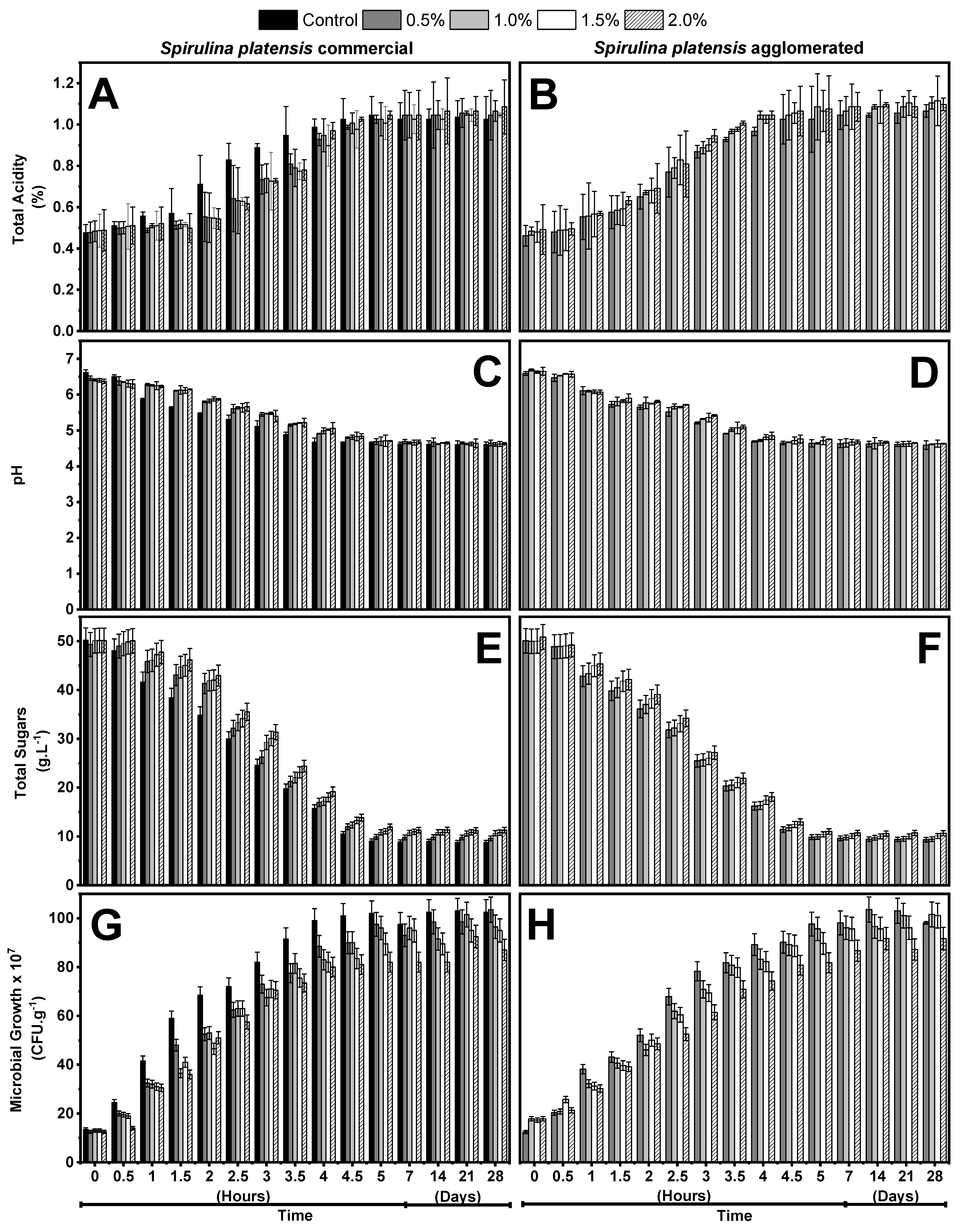
2.1. Kinetics of the Fermentation Process
2.2. Physicochemical Characterization (Nutritional Composition)
2.3. Bioactive Compounds
2.4. Statistical Analyses
3. Results and Discussion
3.1. Kinetics of the Fermentation Process
3.2. Nutritional Composition of the Yogurts
3.3. Bioactive Compounds in the Incorporated Yogurts
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| D50 | D50 size at percentile 50% [μm] |
| DE | dextrose equivalent value |
| LAB | lactic acid bacteria |
| pH | hydrogen potential |
| CFU | Colony Forming Units per gram [CFU.g−1] |
| v | volume of a sample or sodium hydroxide solution used in the titration [mL] |
| N | normality of the sodium hydroxide solution |
| f | standardization factor of the sodium hydroxide solution |
| w | weight of asample |
| eq | gram equivalent expressed in acid |
References
- Çelekli, A.; Özbal, B.; Bozkurt, H. Challenges in functional food products with the incorporation of some microalgae. Foods 2024, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Ruiz, F.E.; Andrade-Bustamante, G.; Holguín-Peña, R.J.; Renganathan, P.; Gaysina, L.A.; Sukhanova, N.V.; Puente, E.O.R. Microalgae as Functional Food Ingredients: Nutritional Benefits, Challenges, and Regulatory Considerations for Safe Consumption. Biomass 2025, 5, 25. [Google Scholar] [CrossRef]
- Algae Product Market Report. Available online: https://www.factmr.com/report/845/algae-products-market (accessed on 20 April 2025).
- Beheshtipour, H.; Mortazavian, A.M.; Haratiano, P.; Khosravi-Darani, K. Effects of the addition of Chlorella vulgaris and Arthrospira platensis on the viability of probiotic bacteria in yogurt and their biochemical properties. Eur. Food Res. Technol. 2012, 235, 719–728. [Google Scholar] [CrossRef]
- Bullock, Y.; Gruen, I. Effect of strained yogurt on the physicochemical, textural and sensory properties of low-fat frozen desserts. Food Chem. Adv. 2023, 2, 100161. [Google Scholar] [CrossRef]
- Devnani, B.; Ong, L.; Kentish, S.; Scales, P.J.E.; Gras, S.L. Physicochemical and rheological properties of commercial almond-based yogurts as alternatives to dairy and soy yogurts. Future Foods 2022, 6, 100185. [Google Scholar] [CrossRef]
- Fan, X.; Yu, L.; Shi, Z.; Li, C.; Zeng, X.; Wu, Z.; Pan, D. Characterization of a novel flavored yogurt enriched in γ-aminobutyric acid fermented by Levilactobacillus brevis CGMCC1.5954. J. Dairy Sci. 2022, 106, 852–867. [Google Scholar] [CrossRef]
- Mesbah, E.; Matar, A.; Karam-Allah, A. Functional Properties of Yogurt Fortified with Spirulina platensis and Concentrated Milk Protein. J. Food Dairy Sci. 2022, 13, 1–7. [Google Scholar] [CrossRef]
- Aktar, T. Physicochemical and sensorial characterization of different yogurt production methods. Int. Dairy J. 2021, 125, 105245. [Google Scholar] [CrossRef]
- Jaeger, S.R.; Cardello, A.V.; Jin, D.; Ryan, G.S.; Giacalone, D. Consumer perception of plant-based yogurt: Sensory drivers of taste and emotional, holistic and conceptual associations. Int. Food Res. J. 2023, 167, 112666. [Google Scholar] [CrossRef]
- Aleman, R.S.; Cedillos, R.; Page, R.; Olson, D.; Aryana, K. Physicochemical, microbiological and sensory characteristics of yogurt affected by various ingredients. J. Dairy Sci. 2023, 106, 3868–3883. [Google Scholar] [CrossRef]
- Hernández, H.M.; Nunes, M.T.; Prista, C.; Raymundo, A. Innovative and healthier dairy products through the addition of microalgae: A review. Foods 2021, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.A.B.; Parvin, M.; Huntington, T.C.; Hasan, M.R. A Review on Culture, Production and Use of Spirulina platensis as Food for Humans and Feeds for Domestic Animals and Fish; FAO Fisheries and Aquaculture Circular No. 1034; FAO: Rome, Italy, 2008; pp. 1–33. [Google Scholar]
- Kraus, A. Factors influencing the decisions to buy and consume functional food. Br. Food J. 2015, 117, 1622–1636. [Google Scholar] [CrossRef]
- Pan-Utai, W.; Atkonghan, J.; Onsamark, T.; Imthalay, W. Effect of Arthrospira Microalga Fortification on the Physicochemical Properties of Yogurt. Curr. Res. Nutr. Food Sci. 2020, 8, 531–540. [Google Scholar]
- Shimamatsu, H. Mass production of Spirulina platensis an edible microalga. Hydrobiology 2004, 512, 39–44. [Google Scholar] [CrossRef]
- López, E.P. Superalimento para un mundo en crisis: Spirulina platensis a bajo costo. Idesia 2013, 31, 135–139. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef]
- Fröhlich, J.A.; Ruprecht, N.A.; Hinrichs, J.; Kohlus, R. Nozzle zone agglomeration in spray dryers: Effect of powder addition on particle coalescence. Powder Technol. 2020, 374, 223–232. [Google Scholar] [CrossRef]
- Albuquerque, R.C.V.; Silva, C.E.D.F.; Carneiro, W.D.S.; Andreola, K.; Gama, B.M.V.; Silva, A.E.D. Incorporation of Cyanobacteria and Microalgae in Yogurt: Formulation Challenges and Nutritional, Rheological, Sensory, and Functional Implications. Appl. Microbiol. 2024, 4, 1493–1514. [Google Scholar] [CrossRef]
- Avila-Leon, I.; Matsudo, M.C.; Sato, S.; De Carvalho, J.C.M. Arthrospira platensis biomass with high protein content cultivated in continuous process using urea as nitrogen source. J. Appl. Microbiol. 2012, 6, 1086–1094. [Google Scholar] [CrossRef]
- ANVISA—Agência Nacional de Vigilância Sanitária. Instrução Normativa—In N° 28, de 26 de Julho de 2018. Diário Oficial da União, 144th ed.; Seção: 1; ANVISA: Brasília, Brazil, 2018; 141p.
- Ahmad, I.; Hao, M.; Li, Y.; Zhang, J.; Ding, Y.; Lyu, F. Fortification of yogurt with bioactive functional foods and ingredients and associated challenges—A review. Trends Food Sci. 2022, 129, 558–580. [Google Scholar] [CrossRef]
- Lafarga, T. Effect of Microalgal Biomass Incorporation into Foods: Nutritional and Sensorial Attributes of the End Products. Algal Res. 2019, 41, 101566. [Google Scholar] [CrossRef]
- Robertson, R.C.; Mateo, M.R.G.; O’Grady, M.N.; Guihéneuf, F.; Stengel, D.B.; Ross, R.P.; Fitzgerald, G.F.; Kerry, J.P.; Stanton, C. An assessment of the techno-functional and sensory properties of yoghurt fortified with a lipid extract from the microalga Pavlova lutheri. Innov. Food Sci. Emerg. Technol. 2016, 37, 237–246. [Google Scholar] [CrossRef]
- Bchir, B.; Felfoul, I.; Bouaziz, M.A.; Garred, T.; Yaich, H.; Numi, E. Investigation of the physicochemical, nutritional, textural and sensory properties of yogurt fortified with fresh and dried Spirulina (Arthrospira platensis). Int. Food Res. 2019, 1, 65–76. [Google Scholar]
- Vo, H.H.; Tran, K.D.; Le-Thi, L.; Nguyen-Thi, N.N.; Nguyen-Van, T.; Dinh-Thi, T.V.; Pham, T.A.; Nguyen-Thi, T.; Vu-Thi, T. The Effect of the Addition of Spirulina spp. on the Quality Properties, Health Benefits, and Sensory Evaluation of Green Tea Kombucha. Food Biophys. 2024, 19, 911–922. [Google Scholar] [CrossRef]
- Nourmohammadi, N.; Soleimanian-Zad, S.; Shekarchizadeh, H. Effect of Spirulina (Arthrospira platensis) microencapsulated in alginate and whey protein concentrate addition on physicochemical and organoleptic properties of functional stirred yogurt. J. Sci. Food Agric. 2020, 100, 5260–5268. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.K.F.; Moreira, J.B.; Costa, J.A.V.; De Souza, C.K.; Bertoli, S.L.; Carvalho, L.F.D. Evaluation of Adding Spirulina to Freeze-Dried Yogurts Before Fermentation and After Freeze-Drying. Ind. Biotechnol. 2019, 15, 89–94. [Google Scholar] [CrossRef]
- Luwidharto, J.C.N.; Rahayu, E.S.; Suroto, D.A.; Wikandari, R.; Ulfah, A.; Utami, T. Effects of Spirulina platensis addition on growth of Lactobacillus plantarum Dad 13 and Streptococcus thermophilus Dad 11 in fermented milk and physicochemical characteristics of the product. Appl. Food Biotechnol. 2022, 9, 205–216. [Google Scholar]
- Carneiro, W.S.; Andreola, K.; Silva, C.E.F.; Gama, B.M.V.; Albuquerque, R.C.V.; Freitas, J.M.D.; Freitas, J.D. Agglomeration Process of Spirulina platensis Powder in Fluidized Bed improves its Flowability and Wetting Capacity. ACS Food Sci. Technol. 2024, 4, 3120–3134. [Google Scholar] [CrossRef]
- Silva, P.I.; Stringheta, P.C.; Teófilo, R.F.; de Oliveira, I.R.N. Parameter optimization for spray-drying microencapsulation of jaboticaba (Myrciaria jaboticaba) peel extracts using simultaneous analysis of responses. J. Food Eng. 2013, 117, 538–544. [Google Scholar] [CrossRef]
- Özkan, G.; Bilek, S.E. Microencapsulation of natural food colourants. Int. J. Nutr. Food Sci. 2014, 3, 145–156. [Google Scholar] [CrossRef]
- Laureanti, E.J.G.; Paiva, T.S.; de Matos Jorge, L.M.; Jorge, R.M.M. Microencapsulation of bioactive compound extracts using maltodextrin and gum arabic by spray and freeze-drying techniques. Int. J. Biol. Macromol. 2023, 253, 126969. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.A.M.; Soldi, C.L.; Caveião, C.; Sales, W.B. Contagem de bactérias láticas viáveis em leites fermentados. Rev. Univap 2018, 24, 94–104. [Google Scholar] [CrossRef]
- Sousa, T.L.T.L.; Silva, A.M.S.; Silva, M.K.G.; Lima, G.S.; Veloso, R.R.; Shinohara, N.K.S. Counting of lactic bacteria in yogurts and dairy drinks in the metropolitan region of Recife-PE. Res. Soc. Dev. 2022, 11, e157111537002. [Google Scholar] [CrossRef]
- Trevelyan, W.E.; Forrest, R.S.; Harrison, J.S. Determination of yeast carbohydrates with the anthrone reagent. Nature 1952, 170, 626–627. [Google Scholar] [CrossRef]
- Instituto Adolfo Lutz. Métodos Físico-Químicos Para Análise de Alimentos, 4th ed.; Instituto Adolfo Lutz, Ed.; Instituto Adolfo Lutz: São Paulo, Brazil, 2005. [Google Scholar]
- AOAC. Official Method of Analysis, 16th ed.; Association of Official Analytical: Washington, DC, USA, 2002. [Google Scholar]
- Waterhouse, A.L. PoMANYlyphenolics: Determination of total phenolics. In WROLSTAD, R.E. Currente Protocols in Food Analytical Chemistry; Wiley, J., Ed.; EUA: New York, NY, USA, 2002; Volume 1, pp. 11–18. [Google Scholar]
- Nagata, M.; Yamashita, I. Simple method for simultaneous etermination of chlorophyll and carotenoids in tomato fruit. J. Jpn. Soc. Food Sci. Technol. 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Silveira, S.T.; Burkert, J.F.M.; Costa, J.A.V.; Burkert, C.A.V.; Kalil, S.J. Optimization of phycocyanin extraction from Spirulina. platensis using factorial design. Bioresour. Technol. 2007, 98, 1629–16934. [Google Scholar] [CrossRef]
- Deindoerfer, F.H.; West, J.M. Rheological examination of some fermentation broths. J. Biochem. Microb. Technol. Eng. 1960, 2, 165–175. [Google Scholar] [CrossRef]
- Gaden, E.L., Jr. Fermentation process kinetics. J. Biochem. Microb. Technol. Eng. 1959, 1, 413–429. [Google Scholar] [CrossRef]
- Schmidell, W.; de Almeida Lima, U.; Borzani, W.; Aquarone, E. Biotecnologia industrial. In Engenharia Bioquímica; Editora Blucher: São Paulo, Brazil, 2001; Volume 2. [Google Scholar]
- Brasil. Regulamento Técnico de Identidade e Qualidade de Leites Fermentados. Instrução Normativa Nº 46 de 23 de Outubro de 2007. Available online: https://www.studocu.com/pt-br/document/centro-universitario-uniftc/clinica-cirurgica/instrucao-normativa-n-46-de-23-de-outubro-de-2007-leites-fermentados/38234614 (accessed on 7 June 2023).
- CXS 243-2003; Codex Alimentarius. Standard for Fermented Milk. OMC. Food and Agriculture Organization of the United Nations: Rome, Italy, 2003.
- Matos, J.; Afonso, C.; Cardoso, C.; Serralheiro, M.L.; Bandarra, N.M. Yogurt enriched with Isochrysis galbana: An innovative functional food. Foods 2021, 10, 1458. [Google Scholar] [CrossRef]
- Ebid, W.M.; Ali, G.S.; Elewa, N.A. Impact of Spirulina platensis on the physicochemical, antioxidant, microbiological and sensory properties of functional labneh. Discov. Food 2022, 2, 29. [Google Scholar] [CrossRef]
- Atallah, A.; Morsy, O.; Dalia, G. Characterization of functional low-fat yogurt enriched with whey protein concentrate, Ca-caseinate and Spirulina. Int. J. Food Prop. 2020, 23, 78–91. [Google Scholar] [CrossRef]
- Part, N.; Kazantseva, J.; Rosenvald, S.; Kallastu, A.; Vaikma, H.; Kriščiunaite, T.; Pismennõi, D.; Viiard, E. Microbiological, chemical and sensorial characterization of commercially available vegetable yogurt alternatives. Future Foods 2023, 7, 100212. [Google Scholar] [CrossRef]
- Barkallah, M.; Dammak, M.; Louati, I.; Hentati, F.; Hadrich, B.; Mechichi, T.; Ayadi, M.A.; Fendri, I.; Attia, H.; Abdelkafi, S. Effect of Spirulina platensis fortification on the physicochemical, textural, antioxidant and sensory properties of yogurt during fermentation and storage. LWT 2017, 84, 323–330. [Google Scholar] [CrossRef]
- Nazir, F.; Saeed, M.A.; Abbas, A.; Majeed, M.R.; Israr, M.; Zahid, H.; Ilyas, M.; Nasir, M. Development, quality assessment and nutritional enhancement of Spirulina platensis in yogurt paste. Food Sci. Appl. Biotechnol. 2022, 5, 106. [Google Scholar] [CrossRef]
- Zaid, A.A.; Hammad, D.M.; Sharaf, E.M. Antioxidant and Anticancer Activity of S. platensis Water Extracts. Int. J. Pharmacol. 2015, 11, 846–851. [Google Scholar] [CrossRef]
- Jesus, C.S.; Uebel, L.S.; Costa, S.S.; Miranda, A.L.; Morais, E.G.; Morais, M.G.; Costa, J.A.V.; Nunes, I.L.; Ferreira, E.S.; Druzian, J.I. Outdoor pilot-scale cultivation of Spirulina sp. LEB-18 in different geographic locations for evaluating its growth and chemical composition. Bioresour. Technol. 2018, 256, 86–94. [Google Scholar] [CrossRef]
- Abdelhamid, S.M.; Edris, A.E.; Sadek, Z. Novel approach for the inhibition of Helicobacter pylori contamination in yogurt using selected probiotics combined with eugenol and cinnamaldehyde nanoemulsions. Food Chem. 2023, 417, 135877. [Google Scholar] [CrossRef]
- Ahmad, I.; Xiong, Z.; Xiong, H.; Aadil, R.M.; Khalid, N.; Lakhoo, A.B.J.; Zia-Ud-Din, N.A.W.A.Z.A.; Walayat, N.; Khan, R.S. Physicochemical, rheological and antioxidant profile of yogurt prepared from non-enzymatically and enzymatically hydrolyzed potato powder under refrigeration. Food Sci. Hum. Wellbeing 2023, 12, 69–78. [Google Scholar] [CrossRef]
- Chen, C.; Tang, T.; Shi, Q.; Zhou, Z.; Fan, J. The potential and challenge of microalgae as promising future food sources. Trends Food Sci. Technol. 2022, 126, 99–112. [Google Scholar] [CrossRef]
- Jensen, G.S.; Drapeau, C.; Lenninger, M.; Benson, K.F. Clinical safety of a high dose of phycocyanin-enriched aqueous extract from Arthrospira (Spirulina) platensis: Results from a randomized, double-blind, placebo-controlled study with a focus on anticoagulant activity and platelet activation. J. Med. Food. 2016, 19, 645–653. [Google Scholar] [CrossRef]
- Soni, A.; Dubey, M.; Verma, M.; Dhankhar, R.; Kaushal, V.; Atri, R.; Sabharwal, R. Revisiting the role of phycocyanin in current clinical practice. Int. J. Pharm. Sci. Res. 2015, 6, 4588. [Google Scholar]
- Patel, A.; Mishra, S.; Ghosh, P.K. Antioxidant potential of C-phycocyanin isolated from cyanobacterial species Lyngbya, Phormidium and Spirulina spp. Indian J. Biochem. Biophys. 2006, 43, 25–31. [Google Scholar]
- Yamamoto, N.; Shoji, M.; Hoshigami, H.; Watanabe, K.; Takatsuzu, T.; Yasuda, S.; Igoshi, K.; Kinoshita, H. Antioxidant capacity of soymilk yogurt and exopolysaccharides produced by lactic acid bacteria. Biosci. Microbiota Food Health 2019, 38, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kong, X.; Zhang, C.; Hua, Y.; Chen, Y.; Li, X. Comparison of physicochemical properties and volatile flavor compounds of vegetable yogurt and dairy yogurt. Int. Food Res. J. 2023, 164, 112375. [Google Scholar] [CrossRef] [PubMed]
- Castaño Peláez, H.I.; Cortés-Rodríguez, M.; Ortega-Toro, R. Storage stability of a fluidized-bed agglomerated spray-dried strawberry powder mixture. F1000Research 2023, 12, 1174. [Google Scholar] [CrossRef]
- Gallón-Bedoya, M.; Cortés-Rodríguez, M.; Gil-González, J.; Lahlou, A.; Guil-Guerrero, J.L. Influence of storage variables on the antioxidant and antitumor activities, phenolic compounds and vitamin C of an agglomerate of Andean berries. Heliyon 2023, 9, e14857. [Google Scholar] [CrossRef]

| Sample | Total Acidity (%) | pH | Total Sugars (g.L−1) | Microbial Count * × 107 (CFU.g−1) |
|---|---|---|---|---|
| Control | 1.031 ± 0.021 d | 4.64 ± 0.02 a | 8.74 ± 0.36 g | 103 ± 4.24 a |
| Spirulina platensis commercial | ||||
| 0.5% | 1.051 ± 0.007 d | 4.66 ± 0.01 a | 9.61 ± 0.16 de | 103 ± 6.90 a |
| 1.0% | 1.061 ± 0.007 cd | 4.63 ± 0.03 a | 10.66 ± 0.33 bc | 97 ± 4.24 a |
| 1.5% | 1.036 ± 0.014 d | 4.64 ± 0.02 a | 10.77 ± 0.16 ab | 95 ± 7.07 a |
| 2.0% | 1.056 ± 0.014 cd | 4.64 ± 0.01 a | 11.29 ± 0.08 ab | 87 ± 4.24 a |
| Spirulina platensis agglomerated with maltodextrin 30% (w/v) | ||||
| 0.5% | 1.051 ± 0.007 cd | 4.66 ± 0.01 a | 9.32 ± 0.28 eg | 99 ± 3.41 a |
| 1.0% | 1.096 ± 0.013 abc | 4.63 ± 0.04 a | 9.44 ± 0.38 dg | 103 ± 6.90 a |
| 1.5% | 1.110 ± 0.007 a | 4.63 ± 0.04 a | 10.07 ± 0.16 cdf | 102 ± 7.31 a |
| 2.0% | 1.092 ± 0.008 abc | 4.64 ± 0.01 a | 10.65 ± 0.16 abf | 88 ± 8.83 a |
| Sample | Protein (%) | Lipids (%) | Moisture (%) | Ash (%) | Carbohydrates (%) |
|---|---|---|---|---|---|
| Control | 3.96 ± 0.05 d | 3.13 ± 0.21 a | 83.10 ± 1.30 a | 1.10 ± 0.20 d | 8.74 ± 0.28 c |
| Spirulina platensis commercial | |||||
| 0.5% | 5.44 ± 0.26 a | 2.28 ± 0.15 c | 81.48 ± 1.60 a | 1.31 ± 0.10 cd | 9.49 ± 0.70 b |
| 1.0% | 5.74 ± 0.03 a | 2.41 ± 0.15 bc | 80.15 ± 1.86 a | 1.52 ± 0.10 b | 9.26 ± 0.50 b |
| 1.5% | 5.74 ± 0.03 a | 2.52 ± 0.16 bc | 80.25 ± 2.12 a | 1.65 ± 0.14 a | 9.86 ± 0.49 b |
| 2.0% | 5.63 ± 0.54 a | 2.53 ± 0.12 bc | 80.18 ± 2.52 a | 1.86 ± 0.20 a | 10.05 ± 0.32 ab |
| Spirulina platensis agglomerated with maltodextrin 30% (w/v) | |||||
| 0.5% | 4.33 ± 0.30 c | 2.42 ± 0.15 c | 82.66 ± 1.10 a | 1.27 ± 0.05 cd | 9.68 ± 0.14 b |
| 1.0% | 4.81 ± 0.25 bc | 2.64 ± 0.13 bc | 81.14 ± 2.10 a | 1.56 ± 0.12 ab | 10.55 ± 0.16 a |
| 1.5% | 5.41 ± 0.36 a | 2.70 ± 0.18 ab | 80.14 ± 1.85 a | 1.67 ± 0.10 a | 10.43 ± 0.11 a |
| 2.0% | 5.49 ± 0.57 ab | 2.79 ± 0.22 ab | 80.13 ± 1.92 a | 1.87 ± 0.10 a | 10.36 ± 0.13 a |
| Sample | Phenolic Compounds (mg.100 g−1) | Phycocyanin (mg.100 g−1) | β-Carotene (mg.100 g−1) | Chlorophyll a (mg.100 g−1) |
|---|---|---|---|---|
| Control | 5.58 ± 0.02 e | 0.00 ± 0.00 d | 0.00 ± 0.00 g | 0.00 ± 0.00 e |
| Spirulina platensis commercial | ||||
| 0.5% | 2.98 ± 0.02 f | 2.19 ± 0.03 c | 4.73 ± 0.05 f | 12.39 ± 0.04 d |
| 1.0% | 5.63 ± 0.06 e | 3.39 ± 0.15 a | 5.09 ± 0.02 e | 12.70 ± 0.01 d |
| 1.5% | 11.20 ± 0.05 c | 3.57 ± 0.17 a | 5.34 ± 0.06 c | 13.28 ± 0.16 c |
| 2.0% | 13.62 ± 0.02 b | 3.65 ± 0.13 a | 5.63 ± 0.01 b | 14.07 ± 0.03 a |
| Spirulina platensis agglomerated with maltodextrin 30% (w/v) | ||||
| 0.5% | 11.02 ± 0.02 c | 3.07 ± 0.04 b | 5.06 ± 0.03 e | 12.43 ± 0.08 d |
| 1.0% | 12.00 ± 0.42 c | 3.12 ± 0.19 ab | 5.24 ± 0.01 d | 12.48 ± 0.04 d |
| 1.5% | 13.19 ± 0.32 b | 3.06 ± 0.01 b | 5.49 ± 0.11 c | 12.60 ± 0.19 d |
| 2.0% | 14.96 ± 0.13 a | 3.13 ± 0.06 b | 6.37 ± 0.03 a | 13.77 ± 0.12 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albuquerque, R.C.V.; de Farias Silva, C.E.; Silva, M.C.d.S.; Carneiro, W.d.S.; Andreola, K.; Gama, B.M.V.d.; Figueiredo, M.V.A.; Silva, A.E.d.; Silva, J.V.O.N.d. Incorporation of Agglomerated Spirulina platensis Powder in Yogurt: A Strategy for Enhancing Nutritional Quality and Bioactive Compounds. Fermentation 2025, 11, 389. https://doi.org/10.3390/fermentation11070389
Albuquerque RCV, de Farias Silva CE, Silva MCdS, Carneiro WdS, Andreola K, Gama BMVd, Figueiredo MVA, Silva AEd, Silva JVONd. Incorporation of Agglomerated Spirulina platensis Powder in Yogurt: A Strategy for Enhancing Nutritional Quality and Bioactive Compounds. Fermentation. 2025; 11(7):389. https://doi.org/10.3390/fermentation11070389
Chicago/Turabian StyleAlbuquerque, Rosana Correia Vieira, Carlos Eduardo de Farias Silva, Margarete Cabral dos Santos Silva, Wanderson dos Santos Carneiro, Kaciane Andreola, Brígida Maria Villar da Gama, Marcos Vinicius Azevedo Figueiredo, Albanise Enide da Silva, and João Victor Oliveira Nascimento da Silva. 2025. "Incorporation of Agglomerated Spirulina platensis Powder in Yogurt: A Strategy for Enhancing Nutritional Quality and Bioactive Compounds" Fermentation 11, no. 7: 389. https://doi.org/10.3390/fermentation11070389
APA StyleAlbuquerque, R. C. V., de Farias Silva, C. E., Silva, M. C. d. S., Carneiro, W. d. S., Andreola, K., Gama, B. M. V. d., Figueiredo, M. V. A., Silva, A. E. d., & Silva, J. V. O. N. d. (2025). Incorporation of Agglomerated Spirulina platensis Powder in Yogurt: A Strategy for Enhancing Nutritional Quality and Bioactive Compounds. Fermentation, 11(7), 389. https://doi.org/10.3390/fermentation11070389









