A Response Surface Methodology for Sustainable Production of GABA from Black Soybean Okara Using Solid-State Collaborative Fermentation of Rhizopus oligosporus and Yarrowia lipolytica
Abstract
1. Introduction
2. Materials and Methods
2.1. Activation and Cultivation of Microbial Strains
2.2. Okara Fermentation
2.3. Optimizing of GABA Production
2.4. Determination of GABA Content in Fermented Okara
2.5. Determination of Protease Activity in Fermented Okara
2.6. Statistical Analysis
3. Results and Discussion
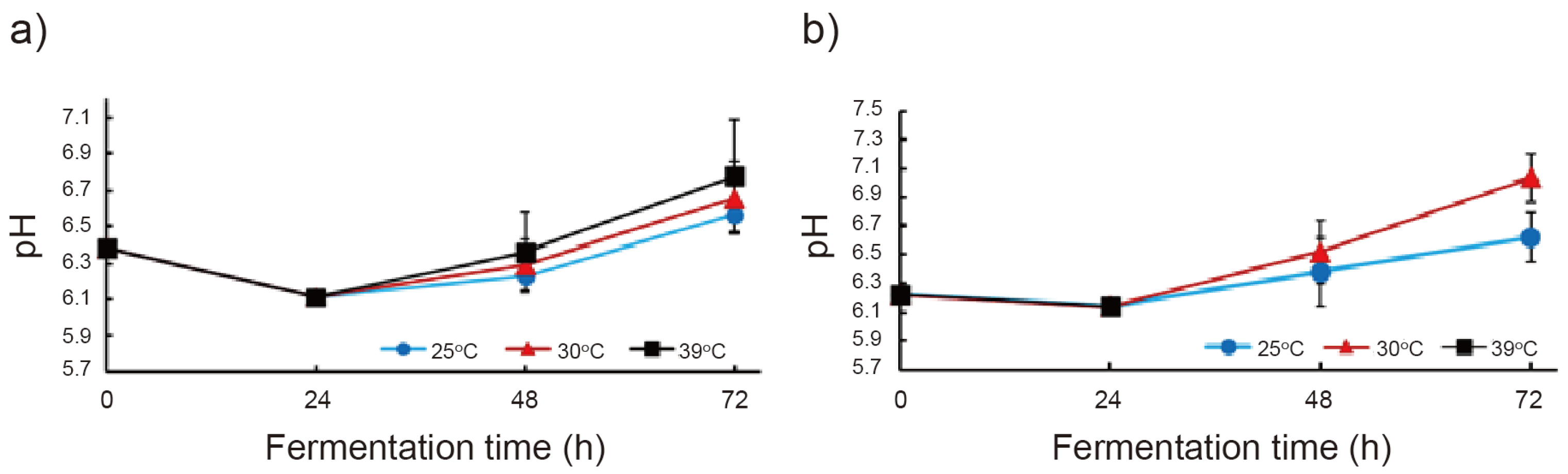
3.1. Solid-State Collaborative Fermentation of Black Soybean Okara
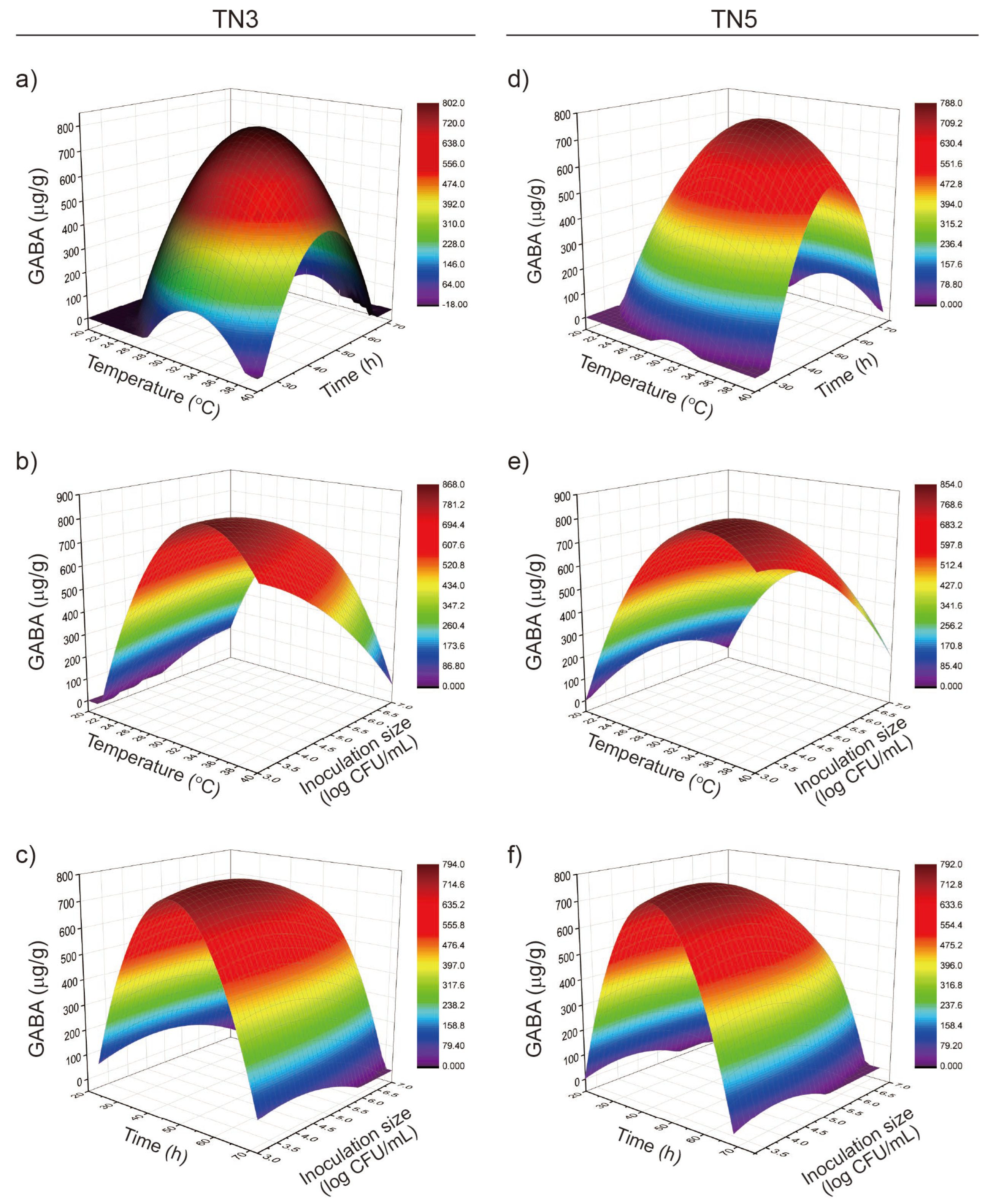
3.2. Response Surface Analysis of GABA Production in TN3 and TN5 Okara Fermentation
3.3. RSM Model Plot Showing the Effect of Variables on GABA Production
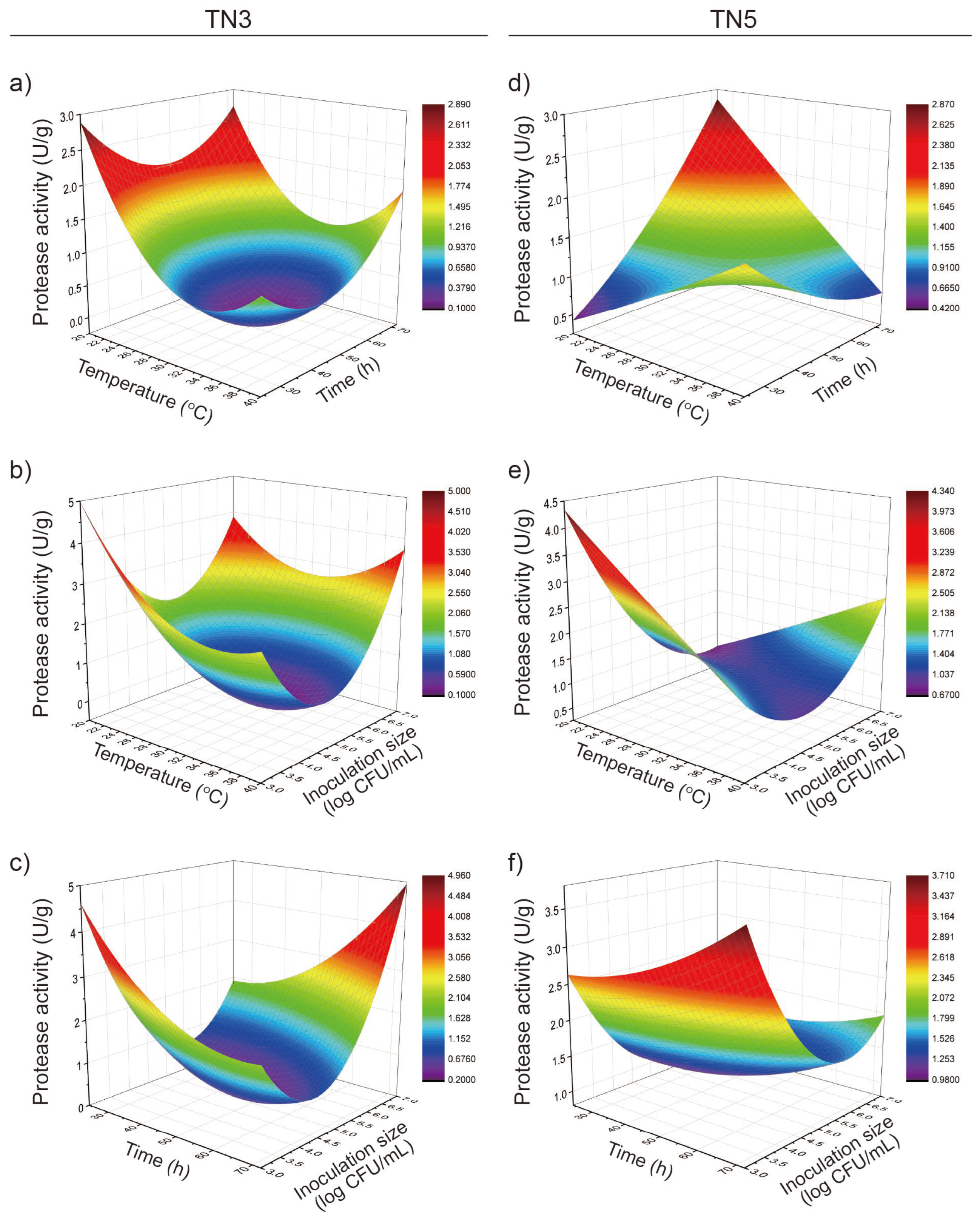
3.4. Response Surface Analysis of Protease Activity in TN3 and TN5 Okara Fermentation
3.5. RSM Model Plot Showing the Effect of Variables on Protease Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Global Food Losses and Food Waste. 2024. Available online: https://wedocs.unep.org/20.500.11822/45230 (accessed on 27 March 2024).
- FAO; IFAD; UNICEF; WFP; WHO. The State of Food Security and Nutrition in the World—Financing to End Hunger, Food Insecurity and Malnutrition in All Its Forms. 2024. Available online: https://doi.org/10.4060/cd1254en (accessed on 24 July 2024).
- Ardra, S.; Barua, M.K. Halving food waste generation by 2030: The challenges and strategies of monitoring UN sustainable development goal target 12.3. J. Clean. Prod. 2022, 380, 135042. [Google Scholar] [CrossRef]
- Tuly, J.A.; Ma, H. Bioconversion of food industrial waste okara by microbial fermentation: Scope of omics study and possibility. Trends Food Sci. Technol. 2024, 146, 104391. [Google Scholar] [CrossRef]
- Vong, W.C.; Liu, S.Q. Biovalorisation of okara (soybean residue) for food and nutrition. Trends Food Sci. Technol. 2016, 52, 139–147. [Google Scholar] [CrossRef]
- Santos, V.A.Q.; Nascimento, C.G.; Schmidt, C.A.; Mantovani, D.; Dekker, R.F.; da Cunha, M.A.A. Solid-state fermentation of soybean okara: Isoflavones biotransformation, antioxidant activity and enhancement of nutritional quality. LWT-Food Sci. Technol. 2018, 92, 509–515. [Google Scholar] [CrossRef]
- Sitanggang, A.B.; Sinaga, W.S.L.; Wie, F.; Fernando, F.; Krusong, W. Enhanced antioxidant activity of okara through solid state fermentation of GRAS Fungi. Food Sci. Technol. 2019, 40, 178–186. [Google Scholar] [CrossRef]
- Qiu, F.; Li, W.; Chen, X.; Du, B.; Li, X.S. Targeted microbial collaboration to enhance key flavor metabolites by inoculating Clostridium tyrobutyricum and Saccharomyces cerevisiae in the strong-flavor Baijiu simulated fermentation system. Food Res. Int. 2024, 190, 114647. [Google Scholar] [CrossRef]
- Vong, W.C.; Hua, X.Y.; Liu, S.Q. Solid-state fermentation with Rhizopus oligosporus and Yarrowia lipolytica improved nutritional and flavour properties of okara. LWT-Food Sci. Technol. 2018, 90, 316–322. [Google Scholar] [CrossRef]
- Ong, A.; Lee, C.L.K. Cooperative metabolism in mixed culture solid-state fermentation. LWT-Food Sci. Technol. 2021, 152, 112300. [Google Scholar] [CrossRef]
- Wang, C.; Qiu, X.; Hou, R.; Liu, J.; Li, L.; Mao, X. Improvement of soybean meal quality by one-step fermentation with mixed-culture based on protease activity. Innov. Food Sci. Emerg. Technol. 2023, 85, 103311. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, B.; Li, Q.; Yang, C.; Yu, P.; Yang, X.; Li, T. Dynamic changes on sensory property, nutritional quality and metabolic profiles of green kernel black beans during Eurotium cristatum-based solid-state fermentation. Food Chem. 2024, 139846. [Google Scholar] [CrossRef]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-aminobutyric acid (GABA): A comprehensive review of dietary sources, enrichment technologies, processing effects, health benefits, and its applications. Crit. Rev. Food Sci. Nutr. 2023, 64, 8852–8874. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.Y.; Lin, H.T.V.; Tsai, G.J. Gamma-aminobutyric acid production in black soybean milk by Lactobacillus brevis FPA 3709 and the antidepressant effect of the fermented product on a forced swimming rat model. Process Biochem. 2013, 48, 559–568. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, P.; Pan, D.; Zeng, X.; Guo, Y.; Zhao, G. Effect of adzuki bean sprout fermented milk enriched in γ-aminobutyric acid on mild depression in a mouse model. J. Dairy Sci. 2021, 104, 78–91. [Google Scholar] [CrossRef]
- Rossetti, V.; Lombard, A. Determination of glutamate decarboxylase by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 1996, 681, 63–67. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.J.; Chen, B.Y. Response surface methodology-optimized co-fermentation of pigeon pea okara by Rhizopus oligosporus and Yarrowia lipolytica and its application for vegetable paste. J. Sci. Food Agric. 2022, 2, 236–246. [Google Scholar] [CrossRef]
- Shih, M.C.; Yang, K.T.; Kuo, S.T. Optimization process of black soybean Natto using response surface methodology. J. Food Sci. 2009, 74, M294–M301. [Google Scholar] [CrossRef]
- Benimana, F.; Potoroko, I.Y.; Bagale, U.; Olivier, M.; Felicien, K. Protease activity in flesh leaves of Bidens pilosa. Int. J. Sci. Technol. Res. 2020, 9, 3108–3112. [Google Scholar]
- Chen, G.; Liu, Y.; Zeng, J.; Tian, X.; Bei, Q.; Wu, Z. Enhancing three phenolic fractions of oats (Avena sativa L.) and their antioxidant activities by solid-state fermentation with Monascus anka and Bacillus subtilis. J. Cereal Sci. 2020, 93, 102940. [Google Scholar] [CrossRef]
- Medwid, R.D.; Grant, D.W. Germination of Rhizopus oligosporus sporangiospores. Appl. Environ. Microbiol. 1984, 48, 1067–1071. [Google Scholar] [CrossRef]
- Barth, G.; Gaillardin, C. Yarrowia lipolytica. In Nonconventional Yeasts in Biotechnology; Wolf, K., Ed.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 10, pp. 313–388. [Google Scholar]
- Tojang, D.; Saparuddin, S.; Alimuddin, A.; Arham, Z. Effects of pretreatment and solid-state fermentation on Tempeh protein content. Orient. J. Chem. 2021, 37, 55–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, R.; Azi, F.; Jiao, L.; Wang, H.; He, T.; Liu, X.; Wang, R.; Lu, B. Solid-state fermentation with Rhizopus oligosporus RT-3 enhanced the nutritional properties of soybeans. Front. Nutr. 2022, 9, 972860. [Google Scholar] [CrossRef] [PubMed]
- Lien, T.J.; Yiu, T.J.; Wu, C.H. The breeding of the new black soybean variety Tainan no. 5. Res. Bull. Tainan Dist. Agric. Res. Ext. Stn. 2001, 38, 1–19. [Google Scholar] [CrossRef]
- Handoyo, T.; Morita, N. Structural and functional properties of fermented soybean (Tempeh) by using Rhizopus oligosporus. Int. J. Food Prop. 2006, 9, 347–355. [Google Scholar] [CrossRef]
- Bankar, A.V.; Kumar, A.R.; Zinjarde, S.S. Environmental and industrial applications of Yarrowia lipolytica. Appl. Microbiol. Biotechnol. 2009, 84, 847–865. [Google Scholar] [CrossRef]

| Independent Variables | Code | Levels | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| Incubation temperature (°C) | X1 | 20 | 25 | 30 | 35 | 40 |
| Incubation time (h) | X2 | 24 | 36 | 48 | 60 | 72 |
| inoculation amount of Y. lipolytica (CFU/mL) | X3 | 103 | 104 | 105 | 106 | 107 |
| Code and Decoded Variables | Output Responses | ||||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | Y1 GABA (μg/g) | Y2 Protease Activity (U/g) | |||
| Temperature (°C) | Incubation Time (h) | Inoculation Size (log CFU/mL) | TN3 | TN5 | TN3 | TN5 | |
| 1 | 1 (35) | 1 (60) | 1 (6) | 118.63 | 294.27 | 1.59 | 1.88 |
| 2 | 1 (35) | 1 (60) | −1 (4) | 171.39 | 189.63 | 0.41 | 1.29 |
| 3 | 1 (35) | −1 (36) | 1 (6) | 262.20 | 181.58 | 1.33 | 1.77 |
| 4 | 1 (35) | −1 (36) | −1 (4) | 235.80 | 230.16 | 1.33 | 1.48 |
| 5 | −1 (25) | 1 (60) | 1 (6) | 234.80 | 132.12 | 1.55 | 1.38 |
| 6 | −1 (25) | 1 (60) | −1 (4) | 210.98 | 203.08 | 0.75 | 2.43 |
| 7 | −1 (25) | −1 (36) | 1 (6) | 751.31 | 811.10 | 1.30 | 0.88 |
| 8 | −1 (25) | −1 (36) | −1 (4) | 746.40 | 588.50 | 2.10 | 1.25 |
| 9 | 2 (40) | 0 (48) | 0 (5) | 189.43 | 97.89 | 0.28 | 0.64 |
| 10 | −2 (20) | 0 (48) | 0 (5) | 730.36 | 595.68 | 2.44 | 1.73 |
| 11 | 0 (30) | 2 (72) | 0 (5) | 272.36 | 175.62 | 1.71 | 1.66 |
| 12 | 0 (30) | −2 (24) | 0 (5) | 594.72 | 666.26 | 0.28 | 1.09 |
| 13 | 0 (30) | 0 (48) | 2 (7) | 176.72 | 349.50 | 2.40 | 1.04 |
| 14 | 0 (30) | 0 (48) | −2 (3) | 147.63 | 274.70 | 2.85 | 3.46 |
| 15 | 0 (30) | 0 (48) | 0 (5) | 840.65 | 787.13 | 0.22 | 1.28 |
| 16 | 0 (30) | 0 (48) | 0 (5) | 884.56 | 828.07 | 0.23 | 1.14 |
| 17 | 0 (30) | 0 (48) | 0 (5) | 912.08 | 794.86 | 0.23 | 0.89 |
| 18 | 0 (30) | 0 (48) | 0 (5) | 790.76 | 826.55 | 0.25 | 1.25 |
| 19 | 0 (30) | 0 (48) | 0 (5) | 781.04 | 852.88 | 0.16 | 1.25 |
| 20 | 0 (30) | 0 (48) | 0 (5) | 801.18 | 777.65 | 0.22 | 1.21 |
| Parameter Intercept | GABA Content | |
|---|---|---|
| TN3 | TN5 | |
| intercept | −10754 * | −7221.245 * |
| X1 | 443.679 *** | 272.262 * |
| X2 | 133.335 * | 105.133 * |
| X3 | 586.905 | 481.306 |
| X12 | −5.517 ** | −3.987 * |
| X22 | −1.199 *** | −1.266 *** |
| X33 | −21.296 | −34.234 |
| X1X2 | −0.559 | 0.528 |
| X1X3 | −12.786 | −7.180 |
| X2X3 | −0.540 | 0.139 |
| R2 | 0.8539 | 0.8318 |
| lack of fit p-value | 0.0037 | 0.0002 |
| Parameter Intercept | Protease Activity | |
|---|---|---|
| TN3 | TN5 | |
| intercept | 42.885 *** | 13.1668 |
| X1 | −0.942 * | −0.1510 |
| X2 | −0.295 * | 0.1081 |
| X3 | −8.251 *** | −4.6010 ** |
| X12 | 0.011 * | 0.0003 |
| X22 | 0.001 | 0.0004 |
| X32 | 0.600 *** | 0.2746 ** |
| X1X2 | 0.001 | −0.0037 |
| X1X3 | 0.029 | 0.0572 |
| X2X3 | 0.029 | −0.0041 |
| R2 | 0.8474 | 0.7477 |
| lack of fit p-value | <0.0001 | 0.0037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, Y.-C.; Yeh, C.-C.; Chen, B.-Y.; Hsieh, J.-F.; Chang, C.-I.; Huang, C.; Kuo, M.-I.; Lu, C.-P. A Response Surface Methodology for Sustainable Production of GABA from Black Soybean Okara Using Solid-State Collaborative Fermentation of Rhizopus oligosporus and Yarrowia lipolytica. Fermentation 2025, 11, 296. https://doi.org/10.3390/fermentation11060296
Lai Y-C, Yeh C-C, Chen B-Y, Hsieh J-F, Chang C-I, Huang C, Kuo M-I, Lu C-P. A Response Surface Methodology for Sustainable Production of GABA from Black Soybean Okara Using Solid-State Collaborative Fermentation of Rhizopus oligosporus and Yarrowia lipolytica. Fermentation. 2025; 11(6):296. https://doi.org/10.3390/fermentation11060296
Chicago/Turabian StyleLai, Yi-Chung, Chien-Cheng Yeh, Bang-Yuan Chen, Jung-Feng Hsieh, Chia-I Chang, Cheng Huang, Meng-I Kuo, and Chun-Ping Lu. 2025. "A Response Surface Methodology for Sustainable Production of GABA from Black Soybean Okara Using Solid-State Collaborative Fermentation of Rhizopus oligosporus and Yarrowia lipolytica" Fermentation 11, no. 6: 296. https://doi.org/10.3390/fermentation11060296
APA StyleLai, Y.-C., Yeh, C.-C., Chen, B.-Y., Hsieh, J.-F., Chang, C.-I., Huang, C., Kuo, M.-I., & Lu, C.-P. (2025). A Response Surface Methodology for Sustainable Production of GABA from Black Soybean Okara Using Solid-State Collaborative Fermentation of Rhizopus oligosporus and Yarrowia lipolytica. Fermentation, 11(6), 296. https://doi.org/10.3390/fermentation11060296









