Enhancing Steamed Bread Quality Through Co-Fermentation of Sourdough with Kazachstania humilis and Lactobacillus plantarum
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Raw Materials
2.2.1. Preparation of MRS Medium and YPD Medium
MRS Medium
YPD Medium
2.3. Statistical Analysis
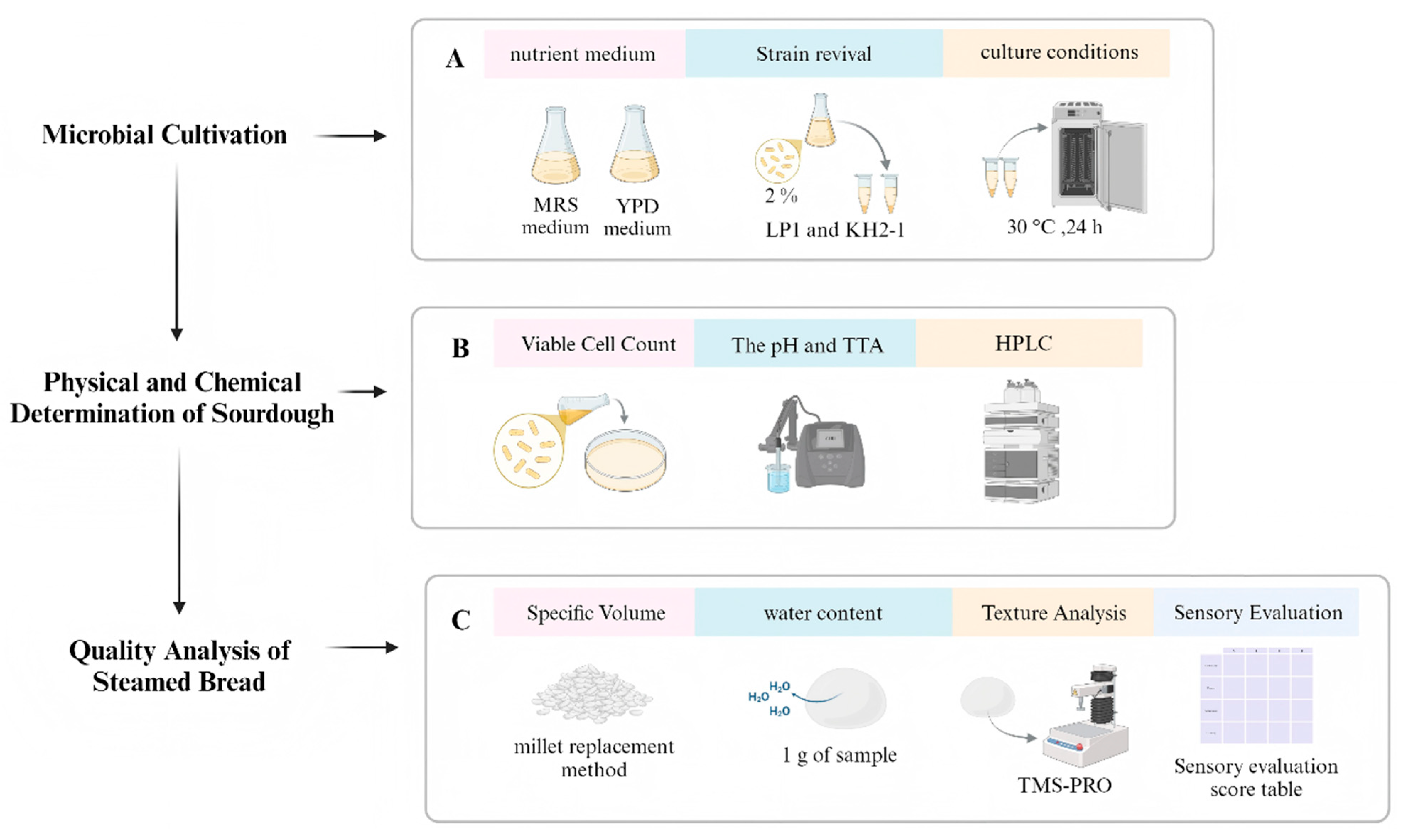
2.3.1. Flowchart Process
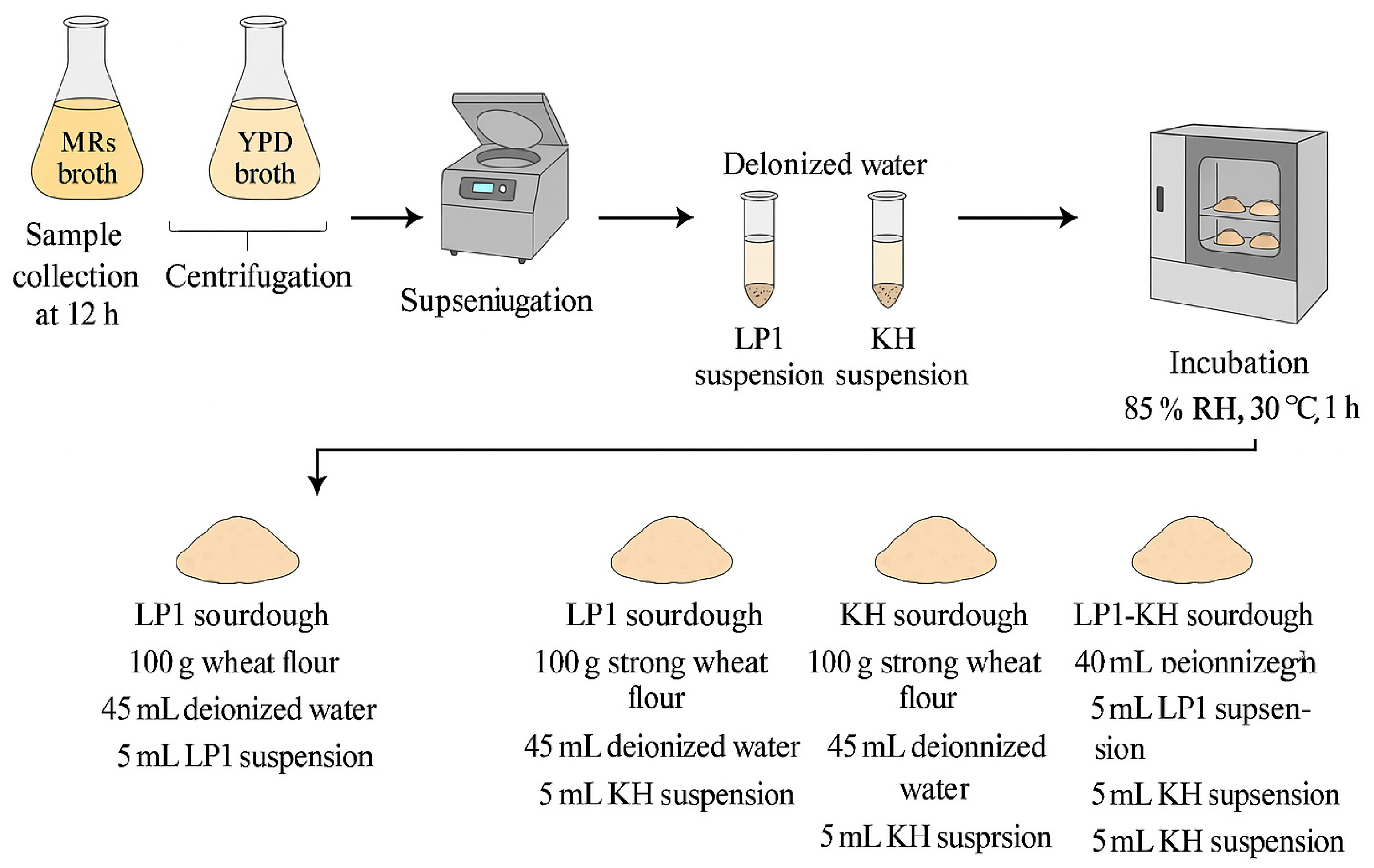
2.3.2. Microbial Cultivation
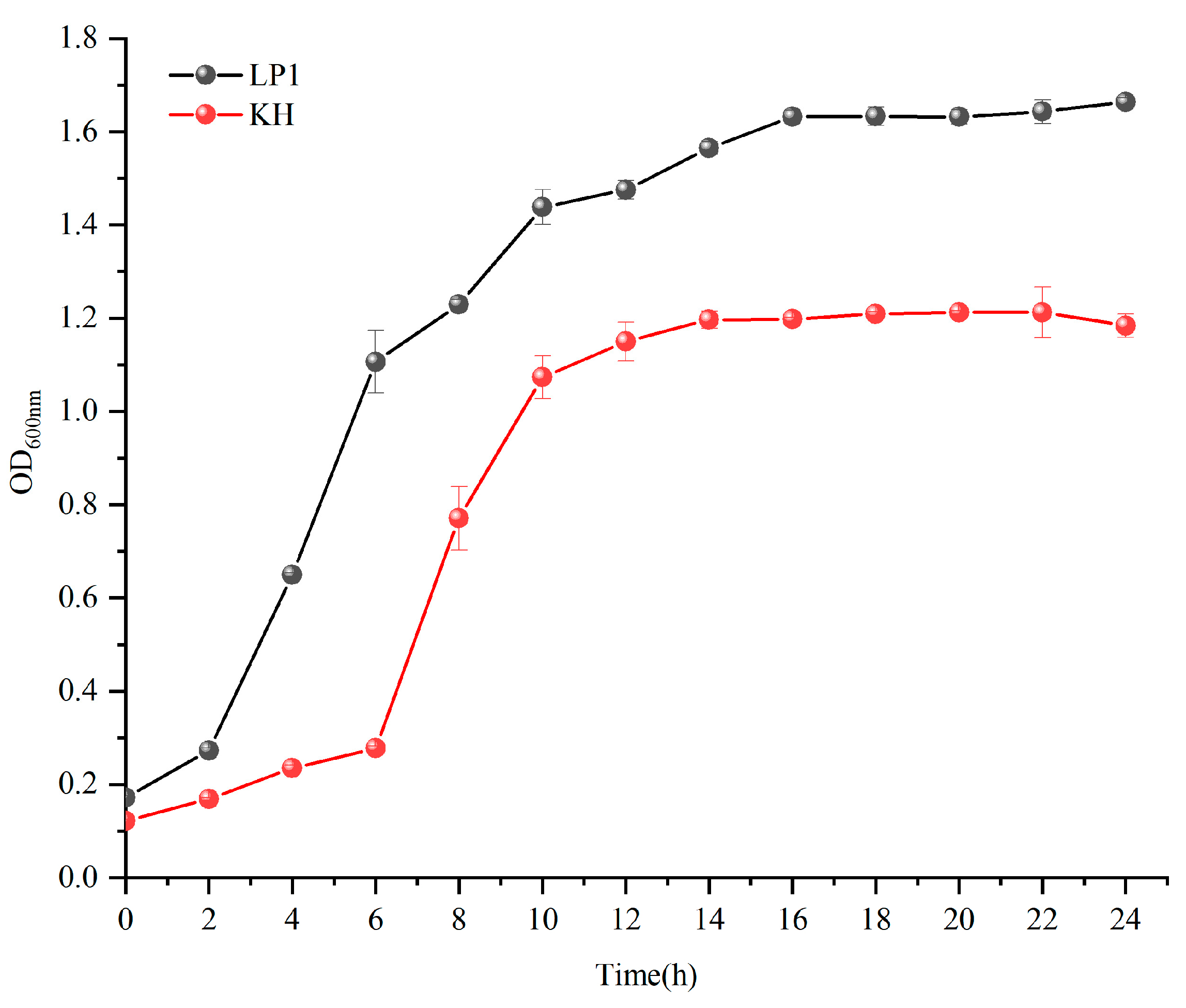
Microbial Starters and Growth Curve
Sourdough Fermentation
2.3.3. Physical and Chemical Determination of Sourdough
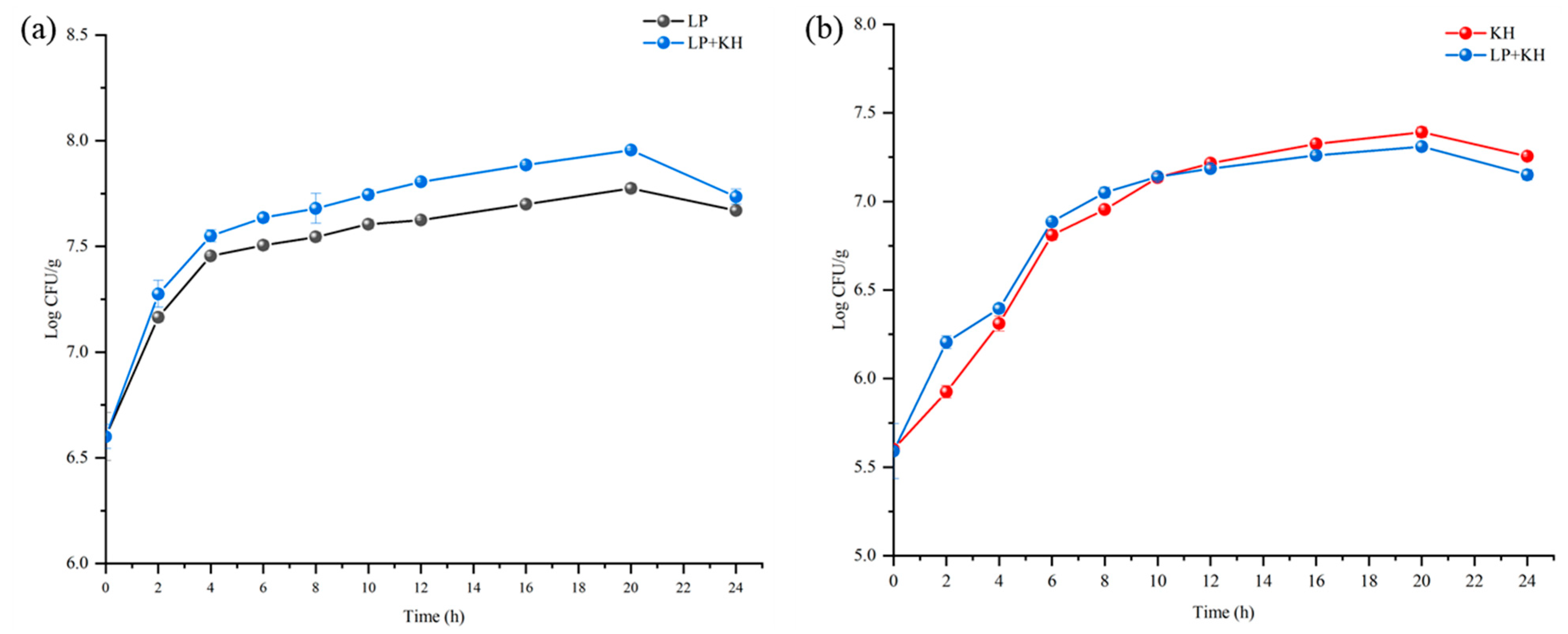
Change of Viable Cell Count
Determination of pH, Total Titratable Acidity, Lactic Acid, and Acetic Acid
2.3.4. Quality Analysis of Steamed Bread
Steamed Bread Performance
Determination of Specific Volume
Determination of Water Content
Determination of Texture
Sensory Evaluation Analysis
3. Results
3.1. Growth Curve and Change of Viable Cell Count During Fermentation of Sourdough
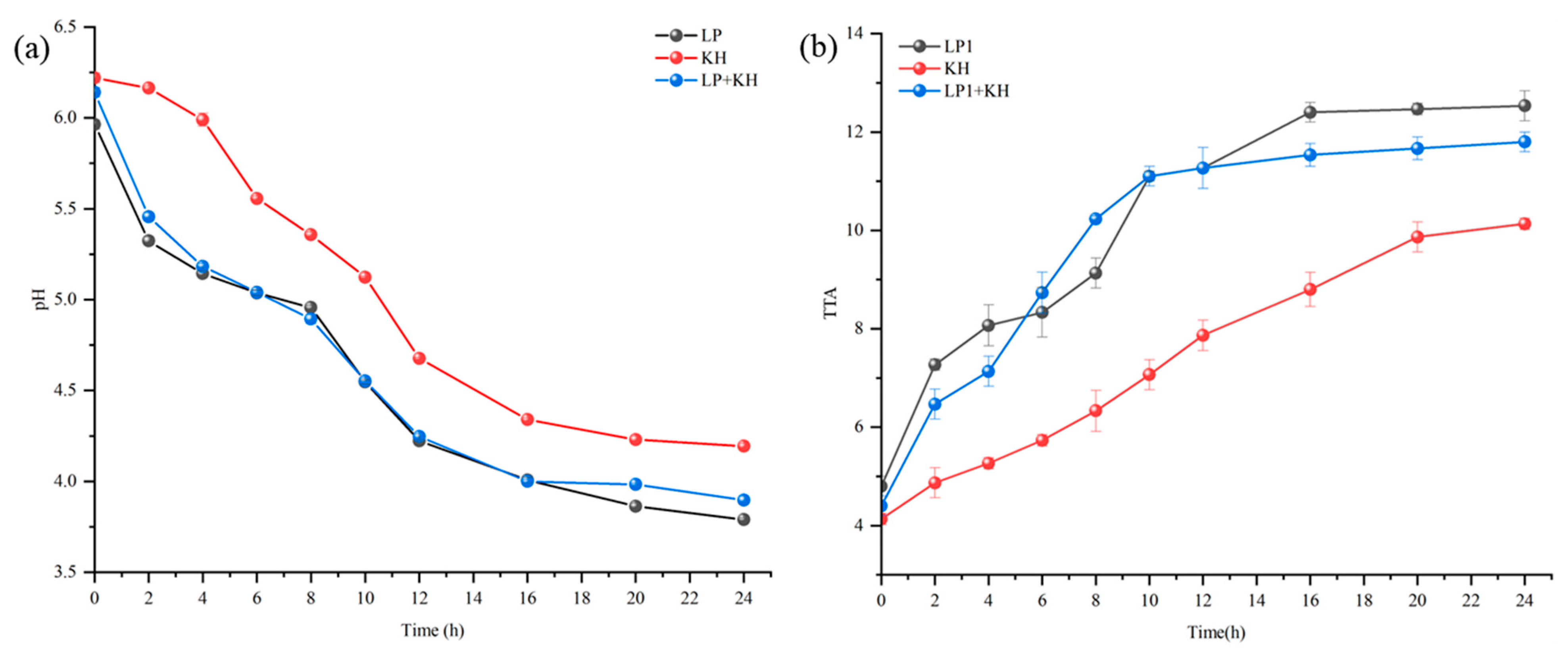
3.2. Changes of pH and TTA During the Fermentation of Sourdough
3.3. Lactic Acid and Acetic Acid Content of Sourdough
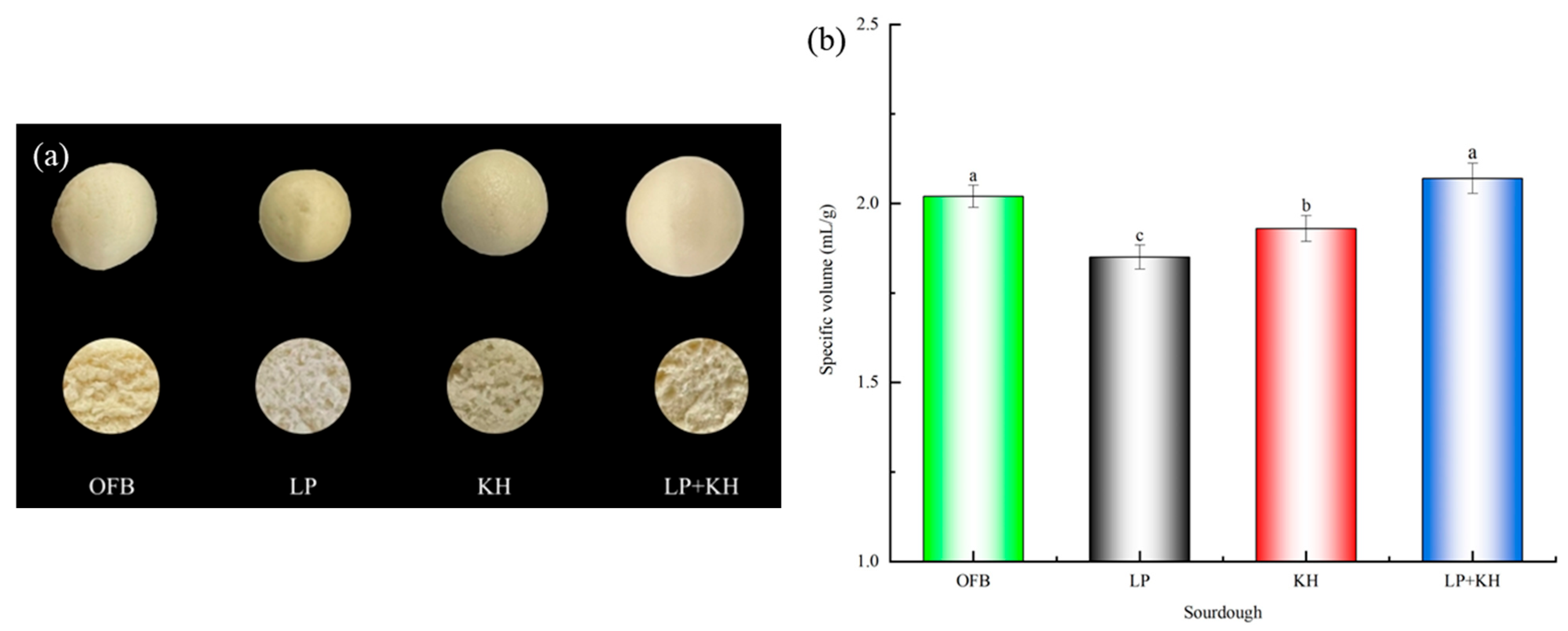
3.4. Appearance, Section and Specific Volume of Steamed Bread
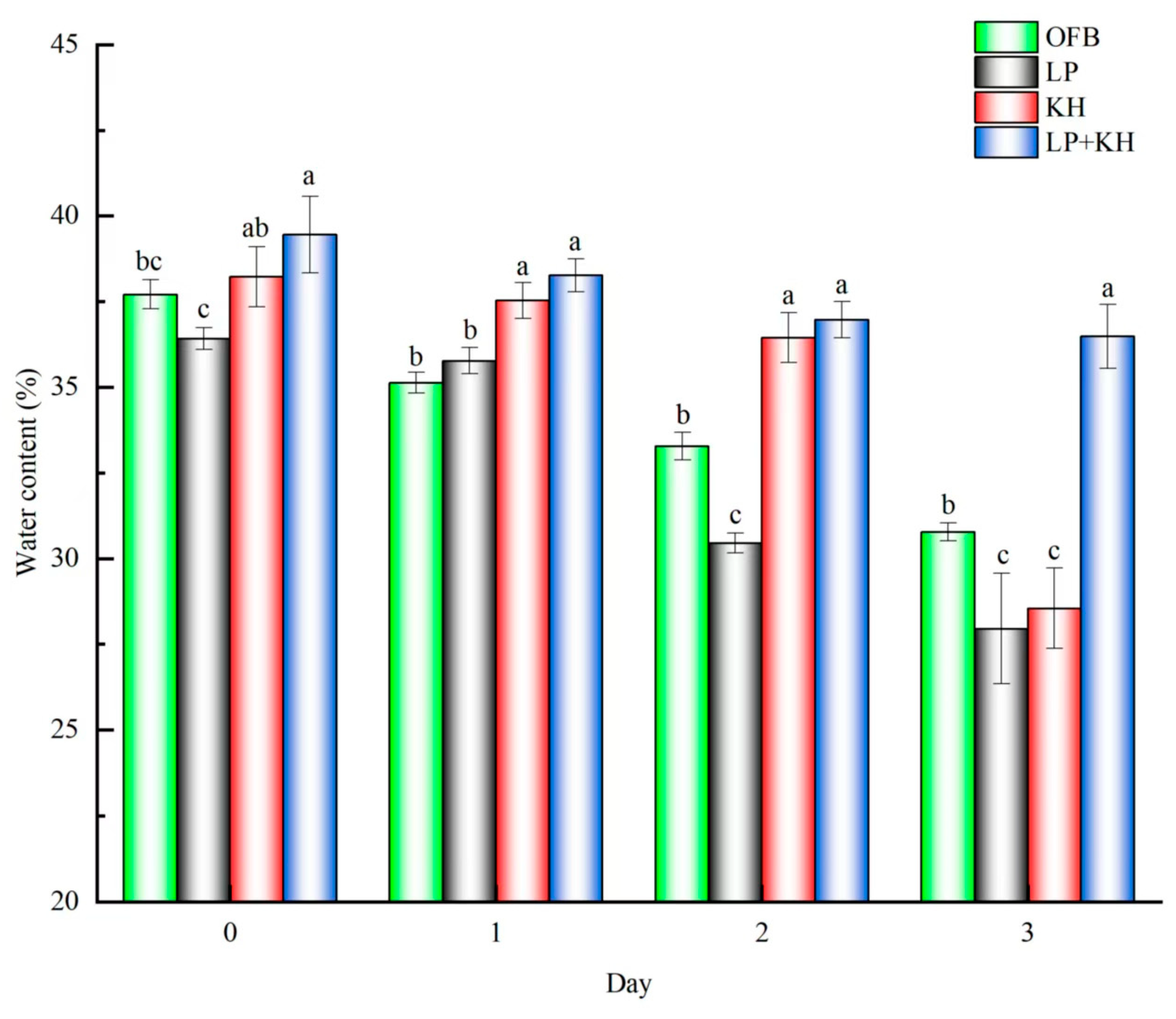
3.5. Changes of Water Content in Steamed Bread
3.6. Texture of Steamed Bread
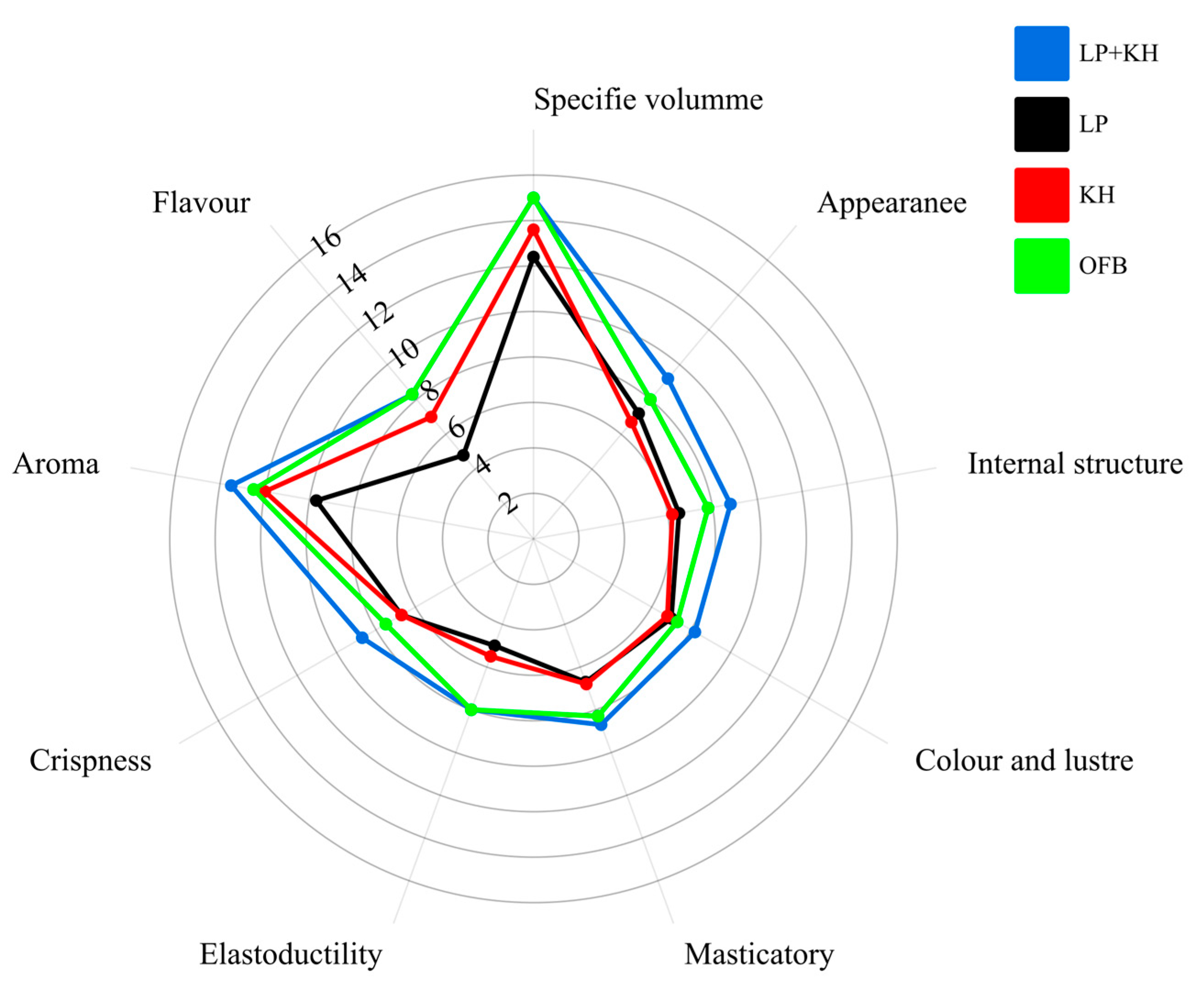
3.7. Sensory Evaluation Results
4. Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KH | Kazachstania humilis |
| LP | Lactobacillus plantarum |
| TTA | total titratable acidity |
References
- Liu, T.; Li, Y.; Chen, J.; Sadiq, F.A.; Zhang, G.; Li, Y.; He, G. Prevalence and diversity of lactic acid bacteria in Chinese traditional sourdough revealed by culture dependent and pyrosequencing approaches. LWT—Food Sci. Technol. 2016, 68, 91–97. [Google Scholar] [CrossRef]
- Akamine, I.T.; Mansoldo, F.R.P.; Vermelho, A.B. Probiotics in the Sourdough Bread Fermentation: Current Status. Fermentation 2023, 9, 90. [Google Scholar] [CrossRef]
- Paramithiotis, S.; Tsiasiotou, S.; Drosinos, E.H. Comparative study of spontaneously fermented sourdoughs originating from two regions of Greece: Peloponnesus and Thessaly. Eur. Food Res. Technol. 2010, 231, 883–890. [Google Scholar] [CrossRef]
- Ribet, L.; Dessalles, R.; Lesens, C.; Brusselaers, N.; Durand-Dubief, M. Nutritional benefits of sourdoughs: A systematic review. Adv. Nutr. 2023, 14, 22–29. [Google Scholar] [CrossRef]
- Wu, C.; Liu, R.; Huang, W.; Rayas-Duarte, P.; Wang, F.; Yao, Y. Effect of sourdough fermentation on the quality of Chinese Northern-style steamed breads. J. Cereal Sci. 2012, 56, 127–133. [Google Scholar] [CrossRef]
- De Vuyst, L.; Neysens, P. The sourdough microflora: Biodiversity and metabolic interactions. Trends Food Sci. Technol. 2005, 16, 43–56. [Google Scholar] [CrossRef]
- Michel, E.; Monfort, C.; Deffrasnes, M.; Guezenec, S.; Lhomme, E.; Barret, M.; Sicard, D.; Dousset, X.; Onno, B. Characterization of relative abundance of lactic acid bacteria species in French organic sourdough by cultural, qPCR and MiSeq high-throughput sequencing methods. Int. J. Food Microbiol. 2016, 239, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wu, S.; Li, W.; Koksel, F.; Du, Y.; Sun, L.; Fang, Y.; Hu, Q.; Pei, F. The effects of cooperative fermentation by yeast and lactic acid bacteria on the dough rheology, retention and stabilization of gas cells in a whole wheat flour dough system-A review. Food Hydrocoll. 2023, 135, 108212. [Google Scholar] [CrossRef]
- Hammes, W.P.; Gänzle, M.G. Sourdough breads and related products. In Microbiology of Fermented Foods; Springer: Boston, MA, USA, 1998; pp. 199–216. [Google Scholar]
- Lombardi, A.; Zilio, F.; Andrighetto, C.; Zampese, L.; Loddo, A. Microbiological characterization of sourdoughs of Veneto region. Ind. Aliment. 2007, 46, 147–151. [Google Scholar]
- Zhang, J.; Liu, W.; Sun, Z.; Bao, Q.; Wang, F.; Yu, J.; Chen, W.; Zhang, H. Diversity of lactic acid bacteria and yeasts in traditional sourdoughs collected from western region in Inner Mongolia of China. Food Control 2011, 22, 767–774. [Google Scholar] [CrossRef]
- Fu, W.; Wang, S.; Xue, W. Mechanism of carbohydrate and protein conversion during sourdough fermentation: An analysis based on representative Chinese sourdough microbiota. Int. J. Food Microbiol. 2024, 410, 110487. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Danial, M.; Liu, L.; Sadiq, F.A.; Wei, X.; Zhang, G. Effects of Co-Fermentation of Lactiplantibacillus plantarum and Saccharomyces cerevisiae on Digestive and Quality Properties of Steamed Bread. Foods 2023, 12, 3333. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhang, Y.; Tang, K.; Hu, Y.; Xu, X.; Gänzle, M.G. Effect of mixed cultures of yeast and lactobacilli on the quality of wheat sourdough bread. Front. Microbiol. 2019, 10, 2113. [Google Scholar] [CrossRef] [PubMed]
- Vogelmann, S.A.; Hertel, C. Impact of ecological factors on the stability of microbial associations in sourdough fermentation. Food Microbiol. 2011, 28, 583–589. [Google Scholar] [CrossRef]
- Hansen, Å.; Hansen, B. Flavour of sourdough wheat bread crumb. Eur. Food Res. Technol. 1996, 202, 244–249. [Google Scholar] [CrossRef]
- Arendt, E.K.; Ryan, L.A.M.; Bello, F.D. Impact of sourdough on the texture of bread. Food Microbiol. 2007, 24, 165–174. [Google Scholar] [CrossRef]
- Wang, P.; Hou, C.; Zhao, X.; Tian, M.; Gu, Z.; Yang, R. Molecular characterization of water-extractable arabinoxylan from wheat bran and its effect on the heat-induced polymerization of gluten and steamed bread quality. Food Hydrocoll. 2019, 87, 570–581. [Google Scholar] [CrossRef]
- García-Mantrana, I.; Monedero, V.; Haros, M. Myo-inositol hexakisphosphate degradation by Bifidobacterium pseudocatenulatum ATCC 27919 improves mineral availability of high fibre rye-wheat sour bread. Food Chem. 2015, 178, 267–275. [Google Scholar] [CrossRef]
- Lefebvre, D.; Gabriel, V.; Vayssier, Y.; Fontagné-Faucher, C. Simultaneous HPLC determination of sugars, organic acids and ethanol in sourdough process. LWT—Food Sci. Technol. 2002, 35, 407–414. [Google Scholar] [CrossRef]
- Liu, X.L.; Mu, T.H.; Sun, H.N.; Zhang, M.; Chen, J.W. Influence of potato flour on dough rheological properties and quality of steamed bread. J. Integr. Agric. 2016, 15, 2666–2676. [Google Scholar] [CrossRef]
- GB/T 5009.3-2016; National Food Safety Standard—Determination of Moisture in Foods. National Health and Family Planning Commission of the People’s Republic of China: Beijing, China, 2016. (In Chinese)
- Xing, X.; Suo, B.; Yang, Y.; Li, Z.; Nie, W.; Ai, Z. Application of Lactobacillus as adjunct cultures in wheat dough fermentation. J. Food Sci. 2019, 84, 842–847. [Google Scholar] [CrossRef]
- Albagli, G.; Silva, L.N.B.; Nunes, N.M.; Moreira, D.P.; Amaral, P.F.F.; Finotelli, P.V. Sourdough-Based Starter Cultures for Fermentation in Agri-food Industry. In Sourdough Microbiota and Starter Cultures for Industry; Springer International Publishing: Cham, Switzerland, 2024; pp. 281–307. [Google Scholar]
- Alkay, Z.; Durak, M.Z. The effect of combinations of sourdough lactic acid bacteria and yeasts on wheat bread quality: Evaluation in terms of microbiological, rheological, textural and volatile components. Lat. Am. Appl. Res. 2024, 54, 31–38. [Google Scholar] [CrossRef]
- Ferraz, R.; Flores, S.H.; Frazzon, J.; Thys, R.C.S. The effect of co-fermentation on sourdough breadmaking using different viable cell concentrations of Lactobacillus plantarum and Saccharomyces cerevisiae as starter cultures. J. Culin. Sci. Technol. 2021, 19, 1–17. [Google Scholar] [CrossRef]
- Sieuwerts, S.; Bron, P.; Smid, E. Mutually stimulating interactions between lactic acid bacteria and Saccharomyces cerevisiae in sourdough fermentation. LWT—Food Sci. Technol. 2018, 90, 201–206. [Google Scholar] [CrossRef]
- De Vuyst, L.; Van Kerrebroeck, S.; Harth, H.; Huys, G.; Daniel, H.-M.; Weckx, S. Microbial ecology of sourdough fermentations: Diverse or uniform? Food Microbiol. 2014, 37, 11–29. [Google Scholar] [CrossRef]
- Hernández-Figueroa, R.H.; Mani-López, E.; Ramírez-Corona, N.; López-Malo, A. Optimizing Lactic Acid Bacteria Proportions in Sourdough to Enhance Antifungal Activity and Quality of Partially and Fully Baked Bread. Foods 2024, 13, 2318. [Google Scholar] [CrossRef] [PubMed]
- Graça, C.; Edelmann, M.; Raymundo, A.; Sousa, I.; Coda, R.; Sontag-Strohm, T.; Huang, X. Yoghurt as a starter in sourdough fermentation to improve the technological and functional properties of sourdough-wheat bread. J. Funct. Foods 2022, 88, 104877. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Z.; Guo, R.; Hu, Y.; Dong, X.; Shi, Q. Efficient cascade conversion of starch to gluconic acid by a chemoenzymatic system with co-immobilized Au nanoparticles and enzymes. Catal. Sci. Technol. 2023, 13, 991–999. [Google Scholar] [CrossRef]
- Miafo, A.-P.T.; Koubala, B.B.; Kansci, G.; Muralikrishna, G. Free sugars and non-starch polysaccharides–phenolic acid complexes from bran, spent grain and sorghum seeds. J. Cereal Sci. 2019, 87, 124–131. [Google Scholar] [CrossRef]
- Ciska, E.; Honke, J.; Drabińska, N. Changes in glucosinolates and their breakdown products during the fermentation of cabbage and prolonged storage of sauerkraut: Focus on sauerkraut juice. Food Chem. 2021, 365, 130498. [Google Scholar] [CrossRef]
- Gänzle, M.G. Enzymatic and bacterial conversions during sourdough fermentation. Food Microbiol. 2014, 37, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Hsiao, F.S.H.; Wen, C.M.; Wu, C.Y.; Dybus, A.; Yu, Y.H. Mixed fermentation of soybean meal by protease and probiotics and its effects on the growth performance and immune response in broilers. J. Appl. Anim. Res. 2019, 47, 339–348. [Google Scholar] [CrossRef]
- Li, Z.; Li, H.; Deng, C.; Bian, K.; Liu, C. Effect of L actobacillus Plantarum DM 616 on Dough Fermentation and C hinese Steamed Bread Quality. J. Food Process. Preserv. 2015, 39, 30–37. [Google Scholar] [CrossRef]
- Tang, N.; Xing, X.; Li, H.; Suo, B.; Wang, Y.; Ai, Z.; Yang, Y. Co-culture fermentation by Saccharomycopsis fibuligera and lactic acid bacteria improves bioactivity and aroma profile of wheat bran and the bran-containing Chinese steamed bread. Food Res. Int. 2024, 182, 114179. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhang, J.; Wang, S.; Liu, Y.; Li, L.; Gao, M. Lactic acid bacteria synergistic fermentation affects the flavor and texture of bread. J. Food Sci. 2022, 87, 1823–1836. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, T.-T.; Guo, R.-R.; Ye, Q.; Zhao, H.-L.; Huang, X.-H. The regulation of key flavor of traditional fermented food by microbial metabolism: A review. Food Chem. X 2023, 19, 100871. [Google Scholar] [CrossRef]
- Alkay, Z.; Falah, F.; Cankurt, H.; Dertli, E. Exploring the Nutritional Impact of Sourdough Fermentation: Its Mechanisms and Functional Potential. Foods 2024, 13, 1732. [Google Scholar] [CrossRef]

| Ingredient | Content (L−1) |
|---|---|
| Pepton | 10.00 g |
| Beef Powder | 10.00 g |
| Yeast Powder | 5.00 g |
| Glucose | 20.00 g |
| Diammonium Citrate | 2.00 g |
| Sodium Acetate | 3.00 g |
| Dipotassium Hydrogen Phosphate | 2.00 g |
| Magnesium sulfate | 0.20 g |
| Manganese sulfate | 0.04 g |
| Tween-80 | 1.0 mL |
| Agar (Solid Culture medium) | 15.00 g |
| Ingredient | Content (L−1) |
|---|---|
| Yeast extract powder | 10.00 g |
| peptone | 20.00 g |
| Glucose | 20.00 g |
| Agar (solid culture medium) | 20.00 g |
| Project | Scores | Scoring Criteria |
|---|---|---|
| Specific volume | 15 | The specific volume is greater than 2.0 and scores a full 15 points; the specific volume is between 2.0 and 1.4, with 0.2 points deducted for every 0.01 decrease; the minimum score for a specific volume of 1.4 or less is 3 points |
| Appearance | 10 | The steamed bread is upright, full, and smooth (7–10); the steamed bread is slightly collapsed and creased (3–6); the steamed bread collapsed and atrophied seriously (0–2) |
| Internal structure | 10 | The stomata structure is fine, the distribution is uniform, the skin and flesh are not separated (7–10); there are more pores, but the size of pores is different (3–6); the epidermis was seriously separated, the stomata were unevenly distributed (0–2) |
| Color and luster | 10 | The color is bright yellow, bright, and evenly distributed (7–10); the color is slightly yellow (3–6); the color is dark (0–2) |
| Masticatory effect | 10 | The steamed bread is palatable, soft, and easy to swallow (7–10); the steamed bread is medium, soft, and firm (4–6); the steamed bread is hard to chew and difficult to swallow (0–3) |
| Elasticity | 10 | Rebound is good, pressing more than 1/2 can quickly recover (7–10); rebound is slightly weak, but pressing more than 1/4 can recover (4–6); rebound is poor or nonexistent (0–3) |
| Crispness | 10 | The steamed bread is pleasant to chew and does not stick to your teeth (7–10); the steamed bread is slightly sticky or crumbly when chewed (4–6); the seamed bread chewing sticky teeth or not refreshing debris feeling serious (0–3) |
| Aroma | 15 | The steamed bread has a strong wheat flavor and no bran odor (11–15); the steamed bread has a strong wheat flavor with a light bran odor (6–10); the wheat flavor of steamed bread is light, or the bran odor is serious (0–5) |
| Flavor | 10 | Slightly sour and sweet (7–10); more sour or less sweet (4–6); too acidic or bitter (0–3) |
| Hardness (g) | Cohesiveness | Springiness (%) | Gumminess (g) | Chewiness (g) | Resilience | |
|---|---|---|---|---|---|---|
| OFB | 280.53 ± 14.14 b | 0.67 ± 0.01 b | 0.80 ± 0.03 bc | 187.43 ± 6.91 b | 150.67 ± 7.64 c | 0.27 ± 0.01 ab |
| LP | 329.73 ± 20.48 a | 0.69 ± 0.02 b | 0.87 ± 0.02 a | 228.50 ± 17.72 a | 193.67 ± 9.87 a | 0.22 ± 0.02 c |
| KH | 317.00 ± 20.73 ab | 0.67 ± 0.02 b | 0.79 ± 0.02 c | 212.43 ± 8.19 ab | 168.33 ± 7.02 b | 0.24 ± 0.01 bc |
| LP+KH | 215.33 ± 18.82 c | 0.74 ± 0.03 a | 0.85 ± 0.03 ab | 152.67 ± 17.11 c | 115.67 ± 5.51 d | 0.29 ±0.02 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Fu, A.; Wang, X.; Zhang, G. Enhancing Steamed Bread Quality Through Co-Fermentation of Sourdough with Kazachstania humilis and Lactobacillus plantarum. Fermentation 2025, 11, 298. https://doi.org/10.3390/fermentation11060298
Wang Z, Fu A, Wang X, Zhang G. Enhancing Steamed Bread Quality Through Co-Fermentation of Sourdough with Kazachstania humilis and Lactobacillus plantarum. Fermentation. 2025; 11(6):298. https://doi.org/10.3390/fermentation11060298
Chicago/Turabian StyleWang, Zicheng, Ao Fu, Xin Wang, and Guohua Zhang. 2025. "Enhancing Steamed Bread Quality Through Co-Fermentation of Sourdough with Kazachstania humilis and Lactobacillus plantarum" Fermentation 11, no. 6: 298. https://doi.org/10.3390/fermentation11060298
APA StyleWang, Z., Fu, A., Wang, X., & Zhang, G. (2025). Enhancing Steamed Bread Quality Through Co-Fermentation of Sourdough with Kazachstania humilis and Lactobacillus plantarum. Fermentation, 11(6), 298. https://doi.org/10.3390/fermentation11060298





