Development and Taste Improvement of Polyamine-Containing Sakekasu Beverages Using Highly Polyamine-Producing Bacteria from Fermented Foods
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Sakekasu Extract
2.2. Cultivation of L. brevis FB215 Using Sakekasu Extract
2.3. High-Performance Liquid Chromatography (HPLC) Analysis of Putrescine and Histamine
2.4. Metabolomic Analysis Using Capillary Electrophoresis Time-of-Flight Mass Spectrometry (CE-TOF-MS) of the Culture Supernatant
2.5. Quantification of Carbohydrates in the Culture Supernatant
2.6. Taste Evaluation Test
3. Results
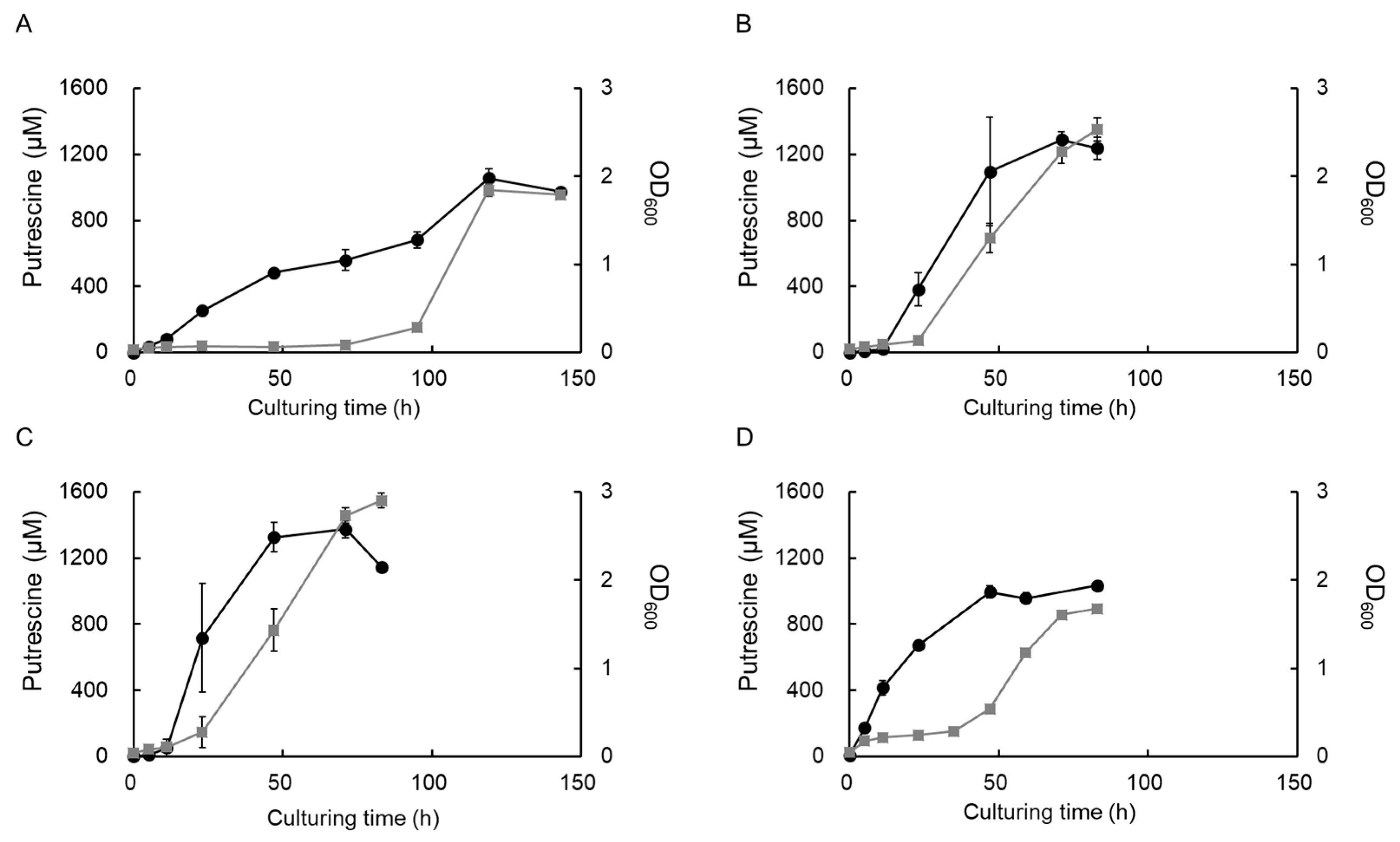
3.1. Effects of Culturing Temperature on Bacterial Growth
3.2. Metabolites in Sakekasu Extract Fermented by FB215
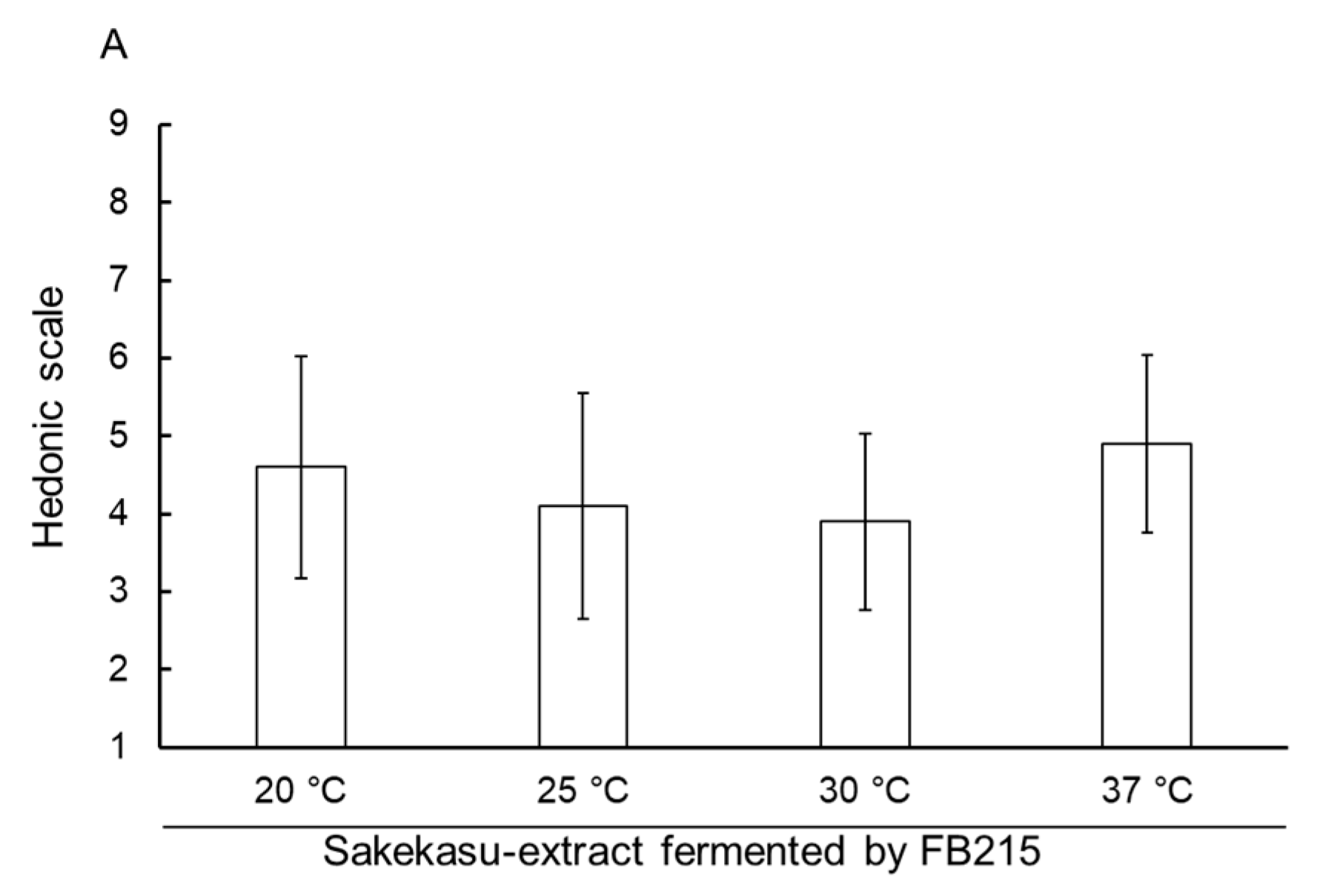
3.3. Sensory Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CE-TOF-MS | Capillary electrophoresis time-of-flight mass spectrometry |
| HPLC | High-performance liquid chromatography |
| m/z | Mass-to-charge ratio |
| MT | Migration time |
References
- Xuan, M.J.; Gu, X.Y.; Li, J.; Huang, D.; Xue, C.; He, Y.T. Polyamines: Their significance for maintaining health and contributing to diseases. Cell Commun. Signal. 2023, 21, 348. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Effects of polyamines on protein synthesis and growth of Escherichia coli. J. Biol. Chem. 2018, 293, 18702–18709. [Google Scholar] [CrossRef]
- Hofer, S.J.; Simon, A.K.; Bergmann, M.; Eisenberg, T.; Kroemer, G.; Madeo, F. Mechanisms of spermidine-induced autophagy and geroprotection. Nat. Aging 2022, 2, 1112–1129. [Google Scholar] [CrossRef] [PubMed]
- Hofer, S.J.; Daskalaki, I.; Bergmann, M.; Friscic, J.; Zimmermann, A.; Mueller, M.I.; Abdellatif, M.; Nicastro, R.; Masser, S.; Durand, S.; et al. Spermidine is essential for fasting-mediated autophagy and longevity. Nat. Cell Biol. 2024, 26, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, S.; Hofer, S.J.; Zimmermann, A.; Pechlaner, R.; Dammbrueck, C.; Pendl, T.; Marcello, G.M.; Pogatschnigg, V.; Bergmann, M.; Muller, M.; et al. Dietary spermidine improves cognitive function. Cell Rep. 2021, 35, 108985. [Google Scholar] [CrossRef]
- Nakamura, A.; Kurihara, S.; Takahashi, D.; Ohashi, W.; Nakamura, Y.; Kimura, S.; Onuki, M.; Kume, A.; Sasazawa, Y.; Furusawa, Y.; et al. Symbiotic polyamine metabolism regulates epithelial proliferation and macrophage differentiation in the colon. Nat. Commun. 2021, 12, 2105. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.L.; Wang, J.N.; Chen, Y.Y.; Wei, W.; Sun, C.H. Association between dietary spermidine intake and depressive symptoms among US adults: National Health and Nutrition Examination Survey (NHANES) 2005–2014. J. Affect. Disord. 2024, 359, 125–132. [Google Scholar] [CrossRef]
- Holbert, C.E.; Cullen, M.T.; Casero, R.; Stewart, T.M. Polyamines in cancer: Integrating organismal metabolism and antitumour immunity. Nat. Rev. Cancer 2022, 22, 467–480. [Google Scholar] [CrossRef]
- Al-Habsi, M.; Chamoto, K.; Matsumoto, K.; Nomura, N.; Zhang, B.; Sugiura, Y.; Sonomura, K.; Maharani, A.; Nakajima, Y.; Wu, Y.; et al. Spermidine activates mitochondrial trifunctional protein and improves antitumor immunity in mice. Science 2022, 378, eabj3510. [Google Scholar] [CrossRef]
- Das, R.; Kanungo, M.S. Activity and modulation of ornithine decarboxylase and concentrations of polyamines in various tissues of rats as a function of age. Exp. Gerontol. 1982, 17, 95–103. [Google Scholar] [CrossRef]
- Nishimura, K.; Shiina, R.; Kashiwagi, K.; Igarashi, K. Decrease in polyamines with aging and their ingestion from food and drink. J. Biochem. 2006, 139, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Soda, K.; Kano, Y.; Sakuragi, M.; Takao, K.; Lefor, A.; Konishi, F. Long-term oral polyamine intake increases blood polyamine concentrations. J. Nutr. Sci. Vitaminol. 2009, 55, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Soda, K.; Uemura, T.; Sanayama, H.; Igarashi, K.; Fukui, T. Polyamine-rich diet elevates blood spermine levels and Inhibits pro-inflammatory status: An interventional study. Med. Sci. 2021, 9, 22. [Google Scholar] [CrossRef]
- Wirth, M.; Benson, G.; Schwarz, C.; Kobe, T.; Grittner, U.; Schmitz, D.; Sigrist, S.J.; Bohlken, J.; Stekovic, S.; Madeo, F.; et al. The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial. Cortex 2018, 109, 181–188. [Google Scholar] [CrossRef]
- Nishibori, N.; Fujihara, S.; Akatuki, T. Amounts of polyamines in foods in Japan and intake by Japanese. Food Chem. 2007, 100, 491–497. [Google Scholar] [CrossRef]
- Zoumas-Morse, C.; Rock, C.L.; Quintana, E.L.; Neuhouser, M.L.; Gerner, E.W.; Meyskens, F.L., Jr. Development of a polyamine database for assessing dietary intake. J. Am. Diet. Assoc. 2007, 107, 1024–1027. [Google Scholar] [CrossRef] [PubMed]
- Buyukuslu, N.; Hizli, H.; Esin, K.; Garipagaoglu, M. A cross-sectional study: Nutritional polyamines in frequently consumed foods of the Turkish population. Foods 2014, 3, 541–557. [Google Scholar] [CrossRef]
- Soda, K.; Dobashi, Y.; Kano, Y.; Tsujinaka, S.; Konishi, F. Polyamine-rich food decreases age-associated pathology and mortality in aged mice. Exp. Gerontol. 2009, 44, 727–732. [Google Scholar] [CrossRef]
- Teratani, T.; Kasahara, N.; Ijichi, T.; Fujimoto, Y.; Sakuma, Y.; Sata, N.; Kitayama, J. Activation of whole body by high levels of polyamine intake in rats. Amino Acids 2021, 53, 1695–1703. [Google Scholar] [CrossRef]
- Gänzle, M.G. Lactic metabolism revisited: Metabolism of lactic acid bacteria in food fermentations and food spoilage. Curr. Opin. Food Sci. 2015, 2, 106–117. [Google Scholar] [CrossRef]
- Kocabay, S. Evaluation of probiotic properties of Levilactobacillus brevis isolated from hawthorn vinegar. Arch. Microbiol. 2023, 205, 258. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Yang, S.J.; Paik, H.D. Probiotic properties of novel probiotic Levilactobacillus brevis KU15147 isolated from radish kimchi and its antioxidant and immune-enhancing activities. Food Sci. Biotechnol. 2021, 30, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Lucas, P.M.; Blancato, V.S.; Claisse, O.; Magni, C.; Lolkema, J.S.; Lonvaud-Funel, A. Agmatine deiminase pathway genes in Lactobacillus brevis are linked to the tyrosine decarboxylation operon in a putative acid resistance locus. Microbiology 2007, 153, 2221–2230. [Google Scholar] [CrossRef]
- Romano, A.; Ladero, V.; Alvarez, M.A.; Lucas, P.M. Putrescine production via the ornithine decarboxylation pathway improves the acid stress survival of Lactobacillus brevis and is part of a horizontally transferred acid resistance locus. Int. J. Food Microbiol. 2014, 175, 14–19. [Google Scholar] [CrossRef]
- Bao, W.; Huang, X.; Liu, J.; Han, B.; Chen, J. Influence of Lactobacillus brevis on metabolite changes in bacteria-fermented sufu. J. Food Sci. 2020, 85, 165–172. [Google Scholar] [CrossRef]
- Saad, M.A.; Abd-Rabou, H.S.; Elkhtab, E.; Rayan, A.M.; Abdeen, A.; Abdelkader, A.; Ibrahim, S.F.; Hussien, H. Occurrence of toxic biogenic amines in various types of soft and hard cheeses and their control by D05-1. Fermentation 2022, 8, 327. [Google Scholar] [CrossRef]
- Coton, E.; Coton, M. Evidence of horizontal transfer as origin of strain to strain variation of the tyramine production trait in. Food Microbiol. 2009, 26, 52–57. [Google Scholar] [CrossRef]
- Tsai, Y.H.; Kung, H.F.; Lin, Q.L.; Hwang, J.H.; Cheng, S.H.; Wei, C.I.; Hwang, D.F. Occurrence of histamine and histamine-forming bacteria in kimchi products in Taiwan. Food Chem. 2005, 90, 635–641. [Google Scholar] [CrossRef]
- Diaz, M.; del Rio, B.; Ladero, V.; Redruello, B.; Fernandez, M.; Martin, M.C.; Alvarez, M.A. Isolation and typification of histamine-producing Lactobacillus vaginalis strains from cheese. Int. J. Food Microbiol. 2015, 215, 117–123. [Google Scholar] [CrossRef]
- Bravo-Lamas, L.; Baroja-Careaga, I.; Olañeta-Jainaga, A.; Sarasua, M. Evaluating bacterial contributions to biogenic amines levels in commercial cheeses. Food Control 2025, 175, 111334. [Google Scholar] [CrossRef]
- Natrella, G.; Vacca, M.; Minervini, F.; Faccia, M.; De Angelis, M. A comprehensive review on the biogenic amines in cheeses: Their origin, chemical characteristics, hazard and reduction strategies. Foods 2024, 13, 2583. [Google Scholar] [CrossRef] [PubMed]
- Burdychova, R.; Komprda, T. Biogenic amine-forming microbial communities in cheese. FEMS Microbiol. Lett. 2007, 276, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Joosten, H.M.; Northolt, M.D. Detection, growth, and amine-producing capacity of lactobacilli in cheese. Appl. Environ. Microbiol. 1989, 55, 2356–2359. [Google Scholar] [CrossRef] [PubMed]
- Nishida, H. Sake Brewing and Bacteria Inhabiting Sake Breweries. Front. Microbiol. 2021, 12, 602380. [Google Scholar] [CrossRef]
- Tsutsui, N.; Yamamoto, Y.; Iwami, K. Protein-nutritive assessment of sake lees obtained by brewing from liquefied rice. J. Nutr. Sci. Vitaminol. 1998, 44, 177–186. [Google Scholar] [CrossRef]
- Mikami, N.; Tsukada, Y.; Pelpolage, S.W.; Han, K.H.; Fukushima, M.; Shimada, K. Effects of Sake Lees (Sake-kasu) supplementation on the quality characteristics of fermented dry sausages. Heliyon 2020, 6, e03379. [Google Scholar] [CrossRef]
- Kim, J.; Bang, J.; Beuchat, L.R.; Kim, H.; Ryu, J.H. Controlled fermentation of kimchi using naturally occurring antimicrobial agents. Food Microbiol. 2012, 32, 20–31. [Google Scholar] [CrossRef]
- Shirasawa, H.; Nishiyama, C.; Hirano, R.; Koyanagi, T.; Okuda, S.; Takagi, H.; Kurihara, S. Isolation of the high polyamine-producing bacterium Staphylococcus epidermidis FB146 from fermented foods and identification of polyamine-related genes. Biosci. Microbiota Food Health 2023, 42, 24–33. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Voss, G.B.; Monteiro, M.J.P.; Jauregi, P.; Valente, L.M.P.; Pintado, M.E. Functional characterisation and sensory evaluation of a novel synbiotic okara beverage. Food Chem. 2021, 340, 127793. [Google Scholar] [CrossRef]
- Hazards, E.P.o.B. Scientific Opinion on risk based control of biogenic amine formation in fermented foods. EFSA J. 2011, 9, 2393. [Google Scholar] [CrossRef]
- Kitada, Y.; Muramatsu, K.; Toju, H.; Kibe, R.; Benno, Y.; Kurihara, S.; Matsumoto, M. Bioactive polyamine production by a novel hybrid system comprising multiple indigenous gut bacterial strategies. Sci. Adv. 2018, 4, eaat0062. [Google Scholar] [CrossRef] [PubMed]
- Ralph, A.; Englyst, K.; Bardócz, S. Polyamine Content of the Human Diet; Kluwer Academic Publishers: London, UK, 1999. [Google Scholar]
- Wójcik, W.; Lukasiewicz, M.; Puppel, K. Biogenic amines: Formation, action and toxicity—A review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ami, Y.; Kodama, N.; Kurihara, S. Development and Taste Improvement of Polyamine-Containing Sakekasu Beverages Using Highly Polyamine-Producing Bacteria from Fermented Foods. Fermentation 2025, 11, 297. https://doi.org/10.3390/fermentation11060297
Ami Y, Kodama N, Kurihara S. Development and Taste Improvement of Polyamine-Containing Sakekasu Beverages Using Highly Polyamine-Producing Bacteria from Fermented Foods. Fermentation. 2025; 11(6):297. https://doi.org/10.3390/fermentation11060297
Chicago/Turabian StyleAmi, Yuta, Narumi Kodama, and Shin Kurihara. 2025. "Development and Taste Improvement of Polyamine-Containing Sakekasu Beverages Using Highly Polyamine-Producing Bacteria from Fermented Foods" Fermentation 11, no. 6: 297. https://doi.org/10.3390/fermentation11060297
APA StyleAmi, Y., Kodama, N., & Kurihara, S. (2025). Development and Taste Improvement of Polyamine-Containing Sakekasu Beverages Using Highly Polyamine-Producing Bacteria from Fermented Foods. Fermentation, 11(6), 297. https://doi.org/10.3390/fermentation11060297




