1. Introduction
Craft beer is an alcoholic beverage obtained through fermentation processes, characterized by its high quality and the diversity of methods used in its production, which allows for a wide range of sensory profiles [
1]. During production, one of the most important components is yeast, especially the species
Saccharomyces cerevisiae for ale-type beers and
Saccharomyces pastorianus for lager-type beers, due to their ability to convert wort sugars into ethanol and carbon dioxide [
2,
3,
4].
The fermentation process requires an initial concentration of approximately 15 million cells per milliliter of wort, which can be achieved through a yeast cultivation process [
5,
6,
7]. The use of a suitable inoculum is essential to guarantee enough viable yeast for efficient fermentation. Achieving this involves establishing optimal cultivation conditions that promote high cell concentrations and improving fermentation performance [
6]. These conditions include key parameters such as temperature, aeration, agitation, and nutrient availability [
8,
9]. However, commercial culture media commonly used for yeast cultivation are expensive, which limits their use on an industrial scale and reduces the economic feasibility of the process [
10,
11].
Beyond optimizing physical parameters, a major challenge lies in developing nutrient media that enables efficient yeast growth at reduced costs. In recent years, several studies have explored alternative low-cost substrates for yeast propagation. For instance, molasses has been used as a carbon source due to its high sugar content, but it often presents compositional variability and requires pretreatment to remove inhibitory compounds [
10,
12]. Agricultural by-products such as corn steep liquor, rice bran, or extruded legumes have been tested as nitrogen sources, offering low cost but limited reproducibility and batch-to-batch consistency [
13,
14]. Similarly, brewery spent grain hydrolysates and vegetable waste extracts have shown promise but often lack the micronutrient balance necessary to sustain high yeast viability and biomass yield [
15,
16]. Several studies have addressed the production and propagation of brewer’s yeast using alternative media, highlighting the importance of optimizing culture conditions to maximize growth and viability, as well as rigorous control of parameters to prevent microbial contamination and loss of active cells [
9,
17,
18]. These studies demonstrate the potential of alternative media but also highlight a persistent limitation: most available low-cost formulations compromise either process reproducibility or biomass productivity compared to synthetic media.
Also, most microbreweries in Ecuador lack dedicated propagation infrastructure and standardized protocols to ensure consistent viability and purity at larger volumes.
Given these limitations, there is a need for a sustainable and reproducible alternative medium that maintains high yeast viability while significantly lowering production costs suitable for small-scale facilities. In this context, yeast waste generated by the brewing industry represents a valuable but underutilized source of nitrogen rich extract [
16], and malt extract from barley provides an inexpensive and compositionally stable carbon source. The combined use of these two brewery-derived by-products offers an innovative and circular-economy approach that minimizes costs and valorizes industrial residues.
Therefore, this study was designed to address the technical bottleneck associated with the high cost and limited reproducibility of current low-cost media for yeast propagation. We hypothesize that a medium formulated from brewery-derived yeast extract and malt extract can support yeast growth comparable to that obtained with commercial synthetic media while reducing production costs and maintaining scalability.
To test this hypothesis, we developed and optimized bioprocess for S. cerevisiae biomass production intended for craft beer fermentation. The optimization involved identifying and adjusting key culture parameters to maximize yeast growth and viability, followed by kinetic and scaling evaluations to validate the process under laboratory and pilot conditions.
3. Results
Optimization of the culture medium significantly improved yeast growth and viability. Through a Plackett–Burman experimental design, key factors influencing cell growth were identified, including maltose concentration, yeast extract, zinc sulfate, and agitation. Subsequently, the factorial design allowed for maximizing cell density and viability, achieving 100% viability in several treatments.
Validation of the optimized medium at different scales demonstrated efficient growth kinetics, characterized by faster doubling rates and higher biomass production compared to the synthetic medium. At both laboratory and prototype scales, cell concentration and biomass value exceeded those obtained with the conventional medium, highlighting its potential as a viable and cost-effective alternative for yeast propagation.
3.1. Microorganism and Culture Conditions
To achieve effective growth, the yeast was reactivated in YPM medium, which promoted rapid adaptation to the substrate and optimal growth, reaching a cell concentration of 1.28 × 107 cells/mL. These results demonstrate not only efficient utilization of maltose as a carbon source, but also the favorable physiological response of the strain to the modified medium.
3.2. Molecular Identification
After performing, the BLAST analysis of the sequence, a 99.82% identity was obtained for a
Saccharomyces cf.
bayanus/pastorianus (KY104984) and
S. cerevisiae (MZ098694) strains. To confirm the identification, a maximum-likelihood tree was performed and confirmed that the sample was
S. cervisiae with 999 bootstrap values. Additionally, the sample was located within the clade of
S. bayanus and
S. pastorianus, suggesting that it could be lager-type strain (
Figure S2).
3.3. Experimental Designs
3.3.1. Significative Variables Selection by Plackett–Burman Design
Based on the results obtained from the Plackett–Burman design, treatment 4 was identified as having the best performance, achieving 99% cell viability and a density of 4.84 × 10
6 cells/mL (
Tables S1 and S2,
Figure 1). Statistical analysis using ANOVA revealed that the variables with a significant effect on the growth of
S. cerevisiae were maltose (
p = 0.020), commercial yeast extract (
p = 0.031), ZnSO
4 (
p = 0.018), and agitation (
p = 0.011;
Table S2).
3.3.2. Treatment Optimization by Factorial Design 24
The factorial design was applied to the significant variables identified in the Plackett–Burman design (
Table S3). It showed 100% cell viability for treatments 9, 10, 12, 13, 15, and 16. Among these, treatment 16 exhibited the highest cell density, reaching 1.89 × 10
7 cells/mL.
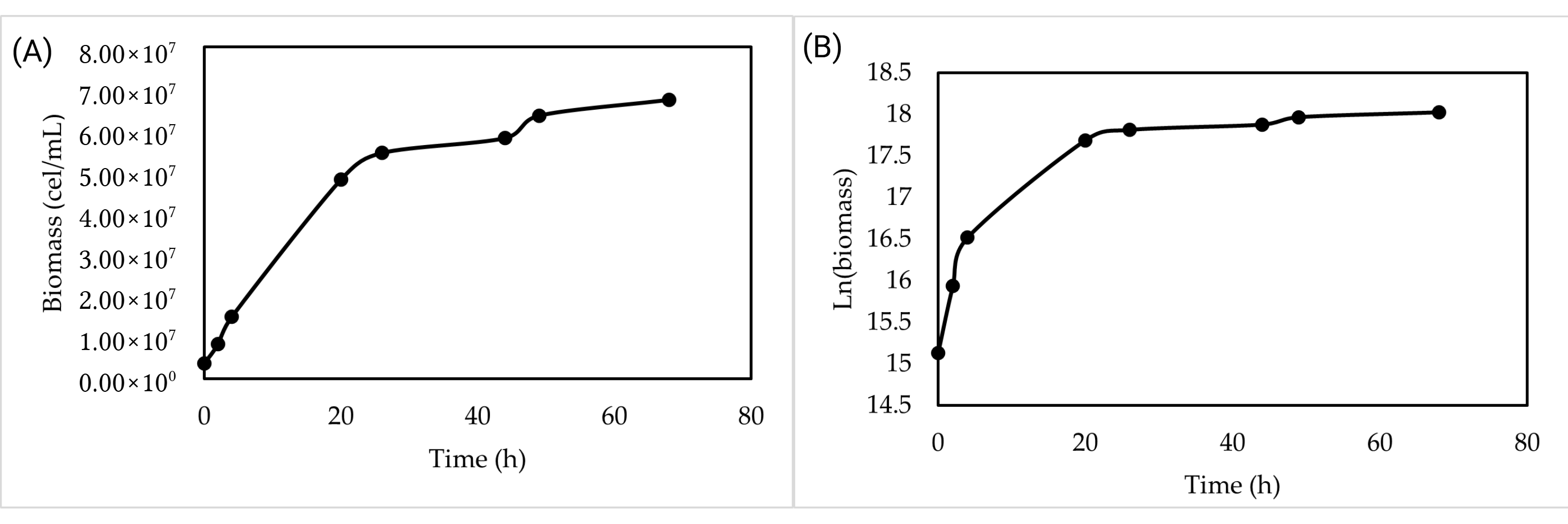
3.4. Validation of Optimized Synthetic Medium at Laboratory Scale
The validation of the optimized synthetic medium at the laboratory scale showed that the yeast growth phases were clearly distinguishable (
Figure 2). A very short lag phase was observed, followed by an exponential phase that lasted up to 24 h. Subsequently, the yeast enters a stationary phase that covers the rest of the process. After 72 h of incubation, a cell concentration of 6.72 × 10
7 cells/mL and a biomass concentration in dry weight of 1.8 g/L were reached.
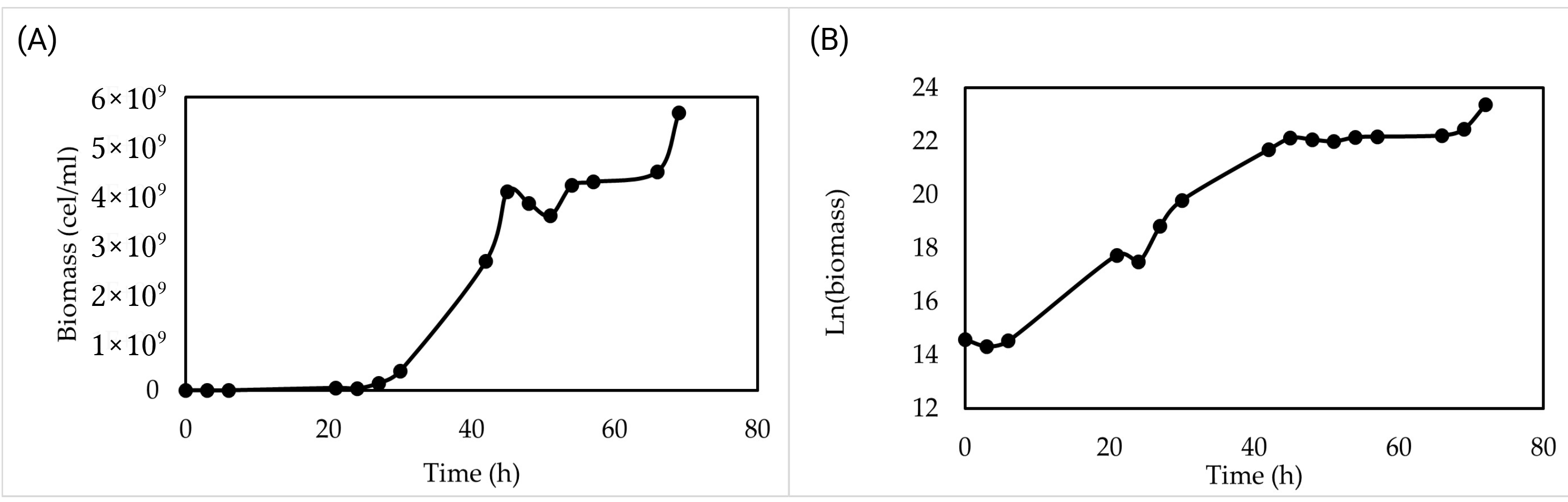
3.5. Validation in Low-Cost Culture Medium at Laboratory and Pilot Scale
Validation at laboratory scale (2 L) revealed the following growth profile: a brief lag phase until hour 6, followed by exponential growth that lasted until hour 45. In the linear plot (
Figure 3A), temporary fluctuations and apparent lag duration are more pronounced, while the steep slope in the semilogarithmic representation (
Figure 3B) clearly shows the exponential phase. From hour 45 onward, the growth rate slowed, reflected in the flattening of the slope in the semilogarithmic plot. After 72 h of incubation, the cell concentration reached 1.4 × 10
10 cells/mL, and the biomass was 10 g/L.
A slight deviation observed around 70 h was attributed to transient variations in mixing conditions during cultivation; however, this did not affect the overall reproducibility or the kinetic parameters derived from the growth curve.
Pilot-scale validation was performed in duplicate in an 83 L bioreactor. For the first validation, the growth kinetics showed a pattern observed at the laboratory scale, identifying the three classic phases of growth, lag, exponential, and stationary. These phases, including the brief lag phase lasting until approximately 6 h, the end of the exponential phase at 42 h, and the transition to the stationary phase after 42 h, are clearly visualized in the semilogarithmic representation (
Figure 4B), which linearizes the exponential growth phase and allows accurate assessment of growth kinetics. In contrast,
Figure 4A shows the same data on a linear scale, where temporary fluctuations appear more pronounced and the growth pattern differs from the second validation. The semilogarithmic plots of the second validation (
Figure 4D) are very similar to the first (
Figure 4B), indicating consistent growth kinetics, whereas the linear plots (
Figure 4C) differ in appearance due to the scale. In the first validation, the final cell concentration was 1.4 × 10
10 cells/mL, and the biomass reached 9.62 g/L.
For the second validation, the growth kinetics showed a pattern like the first, with an exponential phase from 6 h to 42 h, followed by a stationary phase. On this occasion, the final cell density at 72 h was 4.7 × 109 cells/mL, and the biomass reached 8.14 g/L.
3.6. Comparison of Kinetic Parameters
The results obtained from the kinetic parameters revealed differences between the optimized synthetic culture medium and the low-cost alternative medium. In terms of maximum growth rate (µ
max), a notable increase was observed in the laboratory-scale validation of the alternative medium, reaching a value of 0.202 h
−1 at a volume of 2 L, representing an increase of approximately 26% compared to the value of 0.16 h
−1 obtained with the synthetic medium at a volume of 500 mL (
Table S4).
It is important to note that the kinetic results obtained for the synthetic and low-cost media are not directly comparable, as each formulation was optimized and evaluated under specific conditions according to its physicochemical characteristics. The analysis therefore focuses on the reproducibility and scalability of the proposed low-cost medium rather than on direct yield equivalence between both systems.
On a pilot scale, the prototype showed a µmax of 0.244 h−1 in validation 1, which exceeded the maximum growth rate observed in the synthetic medium under the same conditions by 52%. However, in validation 2, the µmax of the alternative medium decreased to 0.185 h−1, reflecting a slight decrease compared to validation 1.
Regarding doubling time, the alternative medium showed significant improvement. At the laboratory scale, the doubling time was reduced by 27% from 4.67 h with the synthetic medium to 3.42 h with the alternative medium. At the prototype level, the doubling times were 2.83 h in the first validation and 3.74 h in the second, compared to 4.67 h for the synthetic medium in the laboratory.
In terms of biomass, the alternative medium showed superior performance under all cultivation conditions. At the laboratory scale, a biomass of 10 g/L was achieved, almost six times more than the 1.8 g/L obtained with the synthetic medium. In industrial-scale validation, the biomass was 9.62 g/L in the first validation, with a slight decrease to 8.14 g/L in the second.
In terms of cell density, the alternative medium also outperformed the synthetic medium. After 72 h of cultivation, a cell density of 1.4 × 1010 cells/mL was obtained in the validation of the alternative medium at 2 L, representing an increase of more than 20 times compared to the 6.72 × 107 cells/mL of the synthetic medium. On an industrial scale, cell densities were equally high, reaching 1.4 × 1010 cells/mL in the first validation, and although in the second validation it was reduced to 4.74 × 109 cells/mL, the figure was still considerably high compared to the values obtained with the synthetic medium.
To place our results in context, we compiled representative KPI values from studies that propagated
S. cerevisiae using common low-cost substrates (molasses, corn steep liquor, whey permeate and spent brewery yeast). The comparison (
Table 3) shows that the biomass achieved with our brewery-derived medium (10 g·L
−1 in laboratory validation) is higher than many reported low-cost formulations and that our observed µ
max values (0.202–0.244 h
−1) are competitive with literature ranges. These differences underscore that direct numeric comparisons must be interpreted cautiously because KPI values depend strongly on strain, inoculum, cultivation time, feeding strategy, and analytical methods; nevertheless, the compiled
Table 3 demonstrates that our brewery-derived medium performs at least as well as many documented low-cost alternatives while offering the additional advantage of valorizing brewery waste.
4. Discussion
To the best of our knowledge, this is the first study to comprehensively evaluate the optimization of a low-cost culture medium for yeast propagation in an industrial context, with emphasis on its performance relative to commercial synthetic media. Yeast growth in liquid cultures is influenced by multiple interrelated factors, including nutrient availability, environmental conditions, and the physicochemical properties of the medium. The optimization of culture media is therefore essential to enhance process efficiency, particularly when aiming for more accessible and sustainable alternatives for industrial applications.
To the best of our knowledge, this is the first study to comprehensively evaluate the optimization of low-cost culture medium for yeast propagation in an industrial context, with emphasis on its performance relative to commercial synthetic media. Yeast growth in liquid cultures is influenced by multiple interrelated factors, including nutrient availability, environment conditions, and the physicochemical properties of the medium. The optimization of culture media is therefore essential to enhance process efficiency, particularly when aiming for more accessible and sustainable alternatives for industrial applications [
34]. This represents a growing need in countries such as Ecuador, where the high cost of imported commercial synthetic media poses a significant challenge for local industries. The use of low-cost media not only provides an economically viable solution but also reduces dependence on imported inputs and promotes local production, thereby generating a positive impact on the national economy.
In this study, several key factors influencing yeast growth and viability were evaluated, including agitation, essential nutrient concentrations, and carbon and nitrogen sources. Agitation played a crucial role in the optimization process, as it not only enhanced the homogeneous distribution of nutrients and oxygen throughout the medium but also contributed to system stability and prevented the formation of gradients that could adversely affect cell viability [
35]. In fact, adequate agitation facilitates the formation of a more homogeneous environment, which improves the metabolic efficiency of yeasts, resulting in faster growth and higher biomass concentration.
In terms of nutrients, zinc, maltose, and yeast extract were identified as essential components promoting both cell growth and viability. These nutrients play key roles in protein synthesis, cell structure formation, and overall metabolic efficiency in yeast [
36].
In particular, the combination of maltose as a carbon source and yeast extract as a nitrogen source proved decisive for maximizing cell growth. While zinc was deliberately included as a key micronutrient due to its enzymatic and metabolic roles, additional trace elements such as magnesium, manganese, copper, and iron were not individually optimized. Following recommendations from experienced brewers, their supplementation was limited to avoid potential toxicity or undesirable sensory effects in the final beer. The intrinsic composition of the yeast extract provided sufficient baseline levels of these elements to sustain balanced and reproducible yeast growth. These findings indicate that the low-cost medium, although simpler in composition, can deliver comparable or even superior performance to commercial synthetic media in terms of biomass production and cell density.
An interesting observation was that the low-cost alternative medium supported a higher growth rate and greater biomass accumulation than the synthetic medium. This result underscores the efficiency of alternative formulations that, despite being less expensive, maintain the quality and robustness of the biological process. Such outcomes are especially significant for industries in developing countries like Ecuador, where high production costs are often driven by the need to import culture media components. Therefore, the optimization of low-cost media represents a viable strategy not only from an economic standpoint but also in terms of long-term environmental and industrial sustainability.
The superior performance of the low-cost medium compared to the synthetic formulation can be attributed to its richer biochemical composition and the synergistic interaction between its carbon and nitrogen sources. The yeast extract recovered from brewery by-products likely retains a complex mixture of amino acids, short peptides, B-group vitamins, and trace minerals that act as essential cofactors in central metabolic pathways, including glycolysis and amino acid biosynthesis. In addition, spent yeast extract has been reported to contain antioxidant molecules such as glutathione and other reducing compounds that protect cells from oxidative stress during propagation, contributing to higher viability and shorter doubling times [
16,
23,
24]. When combined with malt extract, which provides a balanced mixture of fermentable sugars (mainly maltose and maltotriose) and low levels of dextrins, the medium may facilitate more efficient energy metabolism and biomass accumulation. This biochemical richness and synergistic nutrient balance explain the enhanced kinetic behavior observed in the alternative medium relative to the synthetic one [
3].
In addition, the malt extract used in this study was produced from locally sourced Ecuadorian barley, rather than commercial brewing-grade malt. This adaptation substantially reduced raw material costs while maintaining adequate sugar composition for yeast growth. The use of local barley, combined with yeast extract recovered from brewery residues, reinforces the sustainability and economic feasibility of the proposed low-cost propagation medium.
The study demonstrated that agitation was one of the most decisive factors influencing yeast biomass production, playing a key role in enhancing culture performance. Agitation facilitates the uniform distribution of nutrients and oxygen, both essential for the aerobic metabolism of yeast, thereby optimizing growth conditions. Moreover, it prevents the formation of concentration gradients that could compromise cell viability, ensuring a more stable and efficient culture environment [
36,
37]. The importance of agitation in yeast cultures has been widely documented, and it has been proven that optimizing agitation not only improves the availability of these resources, but also increases the stability of the system, which is essential in large-scale industrial processes.
Along with agitation, other factors such as the availability of micronutrients and the selection of appropriate carbon and nitrogen sources significantly influence yeast performance. Zinc, as an essential micronutrient, plays a fundamental role in numerous enzymatic processes critical for cell growth and metabolism [
15]. Additionally, maltose serves as an effective carbon source, providing the energy required for metabolic activity, while yeast extract functions as a nitrogen source that supports protein synthesis and the formation of essential cellular components [
36]. The interaction among these nutritional factors is crucial for optimizing yeast viability and proliferation during cultivation, underscoring the importance of refining growth medium formulation.
The composition and characteristics of both media are key to interpreting comparative results. The synthetic medium was formulated with high-purity inputs, such as maltose monohydrate and commercial yeast extract, and supplemented with zinc sulfate, an essential micronutrient that acts as a cofactor for enzymes involved in central metabolism, protein synthesis, and cell division. Zinc supplementation contributed to enhanced cell growth, viability, and reproducibility, as reflected in the optimization results. Other micronutrients naturally present in yeast extract, including magnesium, trace elements, and B-group vitamins, further supported metabolic activity, although their levels were not independently optimized [
13].
The alternative medium, developed from agro-industrial by-products such as recovered brewery yeast extract, substantially reduced production costs and promoted sustainability [
14]. A key limitation of using brewery-derived by-products as components of low-cost propagation media is their intrinsic compositional variability. Factors such as raw material source, processing conditions, and storage can affect the levels of sugars, amino acids, peptides, vitamins, and trace minerals in yeast extract and malt-derived residues. Such variability may influence yeast growth kinetics, biomass yield, and viability, potentially impacting reproducibility at both laboratory and larger scales. To address this challenge, future research should focus on establishing routine quality control procedures, including rapid assays for key parameters (reducing sugars and protein content) and batch-to-batch consistency checks.
Both media proved suitable for the propagation of
S. cerevisiae; however, from an economic and environmental standpoint, the alternative medium represents a promising option for small and medium-sized breweries seeking greater autonomy in yeast production without compromising microbiological quality (
Table S5).
The results obtained in this study confirm the effectiveness of the commercial medium used as a reference, in which the final biomass exceeded the initial inoculum of 0.5 g/L, reaching values comparable to those reported by Cardozo and Moreno [
12], who achieved a final biomass of 1.861 g/L under similar conditions. Nevertheless, when compared with the results obtained using the proposed alternative medium, a substantial increase in both final cell concentration and biomass was observed. After 72 h of cultivation, the alternative medium reached a cell concentration of 1.4 × 10
10 cells/mL and a biomass of 10 g/L, representing a significant improvement relative to the commercial medium. Although previous studies such as that of Cáceres [
10] reported higher values under specific conditions, particularly when using molasses as the carbon source, the results obtained with the proposed medium remain competitive. This difference can be attributed to the nature of the carbon source: molasses contains simple sugars that are rapidly assimilated by the yeast, whereas the malt extract used in the alternative medium primarily provides disaccharides such as maltose, which require additional metabolic processing time for assimilation.
The scale-up stage demonstrated that the optimized low-cost medium and process parameters were reproducible at pilot scale, confirming the robustness of the proposed bioprocess. Although the transition was made directly from 2 L shake flasks to an 83 L pilot bioreactor, this decision was based on equipment availability and the applied nature of the study. The pilot reactor was adapted from a stainless-steel fermenter with a modified lid, enabling agitation, temperature control, and sampling under semi-controlled conditions. Despite the differences in hydrodynamic behavior between orbital shaking and mechanical agitation, the process maintained comparable cell viability and biomass yields, demonstrating that the optimized conditions can be effectively transferred to larger volumes typical of small-scale brewing operations. These results support the feasibility of implementing the proposed medium and protocol in local craft breweries with limited infrastructure.
The increase in growth rate observed between the laboratory-scale and 58 L validations suggests that the optimized medium retains its effectiveness under higher-volume conditions, although further adjustments may enhance process efficiency. The variations detected in biomass and cell concentration could be attributed to differences in nutrient and oxygen distribution within the bioreactor, particularly at larger scales. As noted by Xia et al. [
38] during bioprocess scale-up, challenges associated with flow heterogeneity and nutrient or oxygen gradients can lead to alterations in cell physiology, ultimately affecting biomass production. These findings highlight the importance of optimizing agitation intensity and dissolved oxygen control systems to ensure process homogeneity and maximize yield.
Although both pilot-scale validation runs followed similar overall growth patterns, differences were observed in the final cell concentration and biomass. Since the initial inoculum was identical in both experiments, variations in inoculum density or physiological state are unlikely to account for these discrepancies. Agitation speed was maintained constant, suggesting a minimal contribution to the observed variation. Nevertheless, subtle differences in oxygen transfer within the 83 L bioreactor may have influenced growth kinetics and biomass accumulation, as even minor changes in mixing or aeration efficiency can significantly impact yeast proliferation at pilot scale.
Additionally, the alternative culture media were manually prepared in separate batches, which may have introduced slight batch-to-batch variability in nutrient composition and quality, further contributing to the observed differences. Collectively, these factors underscore the inherent variability of pilot-scale operations, particularly when using locally prepared, alternative media, and provide a plausible explanation for the discrepancies observed between the two runs.
In summary, the results of this study provide a strong foundation for the use of low-cost alternative media in yeast production. The optimized culture medium demonstrated high effectiveness in terms of growth rate, biomass concentration, and cell viability, outperforming the commercial synthetic medium across several key parameters. These findings are particularly relevant for countries such as Ecuador, where the cost of imported synthetic media remains a significant barrier. The adoption of alternative media not only has the potential to reduce production expenses but also contributes to the sustainability and self-sufficiency of local industries, fostering a more accessible and sustainable model of biotechnology in the long term.