Abstract
Saline–alkaline soils are becoming prevalent across the globe, decreasing the availability of forage for animals and threatening sustainable animal production. This study evaluated the effects of a NaCl-supplemented alfalfa-based total mixed ration, simulating saline–alkaline soil conditions, on intake, the utilization of nutrients, antioxidant levels, and rumen fermentation. A 60-day feeding trial with 24 AOHU lambs (Australian White × Hu) compared a control diet (0.43% NaCl) with the NaCl-supplemented group (1.71% NaCl). Digestibility trials were conducted in metabolic cages for the collection of total feces and urine. Blood samples were taken at 0, 30, and 60 days for serum analysis, and slaughter samples (liver, kidney, rumen tissue, and rumen fluid) were taken for physiological, biochemical, and histological evaluation. The NaCl alfalfa-based TMR markedly increased liver and kidney weights. The rumen muscle layer thickened in the NaCl group. The ruminal ammonia nitrogen (NH3-N), ruminal microbial crude protein (MCP) synthesis, and glucogenic/branched-chain VFAs increased, indicating enhanced proteolysis, microbial protein synthesis, and energetically efficient fermentation. Serum total protein and albumin also rose over time in the NaCl group, reflecting increased nitrogen retention, while superoxide dismutase and glutathione peroxidase activity rose considerably by day 60, reflecting increased antioxidant defense. Furthermore, nitrogen intake, digestibility, and retention were improved in the NaCl group along with augmented digestible and metabolizable energy (28.47 vs. 13.93 MJ/d and 24.68 vs. 11.58 MJ/d, respectively) and gross energy digestibility (78.13% vs. 67.10%). Although NaCl-based alfalfa TMR cannot fully emulate naturally salt-stressed forages, these results indicate that the NaCl alfalfa-based diets improved rumen fermentation, energy yields, and antioxidant enzyme activity without impairing electrolyte balance. These findings suggest that NaCl-supplemented alfalfa-based TMRs, with a salt content comparable to that of alfalfa hay grown under saline–alkaline conditions, could support environmentally sustainable meat production in salt-stressed regions.
1. Introduction
As global demand for high-quality animal protein, particularly from ruminants, continues to grow, there is a need to maximize feed efficiency in a sustainable way [1,2]. Immunological responses, metabolic function, and general animal well-being all depend heavily on nutrition [3,4,5]. Nutrition directly supports sustainable animal production by increasing digestion, maximizing nutrient usage, and boosting resistance to environmental stressors [6]. Proper management and utilization of arid and semi-arid regions is essential to fulfilling these demands and alleviating gaps in food production and security. Over 952 million hectares are salinized worldwide, with the most affected regions in Africa, Australia, and Asia, including China, Kazakhstan, and Iran [7]. Feeding systems that are compatible with saline–alkaline conditions must be developed to increase livestock productivity in these regions.
Alfalfa (Medicago sativa L.) is highly nutritious and hardy and can tolerate saline–alkaline soil. Its tolerance to saline–alkaline soils qualifies it as a potential candidate for feed in marginal land [8,9], yet little has been investigated on how such stress-imposed alterations influence animal performance. Alfalfa cultivated under saline conditions can accumulate sodium chloride (NaCl), potentially altering its nutritional composition and affecting animal metabolism. Salt has been found to influence feed intake, water balance, and digestion [10,11,12,13].
As a critical element of sustainable ruminant nutrition, sodium plays a crucial role in osmotic regulation, neuromuscular function, and palatability of feed, thus having a direct influence on animal health and productivity [12,13]. Salt and mineral imbalances are reported to affect oxidative stress markers and metabolic efficiency in ruminants [14,15,16]. The maximum tolerable level of NaCl for sheep is up to 2.0% in the total diet, provided that fresh water is freely available [17].
Although saline–alkaline soil is increasingly common, and salt-tolerant alfalfa has the potential to sustain ruminant production, little is known regarding NaCl-induced effects on antioxidant enzymatic activity, nutrient utilization, and rumen fermentation in sheep, particularly in salt-stressed areas, where alfalfa hay is grown under saline–alkaline conditions. The initial objective of this research was to compare low-salinity and high-salinity alfalfa; shortages in forage availability necessitated our use of a simulation model. Specifically, 1.71% NaCl was added to an alfalfa-based total mixed ration to mimic the most common salt concentration found in alfalfa from saline–alkaline soils [18]. To our knowledge, the present study is the first to consider these parameters in AOHU sheep, combining metabolic, morphometric, and fermentation markers to guide sustainable feeding in salt-stressed environments.
2. Methods
2.1. Experimental Animals and Feeding Management
This experiment was approved by the Animal Ethics Committee of the Chinese Academy of Agricultural Sciences (IFR-CAAS-2024-07-22), and the procedures followed institutional policy regarding the use and care of experimental animals. The experiment was conducted for 60 days (August to October 2024) at the Nankou experimental site, Beijing, China. The completely randomized design (CRD) consisted of a control group fed the alfalfa cultivated and harvested from non-saline–alkaline soil supplemented with 0.43% NaCl and the NaCl group that received the same alfalfa-based ration supplemented with 1.71% NaCl to achieve the NaCl content of alfalfa as planted in heavily saline–alkaline soil. Twenty-four weaned male lambs aged 2–3 months, which were not castrated, were randomly assigned into the two treatment groups based on their initial body weight (control group: 22.64 ± 4.4 kg; NaCl group: 22.36 ± 4.05 kg). Each pen housed four sheep (12 m2 per pen) and was equipped with two drinkers. The experiment duration was split into three phases: a 20-day adaptation phase, during which the lambs adapted to the housing and automatic feed system conditions; a 10-day initial feeding phase, during which animals adapted to the test diets and the level of feed consumption was recorded (the records of these measurements were not analyzed); and a 60-day feeding trial, which was the main experiment period when growth performance, nutrient use, activity of antioxidant enzymes, and rumen fermentation properties were measured (Supplemental Figure S1). During the period of the trial, all lambs were immunized with the Aftosa vaccine against foot-and-mouth disease (FMD). No additional anthelmintic or antiparasitic treatment was administered as only pre-dewormed sheep were used in the trial. The barn was disinfected every week using glutaral and deciquam solutions. The lambs were adapted to the automatic feeding system before the experiment, and they were maintained under identical conditions. Throughout the experimental period, no cases of illness or mortality were observed.
The feed was formulated in accordance with guidelines [17] (Table 1) to provide adequate nutrition during growth in lambs. The salt was mixed uniformly in the mixer machine with the roughage. For every 82.33 kg of TMR, 2 L of water was added to slightly moisten the feed, reduce dust, and improve mix uniformity. The so-prepared TMR was stored and used for 4 days with the necessary precautions against spoilage and consistency of the feed. The animals had free access to drinking water and received diets twice a day at 07:00 and 17:00 in equal portions of a TMR with 85.41% DM (Supplemental Figure S1). The automatic feeder (model YMT-SF-02, Tai’an Yimeite Machinery Co., Ltd., Tai’an, China) used an integrated RFID antenna (ISO 11784/5 [19,20] FDX-B) to read each sheep’s ear tag and record the exact individual feed intake while dispensing the programmed ration.

Table 1.
Proportions and nutrient contents of test diets (%, dry matter basis).
2.2. Experimental Feed Chemical Composition Analysis
The dried samples were ground in a Wiley mill fitted with a 40-mesh screen, after which nutritional analysis of the feed was carried out. The dry matter (DM) content was established by oven-drying samples at 105 °C for a period of four hours [21]. The ash content was determined through combustion of the samples in a muffle furnace at 550 °C to yield the inorganic ash residue [21]. The nitrogen content (N) of the different samples was determined using the Kjeldahl methods of extraction [21], while the N values obtained were multiplied by a factor of 6.25 to estimate crude protein (CP) content. The ether extract was the crude fat, determined with the Soxhlet extraction method, using petroleum ether as solvent [21]. Calcium was quantified through the titration method, with acid digestion of the sample [21]. Phosphorus (P) was determined using the molybdovanadate method, which forms a yellow phosphomolybdate complex for quantification [21]. Gross energy was measured with an adiabatic bomb calorimeter and expressed in megajoules per kilogram (MJ/Kg). The neutral detergent fiber (NDF) and acid detergent fiber (ADF) content were determined according to the procedure described by [22]. NaCl content was computed from sodium following closed-vessel acid digestion (8 mL 65% HNO3 + 2 mL 30% H2O2, 180 °C, 20 min) [23] on a Raykol automated microwave (Raykol Group, Xiamen, China); sodium was determined using a BIOBASE BK-FP640 flame photometer (BIOBASE, Shandong, China) and NaCl (%) = Na (%) × 2.54 [24].
2.3. Total Feed, Water and Salt Intake and Growth Performance
The feed intake of each sheep fed an alfalfa-based total mixed ration was evaluated using an automatic feeding system (model YMT-SF-02, Tai’an Yimeite Machinery Co., Ltd., Shandong, China) throughout the experimental period. This system was used to monitor the feed intake daily and, hence, guarantee accurate and consistent data collection. This system was able to record in real-time the amount of feed being offered, and hence it was possible to calculate the actual feed intake. The obtained data were used to calculate the performance indicators, such as body weight change in 60 days. The average daily gain (ADG) (the total weight gain divided by the number of days in the experimental period), feed conversion ratio (FCR) (the total feed intake divided by the total weight gain), and water intake were recorded per pen and expressed as pooled values for each treatment group (12 sheep). Water was provided ad libitum. Measurements were taken twice daily at 07:00 and 17:00, and consumption was calculated as the difference in the trough volume at the two intervals. The effect of salt supplementation on total NaCl intake was determined using the following formula: Total NaCl intake (g) = Total feed intake (kg) × NaCl concentration (%).
2.4. Total Fecal and Urine Excretion, Urinary and Fecal Nitrogen, and Energy Metabolism
Total fecal and urine output were measured in a 7-day digestibility trial during the final week of the 60-day experimental period, with the first 2 days as adaptation to the metabolic cages. Intake data (feed, water, and NaCl) for the entire 60-day period were also included for the calculation of total nutrient and salt intake. Six sheep per treatment were placed in metabolic cages equipped with separate feces and urine collection systems. Feces were collected daily in trays placed under the cages; urine was diverted into containers that contained 50 mL of 10% H2SO4 (v/v) to prevent nitrogen volatilization. Representative fecal and urine samples were stored at −20 °C until analysis. Samples were thawed only once immediately prior to subsampling; aliquots for combustion (bomb calorimetry) and Dumas analysis were freeze-dried.
Fecal samples for analysis were pooled per sheep across the 5-day collection period, homogenized, and subsampled. Dry matter of feces was determined by oven-drying to constant weight at 105 °C. Fecal gross energy was determined by adiabatic bomb calorimetry (IKA C 6000, IKA-Werke, Staufen im Breisgau, Germany) on homogenized, dried fecal subsamples; results were corrected for capsule blank and expressed as MJ/kg. Urine energy was measured by combusting freeze-dried 2 mL aliquots in gelatin capsules on the same bomb calorimeter (capsule blank corrected; intra-assay CV < 1.2%).
Fecal nitrogen was determined by the Kjeldahl method (AOAC 984.13). Urinary nitrogen was determined by Dumas combustion: 0.4 mL of well-mixed urine was placed in a tin capsule with 50 mg anhydrous cellulose, freeze-dried overnight to form a pellet, and analyzed on an Elementar Vario MAX cube (Elementar, Langenselbold, Germany) (950 °C, pure oxygen). Nitrogen (N2) was quantified by thermal conductivity and calibrated against an EDTA standard (9.57% N). All N analyses were performed in duplicate; EDTA recovery was 99.2% and intra-assay CV was 0.9% (n = 10).
Total fecal output (g/animal) and total urine output (mL/animal) were calculated as the sum of daily collections over the 5-day collection period. Nitrogen intake (g/day) = daily feed intake × dietary N content. Fecal N (g/day) and urinary N (g/day) were obtained from analyses of the pooled samples and adjusted to total fecal and urine output. Nitrogen absorption = N intake − fecal N. Retained N = absorbed N − urinary N. Nitrogen utilization efficiency (%) = (retained N ÷ N intake) × 100. Biological value of nitrogen (BV, %) was calculated as: BV (%) = (retained N ÷ absorbed N) × 100. Urinary N loss was also expressed as a proportion of intake: (urinary N, g/day ÷ N intake, g/day) × 100.
Gross energy intake (GEI, MJ/day) = feed intake × dietary gross energy content. Digestible energy (DE, MJ/day) = GEI − fecal energy loss. Methane energy loss (CH4, MJ/day) was estimated using the Blaxter and Clapperton [25] approach where CH4 energy = GEI × Ym/100, with Ym (%) representing the percentage of GEI lost as methane; CH4 energy was converted using an energy content of 39.54 kJ/L CH4. Methane emission was expressed relative to intake as CH4 per kg DM (MJ/day ÷ DMI, kg/day) and CH4 per kg NDF (MJ/day ÷ NDF intake, kg/day), where NDF intake = DMI × dietary NDF (% DM ÷ 100. Metabolizable energy (ME, MJ/day) was calculated as ME = DE − urinary energy losses − CH4 energy losses. Apparent digestibility of gross energy (GE%) = (DE ÷ GEI) × 100. Metabolizability of digestible energy (DE%) = (ME ÷ DE) × 100.
2.5. Blood Serum Proteins, Antioxidant Enzymes, and Mineral Analysis
The serum parameter experiment was conducted with 8 sheep per group. Samples were collected on 0, 30, and 60 days of the experiment before slaughter. Whole blood was kept at 2–8 °C overnight and then centrifuged at 1050× g for 15 min; serum was harvested and stored at −20 °C (short term) or −80 °C (long term) to prevent degradation. The blood serum profiles were quantified with a Mindray BS-200 auto-analyzer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) using species-validated kits: albumin (ALB) using the bromocresol-green end-point method [26], total protein (TP) via the biuret reaction [27], and the antioxidant enzyme superoxide dismutase [28] and glutathione peroxidase (GSH-Px) with kinetic assays (450 nm and 340 nm, respectively) based on the xanthine–xanthine oxidase–WST-1 and coupled glutathione reductase–NADPH systems [29]. All reactions were run at 37 °C with daily two-level calibration and an intra-assay CV < 5%, ensuring clear, non-hemolyzed samples free of suspended particles.
Serum chloride, sodium, and potassium were quantified using commercially obtained colorimetric and turbidimetric assay kits (catalog C013-2-1, A031-2-1, and C009-2-1, respectively) on a Mindray BS-200 auto-analyzer (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) following the manufacturer’s instructions. The quantifications were performed at 620–630 nm for sodium, 440–450 nm for potassium, and 450 nm for chloride. Serum samples were analyzed with the same protocols, and potassium was normalized to protein content.
2.6. Kidney and Liver Weight
At the end of the experiment, all animals were deprived of feed for 12 h but had free access to water. Then, eight lambs from each treatment group were randomly selected for slaughter following commercial slaughtering procedures (NY 467-2001, Ministry of Agriculture and Rural Affairs, Beijing, China). The lambs were physically restrained and stunned using electrical stunning equipment with specialized machines (model ES-2025, Qingdao Ejoy Farming Machinery Co., Ltd., Qingdao, China) to induce immediate loss of consciousness. Exsanguination was subsequently carried out by cutting the carotid arteries and jugular veins with a sharp knife to enable rapid bleeding. Immediately post-slaughter, the kidney and liver were weighed.
2.7. Rumen Section Length, Width, and Thickness
The rumen wall from the ventral sac (saccus ventralis) of the eight slaughtered sheep in each treatment group was obtained following slaughter and preserved in formalin. The rumen wall was dehydrated, cleared, and embedded in paraffin wax, then cut into sections 5–10 µm thick. The sections were stained using Hematoxylin and Eosin (H&E) to visualize structural details. Using a light microscope (CX23, Olympus, Tokyo, Japan), the thickness of the muscular layer was measured at multiple points (5 measurements per section) and recorded in millimeters (mm). Cuticle thickness was measured at more than one point in the rumen epithelium. Nipple length (from base to tip) and nipple width (widest diameter) of the papillae were measured. Measurements were recorded in millimeters (mm) for all, and average data per treatment group and each sheep were collected.
2.8. Rumen Fluid pH, NH3-N (Ammonia Nitrogen), and MCP (Microbial Crude Protein)
Rumen fluid was taken directly from the rumen of eight sheep/group at slaughtering, and the fluid was filtered through four layers of cheesecloth to eliminate large feed particles. The pH of the rumen fluid was measured immediately using a calibrated digital pH meter (PHS-3C, INESA Scientific Instrument Co., Ltd., Shanghai, China). To determine the concentration of NH3-N (ammonia nitrogen), 5 mL of rumen fluid was centrifuged at 1006× g for 10 min at 4 °C. The supernatant was then added to the phenol-hypochlorite reagent and determined colorimetrically by absorbance at 630 nm [30]. For microbial crude protein (MCP) measurement, 10 mL of rumen fluid was centrifuged at 11,180× g for 10 min at 4 °C. The microbial pellet was washed, recentrifuged, and freeze-dried [30]. MCP levels were then quantified based on total nitrogen measured with the Dumas combustion method and expressed as protein by multiplying the nitrogen content by 6.25, as described previously [31].
2.9. Volatile Fatty Acid Profiles
Rumen fluid was collected at the time of animal slaughtering from two experiment groups (control and NaCl supplementation) with 8 animals per group. The fluid was extracted by applying a liquid–liquid extraction protocol using ether, drying, and reconstitution with an appropriate solvent [32]. The VFAs were analyzed on a Shimadzu GC-2014C gas chromatograph (Shimadzu China, Shanghai, China) with an HP-INNOWAX capillary column (30 m × 0.25 mm × 0.25 µm; Agilent Technologies, Shanghai, China) and a flame-ionization detector and eluted with helium carrier gas and 2-ethylbutyric acid as internal standard.
2.10. Data Analysis
Statistical analysis was performed with GraphPad Prism (version 9.5.1, GraphPad Software, San Diego, CA, USA) for independent two-tailed t-tests and SPSS (version 28.0, IBM Corp., Armonk, NY, USA) for repeated-measures and one-way ANOVA. An a priori power analysis (G*Power 3.1.9.7, two-tailed, two-independent-samples t-test, α = 0.05, power = 0.95, allocation ratio = 1:1) was conducted using an expected Cohen’s d = 0.65 (mean difference divided by the pooled standard deviation reported in previous work). The analysis indicated that 12 animals per group (total n = 24) would provide an exact power of 0.952 to detect the anticipated treatment effect. Data were checked for normal distribution (Shapiro–Wilk test) and homogeneity of variance (Levene’s test) before analysis. Independent t-tests were used for two-group comparisons (e.g., urine and feces excretion, body weight change, and organ weights), while repeated-measures ANOVA was used for time-dependent parameters (blood serum proteins, antioxidant enzymes, and mineral analysis). The results are presented as mean ± SEM, with exact p-values provided. As arithmetic means and least squares were equal when the design was fully balanced and there were no covariates, treatment was considered as a fixed effect and animal as a random effect in the ANOVA models. A significance level of p < 0.05 was considered statistically significant.
3. Results
3.1. Intake, Excretion, Growth Performance, and Organ Weight
Total feed intake was 31% higher in the NaCl-treated group than in the control group (p = 0.003). This was followed by a 50% increase in total water intake (p < 0.001) and more than a fivefold increase in total salt intake (p < 0.001). The gain in body weight was also greater in NaCl-treated sheep than in the control (p = 0.001), which is equivalent to the increased ADG (p = 0.001) and improved FCR (p = 0.04). Excretory output was substantially increased in the NaCl group, with the total urine excretion doubling (p = 0.01) and the fecal excretion increasing by 38% (p = 0.04). Organ weights were also affected, with kidney weight increasing by 27% (p = 0.01) and liver weight increasing by 25% (p = 0.01) (Table 2).

Table 2.
Effects of NaCl supplementation on intake, excretion, body weight change, and organ changes in AOHU sheep, DM basis.
3.2. Rumen Section Length, Width, and Thickness
The muscular layer thickness was 33% greater in the NaCl-treated group than in the control group (p = 0.007). In contrast, there were no statistically significant group differences in cuticle thickness, papillae length, or papillae width (p > 0.05) (Figure 1).
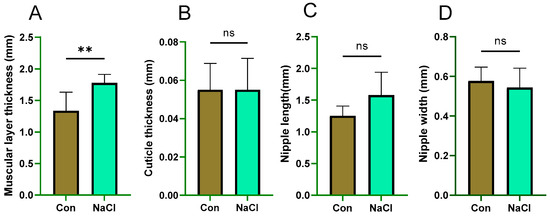
Figure 1.
Effect of NaCl supplementation on rumen sections in sheep. (A): muscular layer thickness; (B): cuticle thickness; (C): nipple length; (D): nipple width. Con: control group; NaCl: 1.71% NaCl group. ** p < 0.01; ns = not significant (p > 0.05). Values are presented as mean ± SEM (n = 8 sheep/group). p-values are based on independent-samples t-test. Con (Control) group: diet containing 0.43% DM NaCl (Low-NaCl). NaCl group: diet containing 1.71% DM NaCl (high-NaCl).
3.3. Rumen Fluid pH, NH3-N (Ammonia Nitrogen), and MCP (Microbial Crude Protein)
Rumen pH in both groups remained unchanged and did not differ significantly between the control and NaCl-treated groups (p > 0.05; Figure 2).
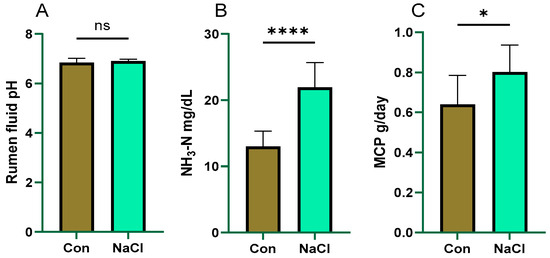
Figure 2.
Effect of NaCl supplementation on rumen fermentation in sheep. (A): rumen fluid pH; (B): ammonia nitrogen milligrams per deciliter (NH3-N mg/dL); (C): microbial crude protein grams per day (MCP g/day). MCP levels were calculated per unit of feed intake. **** p < 0.0001; * p < 0.05; ns = not significant (p > 0.05). Values are presented as mean ± SEM (n = 8 sheep/group). p-values are based on independent-samples t-test. Con (Control) group: diet containing 0.43% DM NaCl (low-NaCl). NaCl group: diet containing 1.71% DM NaCl (high-NaCl).
Nonetheless, the concentration of NH3-N was markedly elevated in the NaCl group, rising by 69% (p = 0.0001). Microbial crude protein (MCP) levels were also higher in the NaCl-treated group, which exhibited a 25% increase (p = 0.037) (Figure 2).
3.4. Blood Serum Mineral Content
Serum electrolyte concentrations did not differ between the NaCl-treated and control groups at 0, 30, or 60 days of the experimental course (p > 0.05; Figure 3).
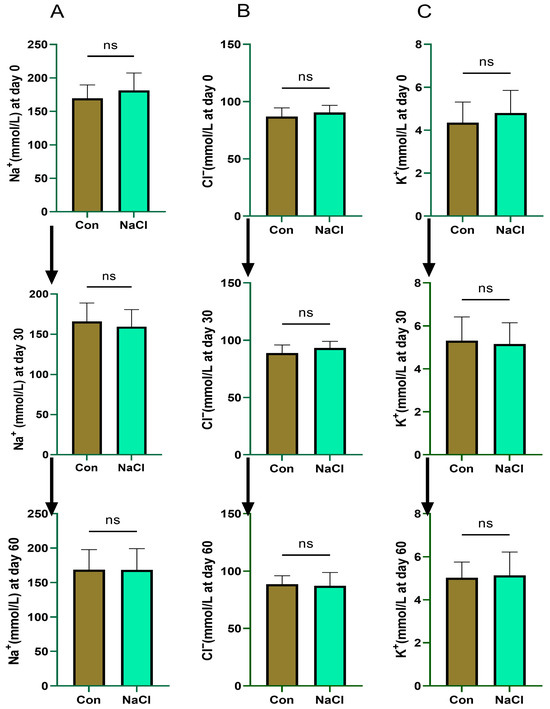
Figure 3.
Effect of NaCl supplementation on blood serum mineral content in sheep. (A): Na+; (B): Cl−; (C): K+ at 0, 30, and 60 days. ns = not significant (p > 0.05). Values are presented as mean ± SEM (n = 8 sheep/group). p-values are based on two-way repeated-measures ANOVA with factors treatment (Con vs. NaCl) and time (days 0, 30, 60). Con (Control) group: diet containing 0.43% DM NaCl (Low-NaCl). NaCl group: diet containing 1.71% DM NaCl (high-NaCl).
3.5. Effect of NaCl on Serum Protein and Antioxidant Enzymes
The albumin (ALB) level did not differ between groups at baseline or day 30 but increased significantly in the NaCl group at day 60. The total protein (TP) level was not different at baseline but was significantly elevated in the NaCl group at days 30 and 60. Likewise, activities of superoxide dismutase and glutathione peroxidase (GSH-PX) were similar at baseline but were significantly elevated in the NaCl group at days 30 and 60 (Figure 4).
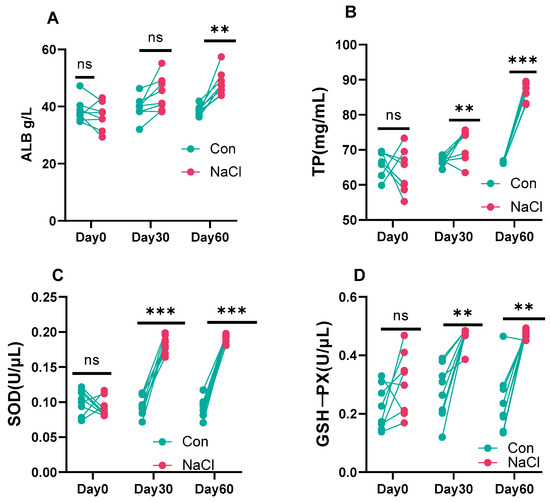
Figure 4.
Effect of NaCl supplementation on blood serum protein and antioxidant enzymes in sheep over time. (A): albumin (ALB); (B): total protein (TP); (C): superoxide dismutase; (D): glutathione peroxidase (GSH-PX). *** p < 0.001; ** p < 0.01; ns = not significant (p > 0.05). Values are presented as mean ± SEM (n = 8 sheep/group). p-values are based on two-way repeated-measures ANOVA with factors treatment (Con vs. NaCl) and time (days 0, 30, 60). Con (Control) group: diet containing 0.43% DM NaCl (Low-NaCl). NaCl group: diet containing 1.71% DM NaCl (high-NaCl).
3.6. Volatile Fatty Acids
NaCl supplementation significantly altered the ruminal VFA profile. The propionic acid concentration was 27% higher in the NaCl group than in the control (p = 0.001), and isobutyric acid (p = 0.002), isovaleric acid (p = 0.001), and valeric acid (p = 0.001) were increased. In contrast, acetic acid was more concentrated within the control group (p = 0.01), and butyric acid was not different between groups (p = 0.6). The total VFA concentration was greater in the NaCl group (p = 0.001). According to VFA molar ratios, NaCl supplementation increased the relative percentage of propionic (p = 0.001), isobutyric (p = 0.008), isovaleric (p = 0.004), and valeric acids (p = 0.001), while acetic acid was higher in the control group (p = 0.001). Consequently, the ratio of acetate to propionate was significantly reduced in the NaCl group (p = 0.001) (Table 3).

Table 3.
Effects of NaCl supplementation on ruminal VFAs profile in sheep.
3.7. Fecal and Urinary Protein and Energy Metabolism
NaCl supplementation highly stimulated protein metabolism. Nitrogen intake was 76% higher for NaCl than the control (p < 0.001), nitrogen absorption was 122% higher (p < 0.001), and retained nitrogen was 292% higher (p < 0.001). Apparent nitrogen digestibility increased from 61% to 77% (p < 0.001), nitrogen utilization efficiency was more than doubled (22% vs. 50%; p = 0.001), and the nitrogen biological value was 76% higher (p = 0.01). Nitrogen excretion in the urine did not vary between groups (p = 0.33). The proportion of nitrogen lost in the urine, however, declined (p < 0.001), and the urinary nitrogen concentration was reduced (p = 0.02). Energy metabolism was improved. Digestible energy intake was roughly twofold (p < 0.001) higher, gross energy digestibility was higher (p < 0.001), and metabolizable energy intake was more than twofold higher (p < 0.001). Metabolizability of gross and digestible energy both increased (p < 0.001). Urinary energy excretion did not differ (p = 0.19), but urinary energy concentration was lower (p = 0.006) (Table 4).

Table 4.
Effects of NaCl on nitrogen and energy metabolism in sheep.
4. Discussion
4.1. Intake, Excretion, Growth Performance, and Organ Weight
The increases in water and feed consumption following the NaCl alfalfa-based TMR supplementation are consistent with [33], where increased sodium intake resulted in thirst and hunger, possibly as a homeostatic mechanism to sustain fluid and electrolyte balance. The significant improvement in growth performance observed in the NaCl-supplemented group can largely be attributed to the role of sodium in stimulating feed intake [34]. The increase in urine excretion is consistent with the diuretic effect of sodium excess, promoting renal sodium excretion to maintain systemic sodium balance [35]. However, it is also possible that elevated urine and fecal excretions partially reflect increases in feed and water intake associated with NaCl supplementation, which can enhance renal and gastrointestinal output. Likewise, the increased fecal output might be due to the osmotic effect of excess luminal salt on intestinal motility and water absorption [35,36]. Furthermore, the noted increase in kidney and liver weights suggests adaptive or stress responses to chronic salt exposure. Epidemiological data have indicated the correlation between high dietary salt and renal hypertrophy because of increased filtration demands [37], and hepatic adaptation may be an expression of broader systemic alterations in fluid balance and metabolic function [38,39]. While the activity of hepatic urea cycle enzymes was not measured in our experiment, the increases in serum urea and excretion of urine nitrogen are consistent with elevated hepatic nitrogen metabolism secondary to augmented ruminal ammonia. Taken together, these findings highlight the multifaceted physiological impact of prolonged salt intake, ranging from fluid control to possibly significant organ function and systemic metabolic alteration.
4.2. Rumen Section Length, Width, and Thickness
The increase in thickness of the muscular layer of the rumen wall in the NaCl-treated group [40] suggests that dietary salt supplementation may lead to structural modifications within smooth muscle tissues, perhaps as a secondary effect of heightened osmotic load. This increase may reflect physiological adjustments within muscle tone and integrity, particularly in tissues responsible for fluid and electrolyte balance.
On the other hand, the absence of any definite variation in cuticle thickness, nipple width, and nipple length implies that the ingestion of NaCl could influence certain types of tissues selectively without influencing others. Such a selective effect implies that tissue types that are more related to osmoregulatory activity would be more susceptible to morphological remodeling under conditions of high sodium intake. The failure to make structure modifications in the cuticle and diameter of the nipple could also signal built-in salt stress resistance or differences in functional pressures not requiring adjustment in morphometrics under applied conditions.
Cumulatively, these findings document the tissue selectivity of adaptation in structure with respect to a salt-based alfalfa diet and indicate the value of exploring the mechanisms by which selectivity is conferred.
4.3. Rumen Fluid pH, NH3-N, and MCP
The results of this study indicated that NaCl alfalfa-based TMR supplementation did not significantly affect rumen fluid pH, in line with previous findings that nutritional salt has a minimal effect on ruminal acidity when consumed in normal physiological quantities [34]. The maintained pH of the two groups reveals that NaCl has no interfering effect on the rumen buffering ability [41], probably due to the strong regulation processes involving bicarbonate secretion and volatile fatty acid absorption. Ruminants have a well-entrenched homeostatic system to maintain rumen pH within optimal levels, and the fact that no change in pH occurred suggests that NaCl did not induce acidosis or alkalosis, confirming its safety at the levels tested. The marked augmentation of NH3-N content within the NaCl-supplemented group is an expression of improved proteolytic action [42] and ruminal nitrogen release that could be related to increased microbial metabolism [43]. The findings are corroborative with previous research in goats and sheep, whereby it was established that supplementation with salt increases nitrogen recycling efficiency, such that more ammonia becomes available to microorganisms [41,44].
Sodium has further been suggested to have a direct function in allowing the passage of amino acids and ammonia through microbial membranes and thus accelerating nitrogen assimilation into microbial biomass [45]. The considerable NH3-N concentrations therefore reflect more effective degradation of the protein diet in the rumen, considering that only 20% of ruminal ammonia derives from endogenous urea, while the majority is produced from feed [46]. The increased MCP yield of the NaCl-treated group is a testament to the beneficial effect of salt on rumen microbe growth and protein synthesis. The higher MCP concentrations may result from increased osmotic conditions in the rumen that facilitate microbial cell metabolism and division [47]. The NaCl supplementation may also be responsible for a favorable shift in the microbial population, primarily because of enriching microbial populations like Prevotella spp. since such populations play significant roles in proteolytic activity as well as in microbial protein synthesis [48]. Enhanced MCP may contribute to higher nitrogen retention through its intestinal digestibility and biological value [49], resulting in more environmentally friendly livestock production systems [50].
Our findings highlight that NaCl supplementation, which mimics the conditions of alfalfa grown in high-saline soil, significantly influences ruminant nitrogen metabolism and microbial protein synthesis. Rising NH3-N and MCP levels signify the augmenting role of dietary NaCl in nitrogen utilization efficiency, presumably leading to improved ruminant growth performance.
4.4. Volatile Fatty Acids
A high-salt diet has been shown to suppress gut microbial populations critical to the generation of anti-inflammatory responses and homeostasis within the gut [51]. For example, overindulgence in sodium consumption has been linked with declines in critical microbial taxa that regulate inflammation and the effectiveness of fermentation [51]. Such microbial structure alteration may lead to significant alterations in the patterns of fermentation as well as VFA production.
In our study, NaCl supplementation raised the content of propionic, isobutyric, isovaleric, and valeric acid, which is an indication of a trend towards secondary end-products of salt-resistant microbial flora. On the other hand, whereas lower acetic acid content reflects lower relative activity of fiber-degrading microbes, increased feed intake in the NaCl-supplemented group must have raised the total quantity of fermentable substrate presented, and this can lead to higher absolute methane emissions despite lower acetate concentration in the rumen. The enhanced concentration of total VFA in the NaCl group reflects heightened fermentative activity when saline is present, and the reduced acetate-to-propionate ratio reflects a shift in the fermentation equilibrium. From the host’s point of view, such a metabolic adaptation translates into greater propionate availability for gluconeogenesis, potentially to augment energy supply, but reduced acetate could restrict lipid synthesis. Augmented branched-chain VFAs also reflect augmented amino acid catabolism and augment microbial protein synthesis but with greater nitrogen turnover [51]. These adjustments put into perspective the potential consequences on host energy investment, nitrogen utilization, and total metabolic function under NaCl supplementation.
4.5. Blood Serum Mineral Content
Our research proved that liver and kidney weights were significantly higher in the NaCl group than in the control group, with a remarkable rise in water intake and urine output but no significant difference in serum electrolyte values between the two groups. The enhanced water intake in the NaCl group is parallel to known physiological responses to sodium surplus ingestion, wherein the body tries to equilibrate osmotic states. Sodium overconsumption raises plasma osmolality, stimulating osmoreceptors in the hypothalamus to stimulate thirst and the regulation of antidiuretic hormone secretion [52]. This homeostatic mechanism is responsible for the well-regulated Na+ levels, as excess fluid consumption dilutes plasma sodium and preserves electrolyte balance. Furthermore, significantly increased urine output facilitates renal compensation for hyperintake of sodium. Osmotic diuresis [39] occurs after excess sodium loss with increased water loss, thereby maintaining sodium and water balance.
The rise in kidney weight observed in the NaCl-treated group is accounted for by renal hypertrophy, a well-known physiological response to augmented renal load due to increased sodium excretion demand [53]. Even though such changes would be favorable in the short term, they have also been associated with long-term adverse renal outcomes [54]. Likewise, the enhanced weight of the liver in the NaCl group demonstrates the role of the liver in sodium management and fluid balance [37]. The liver plays an important part in fluid and nitrogen homeostasis through the regulation of major metabolic pathways according to alterations in sodium and water balance. The hepatomegaly here is likely to be metabolic compensation for elevated salt load, i.e., enhanced urea synthesis and gluconeogenic activity. Although there was no change in serum electrolyte levels throughout the 60 days, the overall physiological shift—i.e., weight gain in the liver and kidneys, increased water intake, and increased urine output—indicates that the body employed hepatic and renal compensation mechanisms to manage excess sodium intake in the form of urea cycle activity for the liver and natriuresis/diuresis for the kidney [55,56].
Perpetuation of serum chloride (Cl−) and potassium (K+) in the NaCl-supplemented sheep illustrates effective homeostatic mechanisms. Chloride, the prominent extracellular anion, plays an essential role in acid-base and osmotic balance. The kidneys and saliva are the significant routes of excess dietary Cl− elimination, with renal regulation inhibiting abnormal serum levels [57]. Potassium, crucial to muscle and nerve function, is maintained tightly despite enhanced urinary excretion secondary to NaCl ingestion [58]. This finding shows that the high level NaCl alfalfa-based TMR is not disruptive to electrolyte balance.
4.6. Effect of NaCl on Serum Protein and Antioxidant Enzymes
The findings of this study indicate that the NaCl supplementation of the diet imposed significant effects on protein metabolism and antioxidant enzyme activity throughout the experimental period. Interestingly, the significant increase in the concentration of serum albumin (ALB) and total protein (TP) by day 60 in the NaCl-supplemented group implies improved protein synthesis and metabolic thrift [59]. Albumin, as a principal hepatic protein, is a synthetic and nutritional biomarker of the capacity of the individual to manage and store ingested nitrogen effectively. Sodium chloride was shown by previous research to improve rumen efficiency for nitrogen absorption and retention in ruminants and optimize ruminal dynamics of fermentation [60]. The noted rise in protein metabolism can be due in part to the regulatory function of NaCl in water–electrolyte homeostasis, indirectly serving enzymatic processes in both protein catabolism and anabolism. It represents the stimulation of protein turnover and not an enhancement of efficiency. A slow but persistent increase in levels of TP and ALB reveals a cumulative effect of NaCl on nitrogen usage, indicating that prolonged exposure may elevate the retention and assimilation of dietary protein.
The significant elevation of superoxide dismutase [28] and glutathione peroxidase (GSH-PX) activity in the NaCl-supplemented group highlights the close relationship between dietary salt intake and modulation of enzymatic antioxidant defenses. SOD catalyzes the dismutation of the superoxide radical to hydrogen peroxide [61], while GSH-Px destroys peroxides, thereby limiting potential oxidative damage [62]. The relationship between a higher salt intake and the increase in antioxidant enzymes conforms to findings from other studies, which have indicated that increased reactive oxygen species (ROS) generation through salinity can lead to oxidative damage, prompting the upregulation of antioxidant enzymes as a defense mechanism [63]. The continued increase in enzyme activities at days 30 and 60 should therefore reflect a chronic physiological adaptation to NaCl intake and, by extension, an upregulation of antioxidant defenses in response to oxidative stress. Elevated concentrations of NaCl may indeed cause damage to cells through different interconnected pathways, primarily by inducing osmotic and oxidative stress. Excess extracellular sodium generates osmotic stress, leading to cell shrinkage and structural alterations [64]. Meanwhile, it upregulates the production of reactive oxygen species (ROS) via NADPH oxidase and mitochondrial-dependent mechanisms, generating oxidative stress that is deleterious to lipids, proteins, and DNA [65], and reduces nitric oxide (NO) bioavailability, increasing endothelial dysfunction [66]. Overconsumption of NaCl also activates pro-inflammatory signaling cascades like NF-κB, which induce tissue injury. Organs such as the kidneys, heart, vasculature, and brain are the most sensitive to salt-induced injury. Enhanced antioxidant enzyme activity may be an adaptive response against NaCl-evoked osmotic stress [67]. Overall, NaCl supplementation, simulating alfalfa grown in high-saline soil, induces both stress and adaptation processes, thereby enhancing nitrogen utilization and antioxidant functions in ruminants.
4.7. Fecal and Urine Protein and Energy Metabolism
The results of this research verify that salt supplementation significantly enhances protein and energy metabolism in AOHU sheep, as supported by previous [11,49] research that validated dietary sodium’s pivotal role in regulating nitrogen and energy balance in ruminants. The enhanced nitrogen intake, absorption, and retention in the NaCl-supplemented group are consistent with earlier findings [44], indicating that sodium can stimulate appetite as well as ruminal fermentation, thereby improving the efficiency of protein digestion and absorption. Notably, our study detected much higher nitrogen use efficiency and nitrogen biological values in the NaCl than in the control group (49.89% and 22.27% and 64.92% and 36.91%, respectively), which suggests the dietary potential of sodium to maximize the metabolic efficiency and protein economy of sheep production units.
The considerably higher digestible and metabolizable energy in the NaCl supplemented group (28.47 MJ/d vs. 13.93 MJ/d and 24.68 MJ/d vs. 11.58 MJ/d, respectively) underscores the profound impact of dietary sodium on AOHU sheep energy metabolism. This rise is most likely driven by sodium’s critical role in maintaining osmotic balance and facilitating active nutrient transport across the intestinal epithelium, leading to greater energy absorption and utilization. Also, the significantly greater gross energy digestibility (78.13% vs. 67.10%) further supports the hypothesis that NaCl supplementation favors a more efficient ruminal environment. Although acetate production decreased, indicative of decreased cellulolytic activity, the overall improvement in the energy digestibility may result from altered patterns of fermentation, such as increased propionate formation or improved microbial efficiency, which can also facilitate better energy extraction from feedstuffs. These findings highlight the outstanding efficacy of our intervention and provide firm evidence of the multifunctional role of sodium in the optimal nutrition of ruminants fed forage grown under saline–alkaline conditions.
The higher methane yield in the NaCl-supplemented group (2.91 MJ/d vs. 1.65 MJ/d) could reflect underlying alterations in ruminal fermentation dynamics due to sodium intake. Although elevated emissions of methane are generally undesirable from an environmental perspective, in terms of the ruminant’s physiology, they may reflect increased fermentative activity and improved carbohydrate degradation. Methane is a microbial fermentation product in the rumen, primarily due to the removal of excess hydrogen formed during the synthesis of volatile fatty acids [68]. Sodium is important in the maintenance of ruminal osmotic balance and microbial ecology, perhaps augmenting processes that promote hydrogen accumulation and methanogenesis. Hence, while NaCl supplementation may shift microbial fermentation patterns toward hydrogen-producing pathways that favor methanogenesis, the comparable methane emissions relative to NDF intake (Table 4) indicate that NaCl did not have a measurable effect on methane release. The shift could be correlated to differences in ruminal energy metabolism and overall nutritional utilization [69]. When methane emission was standardized to intake, there were no group differences (1.99 vs. 2.01 MJ/kg DM; 5.22 vs. 5.49 MJ/kg NDF), indicating that the greater methane yield in the NaCl group was compensated by the increased feed intake and not an actual dietary influence on methane intensity. This supports the notion that NaCl supplementation did not affect the efficiency of methane production per unit of digested feed [70].
Relative to urinary metabolism, the significantly reduced urinary nitrogen concentration in the NaCl-supplemented group (0.008 g/mL versus 0.015 g/mL) should be interpreted considering the elevated urine output of these animals. While the reduced concentration reflects augmented nitrogen retention as well as increased protein utilization, the increased rate of urine production indicates a dilution effect contributing partially to the reduced values. Based on daily total excretion, however, the results are consistent with [71], which reported that excessive sodium intake enhances nitrogen retention by optimizing protein metabolism, and with [72], which demonstrated that sodium supplementation reduces urinary losses of nitrogen as a percentage of intake. Similarly, the reduced urinary energy concentration in the NaCl group (0.005 MJ/mL compared with 0.009 MJ/mL, p < 0.006) indicative of improved metabolic efficiency, with losses of nutrients in excretion being reduced [73]. On the other hand, the percentage intake of urinary nitrogen losses was, more importantly, reduced in the NaCl group (26.7% compared to 38.5%), suggesting reduced waste of dietary nitrogen via urine. The addition of NaCl may enhance nitrogen retention by altering metabolic pathways, thus reducing nitrogen waste through urine. This aligns with findings that dietary modifications can significantly affect nitrogen metabolism and retention [74,75].
5. Conclusions
NaCl supplementation, implemented to mimic alfalfa grown from high-saline soil, improved feed intake, nutrient digestibility, nitrogen utilization, and digestible and metabolizable energy availability in AOHU lambs, without affecting electrolyte balance. Physiological changes included an increase in the mass of visceral organs, the thickening of the rumen wall, and ruminal fermentation profiles redirected toward glucogenic and branched-chain VFAs that were conducive to more efficient energy capture. Antioxidant enzyme activity increased, which may represent a compensatory response to osmotic stress. However, feeding high amounts of NaCl for extended periods is not advisable due to the observed changes in the kidney and liver. Although this simulation cannot precisely replicate the compositional changes (e.g., CP, NDF, ADF, ME, and macro minerals) that occur in naturally salt-stressed forages, the findings suggest that an alfalfa-based TMR with a salt content comparable to that of alfalfa hay grown under saline–alkaline conditions can help sustain physiological equilibrium and optimize rumen function. This strategy introduces a promising approach to improving small ruminant productivity in saline–alkaline regions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11100587/s1, Figure S1: Experimental design of NaCl supplementation in AOHU sheep to mimic alfalfa grown under high-salinity conditions. ADG = Average Daily Gain; FCR = Feed Conversion Ratio; ALB = Albumin; TP = Total Protein; SOD = Superoxide Dismutase; GSH-Px = Glutathione Peroxidase; Na+ = Sodium; Cl− = Chloride; K+ = Potassium; pH = Hydrogen ion concentration; NH3-N = Ammonia Nitrogen; MCP = Microbial Crude Protein.
Author Contributions
H.A.: Conceptualization, Methodology, Validation, Formal Analysis, Investigation, Resources, Data Curation, Writing—Original Draft Preparation, Writing—Review and Editing; R.Y.: Validation, Investigation, Resources, Data Curation, Project Administration; G.W.: Investigation, Resources, Data Curation; X.F.: Investigation, Writing—Review and Editing; Y.T.: Conceptualization, Methodology, Validation, Resources, Data Curation, Writing—Review and Editing, Supervision, Project Administration, Funding Acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Innovation Project of Inner Mongolia (NMKJXM202307).
Institutional Review Board Statement
This study was approved by the Animal Ethics Committee of the Chinese Academy of Agricultural Sciences on 22 July 2024 (Approval Code: IFR-CAAS-2024-07-22) and conducted in accordance with animal welfare standards and the Ministry of Science and Technology’s Guidelines for Experimental Animals (2006, Beijing, China).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to ethical restrictions.
Acknowledgments
We thank the Ruminant Research Team of the Feed Research Institute, CAAS, for the valuable laboratory support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chacko Kaitholil, S.R.; Mooney, M.H.; Aubry, A.; Rezwan, F.; Shirali, M. Insights into the influence of diet and genetics on feed efficiency and meat production in sheep. Anim. Genet. 2024, 55, 20–46. [Google Scholar] [CrossRef]
- Esen, S. Optimizing ruminant nutrition: Insights from a comprehensive analysis of silage composition and in vitro gas production dynamics using nonlinear models. Biosystems 2023, 234, 105062. [Google Scholar] [CrossRef]
- Paul, S.; Dey, A. Nutrition in health and immune function of ruminants. Indian J. Anim. Sci. 2015, 85, 103–112. [Google Scholar] [CrossRef]
- Ingvartsen, K.L.; Moyes, K. Nutrition, immune function and health of dairy cattle. Animal 2013, 7, 112–122. [Google Scholar] [CrossRef]
- Bertoni, G.; Trevisi, E.; Houdijk, J.; Calamari, L.; Athanasiadou, S. Welfare is affected by nutrition through health, especially immune function and inflammation. In Nutrition and the Welfare of Farm Animals; Springer: Cham, Switzerland, 2016; pp. 85–113. [Google Scholar]
- Dotas, V.; Symeon, G.; Dublecz, K. Introducing novel trends in the nutrition of monogastric farm animals for the production of high-quality livestock products. Front. Veter-Sci. 2025, 11, 1514197. [Google Scholar]
- Chanu, P.H. Management of salt-affected soils for increasing crop productivity. In Enhancing Resilience of Dryland Agriculture Under Changing Climate: Interdisciplinary and Convergence Approaches; Springer: Berlin/Heidelberg, Germany, 2023; pp. 113–121. [Google Scholar]
- Guo, R.; Zhou, Z.; Cai, R.; Liu, L.; Wang, R.; Sun, Y.; Wang, D.; Yan, Z.; Guo, C. Metabolomic and physiological analysis of alfalfa (Medicago sativa L.) in response to saline and alkaline stress. Plant Physiol. Biochem. 2024, 207, 108338. [Google Scholar] [CrossRef]
- Abebe, H.; Tu, Y. Impact of salt and alkali stress on forage biomass yield, nutritive value, and animal growth performance: A comprehensive review. Grasses 2024, 3, 355–368. [Google Scholar] [CrossRef]
- Thakur, S.; Tomar, S. In sacco evaluation of total mixed rations. Indian J. Anim. Nutr. 2004, 21, 180–183. [Google Scholar]
- Phillips, C.; Mohamed, M.; Omed, H. The effects of increasing the sodium content of grass or concentrates on the nutrition of sheep. Anim. Sci. 2003, 77, 491–498. [Google Scholar] [CrossRef]
- Sato, Y.; Angthong, W.; Butcha, P.; Takeda, M.; Oishi, K.; Hirooka, H.; Kumagai, H. Effects of supplementary desalted mother liquor as replacement of commercial salt in diet for Thai native cattle on digestibility, energy and nitrogen balance, and rumen conditions. Anim. Sci. J. 2018, 89, 1093–1101. [Google Scholar] [CrossRef]
- Hemsley, J.; Hogan, J.; Weston, R. Effect of high intakes of sodium chloride on the utilization of a protein concentrate by sheep. II.* Digestion and absorption of organic matter and electrolytes. Aust. J. Agric. Res. 1975, 26, 715–727. [Google Scholar] [CrossRef]
- Mukherjee, V.; Priyadarshinee, P. Strategic use of minerals to augment production and reproduction in animals. Pharma Innov. J. 2017, 6, 517–522. [Google Scholar]
- Jakubowski, M.G.T.; Dembele, K. Effect of anionic salts on the metabolism of selected minerals and superoxide dismutase activity in blood of cows. Bull. Vet. Inst. Pulawy 2011, 55, 101–106. [Google Scholar]
- Tripathi, M.; Karim, S. Minerals requirement of small ruminants with special reference to their role in rumen fermentation—A review. Indian J. Small Rumin. 2008, 14, 1–47. [Google Scholar]
- National Research Council Committee on Nutrient Requirements of Domesticated Ruminants. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids; China Legal Publisher: Beijing, China, 2007. [Google Scholar]
- Li, S.; Bao, Y.; Lv, M.; Zhang, L.; Liu, L.; Liu, Y.; Lu, Q. Comparative Na+ and K+ profiling reveals microbial community assembly of alfalfa silage in different saline-alkali soils. Fermentation 2023, 9, 877. [Google Scholar] [CrossRef]
- ISO 11784:2024; Radio Frequency Identification of Animals—Code Structure. International Organization for Standardization: Geneva, Switzerland, 2024.
- ISO 11785:1996; Radio Frequency Identification of Animals—Technical Concept. International Organization for Standardization: Geneva, Switzerland, 1996.
- Association of Official Analytical Chemists. Official Methods of Analysis of the Association of Official Analytical Chemists; The Association: Gaithersburg, MD, USA, 2000; Volume 11. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Rantaša, M.; Majer, D.; Finšgar, M. Preparation of Food Samples Using Homogenization and Microwave-Assisted Wet Acid Digestion for Multi-Element Determination with ICP-MS. J. Vis. Exp. (JoVE) 2023, e65624. [Google Scholar] [CrossRef]
- Montero, E.V.; Trejos, P.S. Validación de un método analítico para la determinación del contenido de sodio en los alimentos. Tecnol. Marcha 2012, 25, 41–49. [Google Scholar]
- Blaxter, K.; Clapperton, J. Prediction of the amount of methane produced by ruminants. Br. J. Nutr. 1965, 19, 511–522. [Google Scholar] [CrossRef]
- Lau, E. Accuracy of Bromocresol Green (BCG) Method for Plasma Albumin; NTNU: Trondheim, Norway, 2020. [Google Scholar]
- Doumas, B.T.; Bayse, D.D.; Borner, K.; Carter, R.; Elevitch, F.; Garber, C.; Graby, R.; Hause, L.; Mather, A.; Peters, T., Jr. A candidate reference method for determination of total protein in serum. II. Test for transferability. Clin. Chem. 1981, 27, 1651–1654. [Google Scholar] [CrossRef]
- Snider, M.A.; Mulakala, B.K.; Driemel, A.W.; Ziegler, S.E.; Darby, H.M.; Soder, K.J.; Brito, A.F.; Greenwood, S.L. 191 Evaluation of Diverse Cool-Season Grass Mixtures with red Clover on Ruminal Fermentation in Continuous Culture. J. Anim. Sci. 2022, 100, 86. [Google Scholar] [CrossRef]
- Lilley, L.M.; Sanche, S.; Moore, S.C.; Salemi, M.R.; Vu, D.; Iyer, S.; Hengartner, N.W.; Mukundan, H. Methods to capture proteomic and metabolomic signatures from cerebrospinal fluid and serum of healthy individuals. Sci. Rep. 2022, 12, 13339. [Google Scholar] [CrossRef]
- Chaney, A.L.; Marbach, E.P. Modified reagents for determination of urea and ammonia. Clin. Chem. 1962, 8, 130–132. [Google Scholar] [CrossRef]
- Makkar, H.; Sharma, O.; Dawra, R.; Negi, S. Simple determination of microbial protein in rumen liquor. J. Dairy Sci. 1982, 65, 2170–2173. [Google Scholar] [CrossRef]
- Devireddy, A. Selection of an Efficient and Sustainable Solvent for Anhydromevalonolactone (AMVL) Extraction and a Techno-Economic Assessment; Southern Illinois University at Edwardsville: Edwardsville, IL, USA, 2025. [Google Scholar]
- Weisinger, R.; Considine, P.; Denton, D.; McKinley, M.; Mouw, D. Rapid effect of change in cerebrospinal fluid sodium concentration on salt appetite. Nature 1979, 280, 490–491. [Google Scholar] [CrossRef]
- De Waal, H.; Baard, M.A.; Engels, E. Effects of sodium chloride on sheep. 2. Voluntary feed intake and changes in certain rumen parameters of young Merino wethers grazing native pasture. S. Afr. J. Anim. Sci. 1989, 19, 34–42. [Google Scholar]
- Rakova, N.; Kitada, K.; Lerchl, K.; Dahlmann, A.; Birukov, A.; Daub, S.; Kopp, C.; Pedchenko, T.; Zhang, Y.; Beck, L. Increased salt consumption induces body water conservation and decreases fluid intake. J. Clin. Investig. 2017, 127, 1932–1943. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.; Webster, M. Food intake, water intake, urine output, growth rate and wool growth of lambs accustomed to high or low intake of sodium chloride. Aust. J. Agric. Res. 1987, 38, 187–194. [Google Scholar] [CrossRef]
- Potter, B. The renal response of sheep to prolonged ingestion of sodium chloride. Aust. J. Agric. Res. 1961, 12, 440–445. [Google Scholar] [CrossRef]
- Rabelink, T.J. Burning calories to excrete salt. Nat. Rev. Nephrol. 2017, 13, 323–324. [Google Scholar] [CrossRef] [PubMed]
- Kitada, K.; Daub, S.; Zhang, Y.; Klein, J.D.; Nakano, D.; Pedchenko, T.; Lantier, L.; LaRocque, L.M.; Marton, A.; Neubert, P. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J. Clin. Investig. 2017, 127, 1944–1959. [Google Scholar] [CrossRef]
- Cui, R.; Chen, D.; Li, N.; Cai, M.; Wan, T.; Zhang, X.; Zhang, M.; Du, S.; Ou, H.; Jiao, J. PARD3 gene variation as candidate cause of nonsyndromic cleft palate only. J. Cell. Mol. Med. 2022, 26, 4292–4304. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.; Araújo, G.; Oliveira, J.; Santos, E.; Perazzo, A.; Pereira, G.; Santos, F.; Zanine, A. Effect of various salt concentrations on the ruminal parameters of goats. S. Afr. J. Anim. Sci. 2020, 50, 635–642. [Google Scholar] [CrossRef]
- Javaid, A.; Sharif, M.; Hamad, M.; Rehman, U.; Zamir, S. Impact of degradable protein on ruminant production: A Review. Transylv. Rev. 2017, 25, 4668–4687. [Google Scholar]
- Demeyer, D.; Fievez, V. Is the synthesis of rumen bacterial protein limited by the availability of pre-formed amino acids and/or peptides? Br. J. Nutr. 2004, 91, 175–176. [Google Scholar] [CrossRef][Green Version]
- Amaning-Kwarteng, K.; Kellaway, R.; Kirby, A. Supplemental protein degradation, bacterial protein synthesis and nitrogen retention in sheep eating sodium hydroxide-treated straw. Br. J. Nutr. 1986, 55, 557–569. [Google Scholar] [CrossRef]
- MacDonald, R.; Lanyi, J. Light-activated amino acid transport in Halobacterium halobium envelope vesicles. Fed. Proc. 1977, 36, 1828–1832. [Google Scholar]
- Lapierre, H.; Berthiaume, R.; Raggio, G.; Thivierge, M.; Doepel, L.; Pacheco, D.; Dubreuil, P.; Lobley, G. The route of absorbed nitrogen into milk protein. Anim. Sci. 2005, 80, 11–22. [Google Scholar] [CrossRef]
- Nack, M.F.; Van Emon, M.L.; Wyffels, S.A.; Manoukian, M.K.; Carlisle, T.J.; Davis, N.G.; Kirkpatrick, T.J.; Kluth, J.A.; DelCurto-Wyffels, H.M.; DelCurto, T. Impact of increasing levels of NaCl in drinking water on the intake and utilization of low-quality forages by beef cattle hand-fed a protein supplement or protein supplement containing 25% salt. Transl. Anim. Sci. 2021, 5, S101–S105. [Google Scholar] [CrossRef]
- Firkins, J.L. Maximizing microbial protein synthesis in the rumen. J. Nutr. 1996, 126, 1347S–1354S. [Google Scholar] [CrossRef]
- Zhang, Z.; Sun, Y.; Zhong, X.; Zhu, J.; Yang, S.; Gu, Y.; Yu, X.; Lu, Y.; Lu, Z.; Sun, X. Dietary crude protein and protein solubility manipulation enhances intestinal nitrogen absorption and mitigates reactive nitrogen emissions through gut microbiota and metabolome reprogramming in sheep. Anim. Nutr. 2024, 18, 57–71. [Google Scholar] [CrossRef]
- Bolam, M.; Connors, M.; McLennan, S.R.; Poppi, D.P. Variability in microbial protein supply under different supplementation strategies. Anim. Prod. Australia. Proc. Aust. Soc. Anim. Prod. 1998, 22, 398. [Google Scholar]
- Hamad, I.; Cardilli, A.; Côrte-Real, B.F.; Dyczko, A.; Vangronsveld, J.; Kleinewietfeld, M. High-salt diet induces depletion of lactic acid-producing bacteria in murine gut. Nutrients 2022, 14, 1171. [Google Scholar] [CrossRef]
- Ramadhan, M.R.; Schlecht, E.; Dickhöfer, U.; Mahgoub, O.; Jörgensen, R.G. Feed digestibility, digesta passage and faecal microbial biomass in desert-adapted goats exposed to mild water restriction. J. Anim. Physiol. Anim. Nutr. 2022, 106, 721–732. [Google Scholar] [CrossRef]
- Mojiminiyi, F.; Anigbogu, C.; Sofola, O.; Adigun, S. Cardiac and kidney weight indices following dietary salt loading and/or chronic nitric oxide synthase inhibition in the hooded (Aguti) rat. Niger. Postgrad. Med. J. 2009, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chandramohan, G.; Bai, Y.; Norris, K.; Rodriguez-Iturbe, B.; Vaziri, N. Effects of dietary salt on intrarenal angiotensin system, NAD (P) H oxidase, COX-2, MCP-1 and PAI-1 expressions and NF-κB activity in salt-sensitive and-resistant rat kidneys. Am. J. Nephrol. 2007, 28, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Zeidel, M.L. Salt and water: Not so simple. J. Clin. Investig. 2017, 127, 1625–1626. [Google Scholar] [CrossRef]
- Vilstrup, H.; Eriksen, P.L.; Kjærgaard, K.; Sørensen, M.; Thomsen, K.L.; Ott, P. Down the road towards hepatic encephalopathy. Urea synthesis-the liver workhorse of nitrogen metabolism. Metab. Brain Dis. 2024, 40, 49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Hartmann, A.-M.; Guo, J. Chloride homeostasis in animal cell physiology. Front. Physiol. 2023, 14, 1227565. [Google Scholar] [CrossRef]
- Zia-ul-Hasan, Z.-u.-H.; Muhammed Sarwar, M.S.; Zafar Iqbal, Z.I.; Sultan Mahmood, S.M. Dietary cation anion balance in the ruminants I-effects during early lactation. Int. J. Agric. Biol. 2001, 3, 243–249. [Google Scholar]
- Tomé, D.; Benoit, S.; Azzout-Marniche, D. Protein metabolism and related body function: Mechanistic approaches and health consequences. Proc. Nutr. Soc. 2021, 80, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.; Croom, W., Jr.; Pond, K.; Hogarth, B.; Leonard, E. High levels of sodium chloride in supplements for growing cattle. Can. J. Anim. Sci. 1986, 66, 423–429. [Google Scholar] [CrossRef]
- Kitiyakara, C.; Chabrashvili, T.; Chen, Y.; Blau, J.; Karber, A.; Aslam, S.; Welch, W.J.; Wilcox, C.S. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J. Am. Soc. Nephrol. 2003, 14, 2775–2782. [Google Scholar] [CrossRef]
- Gomez-Sanchez, E.; Gomez-Sanchez, C.E. The multifaceted mineralocorticoid receptor. Compr. Physiol. 2011, 4, 965–994. [Google Scholar] [CrossRef]
- Miranda, R.; Paula, S.; Araújo, G.; Valença, I.; Miranda, S.; Gomes-Filho, E. NaCl-Priming Mitigates Oxidative Damage and Na+ Accumulation and Enhances Salt Tolerance in Sorghum Plants. Available online: https://icolibri.com.br/public/anais/TC5120931.pdf (accessed on 5 October 2025).
- Della Penna, S.L. Pathophysiological Effects of the Excess of Sodium in Renal and Vascular Tissues. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2013. [Google Scholar]
- Böröcz, K.; Szinger, D.; Simon, D.; Berki, T.; Németh, P. Regulators and Conductors of Immunity: Natural Immune System in Health and Autoimmunity. Int. J. Mol. Sci. 2025, 26, 5413. [Google Scholar] [CrossRef] [PubMed]
- Boegehold, M.A.; Drenjancevic, I.; Lombard, J.H. Salt, angiotensin II, superoxide, and endothelial function. Compr. Physiol. 2016, 6, 215–254. [Google Scholar] [CrossRef]
- Rudi, M.J.; Kouyoumdzian, N.M.; Kim, M.; Rukavina Mikusic, N.L.; Lee, H.J.; Galleano, M.; Fernández, B.; Puyó, A.; Choi, M.R. Participación de los canales de cloruro en la salud cardiovascular y renal. Efectos de dietas altas en cloro sobre la presión arterial en un modelo experimental de sobrecarga salina. Rev. Argent. De Cardiol. 2024, 92, 133–141. [Google Scholar] [CrossRef]
- Wilkinson, J. Methane production by ruminants. UK Vet Livest. 2012, 17, 33–35. [Google Scholar] [CrossRef]
- Jouany, J.-P.; Thivend, P. La production de méthane d’origine digestive chez les ruminants et son impact sur le réchauffement climatique. Manag. Avenir 2008, 20, 259–274. [Google Scholar] [CrossRef]
- Arangsri, M.; Pattarajinda, V.; Duangjinda, M.; Mungkalasiri, J.; Angthong, W.; Bernard, J. Impact of fermented total mixed rations on intake, VFA and methane production of dairy heifers. Indian J. Anim. Res. 2019, 53, 1344–1348. [Google Scholar] [CrossRef]
- Letelier, P.; Zanton, G.; Wattiaux, M. Evaluation of protocols to determine urine output and urinary urea nitrogen excretion in dairy cows with and without dietary salt supplementation. J. Dairy Sci. 2024, 107, 6742–6757. [Google Scholar] [CrossRef]
- Ergene, N.; Pickering, E. The effects of reducing dietary nitrogen and of increasing sodium chloride intake on urea excretion and reabsorption and on urine osmolality in sheep. Q. J. Exp. Physiol. Cogn. Med. Sci. Transl. Integr. 1978, 63, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Godwin, I.R.; Williams, V.J. Effects of intraruminal sodium chloride infusion on rumen and renal nitrogen and electrolyte dynamics in sheep. Br. J. Nutr. 1986, 56, 379–394. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.F.; Bruun, T.S.; Trottier, N.L.; Theil, P.K. Nitrogen utilization of lactating sows fed increasing dietary protein. J. Anim. Sci. 2019, 97, 3472–3486. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, V.; Fialho, E.; de Freitas Lima, J.A.; dos Santos Araújo, J. Metabolismo do nitrogênio em suínos alimentados com dietas contendo baixos teores de proteína bruta. Rev. Bras. Agrociencia 2007, 13, 257–260. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).