Abstract
β-glucosidase is a kind of enzyme that can hydrolyze β-glucosidase bonds, and it plays a key role in many fields, such as lignocellulose degradation and wine brewing. The global β-glucosidase market is currently estimated to be USD 40 billion, and more is expected in the future. This trend is mainly due to the demand for enzymes in biofuel processing. At present, β-glucosidase is mainly derived from microorganisms, animals, plants and so on. It has received great attention due to its ease of production, catalytic efficiency and versatility, which have promoted its biotechnology potential in different industries. With the increasing demand for β-glucosidases, various cost-effective methods are being explored to discover, redesign and enhance their production and functional properties. Therefore, this paper reviews the latest progress in the application of β-glucosidase in industry. In this regard, the focus is on the use of recombinant technology, protein engineering and immobilization technology to improve the industrial applicability of the enzyme. In addition, the application status of β-glucosidase in production and life was analyzed.
1. Introduction
β-glucosidase (β-glucosidase, EC 3.2.1.21) is a kind of enzyme that widely exists in nature and belongs to cellulase family. It is an enzyme that catalyzes the hydrolysis of non-reducing β-D-glycosidic bonds at the end and releases glucose residues from glycosides and oligosaccharides. According to its substrate specificity, it is classified into alkylglucosidases, cellobiases, or enzymes that hydrolyze alkyl-β-glycosides and oligosaccharides. As an important hydrolase, it has received extensive attention and in-depth research [1].
Since Liebig and Wohler first discovered β-glucosidase in bitter almond juice in 1837, the enzyme has been found to exist in various organisms. It is widely distributed in plants, microorganisms and animals [1,2], and has a rich source. For example, ginseng and soybean in plants are important sources of β-glucosidase, which play an important role in the physiological process of plants, such as the interaction between plants and insects, hormone activation, defense mechanism and so on [3]. Microorganisms are another important source of β-glucosidase, such as Flavobacterium meningosepticum [4] and Flavobacterium johnsonae [5]. It can be produced by eukaryotic microorganisms: Candida peltata [6], Trichoderma reesei [7] and Aspergillus niger [8] in eukaryotic microorganisms. There are some differences in the properties and functions of β-glucosidase from different microorganisms, which provides a variety of choices for its application in different fields.
In addition, animal-derived β-glucosidase cannot be ignored. Bees, pig livers and pig small intestines contain this enzyme and participate in physiological processes such as glucose metabolism in animals [9]. β-glucosidases from different sources have different structures and enzymatic properties, and these differences make them show their unique advantages and potential in different application fields. For example, in the food industry, β-glucosidase can be used in the preparation of fruit juice, tea juice, fruit wine, etc., to increase the aroma effect and improve the flavor quality of the product [10]. In the feed industry, it can break the cell wall that is rich in fiber, release nutrients and degrade fiber into reducing sugars that can be digested and absorbed by livestock and poultry, thereby improving feed utilization. In the field of medicine, β-glucosidase also has potential application value, such as participating in the metabolic process of some drugs. In agriculture, β-glucosidase can be used for plant protection and crop quality improvement [11]. In addition, β-glucosidase plays a key role in the conversion of biomass into renewable energy (such as biofuels). Therefore, it is of great significance to study the characteristics and application potential of β-glucosidase from different sources in order to optimize and innovate its application in industrial applications. β-glucosidases from different sources have significant differences in catalytic performance, structure, stability and adaptability to environmental conditions. These changes have obvious advantages and limitations in their applications. With the development of biotechnology, the research on β-glucosidase is not only limited to its role in nature, but also includes genetic modification or protein engineering to improve its catalytic activity, thermal stability and acid or alkali resistance, so as to adapt to the strict requirements of industrial production and improve its performance in industrial applications [11]. This requires an in-depth study of β-glucosidases from different sources in order to better understand and utilize their biochemical properties.
The purpose of this paper is to comprehensively summarize the research progress of β-glucosidase from different sources, and to explore its enzymatic properties, structure-function relationship, genetic engineering and protein engineering transformation. Through the comprehensive analysis of these research progress, we hope to provide scientific basis and technical support for the further research and application of β-glucosidase.
2. Classification and Catalytic Mechanism of β-Glucosidase
2.1. Classification and Protein Structure of β-Glucosidase
β-glucosidase is a hydrolase that catalyzes the hydrolysis of β-D-glycosidic bonds, thereby decomposing glycoside compounds into glucose and aglycone. In 1991, Henrissat classified glycoside hydrolases according to the similarity of amino acid sequences [12]. β-glucosidase has been divided into glycoside hydrolase (GH) family according to its amino acid sequence. As of July 2025, the CAZymes database contains 190 glycoside hydrolase (GHs) families. Polysaccharide lyase (PLs) family: 44. Carbohydrate esterase (CEs) family: 20. Glycosyltransferases (GTs) family: 138. Auxiliary active enzyme (AAs) family: 17. Carbohydrate binding modules (CBMs): 106. The enzymes in each family are similar in sequence and structure, but there may be differences in substrate specificity and catalytic mechanism. According to the amino acid sequence classification, β-glucosidase is distributed in GH1, GH3, GH4, GH5, GH9, GH13, GH17, GH30, GH31, GH63, GH97, GH116 and GH122 glycoside hydrolase family. Among them, some of the β-glucosidases currently studied mainly belong to the GH1 and GH3 families. Glycoside hydrolases have a common ancestor and catalytic mechanism. Because they have similar catalytic domain structure and conserved catalytic amino acid family, they are classified as a family [13]. The GH1, GH5 and GH30 families of β-glucosidase belong to the family GH-A, and GH3 and GH9 have not yet been classified into any family.
The chemical structure of β-glucosidase is closely related to its amino acid sequence, protein folding and three-dimensional structure. The three-dimensional structure of β-glucosidase usually includes α-helix and β-sheet. The β-glucosidases of GH1, GH5 and GH30 families usually have (β/α) 8 barrel structure. The active site contains two conserved carboxylic acid residues, which are used as catalytic acid/base and nucleophile, respectively. This is a common glycoside hydrolase structure that can accommodate and act on a variety of substrates. These structures constitute the active site of the enzyme and the region that stabilizes its overall conformation. The enzyme of GH1 has glucosidase activity. There is also a strong galactosidase activity [14]. The β-glucosidase of the GH3 family mainly exists in fungi and bacteria, most of which are in the form of multi-subunits, and the structure is similar. It has a typical (α/β) 8 barrel and (β/α) 6 sandwich-like domain, and the active site is located between these two domains [15]. The active site of β-glucosidase contains key amino acid residues, which are involved in substrate binding and catalytic reactions. The interaction between the enzyme and the substrate leads to the cleavage of the glycosidic bond and the release of glucose and aglycones.
2.2. The Catalytic Mechanism of β-Glucosidase
β-glucosidase catalyzes the cleavage of β-1,4 glycosidic bonds using a retention type catalytic mechanism, that is, the stereochemical configuration of glycosidic bonds remains unchanged after the hydrolysis reaction. As shown in Figure 1, two conserved glutamic acid residues (Glu) are used as the key catalytic sites: one acts as a nucleophile to attack the anomeric carbon (C1) of the substrate, resulting in the cleavage of the glycosidic bond and the formation of a covalent enzyme-substrate intermediate (oxygen–carbon positive ion transition state). The other acts as a proton donor, which promotes the cleavage of the bond by protonating the oxygen atom of the glycosidic bond. Although both the conversion and retention mechanisms use two main catalytic residues, there are differences. The inversion and retention depend on the change in the anomeric carbon configuration of the glucose moiety. At present, the reversal mechanism is only observed for the GH9 enzyme, but the GH1, 2, 3, 4, 5, 30, 39 and 116 families follow the retention mechanism [16]. The retention mechanism of the GH1, GH2, GH3, GH4, GH5, GH30, GH39 and GH116 families includes two consecutive steps of glycosylation and deglycosylation [17].
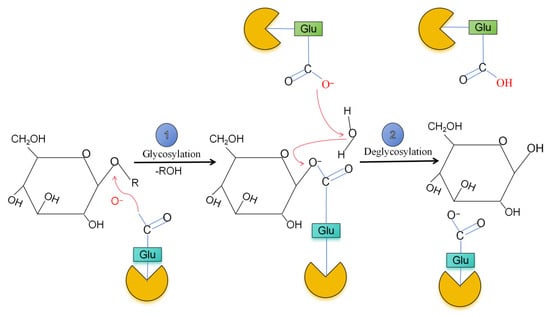
Figure 1.
Two steps hydrolysis mechanism of β-glucosidase.
Different β-glucosidases may be specific to different substrates, and their catalytic mechanisms may involve nucleophilic attack, acid-base catalysis or a combination of the two, depending on their amino acid sequence and three-dimensional structure. For example, the β-glucosidases of GH1, GH3 and GH5 families are different in the organization of active sites and catalytic residues [18,19]. The catalytic mechanism of β-glucosidase involves several key steps, which usually include substrate binding, catalytic hydrolysis, and product release [3]. The β-glucosidase first binds to the substrate. The substrate is usually β-D-glucoside. The active site of the enzyme has specific amino acid residues. These residues form hydrogen bonds and other non-covalent interactions with the substrate, thereby stabilizing the substrate and correctly locating the location of the reaction. At the active site, there are usually two key amino acid residues (such as aspartic acid or glutamic acid) involved in the catalytic process. One of the residues acts as a nucleophilic catalyst, which activates water molecule by obtaining a proton (or losing a hydroxyl) to make it a nucleophile (activated water molecule), which can attack the carbon atom of the substrate to form a glycoside-oxonium ion intermediate [20]. This intermediate is highly unstable and will be quickly attacked by a second nucleophilic catalyst, resulting in the cleavage of the glycosidic bond and the release of glucose molecules. Another residue acts as an acidic/basic catalyst, which promotes the reaction through proton transfer. After the reaction is completed, glucose and aglycone (non-sugar part) are released from the active site of the enzyme to restore the enzyme to its original state and prepare for the next round of catalytic cycle. The catalytic process of β-glucosidase is stereospecific, which can distinguish the α and β isomers of the substrate and catalyze the formation of the corresponding α or β-glucose products [21]. In addition to hydrolytic activity, β-glucosidase can also catalyze the formation of glycosidic bonds through thermodynamically controlled reverse hydrolysis or kinetically controlled transglycosylation. The reverse hydrolysis reaction catalyzed by β-glucosidase usually means that under certain conditions, the enzyme reverses the hydrolysis reaction (such as the hydrolysis of glycosidic bonds) to form glycosidic bonds. The reaction can be carried out under high substrate concentration and high temperature conditions. For example, in 90% glucose solution, the disaccharide yield synthetized by β-glucosidase increased with the increase in glucose concentration and reaction temperature. At 55 °C, the maximum yield of disaccharides can reach 40%, the temperature condition is 40–60 °C. The concentration condition is 85–95% [22]. In the hydrolysis reaction, β-glucosidase catalyzes the cleavage of the glycosidic bond and releases glucose. In the reverse hydrolysis reaction, the enzyme catalyzes the rebinding of glucose molecules to form glycosidic bonds and synthesizes oligosaccharides. The occurrence of reverse hydrolysis reaction depends on thermodynamic conditions. At high temperature and high substrate concentration, the thermodynamic driving force of the reaction system is conducive to the reverse reaction. At this time, the free energy change (ΔG) of oligosaccharide formation is negative, and the reaction is spontaneous. By providing a highly specific catalytic site, β-glucosidase reduces the activation energy of the reaction, thereby accelerating the formation of glycosidic bonds.
The transglycosylation reaction catalyzed by β-glucosidase mainly involves the transfer of glucose (or other sugar molecules) from a donor molecule to a receptor molecule to form a new glycosidic bond. In this process, β-glucosidase acts as a catalyst to catalyze the formation of glycosidic bonds between sugar molecules and alcohol receptors through a nucleophilic reaction mechanism [23]. At the beginning of the reaction, β-glucosidase first binds to its substrate, usually a glucose donor molecule (such as UDP-glucose, glucose-1-phosphate or other activated forms of glucose) and a receptor molecule (such as alcohol). The active site of the enzyme usually contains some amino acid residues, which can form a stable complex with the substrate. The glucose in the donor molecule is activated by binding to the catalytic site of the enzyme. For example, UDP-glucose as a glucose donor, UDP (uridine diphosphate) part will be activated, so that glucose molecules have higher reactivity, prone to transglycosylation reaction. This activation process makes the glycosidic bond of glucose molecules more easily broken, so that the glucose group can be transferred to the alcohol receptor. The sugar group in the activated sugar molecule undergoes a nucleophilic attack process within the catalytic site through the catalysis of the enzyme. Alcoholic groups (such as hydroxyl groups in some molecules) nucleophilically attack the carbon atoms in the sugar donor molecule, usually the carbon atoms that form glycosidic bonds with the sugar group. This reaction usually requires the catalysis of enzymes, which promote this process by positioning and activating the sugar group. The nucleophilic attack causes the sugar group to be transferred to the receptor molecule (alcohols), and the hydroxyl group (-OH) of the alcohol becomes a nucleophilic reagent, attacking the carbon atom of the sugar molecule and generating a new glycosidic bond (such as a glycosidic bond). In this process, the active group of sugar (such as UDP or phosphate) leaves and becomes a free leaving group [24]. Once the glycosidic bond is formed, the product molecule releases the catalytic site and restores the enzyme activity. The catalytic site of the enzyme is restored to the original state and can continue to catalyze the next reaction. At this point, the leaving group (such as UDP) is also released and can continue to participate in other reactions. β-glucosidase has high specificity for its substrate [3]. It is usually catalyzed only by specific sugars (such as glucose) and specific alcohols (such as alcohol, ether or other oxygen-containing groups) to generate specific types of glycosidic bonds (such as β-glycosidic bonds). The structure and catalytic sites of the enzyme limit the type of substrate accept and the direction of the transglycosylation reaction (such as the formation of β-but not α-glycosidic bonds) [25].
3. Biological Characteristics of β-Glucosidase from Different Sources
β-glucosidase is a widely distributed enzyme that exists in a variety of organisms, including plants, animals and microorganisms. There are significant differences in the structure and enzymatic properties of β-glucosidases from different sources. These differences will directly affect their catalytic activity, substrate specificity, stability, and adaptability to environmental conditions. Exploring the biological characteristics of β-glucosidase from different sources can not only deepen the understanding of its mechanism of action, but also provide a solid theoretical basis for selecting the most suitable source of β-glucosidase for different fields. Through the systematic study of different sources of β-glucosidase, it is helpful to develop more efficient, stable and specific β-glucosidase preparations, so as to better meet the growing needs of various industries and promote the innovation and development of related industries.
3.1. β-Glucosidase from Microorganisms
As an important source of β-glucosidase, microorganisms cover a variety of types such as bacteria, fungi and yeast. Bacillus subtilis [26] and Escherichia coli [27] in bacteria, Aspergillus niger [28] and Aspergillus oryzae [29] in fungi, and Saccharomyces cerevisiae [30] in yeast are typical microorganisms that can produce β-glucosidase. Microorganisms have the remarkable characteristics of fast growth and reproduction, easy cultivation and regulation. By optimizing the fermentation conditions, large-scale industrial production of β-glucosidase can be achieved [31]. The production of β-glucosidase by microbial fermentation has many advantages such as low cost, high yield and easy control, and has been widely used in industrial production [32]. There are great differences in the enzymatic properties of β-glucosidase produced by different microorganisms, which provides a rich choice to meet the special needs of different industries. In bacteria and fungi, β-glucosidase is composed of one or more subunits, and the relative molecular weight is generally between 40 and 150 kDa. According to the source of β-glucosidase and the difference in amino acid sequence, the pH and stability of β-glucosidase are also very different [3]. The basic physical and chemical properties of β-glucosidase from different microbial sources are shown in Table 1. It can be seen from the table that the optimum pH of most β-glucosidases in microorganisms is 5~8, and the optimum temperature is distributed within 30~80 °C. From an industrial point of view, the higher the thermal stability of the enzyme, the better. Usually, the storage temperature of β-glucosidase is 0~4 °C, and the pH is 7~8.

Table 1.
Basic physicochemical properties of β-glucosidases from different microbial sources.
3.2. β-Glucosidase from Plant
Compared with microbial-derived β-glucosidases, plant-derived enzymes have unique properties, including high glucose tolerance, broad substrate spectrum and excellent selectivity [41]. In plants, β-glucosidase is composed of one or more subunits, so the relative molecular weight varies greatly, generally between 40~300 kDa. The isoelectric point of most plant-derived β-glucosidases is in an acidic range. According to the different sources and amino acid sequences of β-glucosidase, the pH and stability of β-glucosidase are also very different. The basic physical and chemical properties of β-glucosidase from different plant sources are shown in Table 2. It can be seen from the table that the optimum pH of most β-glucosidases in plants is 4–6, which depends on their source and cell location. The optimum temperature of β-glucosidase in plants is distributed in the range of 20–60 °C. In general, the optimum temperature of β-glucosidase in plants is generally lower than that of microorganisms. From an industrial point of view, the higher the thermal stability of the enzyme, the better.

Table 2.
Basic physicochemical properties of β-glucosidases from different plant sources.
Different physical and chemical properties have significant effects on biosynthesis. In the process of biosynthesis, if the pH value and temperature of the reaction system deviate from the optimum conditions of β-glucosidase, the enzyme activity may be reduced, which will affect the synthesis efficiency. When the enzyme is used to hydrolyze cellulose to produce glucose, too high temperature or pH discomfort will inhibit the enzyme activity, slow down the hydrolysis rate of cellulose, lead to a decrease in glucose production and hinder the biosynthesis process. In the process of using β-glucosidase to hydrolyze cellulose to produce glucose, if the temperature is too high or the pH value is not appropriate, the activity of the enzyme will be inhibited, and the hydrolysis rate of cellulose will be slowed down, which will affect the yield of glucose and the process of biosynthesis. Therefore, in practical applications, it is necessary to optimize the reaction conditions of biosynthesis according to the physical and chemical properties of β-glucosidase, so as to improve the efficiency and yield of biosynthesis.
4. Modification of β-Glucosidase by Genetic Engineering and Protein Engineering
β-glucosidase is a kind of enzyme that can catalyze the hydrolysis of glycosidic bonds and decompose complex carbohydrates into glucose or other monosaccharides. It plays a key role in many fields such as food processing, pharmaceutical synthesis and biomass energy development. However, natural glucosidases often have many limitations, such as low catalytic efficiency, narrow substrate specificity, and poor stability to high temperature or extreme pH environments. These defects seriously restrict their large-scale application in industrial production. The genetic engineering and protein engineering of β-glucosidase are mainly through the optimization of its gene and protein structure to improve its catalytic efficiency, thermal stability, acid and alkali resistance and substrate specificity. In genetic engineering, the β-glucosidase gene is usually modified by gene mutation, site-directed mutagenesis or gene synthesis technology to obtain an enzyme with improved performance. Protein engineering focuses on changing the three-dimensional structure or active site of the enzyme through structural simulation, directed evolution or fusion technology to improve its performance. These modifications can make β-glucosidase more efficient in industrial applications, especially in the fields of biodegradation, food, textile and washing. Therefore, it is of great practical significance to modify β-glucosidases.
4.1. Modification of Enzyme Activity
The way that genetic engineering transforms the target strain is to introduce exogenous genes on the basis of understanding the specific gene sequence of the target product and the synthetic pathway in the strain [50]. By improving the synthetic pathway or increasing the gene dose, the expression of β-glucosidase protein can be improved. Gene modification methods such as codon optimization, overexpression, and competitive pathway knockout are more accurate than non-directional transformation. Guo Jinghan et al. [51] used CRISPR/Cas9 genome editing technology to inactivate the YPS1 and YPS2 genes in the Saccharomyces cerevisiae An-α strain, and then introduced the β-glucosidase expression plasmid. It was found that the β-glucosidase activity and expression level of the obtained strain could be significantly improved. Shen et al. [52] constructed the recombinant plasmid of GH3 amino acid sequence in thermophilic archaea and induced its expression in E. coli competent cells. It was found that the recombinant enzyme TaBgl3 had better thermal stability, and high temperature could activate the β-glucosidase activity of TaBgl3, and the β-glucosidase activity increased by 68% at 80 °C. When using E. coli to express β-glucosidase, the researchers successfully introduced the β-glucosidase gene into E. coli and achieved a high level of expression. The expression level and activity of β-glucosidase were further improved by optimizing the expression conditions, including selecting the appropriate promoter, adjusting the concentration and addition time of the inducer, and optimizing the medium composition [53]. The researchers screened the β-glucosidase Bgl747 from the soil by functional screening method, constructed the recombinant expression plasmid pET-28a-Bgl747, and optimized the expression in E. coli BL21 (DE3). Under the optimal induction conditions (OD600 = 1.0, IPTG concentration 0.6 mmol/L, induction at 37 °C for 10 h), the expression level of Bgl747 reached 1.82 mg/mL, and the enzyme activity was 225.07 U/mg, which had a wide reaction temperature range and good industrial application potential [54]. Researchers screened β-galactosidase-producing strains from fermented food Chhurpi and curd in the Himalayas of Sikkim. Potential isolates exhibiting β-galactosidase activity were selected for comprehensive genomic and biochemical characterization. Genome analysis confirmed that the selected isolates were Lactobacillus fermentum strains. The optimal β-galactosidase activity was recorded at 40 °C and pH 7.0, with a specific activity of 2.46 + 0.70 U/mg. The presence of metal ions such as K+, Ca2+ and Fe2+ was beneficial to the enzyme activity. Genome analysis showed that there are two copies of β-galactosidase gene in Lactobacillus fermentum C2C, which may confer higher β-galactosidase activity than other Lactobacillus fermentum strains [55]. The researchers produced recombinant β-glucosidase from Moniliophtora perniciosa expressed in E. coli cells. After using wheat bran (WB) and carboxymethyl cellulose (CMC) as fungal growth medium, the expression of enzyme coding sequence was confirmed by Sanger sequencing. A synthetic gene betaglyc-GH1 with an optimized codon for E. coli expression was cloned in pET-28a [56]. The β-glucosidase recombinant (GH1chimera) was purified by nickel column and confirmed by mass spectrometry. The apparent molecular weight of the recombinant enzyme on SDS-PAGE was 53.23 kDa. Using p-nitrophenyl-β-D-glucopyranoside (pNPG) as a substrate, the recombinant β-glucosidase showed hydrolytic activity with maximum activity at pH 4.6 and 65 °C.
4.2. Heat-Resistant Modification of Enzyme
According to the different modification methods of β-glucosidase, we divided the thermal stability modification methods of β-glucosidase into three categories. The first category is mutation, including random mutation [57], direct evolution [58], site-directed mutation [59], alanine scanning mutation [60], etc. The second category is hybridization, including DNA family shuffling [61], chimera construction [62], polymerization, and C/N terminal sequence substitution. The substrate preference of the GH1 family β-glucosidase AtBgl1A from A. thermocellus to laminarin was significantly stronger than that of cellobiose, indicating that it was more likely to be involved in the decomposition of laminarin rather than cellobiose in the cytoplasm. The thermal stability of AtBgl1A was significantly improved by introducing three mutations of A17S, S39T and T105V through structure-oriented rational design [63]. The researchers used the innovative strategy of steric hindrance scanning to modify the β-glucosidase BgMd from Microbacterium dextranolyticum. By introducing amino acids with different side chain sizes to construct a mutant library, combined with molecular docking and molecular dynamics (MD) simulation analysis, mutants with significantly improved activity were finally obtained [60]. For the first time, the extreme stability of archaeal BGL was directly related to its C-terminal domain. Chaoneng Ji et al. emphasized that the (α/β) 8 barrel-shaped core of TsBGL2 is highly conserved with eukaryotic BGL, but its C-terminal extension can be used as a specific modification target for protein engineering. This ‘modular’ design idea makes it possible to enhance the thermal stability without affecting the catalytic activity, which lays a foundation for the development of the next generation BGL suitable for high temperature biorefinery process [64]. Researchers have improved the thermal stability, activity and glucose tolerance of Bgl6-M3 by integrating several semi-rational and rational design strategies. The half-life and catalytic efficiency of the best mutant M12 were 20 times and 5.6 times that of M3, respectively. Its glucose tolerance was 200 mM higher than that of M3. The addition of M12 in 1.5 L could increase the glucose production of SCB by 40%. This provides useful guidance for the multi-performance optimization of other enzymes [65].
5. Extraction and Purification of β-Glucosidase
The extraction and purification technology of β-glucosidase is a key step to realize its industrial application. First, it is necessary to break the cells containing β-glucosidase by physical or chemical methods, release the intracellular enzyme, and then extract and purify the β-glucosidase. The ‘purification method’ of β-glucosidase is not a simple subsequent processing, but a core link that determines the final commercial enzyme yield. By (1) optimizing the precipitant type and saturation, (2) shortening the desalination/dialysis time, (3) finely designing the chromatographic gradient and flow rate, and (4) collaborative optimization of the fermentation stage (host selection, induction conditions, glycosylation engineering), the traditional < 20% enzyme activity yield can be increased to 25–30% or even higher, directly amplifying the economic benefits of each batch of fermentation [66]. On the contrary, if the purification scheme is improper, even if the fermentation enzyme activity is high, it may lead to the dilemma of ‘high activity and low yield’ due to early mass inactivation or miscellaneous protein co-precipitation. At present, the purification technology of β-glucosidase mainly includes traditional extraction methods and efficient purification methods [67].
5.1. Traditional Extraction Method
Traditional extraction methods include ammonium sulfate salting out, enzyme-assisted extraction, and three-phase extraction.
Ammonium sulfate salting-out: This is a traditional protein separation technology, commonly used in the extraction process of enzymes such as β-glucosidase [68]. It is based on the decrease in protein solubility at high salt concentration. By increasing the concentration of ammonium sulfate, the solubility of protein can be gradually reduced, resulting in protein precipitation. The concentration of ammonium sulfate was adjusted to achieve the fractional precipitation of the enzyme, so as to initially separate and enrich the β-glucosidase. Firstly, the microbial cell culture medium or plant/animal tissue homogenate containing β-glucosidase was collected, and the pH value of the homogenate was adjusted to near the isoelectric point of the protein, at which time the solubility of the protein was the lowest. Ammonium sulfate is gradually added to the homogenate under continuous stirring until the required saturation (usually 0% to 80% saturation) is reached. With the increase in ammonium sulfate concentration, β-glucosidase and other proteins will gradually precipitate out. The precipitate was separated by centrifugation and the proteins in the precipitate were collected. The solubility of different proteins varies with the concentration of ammonium sulfate. By adjusting the concentration of ammonium sulfate, the fractional precipitation of β-glucosidase can be achieved. The collected precipitate was resuspended in an appropriate buffer and excess ammonium sulfate and other small molecular impurities were removed by dialysis [69]. Ammonium sulfate precipitation has the advantages of simple operation, low cost, and is suitable for the preliminary separation and purification of various proteins. However, the high concentration of ammonium sulfate may have an effect on the activity of some enzymes. For proteins with similar properties, ammonium sulfate salting out may not be able to achieve effective separation and is not suitable for fine separation.
Enzyme-assisted extraction technology: Enzyme-assisted extraction technology is a method of using biological enzymes to improve the extraction efficiency of β-glucosidase from its host cells. Biological enzymes such as cellulase, pectinase and hemicellulase can decompose complex polysaccharide structures such as cellulose, pectin and hemicellulose in plant cell walls. By destroying the cell wall and cell membrane, these enzymes contribute to the release of intracellular β-glucosidase, thereby simplifying the extraction process and improving the recovery rate of the enzyme. First, the cell materials containing β-glucosidase were pretreated, such as grinding or physical crushing, to increase the accessibility of the cell wall. Cellulase, pectinase and hemicellulase were added to the pretreated cell material for enzymatic hydrolysis. The enzymatic hydrolysis led to the decomposition of the cell wall and the release of β-glucosidase. The cell residue was removed by centrifugation or filtration, and the supernatant containing the enzyme was collected for further separation and purification [70]. Enzyme-assisted extraction is usually carried out under mild conditions close to physiological conditions, which helps to maintain the activity of β-glucosidase and significantly improve the release efficiency of β-glucosidase. Compared with traditional chemical extraction methods, enzyme-assisted extraction reduces the use of harmful chemicals. However, the cost of commercial enzymes may be high, especially in large-scale applications, and the enzymes used need to be highly specific to avoid destroying the target β-glucosidase. In addition to β-glucosidase, enzyme-assisted extraction technology is also widely used in the extraction of other intracellular enzymes and metabolites.
Three-phase extraction: Three-phase extraction is a technique used to extract enzymes and other biomolecules, especially when dealing with complex substrates such as plant seeds. It uses organic solvents, salt and water to form three different phases (stages) to achieve enzyme extraction and partial purification in this way. Firstly, the raw materials such as apple seeds were dried and crushed. Organic solvents, such as n-pentanol, were added to the pretreated raw materials to help destroy the cell wall and release the enzyme. Subsequently, ammonium sulfate was added to induce protein precipitation by adjusting the salt concentration of the solution. Under appropriate conditions, the mixture will form three phases: organic phase, salt phase and water phase. The phase containing β-glucosidase was isolated by centrifugation or filtration. The three-phase method is carried out under relatively mild conditions, which helps to maintain the activity of the enzyme. Through the synergistic effect of organic solvents and salts, the cell wall can be effectively destroyed and the enzyme can be released, which is especially suitable for cell materials that are difficult to be broken by physical methods, and helps to improve the extraction efficiency of enzymes such as β-glucosidase. In addition, this method can achieve partial purification of the enzyme while extracting. However, the use of organic solvents may involve the recovery and treatment of solvents. If the operating conditions are improper during the extraction process, the activity of the enzyme may be affected.
5.2. Efficient Purification Technology
High-efficiency purification technologies include ion chromatography, hydrophobic chromatography, aqueous two-phase flotation (ATPF), gel filtration chromatography, ultrafiltration (UF), etc.
Ion chromatography technology: By adjusting the pH value and ionic strength of the buffer, the adsorption and elution conditions of β-glucosidase can be optimized to improve the selectivity of separation. Ion exchange chromatography can provide high-resolution protein separation, which is suitable for separation processes from small-scale to industrial scale, and quaternary aminoethyl-agarose gel has good chemical and mechanical stability. However, in the application of ion exchange chromatography, conditions such as pH and ionic strength need to be carefully optimized to achieve optimal separation. In some cases, extreme pH or ionic strength conditions may also affect enzyme activity. Ion exchange chromatography is usually used as a step in the purification process of β-glucosidase, and may need to be combined with other chromatography techniques (such as gel filtration chromatography or hydrophobic chromatography) to obtain higher purity enzymes. The researchers purified β-glucosidase with high specific activity against isoflavone glycoconjugates from the seeds of Cyamopsis tetragonoloba by ammonium sulfate precipitation, size exclusion and ion exchange chromatography. The optimal pH and temperature for the hydrolysis of β-glucosidase (ICHG) by purified isoflavone conjugates were pH 4.5 and 37 °C, respectively. The enzyme is relatively stable at higher temperatures. The effects of different divalent metal ions were studied, and it was found that cobalt ions and mercury ions completely inhibited enzyme activity. The Km and Vmax of the purified isoflavone conjugate hydrolyzing β-glucosidase (ICHG) were 0.86 mM and 6.6 IU/mg, respectively [71]. Researchers produced β-glucosidase from Penicillium janthinellum NCIM 1171. In the medium optimized by response surface methodology (RSM), the levels of β-glucosidase under submerged and solid-state fermentation conditions were 10.2 ± 0.75 IU/mL and 121 ± 9.3 IU/g, respectively. The supernatant produced by deep fermentation was subjected to ion exchange and gel filtration chromatography. The enzyme was purified in a 44.4-fold manner, and the final recovery rate was 39.75% [52].
Hydrophobic chromatography: The crude extract of β-glucosidase was subjected to appropriate buffer replacement to adapt to the chromatographic conditions. The samples were loaded into the chromatographic column, and the proteins in the samples were separated according to the different hydrophobicity and the binding strength of the medium. Gradient or step elution is performed using an appropriate buffer, changing the salt concentration in the buffer or adding an organic solvent to gradually release the bound protein. Hydrophobic chromatography can effectively separate proteins with similar molecular size but different hydrophobic properties. The chromatography process is carried out in a mild environment close to physiological conditions, which helps to maintain protein activity. However, under certain conditions, especially high concentrations of organic solvents may lead to changes in protein structure or inactivation. At the same time, hydrophobic chromatography media may be relatively expensive, especially in large-scale applications. Some researchers extracted β-glucosidase from cabbage by ammonium sulfate precipitation and hydrophobic interaction chromatography purified from Brassica. The results showed that the enzyme was a dimer (130 kD) composed of a major (80 kD) and a minor subunit (50 kD). The optimum pH was 6.0, and 50% of the original activity of the enzyme was maintained between pH 4.0 and pH 7.0. The optimum temperature was 35 °C, and the activity did not decrease after exposure to this temperature for two hours [72].
Aqueous two-phase flotation (ATPF): This is a flotation technique based on an aqueous two-phase system formed by polymer and salt, which can be used for the separation and purification of β-glucosidase. The system is usually composed of two immiscible water-soluble polymers, such as polyethylene glycol (PEG) and dextran, or a combination of polymer and salt. In the aqueous two-phase system, biomolecules (such as β-glucosidase) are selectively allocated to one of the two phases according to their physical and chemical properties. By reducing the gravity of the system, the separation of light and heavy phases can be achieved, thereby achieving the separation of target molecules. First, two polymers or polymers and salts were mixed to form an aqueous two-phase system, and samples containing β-glucosidase were added to the aqueous two-phase system. The biomolecules in the sample are distributed to different aqueous phases according to their hydrophilicity or hydrophobicity. By reducing the gravity, the light phase rises to the top of the system to achieve the separation of the two phases. Researchers have established a new purification method (ATPF-ITC) that combines aqueous two-phase flotation (ATPF) with reverse transformation cycle (ITC) and used to efficiently purify recombinant β-glucosidase (GLEGB) from cell lysates. Firstly, GLEGB is preferentially adsorbed on the nitrogen bubble interface, depending on the hydrophobicity of the graphene-bound (GB) label and enters the top phase of ATPF. Secondly, based on the thermal sensitivity of the elastin-like peptide (ELP) tag, the further purification of GLEGB was achieved through a round of ITC method. Therefore, the enzyme activity recovery rate of GLEGB was 124.92% ± 0.83%, and the purification coefficient reached 24.26 ± 0.22. The purification results remained stable after 6 polymer cycles, and the process of ATPF-ITC had no negative effect on the structure of the recombinant protein [73].
Gel filtration chromatography: Using porous gel as stationary phase, the pores in the gel allow smaller molecules to enter, while larger molecules are excluded from the pores. Therefore, the separation of molecules is based on their ability to enter the pores of the gel. This technology is carried out under mild conditions and helps to maintain the natural conformation and activity of the protein. Proteins of different sizes can be quickly separated without gradient elution. However, for proteins with similar molecular sizes, the resolution of gel filtration chromatography may not be as good as other chromatographic techniques. At the same time, the loading of the sample is limited by the gel volume and pore size. Gel filtration chromatography is an important tool in the process of protein purification, which is especially suitable for the preliminary purification of enzymes such as β-glucosidase or the removal of high molecular weight impurities in samples. The researchers cultured the newly identified cellulase-producing Chlamydia fusarium HML278 in the solid-state fermentation of bagasse, and recovered two new β-glucosidases (BG FH1, BG FH2) from the fermentation broth by modified non-denaturing active gel electrophoresis and gel filtration. The molecular weights of BG FH1 and BG FH2 were 93 kDa and 52 kDa, respectively, and the enzyme activities were 5.6 U/mg and 11.5 U/mg, respectively. The optimum reaction temperature of the enzyme was 60 °C, which was stable below 70 °C. The optimum pH of the purified enzyme was 6.0, and the enzyme was stable between pH 4–10. The values of K m and V maxpNPG were 2.76 mg/mL and 20.6 U/mg, respectively [63]. A new β-galactosidase gene (Bgal) was cloned from the marine bacterium Alteromonas sp. ML117. Bgal from the genus Alteromonas. ML117 was heterologously expressed in E. coli BL21 (DE3) cells to study the enzymatic properties of recombinant β-galactosidase. Using gel filtration chromatography and SDS-PAGE, the recombinant enzyme was identified as a tetrameric enzyme belonging to the glycoside hydrolase family GH2. The purified recombinant Bgal was able to hydrolyze lactose and o-nitrophenyl-β-d-galactopyranoside (ONPG), and showed maximum activity at pH 8.0 and 30 °C to hydrolyze ONPG, and lactose at 35 °C. When incubated at 35 °C, the activity level of the enzyme decreased rapidly, indicating that it was a cold-adapted variant. At 10 °C, the Km value of the purified enzyme was 1.6 mM for ONPG and 3.8 mM for lactose [74].
Ultrafiltration (UF): For the extraction and purification of enzymes such as β-glucosidase, ultrafiltration provides a fast and efficient method for concentration and desalination. Ultrafiltration is based on the selective permeability of the semi-permeable membrane. The pores on the membrane allow small molecules such as salt, buffer and other small molecular impurities to pass through, while preventing macromolecules such as proteins, viruses and cells from passing through. The appropriate ultrafiltration membrane was selected according to the molecular weight of the target protein. The sample containing β-glucosidase is adjusted to a buffer suitable for ultrafiltration, and the sample is loaded into an ultrafiltration device, which usually includes an ultrafiltration membrane and a collection container. A certain pressure is applied to make the sample pass through the membrane to achieve concentration and desalination.
6. Application of β-Glucosidase
β-glucosidases are a group of heterogeneous hydrolases with good characterization and biological importance. They act on different substrates and have been widely used in biotechnology. They play a diverse role in the industry, from promoting the conversion of cellulose into fermentable sugars in biofuel production, to enhancing flavor and aroma in the food industry, to the synthesis of pharmaceutical ingredients in the pharmaceutical field, and to the application in industries such as feed, cosmetics and environmental governance. It improves production efficiency and promotes product innovation by catalyzing key biochemical reactions. Its application involves many aspects as shown in Figure 2, including biofuel production, application in the food industry, pharmaceutical industry, paper industry, waste treatment, biodegradation, etc.
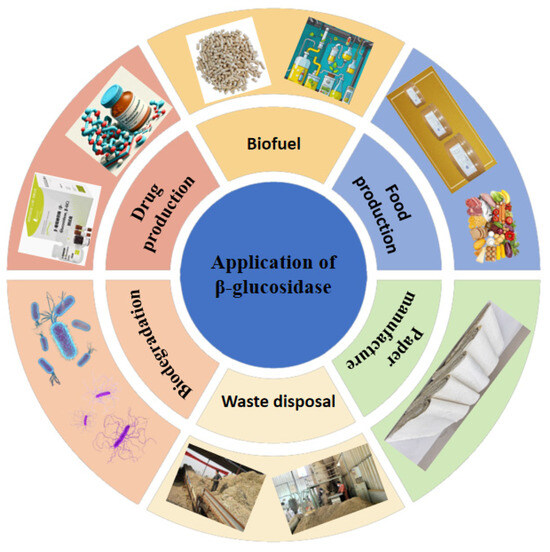
Figure 2.
Application of β-glucosidase in production and life.
6.1. Biofuel Production
β-glucosidase plays an important role in the production of biofuels and has important application prospects. Especially in the process of converting cellulose biomass into renewable energy such as bioethanol, it can help improve energy efficiency and reduce carbon emissions [75,76]. By converting lignocellulose into fermentable sugars to produce biofuels such as ethanol, β-glucosidase plays a key role in this conversion process. The continuous exploitation and continuous use of fossil fuels have increased their burden, and the growth of renewable energy sources such as biofuels and solar energy is alleviating this problem. For example, forest residues, as well as wastes produced by agricultural and industrial activities, can be degraded into biofuels by cellulase. Cellulase is a system composed of three different enzymes, namely exoglucanase, endoglucanase and β-glucosidase. These three enzymes play different roles in the complete hydrolysis of cellulose. Endoglucanases randomly act on the crystal structure of cellulose and cleave the linear chain of glucose, resulting in shorter chains producing two new chain ends. Then, the exoglucanase can act on these two exposed ends to produce cellobiose and some glucose. Finally, β-glucosidase completes the complete degradation of cellulose and decomposes cellobiose and cellooligosaccharides into glucose molecules [77,78,79]. β-glucosidase decomposes insoluble cellulose into soluble sugars by hydrolyzing β-1,4-glycosidic bonds in cellulose, which can be further fermented to produce biofuels. Therefore, β-glucosidase is the final enzyme for lignocellulose degradation, which determines the rate of total conversion of lignocellulose to glucose, and is the rate-limiting enzyme for the conversion of lignocellulosic biomass to sugars into biofuels. It helps to overcome the crystallinity and resistance to degradation of cellulose, thereby improving the conversion efficiency of biomass to sugar. β-glucosidase rapidly hydrolyzes cellobiose into glucose, reducing the inhibition of enzyme activity caused by cellobiose accumulation [80]. The production process from biomass to biofuels is green, sustainable, and an advanced technology to solve the current environmental problems caused by fossil fuels [76].
6.2. Application in Food Industry
β-glucosidase is widely used in the food industry, mainly for improving food flavor or producing active monomer components. β-glucosidase can hydrolyze glycosidic bonds in plants and release aglycones with aromatic odor [81], thereby improving food flavor. This technology is widely used in winemaking, soybean isoflavone hydrolysis, sugar production and juice processing. During the winemaking process, β-glucosidase hydrolyzes glycosides in grapes and releases aroma compounds, thereby improving the aroma and taste of wine [82,83,84,85]. In juice processing, the enzyme can increase the type and content of aroma substances in juice and enhance the flavor of juice [86]. In addition, β-glucosidase can also be used in the dairy industry to enhance the flavor of dairy products by hydrolyzing glycoside compounds in milk. In food processing, β-glucosidase can hydrolyze some bitter glycosides, thereby reducing the bitterness in food [87] and improving the sensory quality of food. With the development of technology, the application potential of β-glucosidase in the food industry is expected to be further developed and applied.
6.3. Applications in the Pharmaceutical Industry
The active ingredients in many natural medicines exist in the form of glycosides, which have low bioavailability and are not easily absorbed by the human body. β-glucosidase can hydrolyze these glycosidic bonds and release bioactive aglycones, which can improve the absorption and utilization efficiency of drugs in the body, thereby enhancing the therapeutic effect of drugs. For example, 97–99% of soybean isoflavones exist in the form of soybean isoflavone glycosides, and the aglycone form is only 3% of the total amount of soybean isoflavones. The physiological activity of soybean isoflavone aglycone is much higher than the activity of its corresponding glycosides. The β-glucosidase can hydrolyze soybean isoflavone glycosides into soybean isoflavone aglycones with high biological activity [88]. In some traditional Chinese medicines, flavonoids, saponins and other components can be transformed into more active forms after the action of β-glucosidase, thereby improving the efficacy of drugs. For example, in the traditional Chinese medicine Scutellaria baicalensis Georgi, baicalin is a precursor drug with relatively weak activity. Through the hydrolysis of β-glucosidase, baicalin can be converted into baicalein, which has stronger pharmacological activities, such as antibacterial, antiviral, antioxidant and so on. Resveratrol is prepared from polydatin. The content of resveratrol in dried rhizome of Polygonum cuspidatum is low, while the content of polydatin is relatively high. The hydrolysis of polydatin by β-glucosidase can prepare high-value resveratrol [89], and has the advantages of mild reaction, environmental friendliness and simple process. In addition, ginsenosides are the main active ingredients in ginseng, and some ginsenosides have low biological activity. However, β-glucosidase can hydrolyze the glycosidic bonds of some ginsenosides to obtain products with higher biological activity. For example, the low-activity ginsenoside Rg3 is hydrolyzed to obtain ginsenoside Rh2 with strong anticancer effect [90]. This provides a new way to develop efficient natural drugs, and also helps to reduce the side effects of drugs. In addition, β-glucosidase can not only improve the efficacy of natural drugs, but also participate in drug synthesis as a biocatalyst, and also play an important role in drug metabolism research and quality control.
A large amount of waste residue will be produced in the pharmaceutical process, which may contain some underutilized pharmaceutical ingredients and excipients. β-glucosidase can degrade cellulose and hemicellulose in pharmaceutical waste residue, reduce the difficulty of waste residue treatment, and recover useful components to reduce resource waste and environmental pollution. With the continuous development of biotechnology, the application prospects of β-glucosidase in the pharmaceutical industry will be broader.
6.4. Application in Paper Industry
In the papermaking process, plant fiber raw materials need to be treated for subsequent processing, and β-glucosidase can be used to pretreat fiber raw materials to promote fiber dissociation. β-glucosidase can act on the cellulose and hemicellulose in the fiber raw material, hydrolyze the β-glycosidic bond in it, weaken the connection between the fibers, thereby promoting the dissociation of the fibers, which helps to improve the accessibility of the fibers. It provides better conditions for the subsequent papermaking pulping process and can reduce the energy consumption and the amount of chemicals used in the pulping process [91,92]. At the same time, β-glucosidase can improve the quality of fibers. Through moderate hydrolysis of fibers, β-glucosidase can remove some impurities and hemicellulose on the surface of fibers, making the surface of fibers cleaner and smoother [92]. In this way, the strength, uniformity and other performance indicators of paper can be improved, and higher quality paper can be produced. In the process of waste paper recycling, deinking is a key step. Traditional deinking methods mainly rely on chemical drugs and mechanical effects, but the effect is limited and will produce certain environmental pollution. β-glucosidase can cooperate with other enzymes (such as lipase, protease, etc.) to hydrolyze the connecting components and adhesives in the ink, so that the ink particles can be more easily separated from the fiber, reduce the amount of residual ink, and improve the whiteness and cleanliness of the paper [93,94,95]. Papermaking wastewater contains a large number of organic substances such as cellulose and hemicellulose. β-glucosidase can degrade these organic substances, reduce the chemical oxygen demand (COD) and biological oxygen demand (BOD) of wastewater [96], improve the biodegradability of wastewater, and facilitate subsequent biological treatment, thereby reducing the pollution of papermaking wastewater to the environment.
6.5. Waste Treatment and Biodegradation
In the process of biomass treatment, β-glucosidase helps to degrade polysaccharide compounds in biomass and improve the utilization rate and degradation rate of waste, which is of great significance for the treatment and resource utilization of biomass waste. β-glucosidase is widely used in waste treatment and biodegradation, including the degradation of agricultural, forestry, industrial waste and urban organic waste. Straw contains a large amount of cellulose and hemicellulose, which are slowly degraded naturally. β-glucosidase can cooperate with other cellulose degrading enzymes to degrade cellulose and hemicellulose in straw into small molecular sugars, so that straw can be converted into available resources. The treated straw can be used to produce organic fertilizer, feed or chemical raw materials. Composting is an effective strategy for agricultural waste recycling, and β-glucosidase plays a vital role in cellulose degradation during composting. The production of cellulase is regulated by differentially expressed glucose/non-glucose-tolerant β-glucosidase genes, which can accelerate cellulose degradation during composting and improve composting efficiency [97]. The β-glucosidase in the soil helps to degrade a variety of organic matter in the soil, thereby maintaining soil quality.
The fruit processing industry produces a large amount of pomace waste. β-glucosidase can decompose the fiber substances in the pomace and convert them into fermentable sugars, which can be used to produce biofuels (such as ethanol) or other bio-based chemicals to increase the added value of the pomace. A large amount of wood scraps, wood chips and other wastes will be produced during forestry processing. β-glucosidase is involved in the degradation process of these lignocellulosic materials, and interacts with other lignin degrading enzymes and cellulases to decompose cellulose and hemicellulose in wood waste into simple sugars, providing raw materials for subsequent biotransformation or energy production.
Municipal solid waste contains a certain amount of cellulose substances, such as paper, plant fiber and so on. The textile industry also produces a large amount of fiber waste, such as waste cloth, fiber products and so on. β-glucosidase can participate in the biological treatment of these domestic waste and fiber waste, accelerate the decomposition of cellulose, reduce the volume and weight of waste, and produce carbohydrates that can provide carbon sources for subsequent microbial fermentation to produce bioenergy (such as biogas) or biofertilizers. Kitchen waste is an important part of urban organic waste. Its main components include food residue, peel, vegetable leaves and so on. β-glucosidase can decompose carbohydrates such as cellulose and hemicellulose in kitchen waste into monosaccharides such as glucose. These monosaccharides can be further utilized by microorganisms and converted into organic acids, alcohols and other substances to realize the reduction and resource treatment of kitchen waste.
7. Future Prospects
With the rapid development of biotechnology, β-glucosidase, as an enzyme with wide application potential, has very broad research prospects. In future, the research on β-glucosidase will focus on the following key directions to achieve its efficient application and industrial development in more fields.
7.1. Optimization of Enzymatic Properties
Although the physical and chemical properties of β-glucosidase have been deeply understood, further improvement of its performance is still one of the focuses of research. In vitro modification of β-glucosidase by protein engineering technology is expected to improve its glycoside hydrolysis activity, thereby accelerating the degradation rate of substrates such as cellulose and improving production efficiency. For example, site-directed mutagenesis was used to change the key amino acid residues of the enzyme, optimize the active center structure of the enzyme, and enhance the affinity of the enzyme to the substrate. At the same time, improving the stability of β-glucosidase in extreme environments (such as high temperature, high pressure, high acid and alkali, etc.) will enable it to better adapt to harsh conditions in industrial production. In addition, changing its substrate specificity so that it can act on more types of substrates and expand its application range is also an important goal of future research.
7.2. Innovative Immobilization Technology
The development of new immobilization carriers and immobilization methods is an important way to improve the activity and stability of β-glucosidase. Nanomaterials have unique physical and chemical properties, such as high specific surface area and good biocompatibility, which can be used as potential immobilization carriers to improve the immobilization efficiency and catalytic efficiency of enzymes. At the same time, more environmentally friendly and economical immobilization methods are explored to meet the needs of industrial production. For example, by optimizing the immobilization conditions, the damage to the enzyme activity during the immobilization process is reduced, the reuse rate of the enzyme is improved, and the production cost is reduced. The interaction mechanism between enzyme molecules and immobilized carrier was studied to provide theoretical support for the further optimization of immobilization technology.
7.3. Biological Reaction Synthesis of Enzyme
In addition to genetic engineering, protein purification and immobilization. By regulating the medium composition, inducer addition and metabolic flow during fermentation, the synthesis efficiency of β-glucosidase can be further improved. The current mainstream regulation strategies include carbon source optimization, inducer regulation and metabolic flux redistribution. Carbon source is the core substrate of strain growth and enzyme synthesis, and the induction effect of different carbon sources on β-glucosidase synthesis is significantly different [97]. Traditional studies have found that cellulose carbon sources (such as microcrystalline cellulose, straw hydrolysate)are efficient inducers of β-glucosidase, and their decomposition products (cellobiose) can activate the expression of enzyme genes. The easy-to-use carbon sources such as glucose inhibit enzyme synthesis through CCR [98]. In recent years, the optimization strategy has focused on the design of ‘mixed carbon source’: glucose (growth carbon source) and microcrystalline cellulose (induced carbon source) are mixed in a certain ratio (such as 1:3), which not only ensures the rapid growth of the strain in the early stage (glucose provides energy), but also induces enzyme synthesis through cellulose in the later stage. In addition, the use of agricultural waste (such as corncob, bagasse) as a cheap carbon source can further reduce the fermentation cost and provide feasibility for industrial production [99].
The addition of exogenous inducers can specifically activate the promoter of β-glucosidase gene and improve the efficiency of enzyme synthesis. At present, the commonly used inducers include small molecule glycosides (such as cellobiose, salicyloside) and non-glycosides (such as sodium benzoate, Tween-80) [100]. Small molecule glycosides: cellobiose is the most effective inducer, but its cost is high; as an alternative inducer, when the concentration of salicyloside was 0.5 g/L, the β-glucosidase production of T.reesei increased by 1.2 times, and the cost was only 1/5 of cellobiose [101]. Non-glycosides: Tween-80 as a surfactant can increase cell membrane permeability and promote enzyme secretion. When 0.1% Tween-80 was added to the fermentation, the enzyme yield increased from 25 U/mL to 30 U/mL; sodium benzoate relieves the inhibition of glucose on enzyme synthesis by inhibiting the activity of CCR-related proteins, which is suitable for the mixed carbon source system containing glucose [102].
By regulating the concentration of nutrients such as nitrogen source and phosphorus source, the metabolic flow of the strain can be changed, and the carbon source is preferentially used for β-glucosidase synthesis, rather than cell proliferation. For example, under the condition of nitrogen limitation (e.g., the concentration of ammonium sulfate decreased from 2 g/L to 1 g/L), the cell biomass of Aspergillus niger decreased by 15%, but the production of β-glucosidase increased by 25%. The reason is that nitrogen limitation inhibits cell division and makes carbon flow more to enzyme synthesis; phosphorus source limitation (e.g., the concentration of potassium dihydrogen phosphate decreased from 0.5 g/L to 0.2 g/L) further increased enzyme production by inhibiting energy metabolism (reduced ATP synthesis) and reducing the conversion of carbon sources to energy storage substances such as glycogen [103]. In addition, metabolic flux redistribution can also be achieved by adding metabolic intermediate product inhibitors (such as fluoroacetic acid inhibiting the tricarboxylic acid cycle), but the concentration of inhibitors needs to be strictly controlled to avoid excessive inhibition leading to strain death.
7.4. Expanding Application Fields
β-glucosidase has shown certain application value in the food industry, pharmaceutical industry and chemical industry, but its application potential in other fields can be further explored in the future. In the field of environmental protection, β-glucosidase can be used to treat organic pollutants to achieve environmental remediation. For example, by its catalytic degradation of certain organic compounds, the harm of pollutants to the environment is reduced. In the field of energy, the application of β-glucosidase in the production of bioenergy, such as participating in the conversion of biomass into biofuels, provides a new way for sustainable energy development. In addition, strengthening the application of β-glucosidase in the field of agriculture, such as the prevention and control of plant diseases and insect pests, and the improvement of crop quality, is also one of the future research directions.
7.5. Promote Industrial Production
Strengthen the industrial application research of β-glucosidase, and promote its wide application and promotion in actual production. This requires strengthening cooperation with the industry to jointly develop technologies and processes suitable for industrial production. For example, optimizing the fermentation process to improve the yield and purity of β-glucosidase. At the same time, the key problems in the industrial production process, such as large-scale preparation of enzymes, storage stability and cost control, are solved. By formulating relevant policy support and strengthening personnel training, it provides guarantee for the industrialization development of β-glucosidase, so that it can play a greater economic and social benefits in various fields.
Author Contributions
H.X.: supervision, conceptualization, methodology, investigation, writing—review and editing; R.C.: supervision, conceptualization, methodology, investigation, writing—review and editing; L.L.: supervision, conceptualization, methodology, investigation, writing—review and editing; H.L.: methodology, investigation. T.L.: investigation; J.M.: methodology, investigation. All authors have read and agreed to the published version of the manuscript.
Funding
This study has not received any funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lu, X.; Li, X.; Zhao, J. Improving enzymatic efficiency of β-glucosidases in cellulase system by altering its binding behavior to the insoluble substrate during bioconversion of lignocellulose. Bioresour. Technol. 2024, 391, 129974. [Google Scholar] [CrossRef]
- Kaenying, W.; Tagami, T.; Suwan, E.; Pitsanuwong, C.; Chomngam, S.; Okuyama, M.; Kongsaeree, P.; Kimura, A.; Kongsaeree, P.T. Structural and mutational analysis of glycoside hydrolase family 1 Br2 β-glucosidase derived from bovine rumen metagenome. Heliyon 2023, 9, e21923. [Google Scholar] [CrossRef]
- Erkanli, M.E.; El-Halabi, K.; Kim, J.R. Exploring the diversity of β-glucosidase: Classification, catalytic mechanism, molecular characteristics, kinetic models, and applications. Enzym. Microb. Technol. 2024, 173, 110363. [Google Scholar] [CrossRef]
- Hao, S.; Liu, Y.; Qin, Y.; Zhao, L.; Zhang, J.; Wu, T.; Sun, B.; Wang, C. Expression of a highly active β-glucosidase from Aspergillus niger AS3.4523 in Escherichia coli and its application in gardenia blue preparation. Ann. Microbiol. 2020, 70, 32. [Google Scholar] [CrossRef]
- Okamoto, K.; Nakano, H.; Yatake, T.; Kiso, T.; Kitahata, S. Purification and some properties of a beta-glucosidase from Flavobacterium johnsonae. Biosci. Biotechnol. Biochem. 2000, 64, 333–340. [Google Scholar] [CrossRef][Green Version]
- Lu, J.; Du, L.; Wei, Y.; Hu, Y.; Huang, R. Expression and characterization of a novel highly glucose-tolerant β-glucosidase from a soil metagenome. Acta Biochim. Biophys. Sin. 2013, 45, 664–673. [Google Scholar] [CrossRef]
- Frassatto, P.A.C.; Casciatori, F.P.; Thoméo, J.C.; Gomes, E.; Boscolo, M.; da Silva, R. β-Glucosidase production by Trichoderma reesei and Thermoascus aurantiacus by solid state cultivation and application of enzymatic cocktail for saccharification of sugarcane bagasse. Biomass Convers. Biorefinery 2021, 11, 503–513. [Google Scholar] [CrossRef]
- Chang, J.; Wang, J.; Li, Z.; Wang, L.; Lu, P.; Zhong, Y.; Liu, H. High-Level Expression of β-Glucosidase in Aspergillus niger ATCC 20611 Using the Trichoderma reesei Promoter Pcdna1 to Enhance Cellulose Degradation. Fermentation 2024, 10, 461. [Google Scholar]
- Cairns, J.R.K.; Esen, A. β-Glucosidases. Cell. Mol. Life Sci. CMLS 2010, 67, 3389. [Google Scholar]
- Juhász, T.; Egyházi, A.; Réczey, K. β-Glucosidase production by Trichoderma reesei. Appl. Biochem. Biotechnol. 2005, 121, 243–254. [Google Scholar] [CrossRef]
- Wang, C.; Wu, G.; Chen, C.; Chen, S. High production of β-glucosidase by Aspergillus niger on corncob. Appl. Biochem. Biotechnol. 2012, 168, 58–67. [Google Scholar] [CrossRef]
- Parafati, L.; Proetto, I.; Palmeri, R.; Pesce, F.; Fallico, B.; Restuccia, C. Reuse of Brewer’s Spent Grain (BSG) for the Induction of Wickerhamomyces anomalus BS91 β-Glucosidase with Bioflavoring Potential. Fermentation 2024, 10, 472. [Google Scholar] [CrossRef]
- Muradova, M.; Proskura, A.; Canon, F.; Aleksandrova, I.; Schwartz, M.; Heydel, J.-M.; Baranenko, D.; Nadtochii, L.; Neiers, F. Unlocking flavor potential using microbial β-glucosidases in food processing. Foods 2023, 12, 4484. [Google Scholar] [CrossRef]
- Yang, W.; Su, Y.; Wang, R.; Zhang, H.; Jing, H.; Meng, J.; Zhang, G.; Huang, L.; Guo, L.; Wang, J.; et al. Microbial production and applications of β-glucosidase-A review. Int. J. Biol. Macromol. 2024, 256, 127915. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B. A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem. J. 1991, 280, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Henrissat, B.; Davies, G. Structural and sequence-based classification of glycoside hydrolases. Curr. Opin. Struct. Biol. 1997, 7, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Meng, C.; Wu, Y.; Xu, J.; Tang, X.; Zhang, X.; Xiao, Y.; Wang, X.; Fang, Z.; Fang, W. An unusual GH1 β-glucosidase from marine sediment with β-galactosidase and transglycosidation activities for superior galacto-oligosaccharide synthesis. Appl. Microbiol. Biotechnol. 2020, 104, 4927–4943. [Google Scholar]
- Gudmundsson, M.; Hansson, H.; Karkehabadi, S.; Larsson, A.; Stals, I.; Kim, S.; Sunux, S.; Fujdala, M.; Larenas, E.; Kaper, T.; et al. Structural and functional studies of the glycoside hydrolase family 3 β-glucosidase Cel3A from the moderately thermophilic fungus Rasamsonia emersonii. Acta Crystallogr. Sect. D Struct. Biol. 2016, 72, 860–870. [Google Scholar] [CrossRef]
- Drula, E.; Garron, M.-L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar]
- Koetzler, M.P.; Robinson, K.; Chen, H.-M.; Okon, M.; McIntosh, L.P.; Withers, S.G. Modulating the Nucleophile of a Glycoside Hydrolase through Site-Specific Incorporation of Fluoroglutamic Acids. J. Am. Chem. Soc. 2018, 140, 8268–8276. [Google Scholar] [CrossRef]
- Xinhan, L.; Fengfei, S.; Pengjun, S. Prokaryotic expression and characterization of bifunctional thermostable β-glucosidase IuBgl3 from Infirmifilum uzonense. Acta Bioeng. 2022, 38, 4644–4657. [Google Scholar]
- Zayulina, K.S.; Elcheninov, A.G.; Toshchakov, S.V.; Kochetkova, T.V.; Novikov, A.A.; Blamey, J.M.; Kublanov, I.V. Novel hyperthermophilic crenarchaeon Infirmifilum lucidum gen. nov. sp. nov., reclassification of Thermofilum uzonense as Infirmifilum uzonense comb. nov. and assignment of the family Thermofilaceae to the order Thermofilales ord. nov. Syst. Appl. Microbiol. 2021, 44, 126230. [Google Scholar] [CrossRef]
- Karkehabadi, S.; Hansson, H.; Mikkelsen, N.E.; Kim, S.; Kaper, T.; Sandgren, M.; Gudmundsson, M. Structural studies of a glycoside hydrolase family 3 β-glucosidase from the model fungus Neurospora crassa. Struct. Biol. Cryst. Commun. 2018, 74, 787–796. [Google Scholar] [CrossRef]
- Santos, C.M.M.; Proença, C.; Freitas, M.; Araújo, A.N.; Silva, A.M.S.; Fernandes, E. Inhibition of the carbohydrate-hydrolyzing enzymes α-amylase and α-glucosidase by hydroxylated xanthones. Food Funct. 2022, 13, 7930–7941. [Google Scholar] [CrossRef]
- Thenchartanan, P.; Wattana-Amorn, P.; Svasti, J.; Kongsaeree, P.T. Improved synthesis of long-chain alkyl glucosides catalyzed by an engineered β-glucosidase in organic solvents and ionic liquids. Biotechnol. Lett. 2020, 42, 2379–2387. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, Y.; Qin, S.; Qin, P.; Chu, J.; He, B. β-Galactosidase BMG without galactose and glucose inhibition: Secretory expression in Bacillus subtilis and for synthesis of oligosaccharide. Int. J. Biol. Macromol. 2018, 120, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Ortega, P.G.; López-Miranda, J.; Rojas-Contreras, J.A.; Ilina, A.; Soto-Cruz, N.O.; Páez-Lerma, J.B. Expression of a β-glucosidase from Trichoderma reesei in Escherichia coli using a synthetic optimized gene and stability improvements by immobilization using magnetite nano-support. Protein Expr. Purif. 2022, 190, 106009. [Google Scholar] [CrossRef]
- Lopez-Trujillo, J.; Medina-Morales, M.A.; Sanchez-Flores, A.; Arevalo, C.; Ascacio-Valdes, J.A.; Mellado, M.; Aguilar, C.N.; Aguilera-Carbo, A.F. Solid bioprocess of tarbush (Flourensia cernua) leaves for β-glucosidase production by Aspergillus niger: Initial approach to fiber–glycoside interaction for enzyme induction. 3 Biotech 2017, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, H.; Kang, K.; Zhang, X.; Fraser, K.; Zhang, F.; Linhardt, R.J. β-Glucosidase on clay minerals: Structure and function in the synthesis of octyl glucoside. Int. J. Biol. Macromol. 2024, 256, 128386. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Y.; Lin, L.; Zhou, W.; Liu, M.; Cheng, K.; Wang, W. Engineering Saccharomyces cerevisiae with the deletion of endogenous glucosidases for the production of flavonoid glucosides. Microb. Cell Factories 2016, 15, 134. [Google Scholar] [CrossRef]
- Magwaza, B.; Amobonye, A.; Bhagwat, P.; Pillai, S. Hyper β-glucosidase producer Beauveria bassiana SAN01—Optimization of fermentation conditions and evaluation of saccharification potential. Biomass Convers. Biorefinery 2025, 15, 9753–9765. [Google Scholar] [CrossRef]
- Paventi, G.; Di Martino, C.; Coppola, F.; Iorizzo, M. β-Glucosidase Activity of Lactiplantibacillus plantarum: A Key Player in Food Fermentation and Human Health. Foods 2025, 14, 1451. [Google Scholar] [CrossRef]
- Xie, Y.; Yan, X.; Li, C.; Wang, S.; Jia, L. Characterization and insight mechanism of an acid-adapted β-Glucosidase from Lactobacillus paracasei and its application in bioconversion of glycosides. Front. Bioeng. Biotechnol. 2024, 12, 1334695. [Google Scholar]
- Romero-Téllez, S.; Lluch, J.M.; González-Lafont, À.; Masgrau, L. Comparing hydrolysis and transglycosylation reactions catalyzed by Thermus thermophilus β-glycosidase. A Comb. MD QM/MM Study. Front. Chem. 2019, 7, 200. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, S.; Saburi, W.; Yu, J.; Matsuura, H.; Cairns, J.R.K.; Yao, M.; Mori, H. Substrate specificity of glycoside hydrolase family 1 β-glucosidase AtBGlu42 from Arabidopsis thaliana and its molecular mechanism. Biosci. Biotechnol. Biochem. 2022, 86, 231–245. [Google Scholar]
- Liew, K.J.; Lim, L.; Woo, H.Y.; Chan, K.-G.; Shamsir, M.S.; Goh, K.M. Purification and characterization of a novel GH1 beta-glucosidase from Jeotgalibacillus malaysiensis. Int. J. Biol. Macromol. 2018, 115, 1094–1102. [Google Scholar] [CrossRef]
- Ahmed, S.; Andaleeb, H.; Aslam, A.; Raza, J.A.; Waseem, S.M.Y.; Javaid, A.; Talib, C. Improved Cellulolytic Activity of Alternaria citri: Optimization and EMS Treatment for Enhanced Cellulase Production. Fermentation 2025, 11, 274. [Google Scholar] [CrossRef]
- Lee, K.W.; Han, N.S.; Kim, J.H. Purification and characterization of beta-glucosidase from Weissella cibaria 37. J. Microbiol. Biotechnol. 2012, 22, 1705–1713. [Google Scholar] [CrossRef]
- Fan, T.; Jing, S.; Zhang, H.; Yang, X.; Jin, G.; Tao, Y. Localization, purification, and characterization of a novel β-glucosidase from Hanseniaspora uvarum Yun268. J. Food Sci. 2022, 87, 886–894. [Google Scholar]
- Del Cueto, J.; Møller, B.L.; Dicenta, F.; Sánchez-Pérez, R. β-Glucosidase activity in almond seeds. Plant Physiol. Biochem. 2018, 126, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Kumar, A.K.; Shah, A. Purification and characterization of novel bi-functional GH3 family β-xylosidase/β-glucosidase from Aspergillus niger ADH-11. Int. J. Biol. Macromol. 2018, 109, 1260–1269. [Google Scholar] [CrossRef]
- Zhong, P.; Xiu, Y.; Zhou, K.; Zhao, H.; Wang, N.; Zheng, F.; Yu, S. Characterization of a novel thermophilic beta-glucosidase from Thermotoga sp. and its application in the transformation of notoginsenoside R1. 3 Biotech 2022, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S.; Lilao, J.; Mateo, J.J. Characterization of a β-glucosidase isolated from an alpeorujo strain of Candida adriatica. Food Biotechnol. 2017, 31, 114–127. [Google Scholar] [CrossRef]
- Vervoort, Y.; Herrera-Malaver, B.; Mertens, S.; Medina, V.G.; Duitama, J.; Michiels, L.; Derdelinckx, G.; Voordeckers, K.; Verstrepen, K.J. Characterization of the recombinant Brettanomyces anomalus β-glucosidase and its potential for bioflavouring. J. Appl. Microbiol. 2016, 121, 721–733. [Google Scholar] [CrossRef]
- Karaulli, J.; Xhaferaj, N.; Coppola, F.; Testa, B.; Letizia, F.; Kyçyk, O.; Kongoli, R.; Ruci, M.; Lamçe, F.; Sulaj, K.; et al. Bioprospecting of Metschnikowia pulcherrima Strains, Isolated from a Vineyard Ecosystem, as Novel Starter Cultures for Craft Beer Production. Fermentation 2024, 10, 513. [Google Scholar] [CrossRef]
- Bi, Y.; Zhu, C.; Wang, Z.; Luo, H.; Fu, R.; Zhao, X.; Zhao, X.; Jiang, L. Purification and characterization of a glucose-tolerant β-glucosidase from black plum seed and its structural changes in ionic liquids. Food Chem. 2019, 274, 422–428. [Google Scholar] [CrossRef]
- Gómez-Anduro, G.; Ceniceros-Ojeda, E.A.; Casados-Vázquez, L.E.; Bencivenni, C.; Sierra-Beltrán, A.; Murillo-Amador, B.; Tiessen, A. Genome-wide analysis of the beta-glucosidase gene family in maize (Zea mays L. var B73). Plant Mol. Biol. 2011, 77, 159–183. [Google Scholar]
- Asati, V.; Sharma, P.K. Purification and characterization of an isoflavones conjugate hydrolyzing β-glucosidase (ICHG) from Cyamopsis tetragonoloba (guar). Biochem. Biophys. Rep. 2019, 20, 100669. [Google Scholar] [CrossRef]
- Rodríguez, A.C.; Esperanza, F.A.S.; Pérez-Campos, E.; Hernández-Huerta, M.T.; Mayoral, L.P.-C.; Matias-Cervantes, C.A.; Barras, A.M.; Mayoral-Andrade, G.; Pineda, L.Á.S.; Barrita, A.J.D.; et al. Aggregation and molecular properties of β-glucosidase isoform II in chayote (Sechium edule). Molecules 2020, 25, 1699. [Google Scholar] [CrossRef]
- Kumar, A.; Gautam, A.; Dutt, D. Co-Cultivation of Penicillium sp. AKB-24 and Aspergillus nidulans AKB-25 as a Cost-Effective Method to Produce Cellulases for the Hydrolysis of Pearl Millet Stover. Fermentation 2016, 2, 12. [Google Scholar] [CrossRef]
- Cameron, R.G.; Manthey, J.A.; Baker, R.A. Purification and characterization of a beta-glucosidase from Citrus sinensis var. Valencia fruit tissue. J. Agric. Food Chem. 2001, 49, 4457–4462. [Google Scholar] [CrossRef]
- Bešić, L.; Ašić, A.; Muhović, I.; Dogan, S.; Turan, Y. Purification and Characterization of β-Glucosidase from Brassica oleracea. J. Food Process. Preserv. 2017, 41, e12764. [Google Scholar] [CrossRef]
- Hao, W.X. Construction of Engineering Strain with High β-Glucosidase Yield and Its Effect on Fermentation of Saccharomyces Cerevisiae WXY12 to Produce Ethanol; Jilin Agricultural University: Changchun, China, 2019. [Google Scholar]
- Guo, J.H.; Lu, H.Y.; Hong, J.F. YPS1/YPS2 inactivation improves β-glucosidase expression in Saccharomyces cerevisiae An-α strain. Microbiol China 2021, 48, 4485–4495. [Google Scholar]
- Shen, F.F.; Miu, T.T.; Zhang, X.X. Prokaryotic expression and characterization of thermoactivated β-glucosidase TaBgl3 from thermophilic archaea Thermofilum adornatum. Acta Microbiol Sin. 2022, 62, 2555–2567. [Google Scholar]
- Tong, X.; Qi, Z.; Zheng, D.; Pei, J.; Li, Q.; Zhao, L. High-level expression of a novel multifunctional GH3 family β-xylosidase/α-arabinosidase/β-glucosidase from Dictyoglomus turgidum in Escherichia coli. Bioorganic Chem. 2021, 111, 104906. [Google Scholar] [CrossRef]
- Shuang, D.; He, L. Directional screening, recombinant expression and enzymatic properties of β-glucosidase Bgl747. Microbiol. Bull. 2021, 48, 2524–2533. [Google Scholar]
- Singh, A.K.; Raheja, T.; Sarkar, P.; Sathaye, S.B.; Rai, A.K.; Singh, S.P. Genetic and functional characterization of a Limosilactobacillus fermentum strain with β-galactosidase activity, isolated from Chhurpi sample of Sikkim. Food Bioprod. Process. 2025, 150, 107–117. [Google Scholar] [CrossRef]
- Britto, D.S.; Pirovani, C.P.; Andrade, B.S.; dos Santos, T.P.; Pungartnik, C.; Cascardo, J.C.M.; Micheli, F.; Gesteira, A.S. Recombinant β-1,3-1,4-glucanase from Theobroma cacao impairs Moniliophthora perniciosa mycelial growth. Mol. Biol. Rep. 2013, 40, 5417–5427. [Google Scholar] [CrossRef]
- Wickramasinghe, G.H.I.M.; Rathnayake, P.P.A.M.S.I.; Chandrasekharan, N.V.; Weerasinghe, M.S.S.; Wijesundera, R.L.C.; Wijesundera, W.S.S. Trichoderma virens β-glucosidase I (BGL I) gene; expression in Saccharomyces cerevisiae including docking and molecular dynamics studies. BMC Microbiol. 2017, 17, 137. [Google Scholar] [CrossRef] [PubMed]
- Larue, K.; Melgar, M.; Martin, V.J.J. Directed evolution of a fungal β-glucosidase in Saccharomyces cerevisiae. Biotechnol. Biofuels 2016, 9, 52. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Ying, Y.; Hao, J. A novel glucose-tolerant GH1 β-glucosidase and improvement of its glucose tolerance using site-directed mutation. Appl. Biochem. Biotechnol. 2020, 192, 999–1015. [Google Scholar] [CrossRef]
- Xia, H.; Zhou, Y.; Zheng, Y.; Xu, Q.; Xue, F. Steric hindrance scanning for activity enhancement of β-glucosidase BgMd and its application in glucoside transformation. Mol. Catal. 2025, 584, 115260. [Google Scholar] [CrossRef]
- Li, J.-J.; Pei, X.-Q.; Zhang, S.-B.; Wu, Z.-L. Improving the thermostability of feruloyl esterase by DNA shuffling and site-directed mutagenesis. Process Biochem. 2015, 50, 1783–1787. [Google Scholar]
- Yücetürk, S.Ç.; Azaz, A.D. Production, purification, and determination of the biochemical properties of β-glucosidase in Trichoderma koningii via solid substrate fermentation. Z. Für Naturforschung C 2025, 80, 9–19. [Google Scholar] [CrossRef]
- Ichikawa, S.; Ichihara, M.; Ito, T.; Isozaki, K.; Kosugi, A.; Karita, S. Glucose production from cellulose through biological simultaneous enzyme production and saccharification using recombinant bacteria expressing the β-glucosidase gene. J. Biosci. Bioeng. 2019, 127, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Nath, P.; Dhillon, A.; Kumar, K.; Sharma, K.; Jamaldheen, S.B.; Moholkar, V.S.; Goyal, A. Development of bi-functional chimeric enzyme (CtGH1-L1-CtGH5-F194A) from endoglucanase (CtGH5) mutant F194A and β-1, 4-glucosidase (CtGH1) from Clostridium thermocellum with enhanced activity and structural integrity. Bioresour. Technol. 2019, 282, 494–501. [Google Scholar] [CrossRef] [PubMed]
- Pitchayatanakorn, P.; Putthasang, P.; Chomngam, S.; Jongkon, N.; Choowongkomon, K.; Kongsaeree, P.; Fushinobu, S.; Kongsaeree, P.T. Structure-Based Engineering to Improve Thermostability of Acetivibrio thermocellus AtBgl1A β-Glucosidase. ACS Omega 2025, 10, 27153–27164. [Google Scholar]
- Chen, A.; Liu, K.; Guo, Y.; Wang, C.; Ji, C. Structural and Functional Insights into β-Glucosidase Derived from Thermoproteus sp. AZ2. Arch. Biochem. Biophys. 2025, 771, 110478. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, L.; Yang, X.; Wu, X.; Xu, S.; Liu, Y. Simultaneously optimizing multiple properties of β-glucosidase Bgl6 using combined (semi-) rational design strategies and investigation of the underlying mechanisms. Bioresour. Technol. 2023, 374, 128792. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S. Production of pharmaceutically important genistein and daidzein from soybean flour extract by using β-glucosidase derived from Penicillium janthinellum NCIM 1171. Process Biochem. 2020, 97, 183–190. [Google Scholar] [CrossRef]
- Han, J.; Fang, S.; He, X.; Wang, L.; Li, C.; Wu, J.; Cai, Y.; Wang, Y. Combination of aqueous two-phase flotation and inverse transition cycling: Strategies for separation and purification of recombinant β-glucosidase from cell lysis solution. Food Chem. 2022, 373, 131543. [Google Scholar] [CrossRef]
- Qin, Y.; Li, Q.; Luo, F.; Fu, Y.; He, H. One-step purification of two novel thermotolerant β-1, 4-glucosidases from a newly isolated strain of Fusarium chlamydosporum HML278 and their characterization. AMB Express 2020, 10, 182. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Sun, J.; Wang, W.; Zhuang, Z.; Liu, J.; Hao, J. A novel cold-adapted β-galactosidase from Alteromonas sp. ML117 cleaves milk lactose effectively at low temperature. Process Biochem. 2019, 82, 94–101. [Google Scholar] [CrossRef]
- Zheng, W.; Qin, Y.; Zhuang, X.; Xiao, H.; Liu, C.; Fu, X.; Lin, Q.; Lan, T. Recycling enzymatic hydrolysis lignin residues saved cellulase in enzymatic hydrolysis of lignocellulose: An insight from cellulase adsorption mechanism. Ind. Crop. Prod. 2024, 208, 117884. [Google Scholar] [CrossRef]
- Srivastava, N.; Rathour, R.; Jha, S.; Pandey, K.; Srivastava, M.; Thakur, V.K.; Sengar, R.S.; Gupta, V.K.; Mazumder, P.B.; Khan, A.F.; et al. Microbial beta glucosidase enzymes: Recent advances in biomass conversation for biofuels application. Biomolecules 2019, 9, 220. [Google Scholar] [CrossRef]
- Boisset, C.; Fraschini, C.; Schülein, M.; Henrissat, B.; Chanzy, H. Interfacial molecular interactions of cellobiohydrolase Cel7A and its variants on cellulose. Biotechnol. Biofuels 2020, 13, 10. [Google Scholar] [CrossRef]
- Gu, B.; Xia, L. High expression of a neutral endo-β-glucanase gene from Humicola insolens in Trichoderma reesei. J. Ind. Microbiol. Biotechnol. 2013, 40, 773–779. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, C.; Wang, G.; Ma, Y.; Zhang, K.; Zou, S.; Zhang, M. Comparison of the expression in Saccharomyces cerevisiae of endoglucanase II from Trichoderma reesei and endoglucanase I from Aspergillus aculeatus. BioResources 2012, 7, 4031–4045. [Google Scholar] [CrossRef]
- Zhang, Y.; Min, Z.; Qin, Y.; Ye, D.-Q.; Song, Y.-Y.; Liu, Y.-L. Efficient Display of Aspergillus niger β-glucosidase on Saccharomyces cerevisiae cell wall for aroma enhancement in wine. J. Agric. Food Chem. 2019, 67, 5169–5176. [Google Scholar] [CrossRef]
- Han, Y.; Du, J.; Song, Z. Effects of the yeast endogenous β-glucosidase on hawthorn (Crataegus pinnatifida Bunge) wine ethyl carbamate and volatile compounds. J. Food Compos. Anal. 2021, 103, 104084. [Google Scholar] [CrossRef]
- Wang, J.; Ma, L.; Gao, H. The effect of commercial β-glucosidase and its immobilization on quality of red wine production. Biocatal. Agric. Biotechnol. 2023, 54, 102965. [Google Scholar] [CrossRef]
- Baffi, M.A.; Tobal, T.; Lago, J.H.G.; Boscolo, M.; Gomes, E.; Da-Silva, R. Wine aroma improvement using a β-glucosidase preparation from Aureobasidium pullulans. Appl. Biochem. Biotechnol. 2013, 169, 493–501. [Google Scholar] [CrossRef]
- González-Pombo, P.; Fariña, L.; Carrau, F.; Batista-Viera, F.; Brena, B.M. A novel extracellular β-glucosidase from Issatchenkia terricola: Isolation, immobilization and application for aroma enhancement of white Muscat wine. Process Biochem. 2011, 46, 385–389. [Google Scholar] [CrossRef]
- Su, H.; Xiao, Z.; Yu, K.; Zhang, Q.; Lu, C.; Wang, G.; Wang, Y.; Liang, J.; Huang, W.; Huang, X.; et al. Use of a purified β-glucosidase from coral-associated microorganisms to enhance wine aroma. J. Sci. Food Agric. 2022, 102, 3467–3474. [Google Scholar] [CrossRef]
- Keerti; Gupta, A.; Kumar, V.; Dubey, A.; Verma, A.K. Kinetic characterization and effect of immobilized thermostable β-glucosidase in alginate gel beads on sugarcane juice. Int. Sch. Res. Not. 2014, 2014, 178498. [Google Scholar]
- Yuksekdag, Z.; Acar, B.C.; Aslim, B.; Tukenmez, U. β-Glucosidase activity and bioconversion of isoflavone glycosides to aglycones by potential probiotic bacteria. Int. J. Food Prop. 2017, 20, S2878–S2886. [Google Scholar] [CrossRef]
- Zhou, J.; Liang, M.; Lin, Y.; Pang, H.; Wei, Y.; Huang, R.; Du, L. Application of β-glucosidase in a biphasic system for the efficient conversion of polydatin to resveratrol. Molecules 2022, 27, 1514. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.-F.; Wang, X.-Z.; Jiang, S.; Liu, J.-S.; Zheng, M.-Z.; Chen, P. Enzymatic transformation of ginsenosides Re, Rg1, and Rf to ginsenosides Rg2 and aglycon PPT by using β-glucosidase from Thermotoga neapolitana. Biotechnol. Lett. 2019, 41, 613–623. [Google Scholar] [CrossRef]
- Garbin, A.P.; Garcia, N.F.L.; Cavalheiro, G.F.; Silvestre, M.A.; Rodrigues, A.; Paz, M.F.D.A.; Fonseca, G.G.; Leite, R.S.R. β-glucosidase from thermophilic fungus Thermoascus crustaceus: Production and industrial potential. An. Acad. Bras. Ciências 2021, 93, e20191349. [Google Scholar] [CrossRef]
- Singh, G.; Verma, A.K.; Kumar, V. Catalytic properties, functional attributes and industrial applications of β-glucosidases. 3 Biotech 2016, 6, 3. [Google Scholar] [CrossRef]
- Tsatsis, D.E.; Papachristos, D.K.; Valta, K.A.; Vlyssides, A.G.; Economides, D.G. Enzymatic deinking for recycling of office waste paper. J. Environ. Chem. Eng. 2017, 5, 1744–1753. [Google Scholar] [CrossRef]
- Saxena, A.; Chauhan, P.S. Role of various enzymes for deinking paper: A review. Crit. Rev. Biotechnol. 2017, 37, 598–612. [Google Scholar] [CrossRef]
- Soni, R.; Nazir; Chadha, B.S.; Saini, H.S. Novel sources of fungal cellulases for efficient deinking of composite paper waste. Bioresources 2008, 3, 234–246. [Google Scholar] [CrossRef]
- Ram, C.; Rani, P.; Gebru, K.A.; Abrha, M.G.M. Pulp and paper industry wastewater treatment: Use of microbes and their enzymes. Phys. Sci. Rev. 2020, 5, 20190050. [Google Scholar] [CrossRef]
- Ruiz-Barba, J.L.; Cortés-Delgado, A.; Sánchez, A.H.; López-López, A.; Montaño, A. Naturally Fermented Gordal and Manzanilla Green Table Olives: Effect of Single Yeast Starters on Fermentation and Final Characteristics of the Products. Fermentation 2024, 10, 439. [Google Scholar] [CrossRef]
- Mallek-Fakhfakh, H.; Fakhfakh, J.; Masmoudi, N.; Rezgui, F.; Gargouri, A.; Belghith, H. Agricultural wastes as substrates for β-glucosidase production by Talaromyces thermophilus: Role of these enzymes in enhancing waste paper saccharification. Prep. Biochem. Biotechnol. 2017, 47, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, R.; Zhang, J.; Liu, H.; Xu, Z.; Jin, M.; Bai, F.; Zhong, Y. Achieving high-concentration lignocellulosic sugars by using the Trichoderma reesei strain with high β-glucosidase activity for inducer synthesis and cellulase production. Chem. Eng. J. 2025, 522, 167764. [Google Scholar] [CrossRef]
- Azevedo, R.; Lopes, J.L.; de Souza, M.M.; Quirino, B.F.; Cançado, L.J.; Marins, L.F. Synechococcus elongatus as a model of photosynthetic bioreactor for expression of recombinant β-glucosidases. Biotechnol. Biofuels 2019, 12, 174. [Google Scholar] [CrossRef]
- Bara Ski, R.; Puddephat, I.J. Tissue specific expression of β-glucuronidase gene driven by heterologous promoters in transgenic cauliflower plants. Acta Physiol. Plant. 2004, 26, 307–315. [Google Scholar] [CrossRef]
- Long, T.; Zhang, P.; Yu, J.; Gao, Y.; Ran, X.; Li, Y. Regulation of β-disaccharide accumulation by β-glucosidase inhibitors to enhance cellulase production in Trichoderma reesei. Fermentation 2022, 8, 232. [Google Scholar] [CrossRef]
- Wang, L.-L.; Tang, W.; Wang, Z.; Wang, Y.-X.; Li, N.; Ren, F.-C. Antimicrobial, Antioxidant, and α-Glucosidase-Inhibitory Activities of Prenylated p-Hydroxybenzoic Acid Derivatives from Oberonia ensiformis. Molecules 2025, 30, 2132. [Google Scholar] [CrossRef] [PubMed]
- Sayyed, R.Z.; Shimpi, G.B.; Chincholkar, S.B. Constitutive production of extracellular glucose isomerase by an osmophillic Aspergillus sp. under submerged conditions. J. Food Sci. Technol. 2010, 47, 496–500. [Google Scholar] [CrossRef][Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).