Abstract
This study investigated the fermentation of beef burgers enriched with varying quantities (5% and 10%) of hazelnut skin powder using Lactobacillus acidophilus and Lactiplantibacillus plantarum cultures. The physicochemical, textural, microbiological, and sensory characteristics of the burgers were examined. The research indicates that incorporating hazelnut skin powder enhances the fermentation process via its prebiotic properties. The addition of hazelnut skin powder and the fermentation process were found to affect the quality characteristics of the burgers. The findings indicated that after cooking, the reductions in weight loss, as well as changes in diameter and height, were inversely related to the quantity of hazelnut skin powder incorporated. With the increase in the amount of added hazelnut skin powder, there was a corresponding decrease in the L*, a*, and b* values of the samples. With an increase in the amount of added powder, there was a corresponding rise in the hardness value; however, it was observed that the hardness value decreased while the chewiness value improved in the fermented samples. The amounts of oleic acid and linoleic acid increased in accordance with the quantity of hazelnut skin powder added. Sample S3 exhibited the highest oleic acid amount at 49.05% and the highest linoleic acid amount at 6.10%. The prebiotic characteristics of hazelnut skin powder enhanced the growth of L. acidophilus and L. plantarum. The highest count of L. acidophilus was 8.90 log cfu/g in sample S6, while the maximum count of L. plantarum was 8.91 log cfu/g in sample S9. As the amount of added hazelnut skin powder increased, the scores for sensory properties decreased. Sample S7 was the most liked in terms of sensory properties. Consequently, it was concluded that the incorporation of hazelnut skin powder into the burgers enhanced specific physicochemical, microbiological, and sensory properties of the products. The addition of hazelnut skin powder was found to enhance the growth of lactic acid bacteria.
1. Introduction
The use of by-products from animal and plant sources in new product formulations has emerged as a prominent and functional subject in the food industry in recent years. By-products from the food industry and agri-food waste are presently used in different food formulations due to their bioactive constituents, including antimicrobial and antioxidant properties [1]. Applications for utilising food industry by-products in various foodstuffs are available in the literature [2,3]. Recent studies indicate that natural preservatives from plant sources, especially those abundant in antioxidants, enhance the shelf life and quality of meat products, aligning with increasing customer demand for healthier options [4]. Furthermore, incorporating plant-based raw materials in meat products aligns with the increasing demand for functional foods that offer extra health benefits [5].
Hazelnut skin has gained significance as a product, owing to its nutritional advantages in the food industry and its possible uses in a variety of food items. Hazelnut skin represents a by-product of the hazelnut industry, comprising roughly 2.5% of the total weight of harvested hazelnuts [6]. This means that approximately 28,750 tonnes of hazelnut skin can be obtained following the annual hazelnut harvest worldwide [7]. This amount offers a substantial opportunity for the assessment of this product within the food industry.
Hazelnut skin contains high levels of bioactive compounds, including phenolics and dietary fibre. These chemicals exhibit significant antioxidant activities that can improve the nutritional quality of food products [8,9]. Research indicates that hazelnut skin extracts may positively influence lipid metabolism and enhance gastrointestinal health by fostering a beneficial microbiota [10,11]. These properties make hazelnut skin not only a versatile ingredient but also a health-promoting additive.
The basic mechanism of fermented meat products entails the incorporation of particular microorganisms, primarily lactic acid bacteria (LAB), which play a significant role in fermentation processes. LABs exhibiting probiotic properties, such as Lactobacillus acidophilus, are commonly used due to their ability to produce acid, which lowers the pH of the environment and inhibits the growth of pathogenic microorganisms, thereby enhancing food safety [12]. Probiotic cultures also contribute to the health benefits associated with fermented meat products, as they can improve gut health and nutritional value [12,13].
There are studies in the literature on adding plant-based additives to meat products or fermenting meat products by adding different microorganisms. However, to the best of our knowledge, there are no studies on producing functionally enhanced burgers by applying both methods together. This study aimed to produce functional burgers with high antioxidant and dietary fibre content by adding hazelnut skin powder and fermenting them with lactic acid bacteria, using formulations determined with beef mince, and to ascertain their quality characteristics.
2. Materials and Methods
2.1. Materials
The mincemeat used in the production of the burgers used in the study was sourced from a butcher operating in Afyonkarahisar, Türkiye. The mincemeat was obtained from the round of beef section of the carcass, and the fat content was set to 10%. The hazelnuts (Corylus avellana) of the Tombul variety were sourced from a local producer operating in the province of Giresun, Turkey. To remove the skin, the hazelnuts were heated at 105 °C for 90 min. As the skin cracked during heating, it was then rubbed off to separate it from the nut. It was then ground into powder using a grinding machine (Sinbo, İstanbul, Türkiye). The salt, onions and spices used in the production of the burgers were sourced from a local market operating in Afyonkarahisar, Türkiye.
Strains Used in the Study
Bacterial strains of Lactobacillus acidophilus (Chr Hansen LA-5®) and Lactiplantibacillus plantarum (Chr Hansen UALp-05TM) were used in the study.
2.2. Methods
2.2.1. Preparation of Lactic Acid Bacteria Stains
MRS broth (Merck, 110661, Darmstadt, Germany) was used to inoculate the lactic acid bacteria (L. plantarum UALp-05TM and L. acidophilus LA-5®) involved in the production process, followed by incubation at 37 °C for 48 h. Following the incubation period, lactic acid bacteria were subjected to centrifugation at 7000 rpm for 10 min at 22 °C, and the pellets were obtained [14].
2.2.2. Production of Burgers
The raw materials of the burger samples (beef meat, 85%; salt, 1.5%; spices, 0.5%; onion, 3%; fat, 10%) were combined and subsequently blended with varying concentrations of dried hazelnut skin powder (5 and 10%) [15]. Additionally, L. acidophilus LA-5® and L. plantarum UALp-05TM strains were adjusted to the 0.5 McFarland standard (107–108 cfu/mL) and added to the samples to be fermented at a rate of 1 mL per 100 g of mixture. The samples were fermented in two different stages. The first stage was conducted in an incubator (Incucell MMM, Gräfelfing, Germany) at 30 °C for 8 h, while the second stage was conducted in the refrigerator at 4 °C for 40 h. The burgers produced were set to be 90 × 15 mm in size and weigh 60 g (Figure 1). The sample codes determined according to the added hazelnut skin powder and the used microorganism strains are shown in Table 1.
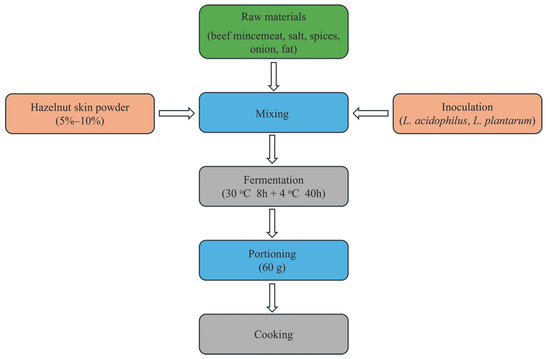
Figure 1.
Flow chart of the burger samples.

Table 1.
Experimental design of the samples.
2.2.3. Physicochemical Analyses
The pH values of the samples were determined using a pH meter (Hanna, Woonsocket, RI, USA, HI 2215 pH/ORP meter), while the dry matter values were determined using a drying oven (Nüve, FN055, Ankara, Türkiye) [16].
2.2.4. Cooking Loss and Dimensional Changes
Beef burgers were cooked for a total of 10 min on an electric griddle (Remta, Istanbul, Türkiye) at 200 °C, 6 min on one side and 4 min on the other. For each batch, the weight, height and diameter of three beef burgers were measured at room temperature before and after cooking to calculate cooking loss and changes in dimensions [17].
The weights of the burger samples before and after baking were measured using a precision balance (Sartorius, CP224S, Göttingen, Germany), and the difference was determined as a percentage. The diameters and heights of the samples were measured before and after baking using a digital calliper (Mitutoyo, Ip-67 0-150, Kawasaki, Japan). The difference was determined as a percentage [18].
2.2.5. Colour Values
The colour measurements of the burgers (L*, a* and b*) were performed using a colourimeter (Minolta CR 300, Minolta Co., Osaka, Japan) that works according to the Hunter Lab system and calibrated with a white tile (Minolta calibration plate, No. 21733001, Y = 92.6, x = 0.3136, y = 0.3196). Measurements were carried out at a 2° viewing angle using a C light source. Three burgers were randomly selected per group, and five measurements were taken from each burger. Hunter L* (lightness; 100 = white, 0 = black), a* (redness; +, red; −, green), and b* (yellowness; +, yellow; −, blue) values were recorded [19].
2.2.6. Texture Profile Analysis (TPA)
Texture profile analysis (TPA) was performed using a texture analyser (TA-XT2, Stable Micro Systems Ltd., Godalming, UK) equipped with a 25 kg load cell and a cylindrical aluminium probe (aluminium cylinder probe P/50, 50 mm diameter). The measuring probe was set to be positioned 20 mm above the samples and then lowered at a speed of 30 mm per minute. A 5 s interval was observed between compressions, and the samples were compressed to 50% of their original height. At the end of the analysis, hardness (g), adhesiveness (g), springiness (g·s), cohesiveness, gumminess, chewiness and resilience values of the burger samples were recorded [20].
2.2.7. Determination of Fatty Acid Composition
The fatty acid analyses of the samples were performed using a GC/MS (Agilent 5975 C Agilent 7890A GC, Santa Clara, CA, USA) analyser. The MSD ChemStation (G1701EA.02.00.493) programme and a DB-WAX column (50 × 0.20 mm, 0.20 µm) were used in the device. The oven starting temperature was set at 80 °C. After a 4 min hold, the temperature was increased at a rate of 13 °C per minute to 175 °C. The temperature was held at this level for 27 min. Then, the temperature was increased at a rate of 4 °C per minute to reach 215 °C, and held at this temperature for 5 min. Subsequently, the temperature was increased at a rate of 4 °C per minute to reach 240 °C, and held at this temperature for 15 min. The injection volume was set to 1 µL, and the detector and injector temperature was set to 240 °C. In the analyses, 1.5 M HCl was used as the derivatisation agent, the derivatisation temperature was set to 80 °C, and the derivatisation time was set to 2 h [21].
2.2.8. Microbiological Analyses
The counts of total mesophilic aerobic bacteria (TMAB), total yeast/mould (TYM), and total coliform group bacteria (TCGB) in the burger samples were determined using the spread plate technique. 10 g was sampled from each burger under sterile conditions, transferred to sterile stomacher bags (174538, Lp Italiana Spa, Milan, Italy), and serial dilutions were prepared up to 10−4 [22]. The TMAB count analysis was performed using Plate Count Agar (PCA) (1.05463, Merck, Germany). Petri dishes containing cultures were incubated aerobically at 30 °C in an incubator (MMM Incucell 55, Germany) for 48–72 h for the TMAB count [23,24]. Potato Dextrose Agar (PDA) (1.10130, Merck, Germany) was used for TYM count analysis, and the cultured Petri dishes were incubated at 22 °C under aerobic conditions for 5–7 days in an incubator [25]. Violet Red Bile Agar (VRB) (1.01406, Merck, Germany) was used for total coliform group bacterial count, and Petri dishes were incubated in an incubator at 30 °C under anaerobic conditions for 24–48 h [26].
2.2.9. L. plantarum and L. acidophilus Counts
In burger samples, L. plantarum count was determined using Cystein Bromophenol Blue-modified de Man, Rogosa and Sharpe Agar (110660, Merck, Germany) (MRS-Cys-BPB) (Ngamsomchat et al., 2022) [27], and for L. acidophilus count, using 0.15% w/v bile-modified de Man, Rogosa and Sharpe Agar (MRS-Bile) [28] were prepared. Analyses were performed according to the spread plate method. The inoculated Petri dishes were incubated at 37 °C for 72 h in jars (116387, Merck, Germany) containing Anaerocult A (113829, Merck, Germany) under anaerobic conditions [29].
2.2.10. Sensory Analyses
Sensory analyses of the samples were conducted using the scoring test technique, as described by Ercan et al. [30]. The samples were scored according to five parameters: colour, odour, flavour, texture and general acceptability. A scale ranging from 1 to 10 points (1: dislike at all; 10: extremely like) was used for the evaluation. Sensory analysis was conducted at Afyon Kocatepe University, Afyon Vocational School, with a group of 20 volunteer panellists, consisting of non-smoking men and women aged between 25 and 45, in accordance with ISO standard guidelines [31,32]. Before the sensory evaluation, the panellists were informed about the characteristics, such as colour, smell, texture, aroma, and flavour, that should be present in a standard burger. Grilled burger samples were presented to the panellists immediately after cooking. The panellists were supplied with filtered water and instructed to cleanse their palate between samples.
2.2.11. Statistical Analysis
Statistical values were calculated using the SPSS V 23.0.0 statistical package programme (SPSS Inc., Chicago, IL, USA). Data obtained from the analyses were evaluated by applying the variance analysis. The significance level was determined using the Duncan test (p < 0.05), and the effect of the results was also determined by calculating the Pearson correlation coefficient.
3. Results and Discussion
3.1. Physicochemical Properties of the Samples
The process interaction for the pH values of the samples was very highly significant, while the process, product type, and the process × product type interactions were very highly significant for the dry matter (%) value of the samples (p < 0.0001; Table 2). Furthermore, the process had a highly negative correlative effect on pH and dry matter values. The addition of hazelnut skin powder and the inoculation of lactic acid bacteria was observed to reduce the pH value in the samples (p < 0.05). In addition, it was also observed that the pH decreased after fermentation, except for samples S1 and S2. In general, the cooking process was also found to affect pH (p < 0.05). The highest pH was determined in the S1 sample after fermentation (5.35), while the lowest pH was determined in the S7 sample after fermentation (4.96). Lactic acid, produced during fermentation due to bacterial activity, is thought to contribute to a lower pH. Lactic acid production during fermentation results in an increase in total acid concentration and a decrease in pH [33]. After analysing the pH values post-cooking, it was noted that cooking increases the pH levels. The increase in pH during cooking can primarily be attributed to the denaturation of proteins. This process results in the loss of acidic amino groups and the subsequent release of more basic residues. Studies indicate that the pH values in cooked meat may increase as a result of the loss of acidic regions, which occurs when proteins undergo structural changes [34]. The percentage of dry matter increased, especially with the addition of hazelnut skin powder (p < 0.05). An increase in the percentage of dry matter content in the samples occurred in conjunction with the amount of hazelnut skin powder added. The use of fibrous and low-moisture additions, such as hazelnut skin powder, may enhance the percentage of dry matter content by binding the free water inside the product [35]. The fermentation process did not have a significant effect on the percentage of dry matter (p > 0.05). A decrease in the rate of dry matter was noted as a function of the cooking process applied (p < 0.05). The fat loss during cooking significantly affects the dry matter percentage in meatballs. The fat contents of products like burgers affect cooking loss, cooking efficiency, and consequently the dry matter content of the products [36].

Table 2.
Physicochemical properties during the production of the burger samples (S1–S9).
3.2. Cooking Loss (%) and Dimensional Changes (%)
The weight loss (%), diameter change (%), and height change (%) of the samples were measured to determine the changes in the product after cooking (Table 3). The weight loss of the samples ranged between 14.58% and 29.25%. The addition of hazelnut skin powder affected weight loss. As the amount of powder added increased, weight loss decreased (p < 0.05). On the other hand, weight loss was higher in samples using lactic acid bacteria compared to the control group. Cooking loss denotes the reduction in weight of burgers during preparation, attributed to the loss of moisture and fat; this loss can be minimised by optimising the properties of the meat combination and the cooking method. The addition of fibre-rich ingredients may reduce cooking losses by increasing water retention capacity [37]. The % diameter changes in the samples were similar. The highest diameter change was found to be 22.83% (S5), while the least diameter change was recorded at 12.66% (S8). The S5 sample exhibited the maximum height change at 38.33%, while the S2 sample exhibited the minimum height change at 25.63%. A study conducted on mixtures containing hazelnut shells demonstrated improved mechanical properties and reduced overall deformation [38]. Ensuring structural integrity while cooking can help preserve the original diameter and height of burgers, thus minimising excessive size changes.

Table 3.
Cooking loss and dimensional changes in the burger samples (S1–S9).
3.3. Colour Values
The L* value, which is an indicator of brightness in foods, changed with the addition of hazelnut skin powder (Figure 2). It was determined that there was a decrease in the L* value depending on the amount of powder added (p < 0.05). The highest L* value was determined in samples without powder addition in all samples before fermentation, after fermentation, and after cooking. Due to the dark colour of the hazelnut skin, a decrease in L* value occurred in samples with skin powder addition. After fermentation, a slight decrease in L* values was determined. Protein denaturation resulting from a decrease in pH during fermentation was thought to contribute to this change. The thermal processes that occurred during cooking affected the changes in the L* value after cooking. Cooking may have promoted Maillard reactions, which are responsible for browning and can subsequently reduce L* values. In cooked samples, the L* value usually appeared to be lowered. In processed foods like burgers, the type and concentration of additives could reduce the myoglobin concentration, thus affecting the L* value [39].
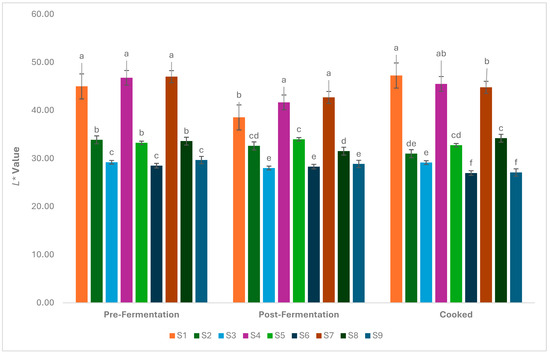
Figure 2.
Change in L* values during the production of the burger samples (S1–S9). a–f: Values with the different lowercase letters on the bars for each analysis differ significantly (p < 0.05).
It was determined that the addition of hazelnut skin powder caused a change in the a* values, which indicate the degree of redness-greenness in foods, similar to the L* values (p < 0.05). The highest a* value was detected in samples without powder addition (Figure 3). However, there was a slight decrease in a* values in the post-fermentation and post-cooking samples. The b* value, which indicates the degree of yellowness and blueness, decreased in parallel with the amount of powder added (p < 0.05). The highest b* values were determined in samples S1, S4, and S7 (without added hazelnut skin powder), while the lowest b* values were determined in samples S3, S6, and S9 (Figure 4). A significant increase in b* values was observed after cooking. The high temperatures associated with frying can increase the Maillard reaction, leading to browning and subsequently more intense yellow tones. Su [40] emphasised that frying causes an increase in b* values in burgers and indicates an increase in yellowness.
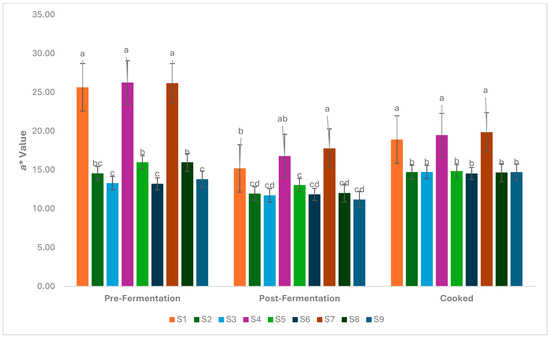
Figure 3.
Change in a* values during the production of the burger samples (S1–S9). a–d: Values with the different lowercase letters on the bars for each analysis differ significantly (p < 0.05).
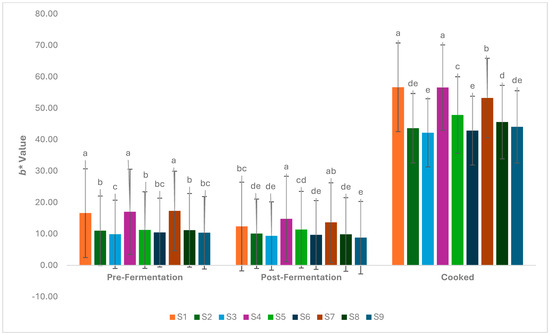
Figure 4.
Changeinb* values during the production of the burger samples (S1–S9). a–e: Values with the different lowercase letters on the bars for each analysis differ significantly (p < 0.05).
3.4. Textural Properties of the Samples
The process, product type, and process × product type interactions were very highly significant for all texture values of the samples (except cohesiveness and resilience) (p < 0.0001). Furthermore, the process had a highly positive correlative effect on adhesiveness, springiness, chewiness and resilience values, while it had a highly negative correlative effect on hardness, cohesiveness, and gumminess values. In addition to this, the product type had a highly positive correlative effect on adhesiveness and resilience values, while it had a negative correlative effect on cohesiveness value.
Changes made in the formulation of three different processes (pre-fermentation, post-fermentation and cooked) affected the hardness values (p < 0.05). The addition of hazelnut skin to the formulation increased the hardness value in all three processes, with an increase in value observed in parallel with the amount added. The inoculation of lactic acid bacteria to the samples reduced the hardness value compared to the control sample (Table 4). The inoculation of L. acidophilus LA-5® from two different cultures resulted in an increase in the hardness value of 7.07 N compared to L. plantarum UALp-05TM (p > 0.05).

Table 4.
Changes in textural properties during the production of the burger samples (S1–S9).
An increase in the dry matter content of the formulation caused an increase in the hardness values of the samples. Moreover, the inoculation of lactic acid bacteria resulted in a decrease in the hardness value of the samples. Lactic acid bacteria produce lactic acid during the fermentation of carbohydrates found in meat, resulting in a reduction in pH value. The decrease in pH is crucial in forming a more acidic environment, which increases the solubility of meat proteins. The protein solubility is one of the key factors affecting the hardness and texture of the final product. LAB’s hydrolytic activity leads to the breakdown of muscle proteins into smaller peptides and amino acids, resulting in a softer texture [41].
The process and the product type significantly affected the adhesiveness values (p < 0.05). An increase in adhesiveness values was observed in proportion to the amount of hazelnut skin powder added to the samples. In samples inoculated with lactic acid bacteria, adhesiveness values did not cause any change in raw (pre-fermentation) samples compared to the control sample (p > 0.05). However, it caused a decrease in pre-cooking (post-fermentation) samples (Table 4). The effect of the species of microorganisms used on the adhesiveness values of the samples was similar (p > 0.05).
The addition of hazelnut skin led to an increase in adhesiveness values due to the resulting increase in dry matter content. The dietary fibre present in hazelnut skin may increase water retention capacity during fermentation in meat products. This increased water retention may contribute to increased adhesiveness by leading to a more cohesive texture [11]. The fibrous texture acts as a binding agent, providing a sticky consistency that allows the fermented meat product to adhere better to surfaces and improves the overall sensation it leaves in the consumer’s mouth [8]. LAB strains used in fermented meat products significantly affect the textural properties of meat. Different strains exhibit different enzymatic profiles, leading to variations in the degree of protein hydrolysis and acid production [42]. Research indicates that adding LAB to meat products not only improves the safety and shelf life of meat products by inhibiting the growth of saprophytic microorganisms but also enhances the textural quality of processed meats by providing a more adhesive texture [43,44].
The process difference significantly affected the springiness values of the samples (p < 0.05). However, the change occurred in parallel with other samples. When the processes performed were evaluated among themselves, there were significant differences between the samples (p < 0.05). As the amount of added hazelnut skin increased in the samples, a decrease in springiness values was again observed. In lactic acid bacteria-inoculated samples, springiness values also decreased compared to the control sample (p < 0.05) (Table 4). The species of the microorganism used affected the adhesiveness values of the samples (p < 0.05).
The addition of hazelnut skin to fermented meat products may have a significant effect on the springiness values of these products. Springiness, a fundamental textural characteristic, is defined as the product’s ability to return to its original shape after deformation, and this is of great importance in terms of consumers’ perception of quality in meat products [45]. The addition of hazelnut skin, which is rich in dietary fibre, altered the springiness values of burgers through various mechanisms by affecting the structure and moisture retention capacity of the products. LAB produces lactic acid during fermentation, lowering the pH level of the meat. The functionality of proteins in the meat matrix can be significantly affected as a result of this decrease in pH. When the pH decreases, the solubility of specific proteins, including myofibrillar proteins, may cause protein coagulation. This coagulation may contribute to a gel-like structure, but excessive acidification may affect elasticity (springiness). Research has shown that excessively acidic conditions can cause the formation of a rigid protein network, which may ultimately lead to a loss of elasticity in the final product [46,47].
The difference in the process significantly affected the cohesiveness values of the samples (p < 0.05). The cohesiveness values of the cooked samples were lower than those of the raw samples (p < 0.05). The increase in the amount of hazelnut skin added to the samples resulted in a decrease in cohesiveness values. The cohesiveness values of the samples with inoculated lactic acid bacteria were also lower than those of the control sample (p < 0.05; Table 4). The species of microorganism used was partially effective on raw samples but was not effective on cooked samples (p > 0.05).
Hazelnut skin is known for its high fibre content, especially in terms of insoluble fibre and polysaccharides [48]. Dietary fibres increase water retention and positively influence textural properties; however, excessive amounts may compromise the integrity of the protein matrix. The presence of large fibrous particles within the meat matrix can reduce cohesiveness by inhibiting the binding capacity of proteins. When incorporated in greater quantities, these fibres may fail to integrate effectively, potentially weakening the interactions between the proteins in the meat [49]. LAB fermentation can trigger the formation of exopolysaccharides (EPSs), which are polysaccharides secreted by specific LAB species. While EPS can yield positive results in terms of textural properties and mouthfeel when present in measured amounts, if not adequately balanced, it can interact with the protein matrix, leading to the formation of a more viscous environment and consequently reducing cohesion values [50].
The difference in the process significantly affected the gumminess values of the samples (p < 0.05). The addition of hazelnut skin to the formulation caused an increase in gumminess values in all three processes, with the increase in value being directly proportional to the amount added. The inoculation of lactic acid bacteria to the samples increased the gumminess value compared to the control sample (Table 4). The post-fermentation values of samples inoculated with lactic acid bacteria were found to be lower than the pre-fermentation values (p < 0.05).
The fat content and protein interactions in meat directly affect textural parameters such as gumminess [51]. The addition of hazelnut skin could modify the overall composition of the product. This significant change can influence texture-related characteristics, such as increased gumminess, as the fibres serve as an alternative structural source. Studies show that dietary fibres can improve the gel-forming properties of meat emulsions and enhance textural characteristics associated with gumminess [52]. The fermentation process itself significantly influences textural properties. During fermentation, lactic acid bacteria produce lactic acid, resulting in a decrease in pH levels that leads to the denaturation and coagulation of proteins in the meat [53].
The production process significantly affected the chewiness values of the samples. The addition of hazelnut skin to the formulation increased the chewiness value in all three processes, with the increase in value found to be directly proportional to the amount added (p < 0.05). The inoculation of lactic acid bacteria to the samples increased the chewiness value compared to the control sample (Table 4). Lactic acid bacteria inoculation of samples with added hazelnut skin reduced chewiness values compared to samples with added hazelnut skin alone (p < 0.05).
The use of hazelnut skins has been linked to an increase in the water retention capacity of meat formulations. This feature is crucial since it can influence the texture and chewiness of the final product. The presence of dietary fibres in hazelnut skins promotes the development of a cohesive protein matrix in meat, yielding a firmer and more chewable texture [54]. LAB plays a vital role in the acidification of meat products. This is essential for flavour development, safety and texture improvement in fermented meat products [55]. The increase in acidity associated with fermentation could influence chewiness by improving the structural integrity of the meat matrix. Specifically, low pH levels can promote protein denaturation and coagulation, resulting in a firmer texture. The influence of LAB on meat proteins through proteolysis leads to the formation of peptides and amino acids that increase the chewiness of the final product [41].
The cooking process had a significant effect on the resilience values of the samples (p < 0.05). In the formulation, the addition of hazelnut skin increased the resilience value in all three processes. This increase was proportional to the amount added. Although the inoculation of lactic acid bacteria to the samples reduced the resilience value compared to the control sample, this increase was not statistically significant (p > 0.05; Table 4). Although the inoculation of L. acidophilus LA-5® from two different cultures resulted in a slight decrease in resilience values compared to L. plantarum UALp-05TM, this change is not statistically significant (p > 0.05).
Studies indicate that the addition of different amounts of hazelnut skin influences product texture differently. Formulations with differing ratios of hazelnut skin maintain optimal textural characteristics. This suggests a synergistic effect in which the combination of meat proteins and hazelnut skins produces an improved textural profile that aligns with customer expectations [18,56]. The increase in dry matter content in the formulation has led to increased resilience values of the samples. The addition of LAB into fermented meat products has a notable impact on the resilience values of the product. The observed effect results from a range of biochemical processes initiated by LAB during fermentation, which encompass acid production, protein interactions, and the synthesis of exopolysaccharides. The reduction in pH that occurs during fermentation leads to the denaturation and aggregation of proteins. This leads to the formation of a more stable protein network, thereby enhancing the resilience value [55].
3.5. Fatty Acid Composition
The fatty acid composition of burgers was examined before and after cooking. Varying proportions of myristic acid (C14:0), palmitic acid (C16:0), palmitoleic acid (C16:1), heptadecanoic acid (C17:0), cis-10-heptadecenoic acid (C17:1), stearic acid (C18:0), oleic acid (C18:1), linoleic acid (C18:2), arachidic acid (C20:0), linolenic acid (C18:3), gadoleic acid (C20:1), and behenic acid (C22:0) were detected in the samples (Table 5). The cooking process had no significant effect on the samples (p > 0.05). However, the addition of hazelnut skin powder increased the concentration of specific fatty acids. In particular, the addition of hazelnut skin powder resulted in a significant increase in the amounts of oleic acid and linoleic acid. The highest levels of oleic acid and linoleic acid were found in sample S3, at 49.05% and 6.10%, respectively. At the same time, the addition of hazelnut skin powder caused a decrease in the amount of stearic acid. The highest stearic acid content was found in sample S1 at 29.66%, while the lowest was in sample S9 at 19.79%. The addition of hazelnut skin powder affected the fatty acid composition. Research has shown that oleic and linoleic acids are the predominant fatty acids in hazelnuts and hazelnut products [9]. The changes in the fatty acid composition of the burger samples with the addition of hazelnut skin powder were consistent with the literature.

Table 5.
Fatty acid composition of the burger samples before (Bfr) and after (Aft) cooking.
3.6. Microbiological Analyses
The process, product type, and process × product type interactions were very highly significant for TMAB, TYM, and TCGB counts (p < 0.0001; Table 6). In addition to this, the process showed a highly positive correlative effect on TMAB, TYM, and TCGB counts. In addition, the product type showed a highly positive correlative effect on TMAB counts, while it showed a highly negative correlative effect on TCGB counts. The product type also showed a positive correlative effect on L. acidophilus LA-5® count.

Table 6.
Changes in microbial counts (log CFU/g) during the production of the burger samples (S1–S9).
The TMAB count increased after fermentation. The addition of hazelnut skin powder and the inoculation of lactic acid bacteria increased the TMAB count to a small extent. The TMAB counts of the pre-fermentation samples ranged from 5.01 to 5.22 log cfu/g. After fermentation, the highest TMAB count was determined to be 5.91 log cfu/g in sample S6, and the lowest TMAB count was 5.21 log cfu/g in sample S1. A slight increase in TYM counts was observed with the addition of hazelnut skin powder. Following fermentation, an increase in TYM counts was observed in the samples compared to pre-fermentation levels. The lowest TYM count was recorded in the pre-fermentation S6 sample at 3.07 log cfu/g, while the highest TYM count was observed in the post-fermentation S1 sample at 4.06 log cfu/g. A decrease in TCGB counts was observed with the addition of powder and the inoculation of lactic acid bacteria (p < 0.05). Although there was a partial increase in TCGB counts after fermentation, TCGB counts remained lower in samples processed with powder and lactic acid bacteria. The fermentation, the species of lactic acid bacteria used, and the amount of added hazelnut skin powder affected TCGB growth.
The addition of hazelnut skin powder had a positive effect on the growth of lactic acid bacteria. After fermentation, a high increase in the counts of L. acidophilus LA-5® and L. plantarum UALp-05TM was determined in the samples to which hazelnut skin powder was added. The highest L. acidophilus LA-5® and L. plantarum UALp-05TM counts were detected in samples S6 and S9, with values of 8.90 log cfu/g and 8.91 log cfu/g, respectively. Hazelnut skin contains fibrous materials that serve as indigestible substrates. The structural composition of hazelnut skin fibres, predominantly including polysaccharides, renders them suitable for fermentation by lactic acid bacteria [57]. Furthermore, the presence of specific compounds such as polyphenols in plant materials positively influences the metabolic activities of LAB, demonstrating potential prebiotic effects [58]. It is thought that the prebiotic properties of hazelnut skin promote the growth of L. acidophilus LA-5® and L. plantarum UALp-05TM.
3.7. Sensory Evaluation
The sensory qualities of the burgers were evaluated based on colour, odour, flavour, texture and general acceptability parameters (Figure 5). The sample that received the highest scores for general acceptability, colour and flavour was sample S7. Sample S4 received the highest scores for odour and texture. The species of lactic acid bacteria inoculated and the amount of skin powder added affected the sensory qualities (p < 0.05). The lowest-scored sample was the S3 sample. When the results were examined, as the amount of added skin powder increased, there was a decrease in sensory qualities. Hazelnut skin powder is also recognised for its richness in phenolic compounds, which are associated with antioxidant properties that can improve sensory characteristics such as freshness and flavour stability [59,60]. For example, Costantini et al. [61] found that adding 5% hazelnut skin powder to lentil crisps improved both their nutritional and sensory properties and supported their potential positive effect in product preparations. Nevertheless, it is advised to use them with caution, since excessive use of such additives may result in sensory decline, and [62] indicated that increasing the amount of plant-based additions resulted in a decreased overall acceptance of sausages.
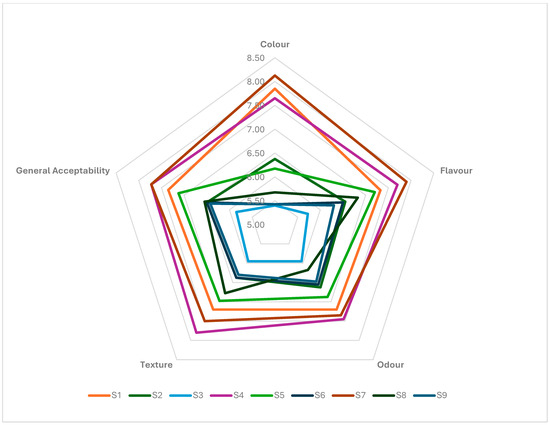
Figure 5.
Sensory properties of the burger samples (S1–S9).
4. Conclusions
The meat industry’s ongoing innovative approaches involve the appropriate integration of by-products and alternative raw materials into food production and the market’s shift towards healthier and more sustainable options. In this study, burgers with varying proportions of hazelnut skin powder added were fermented with L. acidophilus LA-5® and L. plantarum UALp-05TM bacteria. The physicochemical, textural, microbial, and sensory properties of the burgers were examined. The addition of hazelnut skin powder and the fermentation process improved the physicochemical and textural properties of the burgers. The percentage of cooking loss decreased depending on the amount of powder added. Furthermore, the percentage change in diameter and weight was also lower. The addition of hazelnut skin powder caused negative changes in textural properties, but fermentation eliminated this negative effect. The addition of hazelnut skin powder increased the amounts of oleic and linoleic acids. The highest levels of oleic acid and linoleic acid were determined in sample S3, at 49.05% and 6.10%, respectively. The prebiotic properties of hazelnut skin powder contributed to the growth of the cultures used. The highest L. acidophilus LA-5® and L. plantarum UALp-05TM counts were 8.90 log cfu/g and 8.91 log cfu/g, respectively, in samples S6 and S9. Sensory qualities varied depending on the amount of powder added. Analysis of the results suggests that sensory qualities decreased as the amount of skin powder added increased. Consequently, the amount of hazelnut skin powder added affected the basic quality characteristics of the burgers. Furthermore, the fermentation process contributed to the development of these characteristics. The prebiotic properties of hazelnut skin powder have positively influenced the fermentation process and, consequently, the development of lactic acid bacteria. At a time when innovative and functional product development is increasing in the meat industry and consumer demand is shifting towards this area, the findings of this research are expected to make significant contributions. The results of this study will guide researchers planning to work on the use of different plant-based by-products in meat products or the fermentation of meat products using various species of microorganisms.
Funding
This research received no external funding.
Institutional Review Board Statement
The national laws do not require ethical approval for sensory evaluation. There are no human ethics committees’ formal documentation procedures available for sensory evaluation. The experimental protocol involving sensory evaluation was in accordance with the relevant operation specifications in Türkiye.
Informed Consent Statement
Written informed consent for participation was obtained from all subjects involved in the study, in accordance with the General Data Protection Regulation (GDPR) 2016/679. The study was conducted following the principles of the Declaration of Helsinki.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Viola, E.; Badalamenti, N.; Bruno, M.; Tundis, R.; Loizzo, M.R.; Moschetti, G.; Sottile, F.; Naselli, V.; Francesca, N.; Settanni, L.; et al. Reuse of Almond By-Products: Scale-Up Production of Functional Almond Skin Added Semolina Sourdough Breads. Future Foods 2024, 9, 100372. [Google Scholar] [CrossRef]
- Gaglio, R.; Restivo, I.; Barbera, M.; Barbaccia, P.; Ponte, M.; Tesoriere, L.; Bonanno, A.; Attanzio, A.; Di Grigoli, A.; Francesca, N.; et al. Effect on the Antioxidant, Lipoperoxyl Radical Scavenger Capacity, Nutritional, Sensory and Microbiological Traits of an Ovine Stretched Cheese Produced with Grape Pomace Powder Addition. Antioxidants 2021, 10, 306. [Google Scholar] [CrossRef]
- Restivo, I.; Sciurba, L.; Indelicato, S.; Allegra, M.; Lino, C.; Garofalo, G.; Bongiorno, D.; Davino, S.; Avellone, G.; Settanni, L.; et al. Repurposing Olive Oil Mill Wastewater into a Valuable Ingredient for Functional Bread Production. Foods 2025, 14, 1945. [Google Scholar] [CrossRef]
- Kumar, P.; Kaur, S.; Goswami, M.; Singh, S.; Sharma, A.; Mehta, N. Antioxidant and Antimicrobial Efficacy of Giloy (Tinospora cordifolia) Stem Powder in Spent Hen Meat Patties Under Aerobic Packaging at Refrigeration Temperature (4 ± 1 °C). J. Food Process. Preserv. 2021, 45, e15772. [Google Scholar] [CrossRef]
- Nazarova, N.; Lazutina, A.; Lebedeva, T.; Batsyna, Y.; Statuev, A. The Use of Plant Raw Materials in The Production of Meat Pate. IOP Conf. Ser. Earth Environ. Sci. 2022, 1052, 012063. [Google Scholar] [CrossRef]
- Ivanović, S.; Avramović, N.; Dojčinović, B.; Trifunović, S.; Novaković, M.; Tešević, V.; Mandić, B. Chemical Composition, Total Phenols and Flavonoids Contents and Antioxidant Activity as Nutritive Potential of Roasted Hazelnut Skins (Corylus avellana L.). Foods 2020, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO). Crops and Livestock Products. 2023. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 15 August 2025).
- Horoszewicz, J.; Kruk, M.; Król, K.; Jaworska, D.; Hallmann, E.; Trząskowska, M. The Use of Hazelnut Seed Skins for The Fortification of Food with Polyphenols and to Increase Food Safety. Zywnosc Nauka Technol. Jakosc/Food Sci. Technol. Qual. 2022, 29, 102–111. [Google Scholar] [CrossRef]
- Çelik, Ö.; Aktaş, N.; Tugay, M.; Tunçil, Y. Hazelnut (Corylus avellana L.) Skin, a By-Product of Hazelnut Industry, Possesses Oil with High Oxidative and Thermal Stabilities. Int. J. Food Sci. Technol. 2023, 58, 5471–5477. [Google Scholar] [CrossRef]
- Dinkçi, N.; Aktaş, M.; Akdeniz, V.; Sîrbu, A. The Influence of Hazelnut Skin Addition on Quality Properties and Antioxidant Activity of Functional Yogurt. Foods 2021, 10, 2855. [Google Scholar] [CrossRef]
- Zeppa, G.; Belviso, S.; Bertolino, M.; Cavallero, M.C.; Dal Bello, B.; Ghirardello, D.; Giordano, M.; Giorgis, M.; Grosso, A.; Rolle, L.; et al. The Effect of Hazelnut Roasted Skin from Different Cultivars on the Quality Attributes, Polyphenol Content and Texture of Fresh Egg Pasta. J. Sci. Food Agric. 2015, 95, 1678–1688. [Google Scholar] [CrossRef] [PubMed]
- Mani-López, E.; Hernández-Figueroa, R.; López-Malo, A.; Morales-Camacho, J. Viability and Functional Impact of Probiotic and Starter Cultures in Salami-Type Fermented Meat Products. Front. Chem. 2024, 12, 1507370. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Pateiro, M.; Tomašević, I.; Domínguez, R.; da Silva Barretto, A.C.; Santos, E.M.; Lorenzo, J. Functional Fermented Meat Products with Probiotics—A Review. J. Appl. Microbiol. 2022, 133, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Goktas, H.; Dikmen, H.; Bekiroglu, H.; Cebi, N.; Dertli, E.; Sagdic, O. Characteristics of Functional Ice Cream Produced with Probiotic Saccharomyces boulardii in Combination with Lactobacillus rhamnosus GG. LWT 2022, 153, 112489. [Google Scholar] [CrossRef]
- Abdel-Moatamed, B.R.; El-Fakhrany, A.E.M.; Elneairy, N.A.; Shaban, M.M.; Roby, M.H. The Impact of Chlorella vulgaris Fortification on The Nutritional Composition and Quality Characteristics of Beef Burgers. Foods 2024, 13, 1945. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists, Official Methods of Analysis, 20th ed.; AOAC: Washington, DC, USA, 2016. [Google Scholar]
- Turhan, S.; Sagir, I.; Ustun, N.S. Utilization of Hazelnut Pellicle in Low-Fat Beef Burgers. Meat Sci. 2005, 71, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Longato, E.; Meineri, G.; Peiretti, P.G.; Gai, F.; Viuda-Martos, M.; Pérez-Álvarez, J.Á.; Fernández-López, J. Effects of Hazelnut Skin Addition on The Cooking, Antioxidant and Sensory Properties of Chicken Burgers. J. Food Sci. Technol. 2019, 56, 3329–3336. [Google Scholar] [CrossRef] [PubMed]
- Amalia, L.; Yuliana, N.D.; Sugita, P.; Arofah, D.; Syafitri, U.D.; Windarsih, A.; Rohman, A.; Bakar, N.K.A.; Kusnandar, F. Volatile Compounds, Texture, and Color Characterization of Meatballs Made from Beef, Rat, Wild Boar, and Their Mixtures. Heliyon 2022, 8, e10882. [Google Scholar] [CrossRef]
- Romero de Ávila, D.M.; Isabel Cambero, M.; Ordóñez, J.A.; de la Hoz, L.; Herrero, A.M. Rheological Behaviour of Commercial Cooked Meat Products Evaluated by Tensile Test and Texture Profile Analysis (TPA). Meat Sci. 2014, 98, 310–315. [Google Scholar] [CrossRef]
- Bardakçı, S.; Secilmis, H. Investigation of The Chemical Content of Rose Oil in Isparta Region by GC-MS and FTIR Spectroscopy Technique. SDU Fac. Arts Sci. J. Sci. 2006, 1, 64–69. [Google Scholar]
- Halkman, K.; Sağdaş, Ö.E. Food Microbiology Applications; Prosigma Printing and Promotion Services: Ankara, Turkey, 2011. [Google Scholar]
- ISO 4833-2:2013; Horizontal Method for the Enumeration of Microorganisms. Part 2: Colony Count at 30 Degrees C by the Surface Plating Technique. International Standard Organization: Geneva, Switzerland, 2013.
- ISO 4833-1:2013; Microbiology of the Food Chain. Horizontal Method for the Enumeration of Microorganisms. Part 1: Colony Count at 30 Degrees C by the Pour Plate Technique. International Standard Organization: Geneva, Switzerland, 2013.
- ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs, Horizontal Method for the Enumeration of Yeasts and Moulds Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. International Standard Organization: Geneva, Switzerland, 2008.
- ISO 4832; General Guidance for the Enumeration of Coliforms Colony Count Technique. International Standard Organization: Geneva, Switzerland, 1991.
- Ngamsomchat, A.; Kaewkod, T.; Konkit, M.; Tragoolpua, Y.; Bovonsombut, S.; Chitov, T. Characterisation of Lactobacillus plantarum of Dairy-Product Origin for Probiotic Chèvre Cheese Production. Foods 2022, 11, 934. [Google Scholar] [CrossRef]
- Vinderola, C.G.; Bailo, N.; Reinheimer, J.A. Survival of Probiotic Microflora in Argentinian Yogurts during Refrigerated Storage. Food Res. Int. 2000, 33, 97–102. [Google Scholar] [CrossRef]
- Mena, B.; Aryana, K.L. Influence of Ethanol on Probiotic and Culture Bacteria Lactobacillus bulgaricus and Streptococcus thermophilus within a Therapeutic Product. Open J. Med. Microbiol. 2012, 2, 70–76. [Google Scholar] [CrossRef]
- Ercan, M.; Akbulut, M.; Çoklar, H.; Demirci, T. Impacts of Sonication on Fermentation Process and Physicochemical, Microbiological and Sensorial Characteristics of Fermented Black Carrot Juice. Fermentation 2025, 11, 475. [Google Scholar] [CrossRef]
- ISO 8589; Sensory Analysis—General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2007.
- ISO 11132; Sensory Analysis—Methodology—Guidelines for Monitoring the Performance of a Quantitative Sensory Panel. ISO: Geneva, Switzerland, 2012.
- Yuliana, N.; Koesoemawardani, D.; Susilawaty, S.; Kurniati, Y. Lactic Acid Bacteria During Fish Fermentation (Rusip). MOJ Food Process. Technol. 2018, 6, 211–216. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, E.; Hong, G. Effects of Temperature and Time on The Cookery Properties of Sous-Vide Processed Pork Loin. Food Sci. Anim. Resour. 2019, 39, 65–72. [Google Scholar] [CrossRef]
- Kalkan, S.; Incekara, K.; Otağ, M.R.; Unal Turhan, E. The Influence of Hazelnut Milk Fortification on Quality Attributes of Probiotic Yogurt. Food Sci. Nutr. 2025, 13, e70235. [Google Scholar] [CrossRef]
- Liang, X.; Zhou, N.; Irfan, M.; Gerard, M.; Wang, Y.; Wang, F. Synergistic Effect of Protease and Cranberry Powder to Enhance the Quality Characteristics of Fried Beef Meatballs. Food Bioeng. 2023, 2, 64–74. [Google Scholar] [CrossRef]
- Dong, P.; Xiao, L.; Fan, W.; Yang, H.; Xu, C.; Qiao, M.; Zhu, K.; Wu, H.; Deng, J. Effect of Fat Replacement by Flaxseed Flour on The Quality Parameters of Pork Meatballs. Food Sci. Technol. Int. 2023, 31, 48–58. [Google Scholar] [CrossRef]
- Ceraulo, M.; Mantia, F.P.L.; Mistretta, M.C.; Titone, V. The Use of Waste Hazelnut Shells as a Reinforcement in The Development of Green Biocomposites. Polymers 2022, 14, 2151. [Google Scholar] [CrossRef]
- Ergezer, H.; Akcan, T.; Serdaroğlu, M. The Effects of Potato Puree and Bread Crumbs on Some Quality Characteristics of Low Fat Meatballs. Korean J. Food Sci. Anim. Resour. 2014, 34, 561–569. [Google Scholar] [CrossRef]
- Su, W.; Zhao, S.; Zhou, J.; Xi, L.; Jin, W.; Abd El-Aty, A.M. Comparative Analysis of The Quality Characteristics and Flavor Volatiles of Lueyang Black-Bone Chicken Meatballs Cooked Via Different Methods. Front. Nutr. 2025, 12, 1629738. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Han, J.; Wang, D.; Gao, F.; Zhang, K.; Tian, J.; Jin, Y. Research Update on the İmpact of Lactic Acid Bacteria on The Substance Metabolism, Flavor, and Quality Characteristics of Fermented Meat Products. Foods 2022, 11, 2090. [Google Scholar] [CrossRef]
- Todorov, S.D.; Stojanovski, S.; Iliev, I.; Moncheva, P.; Nero, L.A.; Ivanova, I.V. Technology and Safety Assessment for Lactic Acid Bacteria Isolated from Traditional Bulgarian Fermented Meat Product “Lukanka”. Braz. J. Microbiol. 2017, 48, 576–586. [Google Scholar] [CrossRef]
- Li, L.; Wen, X.; Wen, Z.; Chen, S.; Wang, L.; Wei, X. Evaluation of The Biogenic Amines Formation and Degradation Abilities of Lactobacillus curvatus from Chinese Bacon. Front. Microbiol. 2018, 9, 1015. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Feng, M.; Sun, J.; Xu, X.; Zhou, G. Screening of Lactic Acid Bacteria with High Protease Activity from Fermented Sausages and Antioxidant Activity Assessment of Its Fermented Sausages. CyTA J. Food 2019, 17, 347–354. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Lee, S.; Ham, S.H.; Lee, M.G.; Hahn, J.; Kim, Y.; Choi, Y.J. Relationship Between Sensory Attributes and Instrumental Texture Properties in Meat Analog Patty System Substituted with Sweet Potato Stem. J. Sci. Food Agric. 2024, 104, 7002–7012. [Google Scholar] [CrossRef]
- Díaz-Vela, J.; Totosaus, A.; Pérez-Chabela, M.L. Integration of Agroindustrial Co-Products as Functional Food Ingredients: Cactus Pear (Opuntia ficus indica) Flour and Pineapple (Ananas comosus) Peel Flour as Fiber source in Cooked Sausages Inoculated with Lactic Acid Bacteria. J. Food Process. Preserv. 2015, 39, 2630–2638. [Google Scholar] [CrossRef]
- Campêlo, M.C.D.S.; Medeiros, J.M.S.D.; Rebouças, M.D.O.; Pereira, J.C.D.S.; Abrantes, M.R.; Oliveira, A.R.M.D.; Lima, P.D.O.; Silva, J.B.A.D. Use of Natural Preservatives in Low Sodium Carne-De-Sol Beef. J. Food Saf. 2017, 37, e12347. [Google Scholar] [CrossRef]
- Yıldız, E.; Demirkesen, İ.; Mert, B. High Pressure Microfluidization of Agro By-Product to Functionalized Dietary Fiber and Evaluation as a Novel Bakery Ingredient. J. Food Qual. 2016, 39, 599–610. [Google Scholar] [CrossRef]
- Durmus, Y.; Anil, M.; Simsek, S. Innovative Use of Hazelnut Skin and Starch Modifications in Sourdough Bread Formulation. J. Food Process Eng. 2024, 47, e14517. [Google Scholar] [CrossRef]
- Suryadi, U.; Hertamawati, R.T.; Imam, S. Fermented Meat and Digestive Tract of Snail as Amino Acid Supplements for Functional Feed of Native Chickens. IOP Conf. Ser. Earth Environ. Sci. 2022, 980, 012020. [Google Scholar] [CrossRef]
- Zhang, N.; Shi, Z.; Hu, Y.; Sun, Y.; Zhou, C.; Xia, Q.; He, J.; Yan, H.; Yu, H.; Pan, D. Effect of Pulsed Electric Field Pretreatment on the Texture and Flavor of Air-Dried Duck Meat. Foods 2025, 14, 1891. [Google Scholar] [CrossRef]
- Choe, J.; Kim, G.; Kim, H. Effects of Green Tea Leaf, Lotus Leaf, and Kimchi Powders on Quality Characteristics of Chicken Liver Sausages. J. Anim. Sci. Technol. 2019, 61, 28–34. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, T.K.; Kim, Y.B.; Sung, J.M.; Jang, Y.; Shim, J.Y.; Han, S.G.; Choi, Y.S. Effect of The Duck Skin on Quality Characteristics of Duck Hams. Korean J. Food Sci. Anim. Resour. 2017, 37, 360. [Google Scholar] [CrossRef]
- Atalar, İ.; Gül, O.; Kurt, A.; Saricaoğlu, F.T.; Gençcelep, H. Effect of Cold-Pressed Hazelnut Cake Incorporation on The Quality Characteristic of Meat Emulsion System and Its Potential Application for Frankfurter-Type Beef Sausages. Food Sci. Technol. Int. 2023, 30, 329–339. [Google Scholar] [CrossRef]
- Bağdatli, A.; Kundakci, A. Optimization of Compositional and Structural Properties in Probiotic Sausage Production. J. Food Sci. Technol. 2016, 53, 1679–1689. [Google Scholar] [CrossRef]
- Alalwan, T.A.; Mohammed, D.; Hasan, M.; Sergi, D.; Ferraris, C.; Gasparri, C.; Rondanelli, M.; Perna, S. Almond, Hazelnut, and Pistachio Skin: An Opportunity for Nutraceuticals. Nutraceuticals 2022, 2, 300–310. [Google Scholar] [CrossRef]
- Yeung, Y.K.; Lee, Y.K.; Chang, Y.H. Physicochemical, Microbial, and Rheological Properties of Yogurt Substituted with Pectic Polysaccharide Extracted from Ulmus Davidiana. J. Food Process. Preserv. 2019, 43, e13907. [Google Scholar] [CrossRef]
- Detti, C.; Nascimento, L.B.D.S.; Gori, A.; Vanti, G.; Amato, G.; Nazzaro, F.; Ferrini, F.; Centritto, M.; Bilia, A.R.; Brunetti, C. Addition of Polyphenolic Extracts of Myrtus communis and Arbutus unedo Fruits to Whey: Valorization of a Common Dairy Waste Product as a Functional Food. J. Sci. Food Agric. 2025, 105, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Karamac, M.; Kosinska, A.; Rybarczyk, A.; Shahidi, F.; Amarowicz, R. Antioxidant Activity of Hazelnut Skin Phenolics. J. Agric. Food Chem. 2009, 57, 4645–4650. [Google Scholar] [CrossRef] [PubMed]
- Del Rio, D.; Calani, L.; Dall’Asta, M.; Brighenti, F. Polyphenolic Composition of Hazelnut Skin. J. Agric. Food Chem. 2011, 59, 9935–9941. [Google Scholar] [CrossRef] [PubMed]
- Costantini, L.; Frangipane, M.T.; Massantini, R.; Garzoli, S.; Merendino, N. Hazelnut Skin Fortification of Dehulled Lentil Chips to Improve Nutritional, Antioxidant, Sensory, and Chemical Properties. Foods 2025, 14, 683. [Google Scholar] [CrossRef]
- Lee, C.W.; Kim, T.K.; Hwang, K.E.; Kim, H.W.; Kim, Y.B.; Kim, C.J.; Cho, Y.S. Combined Effects of Wheat Sprout and Isolated Soy Protein on Quality Properties of Breakfast Sausage. Korean J. Food Sci. Anim. Resour. 2017, 37, 52. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).