Exploring Static Biological Aging as a Method for Producing Low-Alcohol ‘Fino’ Type White Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Wineries and Wine Samples
2.2. Chemical Analysis
2.3. Volatile Compounds Quantification
2.4. Flor Veil Samples and Microbiological Analysis
2.5. Chemicals and Reagents
2.6. Organoleptic Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Oenological Parameters
3.2. Major Volatile Compounds and Polyols
3.2.1. Principal Component Analysis of Major Volatile and Polyols
3.2.2. Footprints of Major Volatile Compounds and Polyols
3.3. Minor Volatile Compounds
3.3.1. PCA of Minor Volatile Compounds
3.3.2. Footprints from the Chemical Families of Minor Volatiles
3.4. Flor Veil Composition in Saccharomyces and Non-Saccharomyces Yeasts
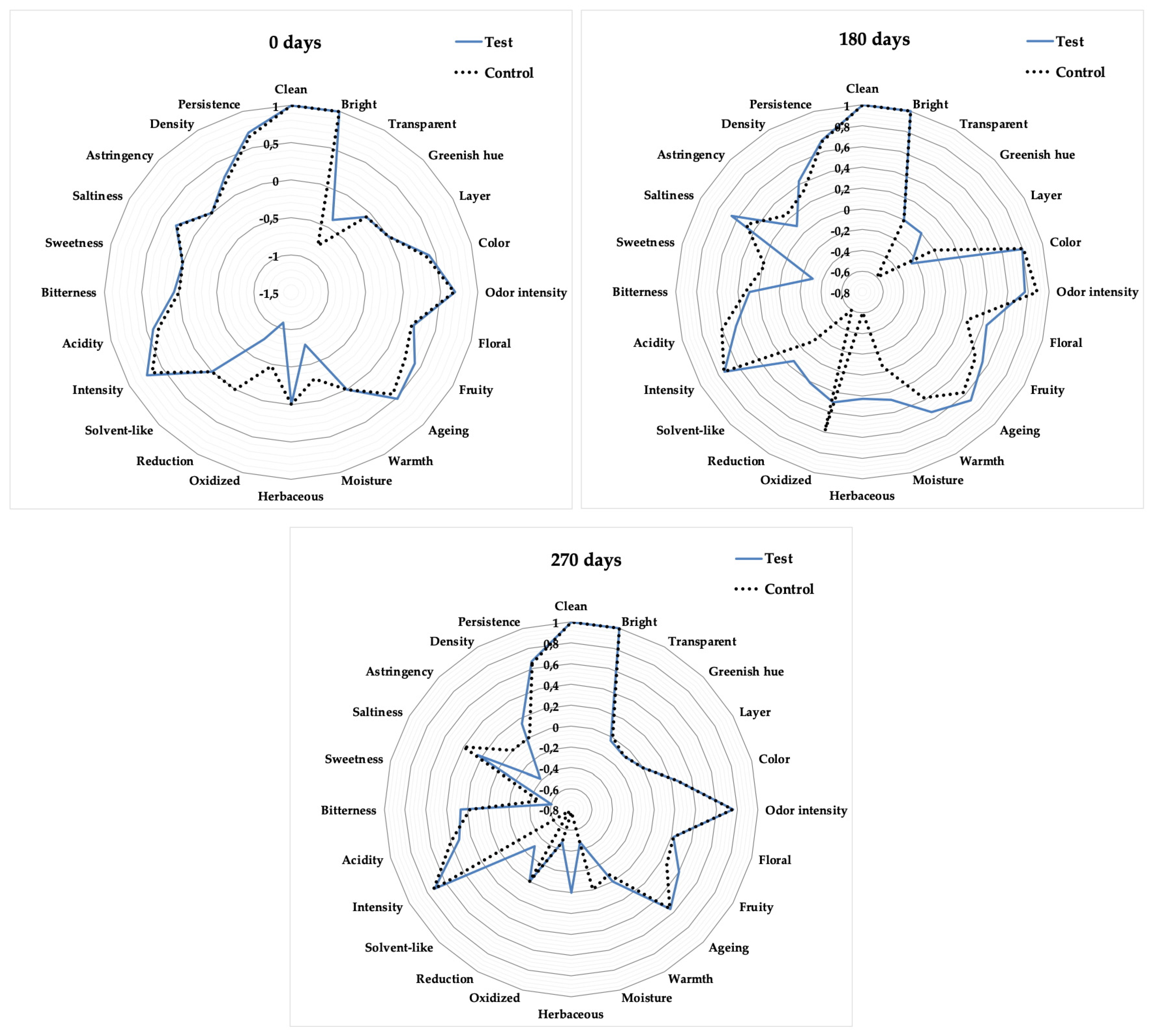
3.5. Organoleptic Profile Changes During Aging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADH2 | Alcohol Dehydrogenase II |
| ADHI | Alcohol Dehydrogenase I |
| ANOVA | Analysis Of Variance |
| CAS | Chemical Abstract Service |
| FID | Flame Ionization Detector |
| GC | Gas Chromatography |
| LSD | Least Significant Difference |
| MALDI | Matrix-Assisted Laser Desorption/Ionization |
| MPS | Multi-Purpose Sampler |
| MS | Mass Spectrometry |
| MVA | Multivariate Analysis |
| NAD+ | Nicotinamide Adenine Dinucleotide |
| OIV | International Organization of Vine and Wine |
| PC | Principal Component |
| PCA | Principal Component Analysis |
| PDMS | Polydimethylsiloxane |
| PDO | Protected Designation of Origin |
| SBSE | Stir Bar Sorptive Extraction |
| SCAI | Central Research Support Service |
| TDU | Thermal Desorption Unit |
| TOF | Time-Of-Flight |
| TPI | Total Polyphenol Index |
References
- International Organisation of Vine and Wine (OIV). Compendium of International Methods of Wine and Must Analysis. 2025, Volume 1. Available online: https://www.oiv.int (accessed on 28 July 2025).
- Esteve-Zarzoso, B.; Peris-Torán, M.J.; García-Maiquez, E.; Uruburu, F.; Querol, A. Yeast population dynamics during the fermentation and biological aging of sherry wines. Appl. Environ. Microbiol. 2001, 67, 2056–2061. [Google Scholar] [CrossRef]
- Legras, J.L.; Moreno-Garcia, J.; Zara, S.; Zara, G.; Garcia-Martinez, T.; Mauricio, J.C.; Mannazzu, I.; Coi, A.L.; Bou Zeidan, M.; Dequin, S.; et al. Flor Yeast: New Perspectives Beyond Wine Aging. Front. Microbiol. 2016, 7, 503. [Google Scholar] [CrossRef]
- Morales, M.L.; Ochoa, M.; Valdivia, M.; Ubeda, C.; Romero-Sánchez, S.; Ibeas, J.I.; Valero, E. Volatile metabolites produced by different flor yeast strains during wine biological aging. Food Res. Int. 2020, 128, 108771. [Google Scholar] [CrossRef] [PubMed]
- Carbonero-Pacheco, J.; García-Jiménez, Á.; García-García, J.C.; Santos-Dueñas, I.M.; García-Martínez, T.; Moreno, J.; Moreno-García, J.; Mauricio, J.C. Use of Wickerhamomyces anomalus Strains from Biologically Aged Wines to Improve the Sensorial Profile of Young White Wines. Appl. Sci. 2025, 15, 1546. [Google Scholar] [CrossRef]
- Benítez, T.; Rincón, A.M.; Codón, A.C. Yeasts associated with biological aging of fortified wines. In Yeasts in the Production of Wine; Romano, P., Ciani, M., Eds.; Springer: New York, NY, USA, 2019; pp. 433–460. [Google Scholar] [CrossRef]
- Durán-Guerrero, E.; Castro, R.; García-Moreno, M.d.V.; Rodríguez-Dodero, M.d.C.; Schwarz, M.; Guillén-Sánchez, D. Aroma of sherry products: A review. Foods 2021, 10, 753. [Google Scholar] [CrossRef] [PubMed]
- Peinado, R.A.; Mauricio, J.C. Biologically aged wines. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer Science+Business Media, LLC: New York, NY, USA, 2009; pp. 81–101. [Google Scholar] [CrossRef]
- Ruiz-Muñoz, M.; Hernández-Fernández, M.; Cordero-Bueso, G.; Martínez-Verdugo, S.; Pérez, F.; Cantoral, J.M. Non-Saccharomyces are also forming the veil of flor in sherry wines. Fermentation 2022, 8, 456. [Google Scholar] [CrossRef]
- Protected Designation of Origin “Jerez-Xérès-Sherry” Home Page. Available online: https://www.sherry.wine/sherry-wine/production/ageing (accessed on 28 July 2025).
- Protected Designation of Origin “Montilla-Moriles” Home Page. Available online: https://www.montillamoriles.es/territorio/la-crianza/ (accessed on 28 July 2025).
- The science of flor yeast. Available online: https://daily.sevenfifty.com/the-science-of-flor/ (accessed on 28 July 2025).
- Cordero-Bueso, G.; Ruiz-Muñoz, M.; González-Moreno, M.; Chirino, S.; Bernal-Grande, M.D.C.; Cantoral, J.M. The Microbial Diversity of Sherry Wines. Fermentation 2018, 4, 19. [Google Scholar] [CrossRef]
- Avdanina, D.; Zghun, A. Sherry Wines: Worldwide Production, Chemical Composition and Screening Conception for Flor Yeasts. Fermentation 2022, 8, 381. [Google Scholar] [CrossRef]
- Moreno, J.; Moreno-García, J.; López-Muñoz, B.; Mauricio, J.C.; García-Martínez, T. Use of a flor velum yeast for modulating colour, ethanol and major aroma compound contents in red wine. Food Chem. 2016, 213, 90–97. [Google Scholar] [CrossRef]
- Valcárcel-Muñoz, M.J.; Guerrero-Chanivet, M.; Rodríguez-Dodero, M.d.C.; García-Moreno, M.d.V.; Guillén-Sánchez, D.A. Analytical and Chemometric Characterization of Fino and Amontillado Sherries during Aging in Criaderas y Solera System. Molecules 2022, 27, 365. [Google Scholar] [CrossRef]
- Silva, P. Low-Alcohol and Nonalcoholic Wines: From Production to Cardiovascular Health, along with Their Economic Effects. Beverages 2024, 10, 49. [Google Scholar] [CrossRef]
- Palenzuela, M.d.V.; López de Lerma, N.; Sánchez-Suárez, F.; Martínez-García, R.; Peinado, R.A.; Rosal, A. Aroma Composition of Wines Produced from Grapes Treated with Organic Amendments. Appl. Sci. 2023, 13, 8001. [Google Scholar] [CrossRef]
- Alcalá-Jiménez, M.T.; García-García, J.C.; Mauricio, J.C.; Moreno, J.; Peinado, R.; García-Martínez, T. Selection of non-Saccharomyces yeasts from extreme oenological environments for potential use in winemaking. Microorganisms 2025, 13, 1260. [Google Scholar] [CrossRef]
- ISO 3591:1977; Sensory Analysis—Apparatus—Wine-Tasting Glass. International Organization for Standardization: Geneva, Switzerland, 1977.
- Marín-Menguiano, M.; Romero-Sánchez, S.; Barrales, R.R.; Ibeas, J.I. Population analysis of biofilm yeasts during fino sherry wine aging in the Montilla-Moriles, D.O. region. Int. J. Food Microbiol. 2017, 244, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Moreno-García, J.; Raposo, R.M.; Moreno, J. Biological aging status characterization of Sherry type wines using statistical and oenological criteria. Food Res. Int. 2013, 54, 285–292. [Google Scholar] [CrossRef]
- Roldán, A.M.; Sánchez-García, F.; Pérez-Rodríguez, L.; Palacios, V.M. Influence of Different Vinification Techniques on Volatile Compounds and the Aromatic Profile of Palomino Fino Wines. Foods 2021, 10, 453. [Google Scholar] [CrossRef]
- Guerrero-Chanivet, M.; García-Moreno, M.V.; Valcárcel-Muñoz, M.J.; Guillén-Sánchez, D.A. Determining the impact of seasoning on the volatile chemical composition of the oak wood of different Sherry Casks® by DTD–GC–MS. Wood Sci. Technol. 2023, 57, 861–878. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.A.; Moreno-Arribas, M.V. Chapter 2—Sherry wines. Adv. Food Nutr. Res. 2011, 63, 17–40. [Google Scholar] [CrossRef]
- Genovese, A.; Caporaso, N.; Moio, L. Influence of yeast strain on odor-active compounds in Fiano wine. Appl. Sci. 2021, 11, 7767. [Google Scholar] [CrossRef]
- Zhang, X.K.; Liu, P.T.; Zheng, X.W.; Li, Z.F.; Sun, J.P.; Fan, J.S.; Ding, Z.Y. The role of indigenous yeasts in shaping the chemical and sensory profiles of wine: Effects of different strains and varieties. Molecules 2024, 29, 4279. [Google Scholar] [CrossRef]
- Carbonero-Pacheco, J.; Rey, M.D.; Moreno-García, J.; Moreno, J.; García-Martínez, T.; Mauricio, J.C. Microbial diversity in sherry wine biofilms and surrounding mites. Food Microbiol. 2023, 116, 104366. [Google Scholar] [CrossRef]
- Marín, J.; Ocete, R.; Pedroza, M.; Zalacain, A.; de Miguel, C.; López, M.A.; Salinas, M.R. Influence of the mite Carpoglyphus lactis (L) on the aroma of pale and dry wines aged under flor yeasts. J. Food Compos. Anal. 2009, 22, 745–750. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, G.J.; Wang, X.J.; Kong, C.L.; Liu, J.; Tao, Y.S. Chemical Profiles and Aroma Contribution of Terpene Compounds in Meili (Vitis vinifera L.) Grape and Wine. Food Chem. 2019; 284, 155–161. [Google Scholar] [CrossRef]
- Florido-Barba, A.; Cordero-Bueso, G.; Cantoral, J.M. Alcoholes alternativos para la fortificación: Impacto en el velo de flor y perfil sensorial de vinos finos de la DO Jerez-Xérès-Sherry. Enólogos 2023, 146, 56–67. [Google Scholar]
- Ailer, Š.; Jakabová, S.; Benešová, L.; Ivanova-Petropulos, V. Wine Faults: State of Knowledge in Reductive Aromas, Oxidation and Atypical Aging, Prevention, and Correction Methods. Molecules 2022, 27, 3535. [Google Scholar] [CrossRef]
- Ailer, Š.; Valšíková, M.; Jedlička, J.; Mankovecký, J.; Baron, M. Influence of Sugar and Ethanol Content and Color of Wines on the Sensory Evaluation: From Wine Competition “Nemčiňany Wine Days” in Slovak Republic (2013–2016). Erwerbs-Obstbau 2020, 62, 9–16. [Google Scholar] [CrossRef]
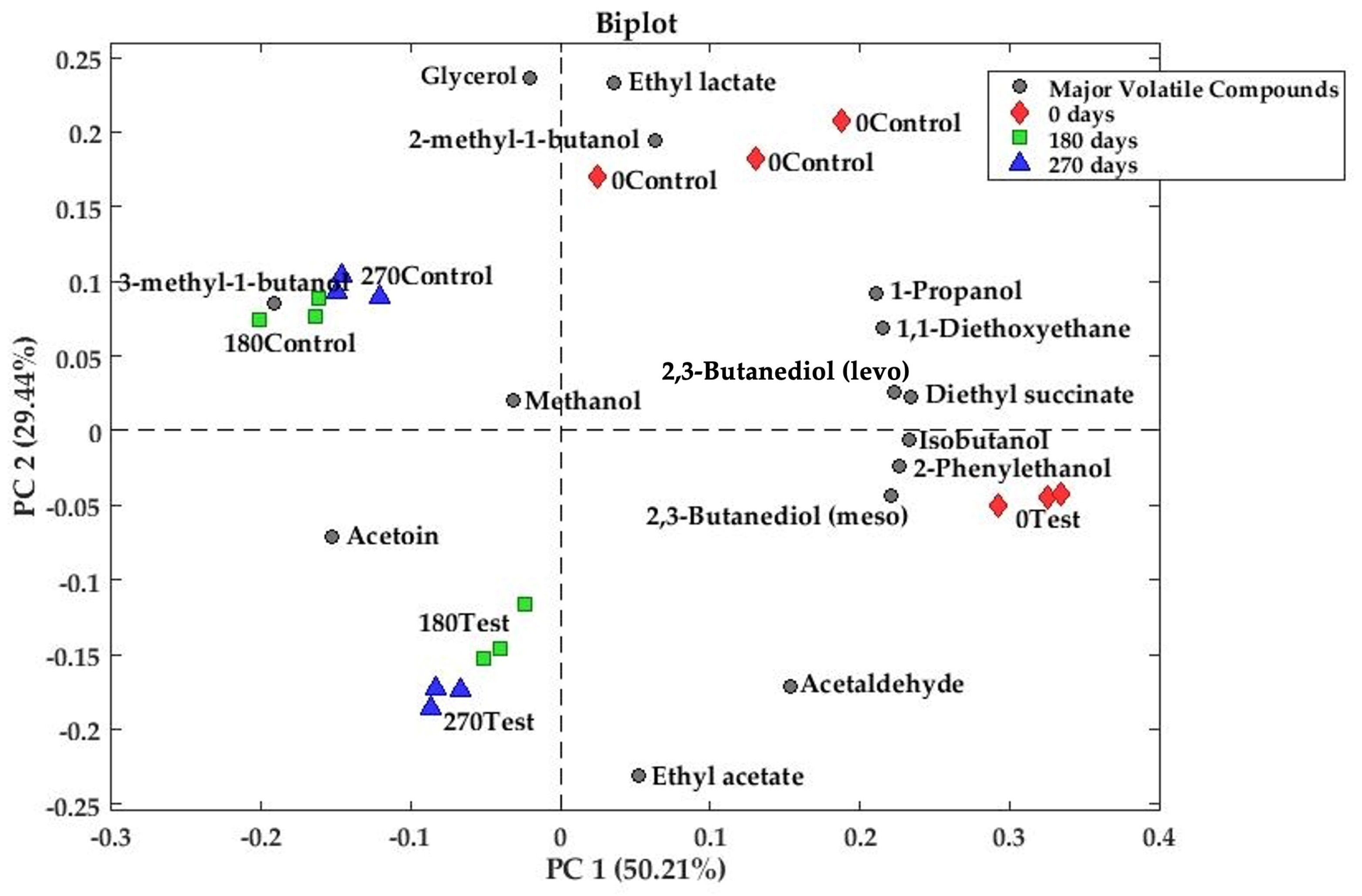

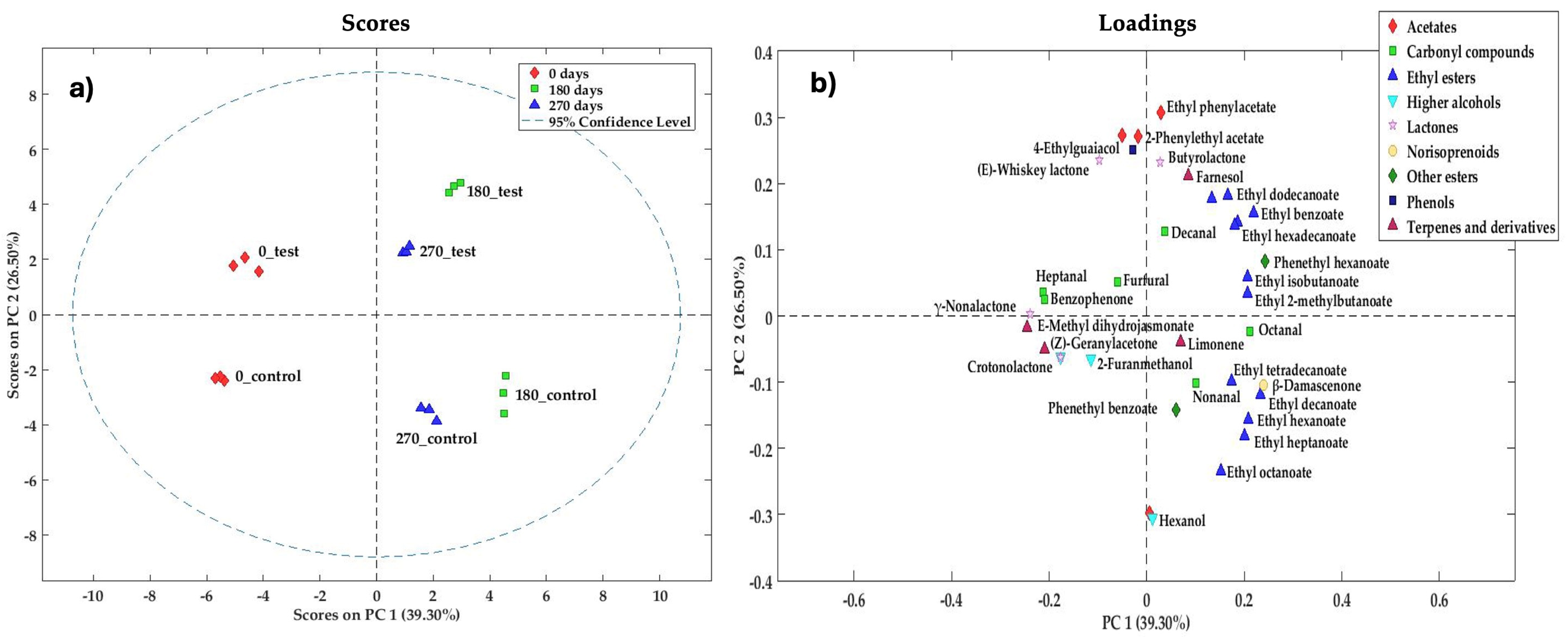
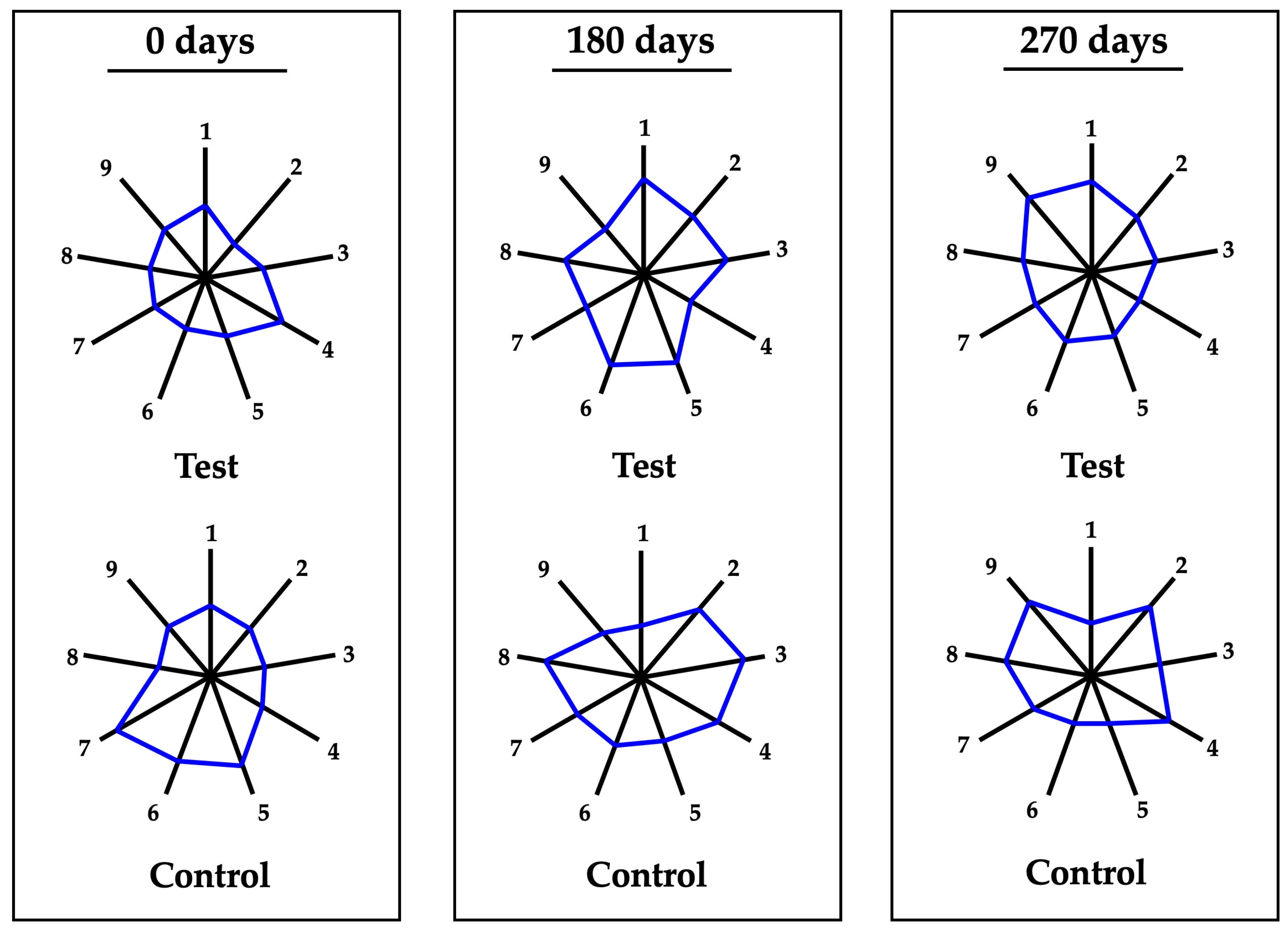
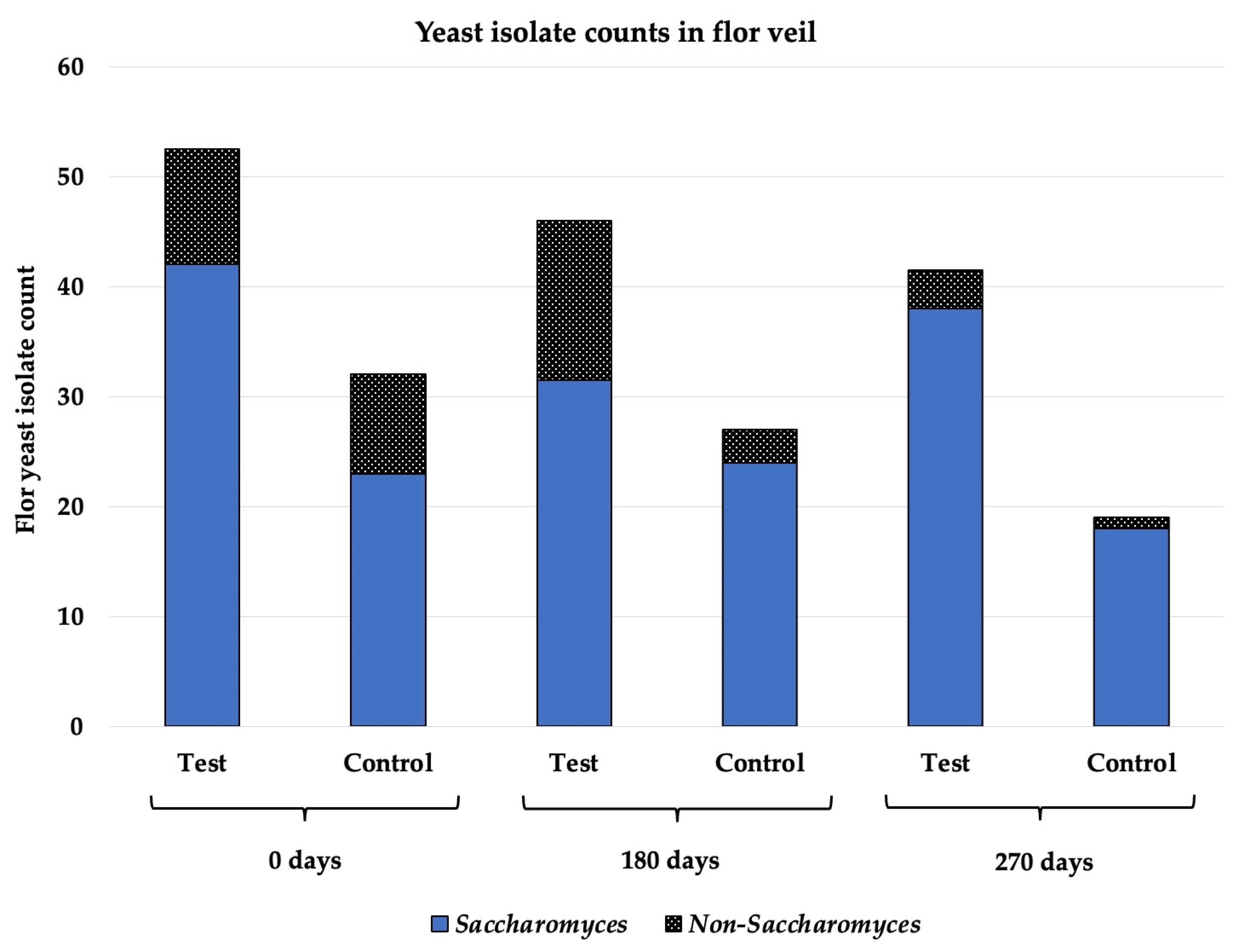
| 0test | 0control | p-Value | 180test | 180control | p-Value | 270test | 270control | p-Value | |
|---|---|---|---|---|---|---|---|---|---|
| pH | 3.47 ± 0.00 | 3.46 ± 0.00 | 0.0161 | 3.13 ± 0.01 | 3.04 ± 0.01 | 0.0000 | 3.33 ± 0.00 | 3.26 ± 0.01 | 0.0000 |
| Volatile acidity (g L−1) | 0.26 ± 0.00 | 0.31 ± 0.00 | 0.0000 | 0.20 ± 0.01 | 0.36 ± 0.00 | 0.0000 | 0.20 ± 0.00 | 0.66 ± 0.00 | 0.0000 |
| Total acidity (g L−1) | 4.31 ± 0.00 | 4.81 ± 0.00 | 0.0000 | 3.59 ± 0.00 | 5.06 ± 0.00 | 0.0000 | 3.63 ± 0.00 | 5.32 ± 0.04 | 0.0000 |
| Ethanol (% v/v) | 13.7 ± 0.1 | 15.7 ± 0.1 | 0.0000 | 13.9 ± 0.1 | 15.0 ± 0.1 | 0.0000 | 13.4 ± 0.1 | 15.1 ± 0.1 | 0.0000 |
| Absorbance 420 nm | 0.159 ± 0.001 | 0.178 ± 0.002 | 0.0001 | 0.186 ± 0.001 | 0.2264 ± 0.0004 | 0.0000 | 0.195 ± 0.002 | 0.230 ± 0.001 | 0.0000 |
| Absorbance 280 nm (TPI) | 8.6 ± 0.1 | 8.18 ± 0.09 | 0.0000 | 14.3 ± 0.4 | 10.28 ± 0.03 | 0.0001 | 11.6 ± 0.2 | 10.0 ± 0.3 | 0.0017 |
| 0 Days | 180 Days | 270 Days | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | CAS | Test | Control | p-Value | Test | Control | p-Value | Test | Control | p-Value |
| Acetaldehyde | 75-07-0 | 397 ± 7 | 283 ± 47 | 0.0138 | 340 ± 10 | 195 ± 13 | 0.0001 | 393 ± 6 | 213 ± 14 | 0.0000 |
| Ethyl acetate | 141-78-6 | 132 ± 4 | 67 ± 2 | 0.0000 | 160 ± 2 | 61 ± 2 | 0.0000 | 159 ± 5 | 80 ± 6 | 0.0001 |
| 1,1-Diethoxyethane | 105-57-7 | 14 ± 1 | 14 ± 2 | 0.8076 | 2.9 ± 0.4 | 2.1 ± 0.5 | 0.1110 | 3.7 ± 0.2 | 1.8 ± 0.5 | 0.0036 |
| Methanol | 67-56-1 | 79 ± 9 | 60 ± 21 | 0.2212 | 77 ± 2 | 77 ± 4 | 0.9204 | 58 ± 2 | 86 ± 2 | 0.0001 |
| 1-Propanol | 71-23-8 | 67 ± 3 | 65 ± 4 | 0.5054 | 54 ± 3 | 50 ± 1 | 0.0975 | 51 ± 1 | 56.8 ± 0.9 | 0.0015 |
| Isobutanol | 78-83-1 | 61 ± 1 | 56 ± 2 | 0.0202 | 52 ± 2 | 47 ± 1 | 0.0118 | 51.5 ± 0.6 | 50.5 ± 0.6 | 0.0949 |
| 2-Methyl-1-butanol | 137-32-6 | 45 ± 0,7 | 54 ± 1 | 0.0003 | 45 ± 2 | 44.4 ± 0.3 | 0.8822 | 43.0 ± 0.9 | 48.28 ± 0.03 | 0.0006 |
| 3-Methyl-1-butanol | 123-51-3 | 260 ± 5 | 298 ± 9 | 0.0037 | 302 ± 11 | 310 ± 6 | 0.3479 | 295 ± 3 | 335 ± 3 | 0.0001 |
| Acetoin | 513-86-0 | 36 ± 1 | 26 ± 10 | 0.2079 | 149.7 ± 0.7 | 72 ± 4 | 0.0000 | 149 ± 8 | 224 ± 12 | 0.0008 |
| Ethyl lactate | 97-64-3 | 265 ± 12 | 431 ± 83 | 0.0269 | 147 ± 3 | 308 ± 14 | 0.0000 | 125 ± 2 | 371 ± 12 | 0.0000 |
| 2,3-Butanediol (levo) | 24347-58-8 | 1494 ± 41 | 1205 ± 347 | 0.2239 | 1020 ± 10 | 772 ± 60 | 0.0021 | 845 ± 38 | 957 ± 58 | 0.0489 |
| 2,3-Butanediol (meso) | 5341-95-7 | 512 ± 18 | 318 ± 69 | 0.0092 | 337 ± 8 | 254 ± 18 | 0.0017 | 286 ± 7 | 296 ± 20 | 0.4930 |
| Diethyl succinate | 123-25-1 | 95 ± 7 | 51 ± 9 | 0.0025 | 26.6 ± 0.6 | 19 ± 2 | 0.0012 | 17.1 ± 0.7 | 21.6 ± 0.6 | 0.0010 |
| 2-Phenylethanol | 60-12-8 | 71 ± 5 | 52 ± 14 | 0.0870 | 50 ± 1 | 34 ± 3 | 0.0012 | 42 ± 2 | 43 ± 3 | 0.6427 |
| Glycerol | 56-81-5 | 2015 ± 50 | 4416 ± 415 | 0.0006 | 526 ± 22 | 4225 ± 245 | 0.0000 | 496 ± 11 | 3704 ± 76 | 0.0000 |
| 0 Days | 180 Days | 270 Days | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compounds | CAS | Test | Control | p-Value | Test | Control | p-Value | Test | Control | p-Value |
| Acetates (4) | ||||||||||
| Butyl acetate | 123-86-4 | 14.1 ± 0.6 | 6 ± 1 | 0.0002 | 4.4 ± 0.5 | 14 ± 1 | 0.0004 | 4.3 ± 0.2 | 14.1 ± 0.4 | 0.0000 |
| Isoamyl acetate | 123-92-2 | 173 ± 11 | 180 ± 8 | 0.4097 | 245 ± 9 | 76 ± 8 | 0.0000 | 269 ± 8 | 87 ± 3 | 0.0000 |
| Ethylphenylacetate | 101-97-3 | 35.2 ± 0.6 | 61 ± 3 | 0.0001 | 99 ± 2 | 31 ± 1 | 0.0000 | 84 ± 5 | 26 ± 1 | 0.0000 |
| 2-Phenylethylacetate | 103-45-7 | 203 ± 3 | 172 ± 2 | 0.0002 | 298 ± 12 | 119 ± 12 | 0.0000 | 252 ± 8 | 104 ± 11 | 0.0001 |
| Ethyl Esters (12) | ||||||||||
| Ethyl isobutyrate | 97-62-1 | 254 ± 8 | 456 ± 16 | 0.0000 | 504 ± 13 | 528 ± 22 | 0.1728 | 529 ± 13 | 544 ± 9 | 0.1722 |
| Ethyl butyrate | 105-54-4 | 140 ± 4 | 253 ± 6 | 0.0000 | 252 ± 6 | 227 ± 10 | 0.0237 | 251 ± 5 | 229 ± 3 | 0.0022 |
| Ethyl 2-methylbutanoate | 7452-79-1 | 59 ± 1 | 122 ± 2 | 0.0000 | 131 ± 3 | 145 ± 5 | 0.0164 | 131 ± 1 | 146 ± 1 | 0.0002 |
| Ethyl 3-methylbutanoate | 108-64-5 | 138 ± 3 | 287 ± 3 | 0.0000 | 324 ± 9 | 301 ± 8 | 0.0287 | 318 ± 6 | 293 ± 2 | 0.0018 |
| Ethyl hexanoate | 123-66-0 | 176 ± 10 | 226 ± 6 | 0.0017 | 320 ± 7 | 529 ± 8 | 0.0000 | 252 ± 2 | 499 ± 5 | 0.0000 |
| Ethyl heptanoate | 106-30-9 | 0.21 ± 0.01 | 0.23 ± 0.01 | 0.0179 | 0.35 ± 0.01 | 1.02 ± 0.03 | 0.0000 | 0.55 ± 0.02 | 0.92 ± 0.01 | 0.0000 |
| Ethyl benzoate | 93-89-0 | 2.25 ± 0.06 | 3.14 ± 0.07 | 0.0001 | 4.33 ± 0.12 | 3.91 ± 0.12 | 0.0131 | 3.8 ± 0.1 | 3.4 ± 0.1 | 0.0144 |
| Ethyl octanoate | 106-32-1 | 0.001 ± 0.000 | 0.001 ± 0.000 | ns | 0.001 ± 0.000 | 225 ± 5 | 0.0000 | 0.001 ± 0.000 | 249 ± 5 | 0.0000 |
| Ethyl decanoate | 110-38-3 | 9.7 ± 0.1 | 9.6 ± 0.9 | 0.9376 | 102 ± 2 | 172 ± 3 | 0.0000 | 59 ± 2 | 155 ± 2 | 0.0000 |
| Ethyl dodecanoate | 106-33-2 | 0.000 ± 0.000 | 0.000 ± 0.000 | ns | 58 ± 2 | 20.9 ± 0.4 | 0.0000 | 63 ± 8 | 23.7 ± 0.6 | 0.0011 |
| Ethyl tetradecanoate | 124-06-1 | 5.6 ± 0.2 | 5.0 ± 0.2 | 0.5844 | 5.7 ± 0.1 | 13.5 ± 0.7 | 0.0001 | 13.0 ± 0.6 | 9.6 ± 0.4 | 0.0014 |
| Ethyl hexadecanoate | 628-97-7 | 3.7 ± 0.5 | 4.2 ± 0.9 | 0.4665 | 24 ± 3 | 18 ± 3 | 0.4846 | 28 ± 2 | 12.7 ± 0.2 | 0.0001 |
| Other esters (2) | ||||||||||
| Phenethyl hexanoate | 101-60-0 | 0.16 ± 0.00 | 0.31 ± 0.02 | 0.0001 | 0.72 ± 0.01 | 0.73 ± 0.05 | 0.5622 | 0.74 ± 0.02 | 0.56 ± 0.01 | 0.0001 |
| Phenethyl benzoate | 94-47-3 | 2.4 ± 0.1 | 2.20 ± 0.08 | 0.1642 | 2.3 ± 0.1 | 2.7 ± 0.1 | 0.0133 | 1.94 ± 0.03 | 2.19 ± 0.01 | 0.0001 |
| Higher alcohols (4) | ||||||||||
| Hexanol | 111-27-3 | 1346 ± 22 | 958 ± 36 | 0.0001 | 831 ± 34 | 1412 ± 44 | 0.0001 | 866 ± 29 | 1403 ± 14 | 0.0000 |
| 2-Ethyl-1-hexanol | 104-76-7 | 0.001 ± 0.000 | 0.001 ± 0.000 | ns | 0.000 ± 0.000 | 0.000 ± 0.000 | ns | 0.001 ± 0.000 | 0.001 ± 0.000 | ns |
| Dodecanol | 112-53-8 | 0.001 ± 0.000 | 0.001 ± 0.000 | ns | 0.000 ± 0.000 | 0.000 ± 0.000 | ns | 0.001 ± 0.000 | 0.001 ± 0.000 | ns |
| 2-Furanmethanol | 98-00-0 | 6.2 ± 0.5 | 12 ± 5 | 0.0975 | 2.1 ± 0.8 | 7 ± 2 | 0.0404 | 5 ± 1 | 6.4 ± 0.6 | 0.1511 |
| Phenols (1) | ||||||||||
| 4-Ethylguaiacol | 2785-89-9 | 443 ± 31 | 787 ± 34 | 0.0002 | 769 ± 51 | 531 ± 15 | 0.0014 | 521 ± 36 | 338 ± 25 | 0.0019 |
| Lactones (4) | ||||||||||
| γ-Butyrolactone | 96-48-0 | 24,137 ± 2022 | 33,606 ± 3532 | 0.0157 | 35,006 ± 1197 | 29,293 ± 5868 | 0.1738 | 29,162 ± 2803 | 23,519 ± 3219 | 0.0839 |
| Crotonolactone | 497-23-4 | 0.001 ± 0.000 | 0.001 ± 0.000 | ns | 0.000 ± 0.000 | 0.000 ± 0.000 | ns | 0.001 ± 0.000 | 0.001 ± 0.000 | ns |
| (E)-Whiskey lactone | 80041-01-6 | 66 ± 7 | 409 ± 7 | 0.0000 | 248 ± 20 | 0.00 ± 0.00 | 0.0000 | 222 ± 13 | 110 ± 8 | 0.0002 |
| γ-Nonalactone | 104-61-0 | 60 ± 3 | 73 ± 3 | 0.0033 | 16 ± 2 | 17 ± 1 | 0.2690 | 15 ± 1 | 13 ± 1 | 0.1517 |
| Carbonyl Compounds (6) | ||||||||||
| Furfural | 98-01-1 | 563 ± 76 | 854 ± 286 | 0.1634 | 597 ± 133 | 644 ± 158 | 0.7183 | 587 ± 65 | 604 ± 73 | 0.7810 |
| Heptanal | 111-71-7 | 0.53 ± 0.08 | 1.2 ± 0.2 | 0.0073 | 0.00 ± 0.00 | 0.00 ± 0.00 | ns | 0.00 ± 0.00 | 0.00 ± 0.00 | ns |
| Octanal | 124-13-0 | 0.90 ± 0.30 | 1.8 ± 0.6 | 0.0813 | 8.2 ± 0.1 | 12.5 ± 0.2 | 0.0000 | 5 ± 2 | 5.4 ± 0.6 | 0.8413 |
| Nonanal | 124-19-6 | 0.84 ± 0.30 | 2.8 ± 0.2 | 0.0974 | 1.1 ± 0.2 | 4.7 ± 0.8 | 0.1878 | 5.0 ± 0.6 | 5.5 ± 0.2 | 0.7020 |
| Decanal | 112-31-2 | 2.6 ± 0.7 | 9 ± 3 | 0.0245 | 9.6 ± 0.3 | 5.6 ± 0.9 | 0.5404 | 5.4 ± 0.6 | 6 ± 1 | 0.8490 |
| Benzophenone | 119-61-9 | 0.36 ± 0.07 | 0.7 ± 0.03 | 0.1583 | 0.00 ± 0.00 | 0.00 ± 0.00 | ns | 0.001 ± 0.000 | 0.001 ± 0.000 | 0.3739 |
| Norisoprenoids (1) | ||||||||||
| β-Damascenone | 23726-93-4 | 1.92± 0.2 | 1.5 ± 0.3 | 0.0860 | 4.8 ± 0.2 | 7.3 ± 0.2 | 0.0001 | 3.5 ± 0.2 | 5.75 ± 0.08 | 0.0000 |
| Terpenes and derivatives (4) | ||||||||||
| Limonene | 5989-27-5 | 0.001 ± 0.000 | 0.000 ± 0.000 | ns | 0.000 ± 0.000 | 0.000 ± 0.000 | ns | 160 ± 4 | 154 ± 7 | 0.2532 |
| (Z)-Geranylacetone | 689-67-8 | 1.9 ± 0.1 | 2.19 ± 0.07 | 0.0191 | 0.00 ± 0.00 | 0.00 ± 0.00 | ns | 1.57 ± 0.06 | 1.61 ± 0.03 | 0.3166 |
| Farnesol | 4602-84-0 | 0.001 ± 0.000 | 0.001 ± 0.000 | ns | 6.6 ± 0.2 | 0.00 ± 0.00 | 0.0000 | 0.001 ± 0.000 | 0.001 ± 0.000 | ns |
| (E)-Methyl dihydrojasmonate | 24851-98-7 | 21 ± 5 | 22 ± 3 | 0.6662 | 0.00 ± 0.00 | 0.00 ± 0.00 | ns | 0.6 ± 0.4 | 1.0 ± 0.2 | 0.2857 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Castells, R.; Vega-Espinar, L.; García-García, J.C.; Alcalá-Jiménez, M.T.; Moreno-García, J.; Lasanta, C.; Moreno, J. Exploring Static Biological Aging as a Method for Producing Low-Alcohol ‘Fino’ Type White Wines. Fermentation 2025, 11, 575. https://doi.org/10.3390/fermentation11100575
Muñoz-Castells R, Vega-Espinar L, García-García JC, Alcalá-Jiménez MT, Moreno-García J, Lasanta C, Moreno J. Exploring Static Biological Aging as a Method for Producing Low-Alcohol ‘Fino’ Type White Wines. Fermentation. 2025; 11(10):575. https://doi.org/10.3390/fermentation11100575
Chicago/Turabian StyleMuñoz-Castells, Raquel, Lourdes Vega-Espinar, Juan Carlos García-García, Maria Trinidad Alcalá-Jiménez, Jaime Moreno-García, Cristina Lasanta, and Juan Moreno. 2025. "Exploring Static Biological Aging as a Method for Producing Low-Alcohol ‘Fino’ Type White Wines" Fermentation 11, no. 10: 575. https://doi.org/10.3390/fermentation11100575
APA StyleMuñoz-Castells, R., Vega-Espinar, L., García-García, J. C., Alcalá-Jiménez, M. T., Moreno-García, J., Lasanta, C., & Moreno, J. (2025). Exploring Static Biological Aging as a Method for Producing Low-Alcohol ‘Fino’ Type White Wines. Fermentation, 11(10), 575. https://doi.org/10.3390/fermentation11100575







