Advances in Algae-Based Bioplastics: From Strain Engineering and Fermentation to Commercialization and Sustainability
Abstract
1. Introduction
1.1. Plastic Pollution and the Rise in Bioplastics
1.2. Limitations of Starch and Corn-Based Bioplastics
1.3. Algae as a Carbon-Neutral, Fast-Growing, Nutrient-Rich Feedstock
1.4. Scope and Novelty of the Review
2. Methodology
2.1. Literature Search Strategy
2.2. Data Extraction and Classification
- Bioplastic type: PHA, PLA, nanocellulose, or blended composites.
- Production pathway: direct synthesis, fermentation, or chemical conversion.
- Scale: laboratory, pilot, or commercial.
2.3. Comparative Analysis
- Production cost (USD/kg).
- Greenhouse gas emissions (kg CO2-eq/kg).
- Water consumption (L/kg).
- Biodegradation rate (% mass loss over a defined period).
2.4. Technology Readiness Level (TRL) Assessment
2.5. Quality Assurance and Limitations
2.6. Scope Clarification
3. Types of Algal Bioplastics
3.1. Polyhydroxyalkanoates (PHAs)
3.1.1. Direct PHA Production in Algal and Cyanobacterial Systems
3.1.2. Algal Biomass as Feedstock for Bacterial PHA Production
3.1.3. Genetic Engineering and Future Potential
3.2. Polylactic Acid (PLA)
3.2.1. Algal Biomass as Alternative PLA Feedstock
3.2.2. Performance Characteristics and Environmental Considerations
3.2.3. Research Gaps and Development Priorities
3.3. Algal Nanocellulose
3.4. PLA-Based Blends: Comparative Analysis
Comparative Analysis
3.5. Challenges and Industrial Potential
3.6. Comparative Performance Metrics and Data Limitations
| Parameter | Value | Source/Context | Reference |
|---|---|---|---|
| PHB/PHA Production | |||
| PHB content in Chlorogloea fritschii | 23% DCW | With sodium acetate supplementation | [144] |
| PHB concentration in cyanobacteria | 1.2 g/L | Under optimized saline conditions | [145] |
| PHA content range | 10–20% DCW | Current algal systems (stated in text) | Section 4.1 |
| PHA degradation time | 1.5–3.5 years | Ambient conditions | [48] |
| Lipid/TAG Production | |||
| TAG content in P. tricornutum | 15% → 43% DCW | After TALEN mutagenesis | [146] |
| Grazing loss reduction | 70% | B. braunii engineering | [147] |
| Hydrocarbon yield increase | 35% | B. braunii engineering | [147] |
| Biomass Productivity | |||
| Chlamydomonas reinhardtii | >80 mg/L/day | iMAP platform screening | [148] |
| Carbohydrate Content | |||
| Chlorella vulgaris starch | 12–17% | Section 3.2 | |
| Cladophora cellulose | 20–30% DW | Stated in text | [56] |
| Mechanical Properties | |||
| Algal films tensile strength | 12–25 MPa | From E. cottonii study | [103] |
| PLA tensile strength | 50–65 MPa | Conventional PLA (for comparison) | [53] |
| Nanocellulose tensile strength | ~150 MPa | Laboratory-produced films | [56] |
| Biodegradation | |||
| PLA composting | 60–90 days | Industrial conditions | [56] |
| PHB marine degradation | 46% mass loss in 160 days | At 29 °C | [149] |
| Technology Readiness | |||
| Algal PHA TRL | 5–6 | Current status | [150] |
| Algal PLA TRL | 4–5 | Current status | [150] |
| Economic Data | |||
| Bacterial PHA production cost | $4–6/kg | Not algal, but cited for comparison | Text states |
| Algal biomass production cost | $1.13–2.04/kg | General biomass, not bioplastic | [142,143] |
4. Algal Strain Engineering for Bioplastic Production
4.1. Genetic Modifications for PHA/PLA Precursors
4.2. CRISPR/Cas and Synthetic Biology Tools in Microalgae
4.3. Overexpression of Key Pathways
4.4. Case Studies in Algal Strain Engineering for Bioplastic Production
4.4.1. Enhanced Lipid Production Through Targeted Mutagenesis
4.4.2. Multi-Species Synergistic Engineering for Algal Grazing Protection
4.4.3. Rapid Strain Selection via Integrated Microalgae Analysis Photobioreactor (iMAP)
4.4.4. Novel Bioplastic Formulation from Marine Alga
4.4.5. Polyhydroxybutyrate Production from Microalgae
4.4.6. Advances in Biopolymer Research and Development
5. Fermentative Bioplastic Production Routes
5.1. Heterotrophic Fermentation
5.2. Dark Fermentation
5.3. Algae as Host Organisms for Direct Bioplastic Synthesis
5.4. Comparative Assessment of Fermentative Routes for Bioplastic Production
5.5. Process Optimization Strategies for Fermentative Routes
5.6. Integration into Circular Bioeconomy Frameworks
6. Downstream Processing
6.1. Extraction of PHAs and PLAs from Algal Biomass
6.2. Solvent-Based, Enzymatic, and Mechanical Recovery Approaches
6.3. Purity, Scalability, and Cost-Efficiency
6.4. Waste Valorization Options
7. Industrial Applications and Products of Algal Fermentation in Bioplastics
7.1. Bioplastic Packaging
7.2. Biomedical Applications
7.3. 3D Printing, Films, and Automotive Parts
7.4. Case Studies
7.4.1. Algix
7.4.2. Loliware
7.4.3. Cladophora Bioplastics
8. Techno-Economic Analysis and Life Cycle Assessment
8.1. Cost Comparison: Algae-Based vs. Petroplastic vs. Other Bioplastics
8.2. Key Metrics: GHG Emissions, Land/Water Use, Energy Consumption
8.3. Challenges in Feedstock Availability, Yield, Productivity
8.4. Policy and Market Incentive
| Metric | Algae-Based Bioplastics (TRL 6–7) | Petrochemical Plastics (TRL 9) | Other Bioplastics (TRL 8–9) |
|---|---|---|---|
| Production Cost ($/kg) | $1.30–$3.50 | $0.90–$2.20 | $2.0–$4. |
| GHG Emissions (kg CO2-eq/kg) | 2–3 | 6.0–9.0 | 2.5–4.0 |
| Water Usage (L/kg plastic) | 15–20 | 45–120 | 30–80 |
| Energy Consumption (MJ/kg) | 5–10 | 20–25 | 12–18 |
| Commercial Maturity (TRL) | 6–7 (Pilot to early commercial) | 9 (Fully commercial) | 8–9 (Late-stage commercial) |
8.5. Regional Policy Landscape
9. Challenges and Limitations
9.1. Economic & Technical Barriers
9.2. Biological & Material Stability Challenges
9.3. Policy, Infrastructure & Market Integration
9.4. Environmental Fate & Marine Biodegradability
9.5. Environmental and Biodiversity Considerations
10. Future Prospects and Recommendations
10.1. Integrated Biorefineries and Wastewater Synergies
10.2. Digital Optimization and AI-Driven Strain Development
10.3. Collaborative Ecosystem for Commercialization
11. Conclusions
11.1. Synthesis of Key Findings
11.2. Critical Limitations
- −
- Economic: Current production costs ($4–10/kg) exceed petroplastics.
- −
- Technical: Genetic instability reduces yields by 30–50% over 20 generations.
- −
- Scalability: No facility exceeds 1000 tonnes/year production.
- −
- Regulatory: Absence of international standards for marine biodegradability.
11.3. Prioritized Research Roadmap
- Immediate (1–2 years): Standardize testing protocols and improve genetic stability.
- Short-term (3–5 years): Demonstrate stable pilot-scale production (>100 tonnes/year).
- Medium-term (5–10 years): Achieve cost parity through integrated biorefineries.
- Long-term (10+ years): Establish circular economy infrastructure.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cifuentes, I.E.M.; Werner, J.; Heuer, A.; Jehmlich, N.; Öztürk, B. Synergistic Degradation of a Biodegradable Plastic Film by a Marine Microbial Community. Access Microbiol. 2020, 2. [Google Scholar] [CrossRef]
- Filiciotto, L.; Rothenberg, G. Biodegradable Plastics: Standards, Policies, and Impacts. Chemsuschem 2020, 14, 56–72. [Google Scholar] [CrossRef] [PubMed]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly(glycolic Acid) (PGA): A Versatile Building Block Expanding High Performance and Sustainable Bioplastic Applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Weinstein, J.E.; Dekle, J.L.; Leads, R.R.; Hunter, R.A. Degradation of Bio-Based and Biodegradable Plastics in a Salt Marsh Habitat: Another Potential Source of Microplastics in Coastal Waters. Mar. Pollut. Bull. 2020, 160, 111518. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of Plastics: Current Scenario and Future Prospects for Environmental Safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Wemyss, A.M.; Iacovidou, E.; Wan, C. Design and Control of Compostability in Synthetic Biopolyesters. Acs Sustain. Chem. Eng. 2021, 9, 9151–9164. [Google Scholar] [CrossRef]
- Mercier, A.; Gravouil, K.; Aucher, W.; Brosset-Vincent, S.; Kadri, L.; Colas, J.; Bouchon, D.; Ferreira, T. Fate of Eight Different Polymers Under Uncontrolled Composting Conditions: Relationships Between Deterioration, Biofilm Formation, and the Material Surface Properties. Environ. Sci. Technol. 2017, 51, 1988–1997. [Google Scholar] [CrossRef]
- Ratnawati, R.; Hamidah, N.Y.; Pradana, M.A.; Prasetyaningrum, A. Biodegradable Plastic from Cross-Linked Rice Flour: Effect of Cross-Linking Agent and Plasticizer. Int. J. Appl. Sci. Eng. 2024, 21, 2023061. [Google Scholar] [CrossRef]
- Sowmya, S.; Sayre, R.T. The Right Stuff; Realizing the Potential for Enhanced Biomass Production in Microalgae. Front. Energy Res. 2022, 1, 979747. [Google Scholar] [CrossRef]
- Breuer, F.; Janz, P.; Farrelly, E.; Ebke, P. Seasonality of Algal Communities in Small Streams and Ditches in Temperate Regions Using Delayed Fluorescence. J. Freshw. Ecol. 2016, 31, 393–406. [Google Scholar] [CrossRef][Green Version]
- Quinn, J.C.; Davis, R. The Potentials and Challenges of Algae Based Biofuels: A Review of the Techno-Economic, Life Cycle, and Resource Assessment Modeling. Bioresour. Technol. 2015, 184, 444–452. [Google Scholar] [CrossRef]
- Widyastuti, S.; Utomo, Y.; Firdayanti, A.; Ratnawati, R.; Solikah, U. Bioplastic from Tapioca Starch Waste and Rice Waste. Indones. J. Urban Environ. Technol. 2024, 7, 89–105. [Google Scholar] [CrossRef]
- Saputri, C.A.; Julyatmojo, F.A.; Harmiansyah, H.; Febrina, M.; Mahardika, M.; Maulana, S. Characteristics of Bioplastics Prepared from Cassava Starch Reinforced with Banana Bunch Cellulose at Various Concentrations. IOP Conf. Ser. Earth Environ. Sci. 2024, 1309, 012006. [Google Scholar] [CrossRef]
- Cordeiro, P.N.; Caetano, S.T.; De Carvalho, R.M.M. Production of Bioplastic from Potato Starch. South. Braz. J. Chem. 2019, 27, 30–34. [Google Scholar] [CrossRef]
- Rendón-Villalobos, R.; Lorenzo-Santiago, M.A.; Olvera-Guerra, R.; Trujillo-Hernández, C.A. Bioplastic Composed of Starch and Micro-Cellulose from Waste Mango: Mechanical Properties and Biodegradation. Polímeros 2022, 32, e2022026. [Google Scholar] [CrossRef]
- Gadhave, R.V.; Das, A.; Mahanwar, P.A.; Gadekar, P.T. Starch Based Bio-Plastics: The Future of Sustainable Packaging. Open J. Polym. Chem. 2018, 8, 21–33. [Google Scholar] [CrossRef]
- Chakraborty, I.; Pooja, N.; Banik, S.; Govindaraju, I.; Das, K.; Mal, S.S.; Zhuo, G.Y.; Rather, M.A.; Mandal, M.; Neog, A.; et al. Synthesis and Detailed Characterization of Sustainable Starch-based Bioplastic. J. Appl. Polym. Sci. 2022, 139, e52924. [Google Scholar] [CrossRef]
- Mahreni, M.; Ristianingsih, Y.; Saefudin, A.; Akmal, A.A.; Narullita, A.H. Bioplastic Production from Eucheuma cottoni. RSF Conf. Ser. Eng. Technol. 2021, 1, 661–668. [Google Scholar] [CrossRef]
- Iyer, H.; Grandgeorge, P.; Jimenez, A.M.; Campbell, I.; Parker, M.; Holden, M.; Venkatesh, M.; Nelsen, M.; Nguyen, B.H.; Roumeli, E. Fabricating Strong and Stiff Bioplastics from Whole Spirulina Cells. Adv. Funct. Mater. 2023, 33, 2302067. [Google Scholar] [CrossRef]
- Sriamini, S.; Susilowati, R. Biodiesel Production from Microalgae Botryococcus braunii. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2010, 5, 23. [Google Scholar] [CrossRef][Green Version]
- Soedarmodjo, T.P.; Rachma, F.A.; Aparamarta, H.W.; Widjaja, A. Study of UV-B Mutation Effect on pH Resistance and Lipid Production of Microalgae Botryococcus braunii. IPTEK J. Technol. Sci. 2019, 30, 101. [Google Scholar] [CrossRef]
- Razaghi, A.; Godhe, A.; Albers, E. Effects of nitrogen on growth and carbohydrate formation in Porphyridium cruentum. Open Life Sci. 2014, 9, 156–162. [Google Scholar] [CrossRef]
- Lutzu, G.A.; Zhang, L.; Zhang, Z.; Liu, T. Feasibility of attached cultivation for polysaccharides production by Porphyridium cruentum. Bioprocess Biosyst. Eng. 2017, 40, 73–83. [Google Scholar] [CrossRef]
- Tóth, G.S.; Siitonen, V.; Nikkanen, L.; Sovic, L.; Kallio, P.T.; Kourist, R.; Kosourov, S.; Allahverdiyeva, Y. Photosynthetically Produced Sucrose by Immobilized synechocystis sp. PCC 6803 Drives Biotransformation in E. coli. Biotechnol. Biofuels Bioprod. 2022, 15, 146. [Google Scholar] [CrossRef] [PubMed]
- Solé-Bundó, M.; Eskicioğlu, Ç.; Garfí, M.; Carrere, H.; Ferrer, I. Anaerobic Co-Digestion of Microalgal Biomass and Wheat Straw with and Without Thermo-Alkaline Pretreatment. Bioresour. Technol. 2017, 237, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Raj, R.; Ghangrekar, M.M. Efficient Algal Lipid Extraction via a Green Bio-Electro-Fenton Process and Its Conversion into Biofuel and Bioelectricity with Concurrent Wastewater Treatment in a Photosynthetic Microbial Fuel Cell. Green Chem. 2023, 25, 7166–7182. [Google Scholar] [CrossRef]
- Alcântara, J.M.G.; Distante, F.; Storti, G.; Moscatelli, D.; Morbidelli, M.; Sponchioni, M. Current Trends in the Production of Biodegradable Bioplastics: The Case of Polyhydroxyalkanoates. Biotechnol. Adv. 2020, 42, 107582. [Google Scholar] [CrossRef]
- Koller, M. A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxyalkanoate (PHA) Biopolyesters. Fermentation 2018, 4, 30. [Google Scholar] [CrossRef]
- Sharma, A.; Singholi, A.K.S. Shape Memory and Mechanical Characterization of Polylactic Acid Wood Composite Fabricated by Fused Filament Fabrication 4D Printing Technology. Mater. Werkst. 2021, 52, 635–643. [Google Scholar] [CrossRef]
- Nagarajan, D.; Senthilkumar, G.; Chen, C.W.; Karmegam, N.; Praburaman, L.; Kim, W.; Dong, C.D. Sustainable Bioplastics from Seaweed Polysaccharides: A Comprehensive Review. Polym. Adv. Technol. 2024, 35, e6536. [Google Scholar] [CrossRef]
- Casalini, T.; Bassas-Galià, M.; Girard, H.; Castrovinci, A.; Carolis, A.D.; Brianza, S.; Zinn, M.; Perale, G. A Systematic Experimental and Computational Analysis of Commercially Available Aliphatic Polyesters. Appl. Sci. 2019, 9, 3397. [Google Scholar] [CrossRef]
- NASA. 2020 NASA Technology Taxonomy; National Aeronautics and Space Administration: Washington, DC, USA, 2020. [Google Scholar]
- Koller, M. Biodegradable and Biocompatible Polyhydroxy-Alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef]
- Kourmentza, K.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A. Recent Advances and Challenges Towards Sustainable Polyhydroxyalkanoate (PHA) Production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef]
- da Costa e Silva, P.E.; Michelle, H.L. Obtainment of Polyhydroxyalkanoates (PHAs) from Microalgae Supplemented with Agro-Industry Residue Corn Steep Liquor. J. Bot. Res. 2022, 5, 17. [Google Scholar] [CrossRef]
- Obruča, S. Use of Lignocellulosic Materials for PHA Production. Chem. Biochem. Eng. Q. 2015, 29, 135–144. [Google Scholar] [CrossRef]
- Haase, S.M.; Huchzermeyer, B.; Rath, T. PHB Accumulation in Nostoc muscorum Under Different Carbon Stress Situations. J. Appl. Phycol. 2011, 24, 157–162. [Google Scholar] [CrossRef]
- Ansari, S.; Fatma, T. Cyanobacterial Polyhydroxybutyrate (PHB): Screening, Optimization and Characterization. PLoS ONE 2016, 11, e0158168. [Google Scholar] [CrossRef]
- Singh, M.K.; Pradeep, K.; Rai, A.; Singh, S.; Singh, J.S. Poly-Β-Hydroxybutyrate Production by the Cyanobacterium Scytonema geitleri Bharadwaja Under Varying Environmental Conditions. Biomolecules 2019, 9, 198. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Escalante, F.M.E.; de-Bashan, L.E.; Bashan, Y. Heterotrophic cultures of microalgae: Metabolism and potential products. Water Res. 2011, 45, 11–36. [Google Scholar] [CrossRef]
- Perez-Garcia, O.; Bashan, Y. Microalgal Heterotrophic and Mixotrophic Culturing for Bio-refining: From Metabolic Routes to Techno-economics. In Algal Biorefineries: Volume 2: Products and Refinery Design; Prokop, A., Bajpai, R.K., Zappi, M.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 61–131. [Google Scholar]
- El-Mekkawi, S.A.; Abdo, S.M.; Samhan, F.A.; Ali, G.H. Optimization of some fermentation conditions for bioethanol production from microalgae using response surface method. Bull. Natl. Res. Cent. 2019, 43, 164. [Google Scholar] [CrossRef]
- Vastano, M.; Corrado, I.; Sannia, G.; Solaiman, D.K.Y.; Pezzella, C. Conversion of No/Low Value Waste Frying Oils into Biodiesel and Polyhydroxyalkanoates. Sci. Rep. 2019, 9, 13751. [Google Scholar] [CrossRef] [PubMed]
- Afreen, R.; Tyagi, S.; Singh, G.P.; Singh, M. Challenges and Perspectives of Polyhydroxyalkanoate Production from Microalgae/Cyanobacteria and Bacteria as Microbial Factories: An Assessment of Hybrid Biological System. Front. Bioeng. Biotechnol. 2021, 9, 624885. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Pineda, P.A.; López-Pacheco, I.Y.; Villalba-Rodríguez, A.M.; Godínez-Alemán, J.A.; González-González, R.B.; Parra-Saldívar, R.; Iqbal, H.M. Enhancing the Production of PHA in Scenedesmus sp. By the Addition of Green Synthesized Nitrogen, Phosphorus, and Nitrogen–phosphorus-Doped Carbon Dots. Biotechnol. Biofuels Bioprod. 2024, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- Kamravamanesh, D.; Lackner, M.; Herwig, C. Bioprocess Engineering Aspects of Sustainable Polyhydroxyalkanoate Production in Cyanobacteria. Bioengineering 2018, 5, 111. [Google Scholar] [CrossRef]
- Sawant, S.S.; Salunke, B.K.; Taylor, L.E.; Kim, B.S. Enhanced Agarose and Xylan Degradation for Production of Polyhydroxyalkanoates by Co-Culture of Marine Bacterium, Saccharophagus degradans and Its Contaminant, Bacillus Cereus. Appl. Sci. 2017, 7, 225. [Google Scholar] [CrossRef]
- Troschl, C.; Meixner, K.; Drosg, B. Cyanobacterial PHA Production—Review of Recent Advances and a Summary of Three Years’ Working Experience Running a Pilot Plant. Bioengineering 2017, 4, 26. [Google Scholar] [CrossRef]
- Pinnell, L.J.; Conkle, J.L.; Turner, J.W. Microbial Succession During the Degradation of Bioplastic in Coastal Marine Sediment Favors Sulfate Reducing Microorganisms. Front. Mar. Sci. 2022, 9, 945822. [Google Scholar] [CrossRef]
- Surendran, A.; Lakshmanan, M.; Chee, J.Y.; Sulaiman, A.M.; Thược, Đ.V.; Sudesh, K. Can Polyhydroxyalkanoates Be Produced Efficiently from Waste Plant and Animal Oils? Front. Bioeng. Biotechnol. 2020, 8, 169. [Google Scholar] [CrossRef]
- Abdelshafy, A.; Hermann, A.; Herres-Pawlis, S.; Walther, G. Opportunities and Challenges of Establishing a Regional Bio-based Polylactic Acid Supply Chain. Glob. Chall. 2023, 7, 2200218. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.F.; Robson, G.D. Abiotic and Biotic Environmental Degradation of the Bioplastic Polymer Poly(lactic Acid): A Review. Polym. Degrad. Stab. 2017, 137, 122–130. [Google Scholar] [CrossRef]
- Neeti, K.; Gaurav, K.; Singh, R. The Potential of Algae Biofuel as a Renewable and Sustainable Bioresource. Eng. Proc. 2023, 37, 22. [Google Scholar] [CrossRef]
- Tahir, F.; Ashfaq, H.; Khan, A.Z.; Amin, M.; Akbar, I.; Malik, H.A.; Abdullah, M.; Alessa, A.H.; Alsaigh, A.A.; Ralph, P.J.; et al. Emerging trends in algae farming on non-arable lands for resource reclamation, recycling, and mitigation of climate change-driven food security challenges. Rev. Environ. Sci. Bio/Technol. 2024, 23, 869–896. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, C.; Sun, Y.; Chen, W.; Tan, H.; Cao, X.; Xue, S.; Yin, H. Production and Structural Characterization of a New Type of Polysaccharide from Nitrogen-Limited Arthrospira Platensis Cultivated in Outdoor Industrial-Scale Open Raceway Ponds. Biotechnol. Biofuels 2019, 12, 131. [Google Scholar] [CrossRef]
- Bautista, E.G.; Laroche, C. Arthrospira Platensis as a Feasible Feedstock for Bioethanol Production. Appl. Sci. 2021, 11, 6756. [Google Scholar] [CrossRef]
- Μάρκου, Γ.; Angelidaki, İ.; Georgakakis, D. Microalgal Carbohydrates: An Overview of the Factors Influencing Carbohydrates Production, and of Main Bioconversion Technologies for Production of Biofuels. Appl. Microbiol. Biotechnol. 2012, 96, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Rodríguez, Y.Y.; Herazo-Cárdenas, D.S.; Vallejo-Isaza, A.; Pompelli, M.F.; Jarma-Orozco, A.; Jaraba-Navas, J.d.D.; Cordero-Ocampo, J.D.; González-Berrio, M.; Arrieta, D.V.; Pico-González, A.I.; et al. Optimal Laboratory Cultivation Conditions of Limnospira maxima for Large-Scale Production. Biology 2023, 12, 1462. [Google Scholar] [CrossRef]
- Kruger, J.S.; Christensen, E.; Dong, T.; Wychen, S.V.; Fioroni, G.M.; Pienkos, P.T.; McCormick, R.L. Bleaching and Hydroprocessing of Algal Biomass-Derived Lipids to Produce Renewable Diesel Fuel. Energy Fuels 2017, 31, 10946–10953. [Google Scholar] [CrossRef]
- Ahmed, B.; Sultana, S. A critical review on PLA-algae composite: Chemistry, mechanical, and thermal properties. J. Text. Sci. Eng. 2020, 10. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, C.; Li, Y.; Yu, Z.; Chen, Z.; Ye, T.; Wang, X.; Hu, Z.; Liu, S.; Xiao, B.; et al. Cultivation of Algal Biofilm Using Different Lignocellulosic Materials as Carriers. Biotechnol. Biofuels 2017, 10, 115. [Google Scholar] [CrossRef]
- Rocca, S.; Agostini, A.; Giuntoli, J.; Marelli, L. Biofuels from Algae: Technology Options, Energy Balance and GHG Emissions. Insights from a Literature Review; Publications Office of the European Union: Luxembourg, Belgium, 2015; pp. 1831–9424. [Google Scholar]
- Wellenreuther, C.; Wolf, A.; Zander, N. Cost competitiveness of sustainable bioplastic feedstocks—A Monte Carlo analysis for polylactic acid. Clean. Eng. Technol. 2022, 6, 100411. [Google Scholar] [CrossRef]
- Nanda, N.; Bharadvaja, N. Algal bioplastics: Current market trends and technical aspects. Clean Technol. Environ. Policy 2022, 24, 2659–2679. [Google Scholar] [CrossRef] [PubMed]
- Al-Badaani, A.A.; Hifney, A.F.; Adam, M.S.; Gomaa, M. Low-Cost Biosorption of Fe(II) and Fe(III) from Single and Binary Solutions Using Ulva Lactuca-Derived Cellulose Nanocrystals-Graphene Oxide Composite Film. Sci. Rep. 2023, 13, 6422. [Google Scholar] [CrossRef] [PubMed]
- Petousis, M.; Vidakis, N.; Mountakis, N.; Papadakis, V.; Kanellopoulou, S.; Gaganatsiou, A.; Stefanoudakis, N.; Kechagias, J.D. Multifunctional Material Extrusion 3d-Printed Antibacterial Polylactic Acid (PLA) with Binary Inclusions: The Effect of Cuprous Oxide and Cellulose Nanofibers. Fibers 2022, 10, 52. [Google Scholar] [CrossRef]
- Persincula, M.R.F.; Madrazo, C.F. Cellulose Extraction from Cladophora Rupestris for Extraction of Nanomaterials. IOP Conf. Ser. Mater. Sci. Eng. 2024, 1318, 012002. [Google Scholar] [CrossRef]
- Wahlström, N.; Edlund, U.; Pavia, H.; Toth, G.B.; Jaworski, A.; Pell, A.J.; Choong, F.X.; Shirani, H.; Nilsson, K.P.R.; Richter-Dahlfors, A. Cellulose from the Green Macroalgae Ulva Lactuca: Isolation, Characterization, Optotracing, and Production of Cellulose Nanofibrils. Cellulose 2020, 27, 3707–3725. [Google Scholar] [CrossRef]
- Bras, D.L.; Strømme, M.; Mihranyan, A. Characterization of Dielectric Properties of Nanocellulose from Wood and Algae for Electrical Insulator Applications. J. Phys. Chem. B 2015, 119, 5911–5917. [Google Scholar] [CrossRef]
- Trivedi, N.; Baghel, R.S.; Bothwell, J.H.; Gupta, V.; Reddy, C.R.K.; Lali, A.; Jha, B. An Integrated Process for the Extraction of Fuel and Chemicals from Marine Macroalgal Biomass. Sci. Rep. 2016, 6, 30728. [Google Scholar] [CrossRef]
- Ferraz, N.; Carlsson, D.; Hong, J.; Larsson, R.; Fellström, B.; Nyholm, L.; Strømme, M.; Mihranyan, A. Haemocompatibility and Ion Exchange Capability of Nanocellulose Polypyrrole Membranes Intended for Blood Purification. J. R. Soc. Interface 2012, 9, 1943–1955. [Google Scholar] [CrossRef]
- Tuancharoensri, N.; Ross, G.M.; Kongprayoon, A.; Mahasaranon, S.; Pratumshat, S.; Viyoch, J.; Petrot, N.; Ruanthong, W.; Punyodom, W.; Topham, P.D.; et al. In Situ Compatibilized Blends of PLA/PCL/CAB Melt-Blown Films with High Elongation: Investigation of Miscibility, Morphology, Crystallinity and Modelling. Polymers 2023, 15, 303. [Google Scholar] [CrossRef]
- Samadian, H.; Farzamfar, S.; Vaez, A.; Ehterami, A.; Bit, A.; Alam, M.; Goodarzi, A.; Darya, G.; Salehi, M. A Tailored Polylactic Acid/Polycaprolactone Biodegradable and Bioactive 3D Porous Scaffold Containing Gelatin Nanofibers and Taurine for Bone Regeneration. Sci. Rep. 2020, 10, 13366. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.; Katare, R.; Ali, A. Antibacterial, Antioxidant, and Anti-inflammatory Surgical Sutures Fabricated by Melt Mixing of a Polymer Blend with Curcumin-loaded Microspheres. Polym. Eng. Sci. 2025, 65, 2436–2454. [Google Scholar] [CrossRef]
- Tambrchi, P.; Mahdavi, A.H.; Joupari, M.D.; Soltani, L. Polycaprolactone-co-polylactic Acid Nanofiber Scaffold in Combination with 5-azacytidine and Transforming Growth Factor-β to Induce Cardiomyocyte Differentiation of Adipose-derived Mesenchymal Stem Cells. Cell Biochem. Funct. 2022, 40, 668–682. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.K.; Pal, N.; Mishra, P.K.; Srivastava, P.; Mohanty, S.; Maiti, P. 5-Florouracil-loaded Poly(lactic Acid)-poly(caprolactone) Hybrid Scaffold: Potential Chemotherapeutic Implant. J. Biomed. Mater. Res. Part A 2013, 102, 2600–2612. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Kang, D.J.; Kim, M.P.; Cho, C.H.; Kim, B.J. Synthesis of Biodegradable and Flexible, Polylactic Acid Based, Thermoplastic Polyurethane with High Gas Barrier Properties. Polym. Int. 2014, 63, 1620–1626. [Google Scholar] [CrossRef]
- Zhang, K.; Mohanty, A.K.; Misra, M. Fully Biodegradable and Biorenewable Ternary Blends from Polylactide, Poly(3-Hydroxybutyrate-Co-Hydroxyvalerate) and Poly(butylene Succinate) with Balanced Properties. Acs Appl. Mater. Interfaces 2012, 4, 3091–3101. [Google Scholar] [CrossRef]
- Kim, D.K.; Cho, D. Processing and Mechanical, Thermal and Morphological Properties of Poly(lactic Acid)/Poly(butylene Succinate) Blends. Adhes. Interface 2014, 15, 14–21. [Google Scholar] [CrossRef]
- Chaiwutthinan, P.; Pimpan, V.; Chuayjuljit, S.; Leejarkpai, T. Biodegradable Plastics Prepared from Poly(lactic Acid), Poly(butylene Succinate) and Microcrystalline Cellulose Extracted from Waste-Cotton Fabric with a Chain Extender. J. Polym. Environ. 2014, 23, 114–125. [Google Scholar] [CrossRef]
- Boey, J.Y.; Kong, U.; Lee, C.K.; Lim, G.K.; Oo, C.W.; Tan, C.; Ng, C.Y.; Aziz, A.A.; Tay, G.S. The Effect of Filler Loading, Biological Treatment, and Bioplastic Blend Ratio on Flexural and Impact Properties of Blended Bioplastic Reinforced with Spent Coffee Ground. Polym. Eng. Sci. 2024, 64, 3319–3333. [Google Scholar] [CrossRef]
- Vozniak, I.; Hosseinnezhad, R.; Morawiec, J.; Gałęski, A. Nanofibrillar Green Composites of Polylactide/Polyhydroxyalkanoate Produced in Situ Due to Shear Induced Crystallization. Polymers 2019, 11, 1811. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Álvarez, V.A. Polyhydroxyalkanoate (PHA): Review of Synthesis, Characteristics, Processing and Potential Applications in Packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Siakeng, R.; Jawaid, M.; Ariffin, H.; Sapuan, S.M.; Asim, M.; Saba, N. Natural Fiber Reinforced Polylactic Acid Composites: A Review. Polym. Compos. 2018, 40, 446–463. [Google Scholar] [CrossRef]
- Momeni, S.; Craplewe, K.; Safder, M.; Luz, S.M.; Sauvageau, D.; Elias, A. Accelerating the Biodegradation of Poly(lactic Acid) Through the Inclusion of Plant Fibers: A Review of Recent Advances. Acs Sustain. Chem. Eng. 2023, 11, 15146–15170. [Google Scholar] [CrossRef] [PubMed]
- Kanakannavar, S.; Pitchaimani, J.; Thalla, A.K.; Rajesh, M. Biodegradation Properties and Thermogravimetric Analysis of 3D Braided Flax PLA Textile Composites. J. Ind. Text. 2021, 51, 1066S–1091S. [Google Scholar] [CrossRef]
- Badía, J.D.; Ribes-Greus, A. Mechanical Recycling of Polylactide, Upgrading Trends and Combination of Valorization Techniques. Eur. Polym. J. 2016, 84, 22–39. [Google Scholar] [CrossRef]
- Vázquez-Núñez, E.; Avecilla-Ramírez, A.M.; Vergara-Porras, B.; del Rocío López-Cuellar, M. Green Composites and Their Contribution Toward Sustainability: A Review. Polym. Polym. Compos. 2021, 29, S1588–S1608. [Google Scholar] [CrossRef]
- Valente, B.F.A.; Silvestre, A.J.D.; Neto, C.P.; Vilela, C.; Freire, C.S.R. Improving the Processability and Performance of Micronized Fiber-Reinforced Green Composites Through the Use of Biobased Additives. Polymers 2022, 14, 3451. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Mu, W.; Zheng, Z.; Yang, B.; Wang, J.; Zhang, R.; Zhou, K.; Chen, L.; Ying, J.; et al. Mechanical Properties of 3D Printed Micro-nano Rice Husk/Polylactic Acid Filaments. J. Appl. Polym. Sci. 2022, 139, e52619. [Google Scholar] [CrossRef]
- Mukaila, T.; Adeniyi, A.; Bello, I.; Sarker, N.C.; Monono, E.; Hammed, A. Synthesis and Characterization of Polylactic Acid-lecithin-starch Bioplastic Film. J. Appl. Polym. Sci. 2024, 141, e55345. [Google Scholar] [CrossRef]
- Awale, R.J.; Ali, F.; Azmi, A.S.; Puad, N.I.M.; Anuar, H.; Hassan, A. Enhanced Flexibility of Biodegradable Polylactic Acid/Starch Blends Using Epoxidized Palm Oil as Plasticizer. Polymers 2018, 10, 977. [Google Scholar] [CrossRef]
- Shin, B.Y.; Jang, S.H.; Kim, B.S. Thermal, Morphological, and Mechanical Properties of Biobased and Biodegradable Blends of Poly(lactic Acid) and Chemically Modified Thermoplastic Starch. Polym. Eng. Sci. 2011, 51, 826–834. [Google Scholar] [CrossRef]
- Jalalvandi, E.; Majid, R.A.; Ghanbari, T.; Ilbeygi, H. Effects of Montmorillonite (MMT) on Morphological, Tensile, Physical Barrier Properties and Biodegradability of Polylactic Acid/Starch/MMT Nanocomposites. J. Thermoplast. Compos. Mater. 2013, 28, 496–509. [Google Scholar] [CrossRef]
- Suryani; Rihayat, T.; Fitria, F.; Sariadi; Yunus, M.Y.; Hasanah, U.; Safitri, A. The Impact of Chitosan Incorporation on the Mechanical Characteristics of Biodegradable Packaging Based on PLA/PCL Blend. E3s Web Conf. 2024, 503, 08004. [Google Scholar] [CrossRef]
- Altuntaş, E.; Aydemir, D. Effects of Wood Flour on the Mechanical, Thermal and Morphological Properties of Poly (L-Lactic Acid)-Chitosan Biopolymer Composites. Maderas Cienc. Tecnol. 2019, 21, 611–618. [Google Scholar] [CrossRef]
- Halim, A.S.; Nor, F.M.; Saad, A.Z.M.; Nasir, N.A.M.; Norsa’adah, B.; Ujang, Z. Efficacy of Chitosan Derivative Films Versus Hydrocolloid Dressing on Superficial Wounds. J. Taibah Univ. Med. Sci. 2018, 13, 512–520. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Luo, J.; Cai, C.; Song, G.L.; Tang, G.Y. Fabrication of Silk Fibroin Film with Properties of Thermal Insulation and Temperature Monitoring. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 1846–1852. [Google Scholar] [CrossRef]
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials Fabrication from Bombyx Mori Silk Fibroin. Nat. Protoc. 2011, 6, 1612–1631. [Google Scholar] [CrossRef]
- Howard, F.; Gao, Z.; Mansor, H.B.; Yang, Z.; Muthana, M. Silk Fibroin Nanoparticles: A Biocompatible Multi-Functional Polymer for Drug Delivery. In Biotechnology—Biosensors, Biomaterials and Tissue Engineering Annual Volume 2023; InTechOpen: Rijeka, Croatia, 2023. [Google Scholar] [CrossRef]
- Bhagabati, P.; Bhasney, S.M.; Bose, D.; Remadevi, R.; Setty, M.; Rajkhowa, R.; Katiyar, V. Silk and Wool Protein Microparticle-Reinforced Crystalline Polylactic Acid Biocomposites with Improved Cell Interaction for Targeted Biomedical Applications. ACS Appl. Polym. Mater. 2020, 2, 4739–4751. [Google Scholar] [CrossRef]
- Yu, B.; Li, Y.; Lin, Y.; Zhu, Y.; Teng, H.; Wu, Y.; Sun, Z.; Yang, X.; Xu, H. Research Progress of Natural Silk Fibroin and the Application for Drug Delivery in Chemotherapies. Front. Pharmacol. 2023, 13, 1071868. [Google Scholar] [CrossRef]
- Gang, H.; Wang, H.; Ma, S.; Wang, C.; Bian, L.; Wang, Z.; Zhou, Y.; Gu, S.; Liu, X.; Xu, W.; et al. Polylactic Acid/Silk Fibroin Composite Hollow Fibers as Excellent Controlled Drug Release Systems. Polym. Adv. Technol. 2022, 34, 261–269. [Google Scholar] [CrossRef]
- Jameel, Y.; Firdaus, A.H.M.; Sapuan, S.M.; Asmawi, N. Mechanical and Thermal Properties of Graphene Reinforced Poly (Lactic Acid) Composites for Battery Casing in Electric Vehicles. Phys. Sci. Rev. 2025, 10, 395–406. [Google Scholar] [CrossRef]
- Chieng, B.W.; Ibrahim, N.A.; Yunus, W.M.M.; Hussein, M.Z.; Then, Y.Y.; Loo, Y.Y. Effects of Graphene Nanoplatelets and Reduced Graphene Oxide on Poly(lactic Acid) and Plasticized Poly(lactic Acid): A Comparative Study. Polymers 2014, 6, 2232–2246. [Google Scholar] [CrossRef]
- Gong, M.; Zhao, Q.; Dai, L.; Li, Y.; Jiang, T. Fabrication of Polylactic Acid/Hydroxyapatite/Graphene Oxide Composite and Their Thermal Stability, Hydrophobic and Mechanical Properties. J. Asian Ceram. Soc. 2017, 5, 160–168. [Google Scholar] [CrossRef]
- Kannah, R.Y.; Kavitha, S.; Banu, J.R.; Sivashanmugam, P.; Gunasekaran, M.; Kumar, G. A Mini Review of Biochemical Conversion of Algal Biorefinery. Energy Fuels 2021, 35, 16995–17007. [Google Scholar] [CrossRef]
- Sreenikethanam, A.; Bajhaiya, A.K. Algae Based Bio-Plastics: Future of Green Economy. In Biorefineries—Selected Processes; Biernat, K., Ed.; InTechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Semary, N.A.E.; Alsuhail, M.; Amer, K.A.; AlNaim, A. Applications of Algae for Environmental Sustainability: Novel Bioplastic Formulation Method from Marine Green Alga. Front. Mar. Sci. 2022, 9, 1047284. [Google Scholar] [CrossRef]
- Quiroz, D.; Greene, J.M.; Limb, B.J.; Quinn, J.C. Global Life Cycle and Techno-Economic Assessment of Algal-Based Biofuels. Environ. Sci. Technol. 2023, 57, 11541–11551. [Google Scholar] [CrossRef] [PubMed]
- Fabris, M.; Abbriano, R.M.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.; Labeeuw, L.; McCauley, J.I.; Kuzhiuparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279. [Google Scholar] [CrossRef] [PubMed]
- Laurens, L.M.L.; Markham, J.; Templeton, D.W.; Christensen, E.; Wychen, S.V.; Vadelius, E.W.; Chen-Glasser, M.; Dong, T.; Davis, R.; Pienkos, P.T. Development of Algae Biorefinery Concepts for Biofuels and Bioproducts; A Perspective on Process-Compatible Products and Their Impact on Cost-Reduction. Energy Environ. Sci. 2017, 10, 1716–1738. [Google Scholar] [CrossRef]
- Mogany, T.; Bhola, V.; Bux, F. Algal-Based Bioplastics: Global Trends in Applied Research, Technologies, and Commercialization. Environ. Sci. Pollut. Res. 2024, 31, 38022–38044. [Google Scholar] [CrossRef]
- Yin, M.; Zhu, X.; Fu-liang, C. Release Performance and Sustained-Release Efficacy of Emamectin Benzoate-Loaded Polylactic Acid Microspheres. J. Integr. Agric. 2018, 17, 640–647. [Google Scholar] [CrossRef]
- Oliveira, J.; Belchior, A.; da Silva, V.D.; Rotter, A.; Petrovski, Ž.; Almeida, P.L.; Lourenço, N.D.; Gaudêncio, S.P. Marine Environmental Plastic Pollution: Mitigation by Microorganism Degradation and Recycling Valorization. Front. Mar. Sci. 2020, 7, 567126. [Google Scholar] [CrossRef]
- Hudecová, A.; Hricová, M.; Petková, M.; Plavec, R.; Tomčíková, Z.; Ujhelyiová, A. Colour Masterbatches and Their Use in Polylactic Acid Fibres Dyeing. Acta Chim. Slovaca 2023, 16, 62–72. [Google Scholar] [CrossRef]
- Cucina, M.; Nisi, P.D.; Trombino, L.; Tambone, F.; Adani, F. Degradation of Bioplastics in Organic Waste by Mesophilic Anaerobic Digestion, Composting and Soil Incubation. Waste Manag. 2021, 134, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Simon, W.; Klassen, D.; Mottoul, M.; Ponton, S.; Brassard, D.; Raquez, J.M.; Karthikeyan, A.; Dumont, M.J.; Tavares, J.R. Long-Lasting Hydrophilicity Induced by Ultraviolet Light on Surface Modified Hydrophobic Polylactic Acid. J. Appl. Polym. Sci. 2025, 142, e57009. [Google Scholar] [CrossRef]
- Sabalina, A.; Platnieks, O.; Gaidukova, G.; Aunins, A.; Eiduks, T.V.; Gaidukovs, S. Thermomechanical and Mechanical Analysis of Polylactic Acid/Polyhydroxyalkanoate/Poly(butylene Succinate-co-Adipate) Binary and Ternary Blends. RSC Adv. 2025, 15, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka-Komorowska, D.; Kostecka, E.; Bryll, K.; Gawdzińska, K. Analysis of the Decomposition Using the Short Degradation Technique of Polylactic Acid/Halloysite Nanotube Biocomposites. Matec Web Conf. 2022, 357, 05007. [Google Scholar] [CrossRef]
- Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.Ν. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. [Google Scholar] [CrossRef]
- Ammendolia, J.; Walker, T.R. Consistently Inconsistent: The False Promise of ‘Sustainable’ Plastics. Camb. Prism. Plast. 2024, 2, e8. [Google Scholar] [CrossRef]
- Yeo, S.J.; Oh, M.J.; Yoo, P.J. Structurally Controlled Cellular Architectures for High-Performance Ultra-Lightweight Materials. Adv. Mater. 2018, 31, e1803670. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lee, H.-S.; Seo, J.; Kang, Y.-H.; Kim, W.; Kang, T.H. State-of-the-Art of Cellulose Nanocrystals and Optimal Method for Their Dispersion for Construction-Related Applications. Appl. Sci. 2019, 9, 426. [Google Scholar] [CrossRef]
- Du, H.; Liu, C.; Mu, X.; Gong, W.; Lv, D.; Hong, Y.; Si, C.; Li, B. Preparation and Characterization of Thermally Stable Cellulose Nanocrystals via a Sustainable Approach of FeCl3-catalyzed Formic Acid Hydrolysis. Cellulose 2016, 23, 2389–2407. [Google Scholar] [CrossRef]
- Arora, Y.; Sharma, S.; Sharma, V. Microalgae in Bioplastic Production: A Comprehensive Review. Arab. J. Sci. Eng. 2023, 48, 7225–7241. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Blending Polylactic Acid with Polyhydroxybutyrate: The Effect on Thermal, Mechanical, and Biodegradation Properties. Adv. Polym. Technol. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Pérez-Fonseca, A.A.; Herrera-Carmona, V.S.; González-García, Y.; Alan, S.M.d.C.; González-López, M.E.; Ramírez-Arreola, D.E.; Robledo-Ortíz, J.R. Influence of the Blending Method Over the Thermal and Mechanical Properties of Biodegradable Polylactic Acid/Polyhydroxybutyrate Blends and Their Wood Biocomposites. Polym. Adv. Technol. 2021, 32, 3483–3494. [Google Scholar] [CrossRef]
- Gao, L.; Drozdov, A.D. Exploring the Performance of Bio-Based PLA/PHB Blends: A Comprehensive Analysis. Polym. Renew. Resour. 2024, 15, 358–374. [Google Scholar] [CrossRef]
- Çabuk, Y.; Gümüş, H.; Aydemir, D.; Kurt, R.; İmren, E.; Altuntaş, E.; Özan, Z.E. Taguchi-Grey Relational Analysis in Parameter Optimisation of Green Biopolymer Composites. Plast. Rubber Compos. Macromol. Eng. 2023, 52, 375–386. [Google Scholar] [CrossRef]
- Szatkowski, P.; Twaróg, R.; Sowińska, K.; Pielichowska, K. Chemically Modified Pineapple Leaf Fibre as a Filler of Polyurethane-Based Composites. Materials 2025, 18, 386. [Google Scholar] [CrossRef]
- Mishra, V.; Kumar, J.; Negi, S.; Kar, S. 3D Printing of Continuous Metal Fiber-Reinforced Recycled ABS with Varying Fiber Loading. Rapid Prototyp. J. 2024, 30, 1610–1623. [Google Scholar] [CrossRef]
- Głowińska, E.; Datta, J. Bio Polyetherurethane Composites with High Content of Natural Ingredients: Hydroxylated Soybean Oil Based Polyol, Bio Glycol and Microcrystalline Cellulose. Cellulose 2015, 23, 581–592. [Google Scholar] [CrossRef]
- Brzeska, J.; Morawska, M.; Sikorska, W.; Tercjak, A.; Kowalczuk, M.; Rutkowska, M. Degradability of Cross-Linked Polyurethanes Based on Synthetic Polyhydroxybutyrate and Modified with Polylactide. Chem. Pap. 2017, 71, 2243–2251. [Google Scholar] [CrossRef]
- Suki, F.M.M.; Ismail, H.; Hamid, Z.A.A. Preparation and Properties of Polyvinyl Alcohol/Banana Frond Flour Biodegradable Film. Prog. Rubber Plast. Recycl. Technol. 2014, 30, 103–114. [Google Scholar] [CrossRef]
- Suki, F.M.M.; Azahari, N.A.; Othman, N.; Ismail, H.; Sasidharan, S. Biodegradation Studies of Attapulgite Clay Filled Polyvinyl Alcohol/Modified Corn Starch Blend Films: Microbial and Enzymatic. Adv. Mater. Res. 2013, 747, 668–672. [Google Scholar] [CrossRef]
- Pokhrel, S.; Sundari, L. Fabrication and Characterization of Starch-Based Biodegradable Polymer with Polyvinyl Alcohol. J. Nepal Chem. Soc. 2019, 40, 57–66. [Google Scholar] [CrossRef]
- José Augusto de Almeida, N.; dos Santos, A.F.; Silva, I.D.L.; Falcão, E.H.L.; de Britto, D.; Vinhas, G.M. Physico-Chemical, Mechanical and Morphological Properties of Biodegradable Films Based on Arrowroot Starch and Poly(vinyl Alcohol). J. Macromol. Sci. Part B 2021, 60, 1045–1068. [Google Scholar] [CrossRef]
- Samarasinghe, S.A.S.C.; Easteal, A.J.; Edmonds, N.R. Biodegradable Plastic Composites from Corn Gluten Meal. Polym. Int. 2007, 57, 359–364. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Z.; Ren, T. Mechanical and Thermal Properties of Poly(Lactic Acid)/Starch/Montmorillonite Biodegradable Blends. Polym. Polym. Compos. 2007, 15, 633–638. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, N.; Wang, G.Y.; Du, Y.; Isobe, S. Preparation and Properties of Corn Gluten Meal/Poly (Lactic Acid) Composite. Adv. Mater. Res. 2011, 415–417, 1620–1625. [Google Scholar] [CrossRef]
- Bloom_Sustainable_Materials. Products. Available online: https://www.bloommaterials.com/products/ (accessed on 15 August 2025).
- Loliware. Blue Carbon Straw. Available online: https://brilliantideasplanet.com/solution/125-blue-carbon-straw (accessed on 15 August 2025).
- Borrero-de Acuña, J.M.; Poblete-Castro, I. Rational Engineering of Natural Polyhydroxyalkanoates Producing Microorganisms for Improved Synthesis and Recovery. Microb. Biotechnol. 2022, 16, 262–285. [Google Scholar] [CrossRef]
- Yan, J.; Cheng, R.; Lin, X.; You, S.; Li, K.; Rong, H.; Ma, Y. Overexpression of acetyl-CoA synthetase increased the biomass and fatty acid proportion in microalga Schizochytrium. Appl. Microbiol. Biotechnol. 2013, 97, 1933–1939. [Google Scholar] [CrossRef]
- Mehrshahi, P.; Ginnie, T.D.T.N.; Rovira, A.G.; Sayer, A.; Llavero-Pasquina, M.; Sin, M.L.H.; Medcalf, E.J.; Mendoza-Ochoa, G.I.; Scaife, M.A.; Smith, A.G. Development of Novel Riboswitches for Synthetic Biology in the Green Alga Chlamydomonas. ACS Synth. Biol. 2020, 9, 1406–1417. [Google Scholar] [CrossRef]
- Shahid, A.; Rehman, A.U.; Usman, M.; Ashraf, M.U.; Javed, M.R.; Khan, A.Z.; Gill, S.S.; Mehmood, M.A. Engineering the Metabolic Pathways of Lipid Biosynthesis to Develop Robust Microalgal Strains for Biodiesel Production. Biotechnol. Appl. Biochem. 2019, 67, 41–51. [Google Scholar] [CrossRef]
- Choi, K.R.; Lee, S.Y. Protocols for RecET-based Markerless Gene Knockout and Integration to Express Heterologous Biosynthetic Gene Clusters in Pseudomonas putida. Microb. Biotechnol. 2019, 13, 199–209. [Google Scholar] [CrossRef]
- Ali, S.S.; Abdelkarim, E.A.; Elsamahy, T.; Al-Tohamy, R.; Li, F.; Kornaros, M.; Zuorro, A.; Zhu, D.; Sun, J. Bioplastic Production in Terms of Life Cycle Assessment: A State-of-the-Art Review. Environ. Sci. Ecotechnology 2023, 15, 100254. [Google Scholar] [CrossRef]
- Lu, Y.; Bao-zhang, Z.; Wu, K.; Gou, Z.; Li, L.; Chen, G.G.; Cui, L.; Nian-ci, L. Analysis of Operation Conditions for a Pilot-scale Supercritical CO2 Extraction of Diterpenoid from Pteris semipinnata L. Asia-Pac. J. Chem. Eng. 2011, 7, 777–782. [Google Scholar] [CrossRef]
- Dong, Y.; Zhai, K.; Li, Y.; Lv, Z.Z.; Zhao, M.; Gan, T.; Ma, Y. Modification of Glucose Metabolic Pathway to Enhance Polyhydroxyalkanoate Synthesis in Pseudomonas putida. Curr. Issues Mol. Biol. 2024, 46, 12784–12799. [Google Scholar] [CrossRef] [PubMed]
- Mezzina, M.P.; Manoli, M.T.; Prieto, M.A.; Nikel, P.I. Engineering Native and Synthetic Pathways in Pseudomonas putida for the Production of Tailored Polyhydroxyalkanoates. Biotechnol. J. 2020, 16, e2000165. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Y.; Shu, Y.; Hu, X.; Wu, D.; Jiang, H.; Wang, K.; Liu, W.; Fu, W. Recent Progress on Systems and Synthetic Biology of Diatoms for Improving Algal Productivity. Front. Bioeng. Biotechnol. 2022, 10, 908804. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, X.; Kong, A.; Zhao, Y.; Fan, X.; Ma, T.; Gao, W.; Wang, S.; Yang, C. Screening of Endogenous Strong Promoters for Enhanced Production of Medium-Chain-Length Polyhydroxyalkanoates in Pseudomonas Mendocina Nk-01. Sci. Rep. 2019, 9, 1798. [Google Scholar] [CrossRef]
- Tan, F.H.P.; Nadir, N.; Sudesh, K. Microalgal Biomass as Feedstock for Bacterial Production of PHA: Advances and Future Prospects. Front. Bioeng. Biotechnol. 2022, 10, 879476. [Google Scholar] [CrossRef]
- Molina-Besch, K. Use Phase and End-of-Life Modeling of Biobased Biodegradable Plastics in Life Cycle Assessment: A Review. Clean Technol. Environ. Policy 2022, 24, 3253–3272. [Google Scholar] [CrossRef]
- Kong, F.; Yamaoka, Y.; Ohama, T.; Lee, Y.; Li-Beisson, Y. Molecular Genetic Tools and Emerging Synthetic Biology Strategies to Increase Cellular Oil Content in Chlamydomonas reinhardtii. Plant Cell Physiol. 2019, 60, 1184–1196. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Li, Q.; Li, X.; Lin, S.-Y.; Zhao, W.; Liu, Y.; Wu, B.; Huang, Y.; Jia, B.; et al. A Rapid and Reversible Molecular “Switch” Regulating Protein Expression in Chlamydomonas reinhardtii. Plant Cell Environ. 2025, 48, 3913–3924. [Google Scholar] [CrossRef]
- Heng, K.S.; Ong, S.Y.; Sudesh, K. Efficient Biosynthesis and Recovery of Polyhydroxyalkanoate. Malays. J. Microbiol. 2016, 12, 383–398. [Google Scholar] [CrossRef]
- Dhokane, D.; Shaikh, A.; Yadav, A.; Giri, N.; Bandyopadhyay, A.; Dasgupta, S.; Bhadra, B. CRISPR-based Bioengineering in Microalgae for Production of Industrially Important Biomolecules. Front. Bioeng. Biotechnol. 2023, 11, 1267826. [Google Scholar] [CrossRef] [PubMed]
- Shen, R.; Yin, J.; Ye, J.; Xiang, R.; Ning, Z.-Y.; Huang, W.; Chen, G.Q. Promoter Engineering for Enhanced P(3hb-co-4hb) Production by Halomonas bluephagenesis. Acs Synth. Biol. 2018, 7, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.-G.; Le, T.T.; Choi, D.-Y.; Cho, D.-H.; Yun, J.-H.; Choi, H.I.; Kim, H.-S.; Lee, Y.J. Progress and challenges in CRISPR/Cas applications in microalgae. J. Microbiol. 2025, 63, e2501028. [Google Scholar] [CrossRef]
- Huwald, D.; Duda, S.; Gasper, R.; Olieric, V.; Hofmann, E.; Hemschemeier, A. Distinctive structural properties of THB11, a pentacoordinate Chlamydomonas reinhardtii truncated hemoglobin with N- and C-terminal extensions. JBIC J. Biol. Inorg. Chem. 2020, 25, 267–283. [Google Scholar] [CrossRef]
- Hao, X.; Luo, L.; Jouhet, J.; Rébeillé, F.; Maréchal, É.; Hu, H.; Pan, Y.; Tan, X.; Chen, Z.; You, L.; et al. Enhanced Triacylglycerol Production in the Diatom Phaeodactylum Tricornutum by Inactivation of a Hotdog-Fold Thioesterase Gene Using TALEN-based Targeted Mutagenesis. Biotechnol. Biofuels 2018, 11, 312. [Google Scholar] [CrossRef]
- Thomas, P.K.; Arn, F.J.; Freiermuth, M.; Narwani, A. Botryococcus braunii reduces Algal Grazing Losses To Daphnia and Poterioochromonas through Both Chemical and Physical Interference. J. Appl. Phycol. 2024, 36, 3221–3230. [Google Scholar] [CrossRef]
- Hong, S.; Song, M.; Kim, S.; Bang, D.; Kang, T.; Choi, I.; Lee, L.P. Integrated Microalgae Analysis Photobioreactor for Rapid Strain Selection. Acs Nano 2016, 10, 5635–5642. [Google Scholar] [CrossRef]
- Abdo, S.M.; Ali, G.H. Analysis of Polyhydroxybutrate and Bioplastic Production from Microalgae. Bull. Natl. Res. Cent. 2019, 43, 97. [Google Scholar] [CrossRef]
- Price, S.; Kuzhiumparambil, U.; Pernice, M.; Ralph, P.J. Cyanobacterial Polyhydroxybutyrate for Sustainable Bioplastic Production: Critical Review and Perspectives. J. Environ. Chem. Eng. 2020, 8, 104007. [Google Scholar] [CrossRef]
- Li, C.; Du, M.Q.; Han, Y.; Sun, W.; Chen, Z.; Liu, Q.; Zhu, H.; Zhao, L.; Li, S.; Wang, J. Microalgae in Health Care and Functional Foods: Β-Glucan Applications, Innovations in Drug Delivery and Synthetic Biology. Front. Pharmacol. 2025, 16, 1557298. [Google Scholar] [CrossRef]
- Varman, A.M.; Yu, Y.; You, L.; Tang, Y. Photoautotrophic Production of D-Lactic Acid in an Engineered Cyanobacterium. Microb. Cell Factories 2013, 12, 117. [Google Scholar] [CrossRef]
- Wichmann, J.; Baier, T.; Wentnagel, E.; Lauersen, K.J.; Kruse, O. Tailored Carbon Partitioning for Phototrophic Production of (E)-A-Bisabolene from the Green Microalga Chlamydomonas reinhardtii. Metab. Eng. 2018, 45, 211–222. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Hu, G.-R.; Sommerfeld, M.R.; Hu, Q. Inhibition of Starch Synthesis Results in Overproduction of Lipids in Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2010, 107, 258–268. [Google Scholar] [CrossRef]
- Gonçalves, E.; Koh, J.; Zhu, N.; Yoo, M.J.; Chen, S.; Matsuo, T.; Johnson, J.V.; Rathinasabapathi, B. Nitrogen Starvation-induced Accumulation of Triacylglycerol in the Green Algae: Evidence for a Role for ROC40, a Transcription Factor Involved in Circadian Rhythm. Plant J. 2016, 85, 743–757. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zeng, A.P. Engineering a Lysine-on Riboswitch for Metabolic Control of Lysine Production in Corynebacterium glutamicum. Acs Synth. Biol. 2015, 4, 1335–1340. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, A.V.; Henkin, T.M. Riboswitch-Mediated Gene Regulation: Novel RNA Architectures Dictate Gene Expression Responses. Annu. Rev. Microbiol. 2016, 70, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, J.C.; Topp, S.; Sogi, K.M.; Previti, M.L.; Gallivan, J.P.; Bertozzi, C.R. A Riboswitch-Based Inducible Gene Expression System for Mycobacteria. PLoS ONE 2012, 7, e29266. [Google Scholar] [CrossRef]
- Ogawa, A.; Fujikawa, M.; Onishi, K.; Takahashi, H. Cell-Free Biosensors Based on Modular Eukaryotic Riboswitches That Function in One Pot at Ambient Temperature. Acs Synth. Biol. 2024, 13, 2238–2245. [Google Scholar] [CrossRef]
- McRose, D.L.; Guo, J.; Monier, A.; Sudek, S.; Wilken, S.; Yan, S.; Möck, T.; Archibald, J.M.; Begley, T.P.; Reyes-Prieto, A.; et al. Alternatives to Vitamin B1 Uptake Revealed with Discovery of Riboswitches in Multiple Marine Eukaryotic Lineages. Isme J. 2014, 8, 2517–2529. [Google Scholar] [CrossRef]
- Marcano-Velázquez, J.G.; Lo, J.; Nag, A.; Maness, P.C.; Chou, K. Developing Riboswitch-Mediated Gene Regulatory Controls in Thermophilic Bacteria. Acs Synth. Biol. 2019, 8, 633–640. [Google Scholar] [CrossRef]
- Kent, R.; Dixon, N. Systematic Evaluation of Genetic and Environmental Factors Affecting Performance of Translational Riboswitches. Acs Synth. Biol. 2019, 8, 884–901. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, R.-Y.; Xu, T.; Liu, Z.; Li, B.Z.; Yuan, Y.J. Valorizing Lignin and Coprecursors into Homogeneous Polyhydroxyalkanoates by Engineered Pseudomonas putida. Acs Sustain. Chem. Eng. 2024, 12, 8402–8414. [Google Scholar] [CrossRef]
- Meur, S.L.; Zinn, M.; Egli, T.; Thöny-Meyer, L.; Ren, Q. Production of Medium-Chain-Length Polyhydroxyalkanoates by Sequential Feeding of Xylose and Octanoic Acid in Engineered Pseudomonas putida KT2440. BMC Biotechnol. 2012, 12, 53. [Google Scholar] [CrossRef]
- Hao, J.; Wang, X.; Wang, H. Overall Process of Using a Valerate-Dominant Sludge Hydrolysate to Produce High-Quality Polyhydroxyalkanoates (PHA) in a Mixed Culture. Sci. Rep. 2017, 7, 6939. [Google Scholar] [CrossRef]
- Salgaonkar, B.B.; Bragança, J.M. Utilization of Sugarcane Bagasse by Halogeometricum Borinquense Strain E3 for Biosynthesis of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate). Bioengineering 2017, 4, 50. [Google Scholar] [CrossRef]
- Romenesko, T.; Coats, E.R. Cofermenting Algal Biomass with Municipal Primary Solids to Enhance Carboxylate Production. Water Environ. Res. 2018, 90, 1997–2007. [Google Scholar] [CrossRef]
- Narayanasamy, A.; Patel, S.K.S.; Singh, N.; Rohit, M.V.; Lee, J.-K. Valorization of Algal Biomass to Produce Microbial Polyhydroxyalkanoates: Recent Updates, Challenges, and Perspectives. Polymers 2024, 16, 2227. [Google Scholar] [CrossRef]
- Sekoai, P.T.; Ghimire, A.; Ezeokoli, O.T.; Rao, S.; Ngan, W.Y.; Habimana, O.; Yao, Y.; Pu, Y.; Fung, A.H.Y.; Yoro, K.O.; et al. Valorization of Volatile Fatty Acids from the Dark Fermentation Waste Streams-a Promising Pathway for a Biorefinery Concept. Renew. Sustain. Energy Rev. 2021, 143, 110971. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Saratale, G.D.; Kumar, M.; Bharagava, R.N.; Varjani, S.; Kadam, A.A.; Ghodake, G.; Palem, R.R.; Mulla, S.I.; et al. An Overview of Recent Advancements in Microbial Polyhydroxyalkanoates (PHA) Production from Dark Fermentation Acidogenic Effluents: A Path to an Integrated Bio-Refinery. Polymers 2021, 13, 4297. [Google Scholar] [CrossRef]
- Saraphirom, P.; Reungsang, A.; Plangklang, P. Polyhydroxyalkanoates Production from Effluent of Hydrogen Fermentation Process by Cupriavidus sp. KKU38. Environ. Technol. 2013, 34, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Chalima, A.; Oliver, L.; de Castro, L.F.; Karnaouri, A.; Dietrich, T.; Topakas, E. Utilization of Volatile Fatty Acids from Microalgae for the Production of High Added Value Compounds. Fermentation 2017, 3, 54. [Google Scholar] [CrossRef]
- Cai, M.M.; Cui, F.Y.; Zhao, Q.; Chua, H. Influence of Substrate Load on Polyhydroxyalkanoates (Pha) Accumulation by Unenriched Mixed Cultures from Excess Sludge Fermentation Liquid. Adv. Mater. Res. 2012, 455–456, 856–861. [Google Scholar] [CrossRef]
- Gottardo, M.; Crognale, S.; Tonanzi, B.; Rossetti, S.; D’Annibale, L.; Dosta, J.; Valentino, F. Volatile Fatty Acid Production from Hydrolyzed Sewage Sludge: Effect of Hydraulic Retention Time and Insight into Thermophilic Microbial Community. Biomass Convers. Biorefin. 2022, 14, 14921–14932. [Google Scholar] [CrossRef]
- Tasić, M.B.; Bonon, A.J.; Barbosa, M.I.R.; Klein, B.C.; Veljković, V.B.; Filho, R.M. Cultivation of Chlamydomonas reinhardtii in Anaerobically Digested Vinasse for Bioethanol Production. Waste Biomass Valorization 2020, 12, 857–865. [Google Scholar] [CrossRef]
- Lacroux, J.; Seira, J.; Trably, É.; Bernet, N.; Steyer, J.P.; Lis, R.v. Mixotrophic Growth of Chlorella Sorokiniana on Acetate and Butyrate: Interplay Between Substrate, C:N Ratio and pH. Front. Microbiol. 2021, 12, 70361. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, X.; Wang, Q. Enhanced Lipid Production in Chlamydomonas reinhardtii by Co-Culturing with Azotobacter Chroococcum. Front. Plant Sci. 2018, 9, 741. [Google Scholar] [CrossRef]
- Çakmak, Z.E.; Ölmez, T.T.; Çakmak, T.; Menemen, Y.; Tekinay, T. Induction of Triacylglycerol Production in Chlamydomonas reinhardtii: Comparative Analysis of Different Element Regimes. Bioresour. Technol. 2014, 155, 379–387. [Google Scholar] [CrossRef]
- Therien, J.; Zadvornyy, O.A.; Posewitz, M.C.; Bryant, D.A.; Peters, J.W. Growth of Chlamydomonas reinhardtii in Acetate-Free Medium When Co-Cultured with Alginate-Encapsulated, Acetate-Producing Strains of Synechococcus sp. PCC 7002. Biotechnol. Biofuels 2014, 7, 154. [Google Scholar] [CrossRef]
- Fakhimi, N.; Torres, M.J.; Fernández, E.M.; Galván, A.; Dubini, A.; González-Ballester, D. Chlamydomonas reinhardtii and Microbacterium forte sp. Nov., a Mutualistic Association That Favor Sustainable Hydrogen Production. Sci. Total Environ. 2023, 913, 169559. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.L.; Ghamsari, L.; Manichaikul, A.; Hom, E.; Santhanam, B.; Fu, W.; Shen, Y.; Hao, T.; Palsson, B.Ø.; Salehi-Ashtiani, K.; et al. Metabolic Network Reconstruction of Chlamydomonas Offers Insight into Light-driven Algal Metabolism. Mol. Syst. Biol. 2011, 7, 518. [Google Scholar] [CrossRef] [PubMed]
- Iwai, M.; Hori, K.; Sasaki-Sekimoto, Y.; Shimojima, M.; Ohta, H. Manipulation of Oil Synthesis in Nannochloropsis Strain NIES-2145 with a Phosphorus Starvation–inducible Promoter from Chlamydomonas reinhardtii. Front. Microbiol. 2015, 6, 912. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, W.-K.; Lee, B.S.; Seon, G.; Suh, W.I.; Moon, M.; Chang, Y.K. Optimization of Heterotrophic Cultivation of Chlorella sp. HS2 Using Screening, Statistical Assessment, and Validation. Sci. Rep. 2019, 9, 19383. [Google Scholar] [CrossRef]
- Hong, Y.; Chao-Yang, C.; Wu, Y.R. Biobutanol Production from Sulfuric Acid-pretreated Red Algal Biomass by a Newly Isolated Clostridium sp. Strain WK. Biotechnol. Appl. Biochem. 2019, 67, 738–743. [Google Scholar] [CrossRef]
- Ahuja, V.; Singh, P.; Mahata, C.; Jeon, J.-M.; Kumar, G.; Yang, Y.-H.; Bhatia, S.K. A Review on Microbes Mediated Resource Recovery and Bioplastic (Polyhydroxyalkanoates) Production from Wastewater. Microb. Cell Factories 2024, 23, 187. [Google Scholar] [CrossRef]
- Valasara, V.; Ahn, B.; Liu, J.; Yu, B.; Han, S.-M.; Won, W. Coproduction of Drop-in Biofuels and Biodegradable Plastic Monomers: Process Design and Integrative Analyses. Acs Sustain. Chem. Eng. 2025, 13, 6992–7004. [Google Scholar] [CrossRef]
- Ng, I.S.; Tan, S.I.; Kao, P.H.; Chang, Y.K.; Chang, J.S. Recent Developments on Genetic Engineering of Microalgae for Biofuels and Bio-Based Chemicals. Biotechnol. J. 2017, 12, 1600644. [Google Scholar] [CrossRef]
- Malla, A.; Rosales-Méndoza, S.; Phoolcharoen, W.; Vimolmangkang, S. Efficient Transient Expression of Recombinant Proteins Using DNA Viral Vectors in Freshwater Microalgal Species. Front. Plant Sci. 2021, 12, 650820. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Meng, Y.; Jiang, Y.; Zhang, X.; Liu, H.; Reis, M.A.; Qi, Q.; Yang, C.; Liu, R. Systems Metabolic Engineering of Genome-Reduced Pseudomonas putida for Efficient Production of Polyhydroxyalkanoate from p-Coumaric Acid. J. Agric. Food Chem. 2025, 73, 12899–12907. [Google Scholar] [CrossRef]
- Saha, B.C.; Iten, L.B.; Cotta, M.A.; Wu, Y.V. Dilute Acid Pretreatment, Enzymatic Saccharification, and Fermentation of Rice Hulls to Ethanol. Biotechnol. Prog. 2008, 21, 816–822. [Google Scholar] [CrossRef]
- Ahn, J.; Jho, E.H.; Nam, K. Effect of C/N ratio on polyhydroxyalkanoates (PHA) accumulation by Cupriavidus necator and its implication on the use of rice straw hydrolysates. Environ. Eng. Res. 2015, 20, 246–253. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Y.; Guo, H.; Meng, Y.; Xiong, W.; Liu, R.; Yang, C. Establishment of Low-cost Production Platforms of Polyhydroxyalkanoate Bioplastics from Halomonas cupida J9. Biotechnol. Bioeng. 2024, 121, 2106–2120. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Fatima, N.; Rasheed, A.; Riaz, M.; Anwar, F.; Khatoon, Y. Metabolic Engineered Biocatalyst: A Solution for PLA Based Problems. Int. J. Biomater. 2018, 2018, 1963024. [Google Scholar] [CrossRef] [PubMed]
- Balboa, E.M.; Moure, A.; Domínguez, H. Valorization of Sargassum Muticum Biomass According to the Biorefinery Concept. Mar. Drugs 2015, 13, 3745–3760. [Google Scholar] [CrossRef] [PubMed]
- Uma, V.S.; Usmani, Z.; Sharma, M.; Diwan, D.; Sharma, M.; Guo, M.; Tuohy, M.G.; Makatsoris, C.; Zhao, X.; Thakur, V.K.; et al. Valorisation of Algal Biomass to Value-Added Metabolites: Emerging Trends and Opportunities. Phytochem. Rev. 2022, 22, 1015–1040. [Google Scholar] [CrossRef]
- Gamal, N.; Setta, A.; Saber, H.; Bakr, A.; El, A.; El-Dakkak, A.; Galal, H.R. Potentiality of Algae Extracts in Alleviating Stresses Effect on Common Bean Under Upper Egypt Conditions. Adv. Environ. Biol. 2019, 12, 28–37. [Google Scholar] [CrossRef]
- Singh, J.; Dhar, D.W. Overview of Carbon Capture Technology: Microalgal Biorefinery Concept and State-of-the-Art. Front. Mar. Sci. 2019, 6, 29. [Google Scholar] [CrossRef]
- Patnaik, R.; Singh, N.; Bagchi, S.K.; Rao, P.S.; Mallick, N. Utilization of Scenedesmus obliquus Protein as a Replacement of the Commercially Available Fish Meal Under an Algal Refinery Approach. Front. Microbiol. 2019, 10, 2114. [Google Scholar] [CrossRef]
- Roberts, K.; Heaven, S.; Banks, C.J. Comparative Testing of Energy Yields from Micro-Algal Biomass Cultures Processed via Anaerobic Digestion. Renew. Energy 2016, 87, 744–753. [Google Scholar] [CrossRef]
- Torres, A.; Padrino, S.; Brito, A.; Díaz, L. Biogas Production from Anaerobic Digestion of Solid Microalgae Residues Generated on Different Processes of Microalgae-to-Biofuel Production. Biomass Convers. Biorefin. 2021, 13, 4659–4672. [Google Scholar] [CrossRef]
- Khan, M.A.; Hafeez, M.; Zaheer, M.; Hameed, U. Microscopic and Biochemical Identification of Spirulina spp. for Its Biomass Cultivation by Using Different Types of Photobioreactors at Lab and Pilot Scale. Pak. J. Biochem. Biotechnol. 2022, 3, 123–131. [Google Scholar] [CrossRef]
- Olivieri, G.; Moroni, M.; Janssen, M.; Piersanti, L.; Mezza, D.; Bravi, M. Model-Based Prediction of Perceived Light Flashing in Recirculated Inclined Wavy-Bottomed Photobioreactors. Processes 2021, 9, 1158. [Google Scholar] [CrossRef]
- Koller, M.; Mukherjee, A. Polyhydroxyalkanoates—Linking Properties, Applications and End-of-Life Options. Chem. Biochem. Eng. Q. 2020, 34, 115–129. [Google Scholar] [CrossRef]
- Nduko, J.M.; Taguchi, S. Microbial Production of Biodegradable Lactate-Based Polymers and Oligomeric Building Blocks from Renewable and Waste Resources. Front. Bioeng. Biotechnol. 2021, 8, 618077. [Google Scholar] [CrossRef]
- Rokicka, M.; Zieliński, M.; Dudek, M.; Dębowski, M. Effects of Ultrasonic and Microwave Pretreatment on Lipid Extraction of Microalgae and Methane Production from the Residual Extracted Biomass. Bioenergy Res. 2020, 14, 752–760. [Google Scholar] [CrossRef]
- Abraham, J.; Abimbola, T.; Braida, W.; Terracciano, A.; Su, T.L.; Christodoulatos, C.; Koutsospyros, A.; RoyChowdhury, A.; Smolinski, B.; Lawal, A. On-Site Pilot-Scale Microalgae Cultivation Using Industrial Wastewater for Bioenergy Production: A Case Study Towards Circular Bioeconomy. Bioengineering 2023, 10, 1339. [Google Scholar] [CrossRef]
- Gimondo, J.A.; Currey, C.J.; Jarboe, D.H.; Gross, M.; Graves, W.R. Wastewater-Grown Algae Pellets and Paste as Fertilizers for Containerized Crops. Hortscience 2019, 54, 528–536. [Google Scholar] [CrossRef]
- Synani, K.; Abeliotis, K.; Velonia, K.; Maragkaki, A.; Manios, T.; Lasaridi, K. Environmental Impact and Sustainability of Bioplastic Production from Food Waste. Sustainability 2024, 16, 5529. [Google Scholar] [CrossRef]
- Souza, H.K.; Matos, M.; Reis, M.A.; Covas, J.A.; Hilliou, L. Can Biomass Mastication Assist the Downstreaming of Polyhydroxyalkanoates Produced from Mixed Microbial Cultures? Molecules 2023, 28, 767. [Google Scholar] [CrossRef]
- Soyemi, A.; Szilvási, T. Calculated Physicochemical Properties of Glycerol-Derived Solvents to Drive Plastic Waste Recycling. Ind. Eng. Chem. Res. 2023, 62, 6322–6337. [Google Scholar] [CrossRef]
- Cao, Z.; Hu, Y.; Ni, S.; Liu, Z.; Su, X.; Sun, X. Enrichment of Rare Earth from Wasted NdFeB Lotion with the Extraction-Precipitation Method. Ind. Eng. Chem. Res. 2021, 60, 13338–13347. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, M.; Su, J.; Hu, H.; Yang, M.; Huang, Z.; Chen, D.; Wu, J.; Feng, Z. Overcoming Biomass Recalcitrance by Synergistic Pretreatment of Mechanical Activation and Metal Salt for Enhancing Enzymatic Conversion of Lignocellulose. Biotechnol. Biofuels 2019, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hao, L.; Chen, F.; Zhu, T. The Mechanism of Extraction of Peanut Protein and Oil Bodies by Enzymatic Hydrolysis of the Cell Wall. J. Oleo Sci. 2020, 69, 1467–1479. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, G.; Liu, F.; Xie, M.; Zou, Y.; Wang, S.; Guo, Z.; Dong, J.; Ye, J.; Cao, Y.; et al. An Enzyme-Based System for Extraction of Small Extracellular Vesicles from Plants. Sci. Rep. 2023, 13, 13931. [Google Scholar] [CrossRef]
- Ali, I.; Adnan, M.; Ullah, S.; Zhao, Q.; Iqbal, A.; He, L.; Cheng, F.; Muhammad, I.; Ahmad, S.; Wei, S.; et al. Biochar Combined with Nitrogen Fertilizer: A Practical Approach for Increasing the Biomass Digestibility and Yield of Rice and Promoting Food and Energy Security. Biofuels Bioprod. Biorefin. 2022, 16, 1304–1318. [Google Scholar] [CrossRef]
- Wada, O.Z.; Rashid, N.; Wijten, P.; Thornalley, P.J.; McKay, G.; Mackey, H.R. Evaluation of Cell Disruption Methods for Protein and Coenzyme Q10 Quantification in Purple Non-Sulfur Bacteria. Front. Microbiol. 2024, 15, 1324099. [Google Scholar] [CrossRef]
- Sydney, T.; Marshall-Thompson, J.-A.; Kapoore, R.V.; Vaidyanathan, S.; Pandhal, J.; Fairclough, J.P.A. The Effect of High-Intensity Ultraviolet Light to Elicit Microalgal Cell Lysis and Enhance Lipid Extraction. Metabolites 2018, 8, 65. [Google Scholar] [CrossRef]
- Schüler, L.; Gangadhar, K.N.; Duarte, P.; Placines, C.; Molina-Márquez, A.; León, R.; Sousa, V.S.; Varela, J.; Barreira, L. Improvement of Carotenoid Extraction from a Recently Isolated, Robust Microalga, Tetraselmis sp. CTP4 (Chlorophyta). Bioprocess Biosyst. Eng. 2020, 43, 785–796. [Google Scholar] [CrossRef]
- Ruiz, C.A.S.; Kwaijtaal, J.; Peinado, O.C.; van den Berg, C.; Wijffels, R.H.; Eppink, M.H.M. Multistep Fractionation of Microalgal Biomolecules Using Selective Aqueous Two-Phase Systems. Acs Sustain. Chem. Eng. 2020, 8, 2441–2452. [Google Scholar] [CrossRef]
- Hieda, Y.; Choe, H.; Ike, H.; Abe, K.; Kumagai, K.; Takeyama, M.; Kawabata, Y.; Kobayashi, N.; Inaba, Y. Bead-beating Assay During Synovial Fluid DNA Extraction Improves Real-time PCR Accuracy for Periprosthetic Joint Infection. J. Orthop. Res. 2024, 42, 2123–2130. [Google Scholar] [CrossRef]
- Costa, M.M.; Spínola, M.P.; Prates, J.A.M. Combination of Mechanical/Physical Pretreatments with Trypsin or Pancreatin on Arthrospira Platensis Protein Degradation. Agriculture 2023, 13, 198. [Google Scholar] [CrossRef]
- Sarikhani, K.; Jeddi, K.; Thompson, R.B.; Park, C.B.; Chen, P. Adsorption of Surface-Modified Silica Nanoparticles to the Interface of Melt Poly(lactic Acid) and Supercritical Carbon Dioxide. Langmuir 2015, 31, 5571–5579. [Google Scholar] [CrossRef]
- Porta, G.D.; Falco, N.; Reverchon, E. Continuous Supercritical Emulsions Extraction: A New Technology for Biopolymer Microparticles Production. Biotechnol. Bioeng. 2010, 108, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Phan, H.T.; Brunner, G. Extraction of Oil and Minor Compounds from Oil Palm Fruit with Supercritical Carbon Dioxide. Processes 2019, 7, 107. [Google Scholar] [CrossRef]
- Eisenmenger, M.J.; Dunford, N.T.; Eller, F.J.; Taylor, S.L.; Martínez, J.L. Pilot-scale Supercritical Carbon Dioxide Extraction and Fractionation of Wheat Germ Oil. J. Am. Oil Chem. Soc. 2006, 83, 863–868. [Google Scholar] [CrossRef]
- Kunasundari, B.; Sudesh, K. Isolation and Recovery of Microbial Polyhydroxyalkanoates. Express Polym. Lett. 2011, 5, 620–634. [Google Scholar] [CrossRef]
- Elsayed, N.S.; Aboshanab, K.M.; Yassien, M.A.; Hassouna, N.A. Kinetic Modeling, Recovery, and Molecular Characterization of Poly-Beta-Hydroxybutyrate Polymer in Acinetobacter Baumannii Isolate P39. Bioprocess Biosyst. Eng. 2018, 41, 1779–1791. [Google Scholar] [CrossRef]
- Ong, S.Y.; Zainab-L, I.; Pyary, S.; Sudesh, K. A Novel Biological Recovery Approach for PHA Employing Selective Digestion of Bacterial Biomass in Animals. Appl. Microbiol. Biotechnol. 2018, 102, 2117–2127. [Google Scholar] [CrossRef]
- Koturević, B.; Adnadjević, B.; Jovanović, J.R. Isothermal Green Microwave-Assisted Extraction of Caffeine from Guarana: A Kinetic Study. Green Process. Synth. 2017, 6, 555–563. [Google Scholar] [CrossRef]
- Kapoore, R.V.; Butler, T.O.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef]
- Sookjitsumran, W.; Devahastin, S.; Mujumdar, A.S.; Chiewchan, N. Comparative Evaluation of Microwave-assisted Extraction and Preheated Solvent Extraction of Bioactive Compounds from a Plant Material: A Case Study with Cabbages. Int. J. Food Sci. Technol. 2016, 51, 2440–2449. [Google Scholar] [CrossRef]
- Zhang, G.; Tyagi, R.D.; Chen, J.; Li, J.; Zhang, X.; Drogui, P.; Dong, X. Lipid Extraction from Oleaginous Microorganism with Electrochemical Method. Eur. J. Lipid Sci. Technol. 2018, 120, 1800215. [Google Scholar] [CrossRef]
- Juneja, P. Biomass Waste Conversion Technologies for Its Utilization and Energy Generation in India: A Perspective. IOP Conf. Ser. Earth Environ. Sci. 2024, 1285, 012010. [Google Scholar] [CrossRef]
- Bartley, L.; Ronald, P.C. Plant and Microbial Research Seeks Biofuel Production from Lignocellulose. Calif. Agric. 2008, 63, 178–184. [Google Scholar] [CrossRef][Green Version]
- Cinar, S.Ö.; Chong, Z.K.; Küçüker, M.A.; Wieczorek, N.; Cengiz, U.; Kuchta, K. Bioplastic Production from Microalgae: A Review. Int. J. Environ. Res. Public Health 2020, 17, 3842. [Google Scholar] [CrossRef]
- Teno, J.; Pardo-Figuerez, M.; Evtoski, Z.; Prieto, C.; Cabedo, L.; Lagarón, J.M. Development of Ciprofloxacin-Loaded Electrospun Yarns of Application Interest as Antimicrobial Surgical Suture Materials. Pharmaceutics 2024, 16, 220. [Google Scholar] [CrossRef]
- ASTM_International. F748–06: 2010 Standard Practice for Selecting Generic Biological Test Methods for Materials and Devices Astm International. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- Arul, S.; Madhuppriya, M.; Fathima, H.P.; Premalatha, R.; Nithya, R. Microalgae scenedesmus sp. Sar1 as a Potential Source for Bioplastic Production. J. Adv. Sci. Res. 2022, 13, 84–92. [Google Scholar] [CrossRef]
- Fiedler, M.; Schoemig, O.; Fischer, F.; Droeder, K. Technological Evaluation of Algae-Based Fillers for Polymer 3D Printing. Sustainability 2023, 15, 4039. [Google Scholar] [CrossRef]
- Abu Danish Aiman Bin Abu, S.; Lim, H.R.; Manickam, S.; Ang, W.L.; Show, P.L. Towards a Sustainable Circular Economy: Algae-Based Bioplastics and the Role of Internet-of-Things and Machine Learning. Chembioeng Rev. 2023, 11, 39–59. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Monje, B.; Alonso, R.; Agostinis, L.; et al. Bio-Based Packaging: Materials, Modifications, Industrial Applications and Sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef]
- Zeller, M.A.; Hunt, R.W.; Jones, A.; Sharma, S. Bioplastics and Their Thermoplastic Blends from Spirulina and Chlorella Microalgae. J. Appl. Polym. Sci. 2013, 130, 3263–3275. [Google Scholar] [CrossRef]
- Loliware. Loliware SEA Technology. Available online: https://www.loliware.com/post/loliware-introduces-first-of-its-kind-seaweed-pellet-technology-to-replace-plastics (accessed on 29 September 2025).
- Santana, I.; Felix, M.; Bengoechea, C. Seaweed as Basis of Eco-Sustainable Plastic Materials: Focus on Alginate. Polymers 2024, 16, 1662. [Google Scholar] [CrossRef]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. An Overview of the Alternative Use of Seaweeds to Produce Safe and Sustainable Bio-Packaging. Appl. Sci. 2022, 12, 3123. [Google Scholar] [CrossRef]
- Steven, S.; Fauza, A.N.; Mardiyati, Y.; Santosa, S.P.; Shoimah, S.M. Facile Preparation of Cellulose Bioplastic from Cladophora sp. Algae via Hydrogel Method. Polymers 2022, 14, 4699. [Google Scholar] [CrossRef] [PubMed]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.; Udduttula, A.; Fan, Z.S.; Chen, J.H.; Sun, A.R.; Zhang, P. Microbial-Derived Polyhydroxyalkanoate-Based Scaffolds for Bone Tissue Engineering: Biosynthesis, Properties, and Perspectives. Front. Bioeng. Biotechnol. 2021, 9, 763031. [Google Scholar] [CrossRef]
- Lee, Y.; Cho, I.J.; Choi, S.Y.; Lee, S.Y. Systems Metabolic Engineering Strategies for Non-Natural Microbial Polyester Production. Biotechnol. J. 2019, 14, 1800426. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Lee, J.-K. Exploiting Polyhydroxyalkanoates for Biomedical Applications. Polymers 2023, 15, 1937. [Google Scholar] [CrossRef]
- Insomphun, C.; Chuah, J.A.; Kobayashi, S.; Fujiki, T.; Numata, K. Influence of Hydroxyl Groups on the Cell Viability of Polyhydroxyalkanoate (PHA) Scaffolds for Tissue Engineering. Acs Biomater. Sci. Eng. 2016, 3, 3064–3075. [Google Scholar] [CrossRef]
- García, G.P.; Sosa-Hernández, J.E.; Rodas-Zuluaga, L.I.; Castillo-Zacarías, C.; Iqbal, H.M.; Parra-Saldívar, R. Accumulation of PHA in the Microalgae scenedesmus sp. Under Nutrient-Deficient Conditions. Polymers 2020, 13, 131. [Google Scholar] [CrossRef]
- Algix. BLOOM® Sustainable Performance Materials. Available online: https://www.bloommaterials.com/ (accessed on 15 August 2025).
- Yap, X.Y.; Gew, L.T.; Khalid, M.; Yow, Y.-Y. Algae-Based Bioplastic for Packaging: A Decade of Development and Challenges (2010–2020). J. Polym. Environ. 2023, 31, 833–851. [Google Scholar] [CrossRef]
- Quiroz, D.; McGowen, J.A.; Quinn, J.C. Techno-economic analysis of microalgae cultivation strategies: Batch and semi-continuous approaches. Algal Res. 2025, 90, 104109. [Google Scholar] [CrossRef]
- Gundlapalli, M.; Ganesan, S. Polyhydroxyalkanoates (PHAs): Key Challenges in production and sustainable strategies for cost reduction within a circular economy framework. Results Eng. 2025, 26, 105345. [Google Scholar] [CrossRef]
- Guillén-Cuevas, K.; Ortiz-Espinoza, A.P.; Özinan, E.; Jiménez-Gutiérrez, A.; Kazantzis, N.; El-Halwagi, M.M. Incorporation of Safety and Sustainability in Conceptual Design via a Return on Investment Metric. Acs Sustain. Chem. Eng. 2017, 6, 1411–1416. [Google Scholar] [CrossRef]
- Atiwesh, G.; Mikhael, A.; Parrish, C.C.; Banoub, J.; Le, T.A.T. Environmental Impact of Bioplastic Use: A Review. Heliyon 2021, 7, e07918. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. Acs Sustain. Chem. Eng. 2017, 6, 32–48. [Google Scholar] [CrossRef]
- Diaz, C.J.; Douglas, K.J.; Kang, K.; Kolarik, A.L.; Malinovski, R.; Torres-Tiji, Y.; Molino, J.V.D.; Badary, A.; Mayfield, S.P. Developing Algae as a Sustainable Food Source. Front. Nutr. 2023, 9, 1029841. [Google Scholar] [CrossRef]
- Hui, X.; Lee, U.; Coleman, A.; Wigmosta, M.S.; Sun, N.; Hawkins, T.R.; Wang, M. Balancing Water Sustainability and Productivity Objectives in Microalgae Cultivation: Siting Open Ponds by Considering Seasonal Water-Stress Impact Using AWARE-US. Environ. Sci. Technol. 2020, 54, 2091–2102. [Google Scholar] [CrossRef]
- Elkaliny, N.E.; Alzamel, N.M.; Moussa, S.H.; Elodamy, N.I.; Madkor, E.A.; Ibrahim, E.M.; Elshobary, M.E.; Ismail, G.A. Macroalgae Bioplastics: A Sustainable Shift to Mitigate the Ecological Impact of Petroleum-Based Plastics. Polymers 2024, 16, 1246. [Google Scholar] [CrossRef]
- Boneberg, B.S.; Machado, G.; Santos, D.F.; Gomes, F.J.B.; Faria, D.J.; Gomes, L.; Santos, F. Biorefinery of Lignocellulosic Biopolymers. Rev. Eletrônica Científica Uergs 2016, 2, 79. [Google Scholar] [CrossRef]
- Virtue Market Research. Algae Bioplastics Market; Virtue Market Research: Pune, India, 2025. [Google Scholar]
- Statista. Price of High-Density Polyethylene Forecast, Globally; Statista: Hamburg, Germany, 2025. [Google Scholar]
- Project Drawdown. Bioplastics|Solution; Project Drawdown: St. Paul, MN, USA, 2025. [Google Scholar]
- Gerben, H.; Edse, D.; Coco, Z. How the Plastics Industry Can Function Without Fossil Fuels—And at What Cost; ING Think: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Acumen Research and Consulting. Algae Bioplastics Market Overview; Acumen Research and Consulting: Pune, India, 2025. [Google Scholar]
- Acumen Research and Consulting. Algae Bioplastics Market Press Release; Acumen Research and Consulting: Pune, India, 2024. [Google Scholar]
- Chen, G.; Li, J.; Sun, Y.; Wang, Z.; Leeke, G.A.; Moretti, C.; Cheng, Z.; Wang, Y.; Li, N.; Mu, L.; et al. Replacing Traditional Plastics with Biodegradable Plastics: Impact on Carbon Emissions. Engineering 2024, 32, 152–162. [Google Scholar] [CrossRef]
- Sales, J.C.S.; Santos, A.G.; de Castro, A.M.; Coelho, M.A.Z. A critical view on the technology readiness level (TRL) of microbial plastics biodegradation. World J. Microbiol. Biotechnol. 2021, 37, 116. [Google Scholar] [CrossRef] [PubMed]
- Morsi, H.H.; Eladel, H.; Maher, A. Coupling Nutrient Removal and Biodiesel Production by the Chlorophyte Asterarcys Quadricellulare Grown in Municipal Wastewater. Bioenergy Res. 2021, 15, 193–201. [Google Scholar] [CrossRef]
- Kang, C.; Liu, J.J.; Woo, N.-S.; Won, W. Process Design for the Sustainable Production of Butyric Acid Using Techno-Economic Analysis and Life Cycle Assessment. Acs Sustain. Chem. Eng. 2023, 11, 4430–4440. [Google Scholar] [CrossRef]
- Fatmei, S.M.; Khalilzadeh, R.; Azizi, H.R.; Alipouryan, S. Biodiesel Production: Effects of the Feeding Process on Biomass and Lipid Productivity. Chem. Eng. Technol. 2016, 39, 1468–1474. [Google Scholar] [CrossRef]
- Cruz, R.M.; Krauter, V.; Krauter, S.; Agriopoulou, S.; Weinrich, R.; Herbes, C.; Scholten, P.B.V.; Uysal-Unalan, I.; Söğüt, E.; Kopacic, S.; et al. Bioplastics for Food Packaging: Environmental Impact, Trends and Regulatory Aspects. Foods 2022, 11, 3087. [Google Scholar] [CrossRef]
- Garrido, R.; Cabeza, L.F.; Falguera, V. An Overview of Bioplastic Research on Its Relation to National Policies. Sustainability 2021, 13, 7848. [Google Scholar] [CrossRef]
- Malm, M.; Liceaga, A.M.; Martin-Gonzalez, F.S.; Jones, O.G.; García, J.; Kaplan, I. Development of Chitosan Films from Edible Crickets and Their Performance as a Bio-Based Food Packaging Material. Polysaccharides 2021, 2, 744–758. [Google Scholar] [CrossRef]
- Frisvold, G.B.; Moss, S.M.; Hodgson, A.; Maxon, M.E. Understanding the U.S. Bioeconomy: A New Definition and Landscape. Sustainability 2021, 13, 1627. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhao, H.-Y. Initial Analysis of China’s Industrial Economy Planning from the 14th Five Year Plan. In DEStech Transactions on Economics, Business and Management; DEStech Publications, Inc: Lancaster, PA, USA, 2021. [Google Scholar] [CrossRef]
- Yang, F.; Ren, Y.; Zuo, S.; Ju, J.; Yang, M. Allocation of Carbon Intensity Target Reduction Rate for China’s 332 Cities Based on Entropy Method and Improved Equal-Proportion Distribution Method. Res. Sq. preprint. 2025. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, P.; He, L.; Zhang, Y.; Liu, D. Can China Achieve the 2020 and 2030 Carbon Intensity Targets Through Energy Structure Adjustment? Energies 2018, 11, 2721. [Google Scholar] [CrossRef]
- Portner, B.W.; Endres, C.H.; Brück, T.; Garbe, D. Life Cycle Greenhouse Gas Emissions of Microalgal Fuel from Thin-Layer Cascades. Bioprocess Biosyst. Eng. 2021, 44, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Rajvanshi, M.; Sayre, R.T. Recent Advances in Algal Biomass Production. In Biotechnological Applications of Biomass; InTechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Deepa, P.; Sowndhararajan, K.; Kim, S. A Review of the Harvesting Techniques of Microalgae. Water 2023, 15, 3074. [Google Scholar] [CrossRef]
- Gan, X.; Klose, H.; Reinecke, D.L. Optimizing Nutrient Removal and Biomass Production of the Algal Turf Scrubber (ATS) Under Variable Cultivation Conditions by Using Response Surface Methodology. Front. Bioeng. Biotechnol. 2022, 10, 962719. [Google Scholar] [CrossRef] [PubMed]
- Arora, N.; Lo, E.; Legall, N.; Philippidis, G.P. A Critical Review of Growth Media Recycling to Enhance the Economics and Sustainability of Algae Cultivation. Energies 2023, 16, 5378. [Google Scholar] [CrossRef]
- Kruger, J.S.; Wiatrowski, M.; Davis, R.; Dong, T.; Knoshaug, E.P.; Nagle, N.; Laurens, L.M.L.; Pienkos, P.T. Enabling Production of Algal Biofuels by Techno-Economic Optimization of Co-Product Suites. Front. Chem. Eng. 2022, 3, 803513. [Google Scholar] [CrossRef]
- Abdul-Latif, N.-I.S.; Ong, M.Y.; Nomanbhay, S.; Salman, B.; Show, P.L. Estimation of Carbon Dioxide (CO2) Reduction by Utilization of Algal Biomass Bioplastic in Malaysia Using Carbon Emission Pinch Analysis (CEPA). Bioengineered 2020, 11, 154–164. [Google Scholar] [CrossRef]
- Peng, M.; Liang, Z. Degeneration of industrial bacteria caused by genetic instability. World J. Microbiol. Biotechnol. 2020, 36, 119. [Google Scholar] [CrossRef]
- Volova, T.; Boyandin, A.; Vasiliev, A.; Karpov, V.; Prudnikova, S.; Mishukova, O.; Boyarskikh, U.; Filipenko, M.; Rudnev, V.; Xuân, B.B. Biodegradation of polyhydroxyalkanoates (PHAs) in tropical coastal waters and identification of PHA-degrading bacteria. Polym. Degrad. Stab. 2010, 95, 2350–2359. [Google Scholar] [CrossRef]
- Ali, S.; Isha; Chang, Y.-C. Ecotoxicological Impact of Bioplastics Biodegradation: A Comprehensive Review. Processes 2023, 11, 3445. [Google Scholar] [CrossRef]
- Bao, Q.; Zhang, Z.; Luo, H.; Tao, X. Evaluating and Modeling the Degradation of PLA/PHB Fabrics in Marine Water. Polymers 2022, 15, 82. [Google Scholar] [CrossRef]
- US_DoE_(Bioenergy_Technologies_Office). 2023 Billion-Ton Report: 7. Emerging; U.S. Department of Energy: Washington, DC, USA, 2023. [Google Scholar]
- Wiatrowski, M.; Klein, B.C.; Davis, R.W.; Quiroz-Arita, C.; Tan, E.C.D.; Hunt, R.W.; Davis, R.E. Techno-economic assessment for the production of algal fuels and value-added products: Opportunities for high-protein microalgae conversion. Biotechnol. Biofuels Bioprod. 2022, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Agathos, S.N.; Gao, Z. Editorial: Emerging trends in genetic engineering of microalgae. Front. Bioeng. Biotechnol. 2024, 12, 1403711. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, D.; Chen, H.; Wang, Q. Advances in Genetic Engineering in Improving Photosynthesis and Microalgal Productivity. Int. J. Mol. Sci. 2023, 24, 1898. [Google Scholar] [CrossRef] [PubMed]
- Dilkes-Hoffman, L.S.; Lant, P.A.; Laycock, B.; Pratt, S. The rate of biodegradation of PHA bioplastics in the marine environment: A meta-study. Mar. Pollut. Bull. 2019, 142, 15–24. [Google Scholar] [CrossRef]
- Read, T.; Chaléat, C.; Laycock, B.; Pratt, S.; Lant, P.; Chan, C.M. Lifetimes and mechanisms of biodegradation of polyhydroxyalkanoate (PHA) in estuarine and marine field environments. Mar. Pollut. Bull. 2024, 209, 117114. [Google Scholar] [CrossRef]
- All_About_Feed. Algae Cultivation Profitable by 2025. Available online: https://www.allaboutfeed.net/animal-feed/raw-materials/algae-cultivation-profitable-by-2025/ (accessed on 15 August 2025).
- Kumar, G.; Shekh, A.; Jakhu, S.; Sharma, Y.; Kapoor, R.; Sharma, T.R. Bioengineering of Microalgae: Recent Advances, Perspectives, and Regulatory Challenges for Industrial Application. Front. Bioeng. Biotechnol. 2020, 8, 914. [Google Scholar] [CrossRef]
- Procurement Resource. Algae Based Biofuel Production Cost Report; Procurement Resource: Lucknow, India, 2025. [Google Scholar]
- Imarc Group. Bioplastic Manufacturing Plant Project Report. Available online: https://www.imarcgroup.com/bioplastic-manufacturing-plant-project-report (accessed on 15 August 2025).
- Catarci Carteny, C.; Blust, R. Not Only Diamonds Are Forever: Degradation of Plastic Films in a Simulated Marine Environment. Front. Environ. Sci. 2021, 9, 662844. [Google Scholar] [CrossRef]
- Omura, T.; Tsujimoto, S.; Kimura, S.; Maehara, A.; Kabe, T.; Iwata, T. Marine biodegradation of poly[(R)-3-hydroxybutyrate-co-4-hydroxybutyrate] elastic fibers in seawater: Dependence of decomposition rate on highly ordered structure. Front. Bioeng. Biotechnol. 2023, 11, 1303830. [Google Scholar] [CrossRef]
- Park, H.; Lee, C.G. Theoretical Calculations on the Feasibility of Microalgal Biofuels: Utilization of Marine Resources Could Help Realizing the Potential of Microalgae. Biotechnol. J. 2016, 11, 1461–1470. [Google Scholar] [CrossRef]
- Volova, T.G.; Demidenko, A.V.; Kiselev, E.G.; Baranovskiy, S.V.; Shishatskaya, E.I.; Zhila, N.O. Polyhydroxyalkanoate Synthesis Based on Glycerol and Implementation of the Process Under Conditions of Pilot Production. Appl. Microbiol. Biotechnol. 2018, 103, 225–237. [Google Scholar] [CrossRef]
- Spillias, S.; Kelly, R.; Cottrell, R.S.; O’Brien, K.R.; Im, R.-Y.; Kim, J.Y.; Lei, C.; Leung, R.W.S.; Matsuba, M.; Reis, J.A.; et al. The Empirical Evidence for the Social-Ecological Impacts of Seaweed Farming. PLoS Sustain. Transform. 2023, 2, e0000042. [Google Scholar] [CrossRef]
- Xiao, X.; Agustí, S.; Lin, F.; Li, K.; Pan, Y.; Yu, Y.; Zheng, Y.; Wu, J.; Duarte, C.M. Nutrient Removal from Chinese Coastal Waters by Large-Scale Seaweed Aquaculture. Sci. Rep. 2017, 7, 46613. [Google Scholar] [CrossRef]
- Gao, G.; Gao, L.; Jiang, M.; Jian, A.; He, L. The Potential of Seaweed Cultivation to Achieve Carbon Neutrality and Mitigate Deoxygenation and Eutrophication. Environ. Res. Lett. 2021, 17, 014018. [Google Scholar] [CrossRef]
- Bu, D.; Zhu, Q.; Li, J.; Huang, J.; Zhuang, Y.; Yang, W.; Qi, D. Mariculture May Intensify Eutrophication but Lower N/P Ratios: A Case Study Based on Nutrients and Dual Nitrate Isotope Measurements in Sansha Bay, Southeastern China. Front. Mar. Sci. 2024, 11, 1351657. [Google Scholar] [CrossRef]
- Thomas, J.-B.; Potting, J.; Gröndahl, F. Environmental Impacts of Seaweed Cultivation: Kelp Farming and Preservation. In SEAWEED and Microalgae as Alternative Sources of Protein; Burleigh Dodds: Cambridge, UK, 2021; pp. 165–192. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, L.; Cheung, W.W.L.; Sumaila, U.R. Global Estimates of Suitable Areas for Marine Algae Farming. Environ. Res. Lett. 2023, 18, 064028. [Google Scholar] [CrossRef]
- Morais, A.R.C.; Jian, Z.; Dong, H.; Otto, W.G.; Mokomele, T.; Hodge, D.B.; Balan, V.; Dale, B.E.; Bogel-Łukasik, R.; da Costa Sousa, L. Development of an Ammonia Pretreatment That Creates Synergies Between Biorefineries and Advanced Biomass Logistics Models. Green Chem. 2022, 24, 4443–4462. [Google Scholar] [CrossRef]
- Sharma, B.; Brandt, C.C.; McCullough-Amal, D.; Langholtz, M.; Webb, E. Assessment of the Feedstock Supply for Siting Single- and Multiple-feedstock Biorefineries in the USA and Identification of Prevalent Feedstocks. Biofuels Bioprod. Biorefin. 2020, 14, 578–593. [Google Scholar] [CrossRef]
- Silkina, A.; Zacharof, M.P.; Ginnever, N.E.; Gerardo, M.L.; Lovitt, R.W. Testing the Waste Based Biorefinery Concept: Pilot Scale Cultivation of Microalgal Species on Spent Anaerobic Digestate Fluids. Waste Biomass Valorization 2019, 11, 3883–3896. [Google Scholar] [CrossRef]
- Arora, K.; Kaur, P.; Kumar, P.; Singh, A.; Patel, S.K.S.; Li, X.; Yang, Y.H.; Bhatia, S.K.; Kulshrestha, S. Valorization of Wastewater Resources into Biofuel and Value-Added Products Using Microalgal System. Front. Energy Res. 2021, 9, 646571. [Google Scholar] [CrossRef]
- Sarker, N.K.; Salam, P.A. Design of batch algal cultivation systems and ranking of the design parameters. Energy Ecol. Environ. 2020, 5, 196–210. [Google Scholar] [CrossRef]
- Sarker, N.K.; Salam, P.A. Indoor and outdoor cultivation of Chlorella vulgaris and its application in wastewater treatment in a tropical city—Bangkok, Thailand. SN Appl. Sci. 2019, 1, 1645. [Google Scholar] [CrossRef]
- Babbar, S.; Gangwar, T.; Bhasin, N.; Shriya; Thota, K.; Arora, P. AI-Optimized Carbon Capture & Fermentation for Biofuels: A Sustainable Approach. IOSR J. Biotechnol. Biochem. IOSR-JBB 2025, 11, 42–60. [Google Scholar] [CrossRef]
- Abdullah, M.; Malik, H.A.; Ali, A.; Boopathy, R.; Vo, P.H.N.; Danaee, S.; Ralph, P.; Malik, S. AI-Driven Algae Biorefineries: A New Era for Sustainable Bioeconomy. Curr. Pollut. Rep. 2025, 11, 21. [Google Scholar] [CrossRef]
- Alzahmi, A.S.; Daakour, S.; Nelson, D.; Al-Khairy, D.; Twizere, J.-C.; Salehi-Ashtiani, K. Enhancing algal production strategies: Strain selection, AI-informed cultivation, and mutagenesis. Front. Sustain. Food Syst. 2024, 8. [Google Scholar] [CrossRef]
- Imamoglu, E. Artificial Intelligence and/or Machine Learning Algorithms in Microalgae Bioprocesses. Bioengineering 2024, 11, 1143. [Google Scholar] [CrossRef] [PubMed]
- Oruganti, R.K.; Biji, A.P.; Lanuyanger, T.; Show, P.L.; Sriariyanun, M.; Upadhyayula, V.K.K.; Gadhamshetty, V.; Bhattacharyya, D. Artificial intelligence and machine learning tools for high-performance microalgal wastewater treatment and algal biorefinery: A critical review. Sci. Total Environ. 2023, 876, 162797. [Google Scholar] [CrossRef]
- Anli Dino, A.; Kishore, G.; Rokhum, S.L.; Baskar, G. Bioplastic production using waste macroalgal biomass: A holistic review on challenges, prospects, economic viability and sustainability analysis. J. Environ. Chem. Eng. 2025, 13, 116108. [Google Scholar] [CrossRef]
- Chaudry, S.; Hurtado-McCormick, V.; Cheng, K.Y.; Willis, A.; Speight, R.; Kaksonen, A.H. Microalgae to bioplastics—Routes and challenges. Clean. Eng. Technol. 2025, 25, 100922. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Er, A.C.; Aiyub, K.; Mohd Yasin, N.H.; Ngan, S.L.; Chew, K.W.; Khoo, K.S.; Ling, T.C.; Juan, J.C.; Ma, Z.; et al. Current status and perspectives of algae-based bioplastics: A reviewed potential for sustainability. Algal Res. 2023, 71, 103078. [Google Scholar] [CrossRef]
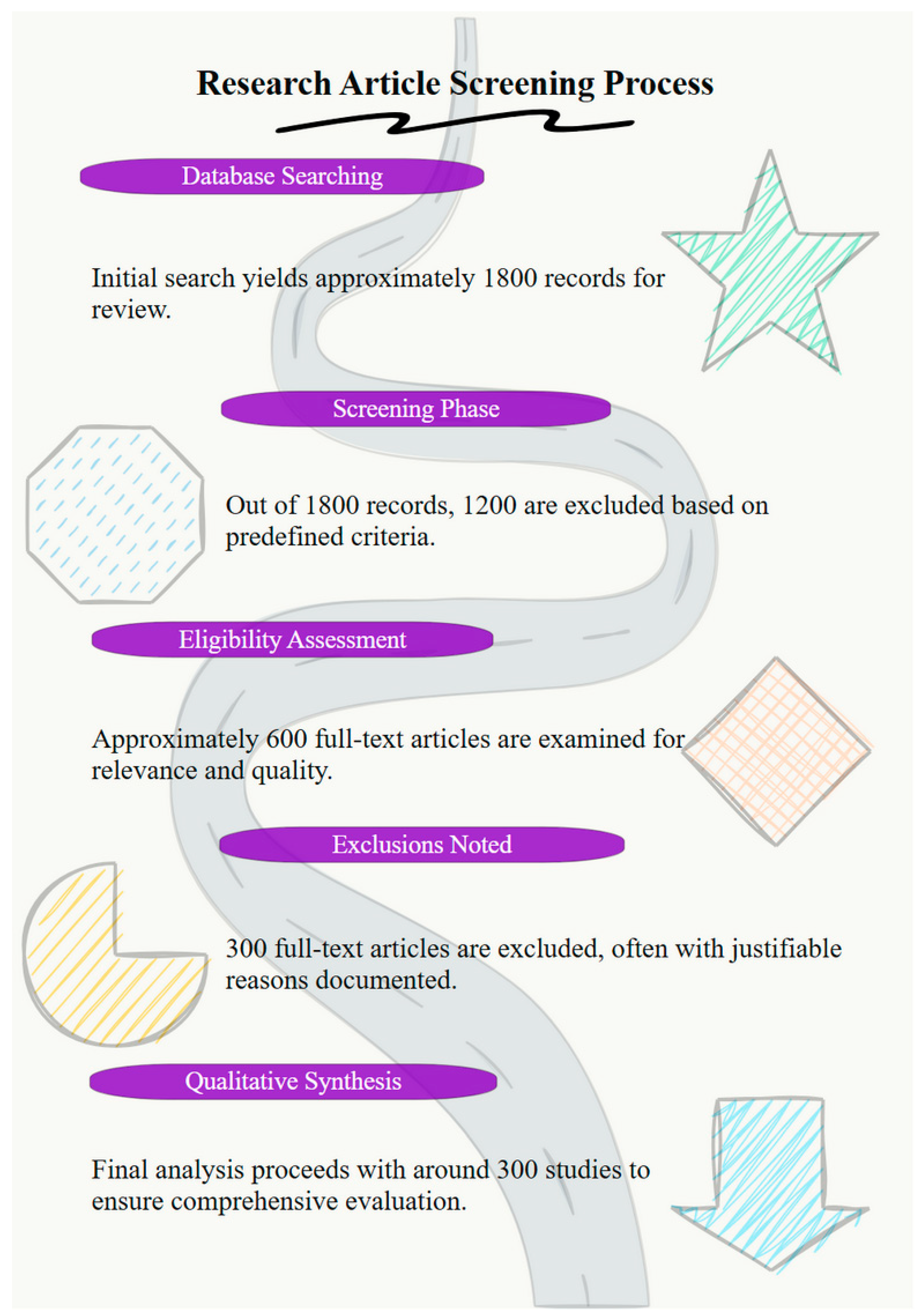

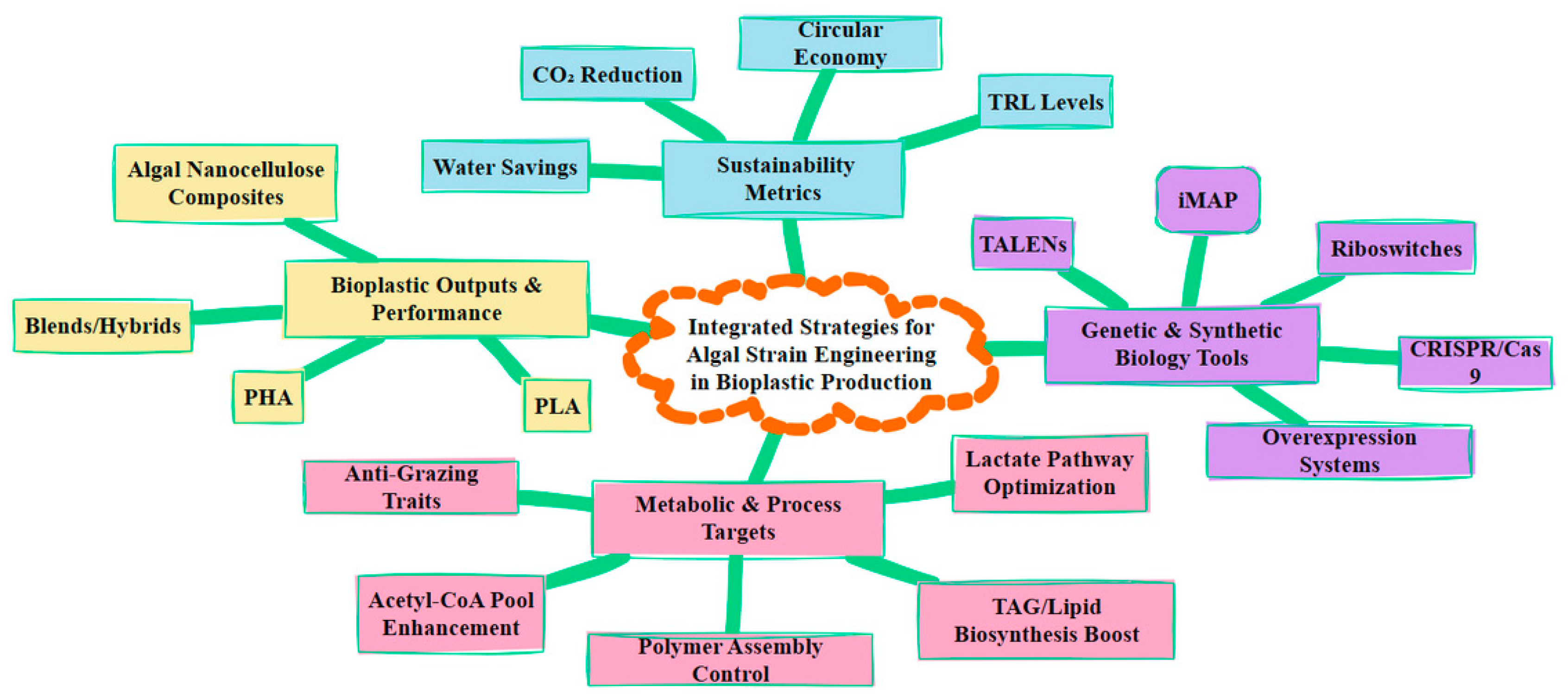
| PLA Blend | Mechanical Properties * | Thermal Properties * | Biodegradation Rate ** | Key Applications | Advantages | Limitations |
|---|---|---|---|---|---|---|
| PLA–PCL | Tensile strength ~150–170 MPa; elongation 100–300% [72,73,74] | Tg: ~55 °C; Tm: ~170 °C (PLA), ~60 °C (PCL) [75] | Moderate; faster than neat PLA in compost/soil [76,77] | Flexible packaging, biomedical scaffolds | High flexibility, good toughness | Lower stiffness, slower biodegradation than other blends |
| PLA–PBS | Tensile strength ~45–50 MPa; elongation 50–150% [78,79,80] | Tg: ~55 °C; Tm: ~114 °C (PBS) [78,79,80] | Faster than PLA in compost [78,79,80] | Packaging films, agricultural mulch | Balanced strength/flexibility | Limited thermal stability |
| PLA–PHA | Tensile strength ~40–55 MPa; elongation 10–50% [81,82] | Tg: ~55 °C; Tm: 150–175 °C (PHA) [81,82] | Enhanced biodegradation in marine/soil [83] | Medical devices, food packaging | Good barrier properties | Brittleness at high PLA ratios |
| PLA–Natural Fibers (e.g., flax, hemp) | Tensile strength 50–70 MPa; elongation 5–15% [84,85] | Tg: ~55 °C; variable Tm [86,87] | Slow in ambient soil, faster in compost [88,89] | Automotive interiors, construction | High stiffness, renewable fillers | Poor impact resistance, moisture sensitivity |
| PLA–Starch | Tensile strength ~20–40 MPa; elongation 2–10% [90,91,92] | Tg: ~55 °C; Tm ~160 °C [90,91,92] | Rapid in compost/soil [93,94] | Disposable cutlery, packaging | Low cost, high biodegradability | Poor water resistance, reduced strength |
| PLA–Chitosan | Tensile strength ~30–50 MPa; elongation 2–20% [95,96] | Tg: ~55 °C; variable Tm [95,96] | Rapid in compost and marine [97] | Antimicrobial films, wound dressings | Antimicrobial activity | Limited processability |
| PLA–Silk Fibroin | Tensile strength ~40–60 MPa; elongation 5–15% [98,99,100,101] | Tg: ~55 °C; Tm ~220 °C (silk) [98,99,100,101] | Moderate in compost [102,103] | Biomedical scaffolds, sutures | High biocompatibility, strength | Higher cost, sourcing constraints |
| PLA–Graphene Oxide | Tensile strength ~60–75 MPa; elongation 2–10% [104,105] | Tg: ~55 °C; improved thermal stability [106] | Similarly to PLA | Electronics, high-strength films | Improved mechanical/thermal properties | Non-biodegradable filler may reduce compostability |
| Type of Algal Bioplastic | Key Properties | Primary Applications | Commercial Potential |
|---|---|---|---|
| Polyhydroxyalkanoates (PHAs) | Biodegradable (degradation time: 1.5–3.5 years) [49]; tensile strength ~20–50 MPa, elongation at break ~5–600%; average productivity 1.33 g/L·h [114,115,116,117] | Packaging, medical devices such as sutures and drug delivery systems | High production costs but efficiency improves with biotechnological advances; promising feedstocks include agricultural residues. |
| Polylactic Acid (PLA) | Compostable in ~60–90 days under industrial conditions; tensile strength ~50–70 MPa, elongation at break ~6–15%; lower carbon footprint from algal feedstock compared to corn [115,118,119,120,121,122] | Food packaging, 3D printing, biomedical tools, disposable cutlery. | Scale limitations in algae cultivation raise costs compared to corn-based PLA. |
| Algal Nanocellulose | High tensile strength ~150 MPa; lightweight (density ~1.5 g/cm3); high crystallinity and thermal stability [123,124,125] | Bioplastic films, reinforced composites, biomedical uses. | Extraction complexity and scalability remain challenges. |
| Algae–Starch Composites | Tensile strength ~25–35 MPa; improved biodegradability compared to plant starch bioplastics [109,113,126] | Flexible packaging, disposable products. | Economically advantageous with minimal resource requirements for algae cultivation. |
| PHB/PLA Hybrids | Tensile strength ~45–70 MPa; improved toughness vs. pure PHB [127,128,129,130] | Packaging and consumer goods. | Potential to reduce brittleness while maintaining biodegradability. |
| Algae–Polyurethane Composites | Tensile strength > 60 MPa; high flexibility; improved biodegradability compared to conventional polyurethane [131,132,133,134] | Packaging films, durable consumer goods | Renewable-resource-based polyurethane with enhanced microbial degradation rates. |
| Algae–Polyvinyl Alcohol (PVA) Blends | Elongation at break up to 667%; high biodegradability [135,136,137,138] | Flexible packaging films. | Requires compatibilization to address miscibility issues. |
| Algae–Corn Gluten Meal Blends | Tensile strength ~40 MPa; improved flexibility and processability [109,139,140,141] | Packaging, agricultural films | Dual waste management benefits using agricultural by-products with algae cultivation. |
| Approach/Tool | Target Organism(s) | Genetic/Process Intervention | Key Outcome(s) | Reported Yield/ Improvement | Reference(s) |
|---|---|---|---|---|---|
| Targeted mutagenesis (TALEN) | Phaeodactylum tricornutum | Inactivation of Hotdog-fold thioesterase; two-phase cultivation (nutrient-rich → nutrient-stress) | Enhanced lipid accumulation for bioplastic precursors | TAG content ↑ from 15% → 43% DCW (~3× increase) | [164] |
| Multi-species engineered protection | Botryococcus braunii (companion for Nannochloropsis) | Genetic modifications to reduce grazer susceptibility | Reduced biomass loss; improved hydrocarbon output | Grazing loss ↓ 70%; hydrocarbon yield ↑ 35% | [165] |
| High-throughput strain screening (iMAP) | Chlamydomonas reinhardtii | Photobioreactor-based integrated analysis for growth & productivity metrics | Rapid identification of top-performing strains | Biomass productivity > 80 mg/L/day | [166] |
| Novel bioplastic blend formulation | Eucheuma cottonii | Biomass + natural latex plasticizers (Artocarpus altilis, Calotropis gigantea) | Balanced mechanical strength & biodegradability | Qualitative mechanical retention with renewable inputs | [109] |
| Nutrient supplementation for PHB | Chlorogloea fritschii | Sodium acetate addition to cultivation medium | Enhanced PHB biosynthesis | PHB ↑ to 23% DCW | [167] |
| Cyanobacterial saline cultivation | Cyanobacteria (various) | Growth in saline & wastewater media | PHB production with reduced freshwater demand | PHB up to 1.2 g/L under saline conditions | [168] |
| Pathway overexpression–acetyl-CoA | Microalgae (engineered) | Overexpression of acetyl-CoA synthetase | Increased precursor availability for PHAs/PLAs | PHA yield ↑ 25–40%; up to 2.2 g/L | [144,147,148,169] |
| Lactate pathway optimization | Microalgae (engineered) | Enzyme modulation for lactate utilization | Balanced biomass & polymer accumulation | Improved PHA titers (quantitative gains context-dependent) | [170] |
| CRISPR/Cas metabolic rerouting | Chlamydomonas reinhardtii | Knockouts to boost acetyl-CoA flux | Increased polymer precursor synthesis | Acetyl-CoA ↑ 1.6×; PHA titer up to 1.5 g/L | [171,172,173] |
| Riboswitch-based pathway control | Microalgae (various) | Conditional gene expression in response to metabolites | Fine-tuned polymer synthesis without growth penalty | Qualitative yield and growth improvements | [174,175,176,177,178,179,180] |
| Route | Feedstock | Reported Product Yields | Key Advantages | Main Challenges | References |
|---|---|---|---|---|---|
| Heterotrophic Fermentation | Algal biomass hydrolysates (enzymatically or chemically pretreated) | ~30 g/L fermentable sugars; PHA titers up to 250 mg/L (C/N ratio dependent) | High sugar conversion efficiency (70–85% glucose recovery); scalable process knowledge from industrial fermentations | Cost of enzymatic pretreatment; potential inhibitory compounds in hydrolysates | [201,202] |
| Dark Fermentation | Co-fermentation of algal biomass with food/agro-waste | 4–7 g/L VFAs; PHA yield ~8 g/L (strain & mode dependent) | Valorizes multiple waste streams; robust mixed-culture metabolism | Optimization of VFA-to-PHA conversion; controlling microbial community dynamics | [183,203] |
| Direct Algal Biosynthesis | Engineered microalgae (e.g., Pseudomonas putida pathways integrated into algal hosts) | PHA content up to 20% CDW; productivity ~1.06 g/L/day | Eliminates need for separate fermentation step; lower substrate preparation costs | Genetic stability of engineered strains; scale-up challenges | [204,205,206,207] |
| Method | Mechanism | Target Polymer(s) | Recovery Yield (%) | Purity (%) | Processing Time (h) | Scalability | Cost-Efficiency | Environmental Impact | TRL * | Residue Valorization Potential | Key Limitation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solvent Extraction | Dissolves target polymers from algal biomass using solvents, followed by separation and precipitation. | PHAs, PLAs | 60–95 [227] | 80–95 | 1–5 | High | Moderate | Moderate; solvent use & toxicity | 5 | Residues usable for fertilizer or biogas [228,229] | Solvent toxicity and disposal issues |
| Enzymatic Digestion | Enzymes degrade cell walls, releasing PHAs/PLAs. | PHAs | 40–70 [230,231] | 85–95 | 6–24 | Moderate | Moderate | Low toxicity | 5 | Animal feed or soil amendment [232,233] | Inconsistent yields between biomass types |
| Mechanical Disruption (e.g., bead milling) | Physical disruption of algal cells to release intracellular polymers. | PHAs, PLAs | 30–50 [234,235] | 70–90 | 1–2 | Moderate | Moderate | High; minimal solvents | 4 | Residues converted to bioenergy [236,237,238,239] | Energy-intensive equipment |
| Supercritical CO2 Extraction | Uses supercritical CO2 to solubilize polymers with minimal solvents. | PHAs, PLAs | 70–95 [150,240,241,242] | 90–98 | 3–6 | High | High | Low emissions | 7 | Residues usable for biofuel/fertilizers [243] | High equipment cost |
| Chemical Digestion (acid/alkali) | Chemical agents degrade biomass to release polymers. | PHAs | 50–80 [244,245] | 70–90 | 2–4 | Moderate | Low | Moderate; hazardous chemicals | 5 | Compost or biogas production [246] | Hazardous chemical handling |
| Microwave-Assisted Extraction | Microwave energy heats and disrupts cells, enhancing polymer release. | PHAs, PLAs | 60–80 [247,248] | 80–95 | 0.5–2 | High | Moderate | Low energy use | 6 | Residues usable as biofertilizers [249] | Specialized equipment required |
| Electrochemical Methods | Electrical energy facilitates polymer release from biomass. | PHAs, PLAs | 50–70 [250,251,252] | 75–90 | 1–3 | Moderate | Moderate | Moderate; energy-intensive | 4 | Feed or compost [250,251] | Limited commercial application |
| Application/ Product Category | Feedstock Source | Key Mechanical Properties | Biodegradation Timeline & Conditions | Approx. Cost Range (USD/kg) | Example Commercial Products | Selected References |
|---|---|---|---|---|---|---|
| Bioplastic Packaging | Microalgae (e.g., Chlorella, Spirulina) | Tensile strength typically 12–25 MPa (varies by formulation); water absorption often <15% (24 h) | 60–180 days in composting (varies by formulation and processing) | Cost varies; estimates suggest higher than petro-plastics; see market reports | Algal-based films, molded packaging | [109,113,227] |
| Biomedical Applications | PHA-producing microalgae; engineered strains | Biodegradation and cell viability depend on polymer type and formulation; tested per ASTM F748 guidance in biomedical contexts | Physiological conditions; in vitro/in vivo results vary (see cited studies) | Higher than commodity plastics; specialized biomedical grade pricing applies | Biodegradable sutures, scaffolds | [266,267,268,269,270] |
| 3D Printing & Automotive Parts | Algal biopolymers, PHA blends | Density ~1.2 g/cm3; tensile modulus typically 0.7–1.5 GPa for PLA blends | ~120 days in composting (PLA blends) | Cost varies; currently above petro-based equivalents | Algal-PLA filaments, lightweight panels | [64,113,253] |
| Algix—BLOOM® Foam | Spirulina, Chlorella (from aquaculture/wastewater) | Comparable to EVA in resilience and elastic recovery | Not biodegradable; biomass content offsets fossil EVA use | Not publicly disclosed | BLOOM® Foam, BLOOM® TPE | [113,271] |
| Loliware | Seaweed (macroalgae) | Stiffness & heat tolerance comparable to conventional cutlery | Home/industrial compostable; company claims partial marine degradability | Not publicly disclosed | Compostable straws, utensils | [143,261,262] |
| Cladophora Bioplastics | Cladophora cellulose | High-crystallinity cellulose composites; improved modulus & tensile strength | Biodegradation varies by formulation; some show rapid mass loss in lab tests | Not publicly disclosed | Specialty packaging, consumer goods | [264,265,272] |
| Challenge Category | Key Issues & Metrics | Representative TRL | Notes/ References |
|---|---|---|---|
| Production Economics | Cultivation & harvesting cost: $0.85–$1.50/kg biomass; total production $1.70–$2.50/kg vs. petroplastics $0.90–$2.20/kg | 5–7 | [311,312] |
| Biological & Technical Reliability | Low transformation efficiency across microalgae species; challenges with stable genetic integration; strain-dependent variability in modification success; limited genetic toolbox compared to other microorganisms | 4–6 | [313,314] |
| Quality Consistency | Variability in mechanical & biodegradability metrics due to biomass source, cultivation conditions; impurity risks from extraction | 5–7 | [315,316] |
| Scale-up Infrastructure | Limited commercial-scale photobioreactors; high capital requirements (250,000–272,000 USD/hectare); energy-intensive processing; labor-intensive installation; biomass costs exceed terrestrial alternatives | 6–8 | [314,317,318] |
| Policy & Market Integration | Lack of harmonized regulations; infrastructure not designed for algal feedstocks; low consumer awareness; disposal confusion | 6–8 | [160,314] |
| Environmental Fate & Marine Biodegradability | PHB: 54–58% loss in 160 days in seawater; PLA: minimal degradation in marine conditions; microplastic risks from incomplete breakdown; biodegradation highly environment-dependent | 4–6 | [319,320,321,322,323,324] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarker, N.K.; Kaparaju, P. Advances in Algae-Based Bioplastics: From Strain Engineering and Fermentation to Commercialization and Sustainability. Fermentation 2025, 11, 574. https://doi.org/10.3390/fermentation11100574
Sarker NK, Kaparaju P. Advances in Algae-Based Bioplastics: From Strain Engineering and Fermentation to Commercialization and Sustainability. Fermentation. 2025; 11(10):574. https://doi.org/10.3390/fermentation11100574
Chicago/Turabian StyleSarker, Nilay Kumar, and Prasad Kaparaju. 2025. "Advances in Algae-Based Bioplastics: From Strain Engineering and Fermentation to Commercialization and Sustainability" Fermentation 11, no. 10: 574. https://doi.org/10.3390/fermentation11100574
APA StyleSarker, N. K., & Kaparaju, P. (2025). Advances in Algae-Based Bioplastics: From Strain Engineering and Fermentation to Commercialization and Sustainability. Fermentation, 11(10), 574. https://doi.org/10.3390/fermentation11100574






