1. Introduction
The growing demand for sustainable and environmentally friendly emulsifying agents has driven the development of bioemulsifiers produced by microorganisms [
1,
2]. Unlike synthetic surfactants, bioemulsifiers are biosynthesized molecules that typically exhibit lower toxicity, higher biodegradability, and, in some cases, stability under challenging conditions such as variations in pH, temperature, and salinity. Among microbial producers, filamentous fungi such as
Aspergillus niger have stood out due to their ability to grow on low-cost substrates and to produce metabolites of biotechnological interest [
3,
4].
Aspergillus niger is a promising species known for its capacity to produce bioemulsifier with high emulsification index and potential applicability in various fields, including environmental remediation, cosmetics, food, and especially controlled drug delivery systems. The production of these compounds via submerged fermentation using agro-industrial residues as carbon sources not only reduces production costs but also contributes to waste valorization, thus supporting a circular economy [
5,
6].
However, it is important to note that some strains of
Aspergillus niger are known to produce mycotoxins, such as ochratoxin A and fumonisins, under certain culture conditions. These secondary metabolites pose significant toxicological risks and are strictly regulated in the food and pharmaceutical industries. Therefore, strain selection, purification strategies, and toxicological screening are critical when considering the use of
Aspergillus-derived compounds in edible or therapeutic products. This underscores the need for rigorous safety assessments to ensure the absence of harmful metabolites in the final product [
7,
8].
Despite progress in small-scale production, transitioning to industrial-scale processes remains a significant challenge. Reproducibility of production parameters, compound stability in pharmaceutical formulations, and regulatory compliance require a systematic approach to enable the successful application of bioemulsifiers in drug delivery systems [
9,
10].
In recent years, microbial bioemulsifiers have gained increasing attention as excipients in pharmaceutical formulations, particularly in controlled drug delivery systems such as nanoemulsions, liposomes, and micellar carriers [
1,
3,
11]. These applications demand critical attributes, including low cytotoxicity, biocompatibility, physicochemical stability under physiological conditions, and the ability to form stable oil-in-water emulsions with reduced droplet size and controlled viscosity [
10,
12]. Glycolipoprotein-type bioemulsifiers have demonstrated the ability to enable sustained drug release and enhance the bioavailability of hydrophobic compounds, highlighting their multifunctional potential [
13,
14]. Although the present study does not include biological assays or formulation testing, the physicochemical profile of the bioemulsifier produced by
Aspergillus niger UCP 1064 supports its potential use in pharmaceutical delivery systems, warranting further experimental investigation [
15,
16].
The aim of this study was to produce and characterize an efficient bioemulsifier using Aspergillus niger UCP 1064 through submerged fermentation, assess its physicochemical stability, evaluate its cytotoxicity, and discuss the main challenges related to its application in pharmaceutical systems and scale-up processes.
2. Materials and Methods
2.1. Microorganisms
The filamentous fungus Aspergillus niger UCP 1064 was isolated from soil samples collected in the Caatinga biome and from the Igarassu River in the state of Pernambuco, Brazil. This strain is part of the Culture Collection UCP (Universidade Católica de Pernambuco) of the Multi-User Center for Analysis and Characterization in Biomolecules and Material Surface (CEMACBIOS), Catholic University of Pernambuco and is registered with the World Federation for Culture Collections (WFCC).
2.2. Agro-Industrial Residues
Cassava wastewater was used as the agro-industrial residue for bioemulsifier production. The residue was obtained from a cassava processing industry located in Cabo de Santo Agostinho (PE), Brazil.
2.3. Alternative Substrate (Soluble Starch)
A commercial corn-derived soluble starch was purchased from a local supplier in Recife (PE), Brazil.
2.4. Production of Bioemulsifier
Aspergillus niger was cultured on Petri dishes containing Potato Dextrose Agar (PDA) (Sigma-Aldrich, St. Louis, MO, USA, purity 98%) and incubated at 28°C for 7 days until sporulation occurred. To prepare the inoculum, young spores were collected and suspended in sterile water to achieve a final concentration of 108 spores/mL. Ten percent of this spore suspension was used to inoculate the production medium.
Bioemulsifier production was conducted in 250 mL Erlenmeyer flasks containing 100 mL of synthetic medium (control), composed of a salt base and glucose (45 g/L) and monosodium glutamate (15 g/L) as described by Gupta et al. [
17] with modifications. The final composition was as follows: KH
2PO
4 (4.0 g/L, Êxodo, São Paulo, Brazil, 98% purity), K
2HPO
4 (5.0 g/L, Sigma-Aldrich, 98% purity), FeSO
4·7H
2O (0.2 g/L, Merck, Darmstadt, Germany, 98% purity), MnSO
4·4H
2O (0.1 g/L, Sigma-Aldrich, 98% purity), MgSO
4·7H
2O (0.1 g/L, Merck, 98% purity), ZnSO
4·7H
2O (0.2 g/L, Sigma-Aldrich, 99% purity), CaCl
2·2H
2O (20 mg/L, Sigma-Aldrich, 99% purity), and (NH
4)
2SO
4 (5.0 mg/L, Sigma-Aldrich, 99% purity).
To identify the inducing source for bioemulsifier production, the carbon (glucose) and nitrogen (monosodium glutamate) sources in the control medium were replaced with renewable agro-industrial residues, specifically cassava wastewater and soluble starch. This new formulation was referred to as the modified medium (cassava wastewater + soluble starch) (
Table 1). In all formulations, the saline base was maintained. pH was adjusted to 6.5, and the flasks were incubated at 28 °C under orbital shaking at 180 rpm for 7 days.
2.5. Surface Tension Measurement
Following cultivation, the surface tension of the cell-free metabolic liquids was measured using the Du Noüy ring method with an automatic tensiometer (Sigma 70 model, KSV Ltd., Helsinki, Finland). All measurements were performed in triplicate according to the protocol described by Kuyukina et al. [
18].
2.6. Emulsification Index Determination
The emulsification capacity was assessed using the emulsification index (EI) after 24 h, following the method of Cooper and Goldenberg [
19]. Hydrophobic substrates used included motor oil, used motor oil, canola oil, castor oil, sunflower oil, soybean oil, and corn oil, all mixed in a 1:1 ratio with the metabolic liquid. Measurements were performed in triplicate.
2.7. Emulsion Type Characterization
The type of emulsion formed was examined using a Zeiss Standard 20 bright-field optical microscope equipped with a digital camera (Oberkochen, Germany). After homogenization, an aliquot of the emulsion was carefully transferred onto a glass slide with a Pasteur pipette and observed at 10× magnification to evaluate the aggregation state and emulsion type.
2.8. Influence on Viscosity of Hydrophobic Compounds
The effect of the bioemulsifier produced by
A. niger UCP 1064 on the viscosity of used motor oil and soybean oil was evaluated. A total of 6 mL of used motor oil was placed in a graduated tube, followed by the addition of 2 mL of cell-free metabolic liquid containing the bioemulsifier. Viscosity was measured at 25 °C using a Brookfield TC 500 viscometer (Middleboro, MA, USA) with spindle No. 42 at 50 rpm. Samples were vortexed for 1 min before analysis, and emulsions were evaluated rheologically as described by Diab et al. [
20]. Results were expressed in centipoise (cP) and percentage (%).
2.9. Bioemulsifier Extraction and Quantification
The bioemulsifier was isolated from the cell-free metabolic liquid by precipitation with 70% ethanol (
v/
v) following the procedure described by Paraszkiewicz et al. [
21]. The mixture was left to rest for 24 h at 4 °C, followed by centrifugation at 4000 rpm for 15 min at 5 °C to collect the precipitate.
For quantification, the recovered precipitate was transferred to pre-weighed aluminum dishes and dried in a hot air oven at 60 °C until constant weight (24–48 h). The final dry weight was calculated by subtracting the weight of the empty dish from that of the dish containing the dried precipitate. All experiments were performed in triplicate. The concentration of the bioemulsifier was expressed as milligrams per milliliter (mg/mL) of the original metabolic liquid.
2.10. Determination of Ionic Charge and Functional Groups
The ionic charge was determined by zeta potential analysis using the Zeta-Meter System 3.0 with Direct Imaging technology (ZM3-DG, ZetaMeter, Inc., Harrisonburg, VA, USA). Functional groups present in the bioemulsifier were identified using Fourier Transform Infrared Spectroscopy (FTIR) on a Mattson 1000 FT-IR instrument (Milton Keynes, UK) within the spectral range of 500–4000 cm−1.
2.11. Determination of Biochemical Constituents
The biochemical composition of the biomolecules was determined by quantifying total protein, total carbohydrate, and total lipid contents. Protein concentration was assessed using a commercial kit from Diagnostic S.A. (Barueri, SP, Brazil), which employs the biuret reagent to detect peptide bonds. In the presence of peptides, copper (II) ions form mauve-colored coordination complexes in an alkaline solution. The alkaline reagent used was 0.2 mol·L
−1 sodium hydroxide (NaOH), adjusted to a pH of approximately 11. Total protein concentration was calculated using the following equation:
Total carbohydrate content was quantified using the phenol-sulfuric acid method described by Dubois et al. [
22], with D-glucose as the standard. Total lipids were determined following the method proposed by Manocha et al. [
23]. Lipids were extracted using chloroform:methanol mixtures in varying volume ratios (1:1, 1:2, and 2:1). The organic phases were pooled, evaporated under vacuum, and quantified gravimetrically.
2.12. Stability of Emulsifying Capacity
The stability of the bioemulsifier’s (BE) emulsifying activity was evaluated by measuring the emulsification index (EI) under various physicochemical conditions. Samples were exposed to a range of pH values (2, 4, 6, 8, 10, and 12), temperatures (5, 10, 20, 40, 80, and 100 °C), and NaCl concentrations (0, 5, 10, 15, and 20%). After treatment, the EI was measured to assess the stability and performance of the BE under these conditions.
2.13. Full-Factorial Design
A 22 full-factorial design was performed to evaluate the main effects and interaction between two variables: cassava wastewater and soluble starch, used as alternative carbon and energy sources for bioemulsifier production by Aspergillus niger UCP 1064.
Subsequently, a central composite design (CCD) with two factors, coupled with response surface methodology (RSM), was applied to model the effects of these variables on emulsifying activity and to explore the response surface.
All statistical analyses were conducted using Statistica® software, version 10.0 (StatSoft Inc., Tulsa, OK, USA), with a significance level of p < 0.05.
2.14. Prospective Scale-Up Assessment of Bioemulsifier Production for Pharmaceutical Applications
A theoretical scale-up analysis was conducted to evaluate the feasibility of applying the bioemulsifier produced by Aspergillus niger UCP 1064 in drug delivery systems. This extrapolation was based on the yield obtained under optimized bench-scale conditions and projected to fermentation volumes of 5 L, 50 L, and 500 L.
All fermentation parameters were maintained constant, including the optimized medium (cassava wastewater and soluble starch), incubation at 28 °C, orbital agitation at 180 rpm, and a 7-day cultivation period. Input requirements such as substrates, mineral salts, water, and ethanol for product recovery were proportionally adjusted.
The assessment also accounted for pharmaceutical-scale requirements, including sterility, process reproducibility, pH and oxygen transfer control, and final product purity. This approach provided preliminary technical and operational insights to support the integration of the bioemulsifier into scalable drug delivery platforms.
3. Results and Discussion
3.1. Bioemulsifier Production Using Agro-Industrial Residues
The production of bioemulsifier by
Aspergillus niger UCP 1064 using agro-industrial residues demonstrated both technical feasibility and biotechnological potential. The formulation containing 2.6% cassava wastewater and 4.5% soluble starch yielded 3.18 g/L of bioemulsifier after 7 days of submerged fermentation under orbital agitation at 180 rpm and 28 °C (
Table 1).
This yield is notably higher than those typically reported in the literature for filamentous fungi under similar conditions, as described by Gupta et al. [
17] and Devale et al. [
4], underscoring the strain’s metabolic capacity to convert low-cost substrates into value-added products.
This approach is particularly relevant within the biorefinery context, as it utilizes regionally abundant by-products such as cassava wastewater, thereby reducing operational costs and promoting the valorization of agro-industrial residues [
24,
25].
The strategic use of these residues aligns with circular economy principles, supporting a sustainable approach with potential for industrial-scale applications [
26,
27]. Bioemulsifier production by
Aspergillus niger, using 10% inoculum and agitation at 180 rpm, resulted in a surface tension of 54 mN/m, an emulsification index (EI
24) of 96%, and a yield of 3.18 g/L. These results indicate high emulsifying efficiency when compared with recent literature. For instance,
A. niger SA1 cultivated on waste cooking oil achieved a maximum yield of 8.02 g/L, EI
24 of 65%, and surface tension reduction to ~35.8 mN/m after statistical optimization (200 rpm, 35 °C, pH 6) [
28]. Although the yield obtained in this study is lower than the maximum reported, the emulsification index is markedly higher, suggesting enhanced emulsion stability under the tested operational conditions. The surface tension value, while higher, remains within the range considered effective for industrial and environmental applications.
Table 1.
Emulsification index (EI24) of the bioemulsifier against different hydrophobic substrates.
Table 1.
Emulsification index (EI24) of the bioemulsifier against different hydrophobic substrates.
| Hydrophobic Substrate | EI24 (%) |
|---|
| Motor oil | 72.0 ± 1.5 |
| Used motor oil | 88.3 ± 2.0 |
| Soybean oil | 50.3 ± 1.2 |
| Corn oil | 62.5 ± 1.7 |
| Canola oil | 55.8 ± 1.3 |
| Castor oil | 76.0 ± 1.1 |
| Sunflower oil | 62.5 ± 1.2 |
3.2. Emulsification Activity and Surface Tension
The emulsifying activity of the bioemulsifier produced by
Aspergillus niger UCP 1064 varied depending on the hydrophobic substrate used (
Table 1).
Among the oils evaluated, used motor oil exhibited the highest emulsification index after 24 h (EI
24), with a value of 88.3 ± 2.0%, followed by castor oil (76.0 ± 1.1%) and fresh motor oil (72.0 ± 1.5%). In contrast, the lowest value was observed for soybean oil (50.3 ± 1.2%), although it still exceeded the 50% threshold established by Lima et al. [
29] and Pele et al. [
30] to characterize compounds with emulsifying potential.
These results suggest that the bioemulsifier has varying affinities for different oil phases, likely due to differences in the chemical composition of the oils, including viscosity, hydrocarbon chain length, and polarity. Similar findings were reported by Santos et al. [
31], who observed higher emulsifying activity against used mineral oil than against vegetable oils. This suggests that complex industrial residues may serve as more suitable models for evaluating the performance of biosurfactants and bioemulsifiers.
Surface tension analysis of the cell-free supernatant further confirmed the compound’s surfactant activity, with values decreasing from 72 mN/m (distilled water) to below 54 mN/m in some formulations. According to Gupta et al. [
17], effective biosurfactants typically reduce surface tension to values below 40 mN/m. Although this threshold was not reached in the present study, the notable reduction observed reinforces the biotechnological potential of the compound produced.
These findings are in line with those of Sundaram et al. [
32], who demonstrated the effectiveness of agro-industrial residues such as whey and potato peels in biosurfactant production by filamentous fungi. Furthermore, the use of cassava wastewater as a carbon source supports the biorefinery concept, contributing to the valorization of abundant and potentially polluting regional by-products and promoting circular economy practices.
Overall, data confirm the potential of A. niger UCP 1064 to produce bioemulsifiers with high stability and efficiency, positioning it as a sustainable and economically viable alternative for industrial processes involving the emulsification of hydrophobic compounds.
3.3. Characterization of Emulsions Formed by the Bioemulsifier Produced by A. niger
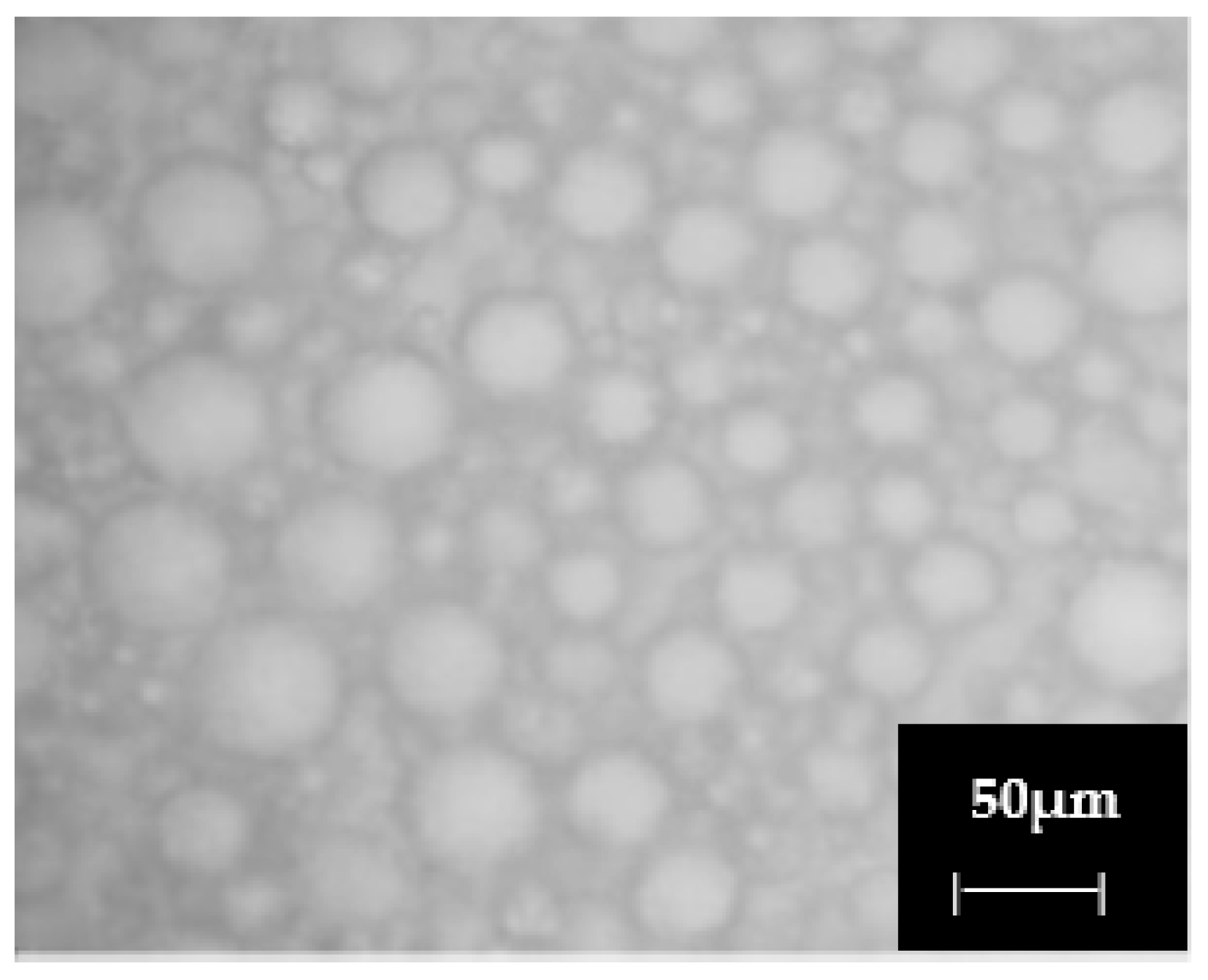
The emulsions formed by the bioemulsifier produced by
A. niger were analyzed using bright-field optical microscopy, focusing on the droplet aggregation state and the type of emulsion formed after using used motor oil as the hydrophobic substrate (
Figure 1).
Optical microscopy confirmed the formation of stable oil-in-water (O/W) emulsions. The emulsified droplets showed a uniform distribution and low levels of coalescence, indicating adequate physical stability.
The emulsifying activity of the bioemulsifier produced by
Aspergillus niger UCP 1064 proved to be highly effective in forming stable O/W emulsions, as evidenced by a notable emulsification index (EI
24) of 88.3 ± 2.0% with used motor oil. This performance is comparable to that reported by Li et al. [
33], who emphasized the influence of oil composition on emulsion stability. According to their findings, oils with shorter and more saturated fatty acid chains, such as corn oil, generally result in more stable emulsions due to their lower interfacial tension.
In addition, the stability of emulsions formed by the
A. niger UCP 1064 bioemulsifier under different environmental conditions—including variations in pH and temperature—further supports its industrial application potential. Similar findings were reported by Barbosa et al. [
2] who observed that bioemulsifiers produced by yeasts such as
Candida subtilis maintained high emulsion stability even under extreme pH and salinity conditions. These observations suggest that the bioemulsifier evaluated in this study may serve as a viable and sustainable alternative to synthetic emulsifiers across various industrial sectors.
Therefore, the results confirm the efficacy of the bioemulsifier produced by A. niger UCP 1064 in forming stable emulsions, with promising applications in the pharmaceutical, cosmetic, and food industries—aligning with current trends toward sustainable, low-impact bioproducts.
3.4. Determination of Ionic Charge and Functional Groups
The bioemulsifier produced by
A. niger exhibited a negative ionic charge, with a zeta potential of −28.7 mV and conductivity of 414.2 µS/cm at 25 °C, indicating an anionic profile. The biochemical composition revealed the presence of proteins, carbohydrates, and lipids, suggesting a glycolipoprotein nature (
Table 2).
Although the biochemical profile indicates a glycolipoprotein nature due to the presence of proteins, carbohydrates, and lipids, further analyses are recommended to investigate the potential presence of low molecular weight metabolites, fermentation by-products, and secondary compounds, including mycotoxins. Some strains of
Aspergillus niger, despite being generally recognized as safe (GRAS), may produce substances such as oxalic acid, gliotoxin, and fumonisins under specific cultivation conditions. Therefore, additional evaluations are essential to ensure the safety, purity, and regulatory compliance of the bioemulsifier for potential pharmaceutical applications, especially in drug delivery systems [
15,
34].
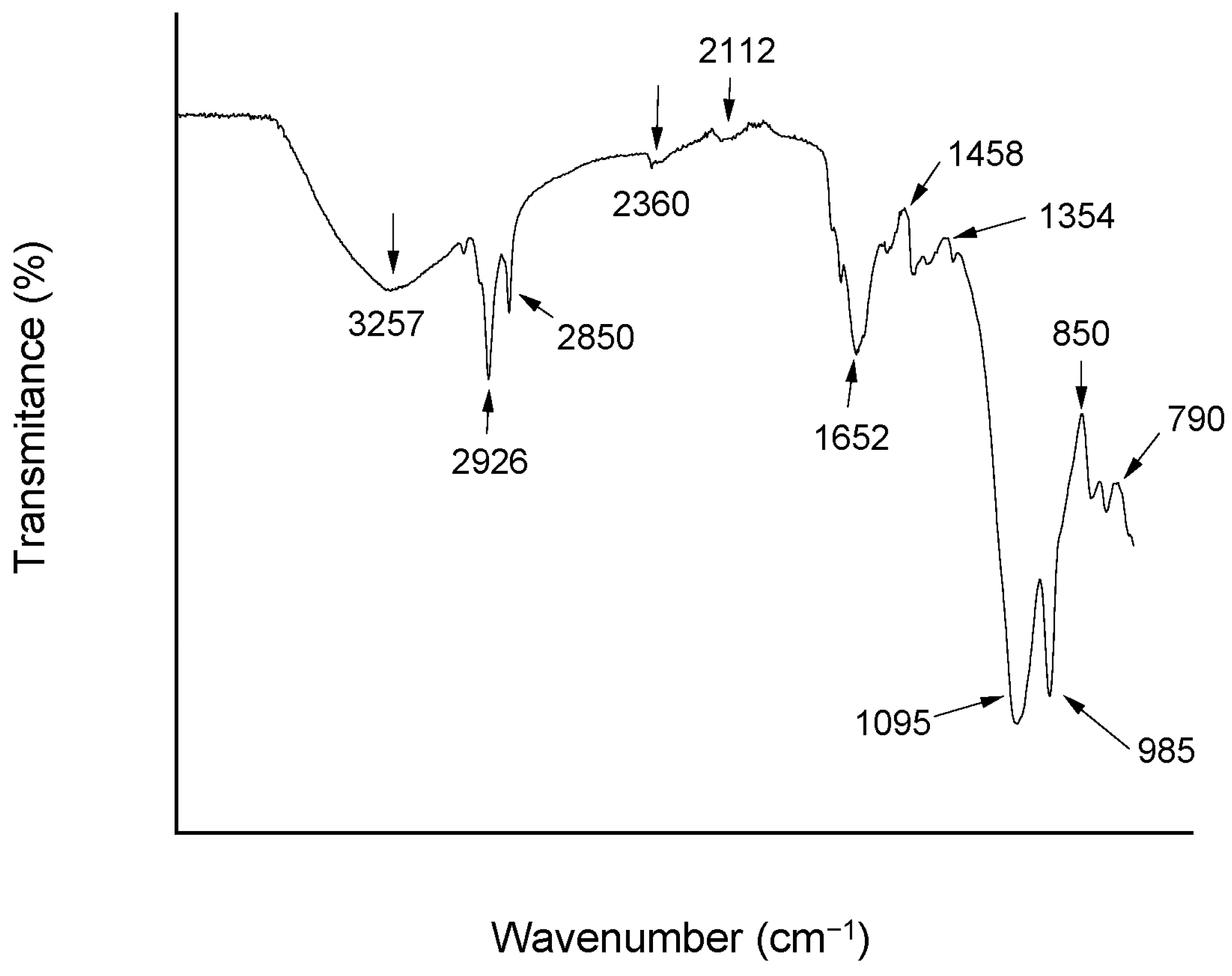
Functional group identification by Fourier-transform infrared spectroscopy (FT-IR), as shown in
Figure 2, revealed absorption bands in the 3400–3200 cm
−1 range corresponding to O–H and N–H stretching vibrations, typically associated with hydroxyl groups from polysaccharides and amine groups from proteins. Bands between 2950 and 2850 cm
−1 indicated C–H stretching vibrations from aliphatic –CH
2 and –CH
3 groups, confirming the presence of long hydrocarbon chains characteristic of lipids or fatty acids and supporting the amphiphilic nature of the molecule [
32,
35,
36]. Additional bands in the 1000–1200 cm
−1 range corresponded to C–O–C and C–O–P stretching vibrations, commonly found in oligosaccharides and polysaccharides. Peaks observed between 1300 and 1500 cm
−1 were attributed to CH
2 and =CH bending vibrations, typical of both lipids and proteins [
37,
38,
39].
These FT-IR spectral features corroborate the biochemical composition, confirming the presence of proteins, carbohydrates, and lipids. Altogether, these results strongly support that the bioemulsifier produced by A. niger UCP 1064 is a glycolipoprotein structurally similar to other bioemulsifying molecules.
These findings are consistent with recent reports describing microbial bioemulsifiers as glycolipoprotein or glycoprotein complexes. For example, Ferreira et al. [
38] described bioemulsifiers produced by
Candida tropicalis strains with similar composition, emphasizing that the combination of proteins and carbohydrates enhances emulsifying capacity and emulsion stability. Likewise, Sankhyan et al. [
13] demonstrated that the lipid fraction interacts with hydrophobic phases, while the protein and polysaccharide fractions confer colloidal stability and inhibit droplet coalescence.
The glycolipoprotein structure of the bioemulsifier produced by
A. niger UCP 1064 provides both surfactant and stabilizing properties, which can be exploited in various industrial applications, particularly in the pharmaceutical and environmental sectors—aligning with current trends in the development of sustainable and multifunctional biosurfactants [
32,
40].
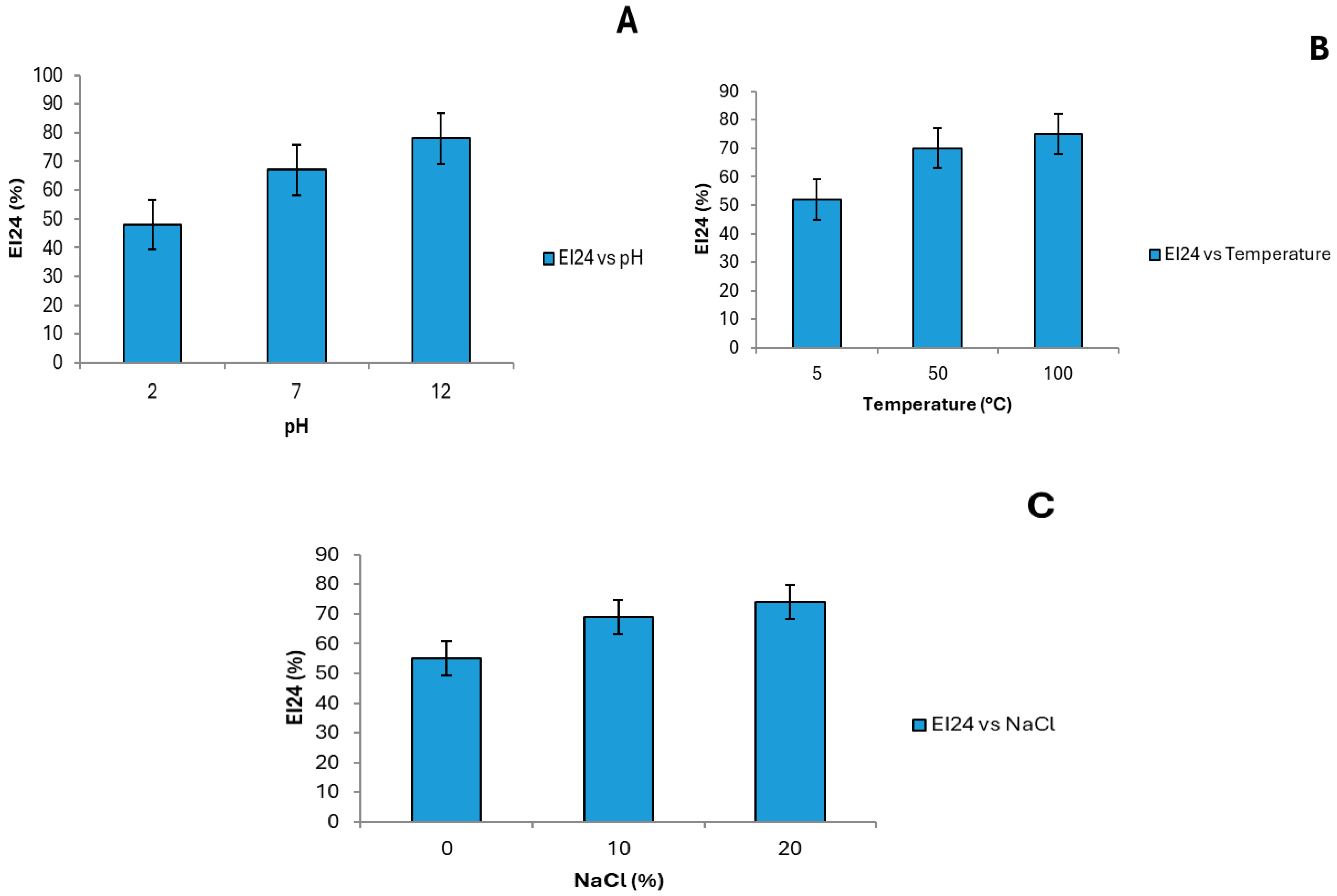
3.5. Bioemulsifier Stability Evaluated by Emulsification Index (EI24)
The stability of the bioemulsifier was assessed by monitoring variations in the emulsification index (EI
24) under different physicochemical conditions. The results are summarized in
Figure 3 and demonstrate that the bioemulsifier retains significant emulsifying activity across a wide range of conditions—an essential feature for industrial applications.
With respect to pH, the bioemulsifier remained stable between pH 2 and 12, with EI
24 values ranging from 48.2% to 77.5%. This broad pH tolerance is consistent with studies by Dias et al. [
39] and Tchakouani et al. [
40], which reported that microbial biosurfactants tend to exhibit enhanced stability in both acidic and alkaline environments—making them suitable for processes involving wide pH fluctuations. The slight reduction in activity at extreme pH levels may be associated with conformational changes in the molecular structure of the bioemulsifier that hinder its interaction with oily phases.
In terms of temperature, the bioemulsifier maintained considerable emulsifying activity from 5 °C to 100 °C, with EI
24 values ranging from 52.3% to 77.1%. This thermostability is advantageous for applications requiring heat resistance, such as in pharmaceutical and food processing. Similar behavior was observed by Zhang et al. [
8], who noted that bioemulsifiers produced by filamentous fungi remained stable at elevated temperatures due to the protective roles of protein and lipid components in preserving molecular structure.
Regarding salinity, the bioemulsifier exhibited tolerance across NaCl concentrations ranging from 0% to 20%, with EI
24 values between 58.1% and 76.5%. This salt resistance is especially relevant for marine applications or industrial processes involving high salinity. Studies by Lima et al. [
29], emphasize the importance of such salt stability for the commercial viability of bioemulsifiers, as resistance to ionic stress ensures consistent emulsification performance in complex systems.
3.6. Effect on the Viscosity of Hydrophobic Compounds
Viscosity measurements revealed a significant reduction in the viscosity of the oils tested following the addition of the bioemulsifier (
Table 3). Specifically, used motor oil showed a decrease from 128.4 cP to 83.7 cP (a 34.8% reduction), and soybean oil decreased from 102.3 cP to 68.1 cP (a 33.4% reduction).
This pronounced decrease in viscosity highlights the bioemulsifier’s effectiveness in modifying the rheological properties of hydrophobic compounds, thereby enhancing fluidity and dispersion. This behavior aligns with the known mechanisms of biosurfactants, which reduce interfacial tension between oil and water phases, promoting emulsification and lowering resistance to flow [
41,
42].
Maia et al. [
37] reported similar findings, demonstrating that microbial biosurfactants significantly reduced the viscosity of crude and vegetable oils, improving processability in applications such as bioremediation and enhanced oil recovery. The observed reduction is also consistent with the amphiphilic glycolipoprotein structure of the bioemulsifier, which enables interactions with oil molecules that disrupt intermolecular forces and reduce cohesion [
43].
Importantly, this reduction in viscosity has valuable implications for pharmaceutical and environmental uses, where lowering the viscosity of lipid-based formulations can enhance drug delivery efficiency and facilitate better dispersion in bioremediation processes [
8,
12].
In conclusion, the significant viscosity reductions observed after the addition of the bioemulsifier confirm its potential as an effective agent for modifying the physical properties of hydrophobic substances, supporting its application across diverse industrial sectors.
3.7. Full-Factorial Design
A factorial experimental design was employed to evaluate the effects of cassava wastewater and soluble starch concentrations on the bioemulsifier production yield by
Aspergillus niger UCP 1064.
Table 4 presents the two factors and their respective levels, each tested at three levels (−1, 0, +1).
The results of the experiments are shown in
Table 5. Both cassava wastewater and soluble starch significantly influenced bioemulsifier production. The lowest yield (16.3%) was observed in the assay where only soluble starch at an intermediate concentration (4.5%) was used without any cassava wastewater (Assay 5). In contrast, the highest yield (26.1%) was achieved with a combination of 1.3% cassava wastewater and 8.0% soluble starch (Assay 8), suggesting that higher starch concentrations strongly favor bioemulsifier biosynthesis.
As shown in
Figure 4, soluble starch (SS%) had the strongest positive effect on the response variable, with a standardized effect value of 7.04, indicating that it was the most influential factor in enhancing bioemulsifier production. Cassava wastewater (CWW%), representing the proportion of liquid effluent derived from cassava processing, also had a significant impact, with a standardized effect of 6.23—reinforcing its potential as a low-cost and sustainable carbon source. In the Pareto chart, the suffix (L) refers to the linear effect of each factor, (Q) denotes the quadratic effect, and 1Lby2L indicates the linear interaction between the two variables. These abbreviations help identify the individual and interactive contributions of each factor to the observed response.
The quadratic effects of cassava wastewater and soluble starch indicate that both variables have a non-linear influence on the response, suggesting that very low or very high concentrations may reduce bioemulsifier production. The interaction between the two factors had the least impact, with a standardized effect of 0.40, and was not statistically significant, indicating that no meaningful synergistic effect was observed within the tested concentration ranges.
These findings are in agreement with those of Santos et al. [
31], who reported that starch-rich substrates and agro-industrial residues promote microbial growth and emulsifier biosynthesis. Similarly, Vučurović et al. [
44] highlight the importance of optimizing substrate concentrations, as excess nutrients may trigger catabolic repression and reduce biosynthetic efficiency.
The ANOVA demonstrated that the regression model exhibited a high coefficient of determination (R2 = 0.960), indicating that 96.0% of the variation in bioemulsifier production was explained by the independent variables, while only 4.0% remained unexplained by the model. The reproducibility of the experimental data was confirmed by the low pure error (0.051) and the value of the adjusted determination coefficient (Adj. R2 = 0.893). The predicted versus observed values plot showed a strong correlation between the experimental results and the values estimated by the model, confirming its suitability for predicting biosurfactant production under the studied conditions.
Figure 5 illustrates the interaction between cassava wastewater and soluble starch in bioemulsifier production. The highest yields were observed at intermediate to high concentrations of both factors, supporting the trends shown in
Figure 3.
The central region of the surface—ranging from 4.5% to 7.0% soluble starch and 1.3% to 2.6% cassava wastewater—exhibited a steep increase in bioemulsifier production, with yields exceeding 26%. In contrast, lower concentrations of both substrates resulted in a marked reduction in yield, likely due to nutrient limitations affecting microbial growth and biosynthetic capacity.
These results are consistent with those of Zhang et al. [
8], who emphasized that balanced formulations of carbon and nitrogen sources enhance secondary metabolism and increase the production of microbial biosurfactants and bioemulsifiers.
3.8. Feasibility Analysis of Bioemulsifier Production Scale-Up for Pharmaceutical Use
Theoretical scale-up of bioemulsifier production, based on a laboratory-scale yield of 3.18 g/L, indicates promising potential for pharmaceutical applications, particularly in drug delivery systems. However, recent studies have shown that large-scale processes rarely maintain proportional productivity per liter. This is primarily due to challenges such as oxygen transfer limitations, inadequate mixing, and the formation of pH or nutrient gradients in large bioreactors [
12,
38].
In aerobic fermentations, maintaining a sufficient volumetric oxygen transfer coefficient (k
la) is essential. For example, Li et al. [
33] demonstrated that increasing agitation speed and aeration rate significantly enhanced k
la and biosurfactant yields in stirred-tank reactors. Similarly, Santos et al. [
31] reported a 20% reduction in biosurfactant yield when scaling up from 5 L to 500 L, due to suboptimal oxygen and nutrient transfer, despite stable temperature and pH conditions.
Considering these technical constraints, a conservative 20% reduction was applied to the theoretical yield projections to better reflect expected performance at larger volumes. The adjusted production estimates are presented in
Table 6.
Recent literature emphasizes that the scalability of bioproducts such as bioemulsifiers is closely linked to the optimization of fermentation parameters—particularly oxygen transfer, agitation, and pH control. These factors are critical for maintaining productivity and ensuring the quality of the final product, as highlighted by Marques et al. [
45] and Li et al. [
33] Therefore, successfully scaling up production depends on integrating these parameters to ensure the bioemulsifier’s efficiency and stability at larger scales.
Furthermore, natural bioemulsifiers have gained increasing attention as biocompatible stabilizers in advanced pharmaceutical formulations, including nanostructured emulsions, liposomes, and nanoparticles for controlled drug release. Their low toxicity, biodegradability, and capacity to enhance drug bioavailability have been well documented in recent studies by Sharma et al. [
10] and Shahiwala et al. [
15].
Despite this promising outlook, further experimental validation is essential at pilot and industrial scales. Future studies should focus on evaluating the bioemulsifier’s stability, functionality, and compatibility with various drug compounds, as well as ensuring the robustness of the production process. This need is reinforced by Rivero et al. [
16] and Barata et al. [
46] who stress the importance of reproducibility and in-depth characterization for clinical applicability.
4. Conclusions
This study demonstrated the efficient production of a bioemulsifier by Aspergillus niger UCP 1064 using agro-industrial residues—specifically cassava wastewater and soluble starch—under submerged fermentation conditions. A maximum yield of 3.18 g/L was achieved after 7 days of cultivation at 28 °C and 180 rpm, using 2.6% cassava wastewater and 4.5% soluble starch as carbon sources.
The bioemulsifier exhibited strong emulsifying activity and physicochemical stability across a wide range of environmental conditions, including pH values from 2 to 12, temperatures between 5 and 100 °C, and NaCl concentrations up to 20%. Biochemical and spectroscopic analyses confirmed its glycolipoprotein composition.
Microscopic and rheological analyses revealed its ability to form stable oil-in-water (O/W) emulsions and significantly reduce the viscosity of hydrophobic substrates. These characteristics are highly relevant for potential applications in various industrial sectors, including environmental remediation and cosmetics.
Although theoretical scale-up projections indicate the feasibility of using this bioemulsifier in pharmaceutical applications, this study did not assess drug release, cytotoxicity, or in vivo biocompatibility. As such, no definitive conclusions can yet be drawn regarding its suitability for use in biological systems or drug formulations. Nevertheless, previous studies have highlighted the promise of glycolipoprotein-based bioemulsifiers as stabilizers in drug delivery systems due to their amphiphilic nature, biodegradability, and surface activity [
10,
12].
Future research should prioritize in vitro and in vivo biological testing, cytotoxicity assessment, formulation stability analysis, and pilot-scale production to confirm the safety and efficacy of this bioemulsifier for pharmaceutical applications.