Abstract
Pleurotus ostreatus is among the most widely cultivated mushroom species on a global scale, valued for its relative ease of cultivation, excellent organoleptic qualities, and notable nutraceutical properties. P. ostreatus could use a wide range of by-products as growth substrates by excreting a potent array of hydrolytic and oxidative enzymes. In this study, a diverse range of agricultural residues and agro-industrial by-products, enriched (or not) with various supplements, was evaluated for the cultivation of five commercial P. ostreatus strains. Key cultivation parameters were assessed, including biological efficiency and productivity. A process analytical technology (PAT) approach, utilizing FTIR spectroscopy in combination with multivariate analysis, was employed to develop a predictive model for biological efficiency based solely on substrate’s spectral profile. Substrates based on wheat and barley straw supplemented with soybean demonstrated superior performance across most strains. The biological efficiency value reached 185% in some cases for a total cultivation period of only 35 days. The resulting model exhibited excellent predictive capability, with a coefficient of determination (R2) of 0.90 and low relative prediction error of just 6%. The developed innovative PAT tool will be beneficial for mushroom growers since it allows the fast and costless evaluation of agro-industrial by-products in respect to their potential exploitation as mushroom substrates.
1. Introduction
Pleurotus (oyster) mushrooms are among the most widely cultivated mushrooms globally, accounting for about 19% of the respective world’s total production, with P. ostreatus to hold the first place among species of this genus [1]. Oyster mushrooms owe their popularity to the relatively low cultivation demands, high mushroom yields, and organoleptic properties; in addition, they possess nutritional and medicinal properties that makes them ideal for consumption by people with blood hypertension, high blood content in low-density lipoprotein, and cholesterol as well as for patients with metabolic diseases and diabetes [2]. In addition, oyster mushrooms are characterized by high contents in protein, minerals, and vitamins and a very low content in fats, while they also contain a broad range of bioactive compounds (e.g., β-glucans, lectins, polyphenols) possessing antioxidant, immunomodulating, antitumor, antimicrobial, and antiviral properties [2,3,4].
Pleurotus species are white-rot fungi capable of efficiently decomposing lignocellulosic biomass, and, most notably, lignin and related structural plant polymers, by employing an arsenal of extracellular enzymes [5]. This allows their cultivation on a wide range of agricultural and forestry residues as well as on agro-industrial by-products [6,7,8], whose further disposal or treatment often causes significant environmental issues. During the last decade, extensive research has been held in relation to the valorization of such streams as substrates for mushroom production, by evaluating the efficacy of several Pleurotus species in decomposing various types of lignocellulosics [9,10]. These studies have also demonstrated the effect of utilizing such substrates at improving the content of mushrooms in bioactive compounds [9,10].
Based on the aforementioned, it is obvious that tools capable of fast, cheap, and accurate determination/prediction of the performance of the Pleurotus cultivation process on a given substrate is of high interest to mushroom growers worldwide. Along this line, process analytical technology (PAT) provides valuable data about biochemical processes to be used for monitoring and optimization purposes that could lead to the development of tools that can rapidly, accurately, and usually non-destructively assess the quality, quantity, and certain functional properties of various materials [11,12]. Fourier transform infrared (FTIR) spectroscopy could serve as an excellent candidate for this purpose, as it can provide detailed information about the molecular structure of the material, while in combination with multivariate analysis, it can lead to the prediction of a substrates’ productivity when this is to be selected and used for the cultivation of edible mushrooms. To date, FTIR spectroscopy has been successfully applied to identify filamentous fungi [13,14], to delimit taxa of the genera Pleurotus, Ganoderma, and Boletus [15,16,17], to discriminate mushroom species on the basis of geographic origin [18], to predict the bioactive compound content of Pleurotus mushrooms [19], or to evaluate the post-harvest quality of Agaricus bisporus mushrooms [20]. To the best of the authors’ knowledge, there is only one previous study reporting the prediction of the biological efficiency of substrates used for mushroom cultivation [21]; however, it focused on another mushroom species, i.e., Cyclocybe cylindracea, with different cultivation requirements, while the PAT tool was calibrated for only a small number of substrates.
Therefore, the objective of the present study was firstly to comparatively assess the performance of various lignocellulosic materials (supplemented or not with several additives), which are widely used in P. ostreatus mushroom cultivation, as substrates for five commercial strains, and secondly, by properly evaluating the data obtained, to develop—for the first time in this field—a fast, low-cost, and reliable tool capable of determining the suitability P. ostreatus cultivation substrates, through the prediction of its biological efficiency.
2. Materials and Methods
2.1. Biological Material and Substrate Preparation
Five of the most popular commercial P. ostreatus strains, including strains more adaptive to colder (n = 3) or warmer (n = 2) climatic conditions, were used for purposes of the present study, and they appear under the code names Strain 1 to Strain 5. Each strain of P. ostreatus was cultivated on substrates consisting of plain wheat straw (WS, control), and its mixtures with other residues and by-products widely used in oyster-mushroom cultivation, i.e., barley straw (BS), corn straw (CS), or grape marc (GM), at rates of 3:1 w/w to WS, thus forming four basic substrates, namely plain WS, WS:GM, WS:BS, and WS:CS. For the preparation of each substrate, materials were chopped into particle sizes of 3–4 cm and soaked in water for 48 h. Surplus water was drained off for 24 h, to obtain a moisture content of ca. 70%, using the loss-on-drying (LOD) method. In addition, three main supplements such as trifolium plant residues (Tr), soybean flour (Soy), and wheat bran (Br) were also added in each basic substrate at three rates as follows: 5, 10, or 20% w/w for Tr and Br; 2, 4, and 8% w/w for Soy, plus substrates with no addition of supplements, thus preparing 10 formulations for each basic substrate, and a total of 40 formulations for each P. ostreatus strain examined. The determination of C and N values of each one of the basic substrates and supplements was performed by a Carlo Erba Elemental Analyzer CHN EA 1108 (Carlo Erba, Milan, Italy) and the recorded C/N ratio is presented in Supplementary Materials, Table S1.
Autoclavable polypropylene bags were filled with 1 kg of each substrate, which were then sterilized for 2 h (121 °C, 1.1 atm). Three bags of each substrate (replicates) were prepared for every commercial strain examined. Prior to sterilization, a small quantity of each substrate was removed, freeze-dried, and milled to a particle size of less than 2 mm, and their mid-infrared spectra were recorded via FTIR spectroscopy. The spawn of the five commercial strains used for this study, as well as the basic substrates and their supplements, were kindly provided by Dirfis Mushrooms IKE (Kathenoi, Euboea, Greece).
2.2. Mushroom Cultivation and Assessment of Cultivation Parameters
Following sterilization, each substrate was inoculated with the commercial strains’ spawn at a rate of 5% w/w. Afterwards the polypropylene bags were sealed and properly mixed to equally distribute the spawn into the substrate. The individual replicates of each treatment (substrate) were arranged in a completely randomized design. The substrates were then incubated at 25 °C, in the absence of light. After substrates’ colonization and in order to initiate the formation of mushroom primordia, the temperature was set at 17 °C and the relative air humidity at 95%, while illumination was also provided (700 lux). During mushroom production, fresh air flow was increased to maintain the CO2 levels under 1200 ppm (photoperiod of 18 h/6 h of light/dark, respectively), while the relative air humidity was decreased to 85%, and illumination increased to 1000 lux. Mushroom harvesting was performed for two production flushes. Set-up and control of the above-mentioned environmental parameters were performed by the Veciap Computer System (VEC-11; DALSEM-VECIAP b.v. Agro-Industries, Horst, The Netherlands)
The suitability of each formulated substrate for the cultivation of P. ostreatus mushrooms was assessed through the following cultivation parameters: (a) earliness (in days), which is the time that has been elapsed from the inoculation of substrates until the primordia formation; (b) total cultivation period (in days); (c) yield (in grams), that is, the total fresh weight of mushrooms harvested in both flashes; (d) biological efficiency (BE, %), calculated as the percentage ratio of yield over the dry weight of the formulated substrate; and (d) productivity, defined as the ratio of biological efficiency over total cultivation period.
2.3. Fourier Transform Infrared (FTIR) Spectroscopic Analysis
The FTIR spectrum of each formulated substrate was recorded using KBr disks by transmittance spectroscopy. Each disk contained 5 mg of substrate mixed with 200 mg of KBr (FT-IR grade) and was compressed into a pellet using a 30-ton press. For each substrate, three disks were prepared, with their spectral recording to take place immediately after their preparation under ambient conditions in the mid-infrared region (4000–500 cm−1). An Ostec IROS 05 spectrometer equipped with the ZaIR 3.5 software (Ostec Enterprise Ltd, Moscow, Russia) in the transmission mode was used for this purpose. The resolution was set to 4 cm−1, while for each spectrum 100 scans were recorded, averaged, and corrected against KBr. The above-described sample preparation ensures very good reproducibility of high-quality spectra.
2.4. Multivariate Analysis and Prediction of Biological Efficiency
Statistically significant differences in the values of cultivation parameters between treatments (combination of substrates and Pleurotus strains) were studied by the analysis of variance (ANOVA) using IBM SPSS Statistics v.20 (IBM corporation, Armonk, New York, USA). A principal component analysis (PCA) was performed on the Savitzky–Golay smoothed (smoothing window of 11 points and zero polynomial), linearly baseline-corrected, and normalized by the mean spectra of the substrates in order to detect any grouping in terms of cultivation substrate and productivity. For this purpose, singular value decomposition (SVD) was applied for 20 principal components using leave-one-out cross validation (CV; models’ self-testing). The partial least square regression (PLSR) analysis was adopted to calibrate a model predicting the biological efficiency of the substrates based on their recorded FTIR spectra. A big number of spectral transformations, as well as different combinations of them, were applied to the recorded spectra (e.g., Savitzky–Golay smoothing, smoothing by the median, linear baseline correction, normalization by the mean, multiplicative scatter correction, standard normal variate, de-trending, first and second derivative, etc.) to ensure the high predictive power and accuracy of the model. Potential outliers were identified by Hotellings’-T2 distribution [22]. To avoid overestimations, the sample set (40 samples) was divided into a calibration (CAL) set containing nine tenths of the samples and an external validation (EV) set with the rest of the samples. For this, every tenth sample following the ascending order of the BE of the substrates was selected to construct the EV set. Afterwards, the CAL set was used to develop the calibration model on which the optimal number of components was chosen based on leave-one-out cross validation, while the EV set was used for the evaluation of the robustness of the developed models. Non-significant variables were removed by the Martens’ uncertainty test [23] to improve models’ stability and robustness. All previously mentioned calibrations (i.e., PCA and PLSR analysis) were performed by The Unscrambler X v.10.5 software (CAMO software, Oslo, Norway).
Models’ performances were determined by R2 (coefficient of determination) values:
where yi represents the measured BE values and fi represents the predicted BE values. The closer R2 is to 1, the better the fit of the measured values (yi) to the regression line.
Models’ precisions were determined by the root mean square error (RMSE) in % of substrates’ BEs:
where yi represents the measured BE values and fi represents the predicted BE values.
The relative error of prediction (REP) [24] given in % was also calculated:
where z is the mean value of the calibration BE values.
3. Results and Discussion
3.1. Evaluation of Agro-Industrial By-Products as Substrates for Pleurotus Mushrooms Cultivation
The summary of the cultivation parameters recorded for the five commercial strains cultivated on 40 substrates is presented in Supplementary Materials, Table S2.
Regarding earliness, statistically significant differences were not observed among the four main substrates (i.e., WS-control, WS:GM, WS:BS, and WS:CS) (Figure 1); P. ostreatus strains cultivated on WS:BS required less time for primordia formation (with an average value among strains of 19.8 ± 3.72 days), followed by WS:GM (20.4 ± 2.55 days), and WS (22 ± 1.73 days), while when cultivated on WS:CS, the highest number of days were required (24.2 ± 2.35 days) (Supplementary Materials, Table S2; Figure 1). On the other hand, supplementation of the four basic substrates revealed a controversial effect on earliness. Hence, the supplementation of WS and WS:CS accelerated the formation of primordia, whereas the opposite effect was observed in the case of WS:BS, with almost all (except soybean) supplements showing an increase in earliness values. In the case of WS:GM, supplementation had no significant effect, with soybean and bran showing a slight increase in the number of days required until primordia formation (Figure 1). The most significant effect of supplementation on the total cultivation period was observed in WS:BS and WS:CS (Figure 1), with soybean supplementation at a rate of 8% presenting the most significant decrease for the former, and trifolium (20%) and bran (20%) for the latter. Finally, regarding WS, supplementation with soybean at a rate of 4% accelerated the completion of the cultivation cycle presenting the most notable effect, albeit not in a statistically significant manner (Figure 1).
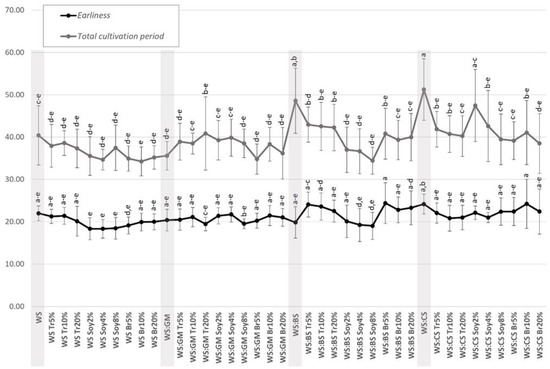
Figure 1.
Earliness and total cultivation period (in days) for the averaged Pleurotus ostreatus commercial strains cultivated on various substrates and supplements. Standard deviation bars represent the variance between strains; absence of common letters indicates statistically significant differences, Duncan’s T-test with p < 0.05.
A general trend could be observed for basic substrates with the exception of WS:GM and WS:BS; hence, WS and WS:CS exhibited relatively higher (albeit not significant) earliness—and, to a lesser degree, total cultivation period—values in respect to their respective treatments with supplementation. Therefore, it seems that addition of N-rich sources favors shorter cultivation phases; however, the time advantage that each one of them could offer is often hardly discernible and depends on the supplement type and rate of application. On the other hand, WS:GM demonstrates low earliness (and total cultivation period) values when used plain, which could be attributed to the higher content of this basic substrate in nitrogen (C/NGM: 19–25:1; Supplementary Materials, Table S1) and in easily assimilable organic compounds (e.g., carbohydrates) [9]. As a result, the potential advantage of using WS:GM with supplements is not evident for these parameters; moreover, in many cases, higher values for earliness were noted (again, not significant) in such treatments probably suggesting the existence of a threshold for nutrient availability, above which there is no pronounced beneficial effect as regards earliness and total cultivation period.
Furthermore, the intraspecific variability observed among the five strains merits further consideration. Each strain presented a different preference for substrate–supplement combination (Supplementary Materials, Table S2); Strain 1 grew faster on WS:BS supplemented with soybean at a rate of 8% (18 days), Strain 2 mainly preferred WS supplemented with soybean at a rate of 4% (15 days), Strain 3 reached primordia formation faster (19 days) when cultivated on WS:GM supplemented with soybean at a rate of 8% or on WS:BS supplemented with soybean at rates of 4 or 8%, Strain 4 presented the lowest earliness among all strains when cultivated on WS supplemented with trifolium at a rate of 20% (14 days), and Strain 5 performed better on WS supplemented with bran at a rate of 5% (18 days). In addition, Strain 5 was the strain that needed the most time for primordia formation (32 days) when cultivated on WS:BS with 5% bran.
Similarly to earliness, intraspecific variability had a considerable effect on total cultivation period, which ranged from 29 to 58 days (Supplementary Materials, Table S2). For instance, Strain 3 required more than 50 days to complete its cultivation period on non-supplemented WS, while Strain 4, for the same substrate, required less than 31 days. The lowest value for the total cultivation period parameter was recorded when strains were cultivated on WS supplemented with 4% soybean, while the combination of Strain 4 cultivated on WS:BS supplemented with 8% soybean presented the shortest cultivation period (Figure 1). Furthermore, the addition of supplements decreased total cultivation period (i.e., for two flushes) for most of the basic substrates, with exception of WS:GM, which could again be attributed to the higher content of this basic substrate in nitrogen and easily assimilable organic compounds (e.g., carbohydrates).
A considerable effect on the yield of mushrooms (total quantity produced in two flushes) was noted as a consequence of substrates’ supplementation (Supplementary Materials, Table S2). Among substrates without supplements, WS:BS was the only one that presented significantly higher yields for most of the strains when compared with the plain WS substrate. A similar outcome was observed when the biological efficiency was taken into account (Figure 2a). In addition, strains exhibited different performances for both parameters examined; Strain 1 recorded the highest values, while Strain 3 and Strain 4 followed for most treatments. With regard to the effect of supplements, the addition of soybean resulted in an overall increase both of yield and of biological efficiency and was followed by the treatment with bran addition. However, supplements’ effect was affected both by the type of the main substrate as well as by the strain examined; the WS substrate presented a higher increase in BE with trifolium than with soybean or bran supplementation. Especially in the case where Strain 5 was used, WS recorded the highest value of BE. On the contrary, it seems that none of the strains preferred WS:CS-based substrates, since they presented the lowest yield and BE values, while Strain 5 failed to produce any mushrooms in half of the treatments. The combination of WS:BS with soybean supplementation provided the best performance among the five strains examined, while the best rate of soybean addition differed slightly among strains. Strains 1 and 3, cultivated on WS:BS supplemented with soybean at a rate of 8% and WS:BS with soybean at a rate of 2%, respectively, presented the highest BE values among the treatments, exhibiting an increase of 46% and 56%, respectively, in comparison with the control (WS). At the same time, this substrate combination (i.e., WS:BS with soybean) was the one that performed remarkably for all strains except Strain 5.
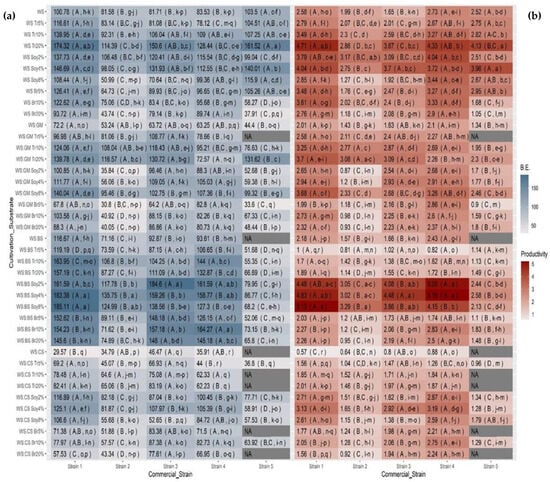
Figure 2.
Heatmaps of (a) biological efficiency (B.E., in blue) and (b) productivity (in red) of each substrate cultivated with five Pleurotus ostreatus commercial strains. Tiles contain the average yield or biological efficiency of three replicates for each substrate and strain. Absence of common letters in parentheses indicates statistically significant differences (Duncan’s T-test, p < 0.05) between strains examined for each substrate (uppercase letters) and between substrates examined for each strain (lowercase letters).
The productivity parameter provides an overall evaluation of the cultivation process since it takes into consideration both BE and the total time length of the cultivation period, i.e., two very important parameters for mushroom growers. By averaging for the strains, it is obvious that the addition of supplements had a beneficial effect in all cases (Figure 2b). Therefore, for the WS substrate, the addition of trifolium (20%) and soybean (4%) significantly increased productivity, while for WS:BS the addition of soybean was the most beneficial, also demonstrating a significant increase when compared with other treatments. Similarly, for WS:CS (which presented the lowest productivity among various substrates), the addition of soybean (2% and 4%) significantly increased productivity (more than three-fold). Regarding WS:GM, the addition of trifolium (20%) and soybean (8%) resulted in the biggest increase, albeit no significant differences were noted.
Koutrotsios et al. [25] examined the intraspecific variability of commercial and wild P. ostreatus strains cultivated on pasteurized wheat straw, and reported a high variation among strains with earliness ranging from 21 to 71 days, BE from 44% to 109%, and productivity from 1.5 to 8.51. Zervakis et al. [26] reported earliness values of 26–40 days and BE values of 45–135% for P. ostreatus strains cultivated on wheat straw or mixtures of wheat straw and raw or composted two-phase olive meal waste. They recorded the highest BE (135.34%) on substrates containing wheat straw and composted two-phase olive mill waste (80%:20%), nevertheless with a slightly delayed appearance of primordia (i.e., earliness value of 28 days). Koutrotsios et al. [10] examined P. ostreatus cultivation performance on a wide range of substrates consisting of agro-industrial by-products, including wheat straw and a mixture of grape marc and cotton gin trash. They reported earliness values of 39 and 34 days, respectively, and BE values of 53 and 137%, respectively.
Koutrotsios et al. [9] on another study examined the effect of WS and WS:GM (1:1 w/w), among other substrates, on the bioactive compound content of mushrooms from three P. ostreatus strains. They recorded earliness values of 25–31 days (WS with 5% bran) and 11–22 days (WS:GM with 5% bran) with total cultivation periods ranging between 34 and 67 days (WS) and 37–54 days (WS:GM). The recorded BE values varied from 38% to 107% for WS and 45% to 98% for WS:GM, while the productivity values were 0.83–2.02 and 1.23–1.92, respectively. Finally, in a similar approach, Doroški et al. [27], reported earliness values of 36 and 39 days, total cultivation periods of 42 and 45 days, and BEs of 19% and 16% for WS and WS:GM (4:1 w/w), respectively. Melanouri et al. [28], cultivating P. ostreatus strains on completely different substrates (i.e., WS, spent mushroom substrate and roots of leafy vegetables, in various rates), reported earliness values between 24 and 30 days, while they achieved BE values between 46% and 88%. Dedousi et al. [29], utilizing substrates consisting of WS, BS, and mixtures with fresh and composted spent mushroom substrate, reported earliness values ranging between 29 and 44 days and BE values between 54% and 133%, for the different substrate mixtures.
In the present study, the best combination of BE (i.e., 185.11% and 184.6%) and earliness values (18 and 22 days) were recorded for Strain 1 and Strain 3, respectively, cultivated on WS:BS with the addition of soybean, while for similar substrates with the previously mentioned studies, i.e., WS Br5% and WS:GM Br5% (3:1 w/w)], earliness values ranged between 16 and 21 and 17–22 days, total cultivation period between 30 and 37 and 29–38 days, BE between 64 and 126% and 31–83% and productivity 1.76–3.48 and 0.91–2.86, respectively, for the different strains. The slightly higher BE values of Koutrotsios et al. [9]’s study on WS:GM might have been due to higher inclusion rate of grape marc in the cultivation substrate that could have provided more available sugars. Finally, a similar intraspecific variability was also observed in the present study, where for WS, earliness reached 23 days for some strains, BE ranged between 82 and 104% and productivity was between 1.65 and 2.73 (Supplementary Materials, Table S2).
3.2. Spectroscopic and Principal Component Analyses
The recorded FTIR spectra of the main substrates (with no supplementation) did not reveal significant qualitative differences among them, probably due to the use of wheat straw as main ingredient (at rates of 75 or 100%). However, small qualitative and mainly quantitative (i.e., in terms of absorption intensity) differences were observed (Figure 3). Significant differences were observed around 3400 cm−1, which is associated with the -OH stretching vibration of alcohols, phenols, or water as well as the N-H stretching of primary amines [30,31,32]. A slight drift in peaks’ position was observed for WS:GM, which is probably related to the higher phenolic content due to the presence of grape marc, while a higher absorption intensity was observed for WS:BS and WS:CS in comparison with WS. Similar quantitative differences could be observed at the peaks located at 2920/2850, 1650, and 1510 cm−1. These peaks are associated with the asymmetric/symmetric C-H stretching (aliphatic methylene), the C=C and C=O stretching (phenolic compounds, carbohydrates, etc.), and N-H and carbonyl C=O bending (amides), as well as aromatic ring C=C stretching, respectively [30,31,32,33,34,35]. The higher absorption intensity at 1650 cm−1 for the substrate containing grape marc (WS:GM) could probably indicate an increased content of phenolic compounds in this substrate [33,36]. The peak at 1734 cm−1 can be assigned to the un-conjugated C=O stretching in ketones, aldehydes, and carboxylic acids, previously associated with hemicellulose in straw [34,37,38]. WS:CS revealed a sharper and more intense peak than the rest of the substrates at 1734 cm−1, in contrary to WS:GM, where the specific peak was almost absent. Furthermore, as anticipated, adding of various N-rich supplements to substrates mostly resulted in changes in absorption intensities in regions related to N-H vibrations, for example, around 3350, 1650, and 1560 cm−1 [31,32].
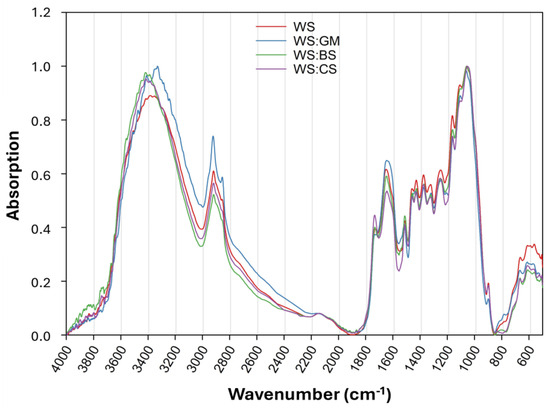
Figure 3.
Recorded FTIR spectra of the Pleurotus ostreatus mushroom cultivation substrates used without supplementation (basic substrates), by marking the differentiated spectral regions.
The principal component analysis (PCA) that was performed on the recorded transformed FTIR spectra (Savitzky–Golay smoothing of 11 points window and zero polynomial and linear baseline correction) of the substrates revealed their satisfactory separation mostly in terms of the used main materials (SPSS). In more details, the two first principal components (PC1 vs. PC2) allowed a clear separation of the substrates consisting of WS or of WS:GM, while a separation was not feasible for WS:CS and WS:BS, pointing out potential spectroscopic similarities between the latter substrates (Figure 4a). The observation of PC1 loadings (Figure 4b), across which the separation is taking place, allowed the detection of a positive correlation of WS:GM with the peaks at 2907, 2825, 1548, and 1471 cm−1. The former two peaks are related to methylene, while the latter two peaks can be assigned to the aromatic ring stretching and the -CH3 deformation vibration [30,31]. All the previously mentioned bond vibrations can be observed in phenolic compounds, such as anthocyanins and flavonols, which are among the main phenolic components of grape marc [36]. On the other hand, the separation of spectra corresponding to the supplemented substrates was not feasible, whereas substrates without supplementation were clearly separated across the PC2 axis (Figure 4c), which could be probably attributed to the high content of these supplements in nitrogen. This can be verified through the observation of PC2 loadings (Figure 4d), across which the separation of supplemented and not-supplemented substrates is taking place; there, a positive correlation of supplemented substrates with spectral regions mostly related to N-H vibrations, such as at 3400 (amines) and 1580 cm−1 (amide I band), could be observed [32].
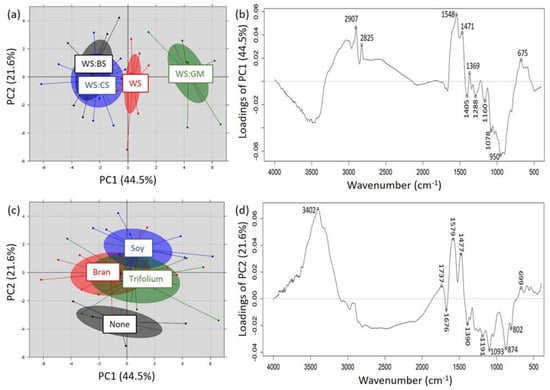
Figure 4.
Score plot of principal component analysis (PC1 vs. PC2) for the discrimination of Pleurotus ostreatus cultivation substrates on the basis of their recorded FTIR spectra, according to (a) the main materials (basic substrates) used or (c) the type of supplementation they received, and the responsible loadings for such discrimination (b,d, respectively).
A PCA was also performed on the recorded FTIR spectra of the substrates (following the above-mentioned spectral transformation) to develop a method capable of discriminating substrates of high or low mushroom productivity. For this purpose, substrates corresponding to the upper 25%—following the Gaussian distribution—of the productivity values (averaged values of the five strains examined) were characterized as “high-productivity substrates”, while substrates belonging on the other end (i.e., the lower 25%) were characterized as “low-productivity substrates”. The first two principal components (PC1 vs. PC2; 51% vs. 20.5%) allowed the discrimination of the recorded substrates’ spectra in two groups following the above-mentioned characterization (i.e., of high or low productivity) (Figure 5a). The interpretation of PC loadings (i.e., PC1 and PC2) allowed the detection of spectral regions responsible for such discrimination (Figure 5b,c). Therefore, spectral regions associated with aliphatic (2903, 2823 cm−1) or aromatic compounds (1600–1450 cm−1) seem to be positively correlated with high-productivity substrates, while spectral regions related to polysaccharides (1200–900 cm−1) seem to positively correlate with low-productivity substrates.
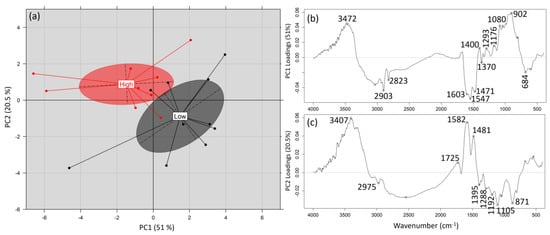
Figure 5.
(a) Score plot of principal component analysis (PC1 vs. PC2) for the discrimination of Pleurotus ostreatus cultivation substrates’ productivity based on their recorded FTIR spectra and (b,c) the respective loadings for such discrimination.
3.3. Prediction of Biological Efficiency
An extensive number of spectral transformations (including different combinations of them or order they were performed) were adopted to develop several tools (more than 50) presenting a wide range of BE predictive power and predictive error. The PAT tools achieving the best possible predictive power and accuracy for each P. ostreatus strain as well as for all strains (pooled), accompanied by the combination and the exact order of the performed spectral transformations, respectively, are presented in Table 1. The extended multiplicative scatter correction (EMSC) exhibited better BE predictions than the rest of spectral transformations for most of the strains examined. No statistically significant difference was observed in any of the pairs (calibration–cross validation–external validation) of R2 and RMSE values (p > 0.05). More particularly, the EMSC transformation of the single model (All, i.e., pooled strains) provided the PAT tool with the best predictive power and accuracy, with an R2EV between the measured and predicted BE value to be 0.90, an RMSEEV value of 6.97%, and a REPEV of only 6.01% (Figure 6). This fact may indicate that the separation of physical light-scattering effects from chemical (or vibrational) light absorbance effects that this spectral transformation offers, could have a significant positive impact since it reduces the variability of the spectra obtained by removing unnecessary information [39,40].

Table 1.
Effect of several indicative spectral transformations in the predictive power and accuracy of biological efficiency for five Pleurotus ostreatus strains (R2: coefficient of determination; RMSE: root mean square value; CAL: calibration; CV: cross validation; EV: external validation).
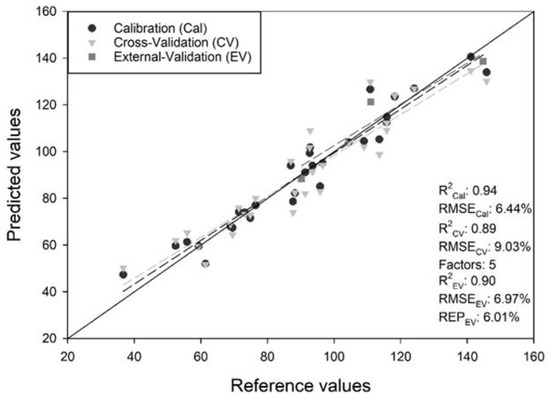
Figure 6.
Correlations between measured and predicted biological efficiency (% BE) values (R2: coefficient of determination; RMSE: root mean square error; REP: relative error of prediction).
Bekiaris et al. [21] have previously achieved R2CV and RMSECV values of 0.70 and 24%, respectively, for a Cyclocybe cylindracea strain cultivated on substrates consisting of various agro-industrial by-products. However, the low number of substrates used in their study (sample set of only 20 substrates) did not allow the creation of an external validation sample set, which could indicate if their developed PAT tool was robust or not. Furthermore, an increased variance of their sample set, which could be achieved with the use of a bigger calibration sample set, could lead to better predictive power and accuracy of this PAT tool. Therefore, a comparison with the PAT tool developed in the present study is not feasible.
4. Conclusions
The present study deals with the optimization of P. ostreatus cultivation using various combinations (and rates) of basic substrates and supplements deriving from various agricultural and agro-industrial activities. Overall, it seems that substrates consisting of mixtures of wheat straw and corn straw (3:1) had a significantly lower performance for the five strains examined, whereas mixtures of wheat straw and barley straw (3:1) supplemented with soybean (especially at a rate of 8%) presented—in most of the cases—the best values for the cultivation parameters studied. Furthermore, FTIR spectroscopy proved to be an ideal PAT tool both for the characterization of substrates and the prediction of biological efficiency values; the model developed for the first time in this study was capable of predicting biological efficiency on the basis of substrates recorded spectra with a R2 value of 0.90 and a very low prediction error (REP) of only 6% BE. This is a very low-cost tool (requiring an inexpensive mobile FTIR device only), which could be beneficial for the mushroom cultivation industry and mushroom growers since evaluation of the suitability of a wide range of mushroom substrates and supplements is feasible without the need of running costly and time-consuming cultivation trials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11100555/s1, Table S1: Recorded C/N ratio values of each of the basic substrates and the supplements used. Table S2: Cultivation parameters exhibited by five Pleurotus ostreatus commercial strains on various substrates supplemented by various supplements. Substrates: WS, wheat straw; GM, grape mark; BS, barley straw; CS, corn straw. Supplements: Tr, trifolium; Soy, soybean; Br, bran. BE: biological efficiency, N.A.: not available/ non-completed cultivation process. Values represent means and standard deviations for three replicates.
Author Contributions
Conceptualization, G.B., A.M., L.L. and G.I.Z.; methodology, G.B., A.M. and G.I.Z.; software, G.B.; validation, G.B., C.S.P., P.A.T. and G.I.Z.; formal analysis, G.B.; investigation, G.B., A.M. and G.I.Z.; resources, G.B. and A.M.; data curation, G.B. and G.I.Z.; writing—original draft preparation, G.B.; writing—review and editing, G.B., C.S.P., A.M., L.L., P.A.T. and G.I.Z.; supervision, G.I.Z.; funding acquisition, G.B. and G.I.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-financed by Greece and the European Union (European Social Fund- ESF) through the Operational Programme “Human Resources Development, Education and Lifelong Learning” in the context of the Programme “Reinforcement of Postdoctoral Researchers—2nd Cycle” (MIS-5033021), organized by the State Scholarships Foundation (ΙΚΥ).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
Authors Athanasios Mastrogiannis and Lefteris Lachouvaris were employed by the company Dirfis Mushrooms. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms; Wiley: Hoboken, NJ, USA, 2017; pp. 5–13. ISBN 978-1-119-14944-6. [Google Scholar]
- Gargano, M.L.; van Griensven, L.J.L.D.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Medicinal Mushrooms: Valuable Biological Resources of High Exploitation Potential. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Pradeep, P.; Manju, V.; Ahsan, M.F. Antiviral Potency of Mushroom Constituents. In Medicinal Mushrooms: Recent Progress in Research and Development; Agrawal, D.C., Dhanasekaran, M., Eds.; Springer: Singapore, 2019; pp. 275–297. ISBN 978-981-13-6382-5. [Google Scholar]
- Badalyan, S.M.; Barkhudaryan, A.; Rapior, S. Recent Progress in Research on the Pharmacological Potential of Mushrooms and Prospects for Their Clinical Application. In Medicinal Mushrooms: Recent Progress in Research and Development; Agrawal, D.C., Dhanasekaran, M., Eds.; Springer: Singapore, 2019; pp. 1–70. ISBN 978-981-13-6382-5. [Google Scholar]
- da Luz, J.M.R.; Nunes, M.D.; Paes, S.A.; Torres, D.P.; de Cássia Soares da Silva, M.; Kasuya, M.C.M. Lignocellulolytic Enzyme Production of Pleurotus Ostreatus Growth in Agroindustrial Wastes. Braz. J. Microbiol. 2012, 43, 1508–1515. [Google Scholar] [CrossRef]
- Das, N.; Mukherjee, M. Cultivation of Pleurotus Ostreatus on Weed Plants. Bioresour. Technol. 2007, 98, 2723–2726. [Google Scholar] [CrossRef] [PubMed]
- Dissasa, G. Cultivation of Different Oyster Mushroom (Pleurotus species) on Coffee Waste and Determination of Their Relative Biological Efficiency and Pectinase Enzyme Production, Ethiopia. Int. J. Microbiol. 2022, 2022, 5219939. [Google Scholar] [CrossRef]
- Ritota, M.; Manzi, P. Pleurotus Spp. Cultivation on Different Agri-Food By-Products: Example of Biotechnological Application. Sustainability 2019, 11, 5049. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Kalogeropoulos, N.; Kaliora, A.C.; Zervakis, G.I. Toward an Increased Functionality in Oyster (Pleurotus) Mushrooms Produced on Grape Marc or Olive Mill Wastes Serving as Sources of Bioactive Compounds. J. Agric. Food Chem. 2018, 66, 5971–5983. [Google Scholar] [CrossRef]
- Koutrotsios, G.; Mountzouris, K.C.; Chatzipavlidis, I.; Zervakis, G.I. Bioconversion of Lignocellulosic Residues by Agrocybe Cylindracea and Pleurotus Ostreatus Mushroom Fungi—Assessment of Their Effect on the Final Product and Spent Substrate Properties. Food Chem. 2014, 161, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Cullen, P.J.; O’Donnell, C.P.; Fagan, C.C. Benefits and Challenges of Adopting PAT for the Food Industry. In Process Analytical Technology for the Food Industry; O’Donnell, C.P., Fagan, C., Cullen, P.J., Eds.; Springer: New York, NY, USA, 2014; pp. 1–5. ISBN 978-1-4939-0311-5. [Google Scholar]
- Roussel, S.; Preys, S.; Chauchard, F.; Lallemand, J. Multivariate Data Analysis (Chemometrics). In Process Analytical Technology for the Food Industry; O’Donnell, C.P., Fagan, C., Cullen, P.J., Eds.; Springer: New York, NY, USA, 2014; pp. 7–59. ISBN 978-1-4939-0311-5. [Google Scholar]
- Lecellier, A.; Mounier, J.; Gaydou, V.; Castrec, L.; Barbier, G.; Ablain, W.; Manfait, M.; Toubas, D.; Sockalingum, G.D. Differentiation and Identification of Filamentous Fungi by High-Throughput FTIR Spectroscopic Analysis of Mycelia. Int. J. Food Microbiol. 2014, 168–169, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Fraga, M.E.; Kozakiewicz, Z.; Lima, N. Fourier Transform Infrared as a Powerful Technique for the Identification and Characterization of Filamentous Fungi and Yeasts. Res. Microbiol. 2010, 161, 168–175. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Li, J.-Q.; Liu, H.-G.; Wang, Y.-Z. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy (ATR-FTIR) Combined with Chemometrics Methods for the Classification of Lingzhi Species. Molecules 2019, 24, 2210. [Google Scholar] [CrossRef]
- Yao, S.; Li, J.; Li, T.; Liu, H.; Wang, Y. Discrimination of Boletaceae Mushrooms Based on Data Fusion of FT-IR and ICP–AES Combined with SVM. Int. J. Food Prop. 2018, 21, 255–266. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Bekiaris, G.; Tarantilis, P.A.; Pappas, C.S. Rapid Strain Classification and Taxa Delimitation within the Edible Mushroom Genus Pleurotus through the Use of Diffuse Reflectance Infrared Fourier Transform (DRIFT) Spectroscopy. Fungal Biol. 2012, 116, 715–728. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, M.-Y.; Yan, Y.; Zhu, S.-B.; Nie, S.-P.; Li, C.; Wang, Y.-X.; Gong, X.-F. Discrimination of Ganoderma Lucidum According to Geographical Origin with near Infrared Diffuse Reflectance Spectroscopy and Pattern Recognition Techniques. Anal. Chim. Acta 2008, 618, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Bekiaris, G.; Tagkouli, D.; Koutrotsios, G.; Kalogeropoulos, N.; Zervakis, G.I. Pleurotus Mushrooms Content in Glucans and Ergosterol Assessed by ATR-FTIR Spectroscopy and Multivariate Analysis. Foods 2020, 9, 535. [Google Scholar] [CrossRef]
- O’Gorman, A.; Downey, G.; Gowen, A.A.; Barry-Ryan, C.; Frias, J.M. Use of Fourier Transform Infrared Spectroscopy and Chemometric Data Analysis To Evaluate Damage and Age in Mushrooms (Agaricus bisporus) Grown in Ireland. J. Agric. Food Chem. 2010, 58, 7770–7776. [Google Scholar] [CrossRef] [PubMed]
- Bekiaris, G.; Koutrotsios, G.; Tarantilis, P.A.; Pappas, C.S.; Zervakis, G.I. FTIR Assessment of Compositional Changes in Lignocellulosic Wastes during Cultivation of Cyclocybe Cylindracea Mushrooms and Use of Chemometric Models to Predict Production Performance. J. Mater. Cycles Waste Manag. 2020, 22, 1027–1035. [Google Scholar] [CrossRef]
- Hotelling, H. The Generalization of Student’s Ratio. Ann. Math. Stat. 1931, 2, 360–378. [Google Scholar] [CrossRef]
- Martens, H.; Martens, M. Modified Jack-Knife Estimation of Parameter Uncertainty in Bilinear Modelling by Partial Least Squares Regression (PLSR). Food Qual. Prefer. 2000, 11, 5–16. [Google Scholar] [CrossRef]
- Olivieri, A.C. The Classical Least-Squares Model. In Introduction to Multivariate Calibration: A Practical Approach; Olivieri, A.C., Ed.; Springer International Publishing: Cham, Switzerland, 2018; pp. 19–38. ISBN 978-3-319-97097-4. [Google Scholar]
- Koutrotsios, G.; Kalogeropoulos, N.; Stathopoulos, P.; Kaliora, A.C.; Zervakis, G.I. Bioactive Compounds and Antioxidant Activity Exhibit High Intraspecific Variability in Pleurotus Ostreatus Mushrooms and Correlate Well with Cultivation Performance Parameters. World J. Microbiol. Biotechnol. 2017, 33, 98. [Google Scholar] [CrossRef] [PubMed]
- Zervakis, G.I.; Koutrotsios, G.; Katsaris, P. Composted versus Raw Olive Mill Waste as Substrates for the Production of Medicinal Mushrooms: An Assessment of Selected Cultivation and Quality Parameters. BioMed Res. Int. 2013, 2013, 546830. [Google Scholar] [CrossRef]
- Doroški, A.; Klaus, A.; Kozarski, M.; Cvetković, S.; Nikolić, B.; Jakovljević, D.; Tomasevic, I.; Vunduk, J.; Lazić, V.; Djekic, I. The Influence of Grape Pomace Substrate on Quality Characterization of Pleurotus ostreatus—Total Quality Index Approach. J. Food Process. Preserv. 2021, 45, e15096. [Google Scholar] [CrossRef]
- Melanouri, E.-M.; Diamantis, I.; Dedousi, M.; Dalaka, E.; Antonopoulou, P.; Papanikolaou, S.; Politis, I.; Theodorou, G.; Diamantopoulou, P. Pleurotus Ostreatus: Nutritional Enhancement and Antioxidant Activity Improvement Through Cultivation on Spent Mushroom Substrate and Roots of Leafy Vegetables. Fermentation 2025, 11, 20. [Google Scholar] [CrossRef]
- Dedousi, M.; Melanouri, E.-M.; Karayannis, D.; Kaminarides, E.-I.; Diamantopoulou, P. Utilization of Spent Substrates and Waste Products of Mushroom Cultivation to Produce New Crops of Pleurotus ostreatus, Pleurotus eryngii and Agaricus bisporus. Carbon Resour. Convers. 2024, 7, 100196. [Google Scholar] [CrossRef]
- Park, Y.-S.; Im, M.H.; Ham, K.-S.; Kang, S.-G.; Park, Y.-K.; Namiesnik, J.; Leontowicz, H.; Leontowicz, M.; Trakhtenberg, S.; Gorinstein, S. Quantitative Assessment of the Main Antioxidant Compounds, Antioxidant Activities and FTIR Spectra from Commonly Consumed Fruits, Compared to Standard Kiwi Fruit. LWT Food Sci. Technol. 2015, 63, 346–352. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2006; ISBN 978-0-470-02731-8. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; JohnWily & Sons Ltd.: Chichester, UK, 2001; ISBN 0-471-85298-8. [Google Scholar]
- Abbas, O.; Compère, G.; Larondelle, Y.; Pompeu, D.; Rogez, H.; Baeten, V. Phenolic Compound Explorer: A Mid-Infrared Spectroscopy Database. Vib. Spectrosc. 2017, 92, 111–118. [Google Scholar] [CrossRef]
- Sills, D.L.; Gossett, J.M. Using FTIR to Predict Saccharification from Enzymatic Hydrolysis of Alkali-Pretreated Biomasses. Biotechnol. Bioeng. 2012, 109, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.K.; Pitman, A.J. FTIR Studies of the Changes in Wood Chemistry Following Decay by Brown-Rot and White-Rot Fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Castellanos-Gallo, L.; Ballinas-Casarrubias, L.; Espinoza-Hicks, J.C.; Hernández-Ochoa, L.R.; Muñoz-Castellanos, L.N.; Zermeño-Ortega, M.R.; Borrego-Loya, A.; Salas, E. Grape Pomace Valorization by Extraction of Phenolic Polymeric Pigments: A Review. Processes 2022, 10, 469. [Google Scholar] [CrossRef]
- Gwon, J.G.; Lee, S.Y.; Doh, G.H.; Kim, J.H. Characterization of Chemically Modified Wood Fibers Using FTIR Spectroscopy for Biocomposites. J. Appl. Polym. Sci. 2010, 116, 3212–3219. [Google Scholar] [CrossRef]
- Kristensen, J.B.; Thygesen, L.G.; Felby, C.; Jørgensen, H.; Elder, T. Cell-Wall Structural Changes in Wheat Straw Pretreated for Bioethanol Production. Biotechnol. Biofuels 2008, 1, 5. [Google Scholar] [CrossRef]
- Martens, H.; Nielsen, J.P.; Engelsen, S.B. Light Scattering and Light Absorbance Separated by Extended Multiplicative Signal Correction. Application to Near-Infrared Transmission Analysis of Powder Mixtures. Anal. Chem. 2003, 75, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Martens, H.; Stark, E. Extended Multiplicative Signal Correction and Spectral Interference Subtraction: New Preprocessing Methods for near Infrared Spectroscopy. Invit. Pap. Int. Symp. Organ. Swed. Acad. Pharm. Sci. 1991, 9, 625–635. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).