Mycomaterials from Agave Bagasse: A Valorization Strategy for Sustainable Tequila Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation and Growth Conditions of Mycelium-Based Biocomposites
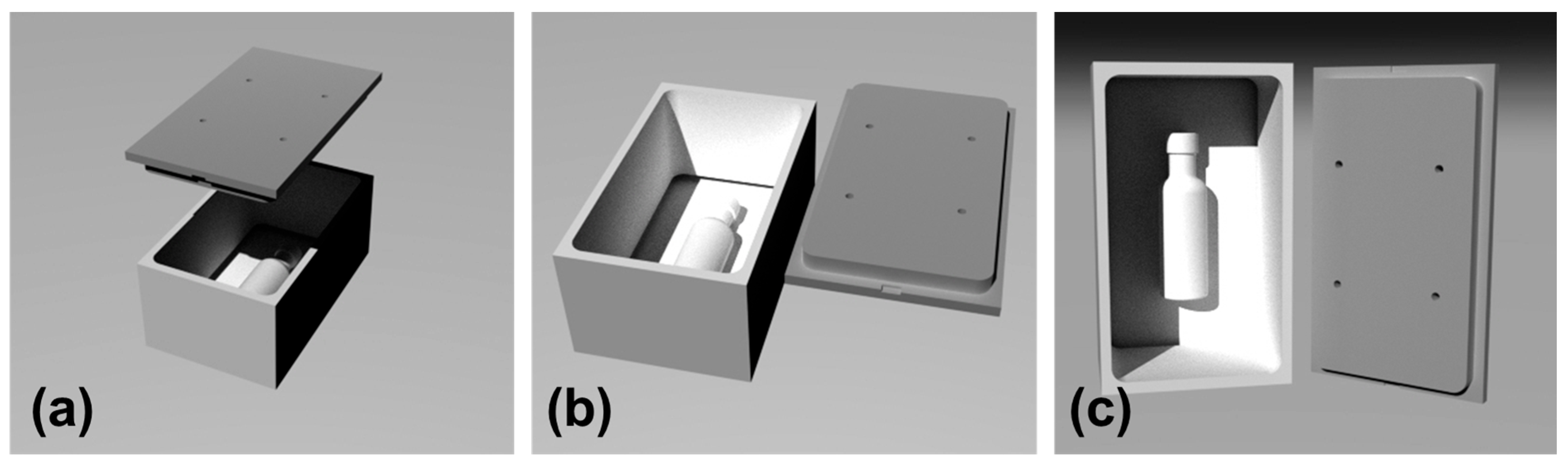
2.2. Methodology for Design and Fabrication of 3D-Printed Mold for Mycelium-Based Packaging
2.3. Methodology for Physical and Mechanical Characterization of the Mycelium-Based Packaging
2.4. Life Cicle Assesment (LCA)
2.4.1. Goal and Scope
2.4.2. Inventory Analysis
2.4.3. Impact Assessment
2.4.4. Interpretation and Comparison Analysis
3. Results and Discussion
3.1. Growth Kinetics
3.2. Growth Characterization of Ganoderma lucidum on Potato Dextrose Agar (PDA) Medium and Lignocellulosic Substrate (Agave Bagasse)
3.3. Design and Fabrication of 3D-Printed Mold for Mycelium-Based Packaging
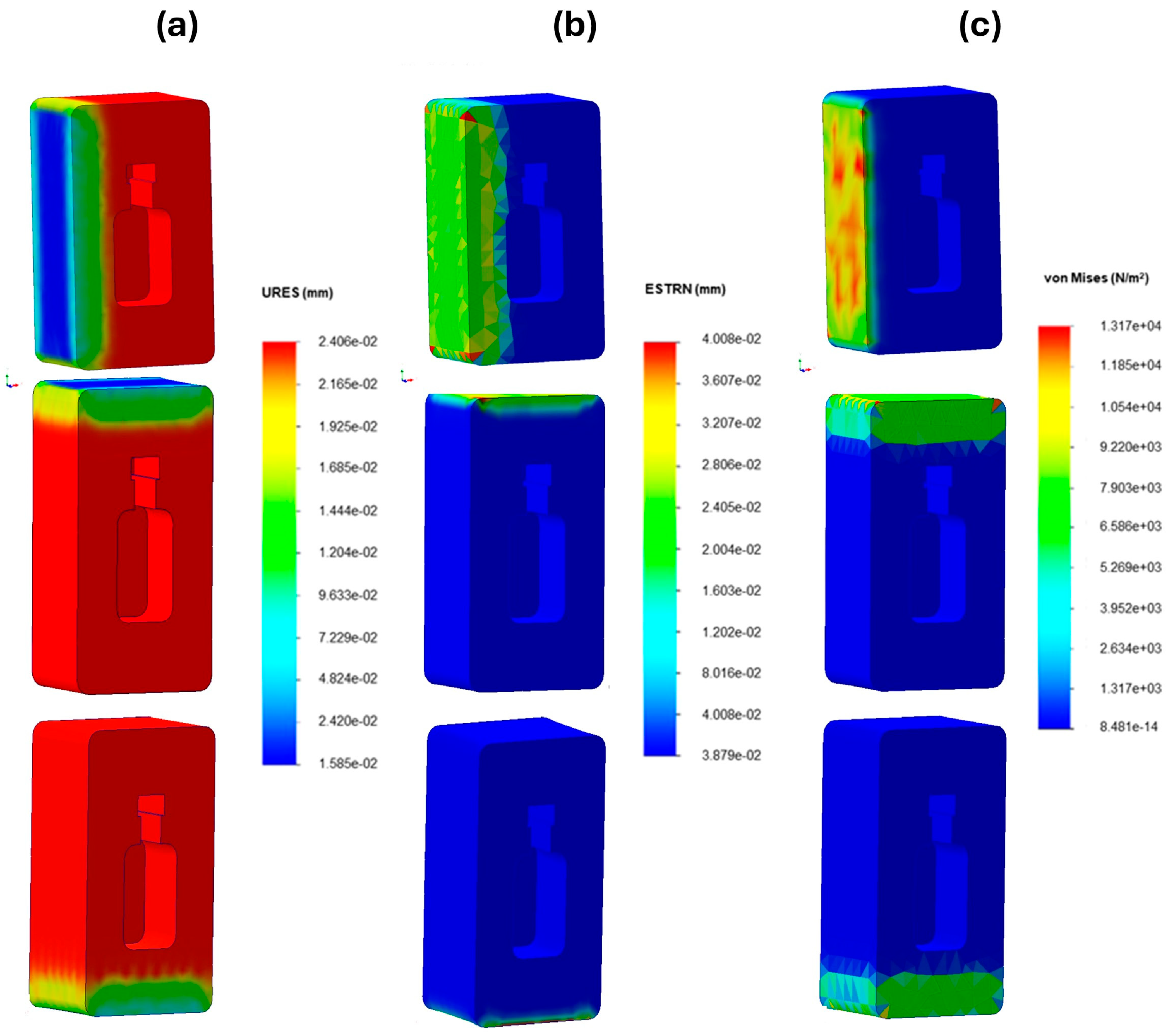
3.4. Physical and Mechanical Characterization of the Mycelium-Based Packaging
Drop Test Simulation
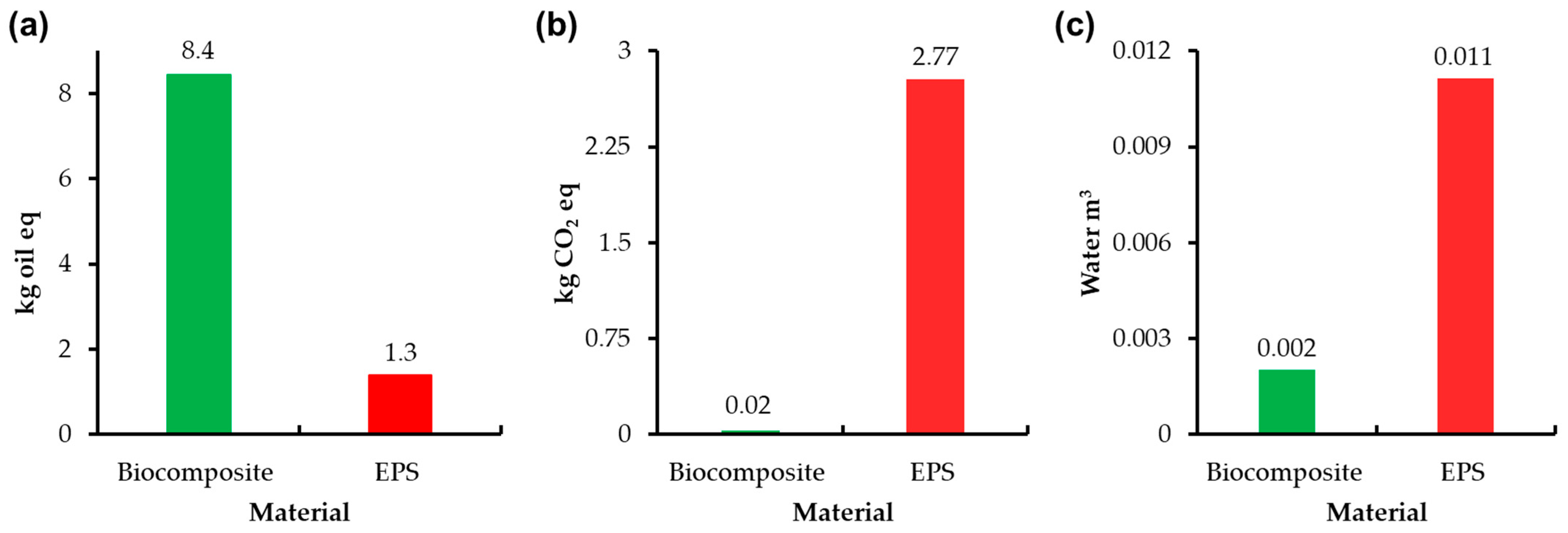
3.5. Lyfe Cycle Assesment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romero-Cano, L.A.; Zárate-Guzmán, A.I.; Nájar-Guzmán, R.; Warren-Vega, W.M.; Campos-Rodríguez, A. Simulation of a Steam Generation Plant Useful in the Tequila Production Process Employing Different Fuels as a Novel Strategy for Environmental Impact Assessment. J. Clean. Prod. 2024, 440, 140983. [Google Scholar] [CrossRef]
- Morales, A.S.; García-Alcaraz, J.L.; Flor-Montalvo, F.J.; Martínez-Cámara, E.; Blanco, J. Sustainability in Tequila Production: A Life Cycle Assessment. Sustain. Dev. 2025. [Google Scholar] [CrossRef]
- Díaz-Vázquez, D.; Carrillo-Nieves, D.; Orozco-Nunnelly, D.A.; Senés-Guerrero, C.; Gradilla-Hernández, M.S. An Integrated Approach for the Assessment of Environmental Sustainability in Agro-Industrial Waste Management Practices: The Case of the Tequila Industry. Front Environ Sci 2021, 9. [Google Scholar] [CrossRef]
- Parsaee, M.; Kiani Deh Kiani, M.; Karimi, K. A Review of Biogas Production from Sugarcane Vinasse. Biomass Bioenergy 2019, 122, 117–125. [Google Scholar] [CrossRef]
- Gómez-Navarro, C.S.; Warren-Vega, W.M.; Serna-Carrizales, J.C.; Zárate-Guzmán, A.I.; Ocampo-Pérez, R.; Carrasco-Marín, F.; Collins-Martínez, V.H.; Niembro-García, J.; Romero-Cano, L.A. Evaluation of the Environmental Performance of Adsorbent Materials Prepared from Agave Bagasse for Water Remediation: Solid Waste Management Proposal of the Tequila Industry. Materials 2022, 16, 8. [Google Scholar] [CrossRef] [PubMed]
- Medellín-Castillo, N.A.; Hernández-Ramírez, M.G.; Salazar-Rábago, J.J.; Labrada-Delgado, G.J.; Aragón-Piña, A. BIOADSORCIÓN DE PLOMO (II) PRESENTE EN SOLUCIÓN ACUOSA SOBRE RESIDUOS DE FIBRAS NATURALES PROCEDENTES DE LA INDUSTRIA IXTLERA (Agave Lechuguilla Torr. Y Yucca Carnerosana (Trel.) McKelvey). Revista Int. Contam. Ambient. 2017, 33, 269–280. [Google Scholar] [CrossRef]
- Garcia-Reyes, R.B.; Rangel-Mendez, J.R. Adsorption Kinetics of Chromium(III) Ions on Agro-Waste Materials. Bioresour. Technol. 2010, 101, 8099–8108. [Google Scholar] [CrossRef]
- Garcia-Reyes, R.B.; Rangel-Mendez, J.R. Contribution of Agro-waste Material Main Components (Hemicelluloses, Cellulose, and Lignin) to the Removal of Chromium (III) from Aqueous Solution. J. Chem. Technol. Biotechnol. 2009, 84, 1533–1538. [Google Scholar] [CrossRef]
- Vigueras-Cortés, J.M.; Villanueva-Fierro, I.; Garzón-Zúñiga, M.A.; de Jesús Návar-Cháidez, J.; Chaires-Hernández, I.; Hernández-Rodríguez, C. Performance of a Biofilter System with Agave Fiber Filter Media for Municipal Wastewater Treatment. Water Sci. Technol. 2013, 68, 599–607. [Google Scholar] [CrossRef]
- Palomo-Briones, R.; López-Gutiérrez, I.; Islas-Lugo, F.; Galindo-Hernández, K.L.; Munguía-Aguilar, D.; Rincón-Pérez, J.A.; Cortés-Carmona, M.Á.; Alatriste-Mondragón, F.; Razo-Flores, E. Agave Bagasse Biorefinery: Processing and Perspectives. Clean. Technol. Environ. Policy 2018, 20, 1423–1441. [Google Scholar] [CrossRef]
- Warren-Vega, W.M.; Fonseca-Aguiñaga, R.; López de la Cruz, C.F.; Campos-Rodríguez, A.; Zárate Guzmán, A.I.; Romero Cano, L.A. Valorization of Agave Bagasse as a Bio-Template for Circular Economy: Applications in Energy Conversion, Metabolite Production, and Sustainable Material Synthesis. Biomass Bioenergy 2025, 197, 107797. [Google Scholar] [CrossRef]
- Kumar, D.; Rajput, M.S.; Jha, A.A.; Kumar, M. Sustainable hybrid composites, constructed from cellulose nanofibrils and wood fungal mycelium. In Fungal Waste Biomass Management for Energy, Environment and Value-Added Products; Pal, D.B., Bansal, S.L., Eds.; Springer: Cham, Switzerland, 2025; pp. 1–26. [Google Scholar]
- Alemu, D.; Tafesse, M.; Mondal, A.K. Mycelium-Based Composite: The Future Sustainable Biomaterial. Int. J. Biomater. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Madusanka, C.; Udayanga, D.; Nilmini, R.; Rajapaksha, S.; Hewawasam, C.; Manamgoda, D.; Vasco-Correa, J. A Review of Recent Advances in Fungal Mycelium Based Composites. Discov. Mater. 2024, 4, 13. [Google Scholar] [CrossRef]
- Rajendran, R.C. Packaging Applications of Fungal Mycelium-Based Biodegradable Composites. In Fungal Biopolymers and Biocomposites; Springer Nature: Singapore, 2022; pp. 189–208. [Google Scholar]
- Abhijith, R.; Ashok, A.; Rejeesh, C.R. Sustainable Packaging Applications from Mycelium to Substitute Polystyrene: A Review. Mater. Today Proc. 2018, 5, 2139–2145. [Google Scholar] [CrossRef]
- Elsacker, E.; Vandelook, S.; Van Wylick, A.; Ruytinx, J.; De Laet, L.; Peeters, E. A Comprehensive Framework for the Production of Mycelium-Based Lignocellulosic Composites. Sci. Total Environ. 2020, 725, 138431. [Google Scholar] [CrossRef] [PubMed]
- Irbe, I.; Loris, G.D.; Filipova, I.; Andze, L.; Skute, M. Characterization of Self-Growing Biomaterials Made of Fungal Mycelium and Various Lignocellulose-Containing Ingredients. Materials 2022, 15, 7608. [Google Scholar] [CrossRef] [PubMed]
- Wattanavichean, N.; Phanthuwongpakdee, J.; Koedrith, P.; Laoratanakul, P.; Thaithatgoon, B.; Somrithipol, S.; Kwantong, P.; Nuankaew, S.; Pinruan, U.; Chuaseeharonnachai, C.; et al. Mycelium-Based Breakthroughs: Exploring Commercialization, Research, and Next-Gen Possibilities. Circ. Econ. Sustain. 2025. [Google Scholar] [CrossRef]
- Bitting, S.; Derme, T.; Lee, J.; Van Mele, T.; Dillenburger, B.; Block, P. Challenges and Opportunities in Scaling up Architectural Applications of Mycelium-Based Materials with Digital Fabrication. Biomimetics 2022, 7, 44. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Atta, O.M.; Yang, G. Synthesis and Applications of Fungal Mycelium-Based Advanced Functional Materials. J. Bioresour. Bioprod. 2021, 6, 1–10. [Google Scholar] [CrossRef]
- Majib, N.M.; Yaacob, N.D.; Ting, S.S.; Rohaizad, N.M.; Azizul Rashidi, A.M. Fungal Mycelium-Based Biofoam Composite: A Review in Growth, Properties and Application. Prog. Rubber Plast. Recycl. Technol. 2025, 41, 91–123. [Google Scholar] [CrossRef]
- Bernabé-González, T.M.; Cayetano-Catarino, G.; Bernabé-Villanueva, A.; Romero-Flores, M.D.; Ángel-Ríos, J. Pérez-Salgado Cultivation of Ganoderma lucidum on Agricultural By-Products in Mexico. Micol. Aplicada Int. 2015, 2, 25–30. [Google Scholar]
- Ramírez-López, C.B.; Pérez-Mayorga, S.; Ramírez Briones, E.; Macias Rodríguez, R.; Salcedo-Pérez, E. Efecto Antifúngico y Estudio Químico de Eysenhardtia Polystachya (Fabaceae) Sobre Phaneroquete Crysosporum y Ganoderma Lucidium. Rev. Mex. Cienc. Agric. 2023, 14, e3208. [Google Scholar] [CrossRef]
- Torres-Torres, M.G.; Ryvarden, L.; Guzmán-Dávalos, L. Ganoderma Subgenus Ganoderma in Mexico. Rev. Mex. Micol. 2015, 41, 27–45. [Google Scholar]
- Jose, J.; Uvais, K.N.; Sreenadh, T.S.; Deepak, A.V.; Rejeesh, C.R. Investigations into the Development of a Mycelium Biocomposite to Substitute Polystyrene in Packaging Applications. Arab. J. Sci. Eng. 2021, 46, 2975–2984. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Byju, S.K.; Prajith, C.; Shaju, J.; Rejeesh, C.R. Development of a Novel Mycelium Bio-Composite Material to Substitute for Polystyrene in Packaging Applications. Mater. Today Proc. 2021, 47, 5038–5044. [Google Scholar] [CrossRef]
- ASTM D5276-19; Standard Test Method for Drop Test of Loaded Containers by Free Fall. ASTM International: West Conshohocken, PA, USA, 2019.
- Böröcz, P.; Németh, Z. Review Paper on the Field Measurement of Parcel Packaging Drop for Testing Purposes. Packag. Technol. Sci. 2025, 38, 345–357. [Google Scholar] [CrossRef]
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- Secretaria de Energía (SENER) 5. Infraestructura Del Sistema Eléctrico. URL. Available online: https://www.cenace.gob.mx/Docs/16_MARCOREGULATORIO/Prodecen/19%202024-2038%20Anexos.pdf (accessed on 15 August 2025).
- Rivas, E.-M.; Gil de Prado, E.; Wrent, P.; de Silóniz, M.-I.; Barreiro, P.; Correa, E.C.; Conejero, F.; Murciano, A.; Peinado, J.M. A Simple Mathematical Model That Describes the Growth of the Area and the Number of Total and Viable Cells in Yeast Colonies. Lett. Appl. Microbiol. 2014, 59, 594–603. [Google Scholar] [CrossRef]
- Brunk, M.; Sputh, S.; Doose, S.; van de Linde, S.; Terpitz, U. HyphaTracker: An ImageJ Toolbox for Time-Resolved Analysis of Spore Germination in Filamentous Fungi. Sci. Rep. 2018, 8, 605. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, L.; Chen, H. Digital Image Analysis and Fractal-Based Kinetic Modelling for Fungal Biomass Determination in Solid-State Fermentation. Biochem. Eng. J. 2012, 67, 60–67. [Google Scholar] [CrossRef]
- Kai, P.M.; de M.N. Soares, F.A.A.; da Costa, R.M.; Felix, J.P.; de Jesus, J.M.I.; da Cunha, M.G. Measurement by Images of Mycelial Growth Of Fungal Colonies On Petri Dishes. In Proceedings of the 2019 IEEE Canadian Conference of Electrical and Computer Engineering (CCECE), Edmonton, AB, Canada, 5–8 May 2019; IEEE: New York, NY, USA, 2019; pp. 1–4. [Google Scholar]
- Ebrahimi, P.; van den Berg, F.; Aunsbjerg, S.D.; Honoré, A.; Benfeldt, C.; Jensen, H.M.; Engelsen, S.B. Quantitative Determination of Mold Growth and Inhibition by Multispectral Imaging. Food Control 2015, 55, 82–89. [Google Scholar] [CrossRef]
- Vidal-Diez de Ulzurrun, G.; Baetens, J.M.; Van den Bulcke, J.; Lopez-Molina, C.; De Windt, I.; De Baets, B. Automated Image-Based Analysis of Spatio-Temporal Fungal Dynamics. Fungal Genet. Biol. 2015, 84, 12–25. [Google Scholar] [CrossRef]
- Gougouli, M.; Koutsoumanis, K.P. Relation between Germination and Mycelium Growth of Individual Fungal Spores. Int. J. Food Microbiol. 2013, 161, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Zervakis, G.; Philippoussis, A.; Ioannidou, S.; Diamantopoulou, P. Mycelium Growth Kinetics and Optimal Temperature Conditions for the Cultivation of Edible Mushroom Species on Lignocellulosic Substrates. Folia Microbiol. 2001, 46, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Nathan, V.K.; Esther Rani, M.; Rathinasamy, G.; Dhiraviam, K.N.; Jayavel, S. Process Optimization and Production Kinetics for Cellulase Production by Trichoderma Viride VKF3. Springerplus 2014, 3, 92. [Google Scholar] [CrossRef]
- Dantigny, P. Applications of Predictive Modeling Techniques to Fungal Growth in Foods. Curr. Opin. Food Sci. 2021, 38, 86–90. [Google Scholar] [CrossRef]
- Chen, J.; Lan, X.; Jia, R.; Hu, L.; Wang, Y. Response Surface Methodology (RSM) Mediated Optimization of Medium Components for Mycelial Growth and Metabolites Production of Streptomyces Alfalfae XN-04. Microorganisms 2022, 10, 1854. [Google Scholar] [CrossRef]
- Jones, M.; Huynh, T.; Dekiwadia, C.; Daver, F.; John, S. Mycelium Composites: A Review of Engineering Characteristics and Growth Kinetics. J. Bionanoscience 2017, 11, 241–257. [Google Scholar] [CrossRef]
- Nussbaum, N.; von Wyl, T.; Gandia, A.; Romanens, E.; Rühs, P.A.; Fischer, P. Impact of Malt Concentration in Solid Substrate on Mycelial Growth and Network Connectivity in Ganoderma Species. Sci. Rep. 2023, 13, 21051. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Huynh, T.; John, S. Inherent Species Characteristic Influence and Growth Performance Assessment for Mycelium Composite Applications. Adv. Mater. Lett. 2018, 9, 71–80. [Google Scholar] [CrossRef]
- Jones, M.; Mautner, A.; Luenco, S.; Bismarck, A.; John, S. Engineered Mycelium Composite Construction Materials from Fungal Biorefineries: A Critical Review. Mater. Des. 2020, 187, 108397. [Google Scholar] [CrossRef]
- Porter, D.L.; Hotz, E.C.; Uehling, J.K.; Naleway, S.E. A Review of the Material and Mechanical Properties of Select Ganoderma Fungi Structures as a Source for Bioinspiration. J. Mater. Sci. 2023, 58, 3401–3420. [Google Scholar] [CrossRef]
- Jones, M.; Bhat, T.; Kandare, E.; Thomas, A.; Joseph, P.; Dekiwadia, C.; Yuen, R.; John, S.; Ma, J.; Wang, C.-H. Thermal Degradation and Fire Properties of Fungal Mycelium and Mycelium—Biomass Composite Materials. Sci. Rep. 2018, 8, 17583. [Google Scholar] [CrossRef]
- Fricker, M.D.; Heaton, L.L.M.; Jones, N.S.; Boddy, L. The Mycelium as a Network. Microbiol Spectr 2017, 5. [Google Scholar] [CrossRef]
- Balaeș, T.; Radu, B.-M.; Tănase, C. Mycelium-Composite Materials—A Promising Alternative to Plastics? J. Fungi 2023, 9, 210. [Google Scholar] [CrossRef]
- Karuppasamy, A.; Rexliene, J.; Dhandapani, A.; Balaji, V.; Praveen, R.; Sridhar, J.; Krishnasamy, S.; Thiagamani, S.M.K.; Muthukumar, C. Recyclability of Lightweight and Sustainable Materials. In Lightweight and Sustainable Composite Materials; Elsevier: Amsterdam, The Netherlands, 2023; pp. 79–96. [Google Scholar]
- Sun, W.; Tajvidi, M.; Hunt, C.G.; Cole, B.J.W.; Howell, C.; Gardner, D.J.; Wang, J. Fungal and Enzymatic Pretreatments in Hot-Pressed Lignocellulosic Bio-Composites: A Critical Review. J. Clean. Prod. 2022, 353, 131659. [Google Scholar] [CrossRef]
- Sreerag, N.K.; Kashyap, P.; Shilpa, V.S.; Thakur, M.; Goksen, G. Recent Advances on Mycelium Based BioComposites: Synthesis, Strains, Lignocellulosic Substrates, Production Parameters. Polym. Rev. 2025, 65, 169–198. [Google Scholar] [CrossRef]
- Islam, M.R.; Tudryn, G.; Bucinell, R.; Schadler, L.; Picu, R.C. Mechanical Behavior of Mycelium-Based Particulate Composites. J. Mater. Sci. 2018, 53, 16371–16382. [Google Scholar] [CrossRef]
- Aiduang, W.; Kumla, J.; Srinuanpan, S.; Thamjaree, W.; Lumyong, S.; Suwannarach, N. Mechanical, Physical, and Chemical Properties of Mycelium-Based Composites Produced from Various Lignocellulosic Residues and Fungal Species. J. Fungi 2022, 8, 1125. [Google Scholar] [CrossRef] [PubMed]
- Pavlík, M.; Fleischer, P.; Fleischer, P.; Pavlík, M.; Šuleková, M. Evaluation of the Carbon Dioxide Production by Fungi Under Different Growing Conditions. Curr. Microbiol. 2020, 77, 2374–2384. [Google Scholar] [CrossRef]
- Desole, M.P.; Gisario, A.; Fedele, L.; Aversa, C.; Barletta, M. Life Cycle Assessment of Secondary Packaging: Expanded Polystyrene versus Bioplastic-Coated Corrugated Cardboard. Sustain. Prod. Consum. 2024, 46, 11–28. [Google Scholar] [CrossRef]
- Bonenberg, A.; Sydor, M.; Cofta, G.; Doczekalska, B.; Grygorowicz-Kosakowska, K. Mycelium-Based Composite Materials: Study of Acceptance. Materials 2023, 16, 2164. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Anda-Rodríguez, F.A.; Corona-Ramírez, M.R.; Patiño-Arévalo, C.D.; Zárate-Navarro, M.A.; Zárate-Guzmán, A.I.; Romero-Cano, L.A. Mycomaterials from Agave Bagasse: A Valorization Strategy for Sustainable Tequila Packaging. Fermentation 2025, 11, 500. https://doi.org/10.3390/fermentation11090500
de Anda-Rodríguez FA, Corona-Ramírez MR, Patiño-Arévalo CD, Zárate-Navarro MA, Zárate-Guzmán AI, Romero-Cano LA. Mycomaterials from Agave Bagasse: A Valorization Strategy for Sustainable Tequila Packaging. Fermentation. 2025; 11(9):500. https://doi.org/10.3390/fermentation11090500
Chicago/Turabian Stylede Anda-Rodríguez, Flavio A., Mariana R. Corona-Ramírez, Carlos D. Patiño-Arévalo, Marco A. Zárate-Navarro, Ana I. Zárate-Guzmán, and Luis A. Romero-Cano. 2025. "Mycomaterials from Agave Bagasse: A Valorization Strategy for Sustainable Tequila Packaging" Fermentation 11, no. 9: 500. https://doi.org/10.3390/fermentation11090500
APA Stylede Anda-Rodríguez, F. A., Corona-Ramírez, M. R., Patiño-Arévalo, C. D., Zárate-Navarro, M. A., Zárate-Guzmán, A. I., & Romero-Cano, L. A. (2025). Mycomaterials from Agave Bagasse: A Valorization Strategy for Sustainable Tequila Packaging. Fermentation, 11(9), 500. https://doi.org/10.3390/fermentation11090500






