Abstract
Due to its high content of lignocellulose, cotton stalk is difficult to degrade naturally and utilize effectively, so it is often regarded as waste. In this study, the effects of Pleurotus ostreatus XH005, Lactiplantibacillus plantarum LP-2, and cellulase enzyme on the cotton stalk substrate under aerobic solid-state fermentation (SSF) conditions were investigated, and the metabolites were analyzed to identify potential functional compounds in the cotton-stalk-fermented feed. Preliminary optimization results obtained through single-factor experiments were as follows: fermentation time 14 days, XH005 inoculum size 8.00% (v/m), material-to-water ratio 1:0.50 (v/m), LP-2 inoculum size 2.00% (v/m), and cellulase addition 0.60% (m/m). Based on these single-factor experimental results, XH005 inoculum size, LP-2 inoculum size, material-to-water ratio, and cellulase addition were selected as independent variables. Through response surface methodology (RSM) optimization experiments, 29 experimental groups were designed. Subsequently, based on Box–Behnken analysis of variance (ANOVA) of lignin and cellulose content, along with contour and response surface plots, the optimal aerobic solid-state fermentation parameters were determined as follows: fermentation time 14 days, XH005 inoculum: 7.00% (v/m), material-to-water ratio: 1:0.55 (v/m), LP-2 inoculum: 2.00% (v/m), and cellulase enzyme addition: 0.65% (m/m). Results showed that compared with the control group (CK), the optimized group exhibited a 27.65% increase in lignin degradation rate and a 47.14% increase in cellulose degradation rate. Crude protein (CP) content increased significantly, while crude fiber (CF), detergent fiber and mycotoxin contents decreased significantly. Non-targeted metabolic analysis indicated that adding cellulase and inoculating Pleurotus ostreatus XH005 and Lactiplantibacillus plantarum LP-2 in aerobic SSF of cotton straw feed produced functionally active substances such as kaempferol (C343), carvone (C709) and trilobatin (C604). Therefore, this study demonstrates that microbial-enzyme co-action SSF significantly enhances the nutritional composition of cotton stalk hydrolysate. Furthermore, this hydrolysate is suitable for the production of functional compounds, endowing the fermented feed with health-promoting properties and enhancing the utilization of cotton processing byproducts in the feed industry.
1. Introduction
Globally, cotton is one of the most widely cultivated crops and the predominant fiber crop [1]. As a major cotton-producing country, China accounts for one-third of the world’s total annual cotton output. Cotton stalk is the primary byproduct of cotton cultivation; therefore, it exists in substantial quantities [2]. Cotton straw also has the basis for being processed into roughage [3]. Despite its certain economic value, many developing countries face difficulties in achieving its sustainable utilization due to a lack of relevant knowledge and technologies. In rural areas, the majority of cotton stalk is treated as waste, with a portion used as fuel and the remainder burned in fields post-harvest. This practice results in substantial environmental pollution and economic losses. This disposal method also limits the potential for comprehensive utilization of cotton stalk, such as conversion into animal feed [4], ethanol production [5], application in road engineering [6], etc. Furthermore, China has imposed a comprehensive ban on open-field burning of crop residues [7].
Solid-state fermentation (SSF) has been demonstrated to enhance the nutritional properties of agricultural byproducts and reduce antinutritional factors through the action of microorganisms and their secreted enzymes, including hydrolytic enzymes and oxidases [8]. Owing to its low energy demands, reduced chemical requirements, and benign environmental profile, SSF represents a viable alternative as a cost-effective and green technology [9]. Although cotton straw has nutritional potential for roughage preparation, its high lignocellulose content results in poor palatability, which limits its application potential as feed [10,11]. Therefore, we can select microorganisms possessing lignocellulose-degrading capability or supplement exogenous enzymes with lignocellulose degradation activities into the fermentation medium, followed by applying SSF technology to achieve lignocellulose degradation. However, the significant biorecalcitrance of lignocellulose may substantially prolong SSF duration and result in low degradation efficiency. Steam explosion pretreatment disrupts the lignocellulosic structure, overcomes biorecalcitrance, reduces lignin’s blocking effect, enhances biodegradability, and creates favorable conditions for subsequent enzymatic hydrolysis or microbial conversion [12]. In previous studies, the pretreatment method for cotton stalk was mechanical comminution [4]. This process primarily alters the particle size and surface area of cotton stalk, yet it fundamentally does not modify the lignocellulosic structure, thereby failing to overcome its inherent biorecalcitrance.
Currently, the main microorganisms that degrade lignocellulose in nature are white-rot fungi [13]. Pleurotus ostreatus, a white-rot fungus, secretes extracellular enzyme systems that specifically degrade lignin in fermentation substrates. These systems include enzymes such as manganese peroxidase and laccase. This process converts lignin into soluble monosaccharides and small-molecule organic compounds, thereby enhancing substrate bioavailability and improving the digestibility of ruminant feed [14]. However, white rot fungi have a long growth cycle and are prone to contamination by undesirable microorganisms, and this adversely affects the fermentation process. Lactic acid bacteria (LAB) rank among the most prevalent microorganisms employed in SSF [15]. By producing metabolites such as lactic acid and acetic acid, LAB suppress pathogenic microorganism growth; they also mitigate protein decomposition and reduce nutrient degradation during the process [16]. Wu et al. also reported similar results, demonstrating that inoculation with Lactobacillus rhamnosus significantly reduced pH, increased lactic acid content, decreased pathogenic bacteria, and altered the microbial community in alfalfa [17]. Nevertheless, LAB are constrained by insufficient secretion of hydrolytic enzymes, restricting their capacity to enhance substrate digestibility [18]. Thus, co-cultivation of Pleurotus ostreatus XH005 and Lactiplantibacillus plantarum LP-2 enables the overcoming of key limitations of monocultures—including low degradation efficiency, extended processing duration, and significant product inhibition—in solid-state fermentation systems. In SSF of agricultural byproducts, enzyme supplementation addresses the challenge of initial insufficient enzyme production by microorganisms, thereby enhancing microbial utilization efficiency of feed macromolecules [19]. Cellulases break down cellulose and other high-molecular-weight carbohydrates in plant cell walls, converting them into small molecules including monosaccharides and potential amino acid precursors. These products provide essential carbon and nitrogen sources for microbial fermentation [20].
Current research on fermented feed utilizing cotton stalk as a substrate remains limited, with insufficient investigation into alterations in nutritional profiles and metabolites post-fermentation. This study utilized steam explosion pretreatment to disrupt the lignocellulosic structure of cotton stalk, followed by aerobic solid-state fermentation employing Pleurotus ostreatus XH005 Lactiplantibacillus plantarum LP-2, and cellulase. We systematically investigated SSF’s impact on the stalk’s nutritional composition and further explored the fermented feed’s functional potential through untargeted metabolomics, thereby establishing a scientific foundation for developing cotton stalk-based fermented feed.
2. Materials and Methods
2.1. Materials and Strains
Cotton stalk and cottonseed meal were purchased from Xinjiang Changji Xuze Biotechnology Co., Ltd. Steam explosion pretreatment was performed using a QB-600 unit (Qingdao Jiuyi Machinery Co., Qingdao, China) with the following parameters: material-water ratio of 35% (w/w), temperature 160 °C, pressure 1.5 MPa, and retention time 3 min.
Wheat bran was sourced from Shandong Dongchen Biological Co., Ltd. (Dongying, China).
Solid-state fermentation bags (gas-exchange membrane, 0.22 μm pore size) and cellulase enzyme (activity: 20,000 U/g) were obtained from Shandong Boffed Technology Co., Ltd. (Zibo, China).
Pleurotus ostreatus XH005 was provided by the Institute of Microbiology, Xinjiang Uygur Autonomous Region Academy of Agricultural Sciences (Xinjiang, China) and is preserved at the China General Microbiological Culture Collection Center under accession number CGMCC 41421.
Lactiplantibacillus plantarum LP-2 was isolated and characterized by the Institute of Microbiology, Xinjiang Uygur Autonomous Region Academy of Agricultural Sciences.
SSF Medium Basal substrate composition: Steam-exploded cotton stalk: 80%; Wheat bran: 15%; Cottonseed meal: 5%.
Pleurotus ostreatus XH005 medium liquid culture: Glucose: 39.5 g/L, Yeast extract: 6.0 g/L, CuSO4·5H2O: 0.85 mmol/L, pH: 5.0 (adjusted prior to sterilization). Spore count: 1 × 106 spores/mL.
MRS Medium (For the cultivation of LP-2): Peptone: 10.00 g/L, Yeast extract: 5.00 g/L, Beef extract: 4.00 g/L, Glucose: 20.00 g/L, Dipotassium phosphate (K2HPO4): 2.00 g/L, Ammonium citrate tribasic: 2.00 g/L, Sodium acetate: 5.00 g/L, Manganese sulfate (MnSO4·H2O): 0.2 g/L, Magnesium sulfate (MgSO4): 0.05 g/L, Polysorbate 80 (Tween 80): 1.0 g/L. Spore count: 1 × 108 CFU/mL.
2.2. Preliminary Experiments
Control Group (CK) was prepared by adding 50 mL of sterile distilled water and SSF Medium Basal substrate into SSF bags (specification: 200 g). The experimental groups were prepared using SSF bags identical to those used for the CK. Experimental group 1 (M): SSF medium basal substrate + 50 mL distilled water + 0.4 g cellulase. Group 2 (P): SSF medium basal substrate + 10 mL XH005 bacterial solution + 0.4 g cellulase + 40 mL distilled water. Group 3 (L): SSF medium basal substrate + 10 mL LP-2 bacterial solution + 40 mL distilled water + 0.4 g cellulase. Group 4 (P+L): SSF medium basal substrate + 8 mL XH005 + 2.0 mL LP-2 bacterial solutions + 40 mL distilled water + 0.4 g cellulase. Each treatment had 3 replicates. Fermentation bags were tied and fermented at room temperature for 14 d; samples were then dried at 65 °C for 24 h, crushed, and sieved through 40-mesh; and lignin and cellulose contents were determined.
2.3. Single-Factor and Response Surface Optimization Experiments
Using cellulose and lignin contents as response indices, single-factor experiments optimized XH005 and LP-2 inoculum amounts, cellulase addition, material-water ratio, and fermentation time. Each condition had 5 levels with 3 replicates. Considering lignocellulose recalcitrance, solid-state fermentation included 5 time gradients (7, 14, 21, 28 and 35 days) under the following initial experiment conditions: material-water ratio (v/m): 1:0.5, LP-2 inoculum (v/m): 1.5%, XH005 inoculum (v/m): 10%, cellulase enzyme (m/m): 0.4%. Material-water ratio gradients (v/m): 1:0.4, 1:0.5, 1:0.6, 1:0.7, 1:0.8. XH005 inoculum (v/m): 6%, 8%, 10%, 12%, 14%. Cellulase enzyme addition (m/m): 0.2%, 0.4%, 0.6%, 0.8%, 1.0%. LP-2 inoculum (v/m): 1.0%, 1.5%, 2.0%, 2.5%, 3.0%.
To determine the optimal fermentation conditions for degrading cellulose and lignin, response surface optimization experiments were conducted. Based on single-factor experiment results, XH005 inoculum size (A), LP-2 inoculum size (B), material-to-water ratio (C), and cellulase addition amount (D) were selected as independent variables, with lignin and cellulose contents as response values. Response surface experiments were performed using the Box–Behnken design, with 3 replicates for each treatment, as shown in Table 1.

Table 1.
The factors and levels used in the response surface experimental design.
2.4. Scanning Electron Microscope Characteristics
Samples were sent to the Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences for scanning electron microscopy (SEM) analysis to assess microstructural changes in the solid-state fermentation medium. Following critical point drying (Quorum K850, Quorum Technologies Ltd., Lewes, UK), the samples were sputter-coated with a thin gold layer using an ion sputter coater (Quorum EMS150TS, Quorum Technologies Ltd., Lewes, UK); they were subsequently imaged with a Hitachi SU8100 (Hitachi High-Tech Corporation, Tokyo, Japan) scanning electron microscope.
2.5. Determination of Nutrient Content Determination
The solid-state fermented substrate was dried at 65 °C for 12 h, ground using a miniature plant grinder, sieved through a 1 mm mesh, and packaged in sealed bags for subsequent nutritional composition analysis. The determination methods for the samples were performed with reference to the following: the contents of crude protein (CP), ether extract (EE), crude fiber (CF), and ash content (Ash) were determined according to the method described by Yang [21]. Cellulose (CEL) and lignin (LIG) were determined using their respective specific assay kits from Nanjing Jicai Biotechnology Co., Ltd. (Nanjing, China). This study utilized the methodology established by Van Soest et al. [22] for the measurement of neutral detergent fiber (NDF) and acid detergent fiber (ADF). pH was measured using a pH meter (PHS-3C, Shanghai Yidian Scientific Instrument Co. Ltd., Shanghai, China). Lactic acid (LA) concentration was analyzed by high-performance liquid chromatography (HPLC; Shimadzu LC-20AT, Shimadzu Corporation, Kyoto, Japan) at a detection wavelength of 210 nm [23].
2.6. Determination of Mycotoxin and Free Gossypol Content
Free gossypol (FG) content was measured using the aniline method [24]. The contents of aflatoxin B1 (AFB1), deoxynivalenol (DON), and zearalenone (ZEN) were determined using ELISA assay kits manufactured by Shanghai Bioengineering Co., Ltd. (Shanghai, China).
2.7. Untargeted Metabolomics
A 100 mg tissue aliquot, pulverized in liquid nitrogen, was transferred to a 1.5 mL microcentrifuge tube. Then, 500 μL of 80% (v/v) methanol was added. After thorough vortexing, the mixture was incubated on ice for 5 min and centrifuged at 12,000 rpm (4 °C) for 20 min. An aliquot of the supernatant was diluted with MS-grade water to 53% (v/v) methanol. After a second centrifugation under the same conditions, the supernatant was collected for LC-MS analysis.
Metabolites were annotated against the KEGG, HMDB, and LIPIDMaps databases. Following transformation, PCA and OPLS-DA were conducted. Differential metabolites were selected with VIP > 1, p < 0.05, and |FC| ≥ 2. Volcano plots were created with ggplot2 (v3.4.3) using VIP, log2(FC), and −log10(p-value). Heatmaps were generated using pheatmap (v1.0.12) on z-score normalized data. KEGG enrichment was analyzed via bubble charts; pathways with x/n > y/n and p < 0.05 were considered significantly enriched.
2.8. Statistical Analysis
Data were processed using Microsoft Excel 2020 (Microsoft Corporation, Redmond, WA, USA). Statistical analysis was performed with IBM SPSS Statistics software (version 22.0; IBM Corp., Armonk, NY, USA). One-way analysis of variance (ANOVA) followed by Duncan’s multiple range test was applied to pre-experimental and single-factor experimental data for screening fermentation conditions. Response surface methodology (RSM) results were evaluated by analysis of variance to determine the optimal fermentation conditions. Parameters such as nutritional components, free gossypol (FG), and lactic acid before and after fermentation were compared using Student’s t-test. Data are presented as mean ± standard deviation (SD) (n = 3 for nutritional components and mycotoxin analysis; n = 3 for metabolomic analysis). A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Comparison of Lignocellulose Degradation Capability in Cotton Straw Among Different Groups
CK represents the control group; M represents the group with cellulase enzyme addition; P represents the group inoculated with XH005 and supplemented with cellulase; P+L represents the group inoculated with both XH005 and LP-2, along with cellulase addition. As shown in Table 2, compared with the CK group, the P, L, and P+L fermentation treatments significantly decreased the lignin and cellulose contents (p < 0.05), demonstrating effective degrading capacity for both components. The M group markedly reduced cellulose content (p < 0.05), while no significant difference was observed in lignin content. Although there was no significant difference in lignin content between the P group and the P+L group, the lignin content was slightly lower under P+L fermentation treatment, with a value of 19.11 ± 0.39%. The P+L group significantly reduced cellulose content, which was measured at 20.34 ± 0.48%. Taking into account factors such as feed storage methods and storage duration, the P+L fermentation method is considered more suitable.

Table 2.
Analysis of Differences in Cellulose and Lignin Content Among Control Group (CK), Cellulase enzyme Group (M), and Microbial-Enzyme Synergistic Groups (P, L, P+L).
3.2. Optimization of Conditions for Synergistic Microbial-Enzymatic Fermentation of Cotton Straw
Initial optimization of five parameters (XH005 and LP-2 inoculation rates, solid-to-water ratio, cellulase addition, and fermentation time) was carried out via single-factor experiments (Table S1), yielding preliminary conditions: XH005 8%, LP-2 2.0%, solid-to-water ratio 1:0.5, cellulase 0.6%, and 14-day fermentation. Further refinement using a Box–Behnken design (Table S2) with 29 experimental sets, along with ANOVA (Tables S3 and S4), contour and surface plots (Figures S1–S12), identified the optimum solid-state fermentation conditions: XH005 7%, LP-2 2.0%, solid-to-water ratio 1:0.55, cellulase 0.65%, and 14 days. The predicted lignin and cellulose contents were 15.73% and 13.27%, respectively. Validation results (Table 3) showed measured values of 15.78% ± 0.32 (lignin) and 13.64% ± 0.66 (cellulose), consistent with predictions (p > 0.05), confirming the optimal process.

Table 3.
Comparison of lignin and cellulose contents between YC and YZ groups.
3.3. Effects of Synergistic Microbial-Enzymatic Fermentation on Cotton Straw
As shown in Table 4, two groups were evaluated: a control (CK) and an optimized group (YH). Nutritionally, the optimized group exhibited a significant 29.69% increase in crude protein (CP) content (p < 0.05), while crude fat (EE) and ash content (Ash) remained unchanged (p > 0.05). Significant reductions were observed in crude fiber (CF), neutral detergent fiber (NDF), and acid detergent fiber (ADF) (p < 0.05), which decreased by 18.36%, 14.88%, and 12.61%, respectively. These results indicate that the optimization effectively enhanced protein content, degraded fibrous components, reduced anti-nutritional factors, and improved feed digestibility. Additionally, the optimized group showed a significantly lower pH and higher lactic acid (LA) content (p < 0.05), suggesting improved shelf life through microbial spoilage and enhanced palatability.

Table 4.
Determination of conventional nutritional components in control group (CK) and optimized group (YH).
As shown in Table 5, the YH group significantly reduced (p < 0.001) the contents of deoxynivalenol (DON), aflatoxin B1 (AFB1), zearalenone (ZEN), and free gossypol by 44.44%, 51.60%, 43.24%, and 56.22%, respectively. The mycotoxin levels in the optimized cotton stalk-based feed were all below the limits specified in GB 13078-2017 “Hygienic Standards for Feeds”. This reduction may be attributed to the degradation of mycotoxins by enzymatic systems produced by microorganisms and/or adsorption by the microbial cells themselves.

Table 5.
Determination of mycotoxins and free gossypol in control group (CK) and optimized group (YH).
Figure 1 presents scanning electron microscopy (SEM) images of cotton straw. As shown in Figure 1a,b, steam explosion pretreatment increased the specific surface area of cotton straw fibers and disrupted the lignocellulosic structure. Figure 1c demonstrates that after steam explosion combined with solid-state fermentation, the surface of cotton straw became loose and porous, indicating the effective degradation of lignocellulose by synergistic microbial-enzymatic action during solid-state fermentation.
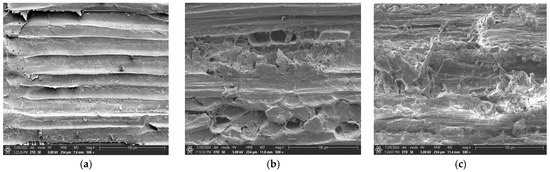
Figure 1.
Scanning electron micrographs of cotton straw. (a): Image of cotton straw before steam explosion treatment. (b): Image of cotton straw after steam explosion treatment. (c): Image of cotton straw inoculated with XH005, LP-2 and cellulase after steam explosion treatment.
3.4. Orthogonal Projections to Latent Structures Discriminant Analysis (OPLS-DA) of Metabolomics Between Control Group (CK) and Optimized Group (YH)
Figure 2 shows the orthogonal projections to latent structures discriminant analysis (OPLS-DA) of CK and YH.
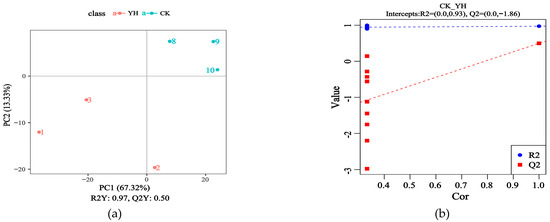
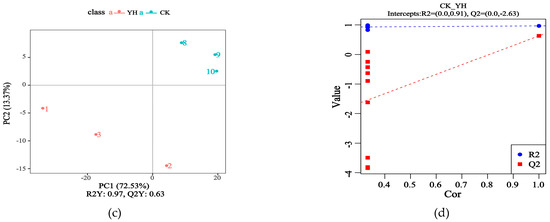
Figure 2.
Metabolomics analysis of fermented feed by PLS-DA. (a,c) depict the analytical results obtained in positive and negative ion mode, respectively. The subsequent OPLS-DA of the identified differential metabolites is displayed as score plots for the positive ion mode (b) and the negative ion mode (d).
Figure 2a displays the OPLS-DA in positive ion mode, where the contribution rates of PC1 and PC2 to sample differences between CK and YH were 80.65%, indicating that the OPLS-DA effectively captured the variations between the groups. Figure 2c presents the OPLS-DA in negative ion mode, with contribution rates of PC1 and PC2 reaching 85.90%, demonstrating significant changes in metabolites between CK and YH.
The OPLS-DA models comparing CK and YH are shown in Figure 2b for positive ion mode and Figure 2d for negative ion mode. The reliability of all models was demonstrated by R2 values that were consistently greater than their corresponding Q2 values, along with a negative Y-axis intercept for the Q2 regression line, confirming their validity and lack of overfitting.
3.5. Identification of Differential Metabolites Between Control Group (CK) and Optimized Group (YH)
The volcano plot in Figure 3 visualizes the distribution of differential metabolites, where the log2 (fold change) and the statistical significance [−log10(p-value)] are mapped on the x- and y-axes, respectively. Red dots denote significantly upregulated metabolites, while blue dots represent significantly downregulated metabolites. A total of 1255 metabolites were detected between CK and YH, among which 64 showed significant differences, including 43 upregulated and 21 downregulated.
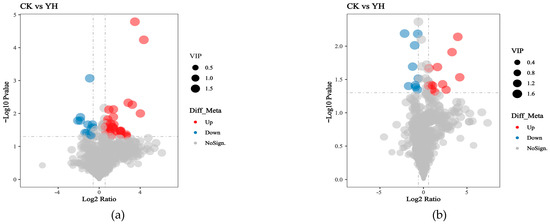
Figure 3.
Volcano plots of differential metabolites. (a): Volcano plot of differential metabolites (positive). (b): Volcano plot of differential metabolites (negative).
Figure 3a presents the volcano plot under positive ion mode, with 532 metabolites detected. Among them, 32 were significantly upregulated and 12 downregulated (see Supplementary Table S5 for details). Figure 3b presents the volcano plot under negative ion mode. A total of 723 metabolites were identified, of which 11 were significantly upregulated and 9 were downregulated (see Supplementary Table S6).
3.6. Hierarchical Clustering Analysis of Differential Metabolites Between Control Group (CK) and Optimized Group (YH)
To better visualize the changes in differential metabolites between CK and YH, a heatmap of the 64 differential metabolites was generated. As shown in Figure 4, the hierarchical clustering results of CK and YH are intuitively displayed, with differential metabolites grouped along the vertical axis and sample information on the horizontal axis. The color scale represents the relative abundance of metabolites, with red and blue corresponding to high and low expression levels, respectively.
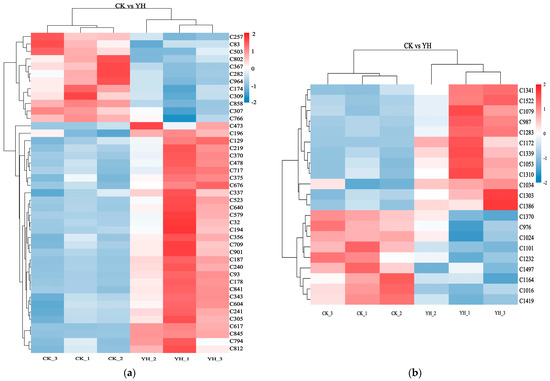
Figure 4.
Cluster heatmap of differential metabolites. The heatmaps visualize the abundance profiles of metabolites identified in the (a) positive and (b) negative ionization modes. Names of differential metabolites are shown in Supplementary Tables S5 and S6.
According to the clustering results, YH and CK samples were distinctly clustered into two separate groups.
Figure 4a shows the hierarchical clustering under positive ion mode. Compared with CK, metabolites significantly increased in YH included tryptamine (C187), Leu-Ala (C187), Glu-Glu-Arg (C841), kaempferol (C343), fisetin (C617), trifolirhizin (C604), and carvone (C709), among others. Metabolites that were significantly higher in CK compared to YH included methylarecoline (C174), sinapine (C858), and ZZNWFORYMOJSKP-UHFFFAOYSA-N (C503).
Figure 4b presents the hierarchical clustering under negative ion mode. Compared with CK, metabolites markedly elevated in YH included 3-hydroxyanthranilic acid (C987), salicylic acid (C1034), L-homocystine (C1303), prolylglycine (C1386), and N-acetyl-L-histidine (C1172). In contrast, metabolites such as DL-α-methoxyphenylacetic acid (C1370), 13(S)-HOTrE (C976), Phe-Asp-Lys (C1232), and LQPJSSVCJFQQDO-IBEHDNSVSA-N (C1419) were significantly higher in CK than in YH.
3.7. Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis of Pathways Between Control Group (CK) and Optimized Group (YH)
Figure 5 shows the KEGG pathway enrichment analysis. Figure 5a displays the enriched pathways in positive ion mode, which mainly include biosynthesis of secondary metabolites, biotin metabolism, flavonoid and flavonol biosynthesis, flavonoid biosynthesis, indole alkaloid biosynthesis, monoterpenoid biosynthesis, phenylpropanoid biosynthesis, and tryptophan metabolism. Figure 5b presents the enriched pathways in negative ion mode, primarily including 2-oxocarboxylic acid metabolism, C5-branched dibasic acid metabolism, cysteine and methionine metabolism, tryptophan metabolism, and alpha-linolenic acid metabolism.
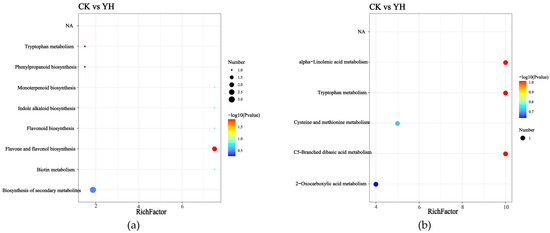
Figure 5.
KEGG enrichment pathway map. (a): KEGG enrichment results of differential pathway (positive). (b): KEGG enrichment results of differential pathway (negative).
4. Discussion
The advantage of synergistic microbial-enzymatic fermentation lies in the fact that substrates hydrolyzed by exogenously added enzymes can serve as carbon or nitrogen sources for microbial utilization, thereby enabling rapid initiation of microbial activity and maximizing the efficiency of substrate conversion by the strains [19]. To achieve the optimal fermentation effect of cotton straw, it is necessary to optimize the fermentation conditions. The results of this study demonstrate that factors such as the inoculum of XH005 and LP-2, and the supplementation level of exogenous enzymes can influence the degradation of lignocellulose. In solid-state fermentation, different inoculum amounts of bacteria and enzymes have a significant impact on the fermentation process [25]. An insufficient inoculation amount reduces substrate degradation efficiency, leading to limited release of carbon and nitrogen sources. This restricts microbial resource utilization, impairs the synthesis of metabolic products, and prolongs fermentation time. Conversely, excessive inoculation rapidly depletes oxygen and nutrients, promotes microbial overgrowth, induces competitive inhibition, and adversely affects fermentation outcomes [26]. Similarly, excessive addition of enzyme preparations is not beneficial, as it may accelerate substrate degradation and subsequently inhibit microbial growth [27]. Water is essential for microbial growth and reproduction, while in solid-state fermentation, it also serves as a critical factor affecting aeration during the process [28]. An excessively low solid-to-water ratio slows substrate decomposition and impairs microbial activity, prolonging fermentation, whereas an excessively high ratio causes substrate clumping, hindering metabolism, increasing costs, and raising contamination risk [29]. The optimal solid-to-water ratio varies depending on the fermentation substrate. Fermentation time is also crucial in microbial solid-state fermentation, and it varies with different fermentation substrates. An insufficient fermentation time will result in incomplete fermentation of the substrate and inadequate improvement of fermentation quality; conversely, an excessively long fermentation time will increase time costs and simultaneously raise the dry matter loss rate [30].
Parameters such as crude protein (CP), crude fiber (CF), neutral detergent fiber (NDF), and acid detergent fiber (ADF) are important components of nutritional composition [31]. The crude protein content in fermented feed is positively correlated with feed quality [32]. Neutral detergent fiber (NDF) and acid detergent fiber (ADF) play crucial roles in regulating the forage-to-concentrate ratio and ensuring rumen health [33]. Lower contents of neutral detergent fiber (NDF) and acid detergent fiber (ADF) are associated with higher digestibility in ruminants and greater nutritional value of fermented feed. Beyzi et al. demonstrated that ensiling reed significantly reduced the contents of acid detergent fiber (ADF) and neutral detergent fiber (NDF), thereby improving its nutritional composition and making it suitable for use as standardized roughage in animal feed [34]. Key indicators for evaluating fermentation quality include pH and organic acid content [21]. In this experiment, the significantly reduced pH in the optimized group indicates that the addition of Lactiplantibacillus plantarum during cotton straw fermentation markedly increased lactic acid production, effectively lowering the pH, which helps inhibit feed spoilage and reduce nutrient loss. In this study, lactic acid content increased significantly, which can be attributed to the fact that Lactiplantibacillus plantarum, as a facultative anaerobe, remains metabolically active and produces lactic acid even under aerobic fermentation conditions.
Feed is prone to contamination by mycotoxins during production and storage, which poses serious risks to the health of livestock and poultry [35]. In this experiment, the optimized group showed a significant reduction in mycotoxin levels, all of which were below the limits specified in GB 13078-2017 Hygienic Standards for Feeds. This may be attributed to the degradation of toxins by enzymes produced by microorganisms or through adsorption by the microbial cells themselves [36]. Guo et al. demonstrated that the CotA laccase produced by Bacillus licheniformis effectively degrades aflatoxin B1 [37]. He et al. also reported that aspergillus niger FS10 can biotransform zearalenone into less toxic metabolites, namely zearalenone-4-sulfate and (E)-zearalenone [38].
This study observed a significant increase in organic acids, amino acids, and their derivatives, including metabolites such as salicylic acid (C1034) and Glu-Glu-Arg (C841). Salicylic acid functions as a natural phytohormone and antioxidant [39]. Hassan et al. demonstrated that impregnating citrus fruits with salicylic acid effectively maintained their postharvest quality during storage, reducing weight loss and decay incidence, thereby extending their storability and marketability [40]. Glu-Glu-Arg, as a bioactive peptide, has become an increasingly important candidate drug due to its certain anticancer and anti-viral properties [41]. Gozde Yilmaz et al. employed molecular docking and molecular dynamics simulations to elucidate the potential anticancer and antiviral activities of Glu-Glu-Arg, and conducted structure-based computational pharmacological predictions, further indicating its promising anticancer and antibacterial properties [42].
Significantly increased phenylpropanoids and polyketides included metabolites such as kaempferol (C343), fisetin (C617), and trifolirhizin (C604). As natural flavonoid and flavonoid-related compounds, kaempferol, fisetin, and trifolirhizin exhibit multiple pharmacological activities, including antioxidant, anti-inflammatory, antimicrobial, and immunomodulatory properties. Amjad et al. revealed that kaempferol exerts anticancer effects by inhibiting cell proliferation and activating both intrinsic and extrinsic apoptotic pathways, involving modulation of JAK/STAT3, PI3K/AKT, and NF-κB pathways, as well as interference with TNF-induced MAPK activation [43]. Muhammad et al. revealed that fisetin, a polyphenol with versatile pharmacological properties, exhibits promising anticancer activity in multiple cancer types by obstructing the cell cycle, inhibiting cellular growth, and promoting apoptosis [44]. Shen et al. demonstrated that trifolirhizin significantly reduced the increase in body weight and liver weight in high-fat diet-induced obese male Sprague-Dawley rats, while also decreasing the accumulation of perirenal, epididymal, and brown adipose tissue, in a dose-independent manner [45].
Additionally, this study observed a significant increase in terpenoid metabolites, including carvone (C709). As a naturally occurring monoterpenoid ketone synthesized via the mevalonate pathway, carvone exhibits antimicrobial, anticancer, and anti-inflammatory activities [46]. Jiang et al. reported that carvone suppresses melanin synthesis and inhibits the proliferation of melanoma cells in a concentration-dependent fashion, a mechanism attributed to the downregulation of cell cycle-associated proteins including cyclin-dependent kinase 1 (CDK1) [47].
5. Conclusions
This study demonstrated that the fermentation of cotton straw significantly enhanced its nutritional profile while effectively reducing the levels of mycotoxins and free gossypol. Metabolomic analysis revealed the generation of functional bioactive compounds such as kaempferol (C343), carvone (C709), and trifolirhizin (C604), which possess anti-inflammatory, antimicrobial, and antioxidant properties. Therefore, this study demonstrates that fermentation of cotton straw with Pleurotus ostreatus XH005, Lactiplantibacillus plantarum LP-2, and cellulase yields a diverse array of bioactive compounds and immunomodulatory substances, offering a theoretical basis for enhancing the nutritional value of cotton straw-derived fermented feed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11100551/s1, Figures S1–S12: Response surface contour plot and surface plot. Table S1: Results of single-factor experiments on lignin and cellulose. Tables S2–S4: Response surface experimental results table and Box–Behnken analysis of variance design. Tables S5 and S6: Significantly differential metabolites detected in both positive and negative ion modes.
Author Contributions
Conceptualization, M.H. and D.D.; methodology, D.D. and M.H.; data research, D.D., H.Y.; data curation, H.Y., Y.L., X.H., M.A., Y.Y. and G.A.; writing—original draft preparation, D.D.; writing—review and editing, M.H. and H.Y.; visualization, D.D. and G.A.; supervision, W.C.; project administration, M.H. and W.C.; funding acquisition, M.H. and W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Autonomous Region Academy of Agricultural Sciences Special Project for Stable Support of Agricultural Science and Technology Innovation, grant number xjnkywdzc-2025003-06-04; the Key Research and Development Task Special Project of Xinjiang Uygur Autonomous Region, grant number 2022B02042; and the sub tasks of the Key Research and Development Program of Xinjiang Uygur Autonomous Region, grant number 2022B02056-2.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All findings and materials essential to this research are provided within the manuscript. Requests for additional details may be directed at the corresponding authors.
Acknowledgments
We extend our grateful acknowledgement to the members of our laboratory for their invaluable contributions to the successful execution of this experiment.
Conflicts of Interest
The authors state no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| XH005 | Pleurotus ostreatus XH005 |
| LP-2 | Lactobacillus plantarum LP-2 |
| SSF | solid-state fermentation |
| YC | Represents the prediction group |
| YZ | Represents the validation group |
| CK | Control Group |
| YH | Optimized Group |
| CP | Crude protein |
| CF | Crude fiber |
| EE | Crude fat |
| Ash | Ash content |
| ADF | Acid detergent fiber |
| NDF | Neutral detergent fiber |
| LA | Lactic acid |
| LAB | Lactic acid bacteria |
| AFB1 | Aflatoxin B1 |
| DON | Deoxynivalenol |
| ZEN | Zearalenone |
| FG | Free gossypol |
| CEL | Cellulose |
| LIG | Lignin |
References
- Li, F.; Bai, J.; Zhang, M.; Zhang, R. Yield estimation of high-density cotton fields using low-altitude UAV imaging and deep learning. Plant Methods 2022, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, S.; Fernandes, Â.; Stojković, D.; Pereira, C.; Taofiq, O.; Di Gioia, F.; Tzortzakis, N.; Soković, M.; Barros, L.; Ferreira, I.C.F.R. Cotton and cardoon byproducts as potential growing media components for Cichorium spinosum L. commercial cultivation. J. Clean. Prod. 2019, 240, 118254. [Google Scholar] [CrossRef]
- Egbuta, M.A.; McIntosh, S.; Waters, D.L.E.; Vancov, T.; Liu, L. Biological Importance of Cotton By-Products Relative to Chemical Constituents of the Cotton Plant. Molecules 2017, 22, 93. [Google Scholar] [CrossRef]
- Li, K.; Xu, Y.; Guo, K.; Cui, W.; Li, Y.; Hou, M. Improving the Nutritional Value and Safety of Cotton Stalk Feed via Response Surface Methodology and Co-Fermentation Techniques. Fermentation 2025, 11, 124. [Google Scholar] [CrossRef]
- Yildirim, O.; Ozkaya, B.; Altinbas, M.; Demir, A. Statistical optimization of dilute acid pretreatment of lignocellulosic biomass by response surface methodology to obtain fermentable sugars for bioethanol production. Int. J. Energy Res. 2021, 45, 8882–8899. [Google Scholar] [CrossRef]
- Yu, X.; Li, G.; Zhao, H.; Ma, Y.; Li, Q.; Chen, Y.; Li, W. Influence of chemically-modified cotton straw fibers on the properties of asphalt mortar. Case Stud. Constr. Mater. 2023, 18, e01787. [Google Scholar] [CrossRef]
- Li, X.; Shi, Z.; Wang, J.; Jiang, R. Review on the Crop Straw Utilization Technology of China. Am. J. Environ. Sci. Eng. 2020, 4, 61–64. [Google Scholar] [CrossRef]
- Garrido-Galand, S.; Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. The potential of fermentation on nutritional and technological improvement of cereal and legume flours: A review. Food Res. Int. 2021, 145, 110398. [Google Scholar] [CrossRef] [PubMed]
- Perwez, M.; Al Asheh, S. Valorization of agro-industrial waste through solid-state fermentation: Mini review. Biotechnol. Rep. 2025, 45, e00873. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Gladysheva, E.K. Liquid Hot Water and Steam Explosion Pretreatment Methods for Cellulosic Raw Materials: A Review. Polymers 2025, 17, 1783. [Google Scholar] [CrossRef]
- Wang, Y.; Gou, C.; Chen, L.; Liao, Y.; Zhang, H.; Luo, L.; Ji, J.; Qi, Y. Solid-State Fermentation with White Rot Fungi (Pleurotus Species) Improves the Chemical Composition of Highland Barley Straw as a Ruminant Feed and Enhances In Vitro Rumen Digestibility. J. Fungi 2023, 9, 1156. [Google Scholar] [CrossRef]
- Atiwesh, G.; Parrish, C.C.; Banoub, J.; Le, T.-A.T. Lignin degradation by microorganisms: A review. Biotechnol. Prog. 2022, 38, e3226. [Google Scholar] [CrossRef] [PubMed]
- Lelis, D.L.; Morenz, M.J.; Paciullo, D.S.; Roseira, J.P.; Gomide, C.A.; Pereira, O.G.; Oliveira, J.S.; Lopes, F.C.; da Silva, V.P.; da Silveira, T.C.; et al. Effects of Lactic Acid Bacteria on Fermentation and Nutritional Value of BRS Capiaçu Elephant Grass Silage at Two Regrowth Ages. Animals 2025, 15, 1150. [Google Scholar] [CrossRef]
- Guo, X.; Xu, D.; Li, F.; Bai, J.; Su, R. Current approaches on the roles of lactic acid bacteria in crop silage. Microb. Biotechnol. 2023, 16, 67–87. [Google Scholar] [CrossRef]
- Wu, B.; Ren, T.; Li, C.; Wu, S.; Cao, X.; Mei, H.; Wu, T.; Yong, M.; Wei, M.; Wang, C. Exploring the Fermentation Products, Microbiology Communities, and Metabolites of Big-Bale Alfalfa Silage Prepared with/without Molasses and Lactobacillus rhamnosus. Agriculture 2024, 14, 1560. [Google Scholar] [CrossRef]
- Yusuf, H.A.; Piao, M.; Ma, T.; Huo, R.; Tu, Y. Effect of lactic acid bacteria and yeast supplementation on anti-nutritional factors and chemical composition of fermented total mixed ration containing cottonseed meal or rapeseed meal. Anim. Biosci. 2022, 35, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; He, T.; Wang, Z.; Mao, J.; Sha, R.J.F.S.; und-Technologie, T.L.-W. The dynamic analysis of non-targeted metabolomics and antioxidant activity of Dendrobium officinale Kimura et Migo by the synergistic fermentation of bacteria and enzymes. LWT 2024, 203, 116354. [Google Scholar] [CrossRef]
- Ma, L.; Li, J.; Zhao, W.; Wang, J.; Li, Y.; Xiong, Y.; He, Y.; Chu, X.; Liu, Q. Key cellulase components synergizing with lactic acid bacteria to degrade alfalfa lignocellulose to improve lactic acid fermentation. Front. Microbiol. 2025, 16, 1566973. [Google Scholar] [CrossRef]
- Yang, S.; Yan, Z.; Fu, T.; Zhang, L.; Xiulan, C.; Li, P.; Gong, C.; Cao, L. Quality and microbial community analysis of solid-state fermented feed with mixed bacteria from corn silage. Anim. Biotechnol. 2025, 36, 2507905. [Google Scholar] [CrossRef]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Han, K.J.; Collins, M.; Vanzant, E.S.; Dougherty, C.T. Bale Density and Moisture Effects on Alfalfa Round Bale Silage. Crop Sci. 2004, 44, 914–919. [Google Scholar] [CrossRef]
- Li, J.; Gao, T.; Hao, Z.; Guo, X.; Zhu, B. Anaerobic solid-state fermentation with Bacillus subtilis for digesting free gossypol and improving nutritional quality in cottonseed meal. Front. Nutr. 2022, 9, 1017637. [Google Scholar] [CrossRef]
- Zhang, A.; He, W.; Han, Y.; Zheng, A.; Chen, Z.; Meng, K.; Yang, P.; Liu, G. Cooperative Fermentation Using Multiple Microorganisms and Enzymes Potentially Enhances the Nutritional Value of Spent Mushroom Substrate. Agriculture 2024, 14, 629. [Google Scholar] [CrossRef]
- Heng, X.; Chen, H.; Lu, C.; Feng, T.; Li, K.; Gao, E. Study on synergistic fermentation of bean dregs and soybean meal by multiple strains and proteases. LWT 2022, 154, 112626. [Google Scholar] [CrossRef]
- Lv, L.; Xiong, F.; Pei, S.; He, S.; Li, B.; Wu, L.; Cao, Z.; Li, S.; Yang, H. Synergistic fermentation of cottonseed meal using Lactobacillus mucosae LLK-XR1 and acid protease: Sustainable production of cottonseed peptides and depletion of free gossypol. Food Chem. 2025, 493, 145848. [Google Scholar] [CrossRef]
- He, D.; Duan, D.; Lv, X.; Xiong, B.; Li, Z.; Zhang, S.; Cai, J.; Qiao, X.; Chen, Q. Optimization of Solid-State Fermentation Process of Radix Ranunculi ternate Using Response Surface Method and Addressing Its Antioxidant and Hypoglycemic Activity. Fermentation 2024, 10, 153. [Google Scholar] [CrossRef]
- Su, J.; Fu, X.; Zhang, R.; Li, X.; Li, Y.; Chu, X. Exploring the Effects of Solid-State Fermentation on Polyphenols in Acanthopanax senticosus Based on Response Surface Methodology and Nontargeted Metabolomics Techniques. J. Food Biochem. 2023, 2023, 6711132. [Google Scholar] [CrossRef]
- Nadeem, F.; Mehmood, T.; Anwar, Z.; Saeed, S.; Bilal, M.; Meer, B. Optimization of bioprocess steps through response surface methodology for the production of immobilized lipase using Chaetomium globosum via solid-state fermentation. Biomass Convers. Biorefinery 2023, 13, 10539–10550. [Google Scholar] [CrossRef]
- Murai, T.; Annor, G.A. Improving the Nutritional Profile of Intermediate Wheatgrass by Solid-State Fermentation with Aspergillus oryzae Strains. Foods 2025, 14, 395. [Google Scholar] [CrossRef]
- Gupta, S.; Lee, J.J.L.; Chen, W.N. Analysis of Improved Nutritional Composition of Potential Functional Food (Okara) after Probiotic Solid-State Fermentation. J. Agric. Food Chem. 2018, 66, 5373–5381. [Google Scholar] [CrossRef] [PubMed]
- Sasu, P.; Attoh-Kotoku, V.; Anim-Jnr, A.S.; Osman, A.; Adjei, O.; Adjei-Mensah, B.; Edinam Aku Akoli, D.; Adjima Tankouano, R.; Kwaku, M.; Obloni Kweitsu, D. Comparative nutritional evaluation of the leaves of selected plants from the Poaceae family (bamboos and grasses) for sustainable livestock production in Ghana. Front. Sustain. Food Syst. 2023, 7, 2263960. [Google Scholar] [CrossRef]
- Beyzi, S.B.; Ülger, İ.; Konca, Y. Chemical, Fermentative, Nutritive and Anti-nutritive Composition of Common Reed (Phragmites australis) Plant and Silage. Waste Biomass Valorization 2023, 14, 927–936. [Google Scholar] [CrossRef]
- Van der Fels-Klerx, H.J.; Adamse, P.; Punt, A.; Van Asselt, E.D. Data Analyses and Modelling for Risk Based Monitoring of Mycotoxins in Animal Feed. Toxins 2018, 10, 54. [Google Scholar] [CrossRef]
- Ndiaye, S.; Zhang, M.; Fall, M.; Ayessou, N.M.; Zhang, Q.; Li, P. Current Review of Mycotoxin Biodegradation and Bioadsorption: Microorganisms, Mechanisms, and Main Important Applications. Toxins 2022, 14, 729. [Google Scholar] [CrossRef]
- Guo, Y.; Qin, X.; Tang, Y.; Ma, Q.; Zhang, J.; Zhao, L. CotA laccase, a novel aflatoxin oxidase from Bacillus licheniformis, transforms aflatoxin B1 to aflatoxin Q1 and epi-aflatoxin Q1. Food Chem. 2020, 325, 126877. [Google Scholar] [CrossRef]
- He, M.; Li, Y.; Pi, F.; Ji, J.; He, X.; Zhang, Y.; Sun, X. A novel detoxifying agent: Using rice husk carriers to immobilize zearalenone-degrading enzyme from Aspergillus niger FS10. Food Control 2016, 68, 271–279. [Google Scholar] [CrossRef]
- Rachappanavar, V.; Padiyal, A.; Sharma, J.K.; Gupta, S.K. Plant hormone-mediated stress regulation responses in fruit crops- a review. Sci. Hortic. 2022, 304, 111302. [Google Scholar] [CrossRef]
- Ennab, H.A.; El-Shemy, M.A.; Alam-Eldein, S.M. Salicylic Acid and Putrescine to Reduce Post-Harvest Storage Problems and Maintain Quality of Murcott Mandarin Fruit. Agronomy 2020, 10, 115. [Google Scholar] [CrossRef]
- Kumar, A.; Kothari, J.; Lokhande, K.B.; Seethamma, T.N.; Venkateswara Swamy, K.; Sharma, N.K. Novel Antiproliferative Tripeptides Inhibit AP-1 Transcriptional Complex. Int. J. Pept. Res. Ther. 2021, 27, 2163–2182. [Google Scholar] [CrossRef]
- Yilmaz, G.; Celik, S.; Erbolukbas Ozel, A.; Akyuz, S. Molecular docking and molecular dynamics studies of Glu-Glu-Arg, Glu-Pro-Arg, and Pro-Arg-Pro tripeptides to reveal their anticancer and antiviral potentials. J. Chin. Chem. Soc. 2024, 71, 1021–1035. [Google Scholar] [CrossRef]
- Amjad, E.; Sokouti, B.; Asnaashari, S. A systematic review of anti-cancer roles and mechanisms of kaempferol as a natural compound. Cancer Cell Int. 2022, 22, 260. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Gilani, S.A.; Shariati, M.A.; Imran, A.; Afzaal, M.; Atif, M.; Tufail, T.; Anjum, F.M. Fisetin: An anticancer perspective. Food Sci. Nutr. 2021, 9, 3–16. [Google Scholar] [CrossRef]
- Shen, H.; Huang, L.; Dou, H.; Yang, Y.; Wu, H. Effect of Trilobatin from Lithocarpus polystachyus Rehd on Gut Microbiota of Obese Rats Induced by a High-Fat Diet. Nutrients 2021, 13, 891. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhou, W.; Bian, C.; Wang, L.; Li, Y.; Li, B. Degradation and Pathways of Carvone in Soil and Water. Molecules 2022, 27, 2415. [Google Scholar] [CrossRef]
- Kang, W.; Choi, D.; Park, S.; Park, T. Carvone Decreases Melanin Content by Inhibiting Melanoma Cell Proliferation via the Cyclic Adenosine Monophosphate (cAMP) Pathway. Molecules 2020, 25, 5191. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).