Organosolv and Hydrothermal Pretreatments of Sugarcane Bagasse and Straw and Enzymatic Hydrolysis of Hemicellulosic Liquor
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Pretreatments
2.2.1. Organosolv Pretreatment
2.2.2. Hydrothermal Pretreatment
2.3. Moisture Content and Mass Yield Calculation
2.4. Characterization of Biomass and Pretreated Pulps
2.4.1. Holocellulose Content
2.4.2. Alpha-Cellulose Content
2.4.3. Hemicellulose Content
2.4.4. Insoluble Lignin Content by Klason Method
2.4.5. Soluble Lignin Content by Klason Method
2.4.6. Lignin Removal from Biomass
2.5. Ash Content
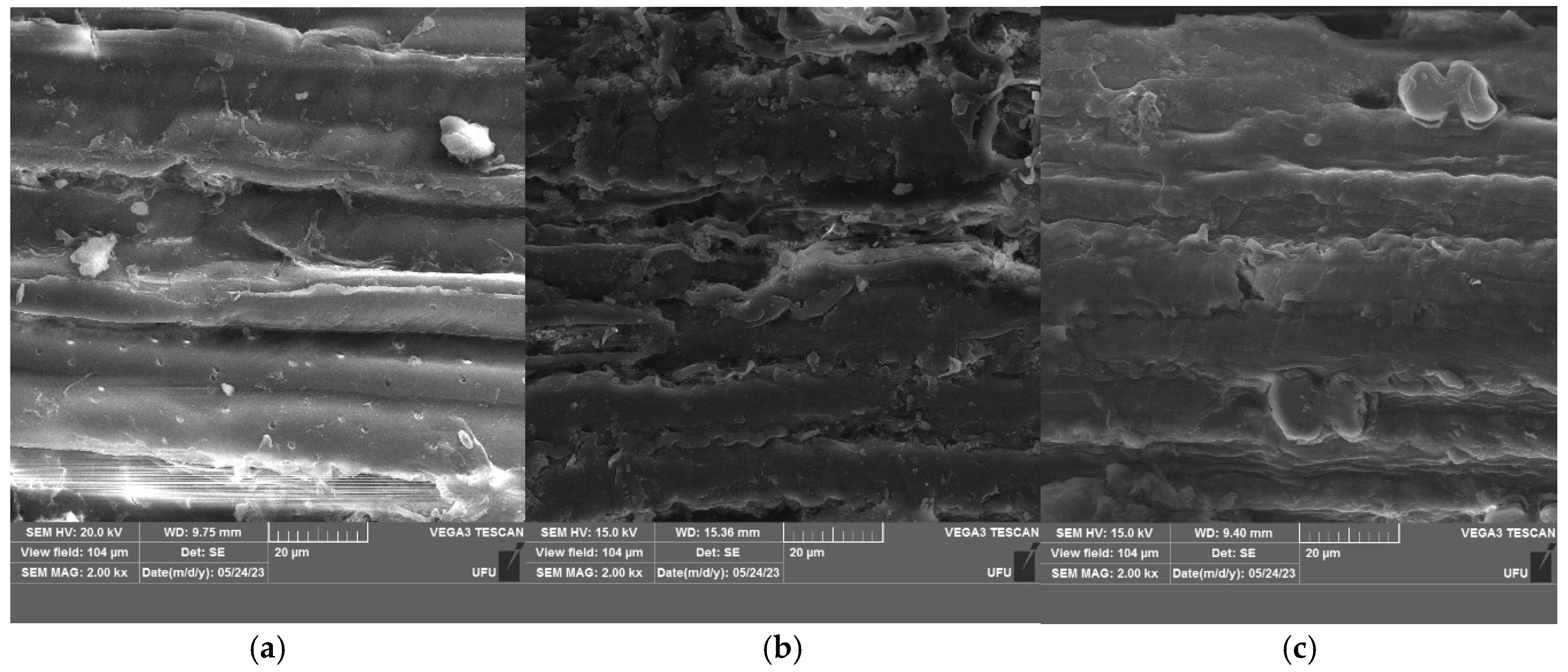
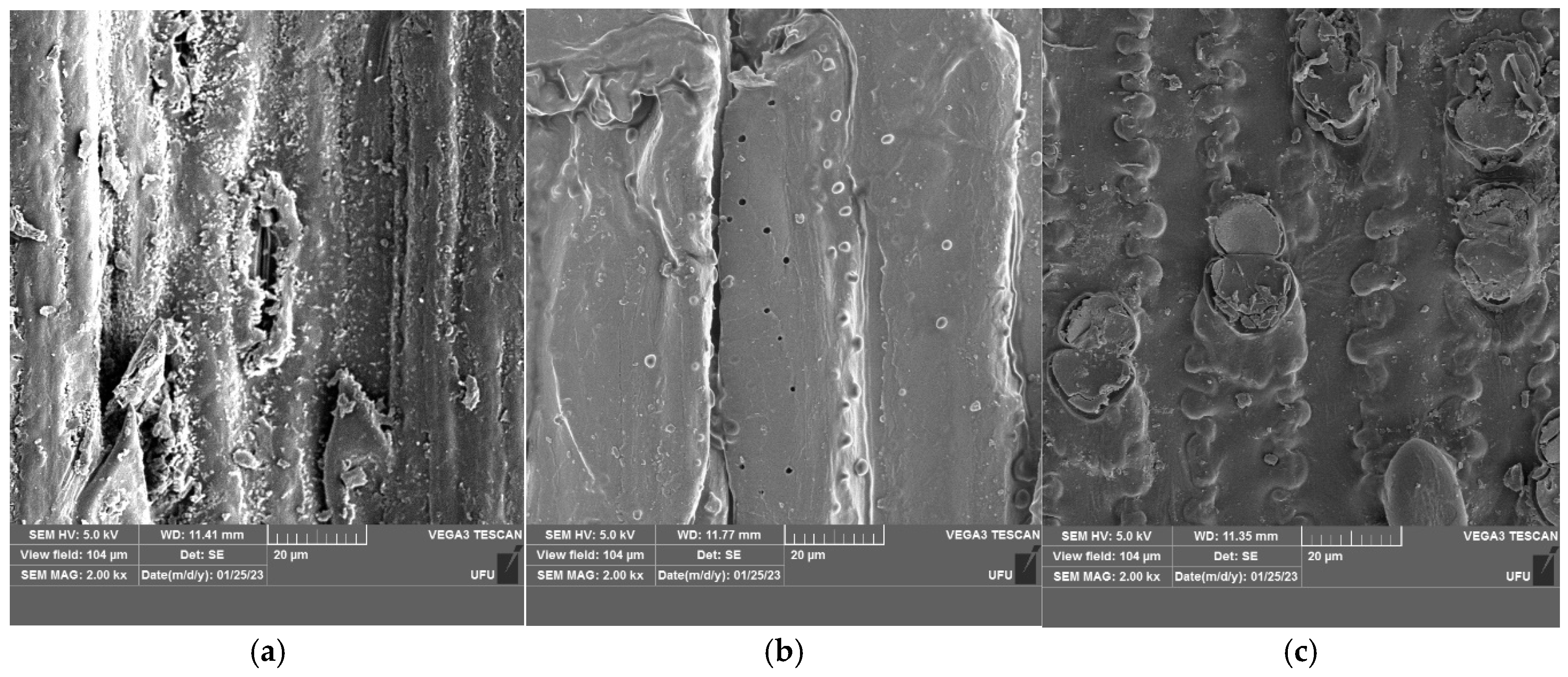
2.6. Analysis of Fibers by Scanning Electron Microscopy (SEM)
2.7. Determination of Crystallinity Index by X-Ray Diffraction (XRD)
2.8. Characterization of Hemicellulosic Liquors
2.8.1. Solids Content in Liquors
2.8.2. Quantification of Total Reducing Sugars (TRSs)
2.8.3. Chemical Composition by HPLC
2.9. Enzymatic Hydrolysis
3. Results and Discussion
3.1. Characterization of Raw and Pretreated Biomasses
3.1.1. Analysis of Fibers by Scanning Electron Microscopy
3.1.2. X-Ray Diffraction
3.2. Characterization of Hemicellulosic Liquors Before and After Enzymatic Hydrolysis
3.2.1. Total Solids Content in the Liquors
3.2.2. Quantification of Total Reducing Sugars (TRSs)
3.2.3. Chemical Composition by HPLC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conab—Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira de Cana-de-Açúcar. 1º Levantamento Safra 2023/2024; Conab—Companhia Nacional de Abastecimento: Brasilia, Brazil, 2023. [Google Scholar]
- Singh, P.; Kiran, U.; Dutta, B.C.; Bhutani, S.; Ghosh, S. Bioconversion of Hemicellulosic Fraction of Wheat Straw Biomass to Bioethanol by Scheffersomyces Stipitis: A kLa-Based Scale-up Study. Ind. Crops Prod. 2024, 214, 118461. [Google Scholar] [CrossRef]
- Hiranobe, C.T.; Gomes, A.S.; Paiva, F.F.G.; Tolosa, G.R.; Paim, L.L.; Dognani, G.; Cardim, G.P.; Cardim, H.P.; Dos Santos, R.J.; Cabrera, F.C. Sugarcane Bagasse: Challenges and Opportunities for Waste Recycling. Clean Technol. 2024, 6, 662–699. [Google Scholar] [CrossRef]
- Agência Nacional do Petróleo, Gás Natural e Biocombustíveis—ANP. RenovaBio; Agência Nacional do Petróleo, Gás Natural e Biocombustíveis: Brasilia, Brazil, 2023. [Google Scholar]
- Brasil. Lei n° 13.576, de 26 de dezembro de 2017. Dispõe sobre a Política Nacional de Biocombustíveis, Brasilia, Brazil. 2017. Available online: https://www.planalto.gov.br/ccivil_03/_ato2015-2018/2017/lei/l13576.htm (accessed on 2 August 2025).
- Ministério de Minas e Energia—MME. Nota Explicativa Sobre a Proposta de Criação da Política Nacional de Biocombustíveis; Ministério de Minas e Energia: Brasilia, Brazil, 2017; pp. 1–138. [Google Scholar]
- Karp, S.G.; Medina, J.D.C.; Letti, L.A.J.; Woiciechowski, A.L.; De Carvalho, J.C.; Schmitt, C.C.; De Oliveira Penha, R.; Kumlehn, G.S.; Soccol, C.R. Bioeconomy and Biofuels: The Case of Sugarcane Ethanol in Brazil. Biofuels Bioprod. Bioref. 2021, 15, 899–912. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Naresh Kumar, A.; Rajesh Banu, J.; Yoon, J.-J.; Kant Bhatia, S.; Yang, Y.-H.; Varjani, S.; Kim, S.-H. Recent Advances in Commercial Biorefineries for Lignocellulosic Ethanol Production: Current Status, Challenges and Future Perspectives. Bioresour. Technol. 2022, 344, 126292. [Google Scholar] [CrossRef]
- Lorenci Woiciechowski, A.; Dalmas Neto, C.J.; Porto De Souza Vandenberghe, L.; De Carvalho Neto, D.P.; Novak Sydney, A.C.; Letti, L.A.J.; Karp, S.G.; Zevallos Torres, L.A.; Soccol, C.R. Lignocellulosic Biomass: Acid and Alkaline Pretreatments and Their Effects on Biomass Recalcitrance—Conventional Processing and Recent Advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef]
- Stagno, V.; Ricci, S.; Longo, S.; Verticchio, E.; Frasca, F.; Siani, A.M.; Capuani, S. Discrimination between Softwood and Hardwood Based on Hemicellulose Content Obtained with Portable Nuclear Magnetic Resonance. Cellulose 2022, 29, 7917–7934. [Google Scholar] [CrossRef]
- Devi, A.; Bajar, S.; Kour, H.; Kothari, R.; Pant, D.; Singh, A. Lignocellulosic Biomass Valorization for Bioethanol Production: A Circular Bioeconomy Approach. Bioenerg. Res. 2022, 15, 1820–1841. [Google Scholar] [CrossRef]
- Lamounier, K.F.R.; Rodrigues, P.D.O.; Pasquini, D.; Dos Santos, A.S.; Baffi, M.A. Ethanol Production and Other Bioproducts by Galactomyces Geotrichum from Sugarcane Bagasse Hydrolysate. Curr. Microbiol. 2020, 77, 738–745. [Google Scholar] [CrossRef]
- Ndubuisi, I.A.; Amadi, C.O.; Nwagu, T.N.; Murata, Y.; Ogbonna, J.C. Non-Conventional Yeast Strains: Unexploited Resources for Effective Commercialization of Second Generation Bioethanol. Biotechnol. Adv. 2023, 63, 108100. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K.K. Pretreatment of Lignocellulosic Biomass: A Review on Recent Advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.A.; Taherzadeh, M.J. Improving the Economy of Lignocellulose-Based Biorefineries with Organosolv Pretreatment. Bioresour. Technol. 2020, 299, 122695. [Google Scholar] [CrossRef]
- Meng, X.; Bhagia, S.; Wang, Y.; Zhou, Y.; Pu, Y.; Dunlap, J.R.; Shuai, L.; Ragauskas, A.J.; Yoo, C.G. Effects of the Advanced Organosolv Pretreatment Strategies on Structural Properties of Woody Biomass. Ind. Crops Prod. 2020, 146, 112144. [Google Scholar] [CrossRef]
- Yue, P.; Hu, Y.; Tian, R.; Bian, J.; Peng, F. Hydrothermal Pretreatment for the Production of Oligosaccharides: A Review. Bioresour. Technol. 2022, 343, 126075. [Google Scholar] [CrossRef]
- Sun, Q.; Chen, W.-J.; Pang, B.; Sun, Z.; Lam, S.S.; Sonne, C.; Yuan, T.-Q. Ultrastructural Change in Lignocellulosic Biomass during Hydrothermal Pretreatment. Bioresour. Technol. 2021, 341, 125807. [Google Scholar] [CrossRef] [PubMed]
- Ilanidis, D.; Stagge, S.; Jönsson, L.J.; Martín, C. Hydrothermal Pretreatment of Wheat Straw: Effects of Temperature and Acidity on Byproduct Formation and Inhibition of Enzymatic Hydrolysis and Ethanolic Fermentation. Agronomy 2021, 11, 487. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Conrad, M.; Sun, S.-N.; Sanchez, A.; Rocha, G.J.M.; Romaní, A.; Castro, E.; Torres, A.; Rodríguez-Jasso, R.M.; Andrade, L.P.; et al. Engineering Aspects of Hydrothermal Pretreatment: From Batch to Continuous Operation, Scale-up and Pilot Reactor under Biorefinery Concept. Bioresour. Technol. 2020, 299, 122685. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Lv, Z.; Chen, G.; Peng, F. Hemicellulose: Structure, Chemical Modification, and Application. Prog. Polym. Sci. 2023, 140, 101675. [Google Scholar] [CrossRef]
- Yuan, Q.; Liu, S.; Ma, M.-G.; Ji, X.-X.; Choi, S.-E.; Si, C. The Kinetics Studies on Hydrolysis of Hemicellulose. Front. Chem. 2021, 9, 781291. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Shaheen, H.; Wu, A.-M. Cell Wall Hemicellulose for Sustainable Industrial Utilization. Renew. Sustain. Energy Rev. 2021, 144, 110996. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, X.; Tang, C.; Chen, Y.; Shen, T.; Zhu, C.; Ying, H. Hydrazine Hydrate and Organosolv Synergetic Pretreatment of Corn Stover to Enhance Enzymatic Saccharification and Co-Production of High-Quality Antioxidant Lignin. Bioresour. Technol. 2018, 268, 677–683. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Rodrigues, P.; Da Silva Barreto, E.; Brandão, R.L.; Gurgel, L.V.A.; Pasquini, D.; Baffi, M.A. On-Site Produced Enzyme Cocktails for Saccharification and Ethanol Production from Sugarcane Bagasse Fractionated by Hydrothermal and Alkaline Pretreatments. Waste Biomass Valor 2022, 13, 95–106. [Google Scholar] [CrossRef]
- Technical Association of the Pulp and Paper Industry—TAPPI. Preparation of Wood for Chemical Analysis; TAPPI T264 OM-88; Tappi Press: Atlanta, GA, USA, 1988. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L.A.P. Determination of Structural Carbohydrates and Lignin in Biomass. Natl. Renew. Energy Lab. 2012, 1–18. Available online: https://docs.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 18 September 2025).
- Technical Association of the Pulp and Paper Industry—TAPPI. Testing for Breaking Length of Paper; TAPPI T13M-54; Tappi Press: Atlanta, GA, USA, 1954. [Google Scholar]
- Alam, A.; Wang, Y.; Liu, F.; Kang, H.; Tang, S.; Wang, Y.; Cai, Q.; Wang, H.; Peng, H.; Li, Q.; et al. Modeling of Optimal Green Liquor Pretreatment for Enhanced Biomass Saccharification and Delignification by Distinct Alteration of Wall Polymer Features and Biomass Porosity in Miscanthus. Renew. Energy 2020, 159, 1128–1138. [Google Scholar] [CrossRef]
- Technical Association of the Pulp and Paper Industry—TAPPI. Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C; TAPPI T211 OM-93; Tappi Press: Atlanta, GA, USA, 1993. [Google Scholar]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Arufe, S.; Rubinos, S.N. Physicochemical Characterization of White, Yellow and Purple Maize Flours and Rheological Characterization of Their Doughs. J. Food Sci. Technol. 2015, 52, 7954–7963. [Google Scholar] [CrossRef]
- Gomes, M.G.; Paranhos, A.G.D.O.; Camargos, A.B.; Baêta, B.E.L.; Baffi, M.A.; Gurgel, L.V.A.; Pasquini, D. Pretreatment of Sugarcane Bagasse with Dilute Citric Acid and Enzymatic Hydrolysis: Use of Black Liquor and Solid Fraction for Biogas Production. Renew. Energy 2022, 191, 428–438. [Google Scholar] [CrossRef]
- Ezequielle Bernardes Costa, B.; Serpa Da Cruz, R.; Cesário Rangel, F.; Margareti Plentz Meneghetti, S. Comparison Between Chemical and Enzymatic Hydrolysis of Lignocellulosic Biomass for Bioethanol Production: A Review. Rev. Virtual Quim. 2021, 13, 242–259. [Google Scholar] [CrossRef]
- Jakob, A.; Likozar, B.; Grilc, M. Model-Assisted Optimization of Xylose, Arabinose, Glucose, Mannose, Galactose and Real Hemicellulose Streams Dehydration To (Hydroxymethyl)Furfural and Levulinic Acid. ChemSusChem 2024, 17, e202400962. [Google Scholar] [CrossRef]
- Lyu, Q.; Dar, R.A.; Baganz, F.; Smoliński, A.; Rasmey, A.H.M.; Liu, R.; Zhang, L. Effects of lignocellulosic biomass-derived hydrolysate inhibitors on cell growth and lipid production during microbial fermentation of oleaginous microorganisms—A review. Fermentation 2025, 11, 121. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Yu, S.; Qin, Y.; Qu, Y.; Zhao, J. Potassium Permanganate Assisted Organosolv Pretreatment Enhances Enzymatic Hydrolysis of Corn Stover. GCB Bioenergy 2021, 13, 665–678. [Google Scholar] [CrossRef]
- Aggarwal, N.; Pal, P.; Sharma, N.; Saravanamurugan, S. Consecutive Organosolv and Alkaline Pretreatment: An Efficient Approach toward the Production of Cellulose from Rice Straw. ACS Omega 2021, 6, 27247–27258. [Google Scholar] [CrossRef] [PubMed]
| Biomass | Cellulose (%) | Hemicelluloses (%) | Total Lignin (%) | Ashes (%) | Yield (%) | Delignification (%) | Mass Balance (%) |
|---|---|---|---|---|---|---|---|
| SB | 41.25 ± 4.60 | 29.30 ± 2.97 | 27.03 ± 1.14 | 2.87 ± 0.59 | - | - | 100.45 ± 5.62 |
| SBO | 56.12 ± 4.07 | 12.86 ± 1.07 | 21.06 ± 4.07 | - | 61.55 | 52.04 | 90.04 ± 5.85 |
| SBH | 54.87 ± 2.32 | 6.64 ± 1.91 | 33.63 ± 6.28 | 0.97 ± 0.59 | 71.21 | 11.40 | 96.11 ± 6.99 |
| SS | 38.31 ± 0.80 | 28.86 ± 3.53 | 25.74 ± 3.04 | 3.42 ± 1.37 | - | - | 96.33 ± 4.92 |
| SSO | 51.23 ± 6.12 | 18.31 ± 6.21 | 22.42 ± 4.75 | - | 50.39 | 56.11 | 91.96 ± 9.93 |
| SSH | 53.71 ± 0.37 | 9.64 ± 0.94 | 27.67 ± 3.21 | 0.46 ± 0.14 | 78.26 | 15.87 | 91.48 ± 3.37 |
| Liquor | Solid Content (%) |
|---|---|
| SBO | 1.420 ± 0.012 |
| SBH | 1.397 ± 0.101 |
| SSO | 2.203 ± 0.012 |
| SSH | 1.873 ± 0.006 |
| Liquor | TRS (g·L−1) |
|---|---|
| SBO | 11.144 ± 0.530 |
| SBO Hydrolyzate | 13.440 ± 0.081 |
| SBH | 16.507 ± 1.679 |
| SBH Hydrolyzate | 22.492 ± 0.074 |
| SSO | 8.560 ± 0.273 |
| SSO Hydrolyzate | 9.478 ± 0.687 |
| SSH | 14.164 ± 0.478 |
| SSH Hydrolyzate | 22.830 ± 0.781 |
| Liquor | Compounds | Concentration in the Liquor (g·L−1) | Concentration in the Hydrolyzed Liquor (g·L−1) |
|---|---|---|---|
| SBO | Cellobiose | ND | 0.268 ± 0.045 |
| Glucose | 0.501 ± 0.037 | 0.204 ± 0.001 | |
| Xylose | 2.030 ± 0.162 | 2.740 ± 0.092 | |
| Arabinose | ND | ND | |
| Formic acid | 0.442 ± 0.033 | 0.278 ± 0.020 | |
| Acetic acid | 1.061 ± 0.024 | 0.880 ± 0.048 | |
| 5-HMF | 0.164 ± 0.015 | 0.095 ± 0.003 | |
| Furfural | 20.714 ± 0.270 | 12.927 ± 0.601 | |
| SBH | Cellobiose | ND | 0.301 ± 0.048 |
| Glucose | 0.185 ± 0.262 | 0.400 ± 0.050 | |
| Xylose | 3.006 ± 0.197 | 6.886 ± 0.755 | |
| Arabinose | 0.362 ± 0.062 | ND | |
| Formic acid | 0.355 ± 0.062 | 0.173 ± 0.176 | |
| Acetic acid | 0.339 ± 0.089 | 0.560 ± 0.599 | |
| 5-HMF | ND | 0.035 ± 0.028 | |
| Furfural | ND | 1.692 ± 0.518 | |
| SSO | Cellobiose | ND | 0.304 ± 0.003 |
| Glucose | 0.473 ± 0.009 | 0.374 ± 0.012 | |
| Xylose | ND | 6.060 ± 1.120 | |
| Arabinose | ND | ND | |
| Formic acid | 0.488 ± 0.049 | 0.400 ± 0.007 | |
| Acetic acid | ND | 0.888 ± 0.080 | |
| 5-HMF | 1.092 ± 0.022 | 0.183 ± 0.012 | |
| Furfural | 3.284 ± 0.204 | 1.526 ± 0.839 | |
| SSH | Cellobiose | 0.139 ± 0.001 | ND |
| Glucose | 2.623 ± 0.057 | 1.983 ± 0.171 | |
| Xylose | 2.728 ± 0.170 | 4.183 ± 0.389 | |
| Arabinose | 1.496 ± 0.076 | 0.573 ± 0.008 | |
| Formic acid | 4.003 ± 0.275 | 2.565 ± 0.180 | |
| Acetic acid | 0.772 ± 0.087 | 1.077 ± 0.031 | |
| 5-HMF | ND | 0.159 ± 0.032 | |
| Furfural | ND | 7.409 ± 3.296 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alves, M.d.S.; Rodrigues, P.d.O.; Baffi, M.A.; Pasquini, D. Organosolv and Hydrothermal Pretreatments of Sugarcane Bagasse and Straw and Enzymatic Hydrolysis of Hemicellulosic Liquor. Fermentation 2025, 11, 550. https://doi.org/10.3390/fermentation11100550
Alves MdS, Rodrigues PdO, Baffi MA, Pasquini D. Organosolv and Hydrothermal Pretreatments of Sugarcane Bagasse and Straw and Enzymatic Hydrolysis of Hemicellulosic Liquor. Fermentation. 2025; 11(10):550. https://doi.org/10.3390/fermentation11100550
Chicago/Turabian StyleAlves, Marlon da Silva, Patrísia de Oliveira Rodrigues, Milla Alves Baffi, and Daniel Pasquini. 2025. "Organosolv and Hydrothermal Pretreatments of Sugarcane Bagasse and Straw and Enzymatic Hydrolysis of Hemicellulosic Liquor" Fermentation 11, no. 10: 550. https://doi.org/10.3390/fermentation11100550
APA StyleAlves, M. d. S., Rodrigues, P. d. O., Baffi, M. A., & Pasquini, D. (2025). Organosolv and Hydrothermal Pretreatments of Sugarcane Bagasse and Straw and Enzymatic Hydrolysis of Hemicellulosic Liquor. Fermentation, 11(10), 550. https://doi.org/10.3390/fermentation11100550






