Abstract
The ongoing occurrence of foodborne diseases and the imperative need for efficient spoilage and pathogen control in food products constitute a critical challenge for the food industry. The rising demands of consumers for safe, healthy, and clean-label food products have led to an increased interest in natural antimicrobial alternatives. Lactic acid bacteria (LAB) have proven their value in the food industry in recent years, also in reason of their antagonistic properties against undesired microbes and their significant related protechnological attributes. The natural antimicrobial compounds produced by LAB exhibit inhibitory effects on pathogens and effectively inhibit the activities of food spoilage-related organisms. Applying secondary metabolites of LAB, notably bacteriocins, organic acids, and others, has found commercial utility across multiple food sectors, effectively preventing the proliferation of undesirable microorganisms and simultaneously enhancing the sensory properties and overall quality of various food products. This review comprehensively explores the natural microbial compounds produced by LAB, specifically focusing on their antimicrobial action in supporting effective and sustainable microbial management. Additionally, it highlights their strategic application across various technological contexts within the food industry.
1. Lactic Acid Bacteria, Food Biocontrol, and the Different Food Categories
Lactic acid bacteria (LAB) are identified as gram-positive and non-spore-forming microorganisms. They are unable to produce catalase and are commonly observed in both bacillus and coccus shapes. Classified as relative or obligatory anaerobes, LAB exhibit adaptability to low-oxygen environments and function effectively in acidic pH conditions. Moreover, they are generally recognized as safe (GRAS) and included in the Qualified Presumption of Safety (QPS) list, which makes them a trusted choice for various food applications. Additionally, their presence in the gut microbiota of human intestines underscores their importance in supporting digestive health [1]. Lactic acid bacteria have also been used in food biopreservation through fermentation for centuries, making it one of the earliest and most traditional biotechnological techniques for preserving various foodstuffs [1].
Fermentation technology holds great significance in the realm of food preservation, as it not only extends the shelf life of food but also enhances the sensory quality and boosts its nutritional value [2]. The technical and microbiological optimization applied in fermentation has further amplified its advantages, particularly in the large-scale commercial production of fermented products. This approach minimizes costs, reduces risks, and ensures the efficient management of consistent physicochemical properties by implementing precise control over specified conditions, promoting the sustainability of food systems and label-cleaning efforts [3].
Consequently, fermentation emerges as a versatile and effective method for preserving food, with time-tested benefits and continual innovations in the food industry [4]. In addition, probiotic fermented products have gained widespread popularity on a global scale. These products, referred to as probiotic food, contain sufficient amounts of live probiotic microorganisms incorporated into a suitable matrix. It is essential for these probiotics to maintain their viability and metabolic functions throughout all stages of food processing, from production to consumption [5]. The regular consumption of probiotic foods, particularly lacto-fermented varieties, has been associated with positive effects on the immune system, reducing the risk of developing certain health conditions [6,7,8]. This improvement is attributed to continuous interactions between probiotic bacteria, which are defined as “live microorganisms that, when administered in sufficient quantities, provide health benefits to the host” [9], and the intestinal microbial composition, which enhances the host immune system. These changes help regulate the pathogenic population while promoting the growth of beneficial microbial populations [10].
While contributing to driving the bioprocesses essential for food fermentation, promoting gut health, and providing various health benefits, LAB exhibit a crucial probiotic trait by actively inhibiting the growth of both spoilage and pathogenic microorganisms in several food ecosystems [7,9,10,11,12,13,14,15] (Figure 1).
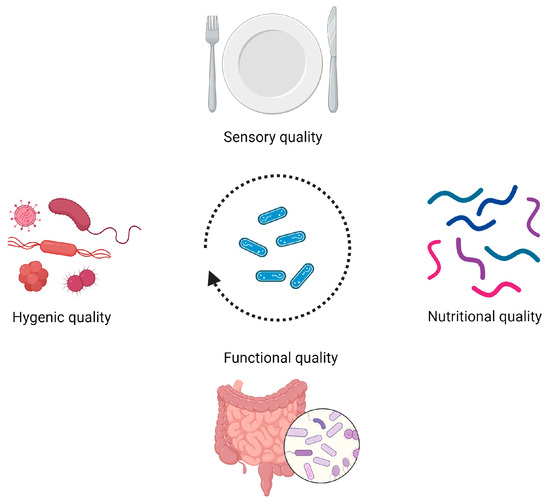
Figure 1.
Examples of impacts of lactic acid bacteria and their antimicrobial properties on different facets of global food quality. Image created using BioRender.com (accessed on 18 April 2024).
This antagonistic property holds great significance in the field of food preservation, as LAB contribute to the production of key metabolites, including organic acids, bacteriocins, reuterin, diacetyl, reutericyclin, acetoin, and hydrogen peroxide. These metabolites act as biopreservative agents, playing a crucial role in inhibiting the growth of harmful microorganisms and ensuring the safety and extended shelf life of various fermented foods [16].
Furthermore, the technological potential of LAB extends beyond food preservation, as they play a crucial role in enhancing the flavours, textures, and other sensory attributes in various fermented products, as well as their nutritional value and functional attributes ([3,17,18,19]). In all these cases, the antimicrobial properties have a crucial effect, as they are the basis of the ecological competition phenomenon that permits LAB to exert a dominance phenomenon that allows these bacteria to be responsible for bio-based modifications that improve the global quality of foodstuffs. The case studies in Table 1 highlight this dual role of antimicrobial properties. On the one hand, we have the immediate valorization of antagonistic attitudes in the design of ‘nature-inspired’ biocontrol solutions. On the other hand, we have studies in which the antimicrobial properties are exploited to allow LAB to perform other biotechnological functions. The case studies are also useful in highlighting how the targets in terms of food categories are extremely broad: we find fermented and non-fermented foods and products of animal origin and plant origin, with a wide diversification within these sectors.

Table 1.
Food biocontrol studies exploiting lactic acid bacteria’s antimicrobial potential in food targets.
This review highlights the significant antimicrobial activity of lactic acid bacteria (LAB) and its crucial role in various biotechnological applications. LAB’s ability to produce essential metabolites, such as bacteriocins, organic acids, and hydrogen peroxide, makes them highly potent biopreservative agents, effectively inhibiting the growth of spoilage and pathogenic microorganisms.
2. Antimicrobial Potential of LAB: Biomolecules and Molecular Mechanisms
LAB’s safety and promising health benefits make them ideal for commercial use. Hence, they have been introduced into different sectors, such as the food industry, where they have been predominantly used (e.g., food biopreservation, improving food quality and safety, and control of biofilms), agriculture, and medicine.
LAB can produce several functional metabolites that have antibacterial, antifungal, and antiviral properties against some challenging pathogens. Some of the substances are bacteriostatic or bactericidal, including organic acids (e.g., lactic, acetic, formic, butyric, and propionic acids), bacteriocins (selected the most studied examples), hydrogen peroxide, lysozymes, CO2, and diacetyl. These compounds (Figure 2) can act alone or in combination to inhibit the growth of pathogenic or spoilage microorganisms in food.
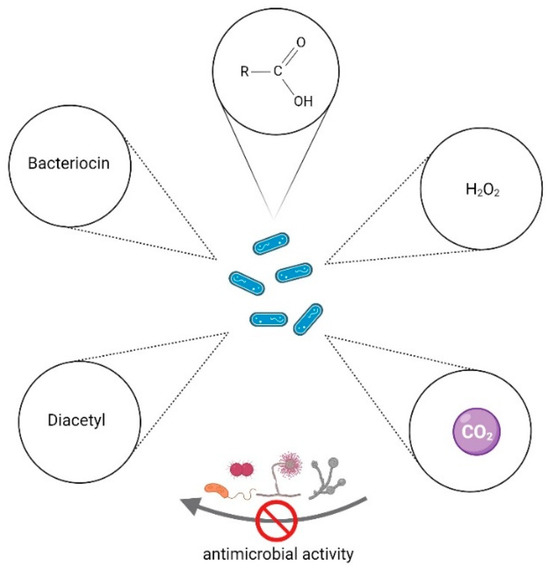
Figure 2.
Examples of metabolites of LAB (i.e., diacetyl, bacteriocin, organic acids, hydrogen peroxide, and carbon dioxide) that have antimicrobial activities against other microorganisms. Image created using BioRender.com (accessed on 16 April 2024).
2.1. Organic Acids
Organic acids represent the major fermentation product made by LAB during their metabolic process [43]. These components of interest have been widely applied in the industry due to their versatile applications. The antimicrobial activity of LAB is mainly due to the production of organic acids, mostly lactic and acetic, but also formic, propionic, butyric, phenyllactic, hydroxyphenyllactic, and indole-3-lactic acids, among others [44]. The quantity and nature of organic acids generated vary based on the species or strain, the composition of the culture, and the growth conditions [24]. Once they are produced, organic acid accumulation engenders a pH reduction, creating an unfavourable environment for pathogenic microorganisms and disrupting their growth. The pH reduction acts mostly on microorganisms like E. coli, Pseudomonas, Salmonella, and Clostridium, which are known for their intolerance to acidic conditions. Due to their lipophilic nature, the undissociated state of organic acids makes them able to pass by the bacterial cell membrane; after entering the more alkaline environment of the bacterial cell, the anions and protons from organic acids increase the osmotic stress and cause cell damage, which will lead to bacterial death [45]. Some researchers have demonstrated the inhibitory action of organic acids on the growth of Gram-positive and Gram-negative bacteria, yeast, and moulds in a wide range of food products [16]. A study conducted by Shehata et al. (2019) revealed that Lactobacillus sp. RM1 exhibited a wide antifungal efficacy against toxigenic fungi [46]. The culture filtrate of this strain demonstrated its capacity to reduce mycotoxins and extend shelf life, using wheat grains as a food model. Moreover, a high concentration of acetic acid inhibits the growth of Melissococcus plutonius and can be used as a biocontrol agent for preventing European Foulbrood disease in honeybees [47]. Overall, organic acids remain a promising option for biopreservation, since they are considered safe for human consumption. They can help to extend the shelf life of food products, as well as enhance their safety and quality.
2.2. Bacteriocins
Since the emergence of antibiotic resistance as a serious threat to the treatment of infectious diseases, bacteriocins have received considerable recognition within the scientific community. These small antimicrobial peptides, produced by various microorganisms such as bacteria and fungi, have shown a broad inhibitory potential against a wide spectrum of microorganisms [48]. As a result, they have emerged as promising biopreservatives in the food industry, replacing the use of chemical additives and antibiotics. Moreover, bacteriocins have generally been recognized as safe (GRAS) for human consumption, making them an attractive alternative to be used as natural food preservatives. The bacteriocin gene clusters are organized into operons that are located on the bacterial chromosome, plasmids, or transposons [49]. A quorum-sensing mechanism regulates the gene clusters and requires the secretion and accumulation of peptides in the extracellular environment for induction. While bacteriocins show considerable promise across various domains, nisin is the only commercially approved bacteriocin to be employed in the food industry [50]. Nisin finds extensive applications as a food additive in processed cheese, dairy products, vegetables, processed meat, and canned foods [51]. This peptide has been included in the list of food additives with the European identifier E234 [52]. Based on their mode of action and their structure, bacteriocins are divided into different classes:
Class I (lantibiotics): These are small peptides (molecular masses < 5 kDa) produced by Gram-positive bacteria that undergo significant post-translational modifications. Lantibiotics are characterized by the presence of unusual amino acids (lanthionine and β methyllanthionine); these residues are the result of the dehydration of serines and threonines, leading to the formation of didehydroalanine and didehydrobutyrine residues. The stability of this peptide is ensured by a thioether bond [53]. The first identified and well-known lantibiotic is nisin, named E 234, a bacteriocin produced by Lactococcus lactis [48] and the only commercialized bacteriocin so far. Based on their structural features, lantibiotics can be sub-divided into three groups: AI, AII, and B, corresponding to bacteriocins with linear, combined, and globular conformations [54]. Nisin, epilancin 15×, and microbisporicin are examples of AI-type lantibiotics; their inhibitory actions are caused by the fixation of the bacteriocin N-terminal domain to Lipid II, engendering the inhibition of cell wall synthesis. Furthermore, the C-terminal extremity (end) attaches to the membrane and causes its perforation, leading to the loss of essential cellular components and ultimately resulting in cell death; this inhibitory effect is known as a double mechanism of action. Mersacidin belongs to type B lantibiotics; they are bacteriocins with a globular secondary structure. They exhibit a bactericidal effect by disrupting (disturbing) enzymatic reactions in the target bacteria, resulting in the inhibition of cell wall formation [55].
Class II (non-lantibiotic): This class includes non-modified, small, and heat-stable peptides that cause the death of the target microorganism. Their inhibitory activity is mainly due to the amphiphilic helical structure that enables them to insert into the membrane of the sensitive cell, inducing depolarization [56]. These bacteriocins can be further subdivided into four subclasses: class IIa (pediocin-like bacteriocins) represent the largest group and are characterized by the presence of the YGNVG consensus sequence [53], including pediocin, sakacin A, and leucocin A. This bacteriocin class exerts its antibacterial activity by forming pores in the cytoplasmic membrane, thereby disrupting the membrane’s integrity [55]. Class IIb, also known as two-peptide, unmodified bacteriocins, includes lactacin F and lactococcin G, which are considered two-component bacteriocins. The antimicrobial effect of these bacteriocins is generated through the synergistic action of two specific peptides. Circular bacteriocins such as gassericin A, circularin A, and carnocyclin A belong to the third subclass, IIc. These peptides possess two transmembrane segments, which are involved in the formation of pores in the target cells [56]. Class IId consists of unmodified, linear, non-pediocin-like bacteriocins [48].
Class III (bacteriocins): These compounds are a type of protein with a high molecular mass > 30 kDA and are easily damaged by heat. Some examples of Class III bacteriocins include helveticins J and lactacin B, which are produced by Lactobacillus helveticus and Lactobacillus acidophilus, respectively. Other examples are enterolysin from Enterococcus faecalis, megacins from Bacillus megaterium, and klebicin from Klebsiella pneumoniae [56]. Bacteriocins are known for their characteristic narrow spectrum of activity, which means that they are only effective on closely related bacterial strains. However, recent studies have challenged this traditional notion and demonstrated that some bacteriocins can have a broader spectrum of activity than previously reported. In their research, Ye et al. (2021) performed a study on a novel bacteriocin called bacteriocin ZFM54, produced by Lacticaseibacillus paracasei ZFM54, and the results of their study showed that bacteriocin ZFM54 exhibited a strong antimicrobial activity through cell permeabilization against a range of pathogenic bacteria, such as Salmonella typhimurium, Micrococcus luteus, and Listeria monocytogenes [57]. Peng et al. (2021), reported in their study that a bacteriocin named plantaricin LP 21-2 produced by Lactiplantibacillus plantarum subsp. plantarum had a broad antimicrobial spectrum on Gram-positive and Gram-negative bacteria as well as fungi, including Staphylococcus aureus, Salmonella typhi, and Saccharomyces cerevisiae. Moreover, the findings demonstrate that LP 21-2 could resist high temperatures and still have 96% antimicrobial activity after being exposed to 121 °C for 15 min, making it a potential option for food preservation applications [58].
Among all bacteriocins, the post-translationally modified class Ia nisin is probably the most widely recognized bacteriocin for its action against Listeria spp. induced by membrane permeabilization of the target microorganism. Recent studies have further shown that various bacteriocins produced by LAB can effectively control the growth of pathogens in diverse food matrices, including dairy products, meat and meat products, fish and seafood products, juices and beverages, fruits, vegetables, and cereals [53].
For instance, Lacticaseibacillus paracasei ZFM54 produces novel bacteriocins with a broad-spectrum inhibitory action against targeted foodborne pathogens, including Listeria monocytogenes, Micrococcus luteus, and Salmonella typhimurium. This inhibitory action is achieved through pore formation in the cell membrane, further enhancing their potential as effective antimicrobial agents in food preservation [57]. In their study, Peng et al. (2021) demonstrated that L. plantarum SHY 21-2, isolated from yak yogurt, produces the novel bacteriocin plantaricin LP 21-2, exhibiting a broad antibacterial spectrum against Gram-negative bacteria, Gram-positive bacteria, and yeast [59]
Notably, LP 21-2 demonstrates resistance to heat and acidity, making it a promising candidate for diverse applications in the food industry. It has been reported that the main antimicrobial compound behind LAB’s antagonistic activity against pathogens is the synthesis of organic acids, predominantly lactic and acetic acids [60]. Organic acids primarily focus on disrupting the bacterial cell wall, cytoplasmic membrane, and specific metabolic functions (e.g., replication and protein synthesis) in pathogenic microorganisms, ultimately leading to their disturbance and death [61].
Indeed, a synergistic effect was observed when a combination of a bacteriocin from Pediococcus acidilactici K10 and succinic acid, lactic acid, and acetic acid was applied, demonstrating enhanced effectiveness against target cells of Escherichia coli O157:H7 both in vivo and in vitro [16]. Thus, the majority of LAB demonstrate inhibitory effects against various foodborne pathogens and spoilage microorganisms through different mechanisms, solidifying their crucial role in safeguarding food safety and quality during fermentation processes.
2.3. Reuterin
Reuterin is a powerful broad-spectrum antimicrobial compound produced by Limosilactobacillus reuteri through the conversion of glycerol. Its composition includes 3-hydroxypropionaldehyde (3-HPA), 3-HPA hydrate, 3-HPA dimer, and acrolein [62]. The 3-PHA content is the main inhibiting factor of reuterin, since it is a highly reactive aldehyde [63]. Reuterin is able to inhibit various spoilage and pathogenic microorganism including E. coli, Salmonella, Shigella, Campylobacter, Clostridium, and Candida [64]. It has been demonstrated by Asare et al. (2023) that reuterin-producing Limosilactobacillus reuteri enhances butyrate production and inhibits the growth of Enterobacteriaceae in the broiler chicken cecal microbiota PolyFermS model [62].
2.4. Hydrogen Peroxide
Hydrogen peroxide is a type of ROS (reactive oxygen species) that can cause oxidative damage to various types of molecules, such as lipids, proteins, and nucleic acids. This damage occurs either directly or indirectly through the formation of highly reactive hydroxyl radicals (OH●) [65]. In living systems, hydrogen peroxide (H2O2) is broken down into water and molecular oxygen by the action of a catalase enzyme. O2 can be released as a gas by both biotic and abiotic reactions [66]. The catalase enzyme plays a critical role in various biological processes, since this enzyme is responsible for maintaining the appropriate levels of H2O2 in cells and tissues, thus avoiding their damage. The toxicity of hydrogen peroxide on microorganisms is widely recognized in the field of biology and medicine; hence, it has been used as an over-the-counter antiseptic against bacteria, fungi, and viruses. Its broad antimicrobial spectrum is due to the production of hydroxyl radicals that damage cell components such as biofilms, cell membranes, and cell walls. Also, the effervescence generated by the released O2 from the degradation of hydrogen peroxide also contributes to its antimicrobial effectiveness [65]. Ibrahim et al. (2021) have reported that H2O2 has a high bactericidal action synergistically combined with heat [16].
2.5. Diacetyl
Diacetyl is an aromatic compound produced by LAB using citrate as a precursor, with a strong buttery aroma that is considered a highly valuable flavour component in dairy products. During lactic acid bacterium-mediated fermentation, diacetyl is produced through a process of non-enzymatic oxidative decarboxylation of alpha-acetolactate. This compound is then converted into acetoin and 2,3-butanediol by the action of 2,3-butanediol dehydrogenase, as described by Li et al. (2023) [67] The antimicrobial potential of diacetyl appears to be effective against yeast, mould, and Gram-negative bacteria [68]. Previous research has shown that the growth of some highly pathogenic microorganisms, such Escherichia coli O157:H7 and Salmonella typhimurium, has been controlled by the action of diacetyl [16]. In their 2020 study, Calvo et al. investigated the antifungal activity of diacetyl produced by Bacillus velezensis strains against postharvest fungal pathogens Penicillium digitatum and Aspergillus niger [69].
2.6. Carbon Dioxide
Carbon dioxide is widely recognized for its remarkable inhibitory effects. It significantly contributes to reducing microbial presence and improving the shelf life of a variety of food products. The inhibitory mechanism operates by extending spoilage microorganisms’ lag phase and generation time, thereby playing a crucial role in maintaining food freshness and safety [70]. Lactic acid bacteria capable of CO2 production have demonstrated their ability to inhibit the proliferation of Gram-positive and Gram-negative psychrotrophic bacteria, such as Enterobacteriaceae and Listeria species. The inhibitory mechanism of CO2 produced by LAB extends beyond the preservation of food freshness, and it also includes the influence on the visual characteristics of specific cheeses. Notably, certain LAB strains, including Lactococcus lactis subsp. lactis biovar. diacetylactis and Leuconostoc spp., play a distinctive role in enhancing the aesthetic appeal of cheeses, particularly in the creation of holes found in several cheeses like Gouda and Cheddar [24].
3. The Biotechnological Significance of LAB and Their Metabolites in the Food Industry: Antimicrobial Features and Related Traits of Interest
The distinctive microbial and metabolic characteristics of LAB have become a significant point of interest, particularly for their pivotal role in the food industry and their recognized probiotic properties. These bacteria can generate a broad spectrum of compounds during their metabolism (Table 2), including bacteriocins, exopolysaccharides, volatile compounds, short-chain fatty acids, vitamins, and amines [71]. Some of these metabolic targets have a direct antimicrobial activity (e.g., bacteriocins); some have biotechnological properties of interest for food but can also show antimicrobial properties (e.g., exopolysaccharides and volatile organic compounds). Other compounds have exclusive properties of interest for food quality (e.g., water-soluble vitamins), but they can use the antimicrobial attributes of the strain, which carries them to improve dominance and, therefore, biotechnological efficacy.

Table 2.
Biotechnological applications and effects of diverse food matrices of lactic acid bacterium metabolites.
3.1. Bacteriocin Applications
Antimicrobial activity is a key characteristic in the selection of probiotic strains of LAB. This intrinsic antagonistic ability amplifies the probiotic potential of LAB and positions them as crucial contributors to the development of functional foods with enhanced microbial defences against foodborne pathogens [16]. The use of probiotic bacteria presents a promising alternative for both prophylactic and therapeutic interventions against pathogens such as S. paratyphi. This approach can be promising against spoilage bacteria and pathogens, preventing biofilm formation, reducing antimicrobial resistance, and extending shelf life [85]. These barriers include substrate competition, the generation of metabolites such as organic acids, aldehydes, and peroxides, and the synthesis of antibacterial peptides [86]. In the control of pathogenic growth, the use of an antimicrobial agent such as nisin, or the synergistic application of two or more antimicrobial agents, proves effective in inhibiting the targeted microorganism and ensuring its complete prevention of growth without compromising the sensory properties of the food matrix [16]. Various studies have highlighted the advantages of the synergistic effects of lactic acid bacterium bacteriocins when combined with other biomolecules. For instance, the combined application of nisin and citric acid has proven effective against Staphylococcus aureus and Listeria monocytogenes. Similarly, enterocin AS-48 and ethambutol have exhibited efficacy in impeding Mycobacterium tuberculosis [61]. Moreover, it has been reported that the antifungal metabolites produced by LAB demonstrate the potential for synergistic interactions. This synergy, particularly between organic acids and other antifungal metabolites, contributes to a higher antifungal activity [87]. The combination of diacetyl produced by certain strains of LAB, along with specific bacteriocins such as reuterin, has been reported as a potent antimicrobial additive effective against Listeria monocytogenes [60].
Several studies have also emphasized the intrinsic antimicrobial properties of lactic acid bacterium bacteriocins, demonstrating their effectiveness against a broad spectrum of foodborne pathogens. For example, leucocin A stands out as a promising candidate, exhibiting anti-Listerial activity and resisting challenging conditions throughout the fermentation process of meat products [23]. Plantaricin P1053, produced by Lactiplantibacillus plantarum subsp. plantarum PBS067, exhibits a wide-ranging antimicrobial effect against both Gram-negative bacteria, including E. coli, and Gram-positive S. aureus [16]. Strong evidence supports the notion that LAB can impart probiotic effects by competitively excluding pathogenic bacteria. Competitive exclusion occurs when introducing a culture containing at least one non-pathogenic bacterium into the gastrointestinal tract of animals [61]. These bacteria compete for limited resources, including nutrients and space, using two distinct competitive strategies: exploitation competition, where they compete for nutrients and space, and interference competition, which involves the production of antimicrobial compounds to target the pathogenic bacteria [88]. Siedler et al. (2020) reported in their studies that the competition for manganese significantly restricts the growth of spoilage organisms in yoghurt supplemented with a bioprotective culture of L. paracasei and Lacticaseibacillus rhamnosus [89]. Additionally, the study emphasized that competitive exclusion constitutes a mechanism that is challenging for spoilage organisms to overcome through spontaneous mutation, making it an ideal mechanism for the bioprotection of food. It has been reported by Vieco-Saiz et al. (2019) that the administration of 1 × 109 CFU/mL of Lactiplantibacillus pentosus HC-2 exerted strong inhibitory effects on the growth of Vibrio parahaemolyticus in the shrimp intestine [61,71,80,90].
3.2. Exopolysaccharide Production
In recent years, the food industry has increasingly recognized the importance of microbial exopolysaccharides, particularly for their significant roles in fermented dairy and bakery products. Notably, these macromolecular compounds, resulting from the polymerization of multiple monosaccharides or their derivatives, offer advantages such as cost-effectiveness and a short production cycle [71,80]. Several species of LAB are documented as producers of EPSs (exopolysaccharides). Notably, strains belonging to streptococci and lactobacilli are primarily producers of heteropolysaccharides (HePSs), while Weissella, Leuconostoc, and Pediococcus spp. are homopolysaccharide (HoPS) producers [90]. LAB and their EPS derivatives (HePSs and HoPSs) find applications as additives in both in situ and ex situ food processing. In situ synthesis primarily relies on fermentation conditions, including factors like medium composition, pH value, temperature, incubation time, and agitation. In contrast, ex situ production involves the direct addition of purified EPSs as an ingredient in food [91]. To enhance products with favourable rheological and sensory attributes, EPSs serve as versatile ingredients, functioning as thickeners, emulsifiers, and stabilizers in the dairy industry [92]. In their study, Aarti and Khusro et al. (2019) proved that the EPS-producing Lactiplantibacillus plantarum subsp. plantarum strain AAS3, isolated from fermented, dry fish-based food, has shown potential as an adjunct or starter culture in the food processing industry [93]. Moreover, the exopolysaccharides derived from Lactiplantibacillus plantarum subsp. plantarum SKT109 exhibit promising potential for applications in the food-processing sector. As adjuncts, they can improve the sensory aspects, texture, rheological properties, and nutritional profile of Cheddar cheese [74]. Similarly, the producer strains can also find applications in the fermentation processes of various foods. For instance, certain starter cultures with the ability to synthesize EPSs, like Streptococcus thermophilus zlw TM11 and Lactobacillus delbrueckii subsp. bulgaricus, have the potential to elevate the exopolysaccharide content in yogurt. This property boosts the yoghurt exopolysaccharide content and enhances its syneresis, texture, and overall sensory qualities [82]. Numerous studies have consistently demonstrated the antibiofilm and antimicrobial properties of exopolysaccharides (EPSs) derived from lactic acid bacteria (LAB), underscoring their potential significance in preventing biofilm-related issues and inhibiting the proliferation of both Gram-positive and Gram-negative spoilage and pathogenic microorganisms. In [94], the researchers investigated the impact of Lactiplantibacillus plantarum-exopolysaccharides (L-EPSs) on biofilm formation by Shigella flexneri CMCC51574. Their findings demonstrated that L-EPSs effectively reduced polysaccharide production within the extracellular polymeric matrix of S. flexneri, thereby inhibiting biofilm formation. Furthermore, L-EPSs contributed to lowering the minimum biofilm elimination concentration (MBEC) of antibiotics against S. flexneri biofilm and inhibited the adhesion and invasion of S. flexneri to HT-29 cell monolayers. In their study, Karaca et al. (2022) found that the antibiofilm potential of Lactiplantibacillus plantarum EIR/IF-1 against oral bacteria is attributed to the EPSs’ ability to inhibit bacterial auto-aggregation and co-aggregation, along with their capacity to bind to hydrocarbons [95]. In another study, Rahnama Vosough et al. (2021) demonstrated that the EPS produced by Enterococcus faecium, exhibiting no in vitro cytotoxicity, shows promise as a natural antioxidant and antibacterial agent. Notably effective against Staphylococcus aureus and Enterococcus faecalis, this EPS offers potential applications in both the food and pharmaceutical industries [96]. According to Riaz Rajoka et al. (2021), Lactobacillus EPSs contain functional groups such as carbonyl, phosphate, and hydroxyl groups, which are considered essential for their antimicrobial and antioxidant properties [97]. Exopolysaccharides (EPSs) serve as crucial adhesins for LAB, enhancing their capacity to colonize the intestinal epithelium. By adhering to intestinal epithelial cells, EPSs facilitate the colonization of LAB in the gastrointestinal tract and prevent their elimination by intestinal peristalsis [98]. Dextran, a type of homopolysaccharide, can act like gluten in terms of texture; they have been exceptionally suitable for the production of gluten-free or low-gluten bakery products. Strains of Weissella cibaria also produce EPSs during sourdough and wheat fermentation, thus allowing for an improvement in the texture, nutritional profile, shelf life, and machinability of gluten-free breads [81]. In a study conducted by Ashfaq et al. (2020), dextran was observed to not only enhance the population of beneficial LAB but also reduce the abundance of pathogenic bacteria such as E. coli, Salmonella, and Enterococcus species in poultry intestines. These results suggest that dextran holds potential as a substitute for antibiotics in poultry feed, effectively controlling the proliferation of common enteric pathogens [99].
Alongside the functions of interest for the development of bio-based food applications, the possibility of polysaccharides exerting a barrier action against the entry of nutrients into undesired microorganisms was studied [100]. Polysaccharides can reduce several phenomena underlying the production of biofilms by eukaryotic microorganisms, reducing the anchoring activity of pathogens [100,101]. Future investigations are necessary for a better valorization of the biotechnological potential, also due to the chemical complexity of this class of biomolecules.
3.3. Volatile Compound Synthesis
Flavour stands out as a crucial aspect for consumers. The fermentative properties of LAB emerge as the key factor in developing the sensory profile of various fermented foods ranging from dairy products and meat to fish, vegetables, as well as alcoholic and non-alcoholic beverages [102]. LAB actively contribute to flavour development by generating a diverse array of volatile compounds, enhancing the overall quality of the final fermented product [103]. Flavour is the intricate combination of taste and smell sensations elicited by a substance within the mouth and is assessed using a sensory analysis. It originates from the recognition of two key elements: water-soluble taste compounds associated with the fundamental tastes (sweet, salty, bitter, sour, and umami) and a multitude of aroma compounds. These aromatic compounds, characterized by their volatile nature, contribute to the diverse array of flavours found in fermented foods [104]. More than 2000 volatile compounds have been identified in fermented foods, including aldehydes, esters, heterocycles, alcohols, acids, terpenes, ketones, and nitrogen and sulfur compounds [105]. Hu et al. (2020) explored the correlation between bacterial communities and volatile compounds in traditional dry sausages from various regions in Northeast China [105]. The results revealed the presence of 120 volatile compounds, predominantly comprising alcohols, acids, aldehydes, ketones, esters, and terpenes. There were positive correlations between Weissella hellenica, Lactobacillus sakei, Lactococcus lactis, Companilactobacillus alimentarius, Lactiplantibacillus plantarum, and carboxylic acids and alcohols. Furthermore, Lactococcus lactis, Companilactobacillus alimentarius, and Lactiplantibacillus plantarum were associated with the production of most esters, aldehydes, and ketones [105]. Zang et al. (2020) investigated the flavour evolution in Chinese traditional fermented fish, known as Suanyu, using a mixed starter culture of Lactiplantibacillus plantarum strain 120 and Saccharomyces cerevisiae strain 31. This study revealed lactobacilli as the dominant starter throughout fermentation, playing a crucial role in flavour development [84]. Notably, Lactobacillus was involved in the production of key flavours, including benzaldehyde, hexanoic and butanoic acids, ethyl octanoate, and ethyl lactate. The unique aroma of yoghurt, characterized by its buttery flavour, is attributed to C4 compounds, including diacetyl, acetoin, and 2,3-butanediol. Several LAB can synthesize these compounds through glycolysis or citrate metabolism, including species belonging to Streptococcus, Lactococcus, Leuconostoc, and Weissella genera and to lactobacilli [106]. Papaioannou et al. characterized the volatile compounds and overall flavour in yoghurts prepared from cow’s and goat’s milks using different commercial starter cultures, both with and without the addition of probiotic bacteria. Their findings indicated that yoghurts made from cow’s milk, using the mild and classic starter culture in the absence of probiotic Bifidobacterium BB-12, and dessert yoghurts from goat’s milk, prepared with the classic and acidic starter culture along with the probiotic Lactobacillus acidophilus LA-5, were most valued during the consumer’ tests. Notably, the respective volatile compounds in these yoghurts were aldehydes, acetaldehyde, ketones, butanoic acid, hexanoic acid, α-pinene, camphene, and limonene [107]. Recently, Xiao and coworkers investigated the microbial communities associated with three traditional Chinese fermented vegetable-based foods (i.e., Jiangxi yancai, Sichuan paocai, and Dongbei suanca). The obtained findings revealed that lactobacilli dominated the microbial composition in all three examined products, with specific strains closely associated with over twenty flavour compounds. Notably, L. sakei exhibited a positive correlation with volatile compounds like ethyl esters, benzoic acid, lactic acid, and (R)-3-hydroxybutyric acid. Additionally, L. acetotolerans demonstrated correlations with flavour compounds such as sinapic acid, linalool, terpinyl acetate, 3-methylhepta-1,6-dien-3-ol, and lactic acid [108].
Due to their diffusive chemical characteristics, mVOCs (microbial VOCs) play a role in various interactions between microorganisms and between micro- and macroorganisms [109]. In the recent literature, there is growing evidence of how VOCs produced by lactic acid bacteria can have an inhibitory effect on the development of filamentous fungi (e.g., Aspergillus niger and Aspergillus flavus) [110,111]. The chemical features of these compounds are also of extreme interest for biotechnological valorization, which makes this type of application promising for the development of new bio-based solutions.
3.4. Vitamins Production
The synthesis of vitamins like folic acid, riboflavin, vitamin C, pyridoxal [112], and cobalamin [113] during the fermentation process of lactic acid bacteria can be regarded as a form of nutritional fortification of the fermented food [82]. Several strains of LAB have the ability to produce vitamin B2, thus enhancing the nutritional profiles of food products and creating novel foods enriched with higher vitamin contents [81]. Riboflavin, also known as vitamin B2, is a water-soluble, heat-stable vitamin synthesized by most plants and microorganisms. It plays a vital role in human and animal growth and reproduction [114]. The industrial production of riboflavin can be achieved through both chemical synthesis and fermentation, but the fermentation process offers a cost-effective advantage by enabling the one-step production of vitamin B2 [115]. Hernández-Alcántara and coworkers reported that strains of Weissella cibaria exhibited the ability to bio-fortify experimental wheat breads in situ with riboflavin [81]. Moreover, incorporating a riboflavin-overproducing Limosilactobacillus fermentum UFG169 strain during soymilk fermentation not only enhanced the nutritional and functional qualities of the resulting product but also increased its riboflavin content [116].
Folate, particularly in the forms of tetrahydrofolate (THF) and methyl-tetrahydrofolate (MTHF), belongs to the water-soluble B-vitamin group [117]. It is pivotal in fundamental biological processes, including DNA synthesis, methylation, and the generation of amino acids, nucleotides, and other vitamins [118]. Folate deficiency in the body may result in conditions such as megaloblastic anaemia, homocysteinemia, cardiovascular diseases, and certain forms of cancer [117]. Recent research suggests that folate-producing lactic acid bacteria (LAB) could serve as a natural substitute for chemical folic acid [119]. Streptococcus, Lactococcus, and lactobacilli are among the lactic acid bacteria identified as folate producers. A study conducted by Carrizo et al. demonstrated that the administration of pasta made with quinoa sourdough and fermented by Lactiplantibacillus plantarum strains, both B2 and B9 producers, led to increased levels of these vitamins in mice blood compared to depleted animals [120]. Their results suggest that the bio-enrichment of quinoa pasta using LAB could be a novel strategy to enhance vitamin and mineral bioavailability in cereal/pseudocereal-derived foods. Albano and collaborators exhibited the potential use of lactobacilli as adjunct cultures for cheese bio-enrichment [121]. The in situ application of LAB strains during cheese manufacturing resulted in a twofold increase in the folate content after 30 days of ripening. Extending the cheese ripening to 60 days led to a further rise, exceeding 100 μg 100 g−1. As a result, the consumption of this bio-enriched cheese can contribute to achieving the recommended daily folate intake of 400 mcg [121]. In this work, we report the in situ, food-grade lactic acid bacterium-based overproduction of vitamins as an example of a trait of biotechnological interest that can benefit from antimicrobial properties to improve dominance. This is to highlight how an antagonistic behaviour against other microorganisms is a phenotype that can directly support other biotechnological features in LAB.
4. Conclusions
Lactic acid bacteria have been extensively employed in various fermentation processes, reaffirming their significant role in the safe production of various bioprocessed foods. These beneficial microorganisms not only offer health benefits but also enhance food safety through the production of natural antimicrobial compounds. The organic acids and bacteriocins produced by LAB inhibit the growth of foodborne pathogens, extend the shelf life of food products, and ensure their safety for human consumption. Additionally, the probiotic properties of several LAB make them valuable for promoting gastrointestinal health. This review highlights their vast potential in different food applications, underlining the importance of antimicrobial attributes, in combination with other features of biotechnological interest, to support the valorization of LAB for the green transition of food systems. Overall, the multifaceted contributions of LAB in food science, including their technological applications, health-promoting properties, and potential for innovative product development, underscore their importance in the food industry and the pursuit of healthier, cleaner, and safer food options for consumers.
Author Contributions
Conceptualization: R.C., V.C. and M.F.; writing R.C.; review and editing: V.C., G.S., Z.B., F.G. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
Giuseppe Spano is supported by funding for the PON project ‘Conservabilità, qualità e sicurezza dei prodotti ortofrutticoli ad alto contenuto di servizio’-POFACS-CUP B74I20000120005. Vittorio Capozzi is supported by the funding of (i) NUTRAGE CNR project FOE-2021 DBA.AD005.225 and (ii) the Next-Generation EU [PNRR], in the framework of the Mission 4 Component 2 Investment 1.3-Award Number: Project code PE00000003, Project title: “ON Foods-Research and innovation network on food and nutrition Sustainability, Safety and Security–Working ON Foods”. Mariagiovanna Fragasso is supported by the funding of the European Union Next-Generation EU [Piano Nazionale di Ripresa e Resilienza (PNRR)—Missione 4 Componente 2, Investimento 1.4—D.D. 1032 17/06/2022, CN00000022] within the Agritech National Research Centre for Agricultural Technologies. Zineb Benmchernene and Cirat Radjaa are supported by DGRSDT in the frame of SUSFOOD2 and CORE Organic co-funds and the PRFU Project (grant number D01N01UN310120220002).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created for this review article.
Acknowledgments
We would like to thank Massimo Franchi of the Institute of Sciences of Food Production—CNR for his skilled technical support provided during the realization of this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of Lactic Acid Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef] [PubMed]
- Qiao, H.; Chen, L.; Yang, J.; Zhi, W.; Chen, R.; Lu, T.; Tan, H.; Sheng, Z. Effect of Lactic Acid Bacteria on Bacterial Community Structure and Characteristics of Sugarcane Juice. Foods 2022, 11, 3134. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Fragasso, M.; Bimbo, F. Microbial Resources, Fermentation and Reduction of Negative Externalities in Food Systems: Patterns toward Sustainability and Resilience. Fermentation 2021, 7, 54. [Google Scholar] [CrossRef]
- Fugaban, J.I.I.; Vazquez Bucheli, J.E.; Park, Y.J.; Suh, D.H.; Jung, E.S.; Franco, B.D.G.d.M.; Ivanova, I.V.; Holzapfel, W.H.; Todorov, S.D. Antimicrobial Properties of Pediococcus acidilactici and Pediococcus pentosaceus Isolated from Silage. J. Appl. Microbiol. 2022, 132, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.G.; Antunes, A.E.C.; Sousa, A.L.O.P.; Faria, J.A.F.; Saad, S.M.I. Ice-Cream as a Probiotic Food Carrier. Food Res. Int. 2009, 42, 1233–1239. [Google Scholar] [CrossRef]
- Maftei, N.-M.; Raileanu, C.R.; Balta, A.A.; Ambrose, L.; Boev, M.; Marin, D.B.; Lisa, E.L. The Potential Impact of Probiotics on Human Health: An Update on Their Health-Promoting Properties. Microorganisms 2024, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.Y.J.; Quek, S.Y. Probiotics: Health Benefits, Food Application, and Colonization in the Human Gastrointestinal Tract. Food Bioeng. 2024, 3, 41–64. [Google Scholar] [CrossRef]
- Selmi, H.; Rocchetti, M.T.; Capozzi, V.; Semedo-Lemsaddek, T.; Fiocco, D.; Spano, G.; Abidi, F. Lactiplantibacillus plantarum from Unexplored Tunisian Ecological Niches: Antimicrobial Potential, Probiotic and Food Applications. Microorganisms 2023, 11, 2679. [Google Scholar] [CrossRef]
- Li, T.; Teng, D.; Mao, R.; Hao, Y.; Wang, X.; Wang, J. A Critical Review of Antibiotic Resistance in Probiotic Bacteria. Food Res. Int. 2020, 136, 109571. [Google Scholar] [CrossRef]
- Castellone, V.; Bancalari, E.; Rubert, J.; Gatti, M.; Neviani, E.; Bottari, B. Eating Fermented: Health Benefits of LAB-Fermented Foods. Foods 2021, 10, 2639. [Google Scholar] [CrossRef]
- Rocchetti, M.T.; Russo, P.; Capozzi, V.; Drider, D.; Spano, G.; Fiocco, D. Bioprospecting Antimicrobials from Lactiplantibacillus plantarum: Key Factors Underlying Its Probiotic Action. Int. J. Mol. Sci. 2021, 22, 12076. [Google Scholar] [CrossRef] [PubMed]
- Sellitto, V.M.; Zara, S.; Fracchetti, F.; Capozzi, V.; Nardi, T. Microbial Biocontrol as an Alternative to Synthetic Fungicides: Boundaries between Pre- and Postharvest Applications on Vegetables and Fruits. Fermentation 2021, 7, 60. [Google Scholar] [CrossRef]
- De Simone, N.; Capozzi, V.; de Chiara, M.L.V.; Amodio, M.L.; Brahimi, S.; Colelli, G.; Drider, D.; Spano, G.; Russo, P. Screening of Lactic Acid Bacteria for the Bio-Control of Botrytis cinerea and the Potential of Lactiplantibacillus plantarum for Eco-Friendly Preservation of Fresh-Cut Kiwifruit. Microorganisms 2021, 9, 773. [Google Scholar] [CrossRef] [PubMed]
- De Simone, N.; Capozzi, V.; Amodio, M.L.; Colelli, G.; Spano, G.; Russo, P. Microbial-Based Biocontrol Solutions for Fruits and Vegetables: Recent Insight, Patents, and Innovative Trends. Recent. Pat. Food Nutr. Agric. 2021, 12, 3–18. [Google Scholar] [CrossRef] [PubMed]
- De Gioia, M.; Russo, P.; De Simone, N.; Grieco, F.; Spano, G.; Capozzi, V.; Fragasso, M. Interactions among Relevant Non-Saccharomyces, Saccharomyces, and Lactic Acid Bacteria Species of the Wine Microbial Consortium: Towards Advances in Antagonistic Phenomena and Biocontrol Potential. Appl. Sci. 2022, 12, 12760. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ayivi, R.D.; Zimmerman, T.; Siddiqui, S.A.; Altemimi, A.B.; Fidan, H.; Esatbeyoglu, T.; Bakhshayesh, R.V. Lactic Acid Bacteria as Antimicrobial Agents: Food Safety and Microbial Food Spoilage Prevention. Foods 2021, 10, 3131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 612285. [Google Scholar] [CrossRef] [PubMed]
- Capozzi, V.; Tufariello, M.; De Simone, N.; Fragasso, M.; Grieco, F. Biodiversity of Oenological Lactic Acid Bacteria: Species- and Strain-Dependent Plus/Minus Effects on Wine Quality and Safety. Fermentation 2021, 7, 24. [Google Scholar] [CrossRef]
- Li, W.; Wu, Q.; Kwok, L.; Zhang, H.; Gan, R.; Sun, Z. Population and Functional Genomics of Lactic Acid Bacteria, an Important Group of Food Microorganism: Current Knowledge, Challenges, and Perspectives. Food Front. 2024, 5, 3–23. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Therapeutic and Dietary Support for Gastrointestinal Tract Using Kefir as a Nutraceutical Beverage: Dairy-Milk-Based or Plant-Sourced Kefir Probiotic Products for Vegan and Lactose-Intolerant Populations. Fermentation 2023, 9, 388. [Google Scholar] [CrossRef]
- Meybodi, N.M.; Mortazavian, A.M.; Arab, M.; Nematollahi, A. Probiotic Viability in Yoghurt: A Review of Influential Factors. Int. Dairy J. 2020, 109, 104793. [Google Scholar] [CrossRef]
- Margalho, L.P.; Jorge, G.P.; Noleto, D.A.P.; Silva, C.E.; Abreu, J.S.; Piran, M.V.F.; Brocchi, M.; Sant’Ana, A.S. Biopreservation and Probiotic Potential of a Large Set of Lactic Acid Bacteria Isolated from Brazilian Artisanal Cheeses: From Screening to in Product Approach. Microbiol. Res. 2021, 242, 126622. [Google Scholar] [CrossRef] [PubMed]
- Poudel, R.; Thunell, R.K.; Oberg, C.J.; Overbeck, S.; Lefevre, M.; Oberg, T.S.; McMahon, D.J. Comparison of Growth and Survival of Single Strains of Lactococcus lactis and Lactococcus cremoris during Cheddar Cheese Manufacture. J. Dairy Sci. 2022, 105, 2069–2081. [Google Scholar] [CrossRef] [PubMed]
- Ben Said, L.; Gaudreau, H.; Dallaire, L.; Tessier, M.; Fliss, I. Bioprotective Culture: A New Generation of Food Additives for the Preservation of Food Quality and Safety. Ind. Biotechnol. 2019, 15, 138–147. [Google Scholar] [CrossRef]
- Barcenilla, C.; Ducic, M.; López, M.; Prieto, M.; Álvarez-Ordóñez, A. Application of Lactic Acid Bacteria for the Biopreservation of Meat Products: A Systematic Review. Meat. Sci. 2022, 183, 108661. [Google Scholar] [CrossRef] [PubMed]
- Casquete, R.; Fonseca, S.C.; Pinto, R.; Castro, S.M.; Todorov, S.; Teixeira, P.; Vaz-Velho, M. Evaluation of the Microbiological Safety and Sensory Quality of a Sliced Cured-Smoked Pork Product with Protective Cultures Addition and Modified Atmosphere Packaging. Food Sci. Technol. Int. 2019, 25, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Arrioja-Bretón, D.; Mani-López, E.; Palou, E.; López-Malo, A. Antimicrobial Activity and Storage Stability of Cell-Free Supernatants from Lactic Acid Bacteria and Their Applications with Fresh Beef. Food Control 2020, 115, 107286. [Google Scholar] [CrossRef]
- Chakchouk-Mtibaa, A.; Smaoui, S.; Ktari, N.; Sellem, I.; Najah, S.; Karray-Rebai, I.; Mellouli, L. Biopreservative Efficacy of Bacteriocin BacFL31 in Raw Ground Turkey Meat in Terms of Microbiological, Physicochemical, and Sensory Qualities. Biocontrol Sci. 2017, 22, 67–77. [Google Scholar] [CrossRef]
- Khouadja, S.; Haddaji, N.; Hanchi, M.; Bakhrouf, A. Selection of Lactic Acid Bacteria as Candidate Probiotics for Vibrio parahaemolyticus Depuration in Pacific Oysters (Crassostrea gigas). Aquac. Res. 2017, 48, 1885–1894. [Google Scholar] [CrossRef]
- Kong, Y.; Gao, C.; Du, X.; Zhao, J.; Li, M.; Shan, X.; Wang, G. Effects of Single or Conjoint Administration of Lactic Acid Bacteria as Potential Probiotics on Growth, Immune Response and Disease Resistance of Snakehead Fish (Channa argus). Fish Shellfish Immunol. 2020, 102, 412–421. [Google Scholar] [CrossRef]
- Gao, P.; Li, L.; Xia, W.; Xu, Y.; Liu, S. Valorization of Nile Tilapia (Oreochromis niloticus) Fish Head for a Novel Fish Sauce by Fermentation with Selected Lactic Acid Bacteria. LWT 2020, 129, 109539. [Google Scholar] [CrossRef]
- Nimalan, N.; Sørensen, S.L.; Fečkaninová, A.; Koščová, J.; Mudroňová, D.; Gancarčíková, S.; Vatsos, I.N.; Bisa, S.; Kiron, V.; Sørensen, M. Supplementation of Lactic Acid Bacteria Has Positive Effects on the Mucosal Health of Atlantic Salmon (Salmo salar) Fed Soybean Meal. Aquac. Rep. 2023, 28, 101461. [Google Scholar] [CrossRef]
- Dong, Q.; Zhang, W.; Guo, L.; Niu, H.; Liu, Q.; Wang, X. Influence of Lactobacillus plantarum Individually and in Combination with Low O2-MAP on the Pathogenic Potential of Listeria monocytogenes in Cabbage. Food Control 2020, 107, 106765. [Google Scholar] [CrossRef]
- López-Salas, D.; Oney-Montalvo, J.E.; Ramírez-Rivera, E.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Fermentation of Habanero Pepper by Two Lactic Acid Bacteria and Its Effect on the Production of Volatile Compounds. Fermentation 2022, 8, 219. [Google Scholar] [CrossRef]
- Tian, M.; Lin, K.; Yang, L.; Jiang, B.; Zhang, B.; Zhu, X.; Ren, D.; Yu, H. Characterization of Key Aroma Compounds in Gray Sufu Fermented Using Leuconostoc mesenteroides subsp. mesenteroides F24 as a Starter Culture. Food Chem. X 2023, 20, 100881. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Liu, T.; Sadiq, F.A.; Yang, H.; Yuan, L.; Zhang, G.; He, G. Biogenic Amines Content and Assessment of Bacterial and Fungal Diversity in Stinky Tofu—A Traditional Fermented Soy Curd. LWT 2018, 88, 26–34. [Google Scholar] [CrossRef]
- Moore, J.F.; DuVivier, R.; Johanningsmeier, S.D. Formation of γ-Aminobutyric Acid (GABA) during the Natural Lactic Acid Fermentation of Cucumber. J. Food Compos. Anal. 2021, 96, 103711. [Google Scholar] [CrossRef]
- Li, S.; Tao, Y.; Li, D.; Wen, G.; Zhou, J.; Manickam, S.; Han, Y.; Chai, W.S. Fermentation of Blueberry Juices Using Autochthonous Lactic Acid Bacteria Isolated from Fruit Environment: Fermentation Characteristics and Evolution of Phenolic Profiles. Chemosphere 2021, 276, 130090. [Google Scholar] [CrossRef]
- Ricci, A.; Marrella, M.; Hadj Saadoun, J.; Bernini, V.; Godani, F.; Dameno, F.; Neviani, E.; Lazzi, C. Development of Lactic Acid-Fermented Tomato Products. Microorganisms 2020, 8, 1192. [Google Scholar] [CrossRef]
- Harlé, O.; Falentin, H.; Niay, J.; Valence, F.; Courselaud, C.; Chuat, V.; Maillard, M.-B.; Guédon, É.; Deutsch, S.-M.; Thierry, A. Diversity of the Metabolic Profiles of a Broad Range of Lactic Acid Bacteria in Soy Juice Fermentation. Food Microbiol. 2020, 89, 103410. [Google Scholar] [CrossRef]
- Väkeväinen, K.; Ludena-Urquizo, F.; Korkala, E.; Lapveteläinen, A.; Peräniemi, S.; von Wright, A.; Plumed-Ferrer, C. Potential of Quinoa in the Development of Fermented Spoonable Vegan Products. LWT 2020, 120, 108912. [Google Scholar] [CrossRef]
- Al-Hindi, R.R.; Abd El Ghani, S. Production of Functional Fermented Milk Beverages Supplemented with Pomegranate Peel Extract and Probiotic Lactic Acid Bacteria. J. Food Qual. 2020, 2020, 4710273. [Google Scholar] [CrossRef]
- Mohamad, N.I.; Abdul Manan, M.; Mohd Lazim, M.I.; Abdullah Sani, N. Antibacterial activity and organic acids formation by Lactobacillus sp. Originated from pickled guava and papaya. J. Sustain. Sci. Manag. 2022, 17, 286–295. [Google Scholar] [CrossRef]
- Guimarães, A.; Santiago, A.; Teixeira, J.A.; Venâncio, A.; Abrunhosa, L. Anti-Aflatoxigenic Effect of Organic Acids Produced by Lactobacillus plantarum. Int. J. Food Microbiol. 2018, 264, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kovanda, L.; Zhang, W.; Wei, X.; Luo, J.; Wu, X.; Atwill, E.R.; Vaessen, S.; Li, X.; Liu, Y. In Vitro Antimicrobial Activities of Organic Acids and Their Derivatives on Several Species of Gram-Negative and Gram-Positive Bacteria. Molecules 2019, 24, 3770. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.G.; Badr, A.N.; El Sohaimy, S.A.; Asker, D.; Awad, T.S. Characterization of Antifungal Metabolites Produced by Novel Lactic Acid Bacterium and Their Potential Application as Food Biopreservatives. Ann. Agric. Sci. 2019, 64, 71–78. [Google Scholar] [CrossRef]
- Mojgani, N.; Bagheri, M.; Moharrami, M.; Toutiaee, S.; Rousta, H.M. Bioactive Metabolites from Autochthonous Lactic Acid Bacteria Inhibit the Growth of Melissococcus plutonius, Causal Agent of European Foulbrood Disease in Honey Bees. Entomol. Exp. Appl. 2023, 171, 386–394. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, Synthesis, Mechanism of Action and Resistance Development in Food Spoilage Causing Bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Dean, S.N.; Rimmer, M.A.; Turner, K.B.; Phillips, D.A.; Caruana, J.C.; Hervey, W.J.; Leary, D.H.; Walper, S.A. Lactobacillus acidophilus Membrane Vesicles as a Vehicle of Bacteriocin Delivery. Front. Microbiol. 2020, 11, 710. [Google Scholar] [CrossRef]
- Mekala, P.M.; Ansari, R.M.H. Biotechnological Potential of Lactic Acid Bacteria Derived Bacteriocins in Sustainable Food Preservation. World J. Biol. Pharm. Health Sci. 2023, 14, 024–035. [Google Scholar] [CrossRef]
- Wu, J.; Zang, M.; Wang, S.; Zhao, B.; Bai, J.; Xu, C.; Shi, Y.; Qiao, X. Nisin: From a Structural and Meat Preservation Perspective. Food Microbiol. 2023, 111, 104207. [Google Scholar] [CrossRef] [PubMed]
- Khelissa, S.; Chihib, N.-E.; Gharsallaoui, A. Conditions of Nisin Production by Lactococcus lactis subsp. lactis and Its Main Uses as a Food Preservative. Arch. Microbiol. 2021, 203, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Hammami, R.; Cotter, P.D.; Rebuffat, S.; Ben Said, L.; Gaudreau, H.; Bédard, F.; Biron, E.; Drider, D.; Fliss, I. Bacteriocins as a New Generation of Antimicrobials: Toxicity Aspects and Regulations. FEMS Microbiol. Rev. 2021, 45, fuaa039. [Google Scholar] [CrossRef] [PubMed]
- Zimina, M.; Babich, O.; Prosekov, A.; Sukhikh, S.; Ivanova, S.; Shevchenko, M.; Noskova, S. Overview of Global Trends in Classification, Methods of Preparation and Application of Bacteriocins. Antibiotics 2020, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Darbandi, A.; Asadi, A.; Mahdizade Ari, M.; Ohadi, E.; Talebi, M.; Halaj Zadeh, M.; Darb Emamie, A.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and Potential Use as Antimicrobials. J. Clin. Lab. Anal. 2022, 36, e24093. [Google Scholar] [CrossRef] [PubMed]
- Negash, A.W.; Tsehai, B.A. Current Applications of Bacteriocin. Int. J. Microbiol. 2020, 2020, 4374891. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Wang, J.; Liu, M.; Li, P.; Gu, Q. Purification and Characterization of a Novel Bacteriocin from Lactobacillus paracasei ZFM54. LWT 2021, 143, 111125. [Google Scholar] [CrossRef]
- Zeng, X.; Zou, Y.; Zheng, J.; Qiu, S.; Liu, L.; Wei, C. Quorum sensing-mediated microbial interactions: Mechanisms, applications, challenges and perspectives. Microbiol. Res. 2023, 127414, 273. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Song, J.; Zeng, W.; Wang, H.; Zhang, Y.; Xin, J.; Suo, H. A Broad-Spectrum Novel Bacteriocin Produced by Lactobacillus plantarum SHY 21–2 from Yak Yogurt: Purification, Antimicrobial Characteristics and Antibacterial Mechanism. LWT 2021, 142, 110955. [Google Scholar] [CrossRef]
- Martín, I.; Rodríguez, A.; Delgado, J.; Córdoba, J.J. Strategies for Biocontrol of Listeria monocytogenes Using Lactic Acid Bacteria and Their Metabolites in Ready-to-Eat Meat- and Dairy-Ripened Products. Foods 2022, 11, 542. [Google Scholar] [CrossRef]
- Vieco-Saiz, N.; Belguesmia, Y.; Raspoet, R.; Auclair, E.; Gancel, F.; Kempf, I.; Drider, D. Benefits and Inputs from Lactic Acid Bacteria and Their Bacteriocins as Alternatives to Antibiotic Growth Promoters during Food-Animal Production. Front. Microbiol. 2019, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Asare, P.T.; Greppi, A.; Geirnaert, A.; Pennacchia, A.; Babst, A.; Lacroix, C. Glycerol and Reuterin-Producing Limosilactobacillus reuteri Enhance Butyrate Production and Inhibit Enterobacteriaceae in Broiler Chicken Cecal Microbiota PolyFermS Model. BMC Microbiol. 2023, 23, 384. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yin, Q.; Bai, H.; Wang, W.; Chen, Y.; Zhou, M.; Zhang, R.; Ding, G.; Xu, Z.; Zhang, Y. Transcriptome Analysis of Glycerin Regulating Reuterin Production of Lactobacillus reuteri. Microorganisms 2023, 11, 2007. [Google Scholar] [CrossRef] [PubMed]
- Özogul, F.; Hamed, I. The Importance of Lactic Acid Bacteria for the Prevention of Bacterial Growth and Their Biogenic Amines Formation: A Review. Crit. Rev. Food Sci. Nutr. 2018, 58, 1660–1670. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.C.; Friedman, A.J. Hydrogen Peroxide and Cutaneous Biology: Translational Applications, Benefits, and Risks. J. Am. Acad. Dermatol. 2019, 81, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Bezsenyi, A.; Sági, G.; Makó, M.; Wojnárovits, L.; Takács, E. The Effect of Hydrogen Peroxide on the Biochemical Oxygen Demand (BOD) Values Measured during Ionizing Radiation Treatment of Wastewater. Radiat. Phys. Chem. 2021, 189, 109773. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Yao, M.; Yang, W.; Han, Y.; Liu, L.; Zhang, J.; Liu, J. Mechanism of Microbial Production of Acetoin and 2,3-Butanediol Optical Isomers and Substrate Specificity of Butanediol Dehydrogenase. Microb. Cell Fact. 2023, 22, 165. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Baskaran, N.; Krishnamoorthy, S.; Sivanandham, V.; Bhavadharani, M. Metabolites of Lactic Acid Bacteria (LAB): Production, Formulation and Potential Applications in Food Industries. In Prospective Research and Technological Advancements in Food and Health Sciences; Skyfox Publishing Group: Thanjavur, India, 2023; pp. 139–228. [Google Scholar]
- Calvo, H.; Mendiara, I.; Arias, E.; Gracia, A.P.; Blanco, D.; Venturini, M.E. Antifungal Activity of the Volatile Organic Compounds Produced by Bacillus velezensis Strains against Postharvest Fungal Pathogens. Postharvest Biol. Technol. 2020, 166, 111208. [Google Scholar] [CrossRef]
- Kimbuathong, N.; Leelaphiwat, P.; Harnkarnsujarit, N. Inhibition of Melanosis and Microbial Growth in Pacific White Shrimp (Litopenaeus vannamei) Using High CO2 Modified Atmosphere Packaging. Food Chem. 2020, 312, 126114. [Google Scholar] [CrossRef]
- Sharma, H.; Ozogul, F.; Bartkiene, E.; Rocha, J.M. Impact of Lactic Acid Bacteria and Their Metabolites on the Techno-Functional Properties and Health Benefits of Fermented Dairy Products. Crit. Rev. Food Sci. Nutr. 2023, 63, 4819–4841. [Google Scholar] [CrossRef]
- Robinson, B.R.; D’Amico, D.J. Hydrogen Peroxide Treatments for the Control of Listeria monocytogenes on High-Moisture Soft Cheese. Int. Dairy J. 2021, 114, 104931. [Google Scholar] [CrossRef]
- Pan, L.; Wang, Q.; Qu, L.; Liang, L.; Han, Y.; Wang, X.; Zhou, Z. Pilot-Scale Production of Exopolysaccharide from Leuconostoc pseudomesenteroides XG5 and Its Application in Set Yogurt. J. Dairy Sci. 2022, 105, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, T.; Fang, X.; Yang, Z. Manufacture of Low-Fat Cheddar Cheese by Exopolysaccharide-Producing Lactobacillus plantarum JLK0142 and Its Functional Properties. J. Dairy Sci. 2019, 102, 3825–3838. [Google Scholar] [CrossRef]
- Fröhlich-Wyder, M.-T.; Guggisberg, D.; Badertscher, R.; Wechsler, D.; Wittwer, A.; Irmler, S. The Effect of Lactobacillus buchneri and Lactobacillus parabuchneri on the Eye Formation of Semi-Hard Cheese. Int. Dairy J. 2013, 33, 120–128. [Google Scholar] [CrossRef]
- Pedersen, T.B.; Vogensen, F.K.; Ardö, Y. Effect of Heterofermentative Lactic Acid Bacteria of DL-Starters in Initial Ripening of Semi-Hard Cheese. Int. Dairy J. 2016, 57, 72–79. [Google Scholar] [CrossRef]
- Tian, H.; Shi, Y.; Zhang, Y.; Yu, H.; Mu, H.; Chen, C. Screening of Aroma-producing Lactic Acid Bacteria and Their Application in Improving the Aromatic Profile of Yogurt. J. Food Biochem. 2019, 43, e12837. [Google Scholar] [CrossRef] [PubMed]
- Cesselin, B.; Henry, C.; Gruss, A.; Gloux, K.; Gaudu, P. Mechanisms of Acetoin Toxicity and Adaptive Responses in an Acetoin-Producing Species, Lactococcus lactis. Appl. Environ. Microbiol. 2021, 87, e01079-21. [Google Scholar] [CrossRef] [PubMed]
- Tsouli Sarhir, S.; Amanpour, A.; Bouseta, A.; Selli, S. Potent Odorants and Sensory Characteristics of the Soft White Cheese “Jben”: Effect of Salt Content. Flavour. Fragr. J. 2022, 37, 243–253. [Google Scholar] [CrossRef]
- Rice, T.; Sahin, A.W.; Lynch, K.M.; Arendt, E.K.; Coffey, A. Isolation, Characterisation and Exploitation of Lactic Acid Bacteria Capable of Efficient Conversion of Sugars to Mannitol. Int. J. Food Microbiol. 2020, 321, 108546. [Google Scholar] [CrossRef]
- Hernández-Alcántara, A.M.; Chiva, R.; Mohedano, M.L.; Russo, P.; Ruiz-Masó, J.Á.; del Solar, G.; Spano, G.; Tamame, M.; López, P. Weissella cibaria Riboflavin-Overproducing and Dextran-Producing Strains Useful for the Development of Functional Bread. Front. Nutr. 2022, 9, 978831. [Google Scholar] [CrossRef]
- Khalili, M.; Rad, A.; Khosroushahi, A.; Khosravi, H.; Jafarzadeh, S. Application of probiotics in folate bio-fortification of yoghurt. Probiotics Antimicrob. Proteins 2020, 12, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Niamah, A.K.; Abdulmuttaleb, A.M.; Hussain, N.A. Antibacterial spectrum of produced reuterin from new isolates of Lactobacillus reuteri. J. Microbiol. Biotechnol. Food Sci. 2020, 10, 134–139. [Google Scholar] [CrossRef]
- Zang, J.; Yu, D.; Li, T.; Xu, Y.; Regenstein, J.M.; Xia, W. Identification of Characteristic Flavor and Microorganisms Related to Flavor Formation in Fermented Common Carp (Cyprinus carpio L.). Food Res. Int. 2022, 155, 111128. [Google Scholar] [CrossRef]
- Divyashree, S.; Ramu, R.; Sreenivasa, M.Y. Evaluation of New Candidate Probiotic Lactobacillus Strains Isolated from a Traditional Fermented Food- Multigrain-Millet Dosa Batter. Food Biosci. 2024, 57, 103450. [Google Scholar] [CrossRef]
- Bungenstock, L.; Abdulmawjood, A.; Reich, F. Evaluation of Antibacterial Properties of Lactic Acid Bacteria from Traditionally and Industrially Produced Fermented Sausages from Germany. PLoS ONE 2020, 15, e0230345. [Google Scholar] [CrossRef]
- Nasrollahzadeh, A.; Mokhtari, S.; Khomeiri, M.; Saris, P.E.J. Antifungal Preservation of Food by Lactic Acid Bacteria. Foods 2022, 11, 395. [Google Scholar] [CrossRef]
- Knipe, H.; Temperton, B.; Lange, A.; Bass, D.; Tyler, C.R. Probiotics and Competitive Exclusion of Pathogens in Shrimp Aquaculture. Rev. Aquac. 2021, 13, 324–352. [Google Scholar] [CrossRef]
- Siedler, S.; Rau, M.H.; Bidstrup, S.; Vento, J.M.; Aunsbjerg, S.D.; Bosma, E.F.; McNair, L.M.; Beisel, C.L.; Neves, A.R. Competitive Exclusion Is a Major Bioprotective Mechanism of Lactobacilli against Fungal Spoilage in Fermented Milk Products. Appl. Environ. Microbiol. 2020, 86, e02312-19. [Google Scholar] [CrossRef]
- Wu, J.; Han, X.; Ye, M.; Li, Y.; Wang, X.; Zhong, Q. Exopolysaccharides Synthesized by Lactic Acid Bacteria: Biosynthesis Pathway, Structure-Function Relationship, Structural Modification and Applicability. Crit. Rev. Food Sci. Nutr. 2023, 63, 7043–7064. [Google Scholar] [CrossRef]
- Korcz, E.; Varga, L. Exopolysaccharides from Lactic Acid Bacteria: Techno-Functional Application in the Food Industry. Trends Food Sci. Technol. 2021, 110, 375–384. [Google Scholar] [CrossRef]
- Pourjafar, H.; Ansari, F.; Sadeghi, A.; Samakkhah, S.A.; Jafari, S.M. Functional and Health-Promoting Properties of Probiotics’ Exopolysaccharides; Isolation, Characterization, and Applications in the Food Industry. Crit. Rev. Food Sci. Nutr. 2023, 63, 8194–8225. [Google Scholar] [CrossRef] [PubMed]
- Aarti, C.; Khusro, A. Functional and Technological Properties of Exopolysaccharide Producing Autochthonous Lactobacillus plantarum Strain AAS3 from Dry Fish Based Fermented Food. LWT 2019, 114, 108387. [Google Scholar] [CrossRef]
- Song, Y.; Sun, M.; Feng, L.; Liang, X.; Song, X.; Mu, G.; Tuo, Y.; Jiang, S.; Qian, F. Antibiofilm Activity of Lactobacillus plantarum 12 Exopolysaccharides against Shigella flexneri. Appl. Environ. Microbiol. 2020, 86, e00694-20. [Google Scholar] [CrossRef] [PubMed]
- Karaca, B.; Haliscelik, O.; Gursoy, M.; Kiran, F.; Loimaranta, V.; Söderling, E.; Gursoy, U.K. Analysis of Chemical Structure and Antibiofilm Properties of Exopolysaccharides from Lactiplantibacillus plantarum EIR/IF-1 Postbiotics. Microorganisms 2022, 10, 2200. [Google Scholar] [CrossRef] [PubMed]
- Rahnama Vosough, P.; Habibi Najafi, M.B.; Edalatian Dovom, M.R.; Javadmanesh, A.; Mayo, B. Evaluation of Antioxidant, Antibacterial and Cytotoxicity Activities of Exopolysaccharide from Enterococcus Strains Isolated from Traditional Iranian Kishk. J. Food Meas. Charact. 2021, 15, 5221–5230. [Google Scholar] [CrossRef]
- Riaz Rajoka, M.S.; Wu, Y.; Mehwish, H.M.; Bansal, M.; Zhao, L. Lactobacillus Exopolysaccharides: New Perspectives on Engineering Strategies, Physiochemical Functions, and Immunomodulatory Effects on Host Health. Trends Food Sci. Technol. 2020, 103, 36–48. [Google Scholar] [CrossRef]
- Yang, S.; Xu, X.; Peng, Q.; Ma, L.; Qiao, Y.; Shi, B. Exopolysaccharides from Lactic Acid Bacteria, as an Alternative to Antibiotics, on Regulation of Intestinal Health and the Immune System. Anim. Nutr. 2023, 13, 78–89. [Google Scholar] [CrossRef]
- Ashfaq, I.; Amjad, H.; Ahmad, W.; Nasir, A.; Ali, A.; Ali, W.R.; Khaliq, S.; Hayat, A.; Ali, H.; Sattar, F.; et al. Growth Inhibition of Common Enteric Pathogens in the Intestine of Broilers by Microbially Produced Dextran and Levan Exopolysaccharides. Curr. Microbiol. 2020, 77, 2128–2136. [Google Scholar] [CrossRef] [PubMed]
- Ponzio, A.; Rebecchi, A.; Zivoli, R.; Morelli, L. Reuterin, Phenyllactic Acid, and Exopolysaccharides as Main Antifungal Molecules Produced by Lactic Acid Bacteria: A Scoping Review. Foods 2024, 13, 752. [Google Scholar] [CrossRef]
- Wang, K.; Niu, M.; Song, D.; Song, X.; Zhao, J.; Wu, Y.; Lu, B.; Niu, G. Preparation, Partial Characterization and Biological Activity of Exopolysaccharides Produced from Lactobacillus fermentum S1. J. Biosci. Bioeng. 2020, 129, 206–214. [Google Scholar] [CrossRef]
- Beauchamp, J.D. Dynamic Flavor: Capturing Aroma Using Real-Time Mass Spectrometry; American Chemical Society: Washington, DC, USA, 2021; Volume 1402, ISBN 9780841297944. [Google Scholar]
- Kouakou-Kouamé, A.C.; N’guessan, F.K.; Montet, D.; Djè, M.K. Production of Flavor Compounds by Lactic Acid Bacteria in Fermented Foods. In Lactic Acid Bacteria as Cell Factories; Elsevier: Amsterdam, The Netherlands, 2023; pp. 239–270. [Google Scholar]
- Thierry, A.; Pogačić, T.; Weber, M.; Lortal, S. Production of Flavor Compounds by Lactic Acid Bacteria in Fermented Foods. In Biotechnology of Lactic Acid Bacteria; Wiley: Hoboken, NJ, USA, 2015; pp. 314–340. [Google Scholar]
- Hu, Y.; Zhang, L.; Liu, Q.; Wang, Y.; Chen, Q.; Kong, B. The Potential Correlation between Bacterial Diversity and the Characteristic Volatile Flavour of Traditional Dry Sausages from Northeast China. Food Microbiol. 2020, 91, 103505. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, S.; Hao, G.; Yu, H.; Tian, H.; Zhao, G. Role of Lactic Acid Bacteria on the Yogurt Flavour: A Review. Int. J. Food Prop. 2017, 20, S316–S330. [Google Scholar] [CrossRef]
- Papaioannou, G.; Kosma, I.; Badeka, A.V.; Kontominas, M.G. Profile of Volatile Compounds in Dessert Yogurts Prepared from Cow and Goat Milk, Using Different Starter Cultures and Probiotics. Foods 2021, 10, 3153. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Huang, T.; Huang, C.; Hardie, J.; Peng, Z.; Xie, M.; Xiong, T. The Microbial Communities and Flavour Compounds of Jiangxi Yancai, Sichuan Paocai and Dongbei Suancai: Three Major Types of Traditional Chinese Fermented Vegetables. LWT 2020, 121, 108865. [Google Scholar] [CrossRef]
- Álvarez-García, S.; Mayo-Prieto, S.; Carro-Huerga, G.; Rodríguez-González, Á.; González-López, Ó.; Gutiérrez, S.; Casquero, P.A. Volatile Organic Compound Chamber: A Novel Technology for Microbiological Volatile Interaction Assays. J. Fungi 2021, 7, 248. [Google Scholar] [CrossRef] [PubMed]
- De Simone, N.; López, L.; Ciudad, C.S.; Scauro, A.; Russo, P.; Rodríguez, J.; Spano, G.; Martínez, B. Antifungal Activity of Lactiplantibacillus plantarum Isolated from Fruit and Vegetables and Detection of Novel Antifungal VOCs from Fungal-LAB Co-Cultures. Food Biosci. 2024, 58, 103824. [Google Scholar] [CrossRef]
- Li, B.; Wang, Z.; Yang, G.; Huang, S.; Liao, S.; Chen, K.; Du, M.; Zalán, Z.; Hegyi, F.; Kan, J. Biocontrol Potential of 1-Pentanal Emitted from Lactic Acid Bacteria Strains against Aspergillus flavus in Red Pepper (Capsicum annuum L.). Food Control 2022, 142, 109261. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Dueñas, M.T.; López, P.; Spano, G. Lactic Acid Bacteria Producing B-Group Vitamins: A Great Potential for Functional Cereals Products. Appl. Microbiol. Biotechnol. 2012, 96, 1383–1394. [Google Scholar] [CrossRef]
- Taranto, M.P.; Vera, J.L.; Hugenholtz, J.; De Valdez, G.F.; Sesma, F. Lactobacillus reuteri CRL1098 Produces Cobalamin. J. Bacteriol. 2003, 185, 5643–5647. [Google Scholar] [CrossRef]
- Suwannasom, N.; Kao, I.; Pruß, A.; Georgieva, R.; Bäumler, H. Riboflavin: The Health Benefits of a Forgotten Natural Vitamin. Int. J. Mol. Sci. 2020, 21, 950. [Google Scholar] [CrossRef]
- Averianova, L.A.; Balabanova, L.A.; Son, O.M.; Podvolotskaya, A.B.; Tekutyeva, L.A. Production of Vitamin B2 (Riboflavin) by Microorganisms: An Overview. Front. Bioeng. Biotechnol. 2020, 8, 570828. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-Y.; Thakur, K.; Feng, J.-Y.; Cai, J.-S.; Zhang, J.-G.; Hu, F.; Russo, P.; Spano, G.; Wei, Z.-J. Riboflavin-Overproducing Lactobacilli for the Enrichment of Fermented Soymilk: Insights into Improved Nutritional and Functional Attributes. Appl. Microbiol. Biotechnol. 2020, 104, 5759–5772. [Google Scholar] [CrossRef] [PubMed]
- Mahara, F.A.; Nuraida, L.; Lioe, H.N. Folate in Milk Fermented by Lactic Acid Bacteria from Different Food Sources. Prev. Nutr. Food Sci. 2021, 26, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Levit, R.; Savoy de Giori, G.; Moreno de LeBlanc, A.; LeBlanc, J.G. Recent Update on Lactic Acid Bacteria Producing Riboflavin and Folates: Application for Food Fortification and Treatment of Intestinal Inflammation. J. Appl. Microbiol. 2021, 130, 1412–1424. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cai, D.; Yang, M.; Hao, Y.; Zhu, Y.; Chen, Z.; Aziz, T.; Sarwar, A.; Yang, Z. Screening of Folate-Producing Lactic Acid Bacteria and Modulatory Effects of Folate-Biofortified Yogurt on Gut Dysbacteriosis of Folate-Deficient Rats. Food Funct. 2020, 11, 6308–6318. [Google Scholar] [CrossRef] [PubMed]
- Carrizo, S.L.; de Moreno de LeBlanc, A.; LeBlanc, J.G.; Rollán, G.C. Quinoa Pasta Fermented with Lactic Acid Bacteria Prevents Nutritional Deficiencies in Mice. Food Res. Int. 2020, 127, 108735. [Google Scholar] [CrossRef]
- Albano, C.; Silvetti, T.; Brasca, M. Screening of Lactic Acid Bacteria Producing Folate and Their Potential Use as Adjunct Cultures for Cheese Bio-Enrichment. FEMS Microbiol. Lett. 2020, 367, fnaa059. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).