Evaluation of the Bio-Protective Effect of Native Candida Yeasts on Sauvignon Blanc Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeasts
2.2. Grape Juice
2.3. Winemaking Assays
- Inoculation of the bio-protective initiator Candida spp. (C. oleophila or C. boidinii) at 1 × 106 cells/mL, without adding SO2 and with inoculation at 72 h with commercial S. cerevisiae yeast at a dose of 1 × 106 cells/mL.
- Inoculation of the bio-protective initiator Candida spp. (C. oleophila or C. boidinii) at 1 × 107 cells/mL, without adding SO2 and inoculation at 72 h with commercial S. cerevisiae yeast at a dose of 1 × 106 cells/mL.
- Inoculation of the mixed bio-protective initiator C. oleophila/C. boidinii 50:50 at 1 × 107 cells/mL, without adding SO2 and inoculation at 72 h with commercial S. cerevisiae yeast at a dose of 1 × 106 cells/mL.
- Initial spontaneous fermentation was initiated by adding 40 ppm of SO2 and inoculating for 72 h with commercial S. cerevisiae at a dose of 1 × 106 cells/mL.
- Initial spontaneous fermentation without addition of SO2 and inoculation at 72 h with commercial S. cerevisiae yeast at a dose of 1 × 106 cells/mL.
2.3.1. Preparation of Initial Inoculum
2.3.2. Experiment Montage
2.3.3. Fermentation Follow-Up
2.4. Microbiological Analysis
2.4.1. Sample Serial Dilution
2.4.2. Microorganism Media
2.4.3. Sample Incubation and Counting Colonies
- is the sum of colonies counted on a plate.
- is the number of replies, in this case 3.
- is the dilution [d = 1 when the undiluted liquid product (test sample) is retained].
- is the volume of the inoculum placed in each plate, in this case 0.1 mL.
2.5. Chemical Composition Analysis
2.6. Volatile Composition Analysis
2.7. Data Analysis
2.7.1. ANOVA Analysis and Post hoc Tukey Test for Chemical Compounds
2.7.2. PCA for Volatile Compounds
3. Results
3.1. Kinetics of Alcoholic Fermentation (AF)
3.2. Population Dynamics of Deterioration Microorganisms
3.2.1. Evolution of Acetic Acid Bacteria during Fermentation
3.2.2. Evolution of Lactic Acid Bacteria (LAB) during Fermentation
3.2.3. Evolution of Brettanomyces bruxellensis Yeasts during Fermentation
3.3. Chemical Compound Analysis via Biosystem Y15 and HPLC
3.4. Volatile Compound Analysis via GC-MS-SPME
4. Discussion
4.1. Candida spp. Yeasts Produce Antimicrobial Effects in Grape Must
4.2. Final Wine Quality Is Comparable to That Obtained with SO2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Strategy | C. oleophila 106 | C. oleophila 107 | C. boidinii 106 | C. boidinii 107 | Mixed Inoculum | SO2 | Antimicrobial-Free | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day | NS | S | NS | S | NS | S | NS | S | NS | S | NS | S | NS | S |
| Must | 90.14 | 9.86 | 88.00 | 12.00 | 78.95 | 21.05 | 78.95 | 21.05 | 92.31 | 7.69 | 88.00 | 12.00 | 90.14 | 9.86 |
| 1 | 94.74 | 5.26 | 95.24 | 4.76 | 84.21 | 15.79 | 89.66 | 10.34 | 97.78 | 2.22 | 75.86 | 24.14 | 95.83 | 4.17 |
| 3 | 77.14 | 22.86 | 78.69 | 21.31 | 60.61 | 39.39 | 70.00 | 30.00 | 87.50 | 12.50 | 16.67 | 83.33 | 19.23 | 80.77 |
| Final | 6.67 | 93.33 | 7.89 | 92.11 | 9.40 | 90.60 | 4.40 | 95.60 | 11.25 | 88.75 | 3.00 | 97.00 | 12.50 | 87.50 |
References
- Fia, G.; Menghini, S.; Mari, E.; Proserpio, C.; Pagliarini, E.; Granchi, L. Replacement of SO2 with an Unripe Grape Extract and Chitosan during Oak Aging: Case Study of a Sangiovese Wine. Antioxidants 2023, 12, 365. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.; De Freitas, V.; Silva, A.M. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Jagatić, A.M.; Biloš, J.; Kozina, B.; Tomaz, I.; Preiner, D.; Jeromel, A. Effect of Different Reducing Agents on Aromatic Compounds, Antioxidant and Chromatic Properties of Sauvignon Blanc Wine. Foods 2020, 9, 996. [Google Scholar] [CrossRef]
- Vally, H.; Misso, N.L.; Madan, V. Clinical effects of sulfite additives. Clin. Exp. Allergy 2009, 39, 1643–1651. [Google Scholar] [CrossRef]
- JECFA. Safety Evaluation of Certain Food Additives and Contaminants; World Health Organization: Geneva, Switzerland, 2006.
- International Organisation of Vine and Wine (OIV). Molecular sulfur dioxide. In Compendium of International Methods of Must and Wine Analysis; OIV: Paris, France, 2019. [Google Scholar]
- Nieto-Villegas, R.; Bernabéu, R.; Rabadán, A. The impact of chemophobia on wine consumer preferences explored through the case of sulfites. J. Agric. Food Res. 2023, 14, 100692. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, K.; Wang, N.; Xiao, X.; Leng, Y.; Fan, J.; Du, Y.; Wang, S. Sulfur dioxide-free wine with polyphenols promotes lipid metabolism via the Nrf2 pathway and gut microbiota modulation. Food Chem. X 2024, 21, 101079. [Google Scholar] [CrossRef]
- Delso, C.; Berzosa, A.; Sanz, J.; Álvarez, I.; Raso, J. Pulsed electric field processing as an alternative to sulfites (SO2) for controlling saccharomyces cerevisiae involved in the fermentation of Chardonnay white wine. Food Res. Int. 2023, 165, 112525. [Google Scholar] [CrossRef]
- Nogueira, D.P.; Jiménez-Moreno, N.; Esparza, I.; Moler, J.A.; Ferreira-Santos, P.; Sagües, A.; Teixeira, J.A.; Ancín-Azpilicueta, C. Evaluation of grape stems and grape stem extracts for sulfur dioxide replacement during grape wine production. Curr. Res. Food Sci. 2023, 6, 100453. [Google Scholar] [CrossRef]
- Hosseini, A.; Koushesh, M.; Watkins, C.B. Microbial antagonists to biologically control postharvest decay and preserve fruit quality. Crit. Rev. Food Sci. Nutr. 2023, 1–13. [Google Scholar] [CrossRef]
- Alexandre, H.; Puyo, M.; Tourdot-Maréchal, R. Bio-protection in Winemaking. In New Advances in Saccharomyces; Morata, A., Loira, I., González, C., Escott, C., Eds.; IntechOpen: London, UK, 2023. [Google Scholar]
- Leyva, M.; Mounier, J.; Valence, F.; Coton, M.; Thierry, A.; Coton, E. Antifungal microbial agents for food biopreservation—A review. Microorganisms 2017, 5, 37. [Google Scholar] [CrossRef]
- Bauer, M.A.; Kainz, K.; Carmona-Gutierrez, D.; Madeo, F. Microbial wars: Competition in ecological niches and within the microbiome. Microb. Cell 2018, 5, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Di Gianvito, P.; Englezos, V.; Rantsiou, K.; Cocolin, L. Bio-protection strategies in winemaking. Int. J. Food Microbiol. 2022, 364, 109532. [Google Scholar] [CrossRef] [PubMed]
- Escribano-Viana, R.; González-Arenzana, L.; Garijo, P.; Fernández, L.; López, R.; Santamaría, P.; Gutiérrez, A.R. Bio-protective Effect of a Torulaspora delbrueckii/Lachancea thermotolerans-Mixed Inoculum in Red Winemaking. Fermentation 2022, 8, 337. [Google Scholar] [CrossRef]
- Simonin, S.; Alexandre, H.; Nikolantonaki, M.; Coelho, C.; Tourdot-Maréchal, R. Inoculation of Torulaspora delbrueckii as a bio-protection agent in winemaking. Food Res. Int. 2018, 107, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Simonin, S.; Roullier-Gall, C.; Ballester, J.; Schmitt-Kopplin, P.; Quintanilla-Casas, B.; Vichi, S.; Peyron, D.; Alexandre, H.; Tourdot-Maréchal, R. Bio-protection as an alternative to sulfites: Impact on chemical and microbial characteristics of red wines. Front. Microbiol. 2020, 11, 1308. [Google Scholar] [CrossRef] [PubMed]
- Windholtz, S.; Redon, P.; Lacampagne, S.; Farris, L.; Lytra, G.; Cameleyre, M.; Barbe, J.C.; Coulon, J.; Thibon, C.; Masneuf-Pomarede, I. Non-Saccharomyces yeasts as bio-protection in the composition of red wine and in the reduction of sulfur dioxide. LWT 2021, 149, 111781. [Google Scholar] [CrossRef]
- Windholtz, S.; Dutilh, L.; Lucas, M.; Maupeu, J.; Vallet-Courbin, A.; Farris, L.; Coulon, J.; Masneuf-Pomarède, I. Population dynamics and yeast diversity in early winemaking stages without sulfites revealed by three complementary approaches. Appl. Sci. 2021, 11, 2494. [Google Scholar] [CrossRef]
- Windholtz, S.; Nioi, C.; Coulon, J.; Masneuf-Pomarede, I. Bio-protection by non-Saccharomyces yeasts in oenology: Evaluation of O2 consumption and impact on acetic acid bacteria. Int. J. Food Microbiol. 2023, 405, 110338. [Google Scholar] [CrossRef]
- Lebleux, M.; Alexandre, H.; Romanet, R.; Ballester, J.; David-Vaizant, V.; Adrian, M.; Tourdot-Maréchal, R.; Rouiller-Gall, C. Must protection, sulfites versus bio-protection: A metabolomic study. Food Res. Int. 2023, 173, 113383. [Google Scholar] [CrossRef]
- Rubio-Bretón, P.; Gonzalo-Diago, A.; Iribarren, M.; Garde-Cerdán, T.; Pérez-Álvarez, E.P. Bio-protection as a tool to free additives winemaking: Effect on sensorial, anthocyanic and aromatic profile of young red wines. LWT 2018, 98, 458–464. [Google Scholar] [CrossRef]
- Puyo, M.; Simonin, S.; Bach, B.; Klein, G.; Alexandre, H.; Tourdot-Maréchal, R. Bio-protection in oenology by Metschnikowia pulcherrima: From field results to scientific inquiry. Front. Microbiol. 2023, 14, 1252973. [Google Scholar] [CrossRef]
- Kántor, A.; Hutková, J.; Petrová, J.; Hleba, L.; Kacániová, M. Antimicrobial activity of pulcherrimin pigment produced by Metschnikowia pulcherrima against various yeast species. J. Microbiol. Biotechnol. Food Sci. 2015, 5, 282. [Google Scholar] [CrossRef]
- Villalba, M.L.; Sáez, J.S.; Del Monaco, S.; Lopes, C.A.; Sangorrín, M.P. TdKT, a new killer toxin produced by Torulaspora delbrueckii effective against wine spoilage yeasts. Int. J. Food Microbiol. 2016, 217, 94–100. [Google Scholar] [CrossRef]
- Shekhawat, K.; Bauer, F.F.; Setati, M.E. Impact of oxygenation on the performance of three non-Saccharomyces yeasts in co-fermentation with Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 2479–2491. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, R.; Xiong, B. Management of postharvest diseases of kiwifruit with a combination of the biocontrol yeast Candida oleophila and an oligogalacturonide. Biol. Control 2021, 156, 104549. [Google Scholar] [CrossRef]
- Ballet, N.; Souche, J.L.; Vandekerckove, P. Efficacy of Candida oleophila, strain O, in preventing postharvest diseases of fruits. In Proceedings of the III International Symposium on Postharvest Pathology: Using Science to Increase Food Availability, Bari, Italy, 7 November 2015. [Google Scholar]
- Droby, S.; Cohen, L.; Daus, A.; Weiss, B.; Horev, B.; Chalutz, E.; Katz, H.; Keren-Tzur, M.; Shachnai, A. Commercial testing of Aspire: A yeast preparation for the biological control of postharvest decay of citrus. Biol. Control 1998, 12, 97–101. [Google Scholar] [CrossRef]
- Sui, Y.; Wisniewski, M.; Droby, S.; Piombo, E.; Wu, X.; Yue, J. Genome sequence, assembly, and characterization of the antagonistic yeast Candida oleophila used as a biocontrol agent against post-harvest diseases. Front. Microbiol. 2020, 11, 295. [Google Scholar] [CrossRef]
- Bonatsou, S.; Panagou, E.Z. Fermentation of cv. kalamata natural black olives with potential multifunctional yeast starters. Foods 2022, 11, 3106. [Google Scholar] [CrossRef] [PubMed]
- Benavides, S.; Franco, W.; Ceppi De Lecco, C.; Durán, A.; Urtubia, A. Evaluation of Indigenous Candida oleophila and Candida boidinii in Monoculture and Sequential Fermentations: Impact on Ethanol Reduction and Chemical Profile in Chilean Sauvignon Blanc Wines. J. Fungi 2022, 8, 259. [Google Scholar] [CrossRef]
- Franco, W.; Benavides, S.; Valencia, P.; Ramírez, C.; Urtubia, A. Native Yeasts and Lactic Acid Bacteria Isolated from Spontaneous Fermentation of Seven Grape Cultivars from the Maule Region (Chile). Foods 2021, 10, 1737. [Google Scholar] [CrossRef]
- International Organization of Vine and Wine (OIV). Microbiological Analysis of Wines and Musts-Detection, Differentiation and Counting of Microorganisms; OIV: Paris, France, 2009. [Google Scholar]
- Perestrelo, R.; Barros, A.S.; Câmara, J.S.; Rocha, S.M. In-depth search focused on furans, lactones, volatile phenols, and acetals as potential age markers of Madeira wines by comprehensive two-dimensional gas chromatography with time-of-flight mass spectrometry combined with solid phase microextraction. J. Agric. Food Chem. 2011, 59, 3186–3204. [Google Scholar] [CrossRef] [PubMed]
- Gujar, A.; Anderson, T.; Cavagnino, D.; Patel, A. Comparative analysis of mass spectral matching for confident compound identification using the Advanced Electron Ionization source for GC-MS. Thermoscientific 2018, 10598, 1–7. [Google Scholar]
- Bosqueiro, A.; Bizarria, R.; Rosa-Magri, M.M. Biocontrol of post-harvest tomato rot caused by Alternaria arborescens using Torulaspora indica. Biocontrol Sci. Technol. 2023, 33, 115–132. [Google Scholar] [CrossRef]
- Xynas, B.; Barnes, C. Yeast or water: Producing wine with lower alcohol levels in a warming climate: A review. J. Sci. Food Agric. 2023, 103, 3249–3260. [Google Scholar] [CrossRef] [PubMed]
- König, H.; Fröhlich, J. Lactic acid bacteria. In Biology of Microorganisms on Grapes, in Must and in Wine; König, H., Unden, G., Fröhlich, J., Eds.; Springer: Cham, Germany, 2017; pp. 3–41. [Google Scholar]
- Balmaseda, A.; Bordons, A.; Reguant, C.; Bautista-Gallego, J. Non-Saccharomyces in wine: Effect upon Oenococcus oeni and malolactic fermentation. Front. Microbiol. 2018, 9, 352041. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sánchez, I.; Suáldea, B.B.; Moreno-Arribas, M.V. Malolactic Fermentation. Red Wine Technology; Academic Press: Cambridge, MA, USA, 2019; pp. 85–98. [Google Scholar] [CrossRef]
- Bartowsky, E.J. Oenococcus oeni and malolactic fermentation–moving into the molecular arena. Aust. J. Grape Wine Res. 2005, 11, 174–187. [Google Scholar] [CrossRef]
- International Organization of Vine and Wine. Maximum acceptable Limits. In International Code of Oenological Practices; OIV: Paris, France, 2015. [Google Scholar]
- Lindsay, M.A.; Granucci, N.; Greenwood, D.R.; Villas-Boas, S.G. Fermentative Production of Volatile Metabolites Using Brettanomyces bruxellensis from Fruit and Vegetable By-Products. Fermentation 2022, 8, 457. [Google Scholar] [CrossRef]
- Ghosh, S.; Kebaara, B.W.; Atkin, A.L.; Nickerson, K.W. Regulation of aromatic alcohol production in Candida albicans. Appl. Environ. Microbiol. 2008, 74, 7211–7218. [Google Scholar] [CrossRef]
- Fujii, T.; Kobayashi, O.; Yoshimoto, H.; Furukawa, S.; Tamai, Y. Effect of aeration and unsaturated fatty acids on expression of the Saccharomyces cerevisiae alcohol acetyltransferase gene. Appl. Environ. Microbiol. 1997, 63, 910–915. [Google Scholar] [CrossRef]
- Wang, M.; Wang, J.; Chen, J.; Philipp, C.; Zhao, X.; Wang, J.; Liu, Y.; Suo, R. Effect of commercial yeast starter cultures on Cabernet Sauvignon wine aroma compounds and microbiota. Foods 2022, 11, 1725. [Google Scholar] [CrossRef]
- Coetzee, C.; Johannes du Toit, W. A comprehensive review on Sauvignon blanc aroma with a focus on certain positive volatile thiols. Food Res. Int. 2012, 45, 287–298. [Google Scholar] [CrossRef]
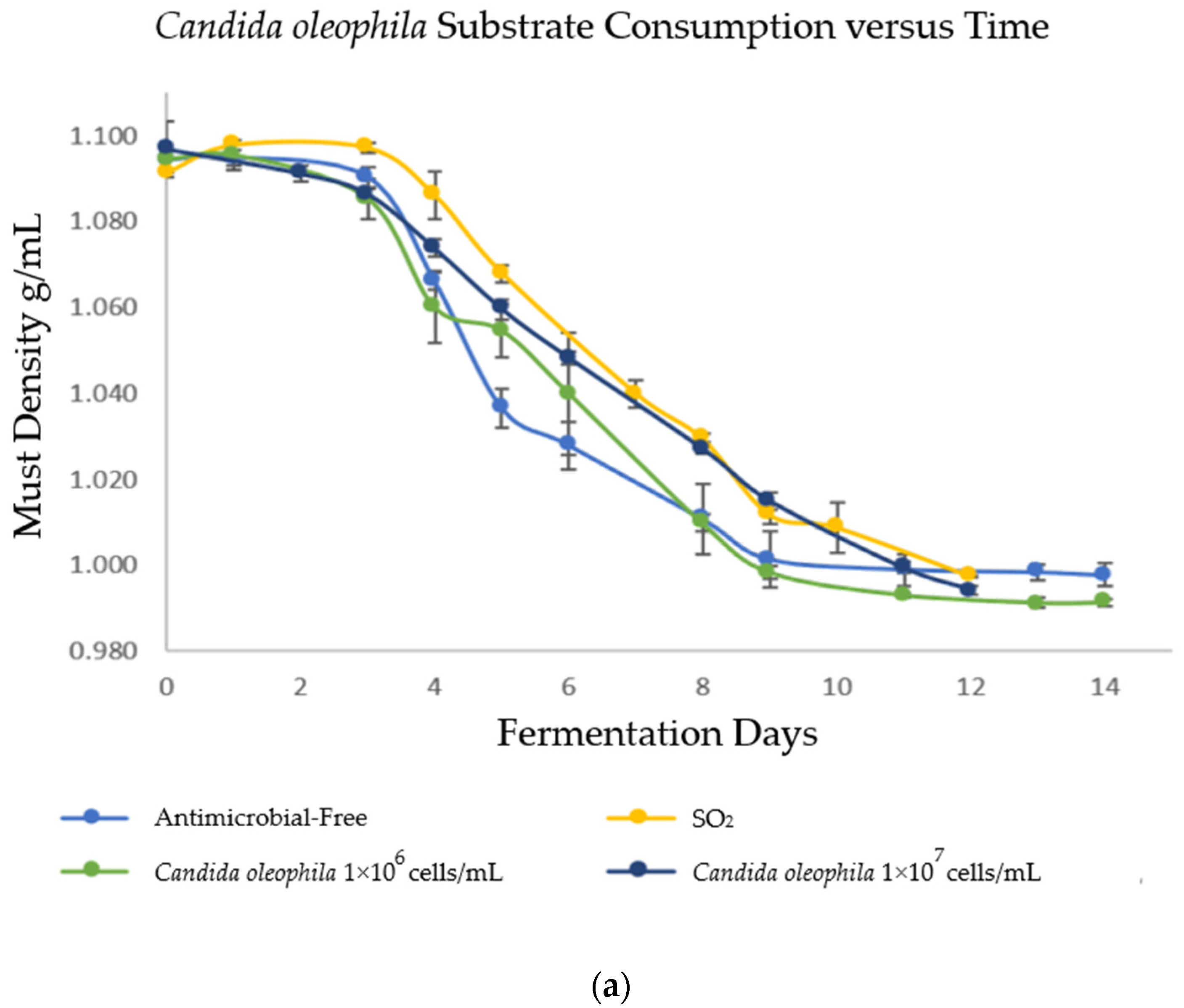
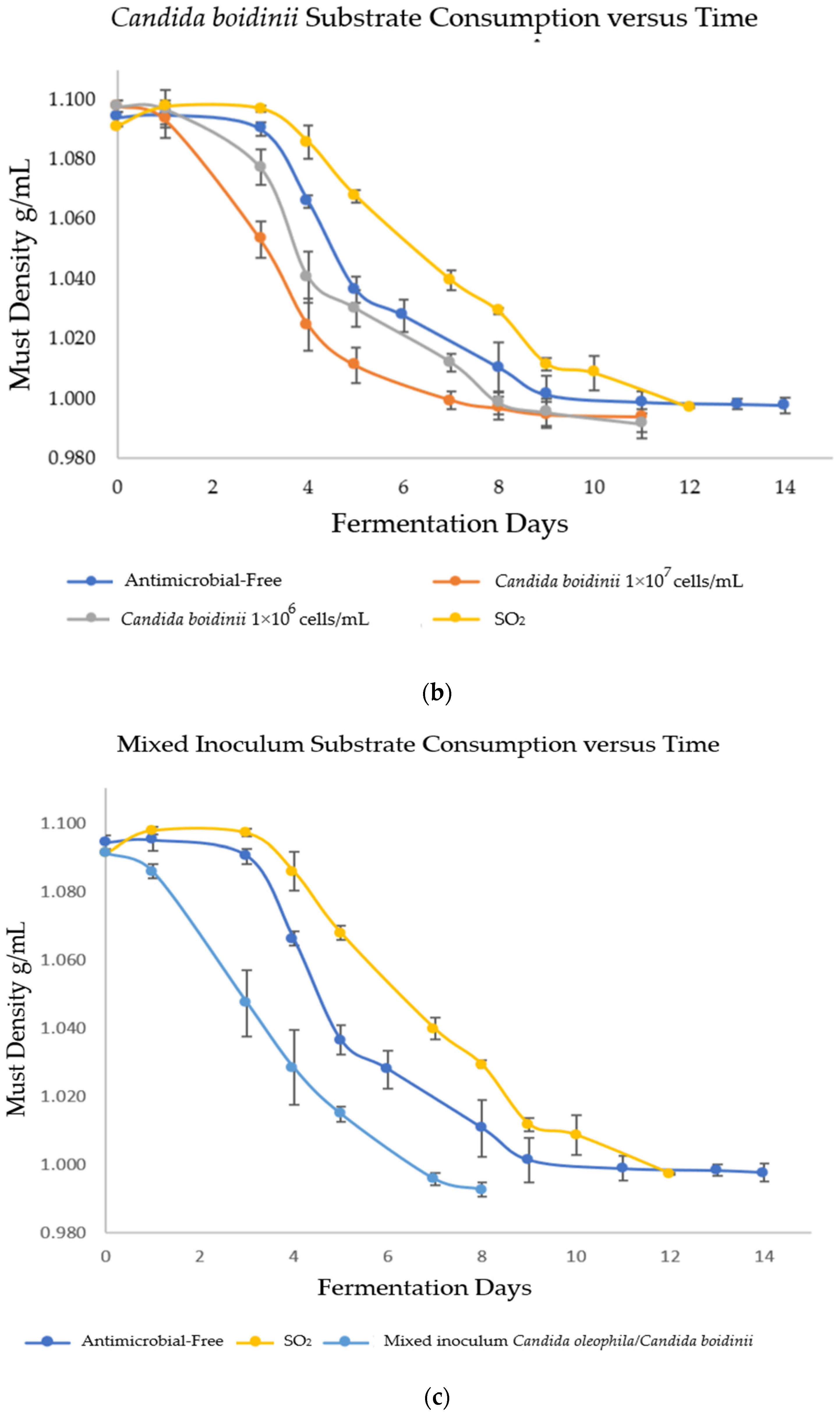
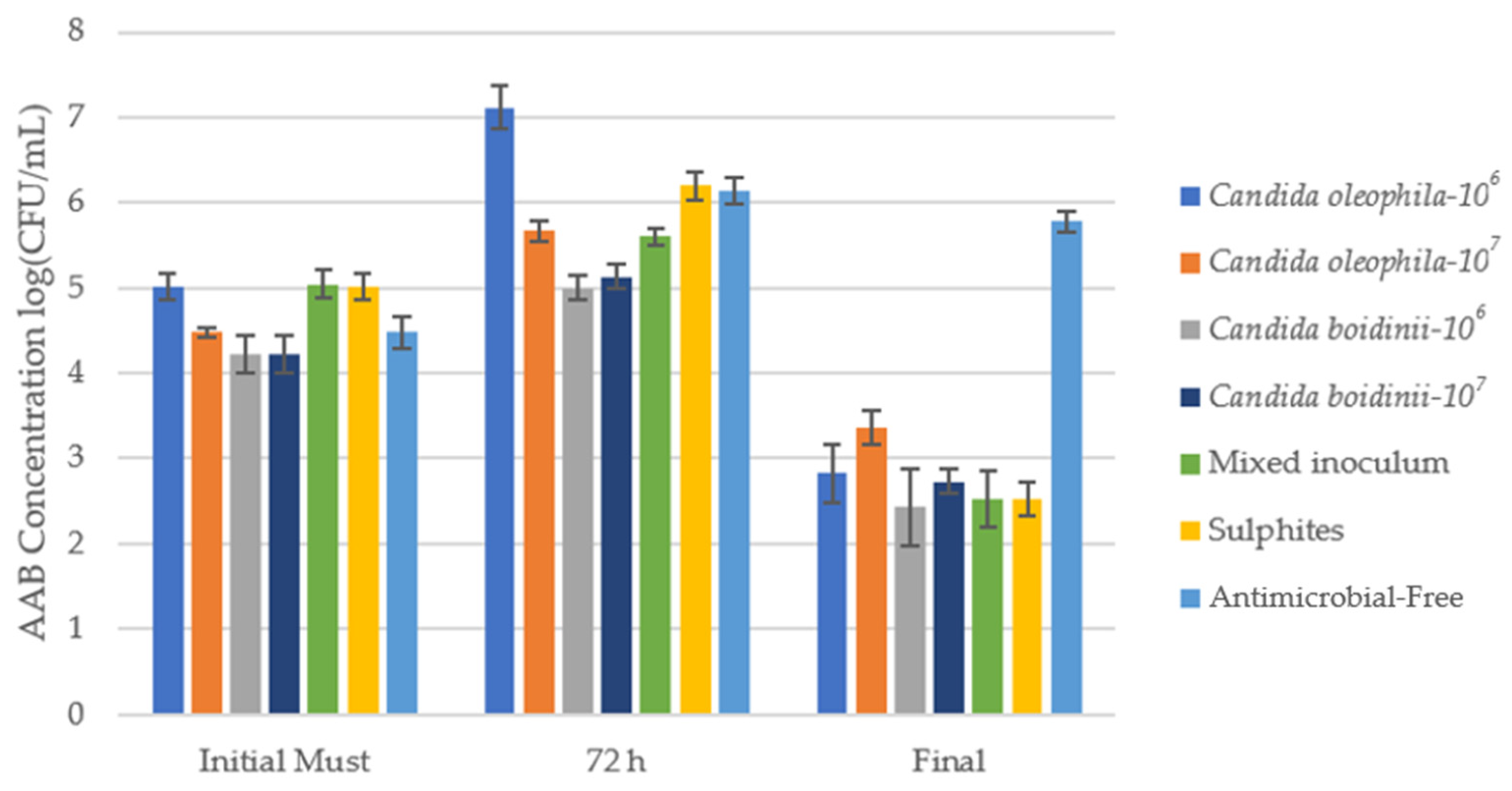
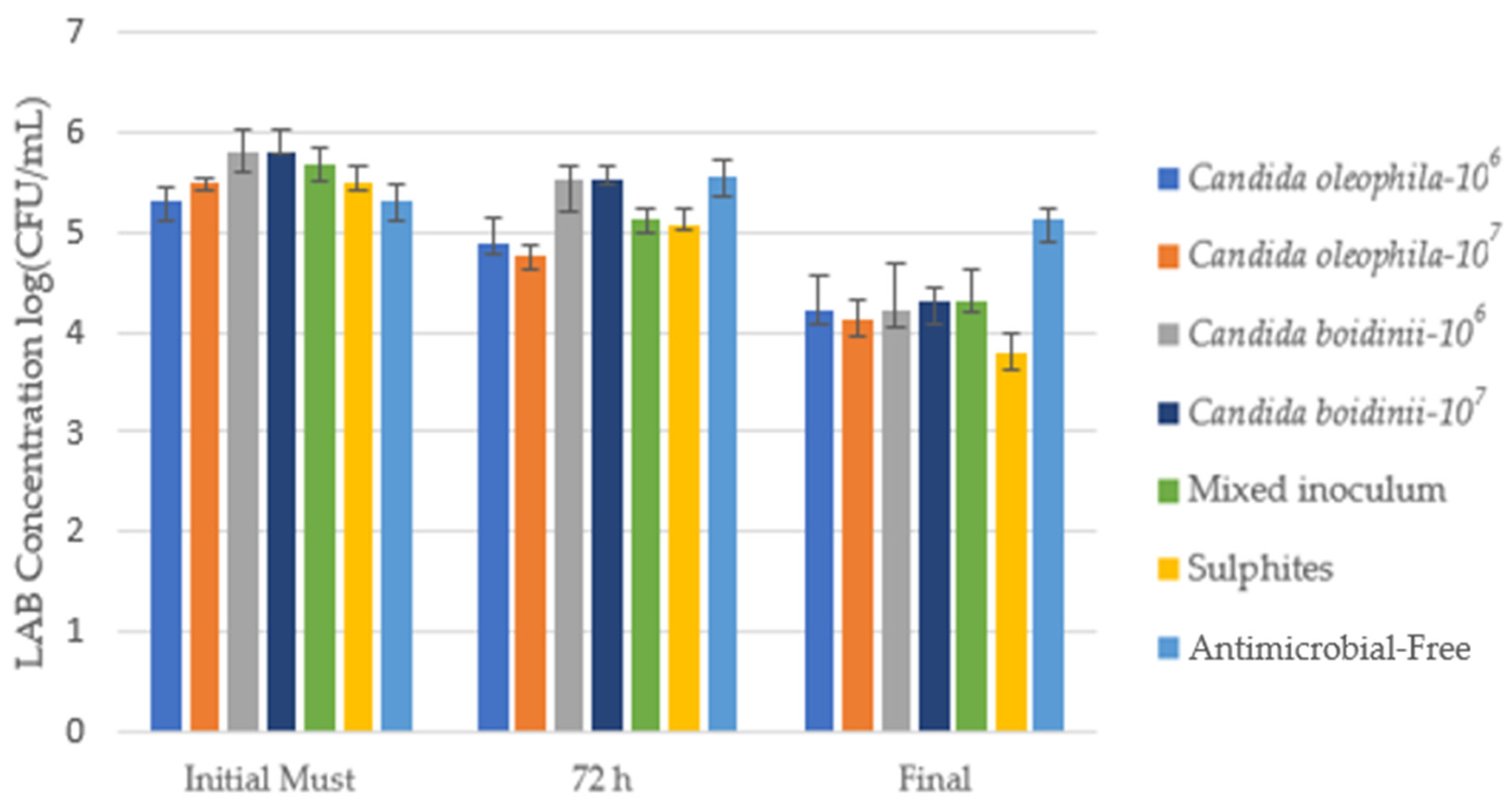
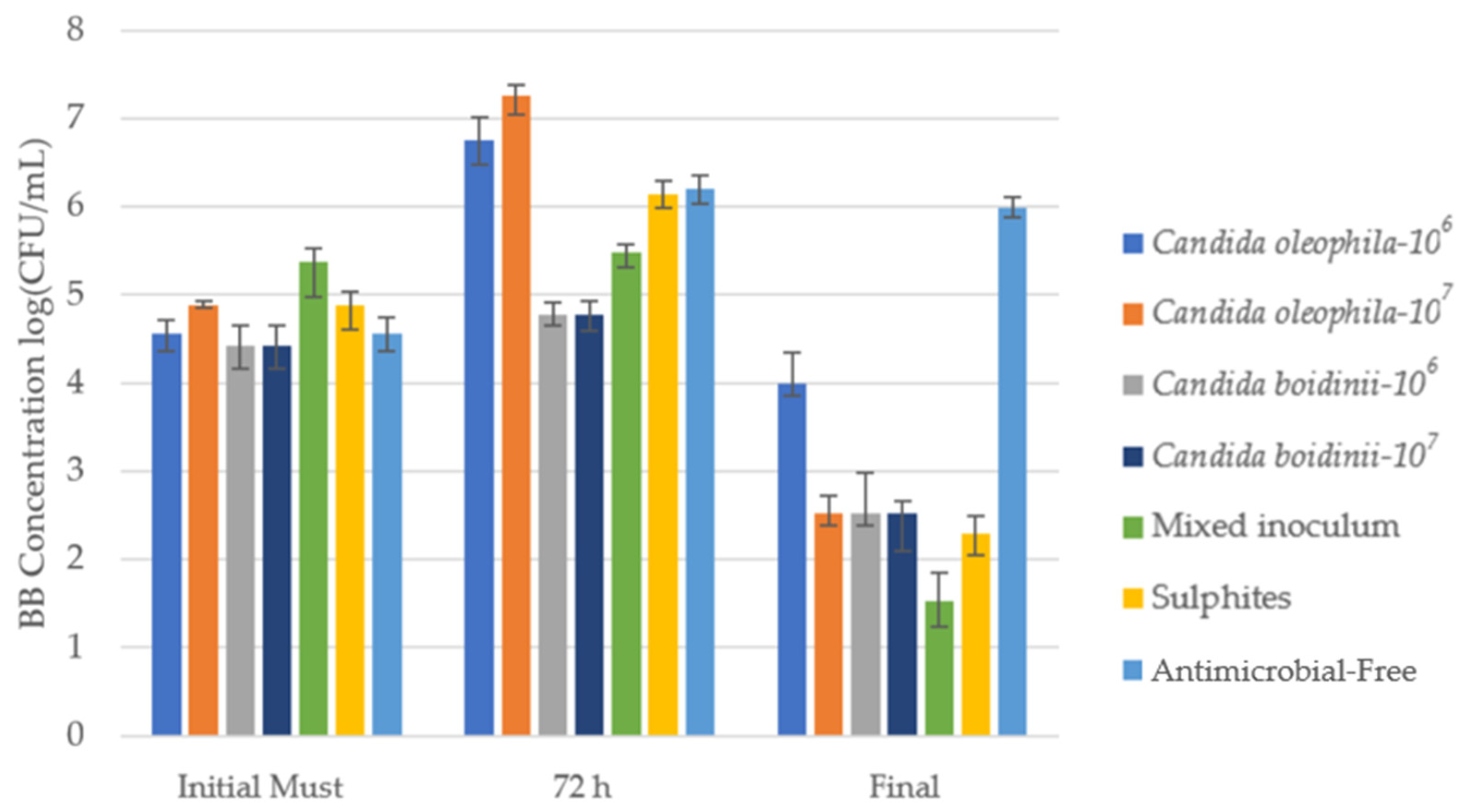
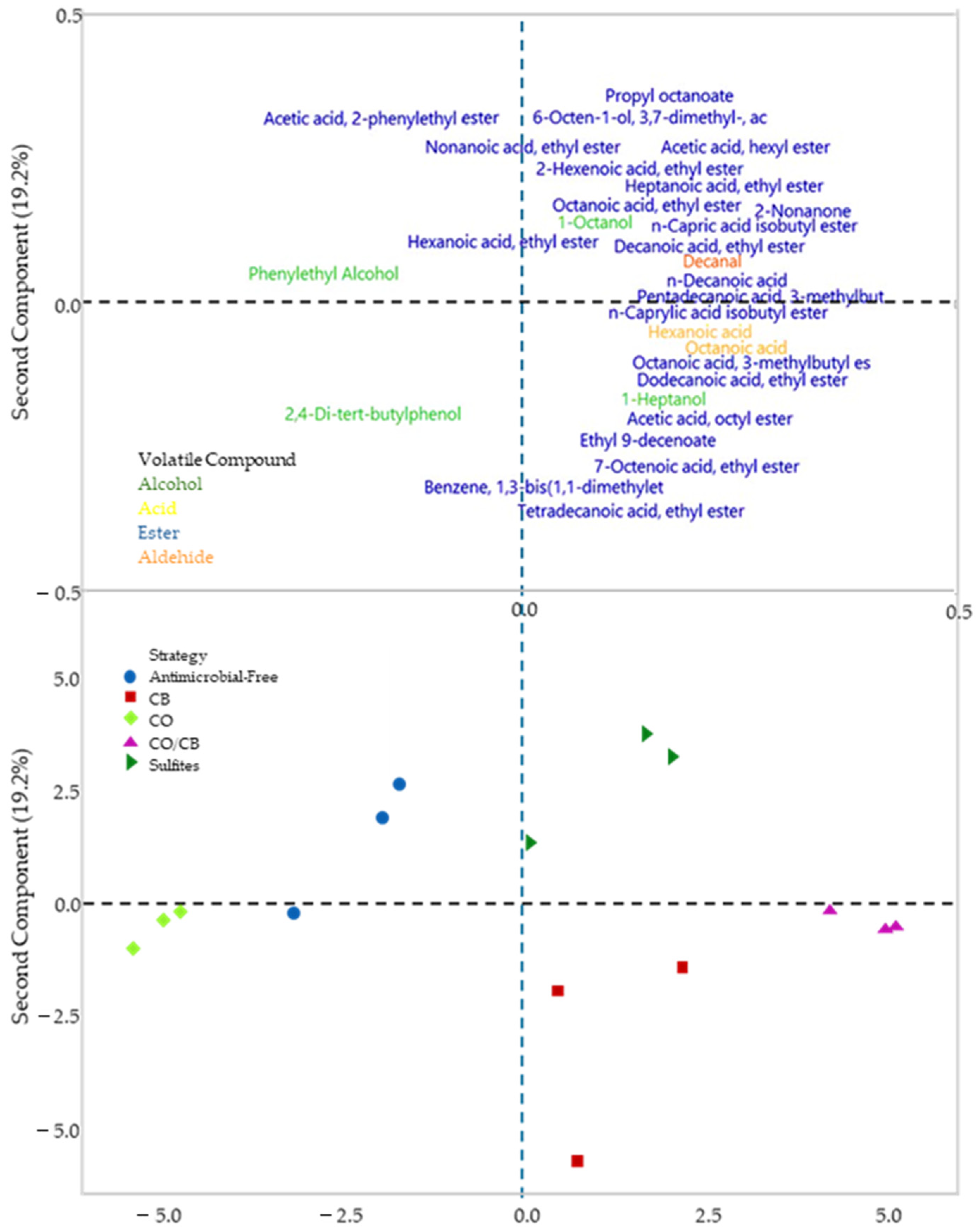
| Strategy | Ranking * | Initial Must | Day 1 | Day 3 | Final |
|---|---|---|---|---|---|
| Candida oleophila-106 | 7° | 1.03 × 105 | 8.33 × 105 b | 1.30 × 107 a | 6.67 × 102 b |
| Candida oleophila-107 | 3° | 3.00 × 104 | 4.00 × 105 b | 4.67 × 105 c | 2.33 × 103 b |
| Candida boidinii-106 | 1° | 1.67 × 104 | 6.33 × 104 de | 1.00 × 105 e | 2.67 × 102 b |
| Candida boidinii-107 | 1° | 1.67× 104 | 3.33 × 104 e | 1.37 × 105 de | 5.33 × 102 b |
| SO2 | 5° | 1.03 × 105 | 1.60 × 105 c | 1.57 × 106 b | 3.33 × 102 b |
| Antimicrobial-free | 4° | 3.00 × 104 | 1.10 × 105 a | 1.37 × 106 b | 6.00 × 105 a |
| Mixed inoculum | 2° | 1.10 × 105 | 1.33 × 105 cd | 4.00 × 105 cd | 3.33 × 102 b |
| Strategy | Ranking * | Initial Must | Day 1 | Day 3 | Final |
|---|---|---|---|---|---|
| Candida oleophila-106 | 2° | 2.00 × 105 | 2.00 × 105 a | 7.67 × 104 bc | 1.67 × 104 b |
| Candida oleophila-107 | 1° | 3.13 × 105 | 7.33 × 104 b | 5.67 × 104 c | 1.33 × 104 ab |
| Candida boidinii-106 | 5° | 6.33 × 105 | 1.33 × 105 ab | 3.33 × 105 a | 1.67 × 104 b |
| Candida boidinii-107 | 6° | 6.33 × 105 | 1.90 × 105 ab | 3.35 × 105 a | 1.97 × 104 b |
| SO2 | 3° | 3.13 × 105 | 2.00 × 105 a | 1.17 × 105 b | 6.00 × 103 a |
| Antimicrobial-free | 7° | 2.00 × 105 | 2.53 × 105 a | 3.67 × 105 a | 1.33 × 105 c |
| Mixed inoculum | 4° | 4.67 × 105 | 3.00 × 105 a | 1.33 × 105 b | 2.00 × 104 b |
| Strategy | Ranking * | Initial Must | Day 1 | Day 3 | Final |
|---|---|---|---|---|---|
| Candida oleophila-106 | 5° | 3.67 × 104 | 1.03 × 106 a | 5.67 × 106 b | 1.00 × 104 b |
| Candida oleophila-107 | 6° | 7.67 × 104 | 8.67 × 105 a | 1.80 × 107 a | 3.33 × 102 d |
| Candida boidinii-106 | 3° | 2.67 × 104 | 6.67 × 104 c | 1.17 × 106 c | 1.33 × 103 c |
| Candida boidinii-107 | 1° | 2.67 × 104 | 5.67 × 104 c | 6.00 × 104 e | 3.33 × 102 d |
| SO2 | 3° | 7.67 × 104 | 2.90 × 105 b | 1.37 × 106 c | 2.00 × 102 d |
| Antimicrobial-free | 4° | 3.67 × 104 | 1.07 × 106 a | 1.57 × 106 c | 9.67 × 105 a |
| Mixed inoculum | 2° | 2.33 × 105 | 1.20 × 105 c | 3.00 × 105 d | 3.33 × 101 e |
| Metabolite/Strategy | SO2 | CO/CB | CO | CB | Antimicrobial-Free | p Value |
|---|---|---|---|---|---|---|
| L-malic acid g/L | 2.75 ± 0.02 a | 2.72 ± 0.03 a | 2.63 ± 0.20 a | 2.59 ± 0.21 a | 2.56 ± 0.17 a | 0.654 |
| Ethanol % v/v | 11.98 ± 0.11 b | 11.37 ± 0.07 c | 11.70 ± 0.15 bc | 12.78 ± 0.15 a | 11.97 ± 0.14 b | 0.000 |
| Acetic acid g/L | 0 ± 0 c | 0.06 ± 0.01 b | 0.27 ± 0.03 a | 0.04 ± 0.01 bc | 0.23 ± 0.01 a | 0.000 |
| Glycerol g/L | 8.64 ± 0.47 a | 7.46 ± 0.29 a | 8.16 ± 1.07 a | 8.46 ± 1.33 a | 7.56 ± 0.47 a | 0.551 |
| L-lactic acid g/L | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.01 ± 0.01 a | 0 ± 0 a | 0.01 ± 0.01 a | 0.231 |
| Tartaric acid g/L | 1.74 ± 0.03 ab | 1.83 ± 0.10 a | 1.67 ± 0.02 ab | 1.61 ± 0.04 b | 1.33 ± 0.01 c | 0.000 |
| Residual sugar g/L | 0.03 ± 0.02 a | 0.02 ± 0.01 a | 0.02 ± 0.01 a | 0.03 ± 0.004 a | 0.02 ± 0.03 a | 0.972 |
| Volatile Compounds | Aroma | CO (%) | CB (%) | CO/CB (%) | Antimicrobial-Free (%) | SO2 (%) |
|---|---|---|---|---|---|---|
| Acids | ||||||
| Hexanoic acid | Sour/greasy/sweet | 0.00 ± 0.00 a | 0.45 ± 0.09 a | 0.51 ± 0.16 a | 0.00 ± 0.00 a | 0.37 ± 0.43 a |
| Octanoic acid | Fat/wax/rancid/cheese | 0.00 ± 0.00 b | 0.90 ± 0.78 b | 2.80 ± 0.16 a | 0.26 ± 0.04 b | 0.86 ± 0.08 b |
| Alcohols | ||||||
| 1-Heptanol | Greasy/pungent/woody/oily | 0.00 ± 0.00 a | 0.06 ± 0.10 a | 0.22 ± 0.19 a | 0.00 ± 0.00 a | 0.00 ± 0.00 a |
| 1-Octanol | Fatty/fungus/pink/green | 0.00 ± 0.00 a | 0.00 ± 0.00 a | 0.03 ± 0.03 a | 0.03 ± 0.05 a | 0.02 ± 0.03 a |
| 2,4-Di-tert-butylphenol | Herbs/green | 0.11 ± 0.03 a | 0.08 ± 0.07 a | 0.04 ± 0.01 a | 0.06 ± 0.01 a | 0.05 ± 0.02 a |
| Phenylethyl Alcohol | Floral/pink/honey | 21.20 ± 2.33 a | 2.50 ± 0.22 c | 3.18 ± 0.18 bc | 6.00 ± 0.47 b | 5.95 ± 1.39 b |
| Aldehyde | ||||||
| Decanal | Sweet/citrus/waxy | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.09 ± 0.01 a | 0.00 ± 0.00 b | 0.03 ± 0.02 b |
| Esters | ||||||
| 2-Hexenoic acid, ethyl ester | Rum/green/sweet | 0.03 ± 0.05 a | 0.06 ± 0.00 a | 0.10 ± 0.00 a | 0.09 ± 0.08 a | 0.08 ± 0.07 a |
| 6-Octen-1-ol, 3,7-dimethyl-, acetate | Floral/green/pink/citrus | 0.00 ± 0.00 b | 0.01 ± 0.01 b | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.06 ± 0.02 a |
| 7-Octenoic acid, ethyl ester | Fruity | 0.00 ± 0.00 b | 0.34 ± 0.07 a | 0.30 ± 0.01 a | 0.00 ± 0.00 b | 0.03 ± 0.03 b |
| Acetic acid, 2-phenylethyl ester | Sweet/honey/pink | 1.21 ± 0.20 a | 0.73 ± 0.16 b | 0.62 ± 0.02 b | 1.26 ± 0.13 a | 1.55 ± 0.19 a |
| Acetic acid, hexyl ester | Green apple/pear/banana/sweet | 1.08 ± 0.33 b | 3.18 ± 0.16 a | 4.43 ± 0.08 a | 4.56 ± 1.06 a | 3.88 ± 1.17 a |
| Acetic acid, octyl ester | Fruity/waxy/mushroom | 0.00 ± 0.00 b | 0.05 ± 0.02 a | 0.07 ± 0.00 a | 0.00 ± 0.00 b | 0.00 ± 0.00 b |
| Decanoic acid, ethyl ester | Sweet/waxy/creamy/floral | 2.43 ± 0.26 c | 12.49 ± 4.32 ab | 12.97 ± 0.79 ab | 8.06 ± 0.51 bc | 16.86 ± 2.41 a |
| Dodecanoic acid, ethyl ester | Sweet/waxy/creamy | 0.25 ± 0.06 c | 3.90 ± 0.46 a | 4.71 ± 0.42 a | 0.80 ± 0.05 c | 2.43 ± 0.38 b |
| Ethyl 9-decenoate | Fruity/fatty | 0.35 ± 0.07 e | 13.58 ± 0.75 a | 6.14 ± 0.19 b | 1.57 ± 0.27 d | 2.72 ± 0.44 c |
| Heptanoic acid, ethyl ester | Fruity/pineapple/banana | 0.00 ± 0.00 b | 0.01 ± 0.21 b | 0.07 ± 0.00 a | 0.02 ± 0.03 ab | 0.05 ± 0.02 ab |
| Hexanoic acid, ethyl ester | Sweet/fruity/pineapple/waxy/banana | 23.60 ± 4.59 a | 14.96 ± 1.52 a | 21.95 ± 0.47 a | 16.90 ± 3.88 a | 16.73 ± 5.12 a |
| n-Capric acid isobutyl ester | Oily/sweet/fermented/cognac | 0.00 ± 0.00 c | 0.01 ± 0.01 bc | 0.04 ± 0.01 ab | 0.00 ± 0.00 c | 0.05 ± 0.02 a |
| n-Caprylic acid isobutyl ester | Green fruity/oily/floral | 0.00 ± 0.00 b | 0.04 ± 0.03 ab | 0.05 ± 0.00 a | 0.00 ± 0.00 b | 0.05 ± 0.00 a |
| n-Decanoic acid | Waxy/fruity/rancid | 0.00 ± 0.00 b | 0.03 ± 0.01 b | 0.13 ± 0.02 a | 0.00 ± 0.00 b | 0.06 ± 0.05 b |
| Nonanoic acid, ethyl ester | Waxy/fruity/rose/wine | 0.05 ± 0.05 a | 0.06 ± 0.02 a | 0.10 ± 0.02 a | 0.22 ± 0.19 a | 0.10 ± 0.03 a |
| Octanoic acid, 3-methylbutyl ester | Fruity green/pineapple/coconut/sweet | 0.05 ± 0.05 c | 0.21 ± 0.02 a | 0.26 ± 0.03 a | 0.08 ± 0.01 b | 0.13 ± 0.03 b |
| Octanoic acid, ethyl ester | Pineapple, floral, strawberry | 12.96 ± 5.28 b | 30.76 ± 4.91 a | 33.06 ± 1.28 a | 33.67 ± 4.43 a | 37.20 ± 6.26 a |
| Pentadecanoic acid, 3-methylbutyl ester | Waxy/banana/cognac | 0.00 ± 0.00 c | 0.08 ± 0.03 b | 0.16 ± 0.02 a | 0.00 ± 0.00 c | 0.12 ± 0.02 ab |
| Propyl octanoate | Sweet/violet/waxy | 0.00 ± 0.00 b | 0.02 ± 0.02 ab | 0.04 ± 0.00 ab | 0.03 ± 0.03 ab | 0.05 ± 0.01 a |
| Tetradecanoic acid, ethyl ester | Waxy/floral/violet/sweet | 0.00 ± 0.00 b | 0.04 ± 0.03 ab | 0.03 ± 0.01 ab | 0.00 ± 0.00 c | 0.01 ± 0.01 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veloso, C.; Mery-Araya, C.; Durán, A.; Urtubia, A. Evaluation of the Bio-Protective Effect of Native Candida Yeasts on Sauvignon Blanc Wines. Fermentation 2024, 10, 223. https://doi.org/10.3390/fermentation10040223
Veloso C, Mery-Araya C, Durán A, Urtubia A. Evaluation of the Bio-Protective Effect of Native Candida Yeasts on Sauvignon Blanc Wines. Fermentation. 2024; 10(4):223. https://doi.org/10.3390/fermentation10040223
Chicago/Turabian StyleVeloso, Camila, Camila Mery-Araya, Angelica Durán, and Alejandra Urtubia. 2024. "Evaluation of the Bio-Protective Effect of Native Candida Yeasts on Sauvignon Blanc Wines" Fermentation 10, no. 4: 223. https://doi.org/10.3390/fermentation10040223
APA StyleVeloso, C., Mery-Araya, C., Durán, A., & Urtubia, A. (2024). Evaluation of the Bio-Protective Effect of Native Candida Yeasts on Sauvignon Blanc Wines. Fermentation, 10(4), 223. https://doi.org/10.3390/fermentation10040223







