Biohydrogen, Volatile Fatty Acids, and Biomethane from Mezcal Vinasses—A Dark Fermentation Process Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Vinasses
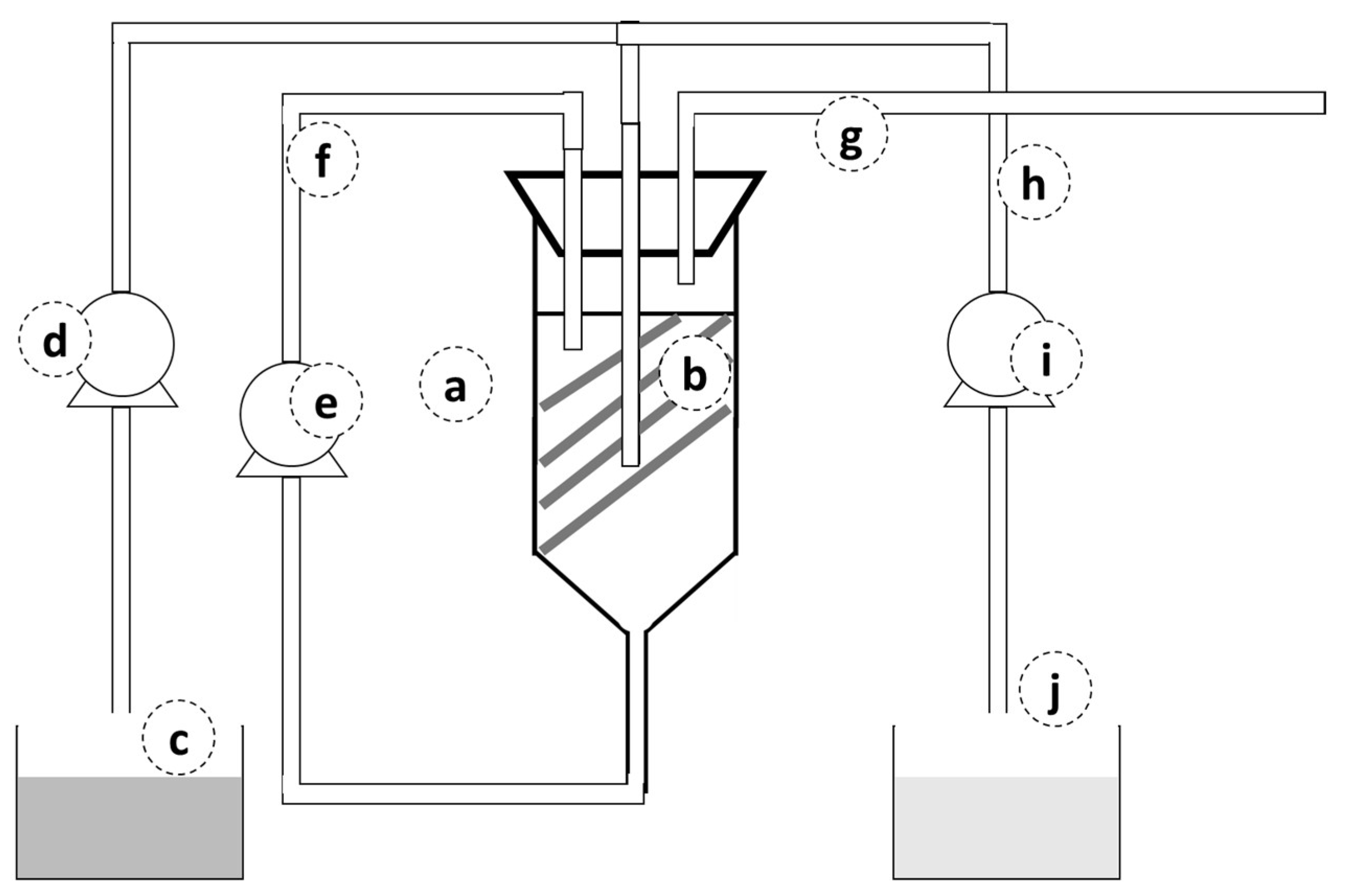
2.2. Experimental Set-Up
2.3. Effect of Reaction Time and Organic Load on a Dark Fermentation Process with Suspended Biomass
2.4. Analytical Tests and Statistical Analysis
3. Results and Discussion
3.1. Influent Characterization
3.2. Effect of Alkali Addition to the Influent
3.3. Effect of Reaction Time and Organic Load in a Dark Fermentation Process
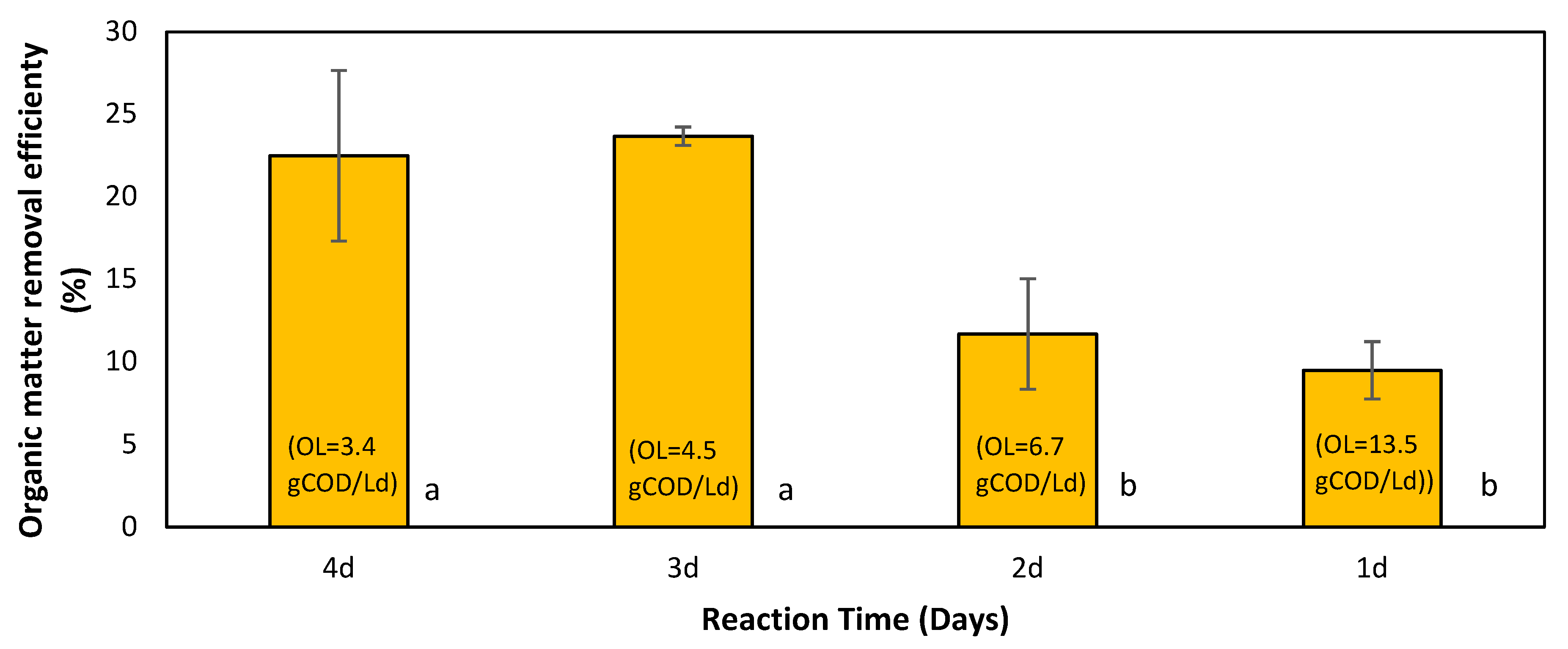
3.3.1. Effect on the Organic Matter Removal (COD)
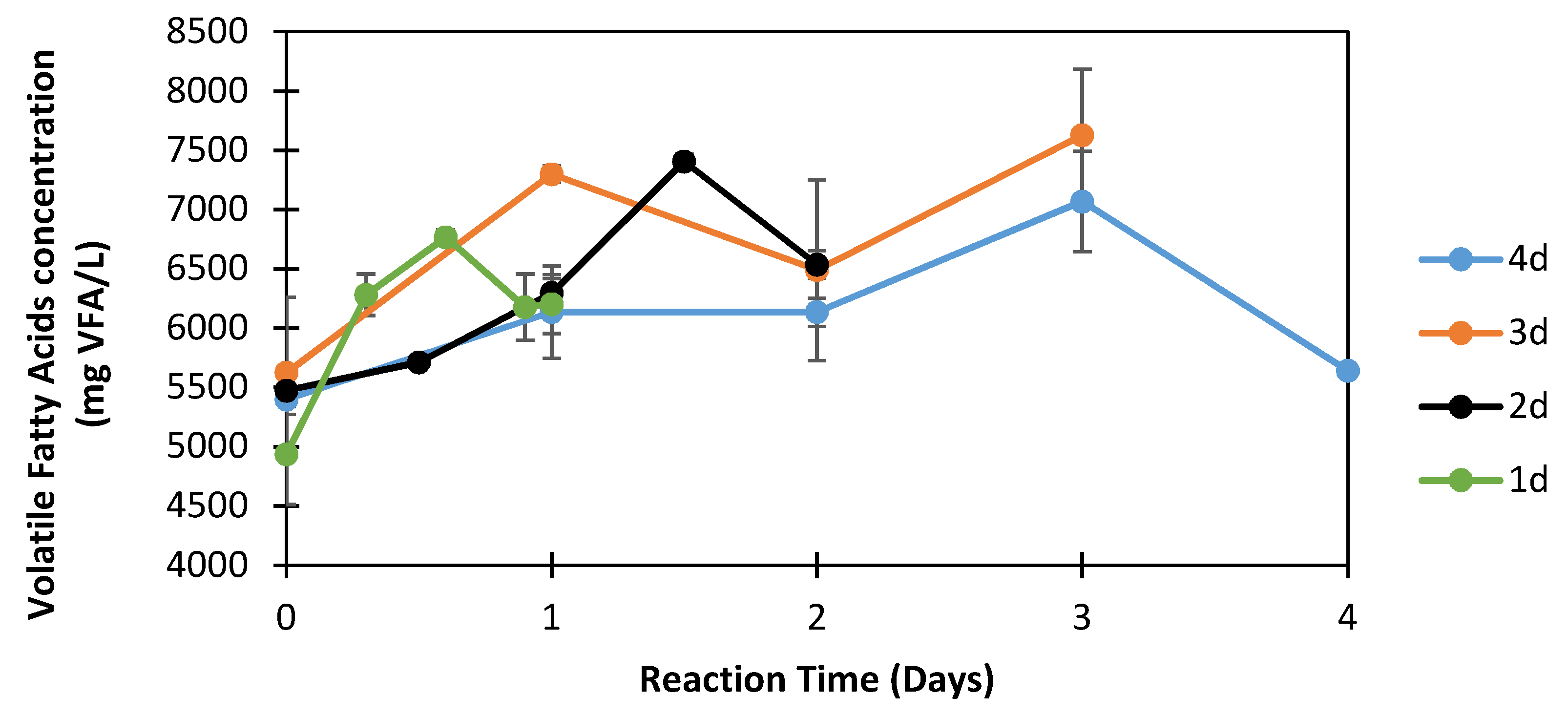
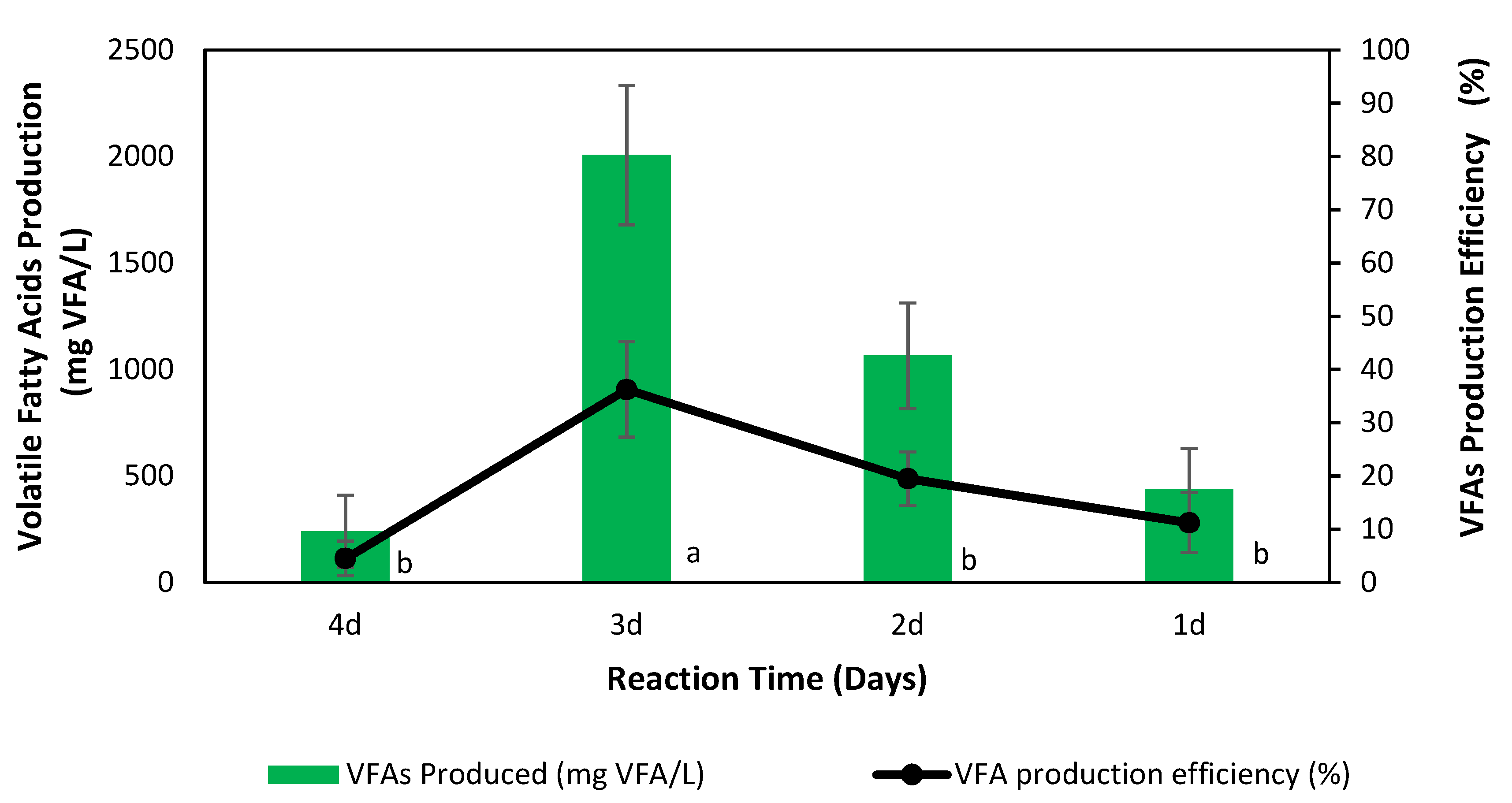
3.3.2. Effect of Reaction Time on the Production of Volatile Fatty Acids
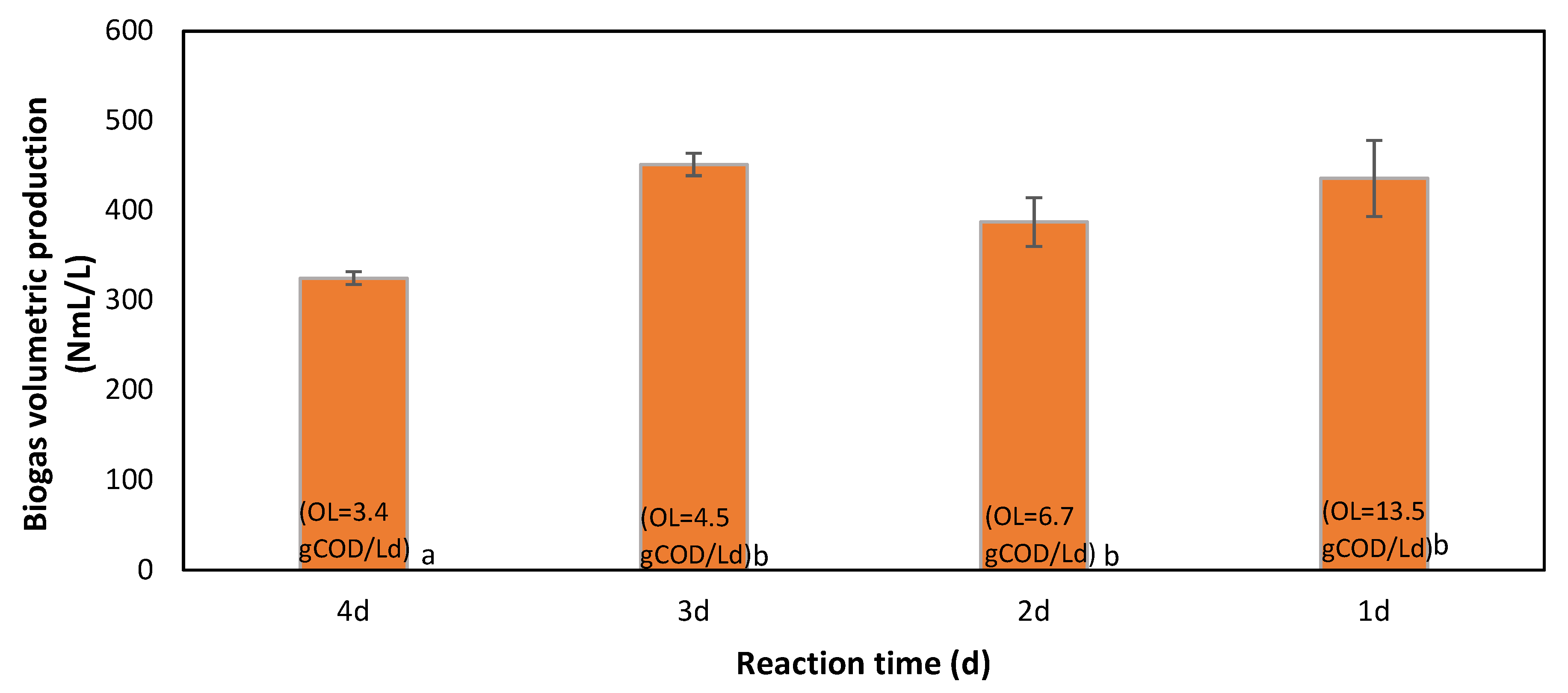
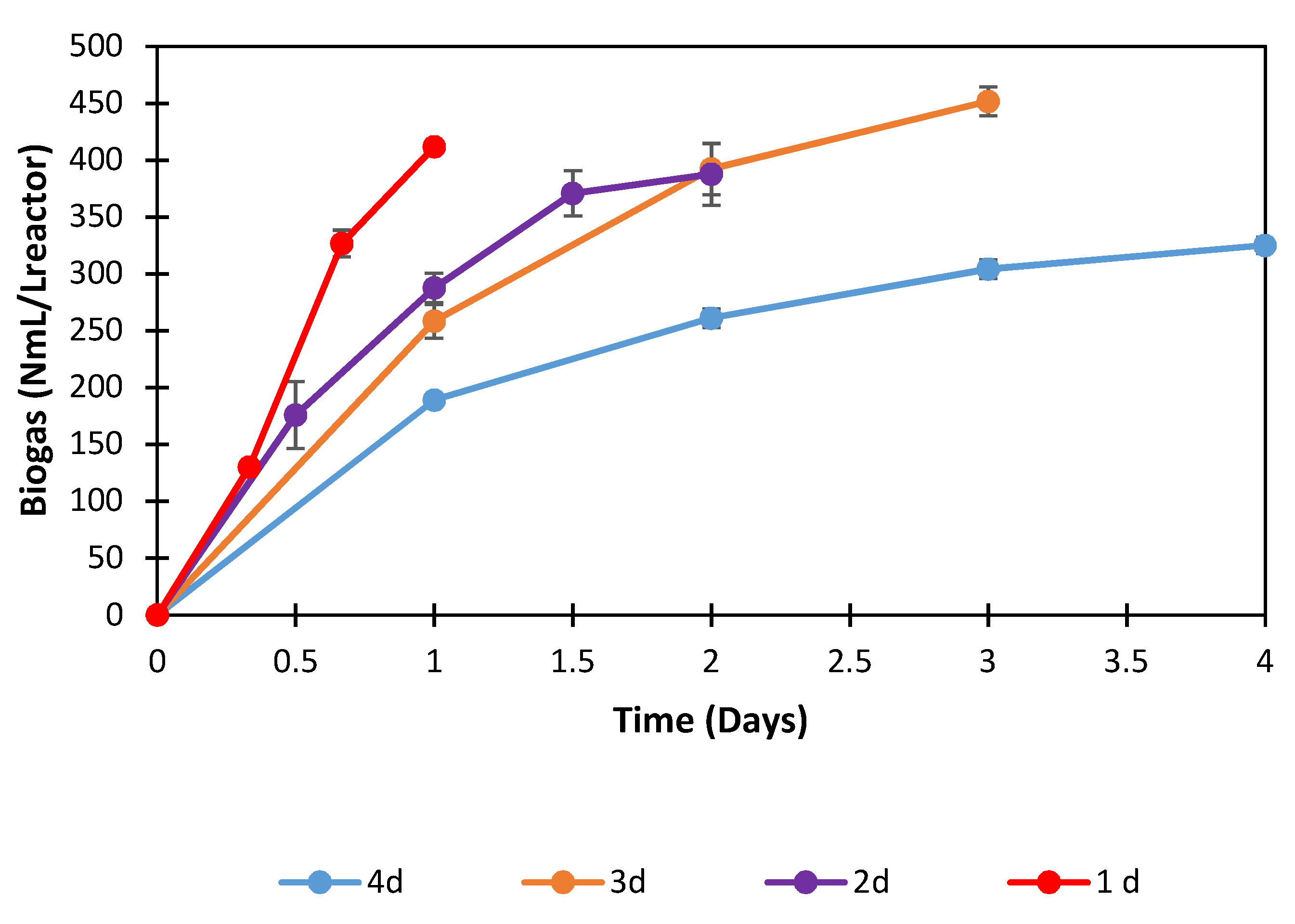
3.3.3. Effect of Reaction Time and Organic Load on Volumetric Biogas Production
3.3.4. Effect of the Reaction Time on the Production of Biohydrogen and Biomethane
3.3.5. Effect of Organic Load and Specific Organic Load on Biohydrogen Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robles-González, V.; Galíndez-Mayer, J.; Rinderknecht-Seijas, N.; Poggi-Varaldo, H.M. Treatment of mezcal vinasses: A review. J. Biotechnol. 2012, 157, 524–546. [Google Scholar] [CrossRef] [PubMed]
- Retes-Pruneda, J.L.; Davila-Vazquez, G.; Medina-Ramírez, I.; Chavez-Vela, N.A.; Lozano-Alvarez, J.A.; Alatriste-Mondragon, F.; Jauregui-Rincon, J. High removal of chemical and biochemical oxygen demand from tequila vinasses by using physicochemical and biological methods. Environ. Technol. 2014, 35, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guerrero. Producción de Biogás a Partir de Bagazo y Vinaza del Agave. angustifolia haw Generada Como Residuo en la Elaboración de Mezcal. Master’s Thesis, Centro Interdisciplinario De Investigación Para El Desarrollo Integral Regional, Unidad Oaxaca. 2014. Available online: http://literatura.ciidiroaxaca.ipn.mx:8080/xmlui/handle/LITER_CIIDIROAX/226 (accessed on 12 December 2023).
- Robles-González, V.; Poggi-Varaldo, H.M.; Galíndez-Mayer, J.; Ruiz-Ordaz, N. Combined Treatment of Mezcal Vinasses by Ozonation and Activated Sludge. Water Environ. Res. 2018, 90, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Alvillo-Rivera, A.J.; Garzón-Zúniga, M.A.; Estrada-Arriaga, E.B.; Buelna, G.; Bahena-Bahena, E.O. Tequila Vinasses treatment using Upflow Anaerobic Sludge Blanket Reactors. In Proceedings of the 4th IWA Mexico Young Water Profesionals Conference, Guanajuato, Gto, Mexico, 27–29 April 2015; pp. 1–10. Available online: https://cimav.repositorioinstitucional.mx/jspui/bitstream/1004/1365/1/proceedingsIWA2015.pdf (accessed on 12 December 2023).
- Martínez, V.L.; García, R. Fermentación oscura, fotofermentación y biofotólisis: Análisis de su aplicación en secuencia para la producción de hidrógeno biológico. Dir. Gen. Investig. Desarro. (DGID) 2010. [Google Scholar] [CrossRef]
- García-Depraect, O.; León-Becerril, E. Fermentative biohydrogen production from tequila vinasse via the lactate-acetate pathway: Operational performance, kinetic analysis and microbial ecology. Fuel 2018, 234, 151–160. [Google Scholar] [CrossRef]
- Buitrón, G.; Prato-Garcia, D.; Zhang, A. Biohydrogen production from tequila vinasses using a fixed bed reactor. Water Sci. Technol. 2014, 70, 1919–1925. [Google Scholar] [CrossRef] [PubMed]
- Sydney, E.B.; Larroche, C.; Novak, A.C.; Nouaille, R.; Sarma, S.J.; Brar, S.K.; Soccol, C.R. Economic process to produce biohydrogen and volatile fatty acids by a mixed culture using vinasse from sugarcane ethanol industry as nutrient source. Bioresour. Technol. 2014, 159, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Y. A bench scale study of fermentative hydrogen and methane production from food waste in integrated two-stage process. Int. J. Hydrogen Energy 2009, 34, 245–254. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Parker, J. Brock Biology of Microorganisms, 10th ed.; Prentice Hall: Madrid, Spain, 2002. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- HACH. DR5000 Spectrophotometer: Procedures Manual, 2nd ed.; Hach Company: Duesseldorf, Germany, 2005. [Google Scholar]
- Díaz-Barajas, S.A.; Garzón-Zúñiga, M.A.; Moreno-Andrade, I.; Vigueras-Cortés, J.M.; Barragán-Huerta, B.E. Acclimation of microorganisms for an efficient production of volatile fatty acids and biogas from mezcal vinasses in a dark fermentation process. Water Sci. Technol. 2021, 83, 2724–2731. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, Y.; Zhou, X. Effect of Ca(OH)2 pretreatment on extruded rice straw anaerobic digestion. Biores. Technol. 2015, 196, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Marino-Marmolejo, E.N.; Corbalá-Robles, L.; Cortez-Aguilar, R.C.; Contreras-Ramos, S.M.; Bolaños-Rosales, R.E.; Davila-Vazquez, G. Tequila vinasses acidogenesis in a UASB reactor with Clostridium predominance. Springerplus 2015, 4, 419. [Google Scholar] [CrossRef]
- Toledo-Cervantes, A.; Villafán-Carranza, F.; Arreola-Vargas, J.; Razo-Flores, E.; Méndez-Acosta, H.O. Comparative evaluation of the mesophilic and thermophilic biohydrogen production at optimized conditions using tequila vinasses as substrate. Int. J. Hydrogen Energy 2020, 45, 11000–11010. [Google Scholar] [CrossRef]
- Chu, C.-Y.; Vo, T.-P.; Chen, T.-H. A novel of biohythane gaseous fuel production from pineapple peel waste juice in two-stage of continuously stirred anaerobic bioreactors. Fuel 2020, 279, 118526. [Google Scholar] [CrossRef]
- Yeshanew, M.M.; Frunzo, L.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Production of biohythane from food waste via an integrated system of continuously stirred tank and anaerobic fixed bed reactors. Bioresour. Technol. 2016, 220, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Meza, A.; Garzón-Zúñiga, M.A.; Moreno-Andrade, I.; Estrada-Arriaga, E.B.; Vigueras-Cortés, J.M.; Barragán-Huerta, B.E.; García-Olivares, J.G. Hydrogen and Methane Production from Tequila Vinasses in a Novel Hybrid Reactor Containing Biofilm and Suspended Biomass. BioEnergy Res. 2021, 15, 1675–1690. [Google Scholar] [CrossRef]
- Buitrón, G.; Kumar, G.; Martinez-Arce, A.; Moreno, G. Hydrogen and methane production via a two-stage processes (H2-SBR + CH4-UASB) using tequila vinasses. Int. J. Hydrogen Energy 2014, 39, 19249–19255. [Google Scholar] [CrossRef]
- García-Depraect, O.; Muñoz, R.; Van Lier, J.B.; Rene, E.R.; Diaz-Cruces, V.F.; León-Becerril, E. Three-stage process for tequila vinasse valorization through sequential lactate, biohydrogen and methane production. Bioresour. Technol. 2020, 307, 123160. [Google Scholar] [CrossRef] [PubMed]
- Fuess, L.T.; Zaiat, M.; Nascimento, C.A. Novel insights on the versatility of biohydrogen production from sugarcane vinasse via thermophilic dark fermentation: Impacts of pH-driven operating strategies on acidogenesis metabolite profiles. Bioresour. Technol. 2019, 286, 121379. [Google Scholar] [CrossRef] [PubMed]


| Parameter | Mezcal (Durango) * | Inoculum (Durango) * | Mezcal (Oaxaca) [1] | Mezcal (Oaxaca) [2] | Mezcal (Oaxaca) [3] |
|---|---|---|---|---|---|
| pH | 3.82 ± 0.16 | 6.63 ± 0.23 | 3.6–3.8 | - | - |
| Temperature (°C) | 70 | 30 | 90 | - | - |
| Volatile Fatty Acids (mg/L) | 5815 ± 714 | - | - | - | |
| COD (mg/L) | 32,966 ± 3088 | 5542 ± 459 | 56,230–122,860 | 42,000 | 35,000–50,000 |
| BOD5 (mg/L) | 11,700 ± 1272 | 26,500–33,600 | 25,576 | 35,000 | |
| Total solids (mg/L) | 43,084 | 25,200 ± 4242 | 26,830–947,130 | 45,860 | - |
| Total Suspended Solids (mg/L) | 31,788 | 7073 ± 1141 | 8400–83,130 | - | - |
| Volatile suspended solids (mg/L) | 920 | 4951 ± 797 | 1130–6850 | - | - |
| Conductivity (mS/cm) | 5.81 | 6.9 | 2.6–4.2 | - | - |
| Reaction Time (d) | Period of Maximum Production of VFAs (d) | Maximum Production of VFAs (mg VFA/L) | VFAs Production Efficiency (%) |
|---|---|---|---|
| 4 | 3 | 1670 ± 552 | 23 ± 6 |
| 3 | 3 | 2007 ± 327 | 36 ± 9 |
| 2 | 1.5 | 1932 ± 198 | 26 ± 2 |
| 1 | 0.66 | 1831 ± 364 | 38 ± 11 |
| Reaction Time (d) | OL (g COD/L d) | H Max (NmL/L) | R (NmL/Lreactorh) | Lag (h) |
|---|---|---|---|---|
| 4 | 3.4 | 119 | 8 | 3 |
| 3 | 4.5 | 146 | 13 | 15 |
| 2 | 6.7 | 145 | 15 | 11 |
| 1 | 13.5 | 170 | 31 | 8 |
| Reaction Time (d) | Biogas Composition at the End of the Cycle (NmL/Lreactor) | ||
|---|---|---|---|
| H2 | CH4 | CO2 | |
| 4 | 0 | 84 ± 2.0 | 240 ± 5 |
| 3 | 17 ± 0.4 | 74 ± 2.0 | 361 ± 10 |
| 2 | 13 ± 1.0 | 81 ± 6.0 | 291 ± 20 |
| 1 | 64 ± 21.0 (15%) | 116 ± 0 (27%) | 250 ± 0 (58%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Barajas, S.A.; Moreno-Andrade, I.; Estrada-Arriaga, E.B.; García-Sánchez, L.; Garzón-Zúñiga, M.A. Biohydrogen, Volatile Fatty Acids, and Biomethane from Mezcal Vinasses—A Dark Fermentation Process Evaluation. Fermentation 2024, 10, 217. https://doi.org/10.3390/fermentation10040217
Díaz-Barajas SA, Moreno-Andrade I, Estrada-Arriaga EB, García-Sánchez L, Garzón-Zúñiga MA. Biohydrogen, Volatile Fatty Acids, and Biomethane from Mezcal Vinasses—A Dark Fermentation Process Evaluation. Fermentation. 2024; 10(4):217. https://doi.org/10.3390/fermentation10040217
Chicago/Turabian StyleDíaz-Barajas, Sergio A., Iván Moreno-Andrade, Edson B. Estrada-Arriaga, Liliana García-Sánchez, and Marco A. Garzón-Zúñiga. 2024. "Biohydrogen, Volatile Fatty Acids, and Biomethane from Mezcal Vinasses—A Dark Fermentation Process Evaluation" Fermentation 10, no. 4: 217. https://doi.org/10.3390/fermentation10040217
APA StyleDíaz-Barajas, S. A., Moreno-Andrade, I., Estrada-Arriaga, E. B., García-Sánchez, L., & Garzón-Zúñiga, M. A. (2024). Biohydrogen, Volatile Fatty Acids, and Biomethane from Mezcal Vinasses—A Dark Fermentation Process Evaluation. Fermentation, 10(4), 217. https://doi.org/10.3390/fermentation10040217





