Differences in the Behavior of Anthocyanin Coloration in Wines Made from Vitis vinifera and Non-vinifera Grapes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Extraction and Partial Purification of Grape Skin Anthocyanins
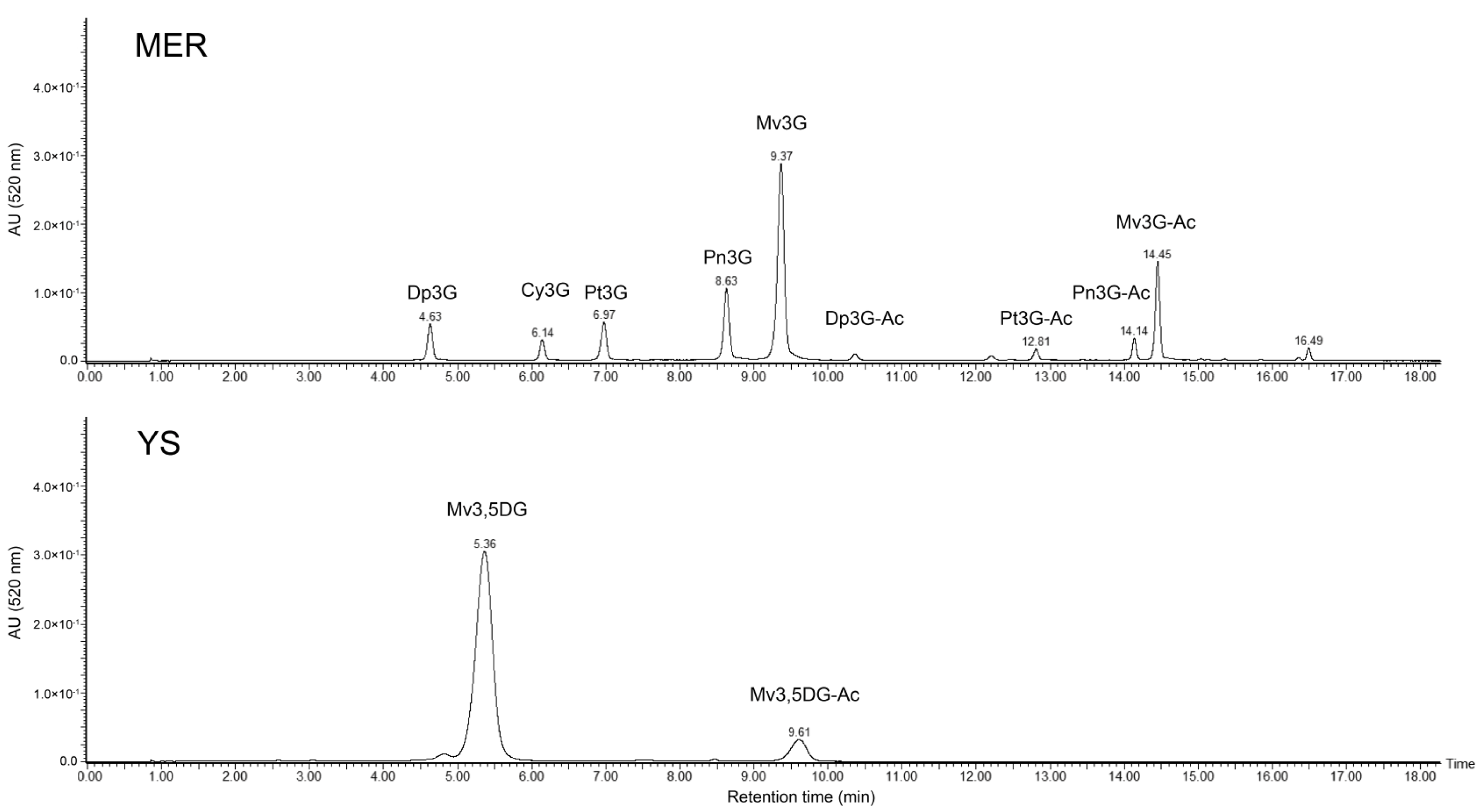
Quantification and Measurement of Anthocyanin Color
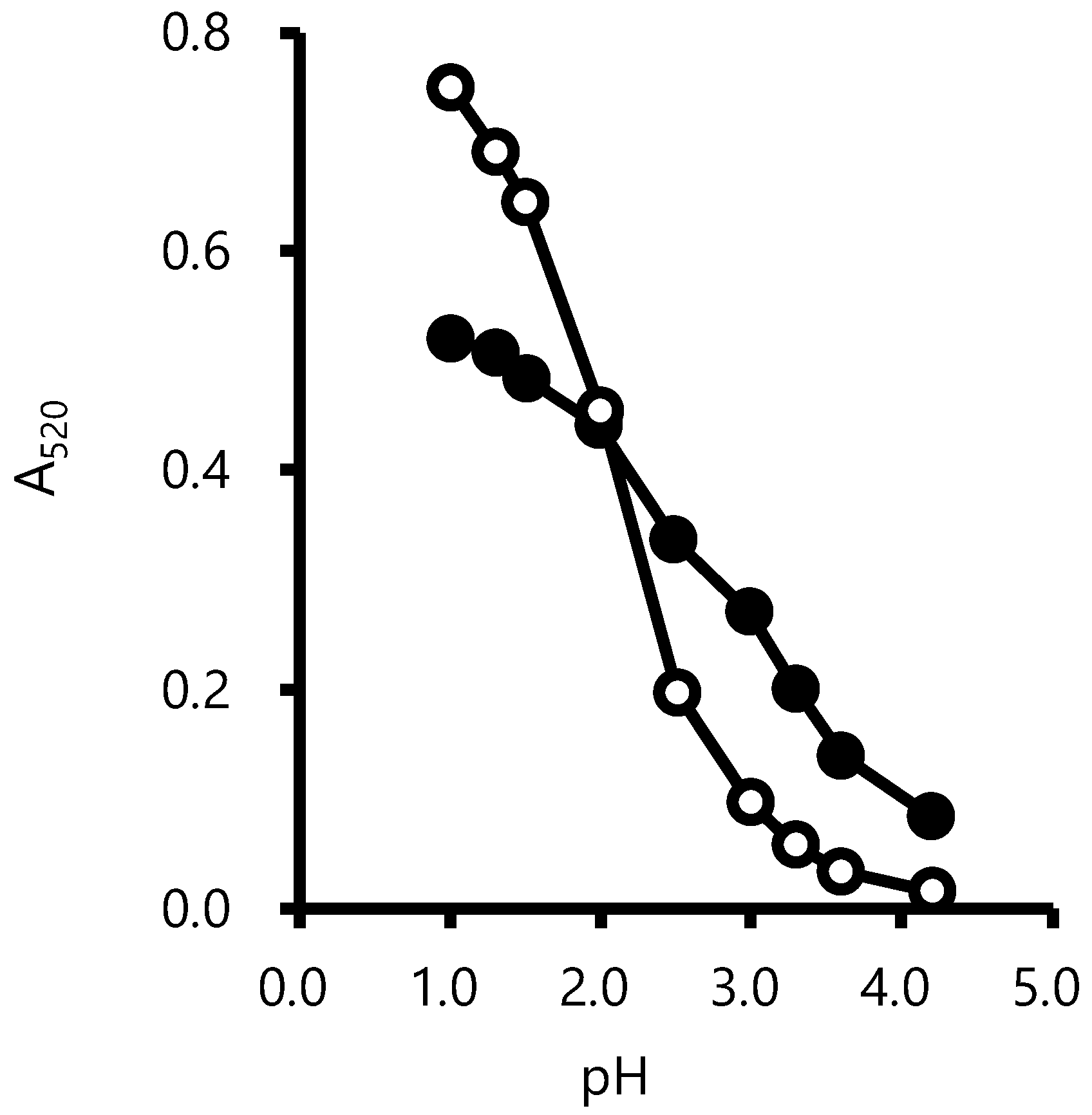
2.3. Calculation of Hydration Constant (pKh)
2.4. Wine Samples
2.5. Measurement of Co-pigmented Anthocyanins, Free Anthocyanins, and Polymeric Pigments
2.6. Statistical Analysis
3. Results
3.1. Effect of pH on Color and Hydration Constant of Grape Anthocyanins
3.2. Differences in Average Percentage of Color Due to Three Classes of Pigments in Cultivars in Commercial Wines
3.3. Changes in the Percentage of Color Due to Three Classes of Pigments during Aging
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kassara, S.; Kennedy, J.A. Relationship between red wine grade and phenolics. 2. Tannin composition and size. J. Agric. Food Chem. 2011, 59, 8409–8412. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and their variation in red wines II. Anthocyanin derived pigments and their color evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef]
- Victor de Freitas, A.P.; Fernandes, A.; Oliveira, J.; Teixeira, N.; Mateus, N. A review of the current knowledge of red wine colour. Oeno One 2017, 51, 1–15. [Google Scholar] [CrossRef]
- Yokotsuka, K.; Nishino, N. Extraction of anthocyanins from muscat bailey A grape skins. J. Ferment. Bioeng. 1990, 69, 328–334. [Google Scholar] [CrossRef]
- Koyama, K.; Kamigakiuchi, H.; Iwashita, K.; Mochioka, R.; Goto-Yamamoto, N. Polyphenolic diversity and characterization in the red–purple berries of East Asian wild Vitis species. Phytochemistry 2017, 134, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.S.; Lee, J.H.; Yoon, S.H.; Oh, C.H.; Choi, D.-S.; Choe, E.; Jung, M.Y. Characterization and quantification of anthocyanins in grape juices obtained from the grapes cultivated in Korea by HPLC/DAD, HPLC/MS, and HPLC/MS/MS. J. Food Sci. 2008, 73, C378–C389. [Google Scholar] [CrossRef] [PubMed]
- Lago-Vanzela, E.S.; Procópio, D.P.; Fontes, E.A.F.; Ramos, A.M.; Stringheta, P.C.; Da-Silva, R.; Castillo-Muñoz, N.; Hermosín-Gutiérrez, I. Aging of red wines made from hybrid grape cv. BRS Violeta: Effects of accelerated aging conditions on phenolic composition, color and antioxidant activity. Food Res. Int. 2014, 56, 182–189. [Google Scholar] [CrossRef]
- Li, S.-Y.; He, F.; Zhu, B.-Q.; Wang, J.; Duan, C.-Q. Comparison of phenolic and chromatic characteristics of dry red wines made from native Chinese grape species and Vitis vinifera. Int. J. Food Prop. 2017, 20, 2134–2146. [Google Scholar] [CrossRef]
- Boulton, R. The co-pigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar] [CrossRef]
- Hermosín Gutiérrez, I. Influence of ethanol content on the extent of co-pigmentation in a Cencibel young red wine. J. Agric. Food Chem. 2003, 51, 4079–4083. [Google Scholar] [CrossRef] [PubMed]
- Dimitrić, M.; Petranović, N.A.; Baranac, J.M. A Spectrophotometric study of the co-pigmentation of malvin with caffeic and ferulic acids. J. Agric. Food Chem. 2000, 48, 5530–5536. [Google Scholar] [CrossRef]
- Galland, S.; Mora, N.; Abert-Vian, M.; Rakotomanomana, N.; Dangles, O. Chemical synthesis of hydroxycinnamic acid glucosides and evaluation of their ability to stabilize natural colors via anthocyanin co-pigmentation. J. Agric. Food Chem. 2007, 55, 7573–7579. [Google Scholar] [CrossRef] [PubMed]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by co-pigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Inoue, E.; Kobayashi, H.; Hoshino, R.; Hisamoto, M.; Watanabe-Saito, F.; Okuda, T. Adsorption properties of grape phenolics to grape insoluble cell wall materials. Food Sci. Technol. Res. 2019, 25, 863–869. [Google Scholar] [CrossRef]
- Talcott, S.T.; Peele, J.E.; Brenes, C.H. Red clover isoflavonoids as anthocyanin color enhancing agents in muscadine wine and juice. Food Res. Int. 2005, 38, 1205–1212. [Google Scholar] [CrossRef]
- Heras-Roger, J.; Díaz-Romero, C.; Darias-Martín, J. What gives a wine its strong red color? Main correlations affecting co-pigmentation. J. Agric. Food Chem. 2016, 64, 6567–6574. [Google Scholar] [CrossRef]
- Yokotsuka, K. Wine making principles. J. Brew. Soc. Jpn. 2000, 95, 318–327. [Google Scholar] [CrossRef]
- González-Manzano, S.; Dueñas, M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T.; Santos-Buelga, C. Studies on the co-pigmentation between anthocyanins and flavan-3-ols and their influence in the colour expression of red wine. Food Chem. 2009, 114, 649–656. [Google Scholar] [CrossRef]
- Darias-Martín, J.; Carrillo-López, M.; Echavarri-Granado, J.F.; Díaz-Romero, C. The magnitude of co-pigmentation in the colour of aged red wines made in the Canary Islands. Eur. Food Res. Technol. 2007, 224, 643–648. [Google Scholar] [CrossRef]
- Boulton, R. A method for the assessment of co-pigmentation in red wines. In Proceedings of the 47th Annual Meeting of the American Society for Enology and Viticulture, Reno, NV, USA, 26–28 June 1996. [Google Scholar]
- Heras-Roger, J.; Pomposo-Medina, M.; Díaz-Romero, C.; Darias-Martín, J. Co-pigmentation, colour and antioxidant activity of single-cultivar red wines. Eur. Food Res. Technol. 2014, 239, 13–19. [Google Scholar] [CrossRef]
- Heras-Roger, J.; Alonso-Alonso, O.; Gallo-Montesdeoca, A.; Díaz-Romero, C.; Darias-Martín, J. Influence of co-pigmentation and phenolic composition on wine color. J. Food Sci. Technol. 2016, 53, 2540–2547. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Ono, K.; Hisamoto, M.; Matsudo, T.; Okuda, T. Concentrations of BSA-binding proanthocyanidins in red wines produced in Japan. Food Sci. Technol. Res. 2011, 17, 335–339. [Google Scholar] [CrossRef]
- Hoshino, T.; Matsumoto, U.; Goto, T. Self-association of some anthocyanins in neutral aqueous solution. Phytochemistry 1981, 20, 1971–1976. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, N.; Wu, G.-F.; He, F.; Lan, Y.-B.; Duan, C.-Q. Intermolecular co-pigmentation between anthocyanidin-3,5-O-diglucosides and three phenolic compounds: Insights from experimental and theoretical studies. Food Chem. Adv. 2022, 1, 100111. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, N.; He, F.; Duan, C. Reactivity comparison of three malvidin-type anthocyanins forming derived pigments in model wine solutions. Food Chem. 2022, 384, 132534. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, I.H.; Lorenzo, E.S.-P.; Espinosa, A.V. Phenolic composition and magnitude of co-pigmentation in young and shortly aged red wines made from the cultivars, Cabernet Sauvignon, Cencibel, and Syrah. Food Chem. 2005, 92, 269–283. [Google Scholar] [CrossRef]
- Schwarz, M.; Quast, P.; von Baer, D.; Winterhalter, P. Vitisin A content in Chilean wines from Vitis vinifera cv. Cabernet Sauvignon and contribution to the color of aged red wines. J. Agric. Food Chem. 2003, 51, 6261–6267. [Google Scholar] [CrossRef] [PubMed]
- Atanasova, V.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Effect of oxygenation on polyphenol changes occurring in the course of wine-making. Anal. Chim. Acta 2002, 458, 15–27. [Google Scholar] [CrossRef]
- Tamura, H.; Yamagami, A. Antioxidative activity of monoacylated anthocyanins isolated from Muscat Bailey A grape. J. Agric. Food Chem. 1994, 42, 1612–1615. [Google Scholar] [CrossRef]

| 2017 | 2016 | 2015 | 2012–2014 | Total | |
|---|---|---|---|---|---|
| MBA | 2 | 12 | 5 | 5 | 24 |
| YS | 5 | 6 | 4 | - | 15 |
| MER | 2 | 3 | 15 | 11 | 31 |
| CS | 1 | 2 | 2 | 5 | 10 |
| Total | 10 | 23 | 26 | 21 | 80 |
| Anthocyanin Conc. (mM) | MER | YS |
|---|---|---|
| 0.02 | 2.92 | 2.11 |
| 0.4 | 3.26 | 2.13 |
| 0.8 | 3.46 | 2.39 |
| 1.2 | 3.76 | 2.73 |
| CA% | Average | 2017 | 2016 | 2015 | 2012–2014 |
|---|---|---|---|---|---|
| MBA | 3.4 ± 3.5 A | 9.7 ± 3.2 Ac | 2.9 ± 2.7 Ab | 3.5 ± 4.0 Ac | 0.2 ± 0.4 Aa |
| YS | 24.0 ± 10.1 B | 33.6 ± 9.7 Bb | 21.2 ± 4.2 Ba | 16.1 ± 8.3 Ba | |
| MER | 2.5 ± 3.2 A | 9.2 ± 3.4 Ac | 4.1 ± 3.2 Abc | 2.3 ± 2.9 Aab | 0.7 ± 0.8 Aa |
| CS | 2.1 ± 3.0 A | 10.0 | 1.6 ± 1.3 Aa | 0 ± 0 Aa | 1.3 ± 1.6 Aa |
| FA% | Average | 2017 | 2016 | 2015 | 2012–2014 |
|---|---|---|---|---|---|
| MBA | 42.7 ± 6.5 B | 50.6 ± 8.7 Aa | 44.7 ± 7.1 Aa | 41.1 ± 5.9 Ba | 41.0 ± 3.4 Ba |
| YS | 48.7 ± 7.9 C | 41.8 ± 6.5 Aa | 54.1 ± 6.5 Bb | 49.1 ± 5.4 Bab | |
| MER | 33.6 ± 8.0 A | 42.5 ± 5.6 Ab | 50.9 ± 9.9 ABb | 30.6 ± 5.5 Aa | 31.6 ± 3.0 Aa |
| CS | 38.4 ± 10.6 BC | 50.0 | 39.8 ± 0.4 ABa | 49.3 ± 1.1 Ba | 31.2 ± 9.7 ABa |
| PP% | Average | 2017 | 2016 | 2015 | 2012–2014 |
|---|---|---|---|---|---|
| MBA | 54.0 ± 7.2 B | 39.7 ± 5.0 Ba | 52.4 ± 6.9 Bb | 55.3 ± 6.1 Bb | 58.8 ± 3.5 Ab |
| YS | 16.1 ± 13.4 A | 24.6 ± 4.9 Aa | 24.7 ± 6.4 Aab | 34.7 ± 7.1 Ab | |
| MER | 63.9 ± 9.0 C | 48.3 ± 9.0 Ba | 45.0 ± 10.3 Ba | 67.1 ± 4.5 Cb | 67.7 ± 2.6 Cb |
| CS | 59.5 ± 11.3 BC | 40.0 | 58.6 ± 1.8 Ba | 50.7 ± 1.1 Ba | 67.7 ± 8.4 BCa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okuda, T.; Maeda, K.; Serizawa, I.; Watanabe-Saito, F.; Hisamoto, M. Differences in the Behavior of Anthocyanin Coloration in Wines Made from Vitis vinifera and Non-vinifera Grapes. Fermentation 2024, 10, 216. https://doi.org/10.3390/fermentation10040216
Okuda T, Maeda K, Serizawa I, Watanabe-Saito F, Hisamoto M. Differences in the Behavior of Anthocyanin Coloration in Wines Made from Vitis vinifera and Non-vinifera Grapes. Fermentation. 2024; 10(4):216. https://doi.org/10.3390/fermentation10040216
Chicago/Turabian StyleOkuda, Tohru, Kyohei Maeda, Itsuki Serizawa, Fumie Watanabe-Saito, and Masashi Hisamoto. 2024. "Differences in the Behavior of Anthocyanin Coloration in Wines Made from Vitis vinifera and Non-vinifera Grapes" Fermentation 10, no. 4: 216. https://doi.org/10.3390/fermentation10040216
APA StyleOkuda, T., Maeda, K., Serizawa, I., Watanabe-Saito, F., & Hisamoto, M. (2024). Differences in the Behavior of Anthocyanin Coloration in Wines Made from Vitis vinifera and Non-vinifera Grapes. Fermentation, 10(4), 216. https://doi.org/10.3390/fermentation10040216






