Abstract
Hydrogen is ideal for replacing fossil fuels because upon combustion it generates only water. Dark fermentation (DF) from lignocellulose might be a competitive process for hydrogen production at the industrial scale. However, lignocellulose must be pretreated to obtain fermentable sugars, which is costly and creates pollution. Microorganisms from bovine rumen efficiently degrade lignocellulose. Unfortunately, they have scarcely been explored for the production of hydrogen. Therefore, deeper studies on the culture conditions have to be undertaken to understand the behavior of microbial consortia from the rumen of bovines (MCRB) during hydrogen production. In this work, we evaluated the production of hydrogen by DF with MCRB by varying the incubation time, two culture media (MB and Rhodospirillaceae), headspace (40 and 80 mL), and thermal treatment. It was found that the production of hydrogen was maximum at 16 h MCRB incubation in MB. An amount of 80 mL headspace resulted in a threefold production of hydrogen as compared to 40 mL; the MCRB without heat treatment had a higher H2 yield. The production of hydrogen with 32 MCRB was highly variable, ranging between 21 and 696 mL. Our findings show a different perspective on the treatment of MCRB for the production of hydrogen and give insights on the impact of the culture conditions for increasing hydrogen production.
1. Introduction
The enormous impact that fossil fuels have on global warming and environmental pollution makes urgent the search for renewable, sustainable, and clean energy sources [1,2]. Hydrogen is a promising candidate as a sustainable fuel due to its high energy content (2.75-fold higher energy as compared to fossil fuels, 122 kJ/g) and clean combustion, which generates only water [3,4]. Although the storage and transportation of hydrogen and hydrogen-based fuels is quite limited, scaling up is key as new applications arise. However, the use of hydrogen is still minimal since it accounts for less than 0.1% of the hydrogen demand in novel applications for heavy industry and long-distance transport. On the other hand, the production of low-emission hydrogen is less than 1% of the total hydrogen production [5]. It is noteworthy that nowadays, despite the great potential to use hydrogen as a clean fuel, it is mainly utilized for the manufacture of ammonia, methanol, flat glass, in metallurgy, and the electronic and oil industries. Moreover, nearly 95% of the H2 produced worldwide is derived from fossil fuels and is associated with significant amounts of CO2 emissions, ranging from 7.5 to 12 tons of CO2 per ton of hydrogen produced. Furthermore, the global increase in CO2 accumulation is nearly ten times faster than any sustained rise in history [6,7]. In this context, the biological production of hydrogen has significant advantages over traditional processes because it requires a low temperature and is carried out at atmospheric pressure, thus implying the use of less energy [8].
Biophotolysis, photo-fermentation, and dark fermentation are alternatives for the production of hydrogen [4]. However, the most promising biological route to produce hydrogen is dark fermentation as the conversion yields (60–70%), which are higher with respect to other biological means, are comparable to the yields obtained by chemical methods, which reach 60 to 75% conversion [9], and DF can be carried out in unsterilized environments [10]. The selection of microorganisms to carry out dark fermentations is essential because these dictate the performance of the process. Many studies have focused on microorganisms that are able to produce H2 by employing axenic or mixed cultures that originate from different ecosystems. Such microorganisms are widely distributed in various natural sources including anaerobic and activated sludges, municipal sewage sludge, soil samples, cow manure, compost, river sediments [11], and bovine rumen, among others [12].
The bovine rumen is an esophageal compartment from the digestive apparatus of ruminates. It can be considered as an anaerobic chamber for methanogenic fermentation that contains an ample spectrum of microorganisms that include bacteria, protozoa, fungus, and archaea [13]. Most rumen microorganisms have the genetic potential to participate in the degradation of waste agricultural materials. Ruminal microbiota has shown excellent results on the biological conversion of lignocellulosic biomass into renewable energy, due to its efficiency to degrade lignocellulose by synergistic interactions among multiple microbiomes [14,15]. Moreover, around ten billion tons of biomass are generated each year worldwide. The efficient utilization of agricultural residues may be key to mitigating both the energy crisis and environmental pollution. However, the majority of agricultural residues are disposed of or burned in the fields, generating waste and creating environmental distress. On the other hand, obtaining fermentable sugars for dark fermentation from lignocellulose requires improving the methods for hydrolysis. Pretreatments used to hydrolyze biomass generate secondary pollution (acid hydrolysis) or are expensive (enzymatic hydrolysis) and the efficiency of hydrolysis is low [16]. By using ruminal microbiota, pretreatments for lignocellulose could be skipped. The microbiome of the rumen is densely populated, highly diverse, and has complex interactions. Owing to the complexity of the ruminal microbiota, a vast field is open for exploration on the production of biohydrogen [17]. Nevertheless, ruminal microorganisms have scarcely been explored for the production of hydrogen. Among the few studies reported to produce H2 with bovine rumen, the enrichment of the ruminal liquid, temperature, pH, and using cellulose as the substrate were studied [12]; the production of H2 from cellulose and ruminal fluid pretreated with perchloric acid (HClO4) was also reported [18]; and hydrogen production was investigated by the acid pretreatment of the ruminal fluid with hydrochloric acid using office paper as the substrate [19]. However, it is necessary to deepen the study of the culture conditions of ruminal microbiota to understand their behavior before its use for the production of hydrogen from lignocellulosic biomass. Therefore, the objective of this study was to evaluate the culture conditions for the production of hydrogen by bovine ruminal microbiota. The novelty of this contribution lies in the following: (a) finding culture conditions that enhance the production of hydrogen, despite the variability in the composition within the ruminal microbiota as well as among the different consortia (different bovines); and (b) identifying conditions that allow the observation of the same tendency for hydrogen production. To the best of our knowledge, there are no reports of the conditions analyzed in this study.
2. Materials and Methods
2.1. Materials
Ruminal samples were collected from slaughtered cattle in the cities of Colotlán and Zapopan, both in the state of Jalisco, México. NaCl, KH2PO4, CaCl2·2H2O, MgCl2·6H2O, MnCl2·4H2O, CoCl2·6H2O, and (NH4)2SO4, K2HPO4, all analytical grade, were purchased from Fermont (Monterrey, México). Yeast extract was purchased from BD Bioxon (Cuautitlán Izcalli, Estado de México, México). Glucose, starch, xylose, acetic acid, propionic acid, butyric acid, n-valeric acid, isovaleric acid, pyridoxine hydrochloride, riboflavin, thiamine hydrochloride, pantothenic acid, p-aminobenzoic acid, vitamin B12, folic acid, and biotin were purchased from Sigma Aldrich (St. Louis, MO, USA).
2.2. General Procedures
The experiments carried out in this study were performed to maximize the production of hydrogen with ruminal microorganisms from bovines. Thirty-two samples were taken from the ruminal liquid of cattle; twelve samples were obtained from the city of Colotlan and the rest from the city of Zapopan, Jalisco, Mexico. A sample or consortium is the ruminal content of a particular bovine and was designated as microbial consortium from the rumen of bovines (MCRB). In order to evaluate the production of hydrogen, each consortium was used to prepare the seeding culture or inoculum. The inoculum was incubated at 39 °C for 12–24 h (the preparation of inoculum is detailed in Section 2.3); after incubation, the inoculum was placed in a culture medium to produce hydrogen at 39 °C; then, gaseous samples were taken to determine the hydrogen content (the procedure to produce and quantify hydrogen is described in Section 2.4). All the experiments were carried out in duplicate, and the results show their average. The conditions analyzed were as follows:
2.2.1. Incubation Time
The incubation time for the inoculum was varied in order to establish the time in which the production of hydrogen was higher and then to select the appropriate incubation time for the following stages. Inocula from MCRB 3, 4, and 7 were incubated at 12, 16, and 24 h; after the incubation time, each inoculum was transferred to the hydrogen production medium, MB [20], and incubated at 39 °C; sampling was performed periodically to determine the production of hydrogen.
2.2.2. Selection of Culture Media and Preparation of Culture Medium
The effect of the culture medium on the production of hydrogen was investigated by testing the culture media MB [20] and Rhodospirillaceae [21], whose composition can be seen in Table 1. Production of hydrogen was carried out, according to the procedure described below (Section 2.3), with consortia 1, 2, and 3. Four conditions for the production of H2 were tested with MCRB 3. In the first treatment (3-a), the nutrients were mixed—with the exception of the ruminal fluid—and sterilized at 121 °C. Then, the ruminal fluid was subjected to UV light for 30 min and then added to the rest of the nutrients. Treatment 3-b consisted firstly of the separation of all the nutrients and their sterilization at 121 °C, and afterwards, they were mixed. In treatment 3-c, all the nutrients were mixed, including the ruminal fluid, and then sterilized at 121 °C. In procedure 3d, all the nutrients were mixed and sterilized at 121 °C, while the ruminal fluid was eliminated. Once the nutrients cooled down, the culture media were inoculated with MCRB3.

Table 1.
Description of components of MB and Rhodospirillaceae culture medium.
2.2.3. Headspace
Two cultures (MCRB 7 and 20) and two volumes of culture (40 and 80 mL) were tested to determine the production of hydrogen. Since culture vials have equal total volume, the volume of the gaseous phase (headspace) was higher with 40 mL culture volume (80 mL gaseous phase or headspace). These experiments were carried out in the conditions selected in previous stages.
2.2.4. Heat Treatment
Before transferring the ruminal consortia to the culture medium to prepare the inoculum, MCRB 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 were subjected to heat treatment at 105 °C in an oven (Heraterm, Thermo Scientific, Waltham, MA, USA) for 20 min, 4, or 24 h, and without thermal treatment. The inoculum was prepared as explained before and then hydrogen was produced in the conditions determined in previous stages to obtain a high H2 volume (incubation time, culture medium, preparation of culture medium, and volume of the culture medium) selected in Section 2.2.1, Section 2.2.2 and Section 2.2.3. Sampling was performed periodically over 48 h to determine the production of hydrogen.
2.2.5. Production and Productivity of Hydrogen
A total of 32 ruminal consortia were evaluated to determine the production of hydrogen (Section 2.3 and Section 2.4) and productivity. These experiments were carried out in the conditions selected in previous stages. Hydrogen productivities (rH2) were obtained by taking the slope of the curve obtained by plotting the accumulated hydrogen versus time.
2.2.6. Cell Growth
Culture conditions to evaluate microbial growth were MB medium (both for inoculum and hydrogen production), 24 h incubation time at 39 °C. Biomass or cell growth for consortia 1 and 2 was determined both by dry weight and by optical density (660 nm, Genesys 10S UV-Vis, Thermo Scientific). For biomass quantification by dry weight, 4 mL of samples was centrifuged at 10,000 rpm (Centrifuge Eppendorf 5810 R) for 15 min, the precipitates washed twice with deionized H2O, and dried at 80 °C for 24 h.
2.3. Inoculum Preparation
Inoculum was prepared for each run as follows: 50 mL of liquid sample (denominated consortium or MCRB) was taken from the rumen of a slaughtered bovine (identified by a number, i.e., 1, 2, 3, 4, 5, etc.), and placed in falcon tubes for transport and filtered with a sterile gauze. An amount of 4 mL from the filtrate was taken and inoculated in 120 mL serological bottles that contained 36 mL of MB culture medium [20] (Table 1), hermetically closed with butyl septa and aluminum rings, and then sterilized at 120 °C for 15 min at pH 6.5. Sterile nitrogen was bubbled for 3 min in order to evacuate oxygen from the bottles, which were then incubated for 24 h at 39 °C (incubator 3522, Thermo Electron Corporation, Marietta, OH, USA).
2.4. Production and Quantification of Hydrogen
Hydrogen was produced by placing 8 mL of a culture obtained from a designated inoculum (Section 2.3) in 72 mL of MB medium at pH 6.5, displacing air by bubbling nitrogen, and then incubating at 39 °C. To evaluate the production of hydrogen and the composition of the biogas, 500 µL biogas was collected with a syringe through the septa in each vial, and directly injected into a gas chromatograph (Perkin Elmer Multisystem XL) equipped with a column (HayeSep D, 100/120, Alltech, Nicholasville, KY, USA) and a thermal conductivity detector at 100 °C; the oven was set at 60 °C and the injector at 120 °C. N2 (20 mL/min) was used as the carrier gas. Biogas samples were taken periodically, and after sampling, the whole headspace was replaced with N2 to avoid inhibition by the product. All the experiments were carried out in duplicate, and the results show their average.
2.5. Massive Sequencing
Massive sequencing was carried out in MCRB 7 to identify the predominant genera, with the Illumina method, using previously extracted, genomic ADN from the microbial community. Massive sequencing was provided by the INRA Transfert Environnement (Narbona, France).
2.6. Statistical Analysis
Significant differences in the production of H2 obtained with the different treatments were evaluated by analysis of variance (ANOVA; Statgraphics Centurion XV.II) using the Fisher method of the Least Significant Difference (LSD) at a 95% confidence level.
3. Results and Discussion
3.1. Incubation Time
To obtain a high quantity of active cells producing H2, it is necessary to know the time of higher microbial activity, which corresponds to the phase that precedes the stationary phase. The optical density of two samples (two consortia from two bovines, 1 and 2) was measured to know the time required to transfer the inoculum of the bovine rumen to the production medium. Figure 1 shows that consortium 1 has a lag phase of 5 h and the stationary phase is achieved at around 10 h. For consortium 2, the lag phase took less than 5 h; however, the stationary phase was reached at around 30 h. This different behavior between both consortia is owed to the ruminal diversity between bovines [22]. It is known that the microbial composition of the bovine rumen is sensitive to changes in diet, age, seasons, geographic location, feeding regime, and in general to the health of the animal [23,24]. Other factors that impact the microbial activity and the diversity of bovine rumen include behavioral and physiological processes, such as rumination, salivation, absorption, passage, and production of volatile gastric acids [17,22,24]; even the ruminal microbiota of dairy cows and steers can be related to different phenotypic characteristics such as milk production and composition [22].
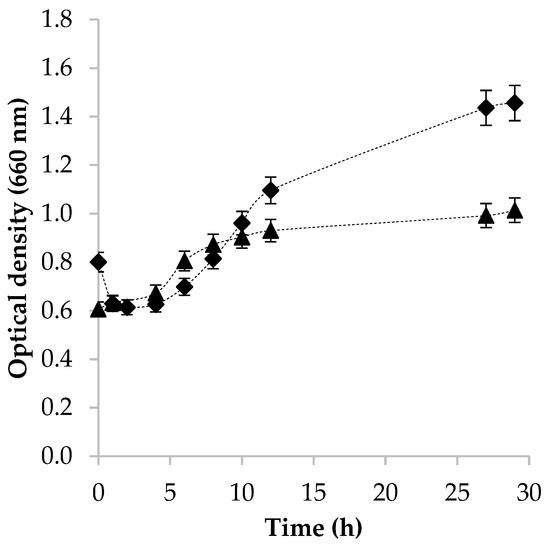
Figure 1.
Growth of bovine rumen microorganisms determined by optical density: (▲) MCRB 1 and (♦) MCRB 2.
Based on the results shown in Figure 1, incubation times at 12, 16, and 24 h were tested for the production of H2 using MCRB 3, 4, and 7 (Figure 2). Consortium 3 produced 32 mL of hydrogen in 11 h, whereas consortia 4 and 7 produced considerably less hydrogen (6.5 and 7 mL, respectively) in 8 h. Such a difference can be due to the fact that the microbiota depends on the nutrition of the different individuals [23,24]. A significant difference in the amount of hydrogen produced can be seen with consortia 3 and 4. However, the incubation of samples for 16 or 24 h resulted in no differences in hydrogen production (p-value = 0.1272), which was the base to select an incubation time of 16 h for subsequent experiments.
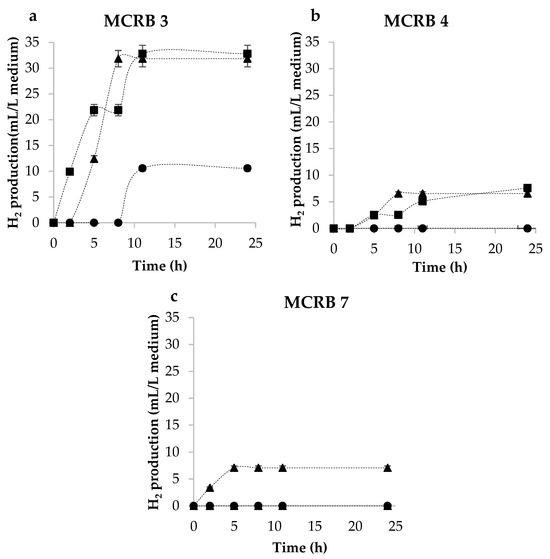
Figure 2.
H2 production with inocula obtained at different incubation times: (●) 12 h, (▲) 16 h, and (■) 24 h on MCRB (a) 3, (b) 4, and (c) 7.
3.2. Culture Media to Produce Hydrogen
3.2.1. Composition of Culture Medium
The composition of the culture medium is crucial for the production of hydrogen. To select an appropriate culture medium for the production of H2 with microorganisms from bovine rumen, a comparison of the culture media Rhodospirillaceae and MB was carried out (Table 1). Rhodospirillaceae medium [21] has been formulated for the conservation of bacteria of the genre Rhodospirillaceae and a high production of hydrogen has been observed with purple non-sulfur bacteria [25]. MB medium, on the other hand, has been developed to enumerate and isolate ruminal bacteria with fibrolytic potential [20]. Three microbial consortiums (1, 2, and 3) were selected to produce hydrogen (Figure 3a) and used to carry out fermentations using both media. It can be observed that the production of H2 in MB was between 36 and 44% higher for the three consortiums. ANOVA shows significant statistical differences between treatments (p-value < 0.05), which can be attributed to the fact that the MB medium has a greater amount and higher concentration of nutrients as compared to the Rhodospirillaceae medium (Table 1), i.e., seven additional vitamins, five different organic acids, and different sources of sugars (glucose, xylose, and starch), yeast extract, and ruminal fluid. Given the higher production of H2, the MB medium was selected.
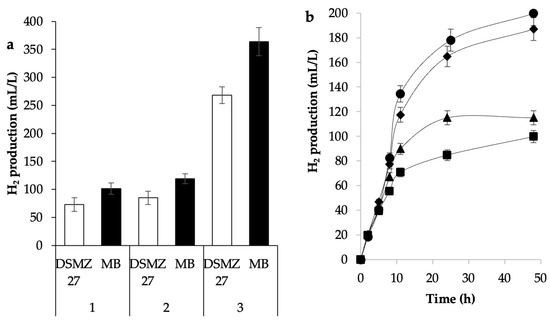
Figure 3.
(a) Comparison between Rhodospirillaceae and MB culture media for H2 production, MCRB 1, 2, and 3. (b) Effect of MB culture medium preparation: (▲) MCRB 3-a, (◆) MCRB 3-b, (●) MCRB 3-c, and (■) MCRB 3-d on H2 production by MCRB 3.
3.2.2. Culture Medium Preparation Conditions
Composition and preparation of the culture medium may influence the production of H2. MB is a culture medium rich in nutrients (Table 1) that under sterilization with heat and pressure may trigger chemical reactions between the components of the culture medium or even degradation of some components, which might be reflected either in the increased or decreased hydrogen production. Also, it is desirable to eliminate the ruminal liquid from the culture because variations in the composition of the ruminal content may affect the industrial production of hydrogen by generating either a higher or lower amount of hydrogen in each run, which results in a variable or uncontrollable process. Moreover, it would be impractical to obtain high amounts of ruminal fluid in order to add it in each production batch. For these reasons, different conditions were evaluated for the preparation of the culture medium.
Figure 3b show the production profile of H2 for each treatment. It is observed that the highest production of H2 was achieved when all nutrients were mixed and sterilized together (treatment MCRB 3-c). Interestingly, no significant statistical difference was found when the nutrients were sterilized separately (treatment MCRB 3-b) as compared to treatment MCRB 3-c (p-value < 0.05), which suggests that the physical–chemical changes undergone in the culture medium (chemical reactions, precipitation, etc.) had no net effect on the production of hydrogen.
On the other hand, the sterilization of the bovine ruminal fluid with UV light significantly reduced the production of H2, probably because the UV light degraded some of its components. It has been reported that the amounts of water- and fat-soluble vitamins in milk are affected by UV treatment. Specifically, vitamins C and E are more sensitive to UV light. Ultraviolet light sensitivities for cow and goat milk samples were in the following order: vitamin C > vitamin E > vitamin A > vitamin B2 [26].
It is worth mentioning that when the ruminal fluid of the culture medium was eliminated (treatment MCRB 3-d), the production of H2 was reduced to half as compared to the medium that contained the ruminal fluid. Bovine ruminal fluid is a metabolically diverse biofluid, with representative metabolites. Specifically, the composition of the bovine ruminal liquid is dominated by phospholipids, inorganic ions and gases (CO2, CH4, N2, and H2), amino acids, dicarboxylic acids, short-chain fatty acids (SCFAs), vitamins, fat, protein, fiber, and carbohydrates [27]. In the absence of such nutrients of the rumen, the microorganisms produced a smaller amount of H2. Finally, it was determined to prepare the culture medium mixing all the components (including bovine ruminal fluid) and sterilize them in the autoclave altogether. Future studies may include the change in ruminal fluid for the addition of the nutrients present in the rumen in order to improve the amount of hydrogen produced.
3.3. Effect of Culture Gas Volume
It has been reported that the partial pressure of the hydrogen (pH2) produced in the reactor’s headspace is autoinhibitory of growth and H2 production [3]. As the gaseous space of microbial cultures is increased, the partial pressure of hydrogen decreases. In order to evaluate the effect of the volume of the ruminal culture gaseous phase, two headspaces (40 and 80 mL) were compared on the production of hydrogen using MCRB 7 and 20 (Figure 4).
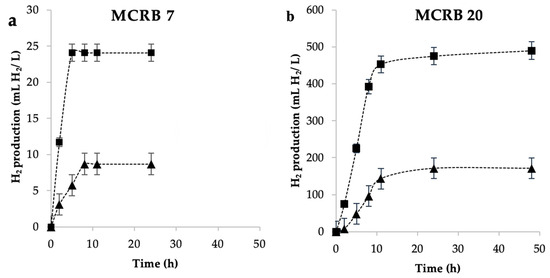
Figure 4.
Effect of the headspace volume (■) 80 mL and (▲) 40 mL on the production of H2 in the cultures of (a) 7 and (b) 20 MCRB.
In a head space of 40 mL, MCRB 20 produced 171 mL/L medium, which was 19-fold more hydrogen as compared to MCRB 7 (8.7 mL/L medium). However, hydrogen production was increased by three times when the head space of the cultures was 80 mL with both consortiums (475 mL/L medium for MCRB 20 and 20 mL/L medium for MCRB 7). ANOVA showed that there are significant statistical differences between treatments (p-value < 0.05). Such behavior can be explained in terms of the partial pressure exerted by the produced hydrogen. Several studies indicate that a high pH2 results in hydrogen dissolution in the medium that modifies the thermodynamic control of the metabolic pathways associated with the production of hydrogen, redirecting the flux to the production of lactate, ethanol, acetone, butanol, and the appearance of homoacetogenic pathways, all of which decrease the production of hydrogen [28,29]. Therefore, further experiments were carried out in 120 mL serological bottles with a headspace of 80 mL.
3.4. Thermal Treatment
H2-producing and H2-consuming organisms (methanogens) frequently coexist in microbial consortia. To suppress the bacterial activity of methanogenic microorganisms while preserving the activity of the H2-producing bacteria [30], consortia are subjected to stress by different treatments such as acidic, alkaline, or thermal, as well as aeration, freezing–thawing, and chloroform [11].
In this study, 10 MCRB from 10 different cows were evaluated to produce H2 at 105 °C for 20 min, 4 h, and 24 h, and without heat treatment (Figure 5).
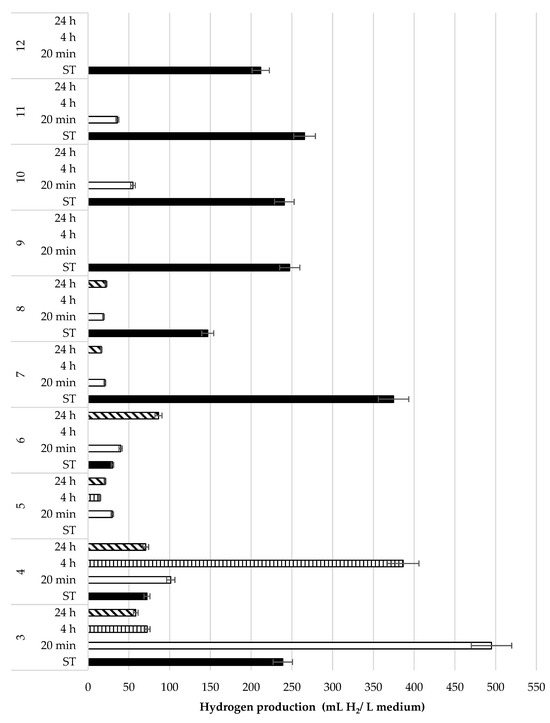
Figure 5.
Hydrogen production with 10 microbial MCRB (3, 4, 5, 6, 7, 8, 9, 10, 11, and 12) from bovine rumen with different thermal treatment time (ST: without treatment; 20 min, 4 h, 24 h).
The highest production of H2 (495 mL) was obtained with 20 min thermal treatment (MCRB 3), followed by the thermal treatment of 4 h (MCRB 4, 387 mL H2/L medium). It should be noted that most of the samples without thermal treatment produced a greater amount of H2 as compared to the heat-treated samples. Thermal treatment is commonly used to eliminate methanogenic microorganisms or other bacteria that interfere with the production of H2 [30]. This procedure is based upon the fact that H2-producing anaerobic bacteria form endospores when unfavorable environmental conditions are encountered; once favorable conditions return, the spores end up as vegetative cells [31]. It has been reported that the heat treatment of MCRB at 105 °C for 24 h before the preparation of inoculum is best to produce H2 [32,33]. In contrast, in the present study, it was observed that most of the MCRB produced a lower amount of H2 when the microbial consortia were subjected to 105 °C for 24 h as compared with other times of exposure to thermal treatment. ANOVA indicates significant statistical differences between treatments (p-value < 0.05); also, the LSD test indicates that the thermal treatments at different times belong to the same group, while the samples without treatment belong to another group.
Interestingly, the highest H2 production was obtained without heat treatment. These results may be owed to the fact that the MCRB without heat treatment were inoculated in a culture medium rich in vitamins and minerals that favored the production of H2. Culture media rich in nutrients eliminate the syntrophy that initially exists between bacteria and archaea, suppressing methanogen microorganisms that results in a greater production of H2 [34]. Also, it is probable that during heat treatment, not only methanogens are eliminated, but also some H2-producing organisms.
With the aim to observe the changes undergone in the microbiota after thermal treatment, MCRB 7 was selected to identify the microbial genera in the cultures employed to produce hydrogen after thermal treatment (105 °C for 24 h) or without thermal treatment (by culturing MCRB 7 in enriched culture MB medium).
MCRB 7 without thermal treatment showed at least twice the variety of genera as compared to the sample with heat treatment. The genera found were Streptococcus sp., Lactobacillus, Clostridium sensu, Prevotella, Incertae Sedis (which might contain Clostridia, Lactobacillus, Thermoanaerobacterales, and Bacillus, all hydrogen producers), Klebsiella, Selenomonas, Enterococcus), and Paenibacillus. On the other hand, the consortium that was thermally treated contained only Enterococcus, Weissella sp., Incertae Sedis, and Rummeliibacillus sp. These findings support the fact that MCRB 7 without heat treatment had a higher production of hydrogen since Prevotella, Klebsiella, Selenomonas, and Paenibacillus have been identified as hydrogen producers [35]
On the other hand, Nissila et al. [12] tested different rumen liquids and found that the optimum temperature and pH to produce H2 were 62 °C and 7.3, respectively; the maximum amount of H2 reported was 1.2 mL H2/L medium obtained with samples without heat treatment. Ratti et al. [18] obtained 829 mL H2/L medium with ruminal fluid pretreated with perchloric acid (HClO4). Botta et al. [19] pretreated the ruminal fluid with hydrochloric acid (HCl) and reported a yield of 61.7 mL H2/L medium using 0.5 g of office paper as the substrate.
These results demonstrate that there are less aggressive treatments, which promote a higher production of H2. To the best of our knowledge, this had not been reported with bovine rumen microorganisms.
3.5. Kinetics of Hydrogen Production
The amount of hydrogen produced depends on the type of microorganisms present in the ruminal consortia. However, the rumen contains a great diversity of microbial species and the synergism and antagonism between the groups of microorganisms and even between the different genera in the same groups is so diverse and complex that it makes it difficult to quantify the activity that each particular group of microorganisms carries out in the rumen [36]. However, our interest lies in the final result of such interactions for the production of hydrogen and, therefore, the analysis of the kinetics of the H2 production of microbial communities of bovine rumen was carried out. Thirty-two microbial communities sampled from thirty-two different bovines were compared to each other. Figure 6a shows the kinetics of H2 production of 10 out of the 32 samples. It can be observed that the behavior is completely different between the samples. MCRB 6 reached the maximum production of H2 at 11 h with a production of 24 mL of H2, while MCRB 7 reached the maximum around 50 h with a maximum production of almost 700 mL of H2.
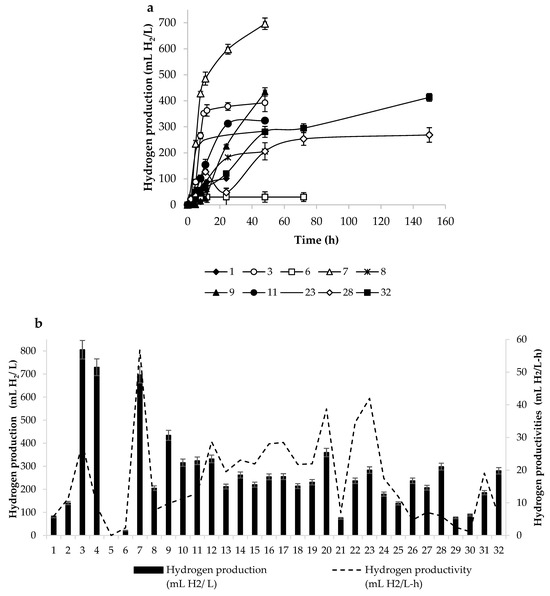
Figure 6.
(a) Kinetics of H2 production with microorganisms from bovine rumen without heat treatment for samples 1, 3, 6, 7, 8, 9, 11, 23, 28, and 32; (b) production and productivity of H2 by microorganisms from bovine rumen.
The reason for knowing the kinetics of H2 production of these selected samples is the variation over time in the H2 production. For instance, the vast majority of the samples produced the largest amount of H2 before 10 h, although some consortia produced larger amounts after 10 h. The maximum time to reach the highest volume of H2 was 150 h (sample 32) and the maximum volume produced was 700 mL (sample 7) in 48 h. On the other hand, it can also be seen (Figure 6) that the amount of H2 kept constant after a certain time, which indicates that there is no presence of methanogen microorganisms that consume H2. This corroborates the elimination of methanogen microorganisms by the medium rich in nutrients.
Figure 6b shows the total production of H2 and the rate of H2 production (productivity) of the 32 consortia. It can be seen that some consortia (30 and 29) produced very little hydrogen at a low rate (88.6 mL/L medium, 1.14 mL/L-h and 76.9 mL/L medium, 2.52 mL/L-h, respectively). The highest productivity was achieved with consortium 7 (56.7 mL/L-h) and 700 mL H2/L, while the highest production of hydrogen was obtained with consortium 3 (805.4 mL H2/L medium) although with a lower productivity (28.3 mL/L-h) as compared to consortium 7. These behaviors in H2 production, productivity, and time can be attributed to the variety of microorganisms present in each sample. Diversity exists due to different factors, mainly cattle feeding, which directly affects the rumen microbiota since feeding depends on the purpose (fattening cattle, dairy cattle, disposal of lignocellulosic materials by region, etc.) [23,24].
Moreover, the microbial composition of the bovine rumen is very sensitive to changes in the diet, although it can also be attributed to age, season of the year, geographical location, diet, and in general to the health of the animal [23,24,35]. Therefore, in order to obtain a high production of hydrogen with ruminal microbiota, it is important to select a consortium able to produce a high amount of hydrogen with a high productivity.
It is worth mentioning that during the experiments carried out in this work, it was observed that, with many subcultures from consortia, the production of hydrogen gradually decreased; therefore, future work will be focused on the study of the conservation of consortia to achieve a constant production of hydrogen through the course of time.
4. Conclusions
In this study, a great variability in the production of hydrogen was found among different ruminal microbial communities (among bovines), a fact that to the best of our knowledge has not been reported before, which suggests that it is necessary to analyze MCRB to select the consortia that have a higher production of hydrogen.
Despite the variability in the production of hydrogen, which can be attributed to the composition within the ruminal microbiota and the diversity of different consortia (different bovines), we were able to find culture conditions that have the same tendency for the production of hydrogen.
It is possible to obtain a higher amount of hydrogen by eliminating methanogenic microorganisms employing an enriched culture medium, as compared to the elimination of methanogenic by thermal treatment at 105 °C. These results demonstrate that there are less aggressive treatments, which promote a higher production of H2. To the best of our knowledge, this has not been reported with microorganisms from the rumen of bovines.
When the ruminal fluid was removed from the MB culture medium, the production of H2 was approximately half the maximum obtained, highlighting the role of ruminal fluid on the production of hydrogen.
This study provides new information to produce hydrogen by microbial consortia and shows that it is possible to obtain high volumes of H2 using microorganisms from bovine rumen. Our findings show a different perspective on the treatment of MCRB for the production of hydrogen and give insights on the impact of the culture conditions on increasing hydrogen production. From the findings in this contribution, future works will focus on analyzing the conditions to reduce variations in the production of hydrogen from ruminal consortia to design culture media with no ruminal fluid to allow keeping a high hydrogen production and the conservation of consortia to achieve a constant production of hydrogen through the course of time.
Author Contributions
R.I.C.-G. and C.P.-O.: conceptualization, methodology, resources, validation supervision, funding acquisition, writing, review, and editing; V.M.G.-A. and G.T.: conceptualization, investigation, methodology, writing original draft; G.M.G.-M. and G.T.: validation, writing, review, and editing; Á.d.J.M.-G.: methodology, visualization, writing, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Universidad de Guadalajara [PROSNI 2019], Jalisco State of México.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.
Acknowledgments
VMGA would like to thank Consejo Nacional de Humanidades, Ciencia y Tecnología (CONAHCYT) for scholarship CVU: 921906.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, V.; Mahata, C.; Das, D. Optimization for simultaneous enhancement of biobutanol and biohydrogen production. Int. J. Hydrogen Energy. 2021, 46, 3726–3741. [Google Scholar] [CrossRef]
- García-Sánchez, R.; Ramos-Ibarra, R.; Guatemala-Morales, G.; Arriola-Guevara, E.; Toriz-González, G.; Corona-González, R.I. Photofermentation of tequila vinasses by Rhodopseudomonas pseudopalustris to produce hydrogen. Int. J. Hydrogen Energy 2018, 43, 15857–15869. [Google Scholar] [CrossRef]
- Teke, G.M.; Anye Cho, B.; Bosman, C.E.; Mapholi, Z.; Zhang, D.; Pott, R.W.M. Towards industrial biological hydrogen production: A review. World J. Microbiol. Biotechnol. 2024, 40, 37. [Google Scholar]
- IEA 50. Hydrogen. Overview. Conference. Last Update on 10 July 2023. Available online: https://www.iea.org/energy-system/low-emission-fuels/hydrogen (accessed on 6 May 2024).
- Katebah, M.; Al-Rawashdeh, M.; Linke, P. Analysis of hydrogen production costs in Steam-Methane Reforming considering integration with electrolysis and CO2 capture. Clean Eng. Technol. 2022, 10, 100552. [Google Scholar] [CrossRef]
- Kayfeci, M.; Keçebaş, A.; Bayat, M. Hydrogen production. In Solar Hydrogen Production: Processes, Systems and Technologies; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 3; pp. 45–83. [Google Scholar] [CrossRef]
- Soares, J.F.; Confortin, T.C.; Todero, I.; Mayer, F.D.; Mazutti, M.A. Dark fermentative biohydrogen production from lignocellulosic biomass: Technological challenges and future prospects. Renew. Sustain. Energy Rev. 2020, 117, 109484. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Ubando, A.T.; Chen, W.H.; Hurt, D.A.; Conversion, A.; Rajendran, S.; Lin, S.L. Biohydrogen in a circular bioeconomy: A critical review. Bioresource Technol. 2022, 366, 128168. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wan, W. Factors influencing fermentative hydrogen production: A review. Int. J. Hydrogen Energy 2009, 34, 799–811. [Google Scholar] [CrossRef]
- Nissila, M.E.; Tähti, H.P.; Rintala, J.A.; Puhakka, J.A. Thermophilic hydrogen production from cellulose with rumen fluid enrichment cultures: Effects of different heat treatments. Hydrogen Energy 2010, 36, 1482–1490. [Google Scholar] [CrossRef]
- DePeters, E.J.; George, L.W. Rumen transfaunation. Immunol. Lett. 2014, 162, 69–76. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, R.; Chang, J.; Chen, L.; Nabi, M.; Zhang, H.; Zhang, G.; Zhang, P. Rumen microbes, enzymes, metabolisms, and application in lignocellulosic waste conversion-A comprehensive review. Biotechnol. Adv. 2024, 71, 108308. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Nabi, M.; Zhang, P.; Zhang, G.; Cai, Y.; Wang, O.; Zhou, Z.; Ding, Y. Promising biological conversion of lignocellulosic biomass to renewable energy with rumen microorganisms: A comprehensive review. Renew. Sustain. Energy Rev. 2020, 134, 110335. [Google Scholar] [CrossRef]
- Liang, J.; Fang, W.; Wang, Q.; Zubair, M.; Zhang, G.; Ma, W.; Cai, Y.; Zhang, P. Metagenomic analysis of community, enzymes and metabolic pathways during corn straw fermentation with rumen microorganisms for volatile fatty acid production. Bioresour. Technol. 2021, 342, 126004. [Google Scholar] [CrossRef] [PubMed]
- Matthews, C.; Crispie, F.; Lewis, E.; Reid, M.; O’Toole, P.W.; Cotter, P.D. The rumen microbiome: A crucial consideration when optimising milk and meat production and nitrogen utilisation efficiency. Gut Microbes 2019, 10, 115–132. [Google Scholar] [CrossRef]
- Ratti, R.P.; Botta, L.S.; Sakamoto, I.K.; Silva, E.L.; Amancio, M.B. Production of H2 from cellulose by rumen microorganisms: Effects of inocula pre-treatment and enzymatic hydrolysis. Biotecnol. Lett. 2014, 36, 537–546. [Google Scholar] [CrossRef]
- Botta, L.S.; Ratti, R.P.; Sakamoto, I.K.; Rodrigues, L.; Silva, E.L.; Amâncio, M.B. Bioconversion of waste office paper to hydrogen using pretreated rumen fluid inoculum. Bioprocess Biosyst. Eng. 2016, 39, 1887–1897. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.; Perelmuter, K.; Valencia, M.J.; Cajarville, C.; Zunino, P. Caracterización de la microbiota bacteriana ruminal de un bovino a pastoreo mediante técnicas clásicas e independientes del cultivo. Veterinaria (Montev.) 2013, 49, 40–55. Available online: https://www.revistasmvu.com.uy/index.php/smvu/article/view/218 (accessed on 15 March 2024).
- DSMZ. DSMZ-German Collection of Microorganisms and Cell Cultures GmbH. [WWW Document]. List Recomm Media Microorg. 2022. Available online: https://www.dsmz.de/collection/catalogue/microorganisms/culture-technology/list-of-media-for-microorganisms (accessed on 26 October 2023).
- Schären, M.; Kiri, K.; Riede, S.; Gardener, M.; Meyer, U.; Hummel, J.; Urich, T.; Breves, G.; Dänicke, S. Alterations in the rumen liquid- particle- and epithelium-associated microbiota of dairy cows during the transition from a silage- and concentrate-based ration to pasture in spring. Front. Microbiol. 2017, 8, 744. [Google Scholar] [CrossRef]
- Ji, S.; Zhang, H.; Yan, H.; Azarfar, A.; Shi, H.; Alugongo, G.; Li, S.; Cao, Z.; Wang, Y. Comparison of rumen bacteria distribution in original rumen digesta, rumen liquid and solid fractions in lactating Holstein cows. J. Anim. Sci. Biotechnol. 2017, 8, 16. [Google Scholar] [CrossRef]
- Petri, R.M.; Forster, R.J.; Yang, W.; McKinnon, J.J.; McAllister, T.A. Characterization of rumen bacterial diversity and fermentation parameters in concentrate fed cattle with and without forage. J. Appl. Microbiol. 2012, 112, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Franco-Leon, J.J.; Arriola-Guevara, E.; Suarez-Hernandez, L.A.; Toriz, G.; Guatemala-Morales, G.; Corona-Gonzalez, R.I. Influence of supplemented nutrients in tequila vinasses for hydrogen and polyhydroxybutyrate production by photofermentation with Rhodopseudomonas pseudopalustris. Bioresour Technol. 2021, 329, 124865. [Google Scholar] [CrossRef]
- Guneser, O.; Karagul Yuceer, Y. Effect of ultraviolet light on water- and fat-soluble vitamins in cow and goat milk. J. Dairy Sci. 2012, 95, 6230–6241. [Google Scholar] [CrossRef] [PubMed]
- Saleem, F.; Bouatra, S.; Guo, A.C.; Psychogios, N.; Mandal, R.; Dunn, S.M.; Ameta, B.N.; Wishart, D.S. The Bovine Ruminal Fluid Metabolome. Metabolomics 2013, 9, 360–378. [Google Scholar] [CrossRef]
- Castelló, E.; Nunes Ferraz, A.D.; Andreani, C.; Anzola-Rojas, M.P.; Borzacconi, L.; Buitrón, G.; Carrillo-Reyes, J.; Gomes, S.D.; Maintinguer, S.I.; Moreno-Andrade, I.; et al. Stability problems in the hydrogen production by dark fermentation: Possible causes and solutions. Renew. Sustain. Energy Rev. 2020, 119, 109602. [Google Scholar] [CrossRef]
- Palomo-Briones, R.; Celis, L.B.; Méndez-Acosta, H.O.; Bernet, N.; Trably, E.; Razo-Flores, E. Enhancement of mass transfer conditions to increase the productivity and efficiency of dark fermentation in continuous reactors. Fuel 2019, 254, 115648. [Google Scholar] [CrossRef]
- Brentner, L.B.; Jordan, P.A.; Zimmerman, J.B. Challenges in developing biohydrogen as a sustainable energy source: Implications for a research agenda. Environ. Sci. Technol. 2010, 44, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Cheong, D.Y.; Hansen, C.L. Bacterial stress enrichment enhances anaerobic hydrogen production in cattle manure sludge. Appl. Microbiol. Biotechnol. 2006, 72, 35–643. [Google Scholar] [CrossRef] [PubMed]
- Arreola-Vargas, J.; Alatriste-Mondragón, F.; Celis, L.B.; Razo-Flores, E.; López-López, A.; Méndez-Acosta, H.O. Continuous hydrogen production in a trickling bed reactor by using triticale silage as inoculum: Effect of simple and complex substrates. J. Chem. Technol. Biotechnol. 2014, 90, 1062–1069. [Google Scholar] [CrossRef]
- Contreras-Dávila, C.A.; Méndez-Acosta, H.O.; Arellano-García, L.; Alatriste-Mondragón, F.; Razo-Flores, E. Continuous hydrogen production from enzymatic hydrolysate of Agave tequilana bagasse: Effect of the organic loading rate and reactor configuration. Chem. Eng. J. 2017, 313, 671–679. [Google Scholar] [CrossRef]
- Arreola-Vargas, J.; Flores-Larios, A.; González-Álvarez, V.; Corona-González, R.I.; Mendez-Acosta, H.O. Single and two-stage anaerobic digestion for hydrogen and methane production from acid and enzymatic hydrolysates of Agave tequilana bagasse. Int. J. Hydrogen Energy 2015, 41, 897–904. [Google Scholar] [CrossRef]
- Hobson, P.N. The Rumen Microbial Ecosystem; Blacki Academic & Professional: Aberdeen, UK, 1997. [Google Scholar] [CrossRef]
- Kamra, D.N. Rumen microbial ecosystem. Curr. Sci. 2005, 89, 124–135. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).