Specific Organic Loading Rate Control for Improving Fermentative Hydrogen Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Fixed-Bed Up-Flow Reactors
2.2. Substrate and Inoculum
2.3. Physical–Chemical Analyses
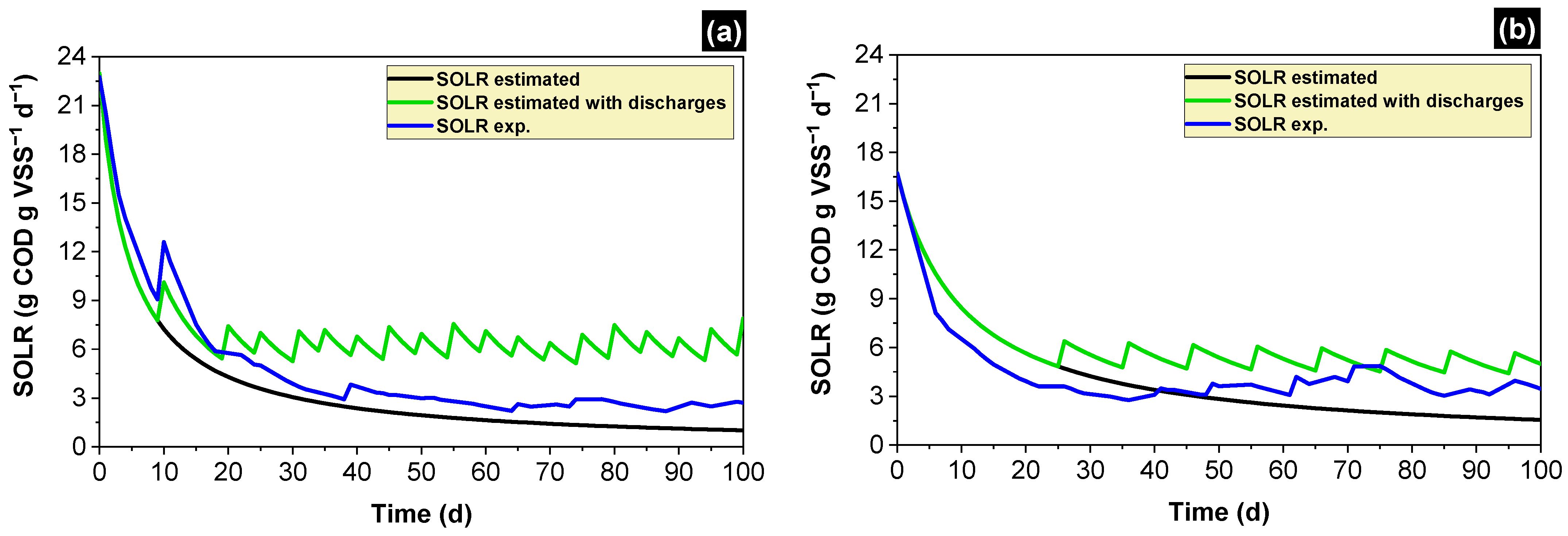
2.4. Calculation of the Specific Organic Loading Rate (SOLR)
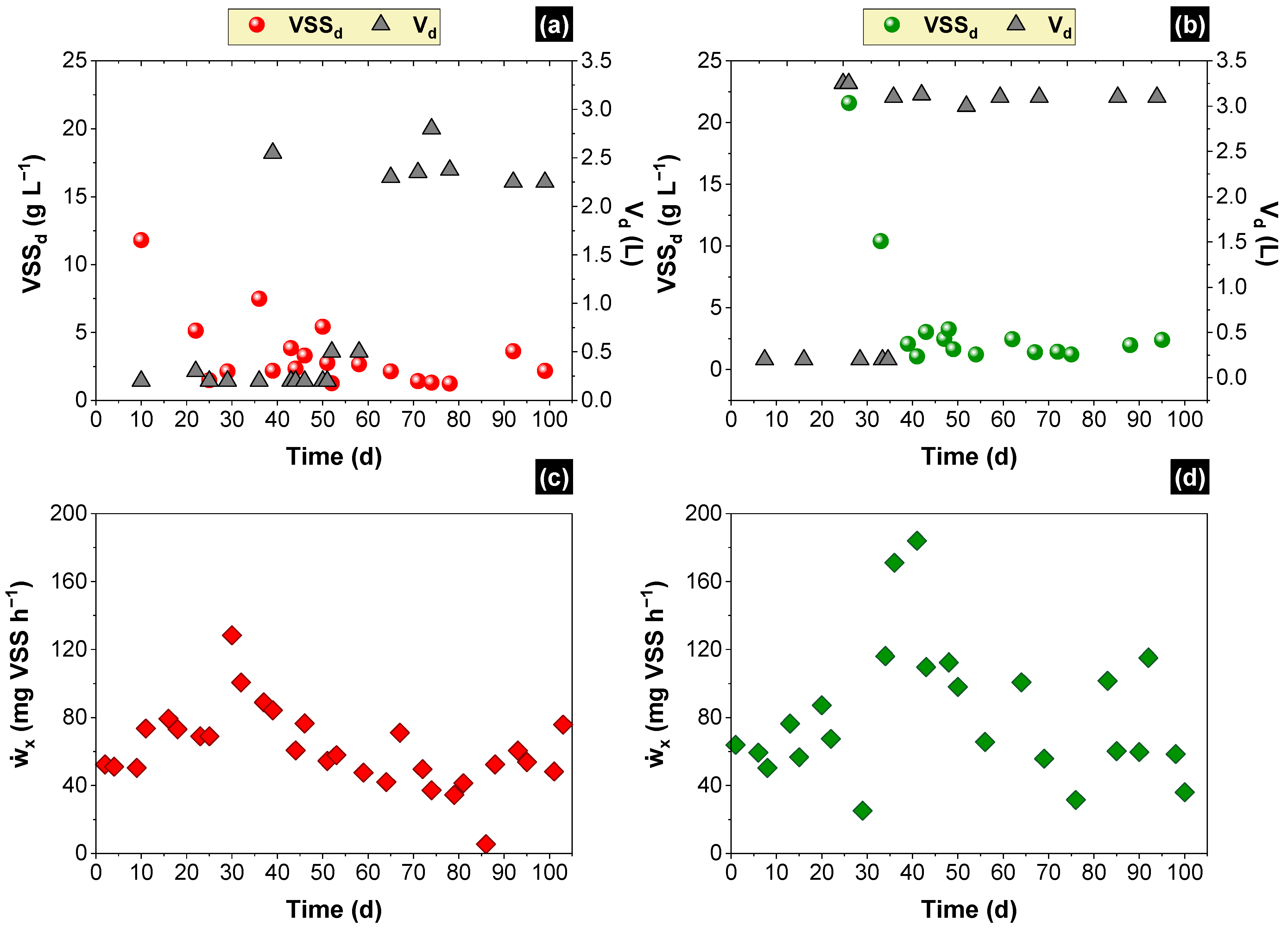
2.5. Estimating the SOLR along the Time and Scheduling Discharges of Biomass
2.6. Quantification of Hydrogen Consumption
3. Results and Discussion
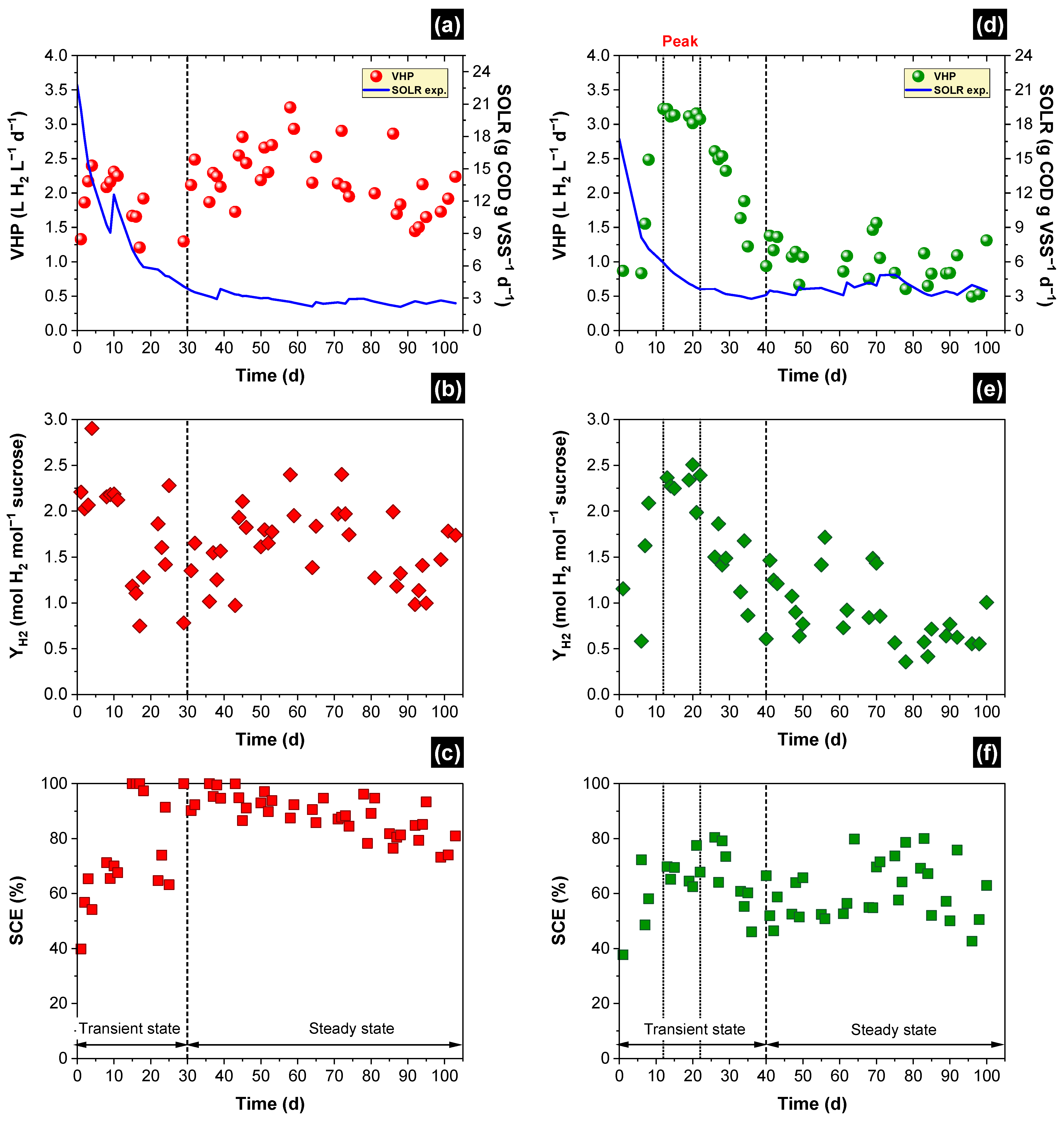
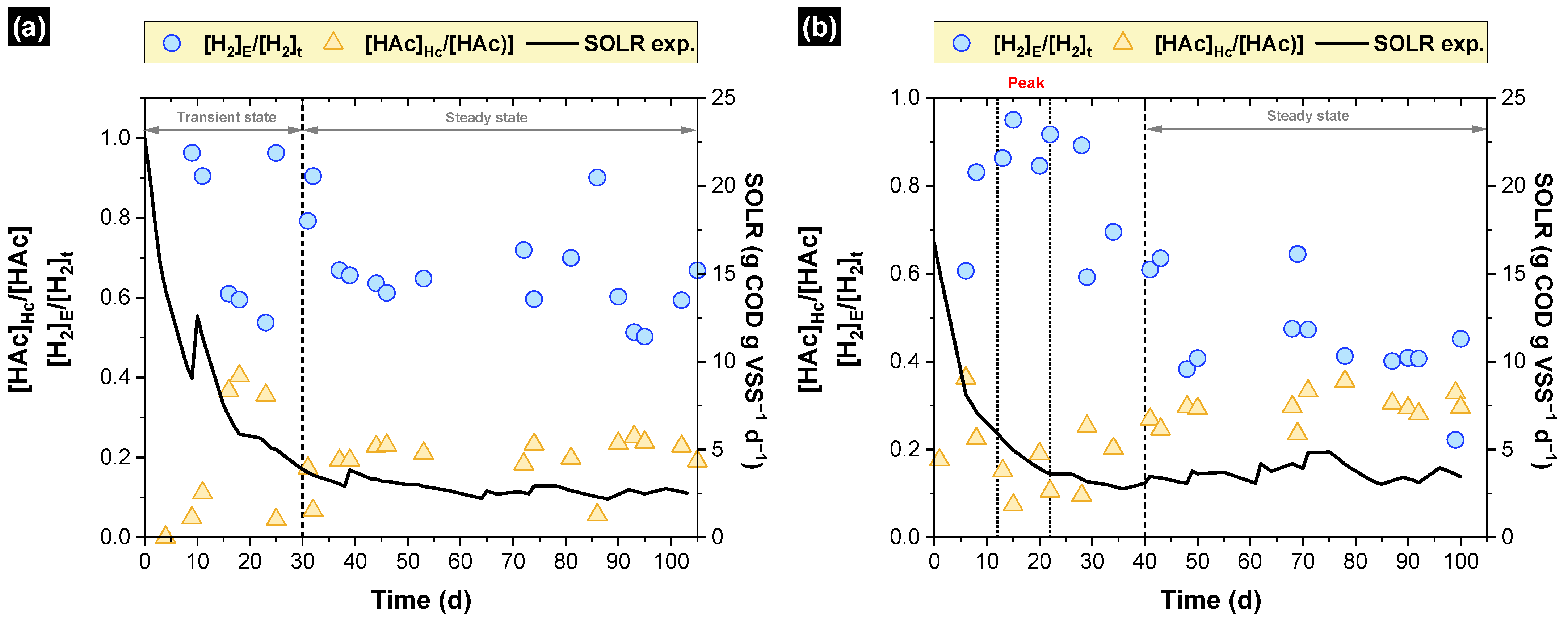
3.1. Simulated and Experimental SOLR
3.2. Hydrogen Production
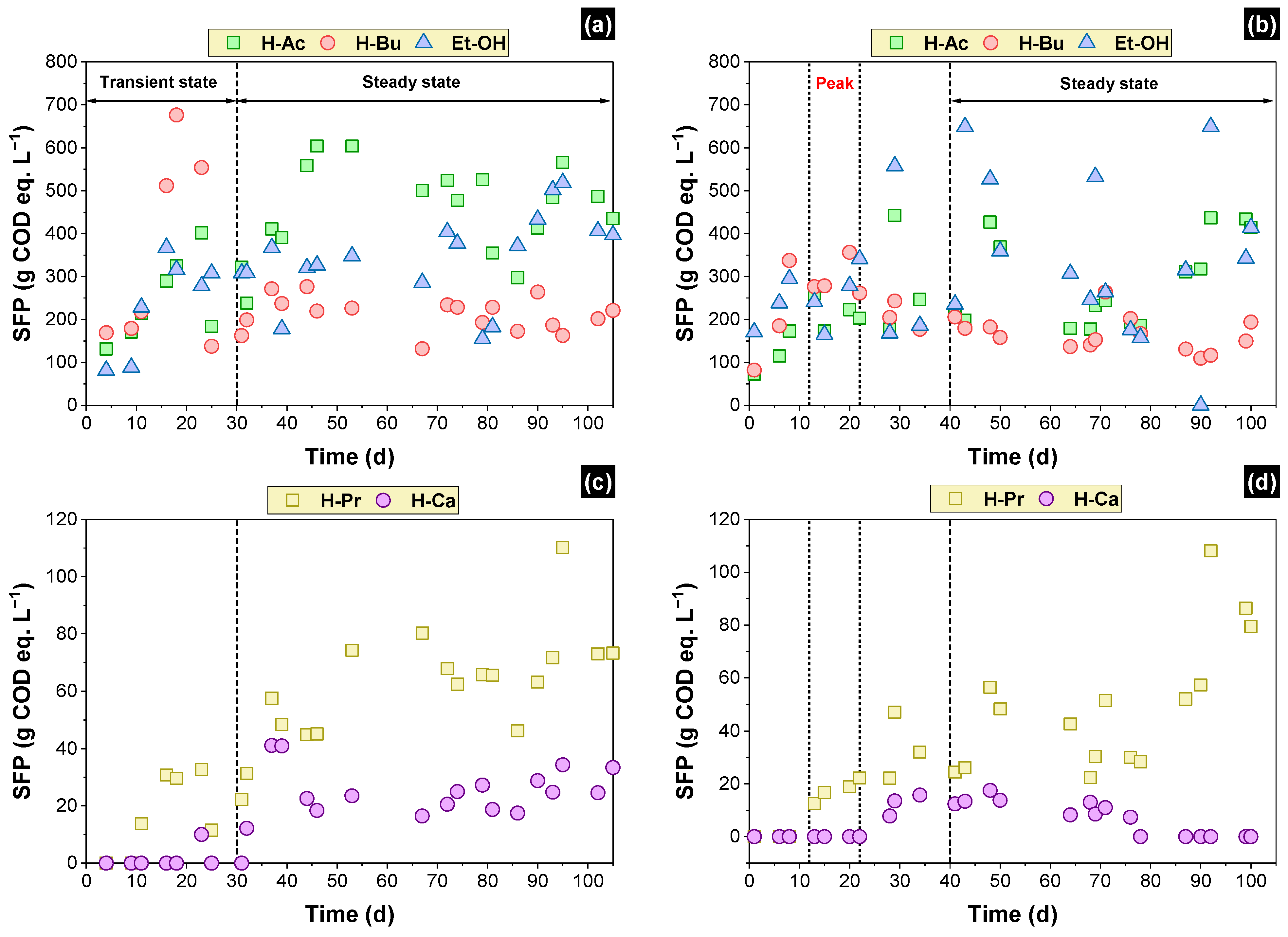
3.3. Soluble Fermentation Products (SFP)
3.4. Hydrogen Consumption
3.5. Unreleased Hydrogen—Where Is the Hydrogen?
3.6. Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC. Climate Change 2014 Synthesis Report; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- IRENA. Geopolitics of the Energy Transformation: The Hydrogen Factor; IRENA: Abu Dhabi, United Arab Emirates, 2022; ISBN 9789292603700. [Google Scholar]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Maggio, G.; Nicita, A.; Squadrito, G. How the Hydrogen Production from RES Could Change Energy and Fuel Markets: A Review of Recent Literature. Int. J. Hydrogen Energy 2019, 44, 11371–11384. [Google Scholar] [CrossRef]
- Ajanovic, A.; Sayer, M.; Haas, R. The Economics and the Environmental Benignity of Different Colors of Hydrogen. Int. J. Hydrogen Energy 2022, 47, 24136–24154. [Google Scholar] [CrossRef]
- IEA. The Future of Hydrogen; IEA: Paris, France, 2019. [Google Scholar] [CrossRef]
- Ntaikou, I.; Antonopoulou, G.; Lyberatos, G. Biohydrogen Production from Biomass and Wastes via Dark Fermentation: A Review. Waste Biomass Valorization 2010, 1, 21–39. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Various Additives for Improving Dark Fermentative Hydrogen Production: A Review. Renew. Sustain. Energy Rev. 2018, 95, 130–146. [Google Scholar] [CrossRef]
- Dahiya, S.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Renewable Hydrogen Production by Dark-Fermentation: Current Status, Challenges and Perspectives. Bioresour. Technol. 2021, 321, 124354. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Y.; Wang, J. Recent Advance in Inhibition of Dark Fermentative Hydrogen Production. Int. J. Hydrogen Energy 2021, 46, 5053–5073. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A Comprehensive Review on Biological Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Saady, N.M.C. Homoacetogenesis during Hydrogen Production by Mixed Cultures Dark Fermentation: Unresolved Challenge. Int. J. Hydrogen Energy 2013, 38, 13172–13191. [Google Scholar] [CrossRef]
- Anzola-Rojas, M.d.P.; Zaiat, M. A Novel Anaerobic Down-Flow Structured-Bed Reactor for Long-Term Stable H2 Energy Production from Wastewater. J. Chem. Technol. Biotechnol. 2016, 91, 1551–1561. [Google Scholar] [CrossRef]
- de Menezes, C.A.; Silva, E.L. Hydrogen Production from Sugarcane Juice in Expanded Granular Sludge Bed Reactors under Mesophilic Conditions: The Role of Homoacetogenesis and Lactic Acid Production. Ind. Crops Prod. 2019, 138, 111586. [Google Scholar] [CrossRef]
- Castelló, E.; Braga, L.; Fuentes, L.; Etchebehere, C. Possible Causes for the Instability in the H2 Production from Cheese Whey in a CSTR. Int. J. Hydrogen Energy 2018, 43, 2654–2665. [Google Scholar] [CrossRef]
- Arooj, M.F.; Han, S.-K.; Kim, S.-H.; Kim, D.-H.; Shin, H.-S. Continuous Biohydrogen Production in a CSTR Using Starch as a Substrate. Int. J. Hydrogen Energy 2008, 33, 3289–3294. [Google Scholar] [CrossRef]
- Luo, G.; Karakashev, D.; Xie, L.; Zhou, Q.; Angelidaki, I. Long-Term Effect of Inoculum Pretreatment on Fermentative Hydrogen Production by Repeated Batch Cultivations: Homoacetogenesis and Methanogenesis as Competitors to Hydrogen Production. Biotechnol. Bioeng. 2011, 108, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Reyes, J.; Celis, L.B.; Alatriste-Mondragón, F.; Razo-Flores, E. Decreasing Methane Production in Hydrogenogenic UASB Reactors Fed with Cheese Whey. Biomass Bioenergy 2014, 63, 101–108. [Google Scholar] [CrossRef]
- Carrillo-Reyes, J.; Celis, L.B.; Alatriste-Mondragón, F.; Montoya, L.; Razo-Flores, E. Strategies to Cope with Methanogens in Hydrogen Producing UASB Reactors: Community Dynamics. Int. J. Hydrogen Energy 2014, 39, 11423–11432. [Google Scholar] [CrossRef]
- Anzola-Rojas, M.d.P.; Da Fonseca, S.G.; Da Silva, C.C.; De Oliveira, V.M.; Zaiat, M. The Use of the Carbon/Nitrogen Ratio and Specific Organic Loading Rate as Tools for Improving Biohydrogen Production in Fixed-Bed Reactors. Biotechnol. Rep. 2015, 5, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, L.; Palomo-Briones, R.; de Jesús Montoya-Rosales, J.; Braga, L.; Castelló, E.; Vesga, A.; Tapia-Venegas, E.; Razo-Flores, E.; Ecthebehere, C. Knowing the Enemy: Homoacetogens in Hydrogen Production Reactors. Appl. Microbiol. Biotechnol. 2021, 105, 8989–9002. [Google Scholar] [CrossRef] [PubMed]
- Thi Nguyen, M.-L.; Hung, P.-C.; Vo, T.-P.; Lay, C.-H.; Lin, C.-Y. Effect of Food to Microorganisms (F/M) Ratio on Biohythane Production via Single-Stage Dark Fermentation. Int. J. Hydrogen Energy 2021, 46, 11313–11324. [Google Scholar] [CrossRef]
- Blanco, V.M.C.; Fuess, L.T.; Zaiat, M. Calcium Dosing for the Simultaneous Control of Biomass Retention and the Enhancement of Fermentative Biohydrogen Production in an Innovative Fixed-Film Bioreactor. Int. J. Hydrogen Energy 2017, 42, 12181–12196. [Google Scholar] [CrossRef]
- Gomes, S.D.; Fuess, L.T.; Mañunga, T.; De Lima Gomes, P.C.F.; Zaiat, M. Bacteriocins of Lactic Acid Bacteria as a Hindering Factor for Biohydrogen Production from Cassava Flour Wastewater in a Continuous Multiple Tube Reactor. Int. J. Hydrogen Energy 2016, 41, 8120–8131. [Google Scholar] [CrossRef]
- Torres, D.G.B.; dal’ Maso Lucas, S.; Andreani, C.L.; de Carvalho, K.Q.; Coelho, S.R.M.; Gomes, S.D. Hydrogen Production and Performance of Anaerobic Fixed-Bed Reactors Using Three Support Arrangements from Cassava Starch Wastewater. Eng. Agric. 2017, 37, 160–172. [Google Scholar] [CrossRef]
- Fuess, L.T.; Fuentes, L.; Bovio-Winkler, P.; Eng, F.; Etchebehere, C.; Zaiat, M.; Oller do Nascimento, C.A. Biohydrogen-Producing from Bottom to Top? Quali-Quantitative Characterization of Thermophilic Fermentative Consortia Reveals Microbial Roles in an Upflow Fixed-Film Reactor. Chem. Eng. J. Adv. 2021, 7, 100125. [Google Scholar] [CrossRef]
- Corbari, S.D.M.L.; Andreani, C.L.; Torres, D.G.B.; Eng, F.; Gomes, S.D. Strategies to Improve the Biohydrogen Production from Cassava Wastewater in Fixed-Bed Reactors. Int. J. Hydrogen Energy 2019, 44, 17214–17223. [Google Scholar] [CrossRef]
- Fuess, L.T.; Mazine Kiyuna, L.S.; Garcia, M.L.; Zaiat, M. Operational Strategies for Long-Term Biohydrogen Production from Sugarcane Stillage in a Continuous Acidogenic Packed-Bed Reactor. Int. J. Hydrogen Energy 2016, 41, 8132–8145. [Google Scholar] [CrossRef]
- Hafez, H.; Nakhla, G.; Naggar, M.H.E.; Elbeshbishy, E.; Baghchehsaraee, B. Effect of Organic Loading on a Novel Hydrogen Bioreactor. Int. J. Hydrogen Energy 2010, 35, 81–92. [Google Scholar] [CrossRef]
- Gomes, S.D.; Fuess, L.T.; Penteado, E.D.; Lucas, S.D.M.; Gotardo, J.T.; Zaiat, M. The Application of an Innovative Continuous Multiple Tube Reactor as a Strategy to Control the Specific Organic Loading Rate for Biohydrogen Production by Dark Fermentation. Bioresour. Technol. 2015, 197, 201–207. [Google Scholar] [CrossRef]
- Stenerson, K. Analysis of Permanent Gases. Report 2004, 3, 3. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association, American Water Works Association: Washington, DC, USA, 2017; ISBN 9780875532875. [Google Scholar]
- Adorno, M.; Hirasawa, J.; Varesche, M. Development and Validation of Two Methods to Quantify Volatile Acids. Am. J. Anal. Chem. 2014, 5, 406–414. [Google Scholar] [CrossRef]
- Maintinguer, S.I.; Fernandes, B.S.; Duarte, I.C.S.; Saavedra, N.K.; Adorno, M.A.T.; Varesche, M.B. Fermentative Hydrogen Production by Microbial Consortium. Int. J. Hydrogen Energy 2008, 33, 4309–4317. [Google Scholar] [CrossRef]
- Matsumoto, M.; Nishimura, Y. Hydrogen Production by Fermentation Using Acetic Acid and Lactic Acid. J. Biosci. Bioeng. 2007, 103, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Dipasquale, L.; d’Ippolito, G.; Fontana, A. Capnophilic Lactic Fermentation and Hydrogen Synthesis by Thermotoga Neapolitana: An Unexpected Deviation from the Dark Fermentation Model. Int. J. Hydrogen Energy 2014, 39, 4857–4862. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.; Chen, M.; Zeng, R.J. Hydrogen Supersaturation in Thermophilic Mixed Culture Fermentation. Int. J. Hydrogen Energy 2012, 37, 17809–17816. [Google Scholar] [CrossRef]
- Ferreira, T.B.; Rego, G.C.; Ramos, L.R.; Soares, L.A.; Sakamoto, I.K.; de Oliveira, L.L.; Varesche, M.B.A.; Silva, E.L. Selection of Metabolic Pathways for Continuous Hydrogen Production under Thermophilic and Mesophilic Temperature Conditions in Anaerobic Fluidized Bed Reactors. Int. J. Hydrogen Energy 2018, 43, 18908–18917. [Google Scholar] [CrossRef]
- Montoya-Rosales, J.d.J.; Ontiveros-Valencia, A.; Esquivel-Hernández, D.A.; Etchebehere, C.; Celis, L.B.; Razo-Flores, E. Metatranscriptomic Analysis Reveals the Coexpression of Hydrogen-Producing and Homoacetogenesis Genes in Dark Fermentative Reactors Operated at High Substrate Loads. Environ. Sci. Technol. 2023, 57, 11552–11560. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mu, Y.; Yu, H.Q. Comparative Performance of Two Upflow Anaerobic Biohydrogen-Producing Reactors Seeded with Different Sludges. Int. J. Hydrogen Energy 2007, 32, 1086–1094. [Google Scholar] [CrossRef]
- Si, B.; Li, J.; Li, B.; Zhu, Z.; Shen, R.; Zhang, Y.; Liu, Z. The Role of Hydraulic Retention Time on Controlling Methanogenesis and Homoacetogenesis in Biohydrogen Production Using Upflow Anaerobic Sludge Blanket (UASB) Reactor and Packed Bed Reactor (PBR). Int. J. Hydrogen Energy 2015, 40, 11414–11421. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, J.; Shen, R.; Zhao, Y.; Lei, C.; Wang, Y. Quantitative Analysis of Hydrogen and Fatty Acid from Homoacetogenesis and Optimization of Glycerol Fermentation Condition. Int. J. Hydrogen Energy 2023, 48, 4182–4192. [Google Scholar] [CrossRef]
- Van Hecke, W.; Bockrath, R.; De Wever, H. Effects of Moderately Elevated Pressure on Gas Fermentation Processes. Bioresour. Technol. 2019, 293, 122129. [Google Scholar] [CrossRef]
- Palomo-Briones, R.; Celis, L.B.; Méndez-Acosta, H.O.; Bernet, N.; Trably, E.; Razo-Flores, E. Enhancement of Mass Transfer Conditions to Increase the Productivity and Efficiency of Dark Fermentation in Continuous Reactors. Fuel 2019, 254, 115648. [Google Scholar] [CrossRef]
- Chang, S.; Li, J.; Liu, F.; Yu, Z. Effect of Different Gas Releasing Methods on Anaerobic Fermentative Hydrogen Production in Batch Cultures. Front. Environ. Sci. Eng. 2012, 6, 901–906. [Google Scholar] [CrossRef]
- Bakonyi, P.; Buitrón, G.; Valdez-Vazquez, I.; Nemestóthy, N.; Bélafi-Bakó, K. A Novel Gas Separation Integrated Membrane Bioreactor to Evaluate the Impact of Self-Generated Biogas Recycling on Continuous Hydrogen Fermentation. Appl. Energy 2017, 190, 813–823. [Google Scholar] [CrossRef]
- Pauss, A.; Andre, G.; Perrier, M.; Guiot, S.R. Liquid-to-Gas Mass Transfer in Anaerobic Processes: Inevitable Transfer Limitations of Methane and Hydrogen in the Biomethanation Process. Appl. Environ. Microbiol. 1990, 56, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
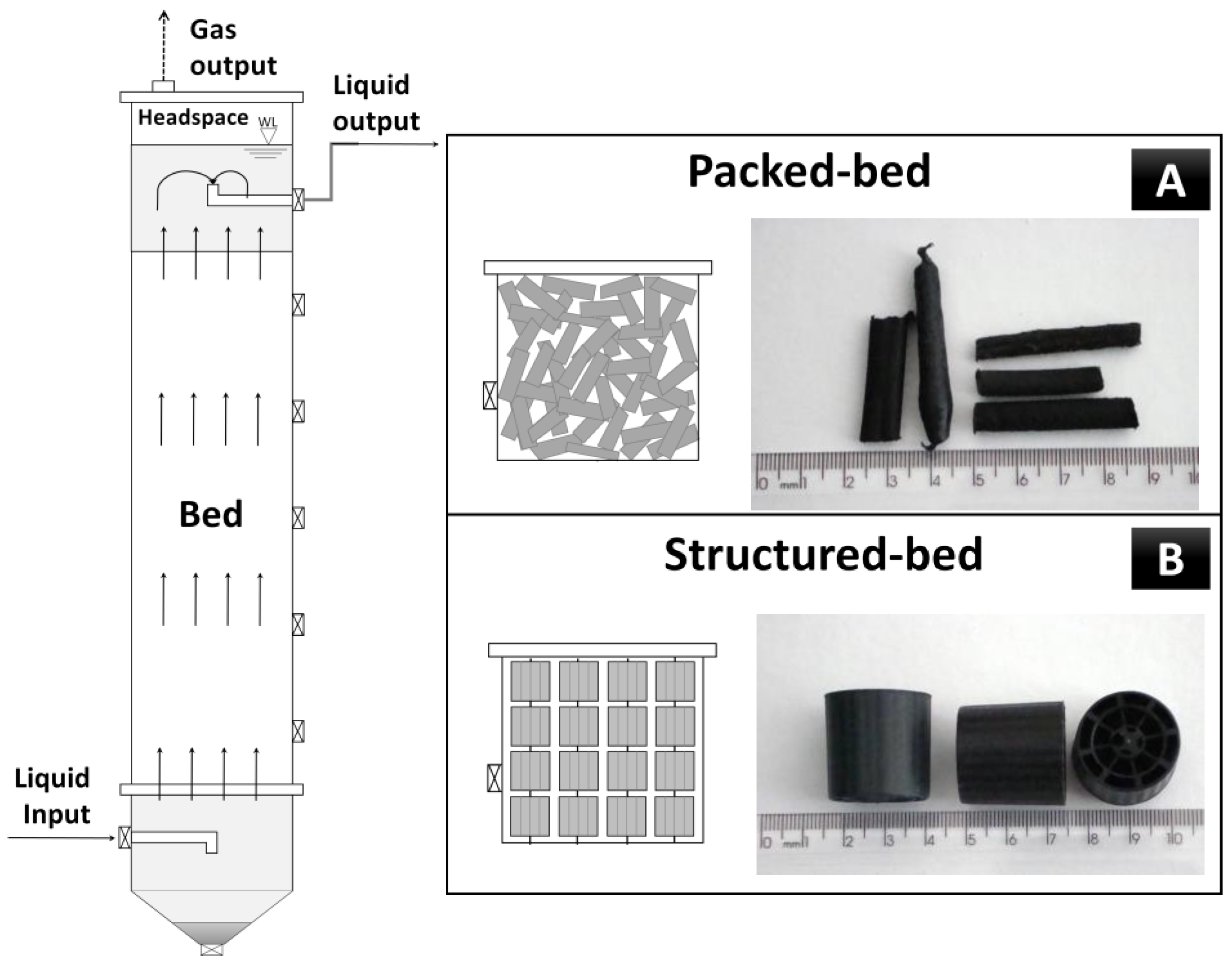
| Features of Reactors | Value | Reference |
|---|---|---|
| for sucrose as substrate | 0.06 g VSS g−1 consumed sucrose | [13] |
| Substrate concentration | 1.79 g sucrose L−1 | This work |
| HRT | 2 h | This work |
| Packed-bed reactor | ||
| Useful volume | 2.8 L | This work |
| Flow | 1.4 L h−1 | This work |
| Substrate conversion (as sucrose concentration) | 80% | [20] |
| Biomass washed out in the effluent | 70% | [20] |
| Structured-bed reactor | ||
| Useful volume | 3.6 L | This work |
| Flow | 1.8 L h−1 | This work |
| Substrate removal (as sucrose concentration) | 70% | [13] |
| Biomass washed out in the effluent | 81% | [13] |
| Biomass/Substrate Count | Packed-Bed Reactor | % | Structured-Bed Reactor | % |
|---|---|---|---|---|
| Retained in the reactor (mg) | 67,417 | 78,507 | ||
| Attached (mg) | 15,504 | 7.1 | 10,207 | 3.8 |
| Suspended (mg) | 5760 | 2.7 | 7502 | 2.8 |
| Discharged frequently (mg) | 46,429 | 21.4 | 60,798 | 22.5 |
| Washed out from the reactor (mg) | 149,612 | 68.8 | 192,282 | 71.0 |
| Total produced biomass (mg) | 217,305 | - | 270,789 | - |
| Total converted sucrose (mg) | 5,000,691 | - | 4,505,748 | - |
| (g VSS g−1 sucrose) | 0.043 | - | 0.060 | - |
| Parameter | Packed-Bed Reactor | Structured-Bed Reactor | ||
|---|---|---|---|---|
| Peak | Steady | |||
| Biogas composition Molar fraction (%) | H2 | 62 ± 2 | 66 ± 1 | 65 ± 4 |
| CO2 | 38 ± 2 | 34 ± 1 | 35 ± 4 | |
| Volumetric hydrogen production—VHP (L H2 L−1 d−1) | Min. | 1.3 | 3.0 | 0.5 |
| Max. | 3.2 | 3.2 | 1.6 | |
| Ave. | 2.2 ± 0.4 | 3.1 ± 0.1 | 1.0 ± 0.3 | |
| St. dev. (%) | 16 | 1.6 | 30 | |
| Hydrogen yield—HY (mole H2 mol−1 sucrose) | Min. | 0.8 | 2.0 | 0.4 |
| Max. | 2.4 | 2.5 | 1.7 | |
| Ave. | 1.6 ± 0.3 | 2.3 ± 0.1 | 0.9 ± 0.3 | |
| St. dev. (%) | 24 | 5 | 30 | |
| Sucrose conversion efficiency—SCE (%) | Min. | 72 | 62 | 43 |
| Max. | 100 | 77 | 80 | |
| Ave. | 89 ± 6 | 68 ± 4 | 61 ± 9 | |
| St. dev. (%) | 7 | 5 | 15 | |
| SOLR (g sucrose g−1 VSS d−1) | Min. | 2.2 | 3.6 | 3.0 |
| Max. | 3.8 | 5.9 | 4.9 | |
| Ave. | 2.8 ± 0.3 | 4.6 ± 0.8 | 3.7 ± 0.4 | |
| St. dev. (%) | 11 | 17 | 12 | |
| Ref. | Fixed-Bed Arrangement/Material (Void Index) | Substrate | Assessed Condition | OLR (g L−1 d−1) | Substrate Conversion (%) | YH2 (mol H2 mol−1 Substrate) | VHP L H2 d−1 L−1 | Steady State Not/Yes (Highlights) |
|---|---|---|---|---|---|---|---|---|
| Anzola-Rojas et al. [20] | Packed-bed RLDP (60%) | Synthetic wastewater based on sucrose | C/N = 40 | 24 | 88.4 ± 5.3 | 1.7 * | ND | NOT (Continuous decrease in H2 production) |
| C/N = 90 | 92.7 ± 7.1 | 3.1 * | ND | |||||
| C/N = 140 | 88.5 ± 5.1 | 3.5 * | ND | |||||
| C/N = 190 | 89.5 ± 9.7 | 2.9 * | ND | |||||
| Anzola-Rojas et al. [13] | Structured-bed and down-flow RLPD (85%) PF (70%) Ceramic (72%) | Synthetic wastewater based on sucrose | RLPD | 24 | 70 ± 11 | 0.4 ± 0.2 | 0.5 ± 0.2 | YES |
| PF | 24 | 71 ± 10 | 0.5 ± 0.2 | 0.6 ± 0.3 | YES | |||
| Ceramic | 24 | 58 ± 14 | 0.1 ± 0.2 | 0.2 ± 0.2 | NOT (H2 production ceased completely after 60 d) | |||
| Same as above with eventual biomass discharges | Synthetic wastewater based on sucrose | RLPD | 24 | 64 ± 11 | 0.6 ± 0.2 | 0.6 ± 0.2 | YES | |
| PF | 24 | 66 ± 9 | 0.6 ± 0.3 | 0.6 ± 0.3 | YES | |||
| Ceramic | 24 | 48 ± 11 | 0.3 ± 0.2 | 0.3 ± 0.2 | YES | |||
| Fuess et al. [28] | Packed-bed Small pieces of RLPD (65%) | Sugarcane stillage | High OLR Eventual biomass discharges ph control between 5.1 and 5.2 | 84.2 | 64 ± 10 | 1.5 ± 1.1 | 0.8 ± 0.6 | NOT (H2 production recovered after operational strategies) |
| Blanco et al. [23] | Structured-bed RLDP (ND) | Synthetic wastewater based on sucrose | COD/Ca = 4423 | 24 | 54 ± 12 | 0.3 ± 0.6 | 0.6 ± 0.6 | NOT (Continuous decrease in H2 production) |
| 2079 | 56 ± 15 | 0.6 ± 0.6 | 1.2 ± 0.5 | |||||
| 1357 | 57 ± 16 | 1.4 ± 1.2 | 1.4 ± 0.6 | |||||
| 1012 | 61 ± 15 | 1.2 ± 1.1 | 1.3 ± 0.4 | |||||
| 884 | 64 ± 15 | 0.2 ± 0.2 | 0.8 ± 0.4 | |||||
| 632 | 67 ± 13 | 0.5 ± 0.4 | 1.3 ± 0.6 | |||||
| Torres et al. [25] | Structured-bed RLDP (ND) Bamboo stems (ND) | Cassava starch wastewater | RLDP | 95 ± 3 | 0.2 | 0.22 | NOT (Continuous decrease in H2 production) | |
| 92 ± 10 | 0.8 | 0.25 | ||||||
| Bamboo stems | 93 ± 11 | 0.15 | 0.17 | |||||
| 93 ± 6 | 0.31 | 0.14 | ||||||
| Corbari et al. [27] | Structured-bed RLDP (76%) | Cassava starch wastewater | Anaerobic Sludge | 10 | 95 ± 3 | 0.2 ± 0.2 | 0.2 ± 0.2 | NOT (Continuous decrease in H2 production) |
| 20 | 90 ± 9 | 0.2 ± 0.2 | 0.5 ± 0.4 | |||||
| Natural fermentation | 20 | 88 ± 12 | 0.3 ± 0.4 | 0.6 ± 0.5 | ||||
| This work | Packed-bed (50%) Structured-bed (85%) | Synthetic wastewater based on sucrose | Controlled biomass discharges | 24 | 89 ± 6 68 ± 4 | 1.6 ± 0.3 0.9 ± 0.3 | 2.2 ± 0.4 1.0 ± 0.3 | YES |
| SFP | Steady State | Peak | ||||
|---|---|---|---|---|---|---|
| Packed-Bed Reactor (mg COD L−1) | COD Eq. (%) | Structured-Bed Reactor (mg COD L−1) | COD Eq. (%) | Structured-Bed Reactor (mg COD L−1) | COD Eq. (%) | |
| Primarily SFP | ||||||
| Acetic acid | 455 ± 87 | 24% | 289 ± 92 | 16% | 214 ± 27 | 12% |
| Butyric acid | 212 ± 32 | 12% | 166 ± 31 | 9% | 293 ± 32 | 16% |
| Ethanol | 344 ± 76 | 18% | 364 ± 127 | 20% | 256 ± 54 | 14% |
| Secondary SFP | ||||||
| Propionic acid | 61 ± 15 | 3% | 50 ± 19 | 3% | 18 ± 3 | 1% |
| Caproic acid | 24 ± 7 | 1% | 12 ± 3 | 1% | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anzola-Rojas, M.d.P.; Fuess, L.T.; Zaiat, M. Specific Organic Loading Rate Control for Improving Fermentative Hydrogen Production. Fermentation 2024, 10, 213. https://doi.org/10.3390/fermentation10040213
Anzola-Rojas MdP, Fuess LT, Zaiat M. Specific Organic Loading Rate Control for Improving Fermentative Hydrogen Production. Fermentation. 2024; 10(4):213. https://doi.org/10.3390/fermentation10040213
Chicago/Turabian StyleAnzola-Rojas, Mélida del Pilar, Lucas Tadeu Fuess, and Marcelo Zaiat. 2024. "Specific Organic Loading Rate Control for Improving Fermentative Hydrogen Production" Fermentation 10, no. 4: 213. https://doi.org/10.3390/fermentation10040213
APA StyleAnzola-Rojas, M. d. P., Fuess, L. T., & Zaiat, M. (2024). Specific Organic Loading Rate Control for Improving Fermentative Hydrogen Production. Fermentation, 10(4), 213. https://doi.org/10.3390/fermentation10040213





