Influence of Cryoextraction and Cold Pre-Fermentative Maceration on the Yeast Microbiota and the Volatile Compounds Profile of Sangiovese Wine
Abstract
1. Introduction
2. Materials and Methods
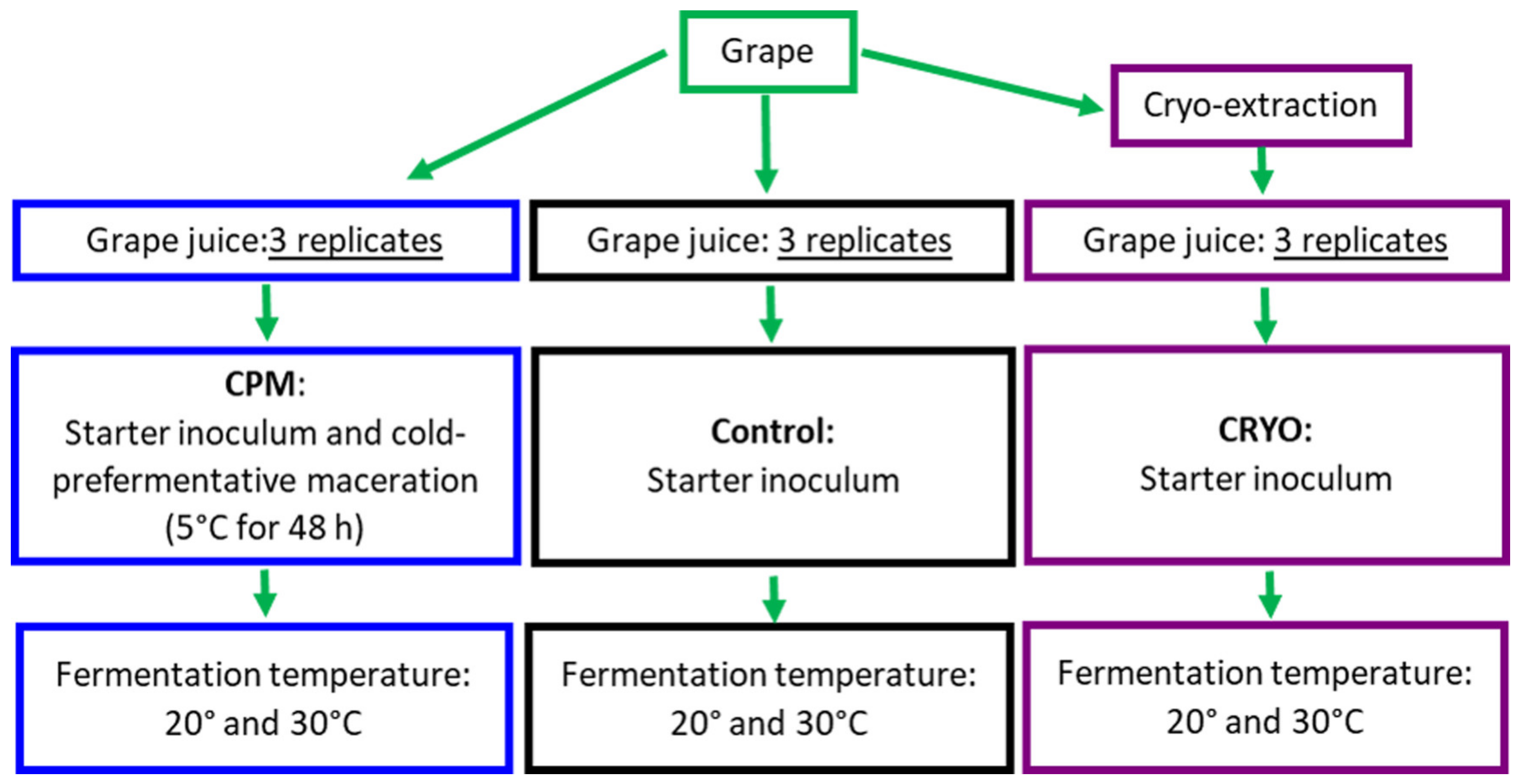
2.1. Winemaking
2.2. Yeast Quantification and Identification
2.3. Chemical Analysis
2.4. Statistical Analysis
3. Results
3.1. Microbiological Analysis of Grapes
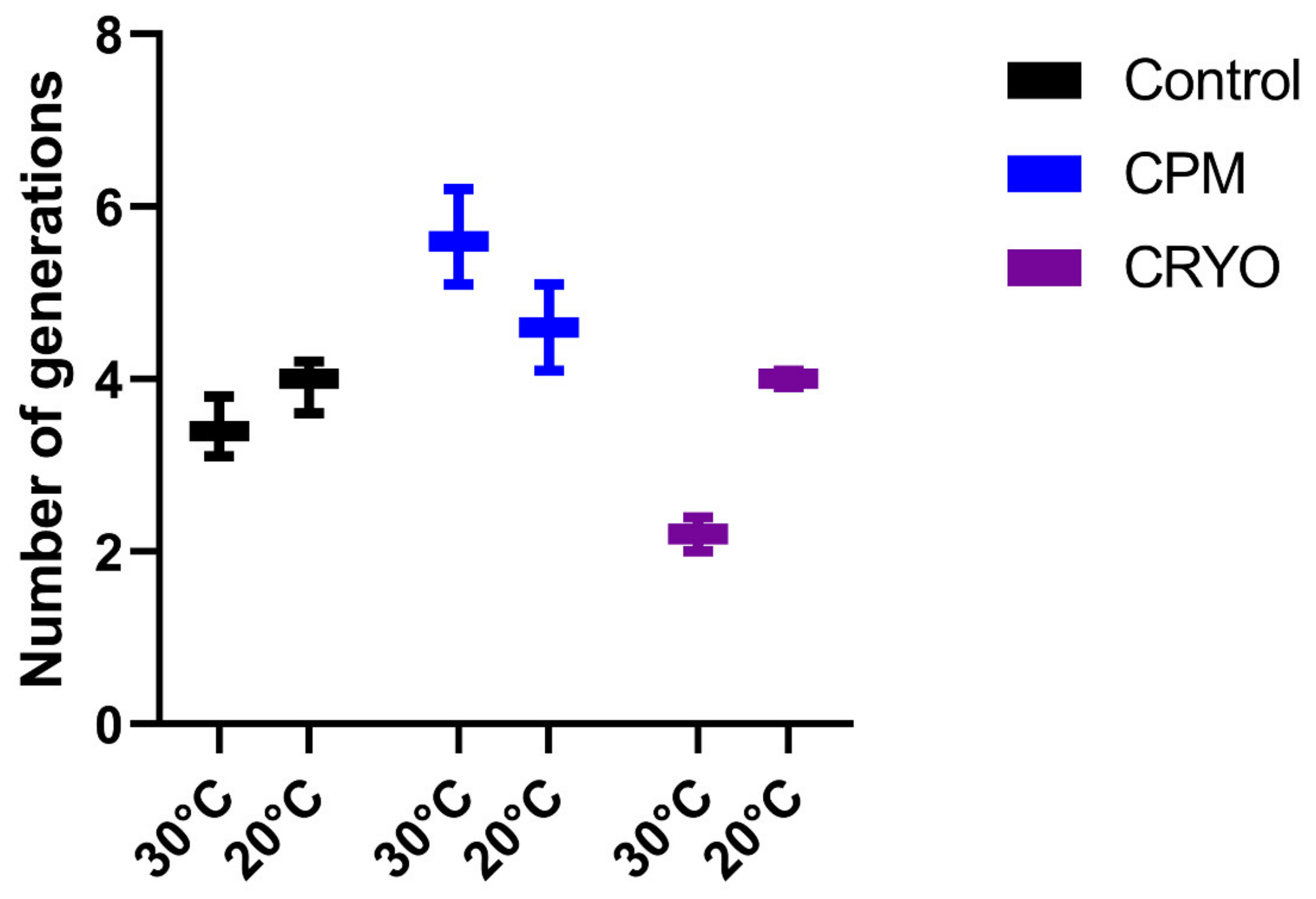
3.2. Fermentation Kinetics
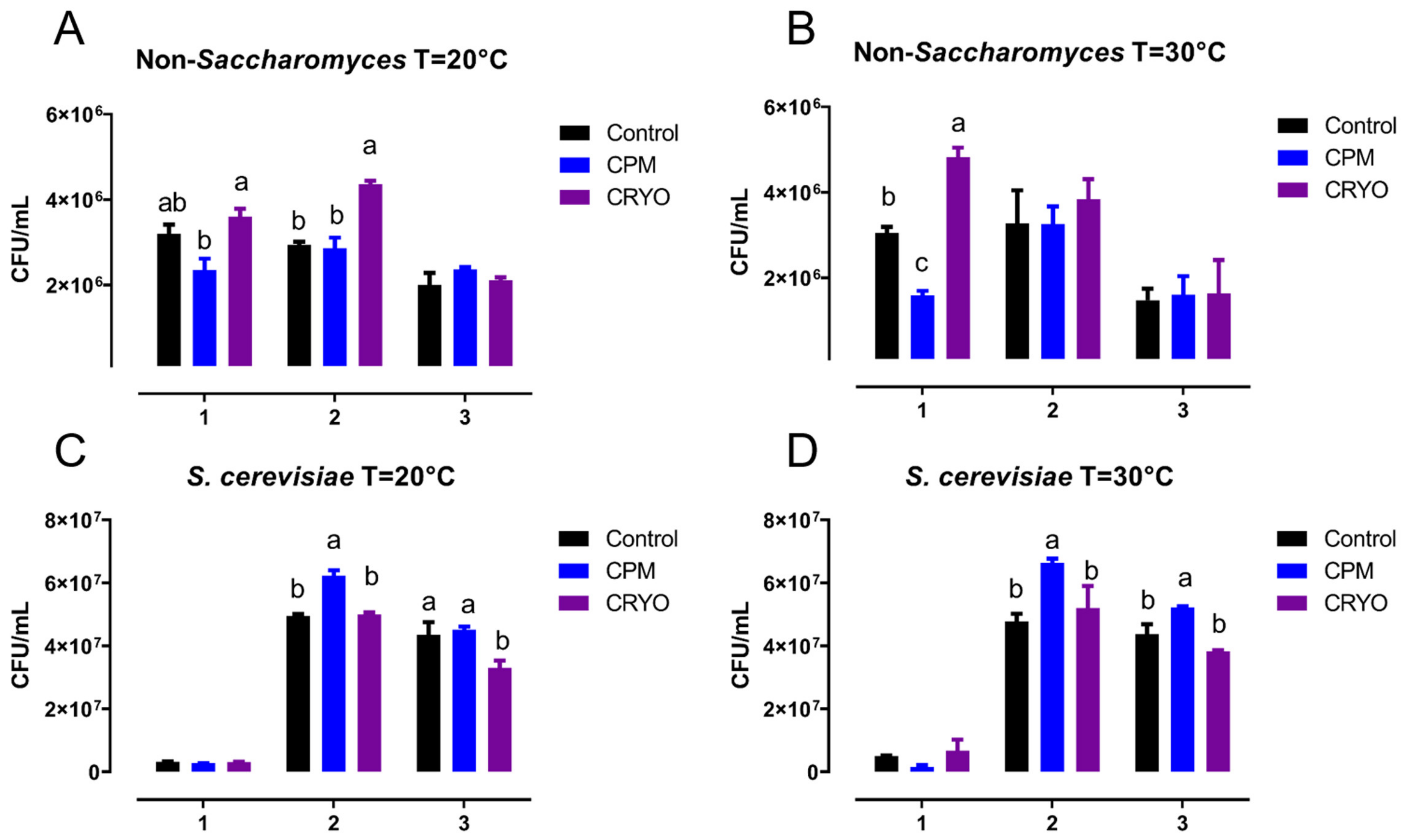
3.3. Yeast Population Dynamics
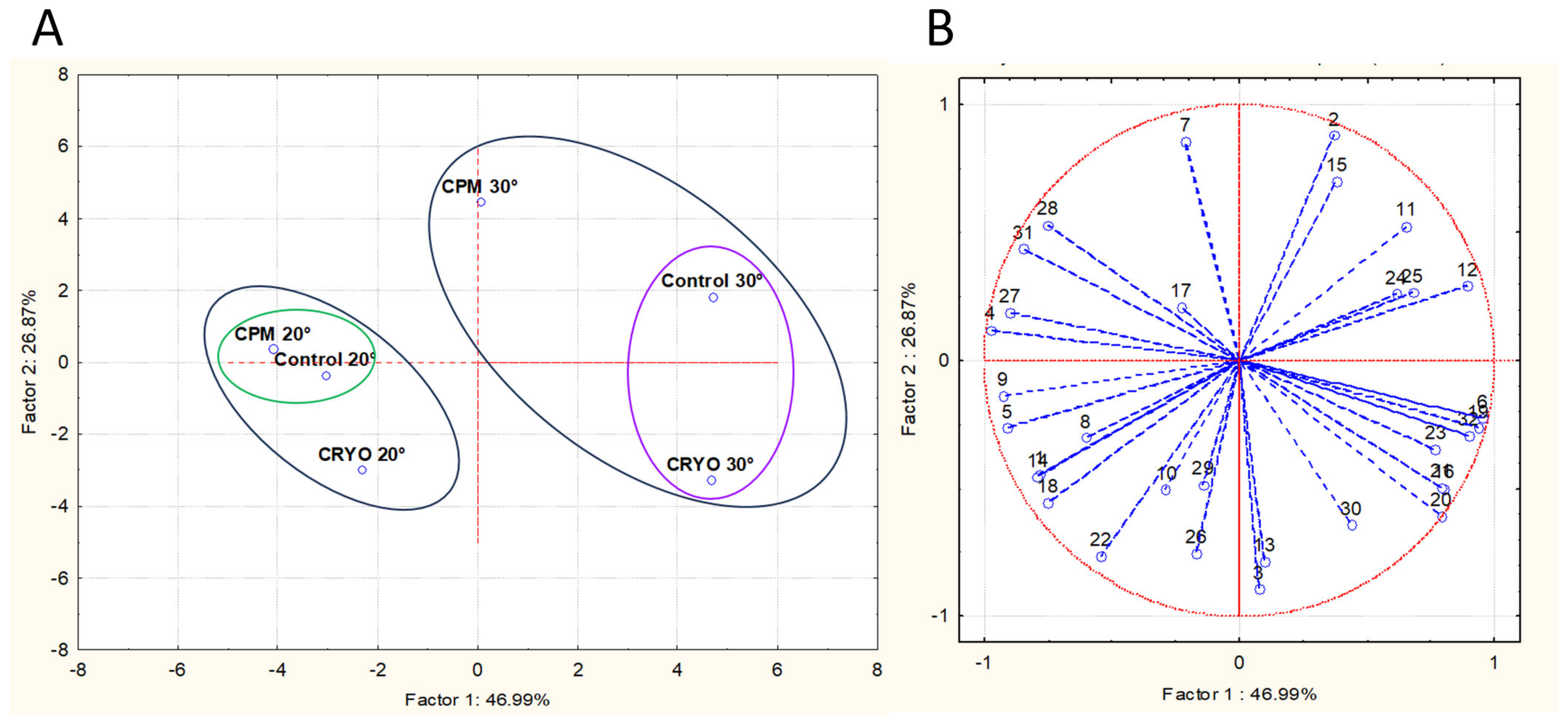
3.4. Volatile Compounds Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, J.; Zhu, B.Q.; Wang, Y.H.; Lu, L.; Lan, Y.B.; Reeves, M.J.; Duan, C.Q. Influence of pre-fermentation cold maceration treatment on aroma compounds of Cabernet Sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef]
- Lasanta, C.; Cejudo, C.; Gómez, J.; Caro, I. Influence of Prefermentative Cold Maceration on the Chemical and Sensory Properties of Red Wines Produced in Warm Climates. Processes 2023, 11, 374–390. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; du Toit, W. Cold maceration application in red wine production and its effects on phenolic compounds: A review. LWT Food Sci. Technol. 2018, 95, 200–208. [Google Scholar] [CrossRef]
- Gil-Muñoz, R.; Moreno-Pérez, A.; Vila-López, R.; Fernández-Fernández, J.I.; Martínez- Cutillas, A.; Gómez-Plaza, E. Influence of low temperature pre fermentative techniques on chromatic and phenolic characteristics of Syrah and Cabernet Sauvignon wines. Eur. Food Res. Technol. 2009, 228, 777–788. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Buica, A.; Nieuwoudt, H.; Aleixandre, J.L.; du Toit, W. Spectrophotometric analysis of phenolic compounds in grapes and wines. J. Agric. Food Chem. 2017, 65, 4009–4026. [Google Scholar] [CrossRef]
- Casassa, L.F.; Harbertson, J.F. Extraction, evolution, and sensory impact of phenolic compounds during red wine maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J. Anthocyanins and their variation in red wines II. Anthocyanin-derived pigments and their colour evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef]
- Smith, P.A.; Mcrae, J.M.; Bindon, K.A. Impact of winemaking practices on the concentration and composition of tannins in red wine. Aust. J. Grape Wine Res. 2015, 21, 601–614. [Google Scholar] [CrossRef]
- Lukić, I.; Budić-Leto, I.; Bubola, M.; Damijanić, K.; Staver, M. Pre-fermentative cold maceration, saignée, and various thermal treatments as options for modulating volatile aroma and phenol profiles of red wine. Food Chem. 2017, 224, 251–261. [Google Scholar] [CrossRef]
- Moreno-Pérez, A.; Fernández-Fernández, J.I.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Martínez-Cutillas, A.; Gil-Muñoz, R. Influence of winemaking techniques on proanthocyanidin extraction in Monastrell wines from four different areas. Eur. Food Res. Technol. 2013, 236, 473–481. [Google Scholar] [CrossRef]
- De Santis, D.; Frangipane, M.T. Effect of Prefermentative Cold Maceration on the Aroma and Phenolic Profiles of a Merlot Red Wine. Ital. J. Food Sci. 2010, 22, 47–53. [Google Scholar]
- Jackson, R. Wine Science; Academic Press: Cambridge, MA, USA, 2008; ISBN 9780123736468. [Google Scholar]
- Schmid, F.; Jiranek, V. Use of fresh versus frozen or blast-frozen grapes for small-scale fermentation. Int. J. Wine Res. 2011, 3, 25–30. [Google Scholar]
- Ruiz-Rodríguez, A.; Durán-Guerrero, E.; Natera, R.; Palma, M.; Barroso, C.G. Influence of Two Different Cryoextraction Procedures on the Quality of Wine Produced from Muscat Grapes. Foods 2020, 9, 1529. [Google Scholar] [CrossRef]
- Hierro, N.; González, Á.; Mas, A.; Guillamón, J.M. Diversity and evolution of non-Saccharomyces yeast populations during wine fermentation: Effect of grape ripeness and cold maceration. FEMS Yeast Res. 2006, 6, 102–111. [Google Scholar] [CrossRef]
- Zott, K.; Miot-Sertier, C.; Claisse, O.; Lonvaud-Funel, A.; Masneuf-Pomarede, I. Dynamics and diversity of non-Saccharomyces yeasts during the early stages in winemaking. Int. J. Food Microbiol. 2008, 125, 197–203. [Google Scholar] [CrossRef]
- Pretorius, I.S. Tailoring wine yeast for the new millennium: Novel approaches to the ancient art of winemaking. Yeast 2000, 16, 675–729. [Google Scholar] [CrossRef]
- Parenti, A.; Spugnoli, P.; Calamai, L.; Ferrari, S.; Gori, C. Effects of cold maceration on red wine quality from Tuscan Sangiovese grape. Eur. Food Res. Technol. 2004, 218, 360–366. [Google Scholar] [CrossRef]
- Magrini, A.; Pantani, O.; Bartolini, A.B.; Stefanini, F.M. On prefermentative maceration techniques: Statistical analysis of sensory descriptors in Sangiovese wine. Biom. Lett. 2016, 53, 1–20. [Google Scholar] [CrossRef]
- Pantani, O.L.; Stefanini, F.M.; Lozzi, I.; Calamai, L.; Biondi Bartolini, A.; Di Blasi, S. Pre-maceration, Saignée and Temperature affect Daily Evolution of Pigment Extraction During Vinification. COBRA Preprint Series, 2014, Working Paper 107. Available online: https://biostats.bepress.com/cobra/art107 (accessed on 10 September 2023).
- Stefanini, F.M.; Pantani, O.L. A Bayesian model to compare vinification procedures. Biom. Lett. 2013, 50, 61–80. [Google Scholar] [CrossRef]
- Granchi, L.; Bosco, M.; Messini, A.; Vincenzini, M. Rapid detection and quantification of yeast species during spontaneous wine fermentation by PCR–RFLP analysis of the rDNA ITS region. J. Appl. Microbiol. 1999, 87, 949–956. [Google Scholar] [CrossRef]
- Cadez, N.; Raspor, P.; de Cock, A.W.; Boekhout, T.; Smith, M.T. Molecular identification and genetic diversity within species of the genera Hanseniaspora and Kloeckera. FEMS Yeast Res. 2002, 1, 279–289. [Google Scholar] [CrossRef]
- Legras, J.L.; Karst, F. Optimisation of interdelta analysis for Saccharomyces cerevisiae strain characterization. FEMS Microbiol. Lett. 2003, 221, 249–255. [Google Scholar] [CrossRef]
- Guerrini, L.; Masella, P.; Angeloni, G.; Calamai, L.; Spinelli, S.; Di Blasi, S.; Parenti, A. Harvest of Sangiovese grapes: The influence of material other than grape and unripe berries on wine quality. Eur. Food Res. Technol. 2018, 244, 1487–1496. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Doneche, B.; Lonvaud, A. Trattato di Enologia II: Chimica Del Vino. Stabilizzazione. Trattamenti, 2nd ed.; Edagricole: Bologna, Italy, 2004. [Google Scholar]
- OIV-MA-AS313-01; Total Acidity (Oeno 551/2015). Compendium of International Methods of Wine and Must Analysis, Volume 1. International Organisation of Vine and Wine (OIV): Paris, France, 2021; pp. 433–435.
- Domizio, P.; Lencioni, L.; Calamai, L.; Portaro, L.; Bisson, L. Evaluation of the Yeast Schizosaccharomyces japonicus for Use in Wine Production. Am. J. Enol. Vitic. 2018, 69, 266–277. [Google Scholar] [CrossRef]
- Dennis, E.G.; Keyzers, R.; Kalua, C.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape Contribution to Wine Aroma: Production of Hexyl Acetate, Octyl Acetate, and Benzyl Acetate during Yeast Fermentation Is Dependent upon Precursors in the Must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef]
- Kheir, J.; Salameh, D.; Strehaiano, P.; Brandam, C.; Lteif, R. Impact of volatile phenols and their precursors on wine quality and control measures of Brettanomyces/Dekkera yeasts. Eur. Food Res. Technol. 2013, 237, 655–671. [Google Scholar] [CrossRef]
- Loureiro, V.; Ferreira, M.M.; Monteiro, S.; Ferreira, R. The Microbial Community of Grape Berry Chapter 12. In The Biochemistry of the Grape Berry; Hernâni Gerós, M., Chaves, M., Delrot, S., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2012; pp. 241–268. [Google Scholar] [CrossRef]
- Zhang, J.; Shang, Y.; Chen, J.; Brunel, B.; Peng, S.; Li, S.; Wang, E. Diversity of non-Saccharomyces yeasts of grape berry surfaces from representative Cabernet Sauvignon vineyards in Henan Province, China. FEMS Microbiol. Lett. 2021, 368, fnab142. [Google Scholar] [CrossRef]
- Nadai, C.; da Silva Duarte, V.; Sica, J.; Vincenzi, S.; Carlot, M.; Giacomini, A.; Corich, V. Starmerella bacillaris Released in Vineyards at Different Concentrations Influences Wine Glycerol Content Depending on the Vinification Protocols. Foods. 2023, 12, 3. [Google Scholar] [CrossRef]
- Rankine, B.C.; Bridson, A.D. Glycerol in Australian wines and Factors influencing its formation. Am. J. Enol. Vitic. 1971, 22, 6–12. [Google Scholar] [CrossRef]
- Canuti, V.; Cantu, A.; Picchi, M.; Lerno, L.A.; Tanabe, C.K.; Zanoni, B.; Heymann, H.; Ebeler, S.E. Evaluation of the Intrinsic and Perceived Quality of Sangiovese Wines from California and Italy. Foods 2020, 9, 1088. [Google Scholar] [CrossRef]
- Borren, E.; Tian, B. The Important Contribution of Non-Saccharomyces Yeasts to the Aroma Complexity of Wine: A Review. Foods 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Molina, A.M.; Swiegers, J.H.; Varela, C.; Pretorius, I.S.; Agosin, E. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl. Microbiol. Biotechnol. 2007, 77, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Baniţă, C.; Antoce, O.A.; Cojocaru, G.A. Evaluation by a GC Electronic Nose of the Differences in Volatile Profile Induced by Stopping Fermentation with Octanoic and Decanoic Acid to Produce Sweet Wines. Chemosensors 2023, 11, 98. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, W.; Wu, Y.; Shi, X.; Yang, X.; Song, Y.; Qin, Y.; Ye, D.; Liu, Y. Pilot-Scale Vinification of Cabernet Sauvignon Using Combined Lactiplantibacillus plantarum and Saccharomyces cerevisiae to Achieve Wine Acidification. Foods 2022, 11, 2511. [Google Scholar] [CrossRef]
- Capozzi, V.; Garofalo, C.; Chiriatti, M.A.; Grieco, F.; Spano, G. Microbial terroir and food innovation: The case of yeast biodiversity in wine. Microbiol. Res. 2015, 181, 75–83. [Google Scholar] [CrossRef]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary Aroma: Influence of Wine Microorganisms in Their Aroma Profile. Foods 2021, 10, 51. [Google Scholar] [CrossRef]


| CFU/mL | Saccharomyces cerevisiae | Non-Saccharomyces | Starmerella bacillaris | Hanseniaspora uvarum | Pichia occidentalis | Issatchenkia terricola |
|---|---|---|---|---|---|---|
| Upon arrival in cellar | (1.60 ± 0.57) × 105 | (3.37 ± 0.11) × 106 a | (1.00 ± 0.14) × 104 a | (1.49 ± 0.58) × 106 | (1.64 ± 0.26) × 106 | (2.35 ± 0.23) × 105 a |
| After cryoextraction | (1.40 ± 0.85) × 105 | (2.42 ± 0.14) × 106 b | (5.00 ± 0.71) × 103 b | (1.30 ± 0.78) × 106 | (1.03 ± 0.52) × 106 | (8.50 ± 0.64) × 104 b |
| μ-Max (Density ∗ Days−1) | Lag Phase (Days) | |
|---|---|---|
| 20 °C | mean ± SD | mean ± SD |
| Control | −21.39 ± 1.07 a | 0.99 ± 0.37 b |
| CPM | −16.49 ± 1.17 b | 3.00 ± 0.30 a |
| CRYO | −19.86 ± 1.07 ab | 1.71 ± 0.27 b |
| 30 °C | ||
| Control | −29.20 ± 0.93 a | 1.17 ± 0.13 b |
| CPM | −22.24 ± 1.10 b | 3.94 ± 0.14 a |
| CRYO | −27.14 ± 1.17 a | 1.80 ± 0.15 b |
| T = 20 °C | Control | CPM | CRYO |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Ethanol (% v/v) | 14.40 ± 0.14 | 14.35 ± 0.35 | 14.20 ± 0.28 |
| Sugars (g/L) | 2.00 ± 1.73 | 1.33 ± 0.58 | 2.67 ± 2.08 |
| Glycerol (g/L) | 7.60 ± 0.44 ab | 6.50 ± 0.40 b | 7.83 ± 0.67 a |
| Malic acid (g/L) | 1.11 ± 0.12 | 1.05 ± 0.10 | 1.12 ± 0.09 |
| Acetic acid (g/L) | 0.17 ± 0.02 | 0.19 ± 0.04 | 0.16 ± 0.02 |
| Total acidity (g/L) | 5.97 ± 0.23 a | 5.53 ± 0.06 b | 6.13 ± 0.06 a |
| SO2 (mg/L) | 64.00 ± 2.83 a | 49.50 ± 0.71 b | 62.00 ± 0.01 a |
| pH | 3.41 ± 0.02 | 3.49 ± 0.03 | 3.42 ± 0.02 |
| T = 30 °C | Control | CPM | CRYO |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Ethanol (% v/v) | 14.30 ± 0.00 | 14.40 ± 0.14 | 14.25 ± 0.07 |
| Sugars (g/L) | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| Glycerol (g/L) | 7.80 ± 0.17 a | 7.00 ± 0.17 b | 8.40 ± 0.28 c |
| Malic acid (g/L) | 0.95 ± 0.02 | 0.88 ± 0.08 | 0.93 ± 0.08 |
| Acetic acid (g/L) | 0.32 ± 0.01 | 0.28 ± 0.04 | 0.28 ± 0.01 |
| Total acidity (g/L) | 6.00 ± 0.10 a | 5.53 ± 0.12 b | 6.25 ± 0.07 a |
| SO2 (mg/L) | 50.50 ± 0.71 | 49.00 ± 1.41 | 48.33 ± 2.52 |
| pH | 3.51 ± 0.02 | 3.54 ± 0.02 | 3.49 ± 0.02 |
| Isolation Frequencies (%) | 20 °C | 30 °C | |||||
|---|---|---|---|---|---|---|---|
| Control | CPM | CRYO | Control | CPM | CRYO | ||
| 1 | Hanseniaspora uvarum | 32 | 36 | 47 | 33 | 12 | 24 |
| Starmerella bacillaris | 61 | 61 | 49 | 65 | 83 | 74 | |
| Pichia occidentalis | 1 | 1 | - | - | 3 | 1 | |
| Issatchenkia terricola | 1 | 1 | 4 | 2 | 2 | 1 | |
| Others | 5 | - | - | - | - | - | |
| 2 | Hanseniaspora uvarum | - | - | 17 | 17 | 7 | 14 |
| Starmerella bacillaris | 93 | 96 | 77 | 77 | 93 | 74 | |
| Others | 7 | 4 | 5 | 7 | - | 12 | |
| 3 | Hanseniaspora uvarum | 6 | 7 | 5 | 3 | 5 | 3 |
| Starmerella bacillaris | 87 | 87 | 94 | 97 | 95 | 92 | |
| Others | 7 | 6 | 1 | - | - | 5 | |
| mg/L | Control | CPM | CRYO | |||
|---|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | ||||
| Esters and Lactones | ||||||
| Ethyl acetate | 30.54 ± 3.26 | 30.45 ± 5.16 | 28.66 ± 7.17 | |||
| Ethyl butyrate | 0.08 ± 0.01 | 0.09 ± 0.04 | 0.07 ± 0.02 | |||
| Ethyl hexanoate | 0.07 ± 0.03 | 0.10 ± 0.04 | 0.08 ± 0.03 | |||
| Ethyl lactate | 0.62 ± 0.05 a | 0.53 ± 0.05 b | 0.60 ± 0.06 ab | |||
| Ethyl octanoate | 0.01 ± 0.01 | 0.01 ± <0.01 | 0.01 ± 0.01 | |||
| Ethyl decanoate | 0.06 ± 0.06 | 0.10 ± 0.03 | 0.08 ± 0.06 | |||
| Ethyl dodecanoate | <0.01 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 | |||
| Ethyl tetradecanoate | <0.01 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 | |||
| Isoamyl acetate | 1.07 ± 0.12 | 1.03 ± 0.27 | 0.98 ± 0.11 | |||
| Hexyl acetate | <0.01 ± <0.01 b | <0.01 ± <0.01 b | 0.64 ± 0.09 a | |||
| Diethyl malonate | 0.00 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 | |||
| Diethyl succinate | 0.15 ± 0.02 | 0.16 ± 0.02 | 0.16 ± 0.03 | |||
| γ-Butyrolactone | 8.23 ± 2.04 | 8.10 ± 2.28 | 9.00 ± 2.95 | |||
| Total esters and lactones | 40.84 ± 3.08 | 40.57 ± 5.18 | 40.28 ± 5.82 | |||
| Fatty acids | ||||||
| Hexanoic acid | 0.73 ± 0.09 b | 0.98 ± 0.13 a | 0.76 ± 0.15 b | |||
| Octanoic acid | 0.35 ± 0.07 | 0.40 ± 0.09 | 0.30 ± 0.07 | |||
| Total acids | 1.08 ± 0.11 | 1.38 ± 0.21 | 1.06 ± 0.21 | |||
| Higher alcohols | ||||||
| 2-Methyl-1-propanol | 43.00 ± 1.12 a | 36.83 ± 1.25 b | 42.76 ± 3.22 a | |||
| 1-Butanol | 2.45 ± 0.12 ab | 2.60 ± 0.13 a | 2.19 ± 0.24 b | |||
| 3-Methyl-1-butanol | 215.50 ± 7.38 | 200.11 ± 5.21 | 208.07 ± 16.27 | |||
| 1-Hexanol | 0.53 ± 0.05 b | 0.54 ± 0.08 b | 1.15 ± 0.13 a | |||
| Benzyl alcohol | 0.18 ± 0.01 | 0.20 ± 0.02 | 0.13 ± 0.11 | |||
| Phenylethyl Alcohol | 44.20 ± 3.49 a | 34.52 ± 2.70 b | 41.74 ± 5.49 a | |||
| Total alcohols | 305.86 ± 9.81 a | 274.80 ± 20.20 b | 296.04 ± 32.60 ab | |||
| Terpenes | ||||||
| Linalool | <0.01 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 | |||
| α-Terpineol | 0.38 ± <0.01 | 0.38 ± <0.01 | 0.38 ± <0.01 | |||
| Geraniol | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | |||
| Total terpenes | 0.40 ± 0.01 | 0.40 ± 0.02 | 0.40 ± 0.01 | |||
| Carbonyl compounds | ||||||
| Diacetyl | 2.86 ± 0.15 | 2.93 ± 0.13 | 2.97 ± 0.23 | |||
| Acetoin | 0.33 ± 0.09 b | 0.46 ± 0.06 a | 0.33 ± 0.06 b | |||
| Benzaldehyde | <0.01 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 | |||
| 4-Ethyl benzaldehyde | <0.01 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 | |||
| 3,4-Dimethyl-benzaldehyde | <0.01 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 | |||
| Total carbonyl compounds | 3.19 ± 0.24 | 3.39 ± 0.17 | 3.30 ± 0.34 | |||
| Other volatile compounds | ||||||
| Acetaldehyde diethyl acetal | 13.87 ± 5.09 | 17.24 ± 4.67 | 11.98 ± 3.63 | |||
| β-Damascenone | 0.01 ± <0.01 | 0.01 ± <0.01 | 0.01 ± <0.01 | |||
| 4-Ethyl-phenol | 0.01 ± 0.01 | 0.01 ± 0.01 | <0.01 ± <0.01 | |||
| Total other volatile compounds | 13.89 ± 5.09 | 17.26 ± 4.67 | 11.99 ± 3.63 | |||
| mg/L | Control | CPM | CRYO |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Esters and Lactones | |||
| Ethyl acetate | 25.53 ± 2.99 | 25.11 ± 1.84 | 26.85 ± 3.14 |
| Ethyl butyrate | 0.05 ± 0.01 ab | 0.07 ± 0.02 a | 0.04 ± 0.01 b |
| Ethyl hexanoate | 0.04 ± 0.02 | 0.07 ± 0.02 | 0.05 ± 0.04 |
| Ethyl lactate | 0.75 ± 0.05 | 0.70 ± 0.05 | 0.70 ± 0.03 |
| Ethyl octanoate | <0.01 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 |
| Ethyl decanoate | 0.02 ± <0.01 b | 0.02 ± 0.01 b | 0.05 ± 0.02 a |
| Ethyl dodecanoate | <0.01 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 |
| Ethyl tetradecanoate | <0.01 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 |
| Isoamyl acetate | 0.66 ± 0.07 | 0.68 ± 0.16 | 0.63 ± 0.16 |
| Hexyl acetate | <0.01 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 |
| Diethyl malonate | <0.01 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 |
| Diethyl succinate | 0.18 ± 0.04 | 0.16 ± 0.03 | 0.18 ± 0.03 |
| γ-Butyrolactone | 9.55 ± 2.67 | 7.86 ± 1.85 | 10.56 ± 2.02 |
| Total esters and lactones | 36.78 ± 2.54 | 34.66 ± 0.88 | 39.07 ± 4.54 |
| Fatty acids | |||
| Hexanoic acid | 0.43 ± 0.07 b | 0.81 ± 0.13 a | 0.50 ± 0.11 b |
| Octanoic acid | 0.22 ± 0.05 b | 0.38 ± 0.09 a | 0.22 ± 0.08 b |
| Total acids | 0.65 ± 0.09 b | 1.19 ± 0.17 a | 0.72 ± 0.18 b |
| Higher alcohols | |||
| 2-Methyl-1-propanol | 54.40 ± 2.79 a | 42.13 ± 1.91 b | 55.12 ± 7.13 a |
| 1-Butanol | 2.53 ± 0.10 a | 2.56 ± 0.13 a | 2.21 ± 0.11 b |
| 3-Methyl-1-butanol | 197.75 ± 10.75 | 181.49 ± 7.33 | 182.74 ± 12.85 |
| 1-Hexanol | 0.49 ± 0.06 b | 0.57 ± 0.06 b | 1.00 ± 0.08 a |
| Benzyl alcohol | 0.10 ± 0.10 | 0.21 ± 0.03 | 0.11 ± 0.10 |
| Phenylethyl Alcohol | 40.02 ± 3.88 a | 31.14 ± 2.02 b | 35.94 ± 5.42 ab |
| Total alcohols | 295.29 ± 11.99 a | 258.10 ± 9.89 b | 277.12 ± 20.47 b |
| Terpenes | |||
| Linalool | 0.01 ± <0.01 a | <0.01 ± <0.01 b | 0.01 ± <0.01 a |
| α-Terpineol | 0.38 ± <0.01 | 0.38 ± <0.01 | 0.38 ± <0.01 |
| Geraniol | 0.29 ± 0.68 | 0.01 ± 0.01 | 0.03 ± 0.02 |
| Total terpenes | 0.68 ± 0.68 | 0.39 ± 0.01 | 0.42 ± 0.02 |
| Carbonyl compounds | |||
| Diacetyl | 2.86 ± 0.08 | 2.82 ± 0.07 | 3.00 ± 0.25 |
| Acetoin | 0.58 ± 0.18 | 0.72 ± 0.08 | 0.63 ± 0.18 |
| Benzaldehyde | <0.01 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 |
| 4-Ethyl benzaldehyde | <0.01 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 |
| 3,4-Dimethyl-benzaldehyde | <0.01 ± <0.01 | <0.01 ± <0.01 | <0.01 ± <0.01 |
| Total carbonyl compounds | 3.44 ± 0.28 | 3.54 ± 0.14 | 3.63 ± 0.41 |
| Other volatile compounds | |||
| Acetaldehyde diethyl acetal | 19.72 ± 5.39 | 21.04 ± 2.07 | 15.23 ± 3.96 |
| β-Damascenone | 0.01 ± <0.01 | 0.01 ± <0.01 | 0.01 ± <0.01 |
| 4-Ethyl-phenol | 0.05 ± 0.04 b | 0.01 ± 0.01 c | 0.09 ± <0.01 a |
| Total other volatile compounds | 19.78 ± 5.36 ab | 21.06 ± 2.0 a | 15.33 ± 3.97 b |
| Point 1 | Point 2 | Point 3 | ||||
|---|---|---|---|---|---|---|
| Non-Sacch. | S. cerevisiae | Non-Sacch. | S. cerevisiae | Non-Sacch. | S. cerevisiae | |
| Compounds Esters and lactones | ||||||
| Ethyl acetate | ns | ns | ns | ns | 0.9062 | ns |
| Ethyl butyrate | ns | ns | ns | ns | 0.8606 | ns |
| Ethyl hexanoate | ns | ns | ns | ns | 0.9525 | ns |
| Ethyl lactate | ns | ns | ns | ns | −0.9932 | ns |
| Ethyl octanoate | ns | ns | ns | ns | 0.8706 | ns |
| Ethyl decanoate | ns | ns | ns | ns | 0.9682 | ns |
| Isoamyl acetate | ns | ns | ns | ns | 0.9080 | ns |
| γ-butyrolactone | 0.8255 | 0.9090 | ns | ns | ns | ns |
| Fatty acids | ||||||
| Octanoic acid | ns | −0.8454 | ns | ns | ns | ns |
| Higher alcohols | ||||||
| 1-butanol | ns | ns | −0.8819 | ns | ns | 0.8795 |
| 1-Hexanol | ns | ns | 0.9310 | ns | ns | −0.8552 |
| Benzyl alcohol | ns | −0.8649 | ns | ns | ns | ns |
| Phenylethyl alcohol | ns | ns | ns | −0.8716 | ns | ns |
| Terpenes | ||||||
| Linalool | ns | 0.9118 | ns | ns | ns | ns |
| Carbonyl compounds | ||||||
| 3,4-dimethyl-benzaldehyde | ns | 0.8507 | ns | ns | ns | ns |
| Other volatile compounds | ||||||
| Acetaldehyde diethyl acetal | ns | ns | ns | ns | ns | 0.8408 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrini, S.; Galli, V.; Mangani, S.; Granchi, L. Influence of Cryoextraction and Cold Pre-Fermentative Maceration on the Yeast Microbiota and the Volatile Compounds Profile of Sangiovese Wine. Fermentation 2024, 10, 148. https://doi.org/10.3390/fermentation10030148
Guerrini S, Galli V, Mangani S, Granchi L. Influence of Cryoextraction and Cold Pre-Fermentative Maceration on the Yeast Microbiota and the Volatile Compounds Profile of Sangiovese Wine. Fermentation. 2024; 10(3):148. https://doi.org/10.3390/fermentation10030148
Chicago/Turabian StyleGuerrini, Simona, Viola Galli, Silvia Mangani, and Lisa Granchi. 2024. "Influence of Cryoextraction and Cold Pre-Fermentative Maceration on the Yeast Microbiota and the Volatile Compounds Profile of Sangiovese Wine" Fermentation 10, no. 3: 148. https://doi.org/10.3390/fermentation10030148
APA StyleGuerrini, S., Galli, V., Mangani, S., & Granchi, L. (2024). Influence of Cryoextraction and Cold Pre-Fermentative Maceration on the Yeast Microbiota and the Volatile Compounds Profile of Sangiovese Wine. Fermentation, 10(3), 148. https://doi.org/10.3390/fermentation10030148







