Aroma Features of Hanseniaspora vineae Hv205 Wines in Sequential and Co-Inoculation Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Winemaking
2.2. Inoculation Protocols
2.3. Fermentation Kinetics
2.4. Fourier-Transform Infrared Spectroscopy (FTIR) Measurement of Must and Wine Basic Chemical Parameters
2.5. GC-MS/MS Analysis of Volatile Compounds
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomyces yeasts in wine production uncovered. FEMS Yeast Res. 2014, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Zironi, R.; Romano, P.; Suzzi, G.; Battistutta, F.; Comi, G. Volatile metabolites produced in wine by mixed and sequential cultures of Hanseniaspora guilliermondii or Kloeckera apiculata and Saccharomyces cerevisiae. Biotechnol. Lett. 1993, 15, 235–238. [Google Scholar] [CrossRef]
- Thomson, J.M.; Gaucher, E.A.; Burgan, M.F.; De Kee, D.W.; Li, T.; Aris, J.P.; Benner, S.A. Resurrecting ancestral alcohol dehydrogenases from yeast. Nat. Genet. 2005, 37, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Piškur, J.; Rozpędowska, E.; Polakova, S.; Merico, A.; Compagno, C. How did Saccharomyces evolve to become a good brewer? TRENDS Genet. 2006, 22, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Comitini, F.; Mannazzu, I.; Domizio, P. Controlled mixed culture fermentation: A new perspective on the use of non-Saccharomyces yeasts in winemaking. FEMS Yeast Res. 2010, 10, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Morata, A.; Loira, I.; González, C.; Escott, C. Non-Saccharomyces as biotools to control the production of off-flavors in wines. Molecules 2021, 26, 4571. [Google Scholar] [CrossRef] [PubMed]
- Hranilovic, A.; Li, S.; Boss, P.K.; Bindon, K.; Ristic, R.; Grbin, P.R.; Van der Westhuizen, T.; Jiranek, V. Chemical and sensory profiling of Shiraz wines co-fermented with commercial non-Saccharomyces inocula. Aust. J. Grape Wine Res. 2018, 24, 166–180. [Google Scholar] [CrossRef]
- Romani, C.; Domizio, P.; Lencioni, L.; Gobbi, M.; Comitini, F.; Ciani, M.; Mannazzu, I. Polysaccharides and glycerol production by non-Saccharomyces wine yeasts in mixed fermentation. Quad. Vitic. Enol. Univ. Torino 2010, 31, 185–189. [Google Scholar]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef]
- Yan, G.; Zhang, B.; Joseph, L.; Waterhouse, A.L. Effects of initial oxygenation on chemical and aromatic composition of wine in mixed starters of Hanseniaspora vineae and Saccharomyces cerevisiae. Food Microbiol. 2020, 90, 103460. [Google Scholar] [CrossRef]
- Viana, F.; Belloch, C.; Vallés, S.; Manzanares, P. Monitoring a mixed starter of Hanseniaspora vineae–Saccharomyces cerevisiae in natural must: Impact on 2-phenylethyl acetate production. Int. J. Food Microbiol. 2011, 151, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shen, J.; Duan, C.; Yan, G. Use of indigenous Hanseniaspora vineae and Metschnikowia pulcherrima co-fermentation with Saccharomyces cerevisiae to improve the aroma diversity of Vidal blanc icewine. Front. Microbiol. 2018, 9, 407852. [Google Scholar] [CrossRef] [PubMed]
- Carrau, F.; Henschke, P.A. Hanseniaspora vineae and the concept of friendly yeasts to increase autochthonous wine flavor diversity. Front. Microbiol. 2021, 12, 702093. [Google Scholar] [CrossRef] [PubMed]
- Comuzzo, P.; Del Fresno, J.M.; Loira, I.; Morata, A. Emerging biotechnologies and non-thermal technologies for winemaking in a context of global warming. Front. Microbiol. 2023, 14, 1273940. [Google Scholar] [CrossRef] [PubMed]
- González-Robles, I.W.; Estarrón-Espinosa, M.; Díaz-Montaño, D.M. Fermentative capabilities and volatile compounds produced by Kloeckera/Hanseniaspora and Saccharomyces yeast strains in pure and mixed cultures during Agave tequilana juice fermentation. Antonie van Leeuwenhoek 2015, 108, 525–536. [Google Scholar] [CrossRef] [PubMed]
- OIV. Compendium of International Methods of Wine and Must Analysis; OIV-MA-INT-01; OIV: Paris, France, 2023; ISBN 978-2-85038-068-6. [Google Scholar]
- Paolini, M.; Tonidandel, L.; Moser, S.; Larcher, R. Development of a fast gas chromatography–tandem mass spectrometry method for volatile aromatic compound analysis in oenological products. J. Mass Spectrom. 2018, 53, 801–810. [Google Scholar] [CrossRef]
- Gallo, A.; Larcher, R.; Cappello, N.; Paolini, M.; Moser, S.; Carrau, F.; Schneider, R.; Roman, T. Insights into the grape must composition effect on Hanseniaspora vineae performance and metabolic aroma compounds in Chardonnay base wine for sparkling wine production. J. Food Compos. Anal. 2023, 123, 105514. [Google Scholar] [CrossRef]
- Torrellas, M.; Pietrafesa, R.; Ferrer-Pinós, A.; Capece, A.; Matallana, E.; Aranda, A. Optimizing growth and biomass production of non-Saccharomyces wine yeast starters by overcoming sucrose consumption deficiency. Front. Microbiol. 2023, 14, 1209940. [Google Scholar] [CrossRef] [PubMed]
- Medina, K.; Boido, E.; Dellacassa, E.; Carrau, F. Growth of non-Saccharomyces yeasts affects nutrient availability for Saccharomyces cerevisiae during wine fermentation. Int. J. Food Microbiol. 2012, 157, 245–250. [Google Scholar] [CrossRef]
- Viana, F.; Taillandier, P.; Valles, S.; Strehaiano, P.; Manzanares, P. 2-phenylethyl acetate formation by immobilized cells of Hanseniaspora vineae in sequential mixed fermentations. Am. J. Enol. Vitic. 2011, 62, 122–126. [Google Scholar] [CrossRef]
- Bisson, L.F. Stuck and sluggish fermentations. Am. J. Enol. Vitic. 1999, 50, 107–119. [Google Scholar] [CrossRef]
- Kemsawasd, V.; Viana, T.; Ardö, Y.; Arneborg, N. Influence of nitrogen sources on growth and fermentation performance of different wine yeast species during alcoholic fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 10191–10207. [Google Scholar] [CrossRef] [PubMed]
- Renault, P.E.; Albertin, W.; Bely, M. An innovative tool reveals interaction mechanisms among yeast populations under oenological conditions. Appl. Microbiol. Biotechnol. 2013, 97, 4105–4119. [Google Scholar] [CrossRef]
- Rollero, S.; Bloem, A.; Ortiz-Julien, A.; Camarasa, C.; Divol, B. Altered fermentation performances, growth, and metabolic footprints reveal competition for nutrients between yeast species inoculated in synthetic grape juice-like medium. Front. Microbiol. 2018, 9, 196. [Google Scholar] [CrossRef]
- Ciani, M.; Capece, A.; Comitini, F.; Canonico, L.; Siesto, G.; Romano, P. Yeast interactions in inoculated wine fermentation. Front. Microbiol. 2016, 7, 555. [Google Scholar] [CrossRef] [PubMed]
- Lleixa, J.; Manzano, M.; Mas, A.; Portillo, M.D.C. Saccharomyces and non-Saccharomyces competition during microvinification under different sugar and nitrogen conditions. Front. Microbiol. 2016, 7, 1959. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhuge, J.; Fang, H.; Prior, B.A. Glycerol production by microbial fermentation: A review. Biotechnology advances. 2001, 19, 201–223. [Google Scholar] [CrossRef] [PubMed]
- van Wyk, N.; Scansani, S.; Beisert, B.; Brezina, S.; Fritsch, S.; Semmler, H.; von Wallbrunn, C. The use of Hanseniaspora occidentalis in a sequential must inoculation to reduce the malic acid content of wine. Appl. Sci. 2022, 12, 6919. [Google Scholar] [CrossRef]
- Ferrando, N.; Araque, I.; Ortis, A.; Thornes, G.; Bautista-Gallego, J.; Bordons, A.; Reguant, C. Evaluating the effect of using non-Saccharomyces on Oenococcus oeni and wine malolactic fermentation. Food Res. Int. 2020, 138, 109779. [Google Scholar] [CrossRef]
- Gallo, A.; Paolini, M.; Castello, D.; Carrau, F.; Schneider, R.; Cappello, N.; Larcher, R.; Roman Villegas, T. Aromatic and fermentative performances of Hanseniaspora vineae in different coinoculation protocols with Saccharomyces cerevisiae for white winemaking. In Proceedings of the 44th World Congress of Vine and Wine, Cadiz/Jerez, Spain, 5–9 June 2023; pp. 699–701. [Google Scholar]
- Del Fresno, J.M.; Escott, C.; Loira, I.; Herbert-Pucheta, J.E.; Schneider, R.; Carrau, F.; Cuerda, R.; Morata, A. Impact of Hanseniaspora vineae in alcoholic fermentation and ageing on lees of high-quality white wine. Fermentation 2020, 6, 66. [Google Scholar] [CrossRef]
- Rollero, S.; Bloem, A.; Ortiz-Julien, A.; Camarasa, C.; Divol, B. Fermentation performances and aroma production of non-conventional wine yeasts are influenced by nitrogen preferences. FEMS Yeast Res. 2018, 18, foy055. [Google Scholar] [CrossRef] [PubMed]
- Rollero, S.; Bloem, A.; Brand, J.; Ortiz-Julien, A.; Camarasa, C.; Divol, B. Nitrogen metabolism in three non-conventional wine yeast species: A tool to modulate wine aroma profiles. Food Microbiol. 2021, 94, 103650. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, H.; Xue, J.; Tang, C.; Duan, C.; Yan, G. Use of Torulaspora delbrueckii and Hanseniaspora vineae co-fermentation with Saccharomyces cerevisiae to improve aroma profiles and safety quality of Petit Manseng wines. LWT 2022, 161, 113360. [Google Scholar] [CrossRef]
- Viana, F.; Gil, J.V.; Vallés, S.; Manzanares, P. Increasing the levels of 2-phenylethyl acetate in wine through the use of a mixed culture of Hanseniaspora osmophila and Saccharomyces cerevisiae. Int. J. Food Microbiol. 2009, 135, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Viana, F.; Gil, J.V.; Genovés, S.; Vallés, S.; Manzanares, P. Rational selection of non-Saccharomyces wine yeasts for mixed starters based on ester formation and enological traits. Food Microbiol. 2008, 25, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Escott, C.; López, C.; Loira, I.; González, C.; Bañuelos, M.A.; Tesfaye, W.; Suárez-Lepe, J.A.; Morata, A. Improvement of must fermentation from late harvest cv. Tempranillo grapes treated with pulsed light. Foods 2021, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, D.; Duan, C.; Yan, G. Synergistic effect enhances 2-phenylethyl acetate production in the mixed fermentation of Hanseniaspora vineae and Saccharomyces cerevisiae. Process Biochem. 2020, 90, 44–49. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Fleet, G.H. Wine yeasts for the future. FEMS Yeast Res. 2008, 8, 979–995. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Escott, C.; del Fresno, J.M.; Bañuelos, M.A.; Suárez-Lepe, J.A. Applications of Metschnikowia pulcherrima in wine biotechnology. Fermentation 2019, 5, 63. [Google Scholar] [CrossRef]
- Morata, A.; Loira, I.; Tesfaye, W.; Bañuelos, M.A.; González, C.; Suárez Lepe, J.A. Lachancea thermotolerans applications in wine technology. Fermentation 2018, 4, 53. [Google Scholar] [CrossRef]
- Dutraive, O.; Benito, S.; Fritsch, S.; Beisert, B.; Patz, C.D.; Rauhut, D. Effect of sequential inoculation with non-Saccharomyces and Saccharomyces yeasts on Riesling wine chemical composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef]
- Martin, V.; Giorello, F.; Fariña, L.; Minteguiaga, M.; Salzman, V.; Boido, E.; Aguilar, P.S.; Gaggero, C.; Dellacassa, E.; Mas, A.; et al. De novo synthesis of benzenoid compounds by the yeast Hanseniaspora vineae increases the flavor diversity of wines. J. Agric. Food Chem. 2016, 64, 4574–4583. [Google Scholar] [CrossRef]
- López, S.; Mateo, J.J.; Maicas, S. Characterisation of Hanseniaspora isolates with potential aroma-enhancing properties in Muscat wines. S. Afr. J. Enol. Vitic. 2014, 35, 292–303. [Google Scholar] [CrossRef][Green Version]
- Hu, K.; Qin, Y.; Tao, Y.S.; Zhu, X.L.; Peng, C.T.; Ullah, N. Potential of glycosidase from non-Saccharomyces isolates for enhancement of wine aroma. J. Food Sci. 2016, 81, M935–M943. [Google Scholar] [CrossRef]
- López, M.C.; Mateo, J.J.; Maicas, S. Screening of β-glucosidase and β-xylosidase activities in four non-Saccharomyces yeast isolates. J. Food Sci. 2015, 80, C1696–C1704. [Google Scholar] [CrossRef]
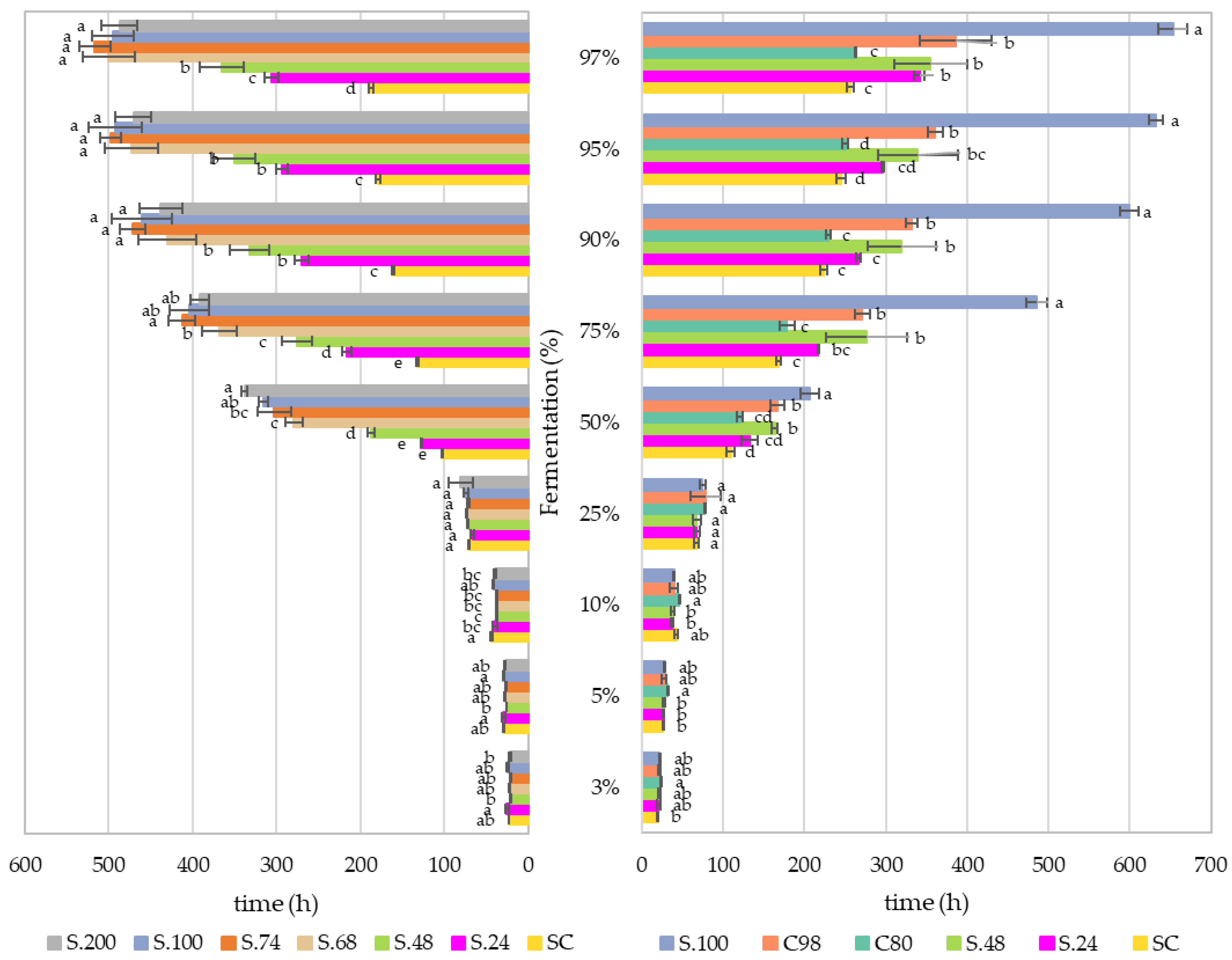

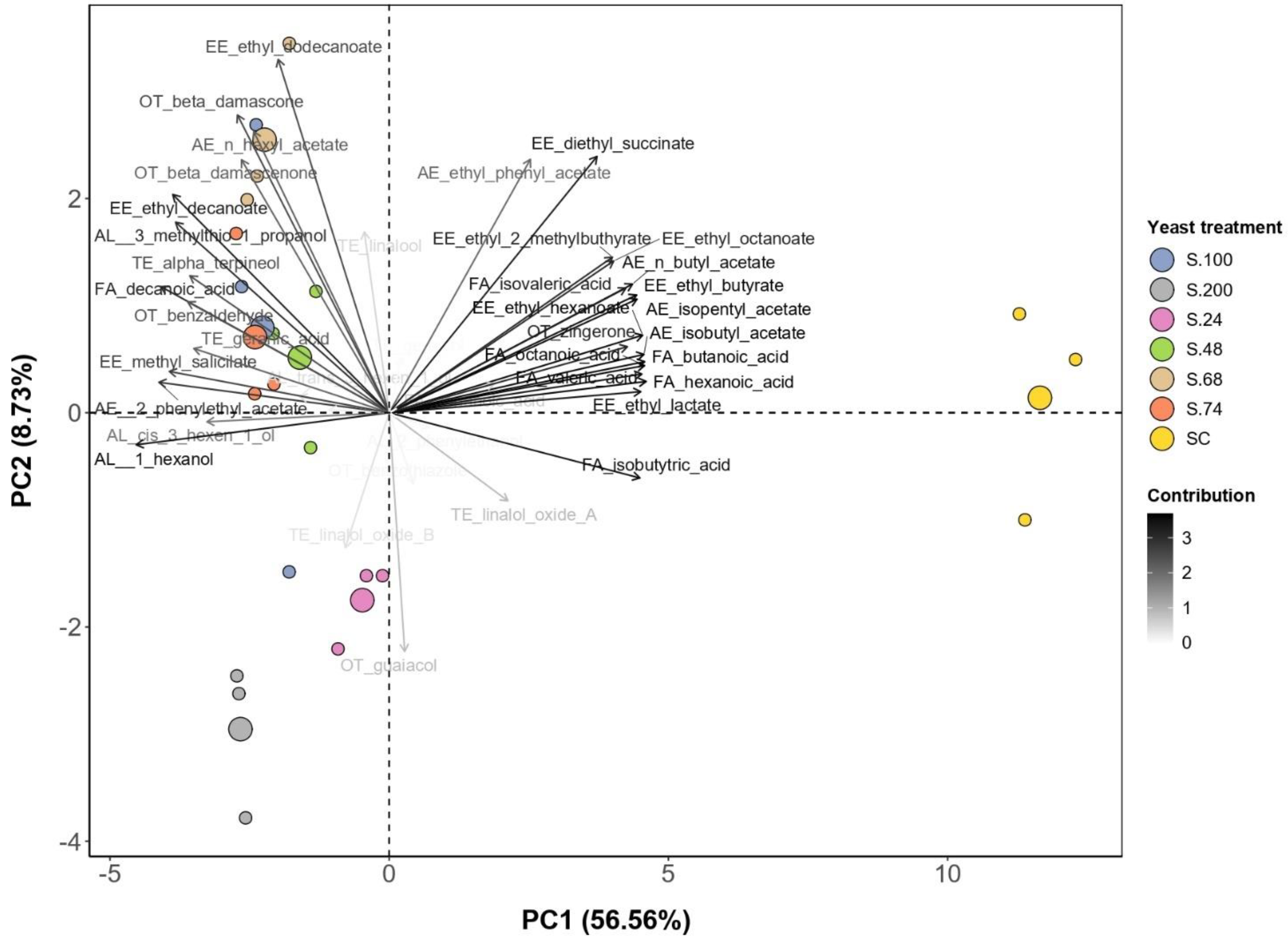
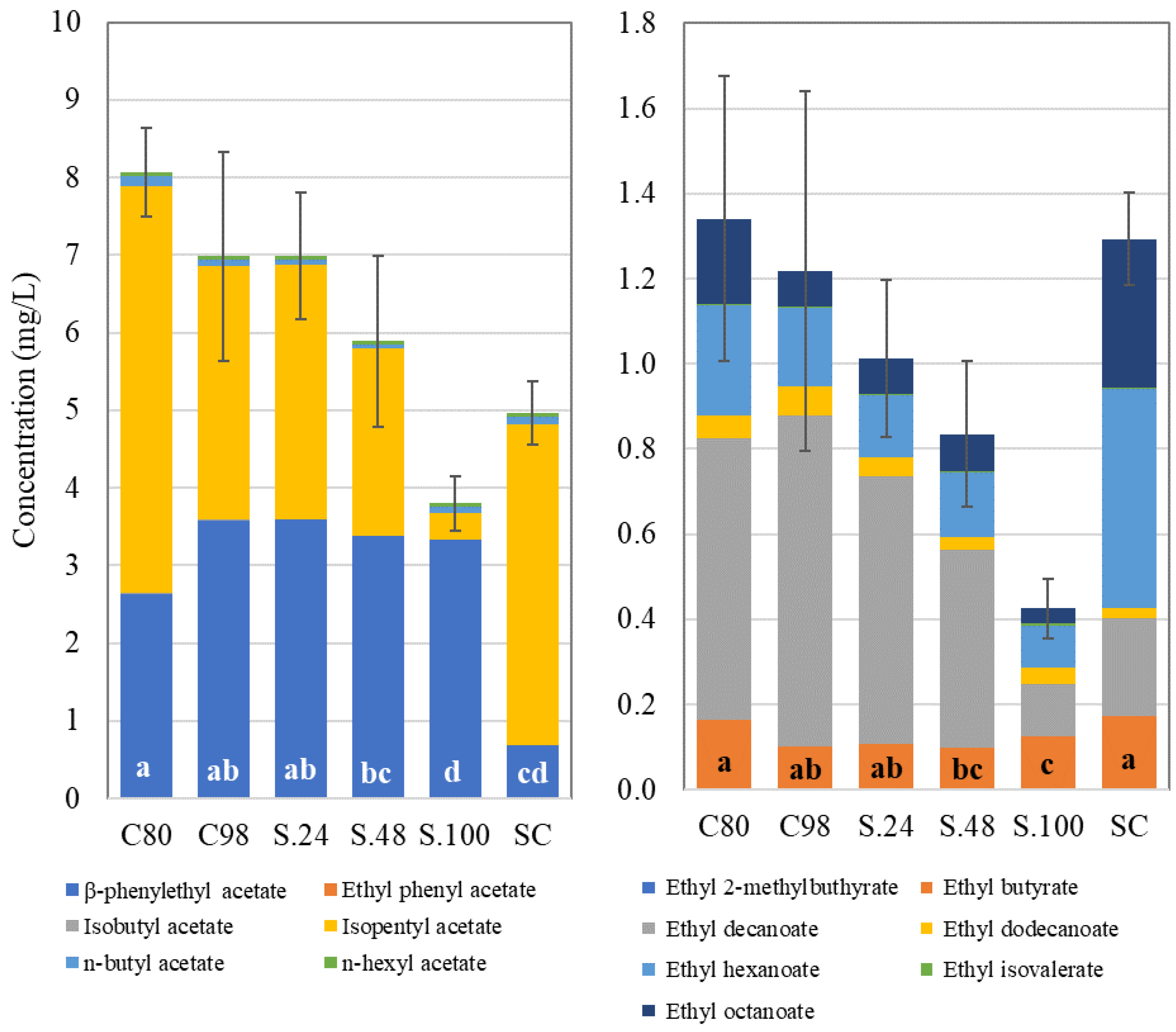

| Grape | Reducing Sugars (g/L) | pH | Titratable Acidity (g/L) | Malic Acid (g/L) | Tartaric Acid (g/L) | Potassium (g/L) | YAN (mg/L) |
|---|---|---|---|---|---|---|---|
| Gerwürztraminer | 230 | 3.53 | 3.46 | 1.58 | 5.22 | 1.86 | 114 |
| Glera | 213 | 3.15 | 4.90 | 2.62 | 3.90 | 0.98 | 223 |
| Abbreviation | Inoculation Strategy | Starter | Time span for S. cerevisiae Sequential Inoculation | Glera Fermentation | Gewürztraminer Fermentation |
|---|---|---|---|---|---|
| Sc | Pure | S. cereviae 100% | • | • | |
| S.24 | Sequential | H.vineae 100% | 24 h | • | • |
| S.48 | Sequential | H.vineae 100% | 48 h | • | • |
| S.68 | Sequential | H.vineae 100% | 68 h | • | |
| S.74 | Sequential | H.vineae 100% | 74 h | • | |
| S.100 | Sequential | H.vineae 100% | 100 h | • | • |
| S.200 | Sequential | H.vineae 100% | 200 h | • | |
| C80 | Coinoculation | H.vineae 80%: S. cerevisiae 20% | • | ||
| C98 | Coinoculation | H.vineae 98%: S. cerevisiae 2% | • |
| SC | S.24 | S.48 | S.68 | S.74 | S.100 | S.200 | |
|---|---|---|---|---|---|---|---|
| Ethanol (%vol) | 12.40 ± 0.05 b | 12.88 ± 0.29 a | 13.00 ± 0.07 a | 12.86 ± 0.08 a | 12.93 ± 0.08 a | 12.85 ± 0.12 a | 12.90 ± 0.09 a |
| Glucose + Fructose (g/L) | <1.0 a | <1.0 a | <1.0 a | 2.53 ± 1.59 a | 2.16 ± 0.86 a | 2.06 ± 1.79 a | 0.66 ± 1.15 a |
| pH | 3.45 ± 0 c | 3.45 ± 0 c | 3.48 ± 0.01 b | 3.5 ± 0.01 ab | 3.51 ± 0 a | 3.5 ± 0 ab | 3.51 ± 0 a |
| Titratable acidity (g/L) | 6.36 ± 0.05 a | 5.46 ± 0.05 b | 5.3 ± 0 c | 5.23 ± 0.05 cd | 5.06 ± 0.05 e | 5.13 ± 0.05 de | 5.03 ± 0.05 e |
| Volatile acidity (g/L) | 0.51 ± 0.01 a | 0.33 ± 0.02 b | 0.28 ± 0 c | 0.28 ± 0 c | 0.29 ± 0 c | 0.27 ± 0.01 c | 0.25 ± 0 c |
| Total dry extract (g/L) | 23.1 ± 0.17 ab | 21.23 ± 0.11 b | 22.13 ± 0.4 ab | 23.8 ± 1.3 a | 23.1 ± 0.62 ab | 23.2 ± 1.3 ab | 22.36 ± 0.92 ab |
| Malic acid (g/L) | 2.86 ± 0.03 a | 2.33 ± 0.03 b | 2.3 ± 0 b | 2.23 ± 0.04 bc | 2.25 ± 0.06 bc | 2.24 ± 0.03 bc | 2.19 ± 0.01 c |
| Lactic acid (g/L) | <Lod | <Lod | <Lod | <Lod | <Lod | <Lod | <Lod |
| Tartaric acid (g/L) | 1.72 ± 0.02 c | 1.81 ± 0.04 b | 1.9 ± 0.02 a | 1.81 ± 0.03 b | 1.83 ± 0.03 ab | 1.81 ± 0.02 b | 1.86 ± 0.01 ab |
| Ashes (g/L) | 2.03 ± 0.05 b | 2.03 ± 0.05 b | 2.13 ± 0.05 ab | 2.16 ± 0.05 ab | 2.16 ± 0.05 ab | 2.16 ± 0.05 ab | 2.2 ± 0 a |
| Glycerol (g/L) | 8.73 ± 0.05 a | 7.26 ± 0.15 b | 7.13 ± 0.05 bc | 6.9 ± 0.17 c | 6.8 ± 0.2 c | 6.86 ± 0.05 c | 6.96 ± 0.05 bc |
| Potassium (g/L) | 0.73 ± 0.01 a | 0.67 ± 0.04 a | 0.72 ± 0.05 a | 0.74 ± 0.02 a | 0.71 ± 0.02 a | 0.73 ± 0 a | 0.75 ± 0.02 a |
| Compounds (µg/L) | SC | S.24 | S.48 | S.68 | S.74 | S.100 | S.200 | |
|---|---|---|---|---|---|---|---|---|
| Acetates | β-phenylethyl acetate | 385.23 ± 153.33 d | 4642.56 ± 153.52 a | 4158.53 ± 198.77 ab | 3823.03 ± 193.24 bc | 3945.8 ± 426.84 abc | 3788.5 ± 532.17 bc | 3206.1 ± 80.12 c |
| ethylphenyl acetate | 0.46 ± 0.05 a | 0.26 ± 0.05 b | 0.33 ± 0.05 ab | 0.43 ± 0.11 ab | 0.3 ± 0 ab | 0.33 ± 0.05 ab | 0.3 ± 0 ab | |
| isobutyl acetate | 35.16 ± 0.81 a | 13.2 ± 1.38 b | 11.46 ± 0.55 b | 11.46 ± 1.48 b | 11.03 ± 1 b | 11 ± 0.5 b | 6.83 ± 1.02 c | |
| isopentyl acetate | 3282.16 ± 114.21 a | 1181.16 ± 177.04 b | 1179.86 ± 6.64 b | 1317.36 ± 259.7 b | 1233.43 ± 107.5 b | 1366.96 ± 108.96 b | 774.26 ± 78.04 c | |
| n-butyl acetate | 75.3 ± 3.26 a | 28.73 ± 4.74 bc | 27.36 ± 1.25 bc | 32.2 ± 7.3 b | 29.06 ± 2.94 bc | 33.06 ± 5.31 b | 18.16 ± 2.92 c | |
| n-hexyl acetate | 25.86 ± 1.25 d | 26.06 ± 1.62 d | 29.73 ± 1.3 bc | 34.03 ± 1.64 a | 35.7 ± 0.43 a | 33 ± 0.85 ab | 27.23 ± 1.44 cd | |
| Alcohols | 1-hexanol | 308.63 ± 18.72 c | 456.93 ± 4.72 b | 462.43 ± 11.79 ab | 482.13 ± 25.53 ab | 477.83 ± 9.86 ab | 478.1 ± 3.78 ab | 497.33 ± 12.84 a |
| 3-methylthio-1-propanol | 259.33 ± 15.59 d | 390.33 ± 2.3 b | 387.56 ± 6.57 b | 437.33 ± 32.81 a | 437.33 ± 4.58 a | 409.63 ± 11.9 ab | 340.43 ± 16.23 c | |
| 2-phenyl ethanol | 13,617.43 ± 754.13 a | 11,896.46 ± 181.11 c | 12,028.33 ± 109.2 bc | 13,364.16 ± 531.06 a | 13,130.1 ± 185.2 ab | 13,434.73 ± 321.76 a | 14,030.13 ± 409.26 a | |
| benzyl alcohol | 2.43 ± 0.7 a | 3.3 ± 0.81 a | 8.83 ± 7.48 a | 4.1 ± 1.44 a | 1.86 ± 0.25 a | 9.26 ± 8.51 a | 5.4 ± 4.47 a | |
| cis-3-hexen-1-ol | 20.3 ± 1.12 b | 22.46 ± 0.2 ab | 23.16 ± 1.87 a | 23.16 ± 0.56 a | 22.36 ± 0.41 ab | 22.3 ± 0.55 ab | 22.73 ± 0.75 ab | |
| trans-3-hexen-1-ol | 5.3 ± 0.91 a | 5.13 ± 0.51 a | 6.46 ± 0.55 a | 6.5 ± 0.34 a | 5.86 ± 0.4 a | 5.9 ± 1.15 a | 5.9 ± 0.52 a | |
| Ethyl esters | diethyl succinate | 38 ± 0.69 a | 25.03 ± 0.92 de | 26.66 ± 1.2 cd | 31.06 ± 2.05 b | 28.66 ± 0.83 bc | 28.36 ± 0.9 bc | 22.9 ± 0.43 e |
| ethyl-2-methylbuthyrate | 0.6 ± 0 a | 0.33 ± 0.05 b | 0.33 ± 0.05 b | 0.33 ± 0.05 b | 0.36 ± 0.05 b | 0.33 ± 0.05 b | 0.23 ± 0.05 b | |
| ethyl butyrate | 201.63 ± 7 a | 94.63 ± 8.3 b | 95.3 ± 8.27 b | 100.3 ± 4.15 b | 95.63 ± 4.01 b | 86.83 ± 2.65 b | 59.13 ± 2.6 b | |
| ethyl decanoate | 160.36 ± 31.94 d | 317.63 ± 9.12 c | 462.23 ± 73.11 ab | 511.23 ± 36.44 a | 429.06 ± 6.63 abc | 403.06 ± 57.18 abc | 385.9 ± 12.11 bc | |
| ethyl dodecanoate | 8.26 ± 1.5 b | 9.5 ± 0.65 b | 14.13 ± 2.77 ab | 18.13 ± 3.36 a | 13.26 ± 2.45 ab | 12.06 ± 2.95 ab | 8.96 ± 0.55 b | |
| ethyl hexanoate | 414.33 ± 44.56 a | 136.86 ± 13.19 b | 132.16 ± 12.26 b | 145.46 ± 4.8 b | 128.96 ± 9.57 b | 124.83 ± 13.82 b | 100.7 ± 1.04 b | |
| ethyl isovalerate | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| ethyl lactate | 932.36 ± 76.31 a | 470.2 ± 32.61 b | 445.06 ± 28.25 bc | 378.06 ± 26.2 bcd | 343.23 ± 28.78 cd | 327.03 ± 31.04 d | 289.96 ± 13.95 d | |
| ethyl octanoate | 424.86 ± 128.97 a | 175.13 ± 10.92 b | 183.96 ± 36.25 b | 233.66 ± 4.3 b | 163.73 ± 11.04 b | 152.56 ± 37.04 b | 142.73 ± 6.03 b | |
| methyl salicilate | 0 ± 0 b | 0.3 ± 0 a | 0.23 ± 0.05 a | 0.26 ± 0.05 a | 0.23 ± 0.05 a | 0.23 ± 0.05 a | 0.2 ± 0 a | |
| Fatty acids | butanoic acid | 459.33 ± 17.35 a | 236.23 ± 11.95 b | 222.13 ± 6.36 bc | 227.53 ± 4.37 bc | 218.63 ± 6.94 bc | 204.56 ± 4.16 cd | 185 ± 8.21 d |
| decanoic acid | 726.03 ± 120.83 c | 1374.63 ± 151.64 b | 1955.46 ± 102.26 a | 1900.2 ± 133.15 a | 1735.16 ± 270.48 ab | 1854.43 ± 284.79 ab | 1844.2 ± 150.23 ab | |
| hexanoic acid | 1572.93 ± 37.16 a | 424.43 ± 34.81 b | 384.06 ± 16.7 bc | 390.53 ± 12.1 bc | 365.23 ± 25.23 bc | 334.36 ± 13.82 cd | 292.93 ± 7.19 d | |
| isobutyric acid | 181.26 ± 3.1 a | 91.3 ± 13.35 b | 77.8 ± 6.5 bcd | 67.1 ± 7.2 cd | 59.7 ± 4.3 d | 68.8 ± 9.4 cd | 84.7 ± 2.92 bc | |
| isovaleric acid | 244.73 ± 9.07 a | 171.46 ± 1.2 bc | 167.3 ± 4.23 bc | 176.46 ± 17.86 b | 166.03 ± 5.16 bc | 149.53 ± 9.76 cd | 139.63 ± 7.84 d | |
| nonanoic acid | 5.6 ± 0.95 a | 5.36 ± 0.32 a | 5.2 ± 0.26 a | 5.03 ± 0.57 a | 4.83 ± 0.6 a | 5.16 ± 0.2 a | 4.76 ± 0.49 a | |
| octanoic acid | 2629.2 ± 77.17 a | 881.2 ± 103.87 b | 863.1 ± 17.54 b | 859.8 ± 51.23 b | 876 ± 93.6 b | 840.73 ± 31.54 b | 718.23 ± 54.7 b | |
| valeric acid | 30.9 ± 0.79 a | 13.86 ± 0.8 b | 12.53 ± 1.1 bc | 10.73 ± 0.65 cd | 12.8 ± 0.87 bc | 10.13 ± 1.17 cd | 8.03 ± 1.88 d | |
| Terpenes | alpha terpineol | 11.76 ± 0.92 d | 15.36 ± 1.44 cd | 17 ± 1.99 bc | 20.73 ± 2.33 ab | 23.23 ± 1.53 a | 20.83 ± 2.41 ab | 20.46 ± 1.06 ab |
| beta citronellol | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| geranic acid | 2.33 ± 0.5 c | 4.9 ± 0.78 bc | 4.96 ± 0.76 bc | 6.66 ± 1.81 ab | 9.16 ± 0.81 a | 8.43 ± 0.7 a | 7.33 ± 0.95 ab | |
| geraniol | 16.36 ± 2.01 a | 16.03 ± 0.92 a | 22.06 ± 5 a | 17.46 ± 4.68 a | 13.73 ± 1.81 a | 14.96 ± 3.54 a | 13.83 ± 1.91 a | |
| linalol oxide A | 22.03 ± 1.05 a | 21.33 ± 1.11 a | 20.5 ± 1.47 a | 20.73 ± 0.56 a | 20.66 ± 1.06 a | 20.5 ± 0.7 a | 21.33 ± 0.49 a | |
| linalol oxide B | 9.93 ± 0.63 a | 9.96 ± 0.96 a | 9.86 ± 0.85 a | 10.36 ± 0.3 a | 10.16 ± 0.55 a | 9.76 ± 0.55 a | 10.93 ± 0.37 a | |
| linalool | 99.36 ± 3.3 a | 103.03 ± 3.58 a | 102.6 ± 2.95 a | 99.53 ± 2.73 a | 98.73 ± 9.84 a | 104.46 ± 5.81 a | 97.33 ± 3.5 a | |
| nerol | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| rose oxide I | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| rose oxide II | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| terpinen 4 ol | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Others | benzaldehyde | 8.56 ± 0.23 b | 19.1 ± 1.03 a | 18.2 ± 6.21 ab | 25.06 ± 1.42 a | 26.23 ± 1.43 a | 20.33 ± 4.74 a | 20.33 ± 4.55 a |
| benzothiazole | 1.73 ± 0.2 a | 1.53 ± 0.05 a | 1.73 ± 0.4 a | 1.9 ± 0.17 a | 1.56 ± 0.32 a | 1.33 ± 0.11 a | 1.86 ± 0.4 a | |
| beta damascenone | 1.43 ± 0.2 b | 1.8 ± 0.34 ab | 1.83 ± 0.15 ab | 2.13 ± 0.2 a | 1.76 ± 0.2 ab | 2.03 ± 0.35 ab | 1.76 ± 0.05 ab | |
| beta damascone | 30.4 ± 5.34 b | 34.56 ± 2.17 ab | 42.56 ± 1.55 ab | 46.13 ± 5.85 a | 37.7 ± 3.48 ab | 45.43 ± 7.26 a | 37.33 ± 2.32 ab | |
| guaiacol | 1.3 ± 0.1 a | 1.23 ± 0.32 a | 1.06 ± 0.25 a | 1.16 ± 0.15 a | 1.13 ± 0.05 a | 1.4 ± 0.36 a | 1.46 ± 0.3 a | |
| zingerone | 0.5 ± 0 a | 0.2 ± 0 b | 0.23 ± 0.05 b | 0.23 ± 0.05 b | 0.2 ± 0 b | 0.26 ± 0.05 b | 0.2 ± 0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallo, A.; Roman, T.; Paolini, M.; Cappello, N.; Guzzon, R.; Carrau, F.; Schneider, R.; Larcher, R. Aroma Features of Hanseniaspora vineae Hv205 Wines in Sequential and Co-Inoculation Strategies. Fermentation 2024, 10, 191. https://doi.org/10.3390/fermentation10040191
Gallo A, Roman T, Paolini M, Cappello N, Guzzon R, Carrau F, Schneider R, Larcher R. Aroma Features of Hanseniaspora vineae Hv205 Wines in Sequential and Co-Inoculation Strategies. Fermentation. 2024; 10(4):191. https://doi.org/10.3390/fermentation10040191
Chicago/Turabian StyleGallo, Adelaide, Tomas Roman, Mauro Paolini, Nicola Cappello, Raffaele Guzzon, Francisco Carrau, Rémi Schneider, and Roberto Larcher. 2024. "Aroma Features of Hanseniaspora vineae Hv205 Wines in Sequential and Co-Inoculation Strategies" Fermentation 10, no. 4: 191. https://doi.org/10.3390/fermentation10040191
APA StyleGallo, A., Roman, T., Paolini, M., Cappello, N., Guzzon, R., Carrau, F., Schneider, R., & Larcher, R. (2024). Aroma Features of Hanseniaspora vineae Hv205 Wines in Sequential and Co-Inoculation Strategies. Fermentation, 10(4), 191. https://doi.org/10.3390/fermentation10040191






