Production with Fermentation Culture and Antioxidant Activity of Polysaccharides from Morchella esculenta
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Strain
2.3. Preparation of Polysaccharides
2.4. M. esculenta Culture in Liquid Shaker
2.4.1. Medium Preparation
2.4.2. M. esculenta Activation
2.4.3. Single-Factor Screening of Liquid Fermentation Cultivation
2.4.4. Determination of Single-Factor Concentration in Liquid Culture
2.4.5. Box–Behnken Center Combination Experiment
2.5. Purification and Extraction of M. esculenta Polysaccharides
2.6. Determination of the Molecular Weight
2.7. Monosaccharide Composition Analysis
2.8. FTIR Analysis
2.9. TG Analysis
2.10. SEM Analysis
2.11. Extracellular Antioxidant Activity Test
2.11.1. Hydroxyl Radical Scavenging Activity
2.11.2. DPPH Radical Scavenging Activity
2.11.3. Fe2+ Chelating Capacity
2.12. Intracellular Antioxidant Activity Test
2.12.1. Estimation of the Oxidative Stress-Induced Intracellular Anti-Apoptotic Potential in Zebrafish Embryos
2.12.2. RT-qPCR Analysis
2.13. Anti-Inflammation Activity Evaluation
3. Results and Discussion
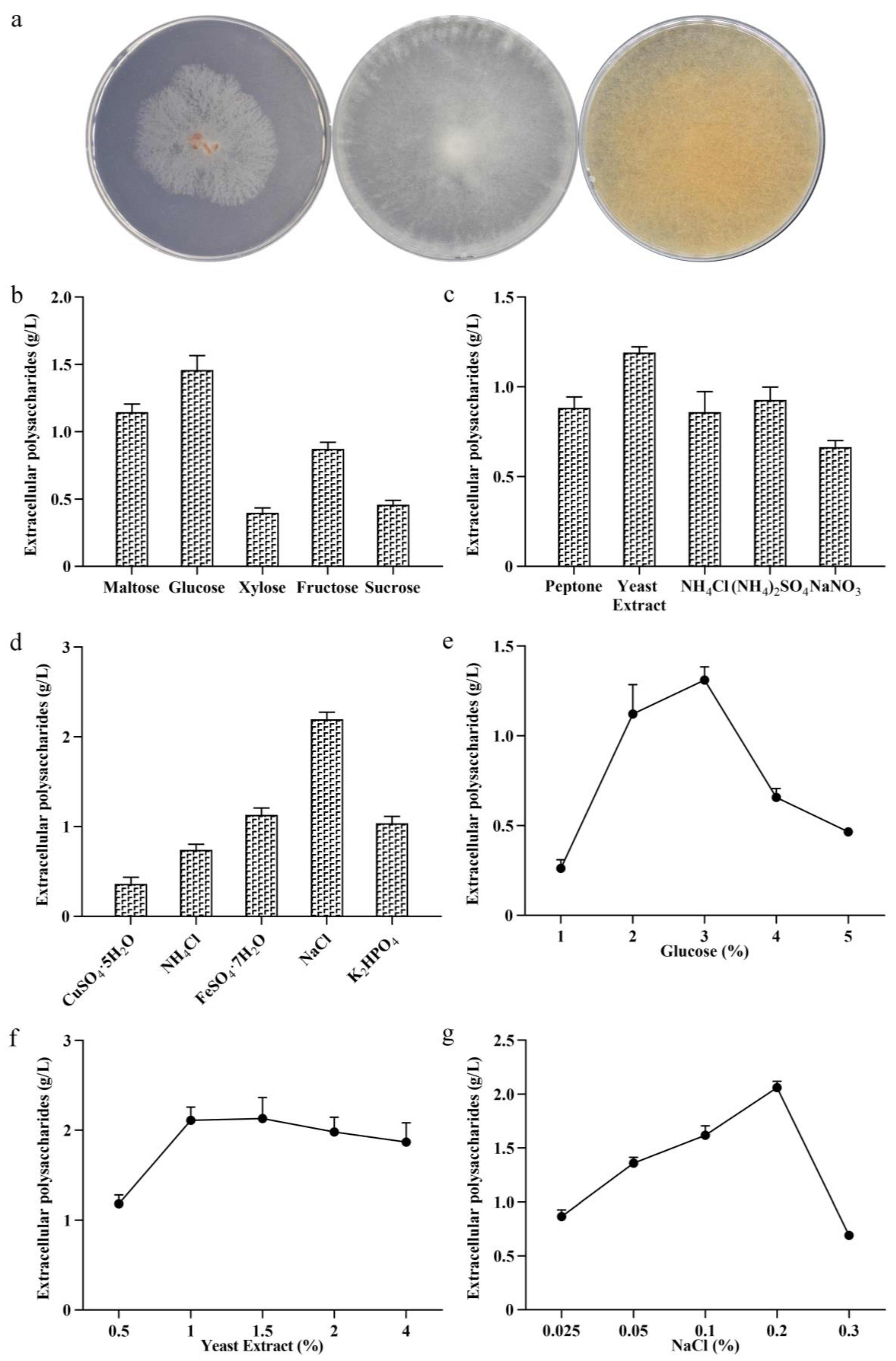
3.1. Strain Identification
3.2. Determination of Liquid Fermentation Medium Conditions
3.2.1. Screening of Single Factors in Medium
3.2.2. Single-Factor Concentration Determination
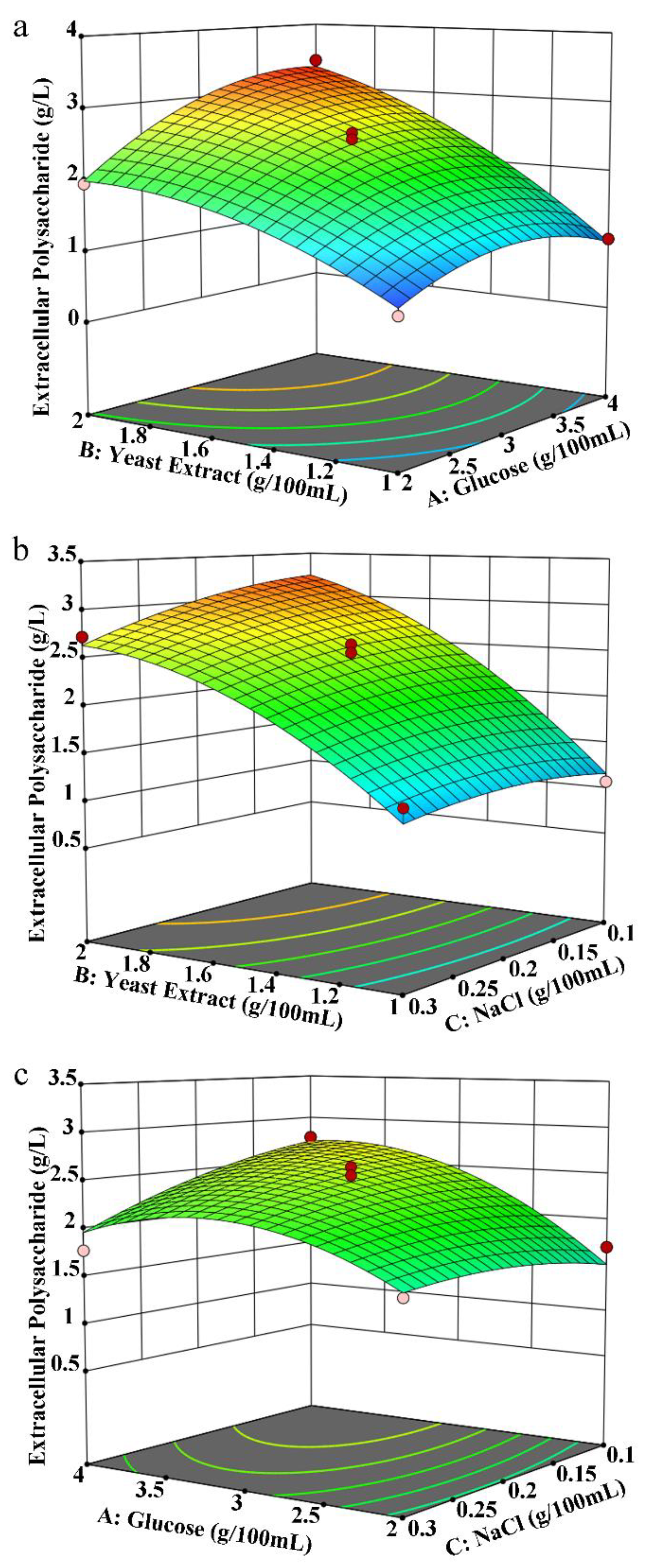
3.2.3. Box–Behnken Central Composite Experiment
3.3. Isolation and Purification of Polysaccharides
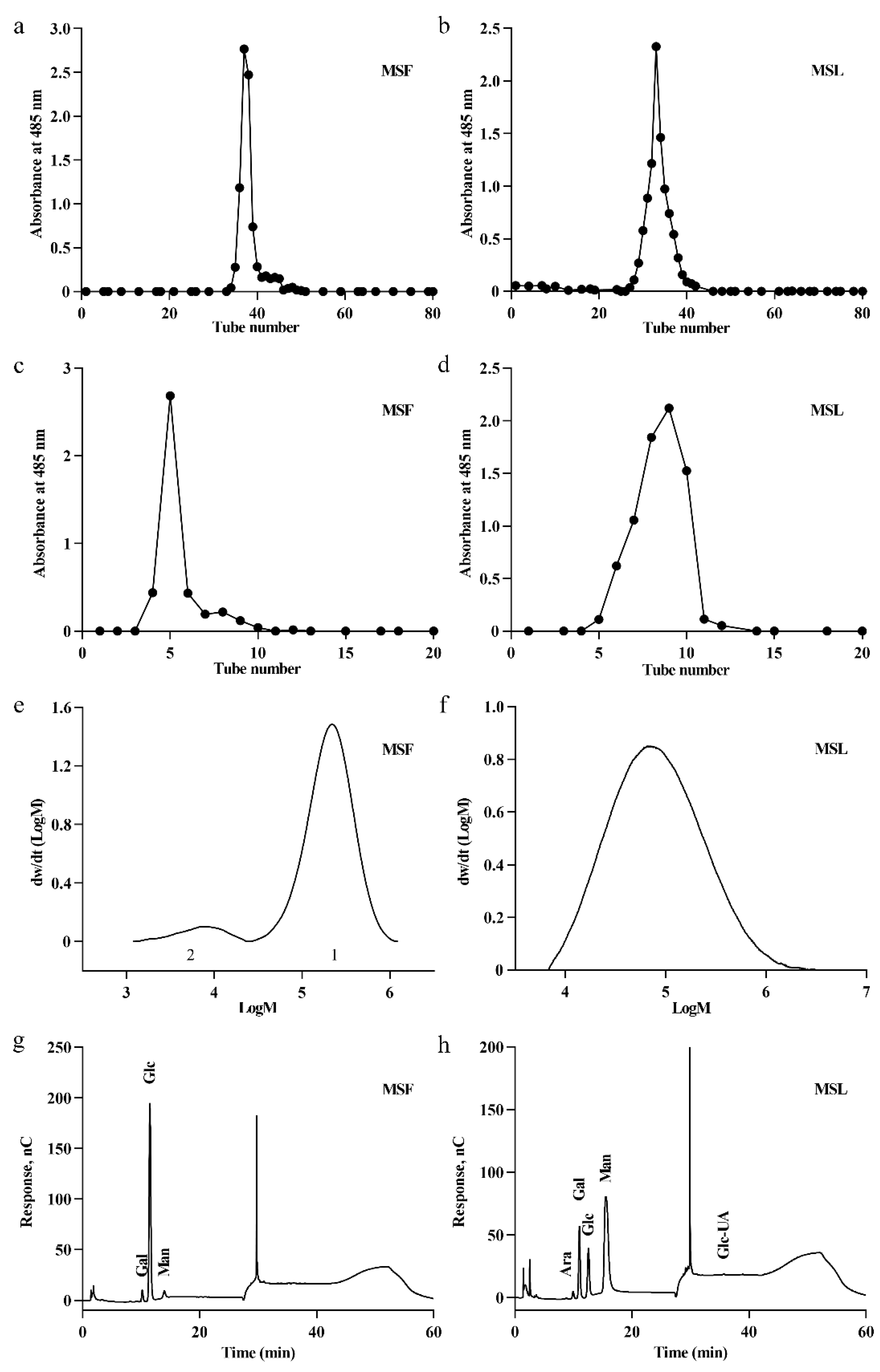
3.4. Analysis of the Molecular Weights of MSF and MSL
3.5. Analysis of the Monosaccharide Composition of MSF and MSL
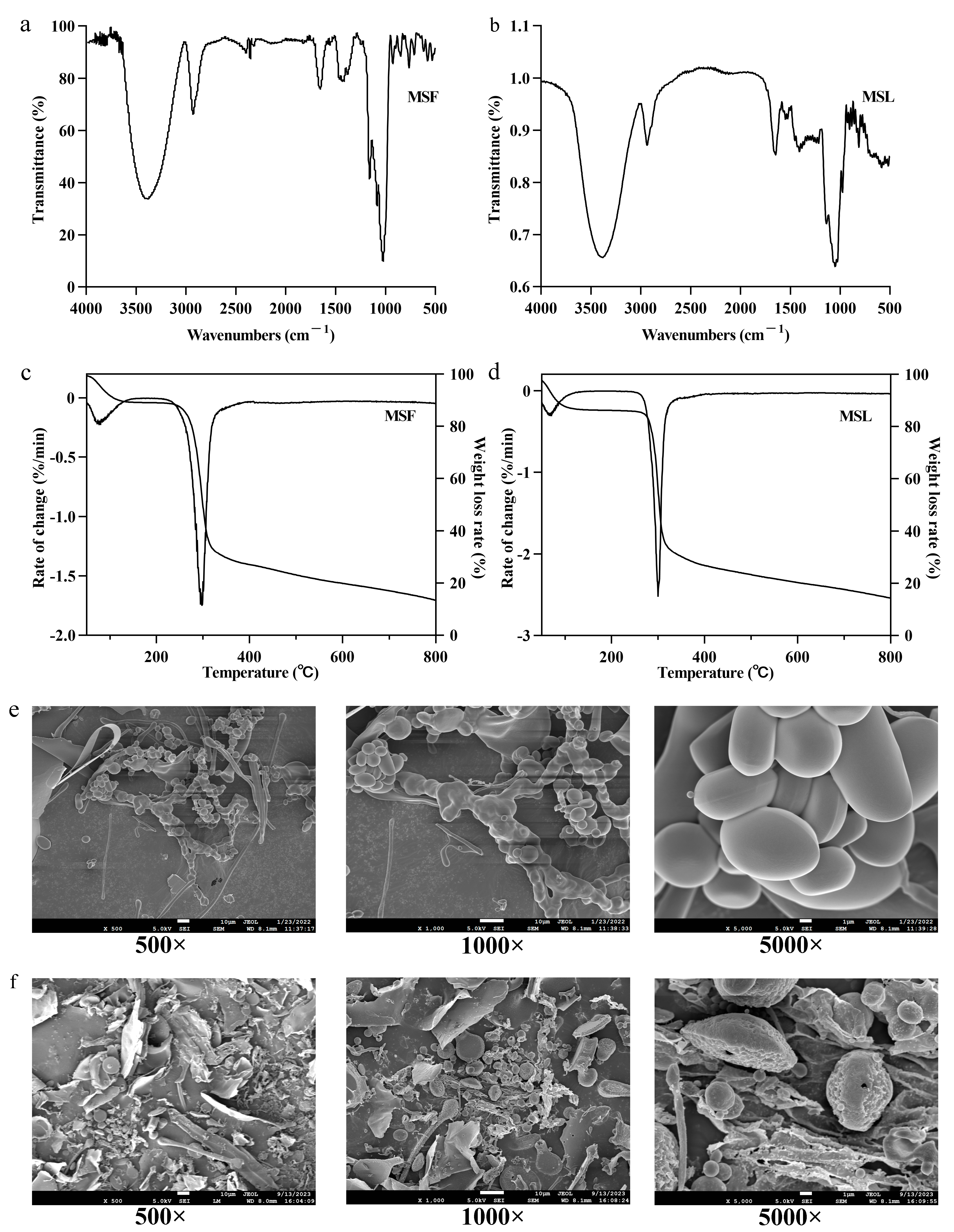
3.6. Analysis of FTIR Spectra of MSF and MSL
3.7. TG of MSF and MSL
3.8. Surface Characterization of MSF and MSL
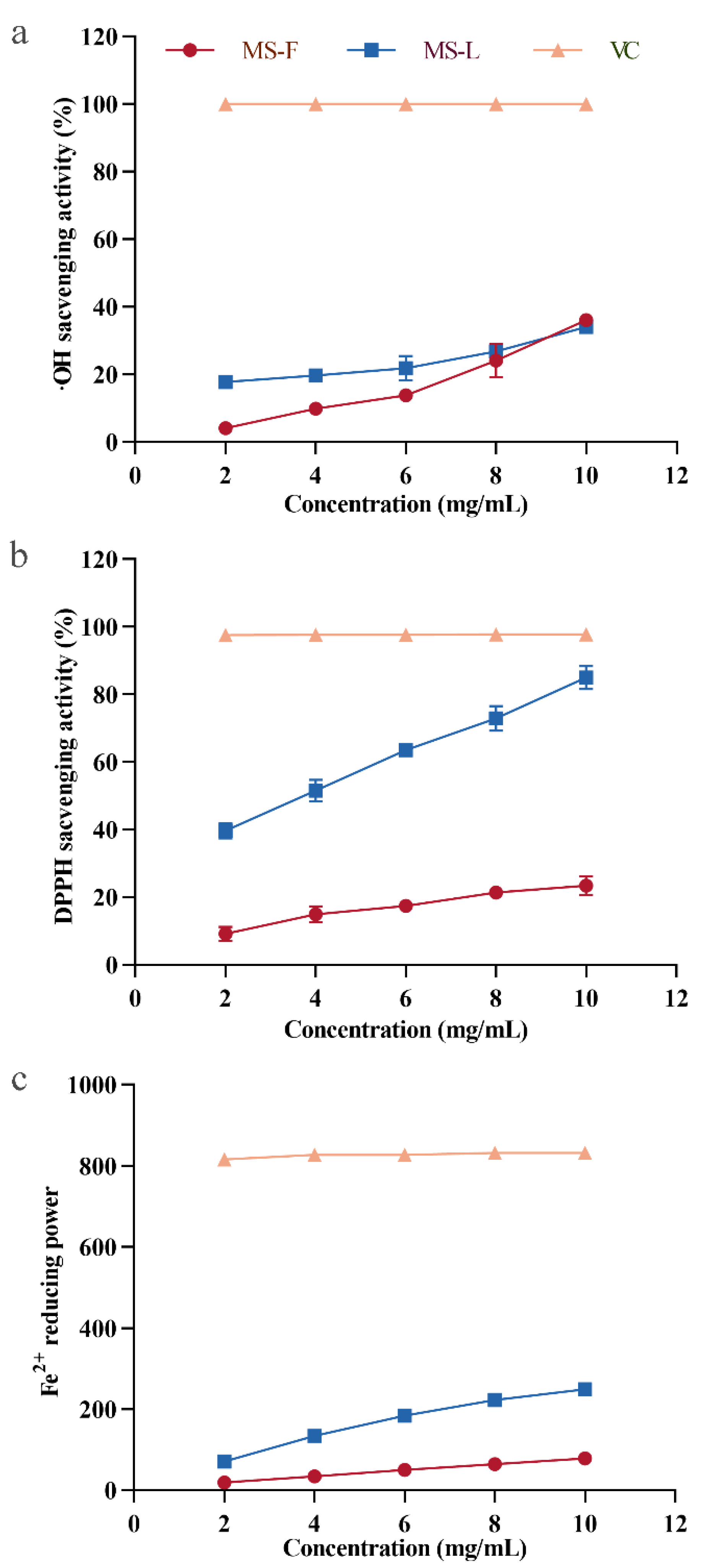
3.9. Antioxidant Activity Analysis of MSF and MSL
3.10. Effect of MSF and MSL Levels and Antioxidant Activities in Metronidazole-Induced Zebrafish Embryos
3.11. Anti-Inflammatory Effects of Polysaccharides MSF and MSL
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunil, C.; Xu, B. Mycochemical profile and health-promoting effects of morel mushroom Morchella esculenta (L.)—A review. Food Res. Int. 2022, 159, 111571. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, H.; Liu, Y.; Nan, J.; Park, H.J.; Chen, Y.; Yang, L. The chemical structure and immunomodulatory activity of an exopolysaccharide produced by Morchella esculenta under submerged fermentation. Food Funct. 2021, 12, 9327–9338. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Roh, H.-S.; Lee, S.; Park, H.B.; Jang, T.S.; Ko, Y.-J.; Baek, K.-H.; Kim, K.H. Bioactivity-guided isolation and chemical characterization of antiproliferative constituents from morel mushroom (Morchella esculenta) in human lung adenocarcinoma cells. J. Funct. Foods 2018, 40, 249–260. [Google Scholar] [CrossRef]
- Longley, R.; Benucci, G.M.N.; Mills, G.; Bonito, G. Fungal and bacterial community dynamics in substrates during the cultivation of morels (Morchella rufobrunnea) indoors. FEMS Microbiol. Lett. 2019, 366, fnz215. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, Y.; Wang, J.; Yang, Y.; Hao, L. Evaluation of the antioxidant activity of extracellular polysaccharides from Morchella esculenta. Food Funct. 2013, 4, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Tseng, M.-H.; Liu, C.-J. Production of polysaccharides from Ganoderma lucidum (CCRC 36041) under limitations of nutrients. Enzym. Microb. Technol. 2006, 38, 109–117. [Google Scholar] [CrossRef]
- Zhang, C.; Song, X.; Cui, W.; Yang, Q. Antioxidant and anti-ageing effects of enzymatic polysaccharide from Pleurotus eryngii residue. Int. J. Biol. Macromol. 2021, 173, 341–350. [Google Scholar] [CrossRef]
- Sun, Y.; Huo, J.; Zhong, S.; Zhu, J.; Li, Y.; Li, X. Chemical structure and anti-inflammatory activity of a branched polysaccharide isolated from Phellinus baumii. Carbohydr. Polym. 2021, 268, 118214. [Google Scholar] [CrossRef]
- Shi, M.; Zhang, Z.; Yang, Y. Antioxidant and immunoregulatory activity of Ganoderma lucidum polysaccharide (GLP). Carbohydr. Polym. 2013, 95, 200–206. [Google Scholar] [CrossRef]
- Liu, X.; Hou, R.; Xu, K.; Chen, L.; Wu, X.; Lin, W.; Zheng, M.; Fu, J. Extraction, characterization and antioxidant activity analysis of the polysaccharide from the solid-state fermentation substrate of Inonotus hispidus. Int. J. Biol. Macromol. 2019, 123, 468–476. [Google Scholar] [CrossRef]
- Yu, J.; Ji, H.-Y.; Liu, A.-J. Alcohol-soluble polysaccharide from Astragalus membranaceus: Preparation, characteristics and antitumor activity. Int. J. Biol. Macromol. 2018, 118, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Wang, W.; Bian, J.; Gao, Y.; Hao, Z.; Tan, J. Recent advances in medicinal and edible homologous polysaccharides: Extraction, purification, structure, modification, and biological activities. Int. J. Biol. Macromol. 2022, 222, 1110–1126. [Google Scholar] [CrossRef]
- Li, W.; Cai, Z.-N.; Mehmood, S.; Wang, Y.; Pan, W.-J.; Zhang, W.-N.; Lu, Y.-M.; Chen, Y. Polysaccharide FMP-1 from Morchella esculenta attenuates cellular oxidative damage in human alveolar epithelial A549 cells through PI3K/AKT/Nrf2/HO-1 pathway. Int. J. Biol. Macromol. 2018, 120, 865–875. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yin, Z.; Ma, L.; Han, L.; Chen, Y.; Pan, W.; Gong, K.; Gao, Y.; Yang, X.; Chen, Y.; et al. Polysaccharide MCP extracted from Morchella esculenta reduces atherosclerosis in LDLR-deficient mice. Food Funct. 2021, 12, 4842–4854. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.-Q.; Feng, J.; Liu, Y.-F.; Hu, D.-M.; Zhang, J.-S. Functional Components from the Liquid Fermentation of Edible and Medicinal Fungi and Their Food Applications in China. Foods 2023, 12, 2086. [Google Scholar] [CrossRef] [PubMed]
- Confortin, F.G.; Marchetto, R.; Bettin, F.; Camassola, M.; Salvador, M.; Dillon, A.J.P. Production of Pleurotus sajor-caju strain PS-2001 biomass in submerged culture. J. Ind. Microbiol. Biotechnol. 2008, 35, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, Z.; Chen, S.-F.; Li, Y.-Q. Optimization for the production of exopolysaccharide from Fomes fomentarius in submerged culture and its antitumor effect in vitro. Bioresour. Technol. 2008, 99, 3187–3194. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, C.; Meng, Q.; Fu, S. Characterization of Secondary Metabolites from Mycelial Cultures of Black Morel Mushroom Morchella importuna (Ascomycota). Int. J. Med. Mushrooms 2023, 25, 49–54. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, X.; Ma, L.; Xiao, D.; Liu, X.; Ying, Z.; Li, Y.; Lin, Y. Transcriptomic and Metabolomic Profiles Provide Insights into the Red-Stipe Symptom of Morel Fruiting Bodies. J. Fungi 2023, 9, 373. [Google Scholar] [CrossRef]
- López-Legarda, X.; Rostro-Alanis, M.; Parra-Saldivar, R.; Villa-Pulgarín, J.A.; Segura-Sánchez, F. Submerged cultivation, characterization and in vitro antitumor activity of polysaccharides from Schizophyllum radiatum. Int. J. Biol. Macromol. 2021, 186, 919–932. [Google Scholar] [CrossRef]
- Zhao, P.; Guan, M.; Tang, W.; Walayat, N.; Ding, Y.; Liu, J. Structural diversity, fermentation production, bioactivities and applications of triterpenoids from several common medicinal fungi: Recent advances and future perspectives. Fitoterapia 2023, 166, 105470. [Google Scholar] [CrossRef] [PubMed]
- He, N.; Yang, X.; Jiao, Y.; Tian, L.; Zhao, Y. Characterisation of antioxidant and antiproliferative acidic polysaccharides from Chinese wolfberry fruits. Food Chem. 2012, 133, 978–989. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, Y.; Lei, L.; Li, F.; Zhang, Y.; Chen, J.; Zhao, G.; Wu, S.; Yin, R.; Ming, J. Carboxymethylation of polysaccharide from Morchella angusticepes Peck enhances its cholesterol-lowering activity in rats. Carbohydr. Polym. 2017, 172, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sang, Y.; Zhu, D.; Yang, Y.; Lei, Z.; Zhang, Z. Optimization of fermentation conditions for crude polysaccharides by Morchella esculenta using soybean curd residue. Ind. Crop. Prod. 2013, 50, 666–672. [Google Scholar] [CrossRef]
- Ge, Y.; Duan, Y.; Fang, G.; Zhang, Y.; Wang, S. Polysaccharides from fruit calyx of Physalis alkekengi var. francheti: Isolation, purification, structural features and antioxidant activities. Carbohydr. Polym. 2009, 77, 188–193. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, G. Extraction, structural analysis and antioxidant activity of aloe polysaccharide. J. Mol. Struct. 2023, 1273, 134379. [Google Scholar] [CrossRef]
- Garai, D.; Kumar, V. A Box–Behnken design approach for the production of xylanase by Aspergillus candidus under solid state fermentation and its application in saccharification of agro residues and Parthenium hysterophorus L. Ind. Crop. Prod. 2013, 44, 352–363. [Google Scholar] [CrossRef]
- Jiang, J.; Kong, F.; Li, N.; Zhang, D.; Yan, C.; Lv, H. Purification, structural characterization and in vitro antioxidant activity of a novel polysaccharide from Boshuzhi. Carbohydr. Polym. 2016, 147, 365–371. [Google Scholar] [CrossRef]
- Zhu, H.; Tian, L.; Zhang, L.; Bi, J.; Song, Q.; Yang, H.; Qiao, J. Preparation, characterization and antioxidant activity of polysaccharide from spent Lentinus edodes substrate. Int. J. Biol. Macromol. 2018, 112, 976–984. [Google Scholar] [CrossRef]
- Xiong, S.L.; Li, A.; Huang, N.; Lu, F.; Hou, D. Antioxidant and immunoregulatory activity of different polysaccharide fractions from tuber of Ophiopogon japonicus. Carbohydr. Polym. 2011, 86, 1273–1280. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Fakhfakh, J.; Athmouni, K.; Mallek-Fakhfakh, H.; Ayedi, H.; Allouche, N. Polysaccharide from Lycium arabicum: Structural Features, in Vitro Antioxidant Activities and Protective Effect against Oxidative Damage in Human Erythrocytes. Chem. Biodivers. 2020, 17, e2000614. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Fang, Y.; Huang, W.; Lei, P.; Xu, X.; Sun, D.; Wu, L.; Xu, H.; Li, S. Effect of surfactants on the production and biofunction of Tremella fuciformis polysaccharide through submerged fermentation. LWT 2022, 163, 113602. [Google Scholar] [CrossRef]
- He, H.; Li, Y.; Fang, M.; Li, T.; Liang, Y.; Mei, Y. Carbon Source Affects Synthesis, Structures, and Activities of Mycelial Polysaccharides from Medicinal Fungus Inonotus obliquus. J. Microbiol. Biotechnol. 2021, 31, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Feng, N.; Tang, Q.-J.; Liu, Y.-F.; Yang, Y.; Liu, F.; Zhang, J.-S.; Lin, C.-C. Optimization of Ganoderma lucidum Polysaccharides Fermentation Process for Large-Scale Production. Appl. Biochem. Biotechnol. 2019, 189, 972–986. [Google Scholar] [CrossRef]
- Ji, X.; Wang, Z.; Hao, X.; Zhu, Y.; Lin, Y.; Li, G.; Guo, X. Structural characterization of a new high molecular weight polysaccharide from jujube fruit. Front. Nutr. 2022, 9, 1012348. [Google Scholar] [CrossRef]
- Li, T.; Yang, Y.; Liu, Y.; Zhou, S.; Yan, M.Q.; Wu, D.; Zhang, J.; Tang, C. Physicochemical characteristics and biological activities of polysaccharide fractions from Phellinus baumii cultured with different methods. Int. J. Biol. Macromol. 2015, 81, 1082–1088. [Google Scholar] [CrossRef]
- Varma, C.A.K.; Kumar, K.J. Structural, functional and pH sensitive release characteristics of water-soluble polysaccharide from the seeds of Albizia lebbeck L. Carbohydr. Polym. 2017, 175, 502–508. [Google Scholar] [CrossRef]
- Zhong, Q.; Wei, B.; Wang, S.; Ke, S.; Chen, J.; Zhang, H.; Wang, H. The Antioxidant Activity of Polysaccharides Derived from Marine Organisms: An Overview. Mar. Drugs 2019, 17, 674. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Fan, L.; Ai, L.; Shan, L. Antioxidant activities of polysaccharides from the fruiting bodies of Zizyphus Jujuba cv. Jinsixiaozao. Carbohydr. Polym. 2011, 84, 390–394. [Google Scholar] [CrossRef]
- Zhang, Z.; Shi, M.; Zheng, H.; Ren, R.; Zhang, S.; Ma, X. Structural characterization and biological activities of a new polysaccharide isolated from Morchella Sextelata. Glycoconj. J. 2022, 39, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, X.; Yan, X.-H.; Zhang, J.-L.; Wang, L.-Y.; Xue, H.; Jiang, G.-C.; Ma, X.-T.; Liu, X.-J. Characterization, hypolipidemic and antioxidant activities of degraded polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2019, 135, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-H.; Dai, X.-Y.; Chen, Q.; Zang, J.-N.; Deng, L.-L.; Liu, Y.-H.; Ying, H.-Z. Hypolipidemic and antioxidant activities of polysaccharides from Rosae Laevigatae Fructus in rats. Carbohydr. Polym. 2013, 94, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Jin, C.; Tong, Z.; Lu, J.; Tian, L.; Chang, Q. Optimization extraction, characterization and antioxidant activities of pectic polysaccharide from tangerine peels. Carbohydr. Polym. 2016, 136, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, S.; Li, T.; Li, X.; Jiao, L.; Zhang, L. Purification, structure and immunobiological activity of a new water-soluble polysaccharide from the mycelium of Polyporus albicans (Imaz.) Teng. Bioresour. Technol. 2008, 99, 900–904. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Sun, H.; Zhang, X.; Huang, F.; Pan, L.; Zhu, Z. Structural characterization and inhibitions on α-glucosidase and α-amylase of alkali-extracted water-soluble polysaccharide from Annona squamosa residue. Int. J. Biol. Macromol. 2020, 166, 730–740. [Google Scholar] [CrossRef]
- Wang, L.C.; Zhang, K.; Di, L.Q.; Liu, R.; Wu, H. Isolation and structural elucidation of novel homogenous polysaccharide from Mactra veneriformis. Carbohydr. Polym. 2011, 86, 982–987. [Google Scholar] [CrossRef]
- Abuduwaili, A.; Nuerxiati, R.; Mutailifu, P.; Gao, Y.; Lu, C.; Yili, A. Isolation, structural modification, characterization, and bioactivity of polysaccharides from Folium Isatidis. Ind. Crop. Prod. 2022, 176, 114319. [Google Scholar] [CrossRef]
- da Rocha, L.B.N.; Sousa, R.B.; dos Santos, M.V.B.; Neto, N.M.A.; Soares, L.L.d.S.; Alves, F.L.C.; de Carvalho, M.A.M.; Osajima, J.A.; Silva-Filho, E.C. Development of a new biomaterial based on cashew tree gum (Anarcadium occidentale L.) enriched with hydroxyapatite and evaluation of cytotoxicity in adipose-derived stem cell cultures. Int. J. Biol. Macromol. 2023, 242, 124864. [Google Scholar] [CrossRef]
- Kong, L.; Yu, L.; Feng, T.; Yin, X.; Liu, T.; Dong, L. Physicochemical characterization of the polysaccharide from Bletilla striata: Effect of drying method. Carbohydr. Polym. 2015, 125, 1–8. [Google Scholar] [CrossRef]
- Li, Q.; Geng, X.; Zhu, L.; Zheng, F.; Chen, E.; Wang, G.; Li, X. Structural characterization and antioxidant properties of a novel polysaccharide isolated from Jiuzao in vitro and in vivo. Food Res. Int. 2022, 162, 111940. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; Seo, E.W.; Jeong, H.J. Effect of extracts from pine needle against oxidative DNA damage and apoptosis induced by hydroxyl radical via antioxidant activity. Food Chem. Toxicol. 2009, 47, 2135–2141. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Haldar, P.K.; Ghosh, A.K. Antioxidant and free-radical-scavenging effects of fruits of Dregea volubilis. J. Nat. Sci. Biol. Med. 2010, 1, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xie, M.-Y.; Nie, S.-P.; Li, C.; Wang, Y.-X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Jo, Y.-J.; Cho, H.-S.; Chun, J.-Y. Antioxidant activity of β-cyclodextrin inclusion complexes containing trans-cinnamaldehyde by DPPH, ABTS and FRAP. Food Sci. Biotechnol. 2021, 30, 807–814. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zhao, Y.; Guo, C.; Yang, X. A comparative study on the antioxidant activities of an acidic polysaccharide and various solvent extracts derived from herbal Houttuynia cordata. Carbohydr. Polym. 2011, 83, 537–544. [Google Scholar] [CrossRef]
- Chen, B.-J.; Shi, M.-J.; Cui, S.; Hao, S.-X.; Hider, R.C.; Zhou, T. Improved antioxidant and anti-tyrosinase activity of polysaccharide from Sargassum fusiforme by degradation. Int. J. Biol. Macromol. 2016, 92, 715–722. [Google Scholar] [CrossRef]
- Zuofa, Z.; Guoying, L.; Meng, S.; Tingting, S.; Juan, P. Protective effect of polysaccharide from the artificially cultivated Sanghuangporus vaninii against H2O2-induced toxicity in vitro and in zebrafish models. Arab. J. Chem. 2023, 16, 105115. [Google Scholar] [CrossRef]
- Cai, Z.-N.; Li, W.; Mehmood, S.; Pan, W.-J.; Wang, Y.; Meng, F.-J.; Wang, X.-F.; Lu, Y.-M.; Chen, Y. Structural characterization, in vitro and in vivo antioxidant activities of a heteropolysaccharide from the fruiting bodies of Morchella esculenta. Carbohydr. Polym. 2018, 195, 29–38. [Google Scholar] [CrossRef]
- Huang, J.; Mckee, M.; Huang, H.D.; Xiang, A.; Davidson, A.J.; Lu, H.A. A zebrafish model of conditional targeted podocyte ablation and regeneration. Kidney Int. 2013, 83, 1193–1200. [Google Scholar] [CrossRef]
- Ko, S.-C.; Kim, D.; Jeon, Y.-J. Protective effect of a novel antioxidative peptide purified from a marine Chlorella ellipsoidea protein against free radical-induced oxidative stress. Food Chem. Toxicol. 2012, 50, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, X.; Gao, D.; Zhang, Y.; Chen, X.; Xia, Q.; Jin, M.; Sun, C.; He, Q.; Wang, R.; et al. Developmental toxicity caused by sanguinarine in zebrafish embryos via regulating oxidative stress, apoptosis and wnt pathways. Toxicol. Lett. 2021, 350, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Gui, Y.-H.; Liu, L.; Wu, W.; Zhang, Y.; Jia, Z.-L.; Shi, Y.-P.; Kong, H.-T.; Liu, K.-C.; Jiao, W.-H.; Lin, H.-W. Discovery of nitrogenous sesquiterpene quinone derivatives from sponge Dysidea septosa with anti-inflammatory activity in vivo zebrafish model. Bioorganic Chem. 2020, 94, 103435. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; He, P.; Shi, X.; Zhang, Y.; Perez-Moreno, J.; Yu, F. Large-Scale Field Cultivation of Morchella and Relevance of Basic Knowledge for Its Steady Production. J. Fungi 2023, 9, 855. [Google Scholar] [CrossRef]


| Peak No. | Mn (KDa) | Mw (KDa) | Mz (KDa) | Mw/Mn |
|---|---|---|---|---|
| MSF-1 | 173 | 243 | 328 | 1.41 |
| MSF-2 | 5.65 | 7.96 | 10.3 | 1.41 |
| MSL | 50.2 | 140 | 396 | 2.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Sun, Q.; Li, S.; Chen, W.; Shi, Z.; Xu, Z.; Xu, L.; Chen, M.; Li, Z. Production with Fermentation Culture and Antioxidant Activity of Polysaccharides from Morchella esculenta. Fermentation 2024, 10, 46. https://doi.org/10.3390/fermentation10010046
Li X, Sun Q, Li S, Chen W, Shi Z, Xu Z, Xu L, Chen M, Li Z. Production with Fermentation Culture and Antioxidant Activity of Polysaccharides from Morchella esculenta. Fermentation. 2024; 10(1):46. https://doi.org/10.3390/fermentation10010046
Chicago/Turabian StyleLi, Xiaobei, Qiuyan Sun, Shuai Li, Wenchao Chen, Zhimin Shi, Ziyin Xu, Lin Xu, Mei Chen, and Zhonghai Li. 2024. "Production with Fermentation Culture and Antioxidant Activity of Polysaccharides from Morchella esculenta" Fermentation 10, no. 1: 46. https://doi.org/10.3390/fermentation10010046
APA StyleLi, X., Sun, Q., Li, S., Chen, W., Shi, Z., Xu, Z., Xu, L., Chen, M., & Li, Z. (2024). Production with Fermentation Culture and Antioxidant Activity of Polysaccharides from Morchella esculenta. Fermentation, 10(1), 46. https://doi.org/10.3390/fermentation10010046





