Urea-Induced Enhancement of Hypocrellin A Synthesis in Shiraia bambusicola GDMCC 60438: Strategies and Mechanisms
Abstract
1. Introduction
2. Materials and Methods
2.1. Production Hypocrellin A Using Submerged Fermentation of S. bambusicola GDMCC60438
2.2. Determination of HA Yield and Mycelium Biomass
2.3. Morphology Observation and Diameter Determination of Mycelium Pellets
2.4. Observation of Mycelium Morphology on the Surface of Mycelium Pellets
2.5. Transcriptome Sequencing and Bioinformatics Analysis
2.6. Identification of Hyaluronic Acid Synthesis and Urea-Pathway-Related Genes by qRT-PCR
2.7. Statistical Analysis
3. Results and Discussion
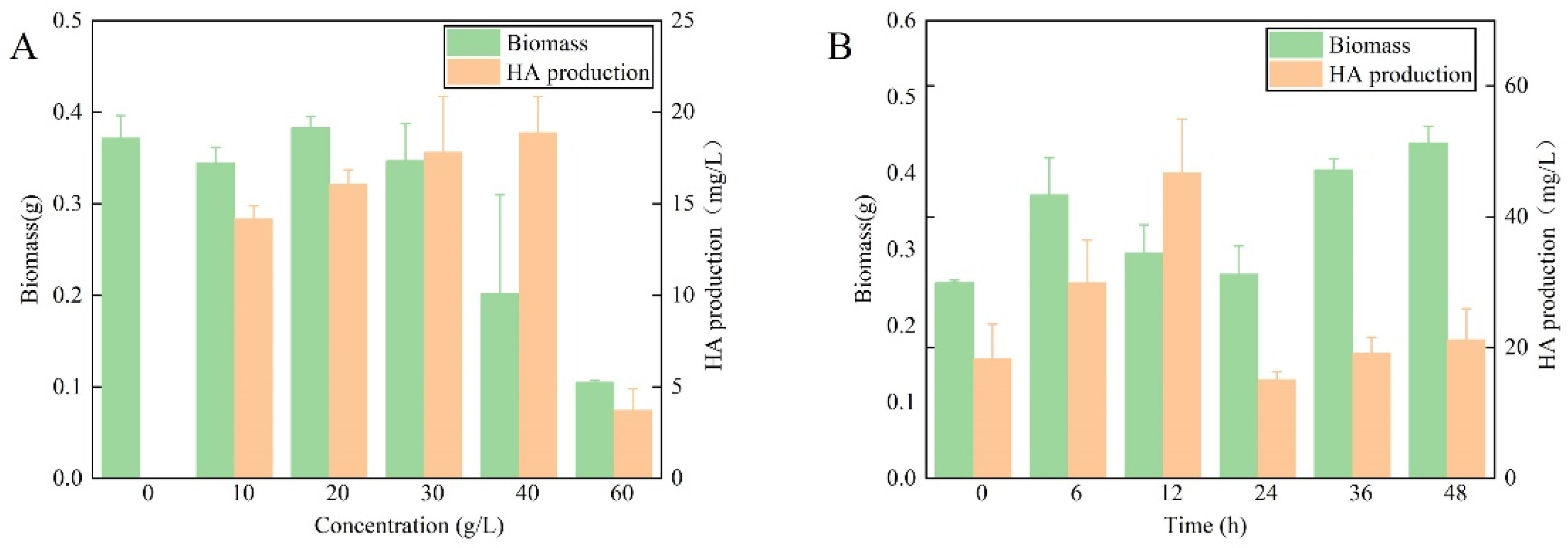
3.1. Impact of Exogenous Urea on Shiraia bambusicola Growth and HA Production
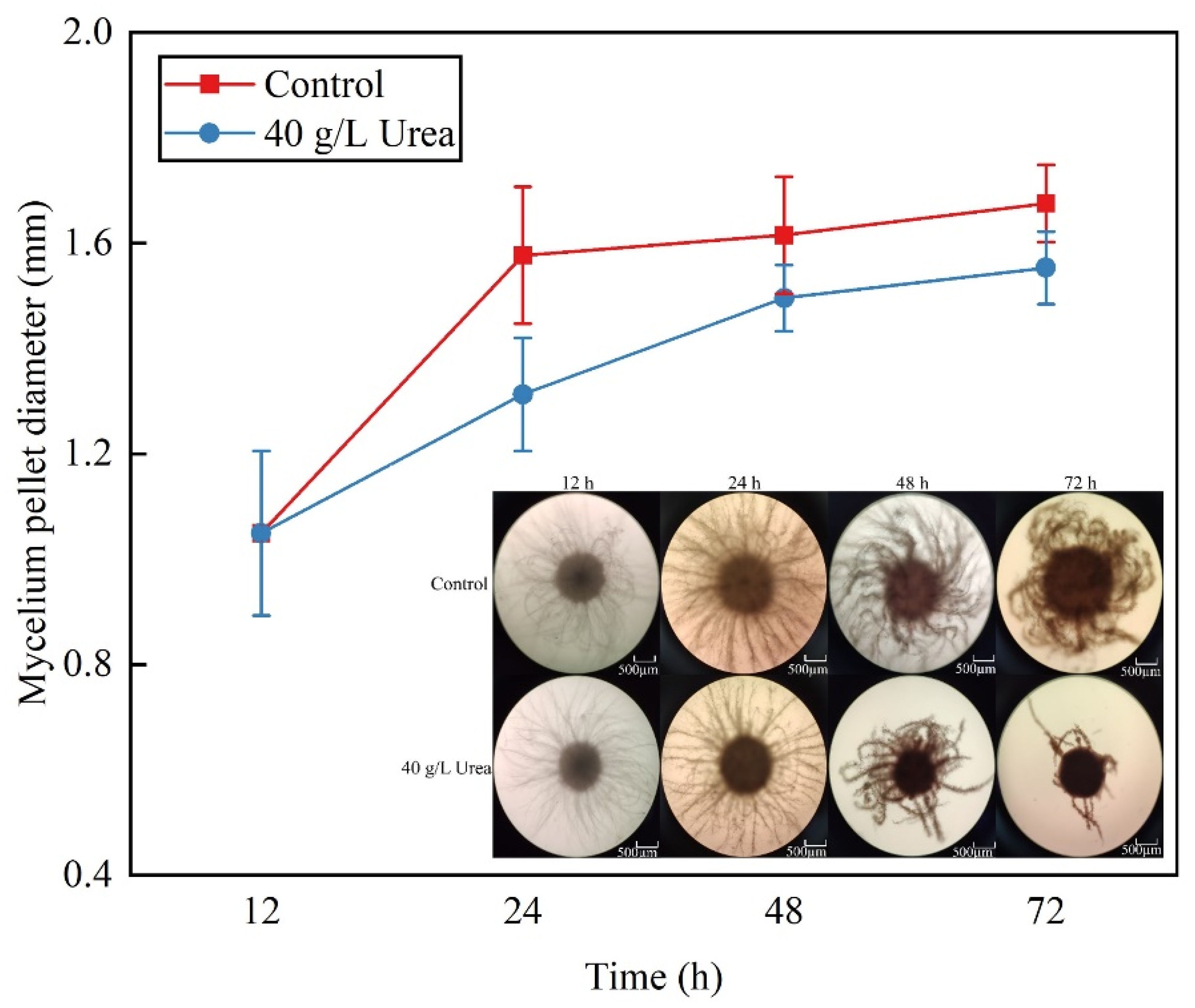
3.2. Mycelium Pellet Size and Morphological Changes Influenced by Urea Supplementation
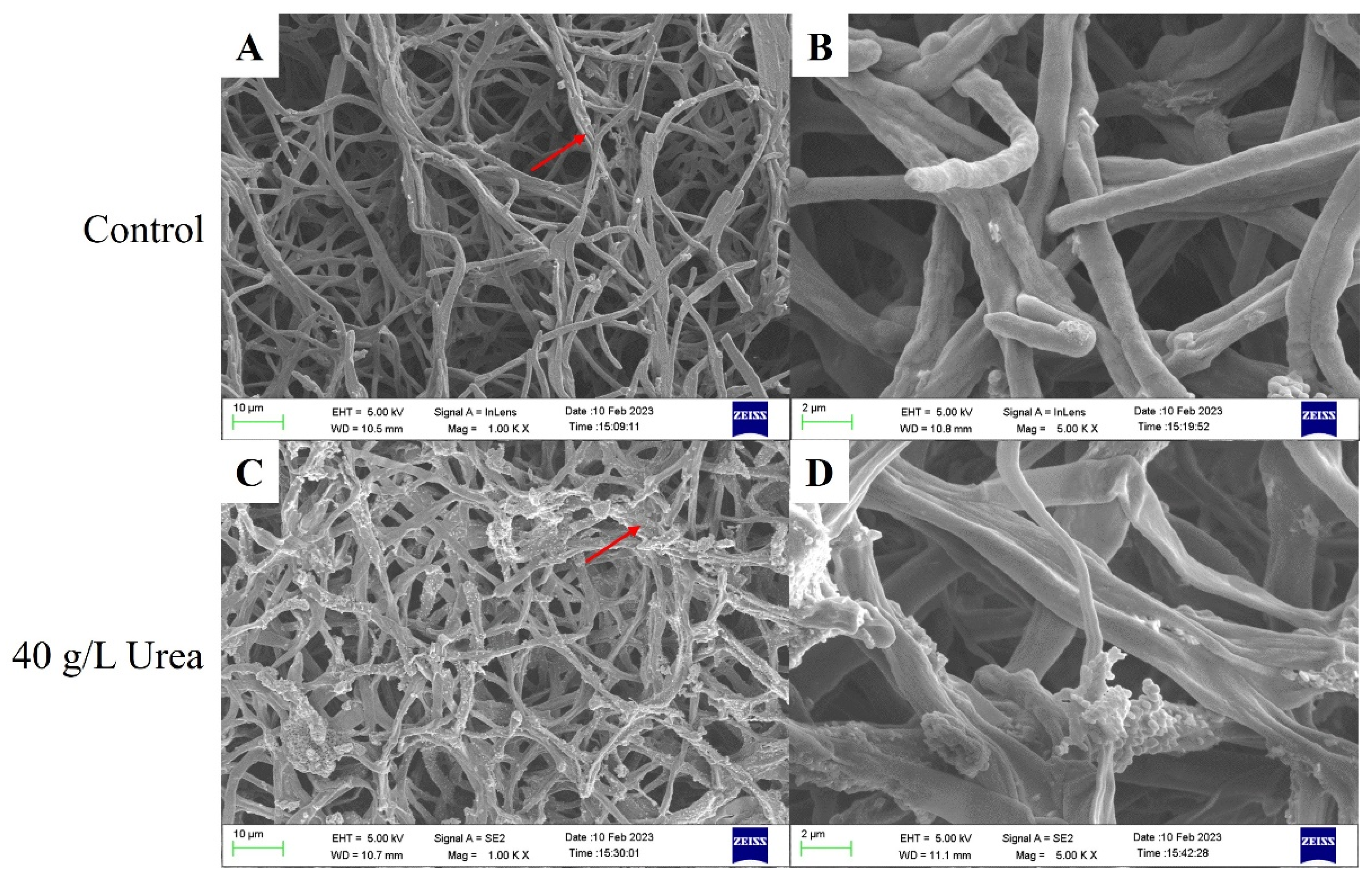
3.3. Scanning Electron Microscopy Analysis of Mycelial Morphology
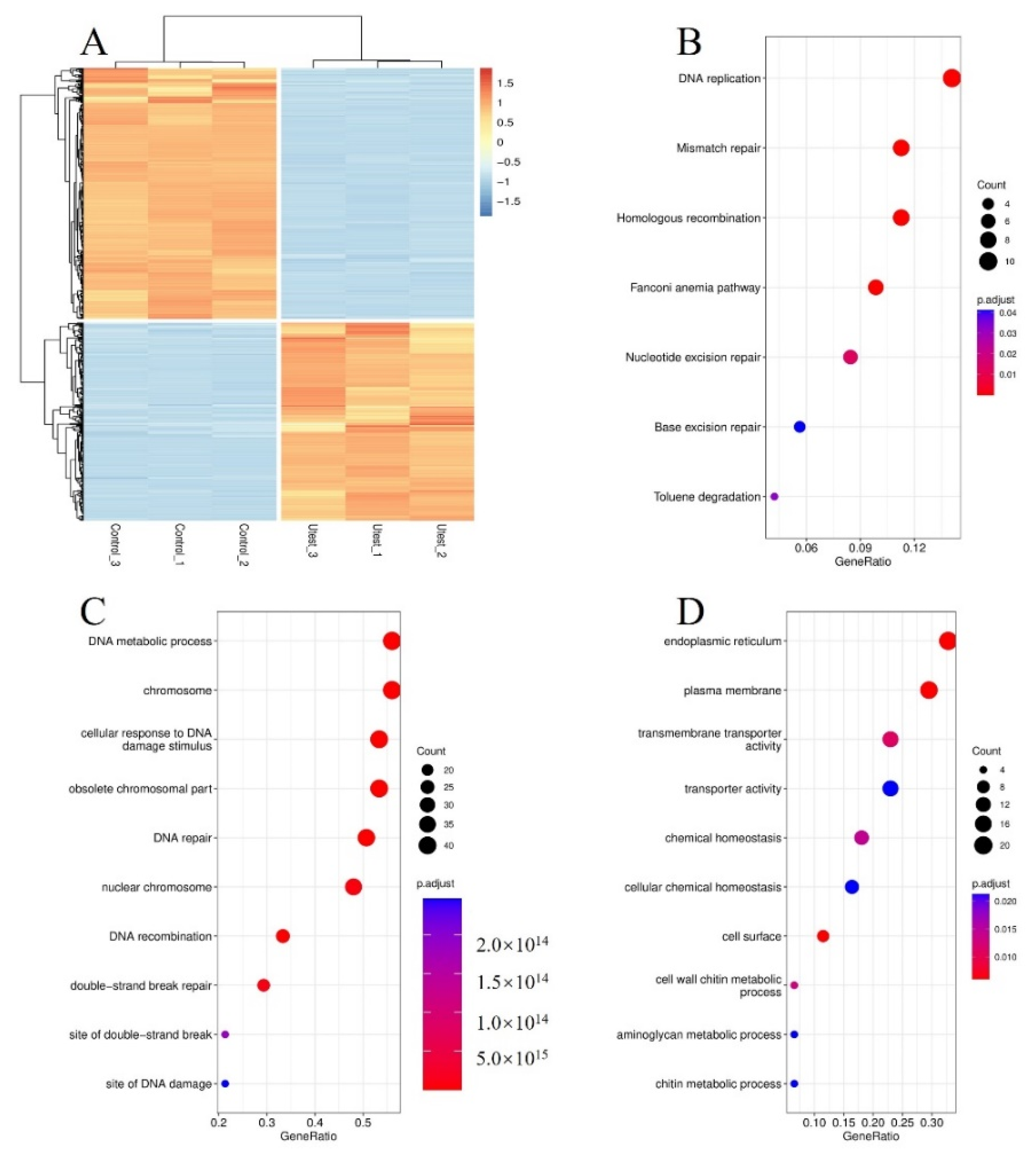
3.4. Transcriptomic Analysis and Differential Expression Profiling
3.5. Acetyl-CoA Accumulation and Its Role in HA Biosynthesis
3.6. Screening and Validation of HA Biosynthesis and Transport Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, S.M.; Olivo, M. Efficacy of hypocrellin pharmacokinetics in phototherapy. Int. J. Oncol. 2002, 21, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Morakotkarn, D.; Kawasaki, H.; Seki, T. Molecular diversity of bamboo-associated fungi isolated from Japan. FEMS Microbiol. Lett. 2007, 266, 10–19. [Google Scholar] [CrossRef]
- Cheng, T.; Jia, X.; Ma, X.; Lin, H.; Zhao, Y. Phylogenetic study on Shiraia bambusicola by rDNA sequence analyses. J. Basic Microbiol. 2004, 44, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiao, C.; Ma, W.; He, G. The production of hypocrellin colorants by submerged cultivation of the medicinal fungus Shiraia bambusicola. Dyes Pigment. 2009, 82, 142–146. [Google Scholar] [CrossRef]
- Estey, E.P.; Brown, K.; Diwu, Z.; Liu, J.; Lown, J.W.; Miller, G.G.; Moore, R.B.; Tulip, J.; Mcphee, M.S. Hypocrellins as photosensitizers for photodynamic therapy: A screening evaluation and pharmacokinetic study. Cancer Chemother. Pharmacol. 1996, 37, 343–350. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, E.H.; Li, J.F.; Zhang, T.C.; Ma, W.J. Photodynamic effects of hypocrellin A on three human malignant cell lines by inducing apoptotic cell death. J. Photochem. Photobiol. B-Biol. 1998, 43, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.Z.; Tang, G.; Gao, B.; Zhang, G. Spectroscopic studies on the excited-state properties of the light-induced antiviral drug hypocrellin A loaded in the mesoporous solid. Chem. Phys. Lett. 2004, 396, 102–109. [Google Scholar] [CrossRef]
- Hudson, J.B.; Zhou, J.; Chen, J.; Harris, L.; Yip, L.; Towers, G.H. Hypocrellin, from Hypocrella bambuase, is phototoxic to human immunodeficiency virus. Photochem. Photobiol. 1994, 60, 253–255. [Google Scholar] [CrossRef]
- Morgan, B.J.; Mulrooney, C.A.; O’Brien, E.M.; Kozlowski, M.C. Perylenequinone natural products: Total syntheses of the diastereomers (+)-phleichrome and (+)-calphostin D by assembly of centrochiral and axial chiral fragments. J. Org. Chem. 2010, 75, 30–43. [Google Scholar] [CrossRef][Green Version]
- O’Brien, E.M.; Morgan, B.J.; Mulrooney, C.A.; Carroll, P.J.; Kozlowski, M.C. Perylenequinone natural products: Total synthesis of hypocrellin A. J. Org. Chem. 2010, 75, 57–68. [Google Scholar] [CrossRef]
- Lei, X.Y.; Zhang, M.Y.; Ma, Y.J.; Wang, J.W. Transcriptomic responses involved in enhanced production of hypocrellin A by addition of Triton X-100 in submerged cultures of Shiraia bambusicola. J. Ind. Microbiol. Biotechnol. 2017, 44, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zhang, Z.; Wang, Y.; Yang, H.; Zeng, Q.; Zhu, D. Efficient strategy for maintaining and enhancing the huperzine A production of Shiraia sp. Slf14 through inducer elicitation. J. Ind. Microbiol. Biotechnol. 2014, 41, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhao, N.; Guo, B.; Lin, X.; Chen, S.; Yan, S. Gentic overexpression increases production of hypocrellin A in Shiraia bambusicola S4201. J. Microbiol. 2019, 57, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Ma, Y.J.; Wang, J.W. Adding bamboo charcoal powder to Shiraia bambusicola preculture improves hypocrellin A production. Sustain. Chem. Pharm. 2019, 14, 100191. [Google Scholar] [CrossRef]
- Liu, X.Y.; Fan, L.; Gao, J.; Shen, X.Y.; Hou, C.L. Global identification of alternative splicing in Shiraia bambusicola and analysis of its regulation in hypocrellin biosynthesis. Appl. Microbiol. Biotechnol. 2020, 104, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Deng, H.; Guan, Z.; Liao, X.; Cai, Y. Enhanced hypocrellin production via coexpression of alpha-amylase and hemoglobin genes in Shiraia bambusicola. AMB Express 2018, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.J.; Sun, C.X.; Wang, J.W. Enhanced Production of Hypocrellin A in Submerged Cultures of Shiraia bambusicola by Red Light. Photochem. Photobiol. 2019, 95, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.X.; Ma, Y.J.; Wang, J.W. Enhanced production of hypocrellin A by ultrasound stimulation in submerged cultures of Shiraia bambusicola. Ultrason. Sonochem. 2017, 38, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Liang, X.; Liao, X.; Ding, Y.; Sun, J.; Li, X. High-Yield Hypocrellin A Production in Solid-State Fermentation by Shiraia sp. SUPER-H168. Appl. Biochem. Biotechnol. 2010, 160, 2275–2286. [Google Scholar] [CrossRef]
- Dos Santos, A.; Santos, E.M.; de Oliveira, J.S.; Ribeiro, O.L.; Perazzo, A.F.; Pinho, R.; Macedo, A.; Pereira, G.A. Effects of urea addition on the fermentation of sorghum (Sorghum bicolor) silage. Afr. J. Range Forage Sci. 2018, 35, 55–62. [Google Scholar] [CrossRef]
- Su, Q.; Zhang, Z.; Liu, X.; Wang, F. The transcriptome analysis on urea response mechanism in the process of ergosterol synthesis by Cordyceps cicadae. Sci. Rep. 2021, 11, 10927. [Google Scholar] [CrossRef]
- Xiang, X. Optimization of Shiraia bambusicola P. Henn. under liquid fermentatio. J. Biotechnol. 2010, 20, 73–75. [Google Scholar]
- Wechgama, K.; Laopaiboon, L.; Laopaiboon, P. Enhancement of batch butanol production from sugarcane molasses using nitrogen supplementation integrated with gas stripping for product recovery. Ind. Crop Prod. 2017, 95, 216–226. [Google Scholar] [CrossRef]
- Deng, H.; Gao, R.; Liao, X.; Cai, Y. Characterisation of a monooxygenase in Shiraia bambusicola. Microbiology 2018, 164, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Gao, R.; Liao, X.; Cai, Y. Characterization of a major facilitator superfamily transporter in Shiraia bambusicola. Res. Microbiol. 2017, 168, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Sitanggang, A.B.; Wu, H.; Wang, S.S.; Ho, Y. Effect of pellet size and stimulating factor on the glucosamine production using Aspergillus sp. BCRC 31742. Bioresour. Technol. 2010, 101, 3595–3601. [Google Scholar] [CrossRef]
- Sun, C.X.; Ma, Y.J.; Wang, J.W. Improved hypocrellin A production in Shiraia bambusicola by light-dark shift. J. Photochem. Photobiol. B-Biol. 2018, 182, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.J.; Li, X.P.; Wang, Y.; Wang, J.W. Nitric oxide donor sodium nitroprusside-induced transcriptional changes and hypocrellin biosynthesis of Shiraia sp. S9. Microb. Cell Fact. 2021, 20, 92. [Google Scholar] [CrossRef]
- Ma, Y.J.; Zheng, L.P.; Wang, J.W. Inducing perylenequinone production from a bambusicolous fungus Shiraia sp. S9 through co-culture with a fruiting body-associated bacterium Pseudomonas fulva SB1. Microb. Cell Fact. 2019, 18, 121. [Google Scholar] [CrossRef]
- Du, W.; Sun, C.; Wang, B.; Wang, Y.; Dong, B.; Liu, J.; Xia, J.; Xie, W.; Wang, J.; Sung, J.; et al. Response mechanism of hypocrellin colorants biosynthesis by Shiraia bambusicola to elicitor PB90. AMB Express 2019, 9, 146. [Google Scholar] [CrossRef]
- Klis, F.M.; Boorsma, A.; De Groot, P.W.J. Cell wall construction in Saccharomyces cerevisiae. Yeast 2006, 23, 185–202. [Google Scholar] [CrossRef]
- Pohling, J.; Hawboldt, K.; Dave, D. Comprehensive review on pre-treatment of native, crystalline chitin using non-toxic and mechanical processes in preparation for biomaterial applications. Green Chem. Int. J. Green Chem. Resour. 2022, 24, 6790–6809. [Google Scholar] [CrossRef]
- Suzuki, K.; Tokoro, A.; Okawa, Y.; Suzuki, S.; Suzuki, M. Effect of N-acetylchito-oligosaccharides on activation of phagocytes. Microbiol. Immunol. 1986, 30, 777–787. [Google Scholar] [CrossRef] [PubMed]
- Wessels, J.G.H.U. Developmental regulation of fungal cell wall formation. Annu. Rev. Phytopathol. 1994, 32, 413–437. [Google Scholar] [CrossRef]
- Navarathna, D.H.M.L.; Harris, S.D.; Roberts, D.D.; Nickerson, K.W. Evolutionary aspects of urea utilization by fungi. FEMS Yeast Res. 2010, 10, 209–213. [Google Scholar] [CrossRef]
- Strope, P.K.; Nickerson, K.W.; Harris, S.D.; Moriyama, E.N. Molecular evolution of urea amidolyase and urea carboxylase in fungi. BMC Evol. Biol. 2011, 11, 80. [Google Scholar] [CrossRef]
- Fan, C.; Glibert, P.M.; Alexander, J.; Lomas, M.W. Characterization of urease activity in three marine phytoplankton species, Aureococcus anophagefferens, Prorocentrum minimum, and Thalassiosira weissflogii. Mar. Biol. 2003, 142, 949–958. [Google Scholar] [CrossRef]
- Solomon, C.M.; Glibert, P.M. Urease activity in five phytoplankton species. Aquat. Microb. Ecol. 2008, 52, 149–157. [Google Scholar] [CrossRef]
- Smith, D.G.; Russell, W.C.; Thirkell, D. Urea-hydrolysis-dependent citrulline synthesis by Ureaplasma urealyticum. FEMS Microbiol. Lett. 1992, 77, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Carrari, F.; Sweetlove, L.J. Respiratory metabolism: Glycolysis, the TCA cycle and mitochondrial electron transport. Curr. Opin. Plant Biol. 2004, 7, 254–261. [Google Scholar] [CrossRef]
- Zhang, S.; Juan, H. Functional Characterization of Citrate Carrier in the Biosynthesis of Juvenile Hormone in Cockroach. Bioprocess 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Brito, D.S.; Agrimi, G.; Charton, L.; Brilhaus, D.; Bitetto, M.G.; Lana-Costa, J.; Messina, E.; Nascimento, C.P.; Feitosa-Araujo, E.; Pires, M.V.; et al. Biochemical and functional characterizationof a mitochondrialcitrate carrier in Arabidopsis thaliana. Biochem. J. 2020, 477, 1759–1777. [Google Scholar] [CrossRef] [PubMed]
- Strijbis, K.; Distel, B. Intracellular Acetyl Unit Transport in Fungal Carbon Metabolism. Eukaryot. Cell 2010, 9, 1809–1815. [Google Scholar] [CrossRef] [PubMed]
- Vorapreeda, T.; Thammarongtham, C.; Cheevadhanarak, S.; Laoteng, K. Alternative routes of acetyl-CoA synthesis identified by comparative genomic analysis: Involvement in the lipid production of oleaginous yeast and fungi. Microbiology 2012, 158, 217–228. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Z.; Shu, S.; Wang, W.; Xu, H.; Ahn, Y.; Wang, M.; Hu, X. Ethanol and Methanol Can Improve Huperzine A Production from Endophytic Colletotrichum gloeosporioides ES026. PLoS ONE 2013, 8, e61777. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Gao, R.; Liao, X.; Cai, Y. Reference genes selection and relative expression analysis from Shiraia sp. SUPER-H168 productive of hypocrellin. Gene 2016, 580, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Gao, R.; Liao, X.; Cai, Y. Genome editing in Shiraia bambusicola using CRISPR-Cas9 system. J. Biotechnol. 2017, 259, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Du, F.; Nong, F.; Li, J.; Huang, P.; Ma, W.; Gu, Y.; Sun, X. Function of the Polyketide Synthase Domains of Schizochytrium sp. on Fatty Acid Synthesis in Yarrowia lipolytica. J. Agric. Food Chem. 2023, 71, 2446–2454. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Liao, B.; Yan, X.; Wu, Z.; Tian, X. Temperature-responsive regulation of the fermentation of hypocrellin A by Shiraia bambusicola (GDMCC 60438). Microb. Cell Fact. 2022, 21, 135. [Google Scholar] [CrossRef]
- Zhao, N.; Li, D.; Guo, B.; Tao, X.; Lin, X.; Yan, S.; Chen, S. Genome Sequencing and Analysis of the Hypocrellin-Producing Fungus Shiraia bambusicola S4201. Front. Microbiol. 2020, 11, 15. [Google Scholar] [CrossRef]
- Li, X.P.; Wang, Y.; Ma, Y.J.; Wang, J.W.; Zheng, L.P. Nitric Oxide and Hydrogen Peroxide Signaling in Extractive Shiraia Fermentation by Triton X-100 for Hypocrellin A Production. Int. J. Mol. Sci. 2020, 21, 882. [Google Scholar] [CrossRef] [PubMed]
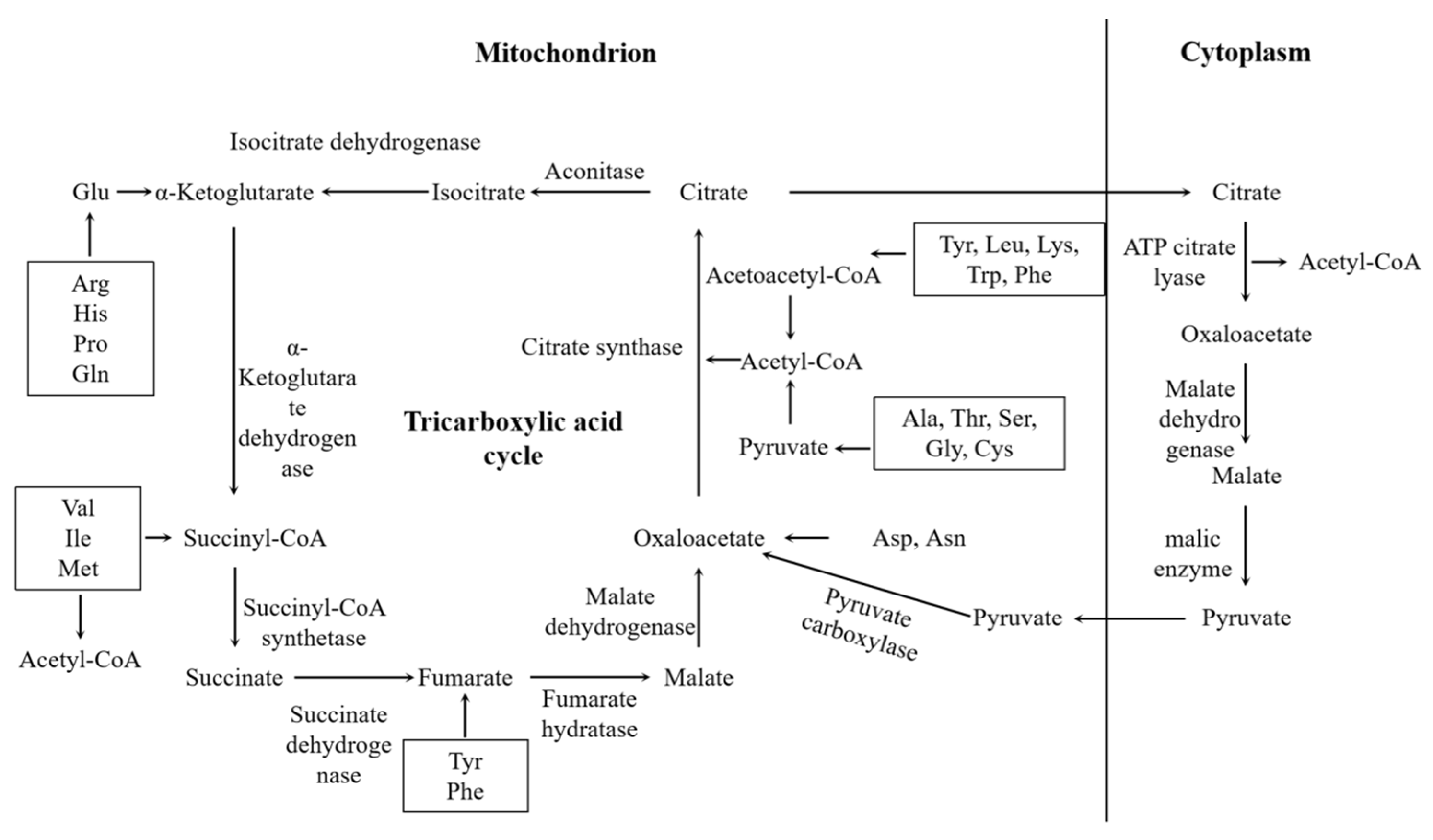

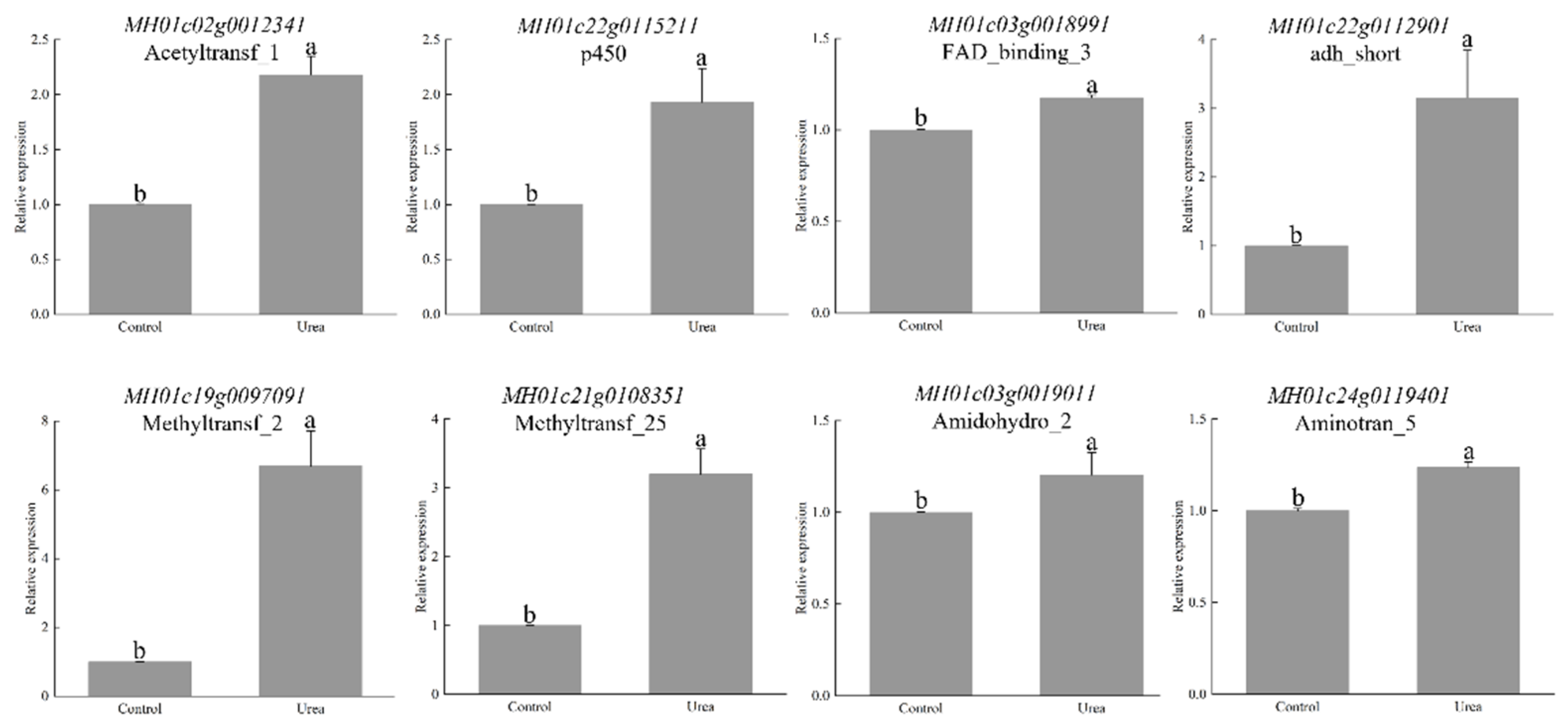
| Pfam ID | Pfam Annotation | Gene ID |
|---|---|---|
| PF04909.13 | Amidohydrolase domain of urease | MH01c03g0019011 |
| PF02666.14 | Phosphatidylserine decarboxylase | MH01c07g0053961 |
| PF00155.20 | Aminotransferase class I and II | MH01c20g0105931 |
| PF00266.18 | Domain of amino transferase or cysteine desulphurase | MH01c24g0119401 |
| PF01179.19 | Copper amine oxidase | MH01c24g0120431 |
| PF04952.13 | Succinylglutamate desuccinylase/Aspartoacylase | MH01c02g0010871, MH01c02g0010881 |
| PF02786.16 | Carbamoyl-phosphate synthase L | MH01c03g0020161, |
| chain | MH01c09g0066391 | |
| PF02629.18 | Succinyl CoA synthetase | MH01c07g0049071 |
| PF00549.18 | ATP-citrate lyase | MH01c07g0049071 |
| PF00285.20 | Citrate synthase | MH01c07g0049071 |
| PF03949.14 | Malic enzyme | MH01c12g0075031 |
| PF00682.18 | Pyruvate carboxylase | MH01c13g0083471 |
| PF17763 | Glutaminase/asparaginase | MH01c18g0092341 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, Y.; Wen, Y.; Zhang, X.; Gao, Q.; Yu, F.; Wu, Z.; Tian, X. Urea-Induced Enhancement of Hypocrellin A Synthesis in Shiraia bambusicola GDMCC 60438: Strategies and Mechanisms. Fermentation 2024, 10, 381. https://doi.org/10.3390/fermentation10080381
Tang Y, Wen Y, Zhang X, Gao Q, Yu F, Wu Z, Tian X. Urea-Induced Enhancement of Hypocrellin A Synthesis in Shiraia bambusicola GDMCC 60438: Strategies and Mechanisms. Fermentation. 2024; 10(8):381. https://doi.org/10.3390/fermentation10080381
Chicago/Turabian StyleTang, Yanbo, Yongdi Wen, Xiang Zhang, Qian Gao, Fuqiang Yu, Zhenqiang Wu, and Xiaofei Tian. 2024. "Urea-Induced Enhancement of Hypocrellin A Synthesis in Shiraia bambusicola GDMCC 60438: Strategies and Mechanisms" Fermentation 10, no. 8: 381. https://doi.org/10.3390/fermentation10080381
APA StyleTang, Y., Wen, Y., Zhang, X., Gao, Q., Yu, F., Wu, Z., & Tian, X. (2024). Urea-Induced Enhancement of Hypocrellin A Synthesis in Shiraia bambusicola GDMCC 60438: Strategies and Mechanisms. Fermentation, 10(8), 381. https://doi.org/10.3390/fermentation10080381







