Abstract
Improper disposal of vegetable waste can cause serious environmental pollution, but because they contain huge water content and organic matter, they are not suitable for disposal by methods such as incineration and landfill. However, vegetable waste contains a large amount of nutrients and have some complementary effects with rice straw in terms of physical structure, nutrients, and moisture. In this experiment, the plant feed (corn husk, peanut shells and sorghum shells) was used as the control group (CON group), and the mixed silage of Chinese cabbage waste and rice straw (mixed silage) was used as the experiment group (TRE group), and its safety performance was evaluated by testing its toxin content, pesticide residues, vitamin contents and feeding experiment of Hu sheep. In the animal experiment, 16 healthy Hu sheep (5.5 months, 39.11 ± 4.16 kg) were randomly divided into two groups of 8 each. The results of the safety performance evaluation showed that the content of mycotoxins, heavy metals, and nitrites as well as pesticide residues in the crude feeds of both groups were within the range of Chinese feed hygiene standards. In addition, the levels of deoxynivalenol (DON) and aflatoxin (AFT) in the CON group were lower, while the content of ochratoxin (OTA) and zearalenone were higher than those in the TRE group (p < 0.05). The levels of plumbum(Pb), chromium (Cr), cadmium (Cd), and nitrite in the CON group were lower than the mixed silage, while the levels of As were higher than the mixed silage (p < 0.05). It is worth noticing that the content of vitamin B2 (VB2) and vitamin C (VC) in the TRE group was higher than the CON group (p < 0.05). The results of the feeding experiment showed that the mixed silage did not affect the growth performance, nutrient digestibility, organ index, and intestinal index of Hu sheep (p > 0.05). In addition, the mixed silage reduced the weight of omasum, the proportion of omasum to live weight before slaughter, the amount of compound stomach, and the proportion of compound stomach to live weight before slaughter, which were higher than those in the TRE group (p < 0.05). The thickness of the basal layer of the rumen abdominal sac, the red blood cell count, the content of IL-10, and TNF-α in the blood, and TNF-α content in the rumen of the Hu sheep in the TRE group were higher than the CON group (p < 0.05). In conclusion, the feed safety index content of the mixed silage did not exceed the Chinese feed hygiene and safety standards and did not cause adverse effects on the growth performance of the Hu sheep, and it improved the immune performance of the body and digestive tract of the sheep to a certain extent and promoted the healthy development of the sheep.
1. Introduction
Rice production is the third most important crop after wheat and maize in the world [1]. And, for each 1 kg of rice produced, 1.0 to 1.5 kg of rice straw is produced [2]. Rice straw is a very important crop by-product, which is widely used in animal silage, bioenergy, organic fertilizer, and other areas, but to a lesser extent [3,4,5]. Vegetables are an essential source of nutrients in people’s daily life, but a large amount of vegetable tails are produced while meeting people’s needs. Supermarkets, food markets, and households are the main places where vegetable tails are produced [6]. To the best of our knowledge, burning of rice straw and abandonment of tailing vegetables are still the main and effective disposal methods, and resource utilization is imminent [7,8]. The seasonal harvest and short-term concentrated outbreak of these wastes put great pressure on the collection and rapid treatment of raw materials [9,10].
There are some complementary effects between vegetable tailing and rice straw in terms of physical structure, nutrient and moisture content. Treatment by ensiling not only improves the nutritional value of rice straw, but also has less environmental impact [11]. Studies have shown that vegetable tails can be mixed with straw for ensiling, for example, mixed silage of broccoli by-products with wheat straw [12], mixed silage of corn straw and cabbage [13], co-storage of sugar beet waste with wheat straw [14]. Partovi et al. [12] replaced 20% of roughage (200 g/kg) in the diet with broccoli by-products mixed with wheat straw silage without affecting the growth performance and rumen fermentation parameters of Fashandy lambs.
Mycotoxins are inanimate, invisible, and toxic secondary metabolites produced by fungi. The presence of mycotoxins can affect crop quality, human health, and animal production, thereby affecting the global economy [15,16]. Moreover, to meet the global demand for crops, people overuse chemical fertilizers, pesticides, etc. Pesticide residues as well as heavy metal content are an increasing threat to ecosystems and human health [17,18]. Therefore, in order to ensure the safety of livestock and poultry, it is necessary to detect mycotoxins, heavy metals, and pesticide residues in the mixed silage.
We hypothesized that the mixed silage of Chinese cabbage waste and rice straw would reduce its toxins content without affecting the health performance of Hu sheep. Therefore, the objective of the research is to evaluate the safety performance of the mixed silage by measuring the vitamins content, mycotoxins content, pesticide residues content, and heavy metals content, and the feeding experiment of Hu sheep.
2. Materials and Methods
2.1. Mixed Silage Production
The rice straw and Chinese cabbage waste used for silage were collected from Suqian City, Jiangsu Province, China. Lactobacillus plantarum (2.0 × 1010 cfu/g) and cellulase (2.0 × 105 U/g) were purchased from Guangzhou Greenfield Biotechnology Co., Ltd. (Guangzhou, China). Prior to silage, Chinese cabbage waste and rice straw were chopped up 2–3 cm. Chinese cabbage waste and rice straw (4:6), lactobacillus plantarum (0.035 g/kg) and cellulase (0.250 g/kg) were then mixed and fermented for 45 d in sealed silage bags. The weight of each silage wrap was 300 kg, resulting in a total production of 15 t.
2.2. Experimental Animals and Feeding Management
A one-way completely randomized group experimental design was used for this experiment. Sixteen healthy Hu sheep were randomly and equally divided into two groups (four rams and four ewes in each group), which were 5.5 months old and of similar weight (39.11 ± 4.16 kg). The treatment (TRE) group of Hu sheep was fed with mixed silage as roughage and the control (CON) group was fed with conventional common feed (peanut seedlings, corn husks and high grain husks) as roughage. The diets of Hu sheep were configured according to the nutritional requirements of sheep weighing 40 kg and gaining 400 g per day [19]. The ratio of concentrate to roughage was 50:50 for all groups (based on the dry matter (DM)). Their nutrient composition is shown in Table 1. The experiment consisted of a pre-feeding period (7 d) and a regular feeding period (28 d), for a total of 35 days. The sheep houses were cleaned and sterilized before the experiment. All sheep were uniformly dewormed and immunized. Hu sheep were kept in a pen. The sheep were fed twice at 7:00 and 19:00 with free access to food and water. Sheep houses were cleaned and disinfected daily to maintain cleanliness and hygiene.

Table 1.
Experimental diet formula and nutrition composition (DM Basis/%).
2.3. Sampling
After the mixed silage was completed, six different wrapped silage samples were randomly selected for sampling and sent to the laboratory with the mill feed for crushing (40 mesh) for feed safety assessment tests. Peanut seedlings, corn husks and sorghum hulls were obtained from the mill feed of a sheep farm in Suqian, Jiangsu Province.
At the end of the feeding experiment, we randomly selected six Hu sheep (three rams and three ewes) in each of the CON group and the TRE group for slaughter. Feed and water were fasted for 24 h before slaughtering, and slaughtered after weighing. After the sheep were stunned by CO2 gas, they were slaughtered by bloodletting from the jugular vein and their viscera were weighed. Blood, heart, liver, spleen, kidney, rumen small intestine (duodenum, jejunum, ileum), and large intestine tissues were collected from Hu sheep during slaughter.
2.4. Safety Performance Assessment
This test is to determine the content of four common mycotoxins in feed, namely DON, AFT, OTA and Zearalenone (ZEN). The four mycotoxins in the feed samples were determined using an enzyme-linked immunosorbent assay (ELISA) kit produced by Shanghai Amperexamination Technology Co., Shanghai, China. The detection of pesticide residues (hexachlorobenzene, dichlorodiphenyltrichloroethane (DDT), hexachlorobenzene, deltamethrin, dimethoate and dichlorvos) and common heavy metal ions (As, Pb, Hg, Cr, Cd and nitrite) and nitrite in the feed samples was tested by Qingdao Stander Testing Company Co., Ltd. (Qingdao, China). The contents of VA, VB2, VC and VE in the feeds were determined by biochemical kits purchased from Shanghai Enzyme-linked Biotechnology Co., Shanghai, China.
2.5. Growth Performance Determination
At the beginning of the experiment and at the end of the experiment, each sheep was weighed on an empty stomach and the initial weight (IW) and final weight (FW) was recorded. The amount of feed fed and the amount remaining per sheep per day during the experiment were recorded, and the dry matter intake (DMI), the average daily gain (ADG) and the feed-to-weight ratio (F/W) were calculated at the end of the experiment using the following formula.
ADG = (FW − IW)/28
DMI = (Feeding rate × DM content) − (Residual feed rate × Residual DM content)
F/W = DMI/ADG
2.6. Determination of the Apparent Digestibility
One week before the end of the feeding experiment, the amount of feed and residual were accurately recorded. Feces were collected for five consecutive days by the whole feces method and weighed after collection. 20% of the daily manure sample was added to 10 mL 10% sulfuric acid and stored at −20 °C. The fresh manure samples were brought back to the laboratory for drying (65 °C), crushing, passing through 40 mesh sieve and then stored for testing.
DM, organic matter (OM), and CP of the manure samples and feed samples were determined using. The content of NDF and ADF was determined using the Van’s washing method. DM digestibility (DMD), OM digestibility (OMD), CP digestibility (CPD), NDF digestibility (NDFD) and ADF digestibility (ADFD) were calculated using the following formula.
Apparent digestibility of a nutrient = 1 − (whole manure/feed intake) × (content of a nutrient in the manure/content of a nutrient in the ration) × 100%
2.7. Blood Physiological and Biochemical Measurements
Blood was collected from the jugular vein of 12 (6 per group) sheep prior to slaughter. Three blood samples were collected from each sheep slaughtered. One of them was packed in EDTA anticoagulation tube, and the PE-6800VET fully automatic animal blood cell analyzer (Procan Electronics Co., Ltd, Shenzhen, China) was used to detect routine blood indexes, including white blood cell count, red blood cell count, hemoglobin concentration, platelets, absolute basophil value, basophil percentage, red blood cell pressure, mean red blood cell volume, mean hemoglobin content, mean hemoglobin concentration, erythrocyte distribution width, and standard deviation of erythrocyte distribution. The other one was divided into lithium heparin anticoagulation tubes, and the SMT-120V automatic biochemical analyzer (Seamaly, Chengdu, China)was used to test blood biochemical parameters, including total protein, albumin, globulin, albumin-to-globulin ratio, alkaline phosphatase, lactate dehydrogenase, creatinine, urea nitrogen, total cholesterol, triglycerides, high-density lipoprotein, and low-density lipoprotein. The remaining portion was divided into common tubes, placed at room temperature for 2 h, and left at 4 °C for 2–3 h. After the blood clotted and contracted, the supernatant was centrifuged for 10 min at 3000 r/min, and the supernatant was collected, and the contents of IL-1β, IL-6, IL-8, IL-10, TNF-α, IgA and IgM in the serum were determined by colorimetric method using a Huawei Delong DR-200BS enzyme standard analyzer (Beijing Huaying Biotechnology Research Institute, Beijing, China).
2.8. Determination of Organ Indices
During the slaughter process, the heart, liver, spleen, lungs, kidneys, and digestive tract were sampled and weighed in their entirety to calculate the organ index. The contents of the digestive tract were removed and cleaned before the rumen, reticulum, flap, wrinkled stomach, small intestine and large intestine were weighed and the organ indices were calculated. The organ index is calculated using the following formula.
Organ index = organ weight (g)/live weight before slaughter (kg) × 100%
Complex stomach index = weight of complex stomach (g)/live weight before slaughter (kg) × 100%
Intestinal index = intestinal weight (g)/live weight before slaughter (kg) × 100%
2.9. Measurement of Immune Performance
Samples of liver, spleen, kidney, rumen and small intestine (duodenum, jejunum and ileum) tissues as described above were homogenized to prepare homogenates. The levels of IL-1β, IL-6, IL-8, IL-10, TNF-α, IgA and IgM in serum as well as other tissue homogenates were determined by ELISA kits.
2.10. Data Analysis
We tested the data for normal distribution and homogeneity using SPSS Statistics V20.0 software (IBM Corporation, Armonk, NY, USA). All the data were subjected to independent samples t-test in SPSS. p < 0.05 indicates a significant difference and p ≥ 0.05 indicates a non-significant difference. Visualization of growth performance and nutrient digestibility, development of the rumen epithelium and indicators of immune performance is done with GraphPad Prism 8.0 software.
3. Results
3.1. Safety Performance Assessment
As shown in Table 2, the contents of DON and AFT in the CON group were lower than the TRE group (p < 0.05), while the levels of OTA and ZEN were higher than the TRE group (p < 0.05). Hexachlorocyclohexane, DDT, dichlorvos and hexachlorobenzene were not detected in the diets of both groups. However, paclobutrazol was detected in the CON group and deltamethrin was detected in the TRE group diets. For heavy metals and nitrite content, the contents of Pb, Cr, Cd and nitrite in the CON feed were lower than the TRE group, while the content of As were higher than the TRE group (p < 0.05). In addition, the VB2 and VC contents of the TRE group were higher than the CON group (p < 0.05).

Table 2.
Assessment of safety performance of the mixed silage.
3.2. Growth Performance and Nutrient Digestibility of Hu Sheep
As can be seen from Figure 1, there were no differences between the CON group and the TRE group in terms of ADG (Figure 1A), DMI (Figure 1B) and F/G (Figure 1C) (p > 0.05). There was no effect on DMD, OMD, CPD, NDFD and ADFD of Hu sheep between the CON and TRE group (p > 0.05) (Figure 1D).
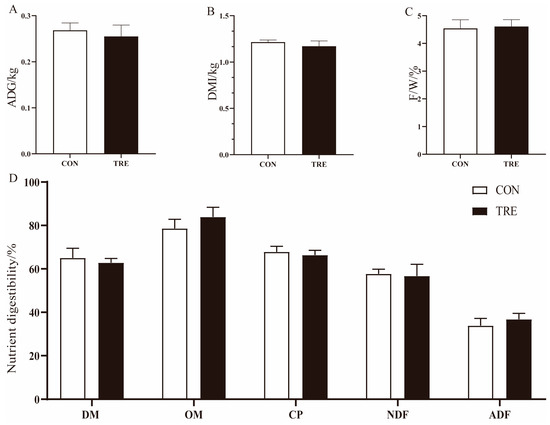
Figure 1.
Effect of mixed silage on growth performance and nutrient digestibility of Hu sheep. (A) Average daily gain. (B) Dry matter intake. (C) the feed-to-weight ratio. (D) Nutrient digestibility. CON: Based on peanut seedling, corn husk and sorghum shell for roughage; TRE: Based on the mixed silage for roughage. DMI: Dry matter intake; ADG: the average daily gain; F/W: the feed-to-weight ratio; DM: Dry matter; OM: Organ matter; CP: Crude protein; NDF: Neutral Detergent Fiber; ADF: Acid Detergent Fiber.
3.3. Organs Index
As can be seen from Table 3, the organ indices of Hu sheep in the CON group were not different from those of the CON group (p > 0.05).

Table 3.
Effects of the mixed silage on organ indices of Hu sheep.
3.4. Complex Stomach Development
The basal and granular layers of the rumen in the TRE group were higher than those in the CON group (p < 0.05) (Figure 2). It can be seen from Table 4 that the weight of omasum, the ratio of omasum to live weight before slaughter, the amount of compound stomach, and the ratio of compound stomach to live weight before slaughter were higher in the CON group (p < 0.05).
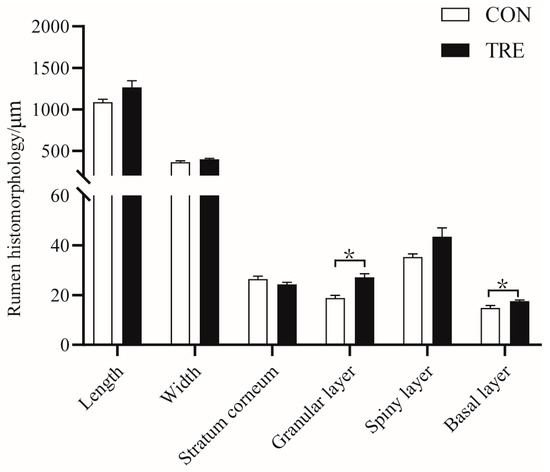
Figure 2.
Effects of the mixed silage feed on rumen epithelium of Hu sheep. CON: Based on peanut seedling, corn husk, and sorghum shell for roughage; TRE: Based on the mixed silage for roughage. * indicates a significant difference between the two groups.

Table 4.
Effects of the mixed silage on the development of compound stomach in Hu sheep.
3.5. Gut Development
The results in Table 5 showed that there was no difference between the CON group and the TRE group in the weight and index of duodenum, jejunum, ileum, cecum, colon, and rectum (p > 0.05).

Table 5.
Effects of the mixed silage on intestinal development of Hu sheep.
3.6. Blood Physiological and Biochemical Indicators
The effects of the mixed silage on blood physiological and biochemical indicators of Hu sheep are shown in Table 6. The results showed that the number of red blood cells in the blood of the TRE group was higher than the CON group (p < 0.05). The numbers of red blood cells in the CON group and the TRE group were both within the normal range.

Table 6.
Effects of the mixed silage on the physicochemical indexes of the blood of Hu sheep.
3.7. Immunological Performance
As can be seen from Figure 3, the levels of IL-10 and TNF-α in the serum of the TRE group were higher than the CON group (p < 0.05). From Figure 2, it can be seen that the content of IL-6 and IgA in the duodenum of the TRE group was higher than that of the CON group (p < 0.05), and the content of TNF-α in the rumen was higher than the CON group (p < 0.05).
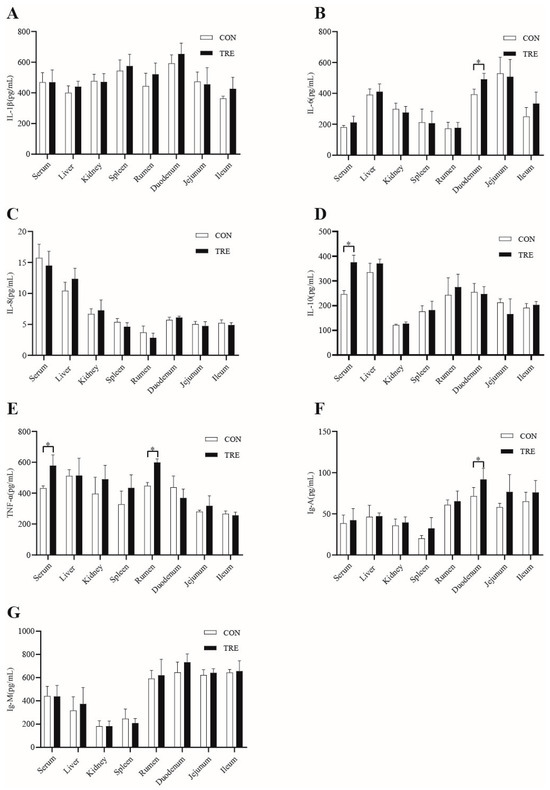
Figure 3.
Effects of the mixed silage on immune performance of Hu sheep. (A) IL-1β. (B) IL-6. (C) IL-8. (D) IL-10. (E) TNF-α. (F) Ig-A. (G) Ig-M. CON: Based on peanut seedling, corn husk and sorghum shell for roughage; TRE: Based on the mixed silage for roughage. * indicates a significant difference between the two groups.
4. Discussion
Fungal spoilage and mycotoxin contamination are among the greatest risks of silage. Spoilage caused by fungi may lead to mold and heat, reduced palatability and loss of nutritional value of the feed [21]. Mycotoxins are anti-nutritional factors present in livestock and poultry feeds that cause mold-infected diseases and can directly harm animal health and performance [22]. In addition, in order to significantly increase the yield of crops, pesticides may be used in large quantities during cultivation, which may result in drug residues in crops [23]. Feeding livestock with high pesticide residue content may seriously endanger the lives of livestock [24]. Before silage, we also considered that Chinese cabbage has a high water content and is prone to degradation and spoilage, and its mixed storage with rice straw may be susceptible to spoilage due to high water content [25]. Therefore, our evaluation of the safety performance of mixed silage includes mycotoxins, drug residues, heavy metals, etc. The risk of fungal contamination exists during growth, before and after mowing, ensiling, transport and storage, but the level of mycotoxins can be reduced by silage [26,27,28]. Therefore, the mixed silage may be due to the reduction of OTA and ZEN in the feed by means of silage compared to the CON group. In addition, the levels of Pb, Cr, Cd and nitrite in the feeds of the TRE group were higher than the CON group. This may be caused by the excessive use of pesticides and nitrogen fertilizers in the process of production of rice and vegetables in the excessive pursuit of yield. The content of mycotoxins, drug residues, heavy metals and nitrite in the mixed silage were within the Chinese feed hygiene standards (GB13078-2017). It is worth noting that the VC and VB content of the mixed silage was significantly higher than the CON group, probably due to the higher vitamin content in Chinese cabbage [29,30].
Feeding experiments are necessary for the evaluation of forage resources and the response of the animal organism is the most realistic and reliable. The nutritional value of a forage is mainly determined by the digestibility of the ruminant, which depends mainly on the nutrient content of the forage [31,32]. In the present experiment, we determined the effects of the mixed silage on growth performance, digestibility, organ index, intestinal development and immune performance of Hu sheep in feeding experiment.
Complex stomach development is a major challenge for ruminants from birth, and much of this revolves around the development of the rumen [33]. The rumen epithelium (including the stratum corneum, stratum basale, stratum granulosum and stratum spinosum) has the function of absorbing fatty acids, providing the animal with animal metabolic energy, and is an important regulatory mechanism for stabilizing the rumen environment [34,35]. In this experiment, the granular and basolateral layers were higher in the CON group. The basal layer has functional mitochondria and has the metabolic properties of the rumen epithelium, which produces ketones mainly from SCFA [36]. The granular layer is characterized by a linking complex called bridging granules, which act as an osmotic barrier for the rumen epithelium [37,38,39]. The results from Li et al. [20] show that the mixed silage of Chinese cabbage waste and rice straw increased the rumen butyric acid. Butyric acid can effectively stimulate the proliferation and growth of rumen epithelial cells [40].
Anti-inflammatory factors (IL-10) play an important role in the termination of neuroinflammation, while pro-inflammatory cytokines (IL-1, IL-6, TNF-α, etc.) are involved in the early response to inflammation and can lead to decreased exercise capacity, loss of appetite, diminished diuretic effect, and other adverse effects [41,42]. IL-6 is a pro-inflammatory factor that promotes the activation of T and B cells and is an important effector molecule in the acute inflammatory response [43]. IL-10 is a cytokine with anti-inflammatory properties that plays a central role in infection by limiting the immune response to pathogens, thereby preventing damage to the host [44]. Our result showed that IL-6 content in the duodenum of the TRE group was higher, and IL-10 and TNF-α content in the serum of the TRE group was higher. In addition, the TNF-α content in the rumen of the TRE group was significantly higher. These results suggest that the mixed silage can reduce the damage caused by inflammatory reactions to the body of Hu sheep to some extent, probably due to the higher vitamin content of the mixed silage. It was found that the maturation, proliferation and cellular activity of lymphocytes were inhibited in mice fed a diet deficient in VB, while the addition of VB completely eliminated this inhibition [45].
Immunoglobulins, as part of the immune system, have a very important role in immune regulation and mucosal defense of the host [46]. Serum immunoglobulins can represent key information about host immunity, including IgM, IgA and IgG [47,48]. In our study, the mixed silage significantly increased the level of IgA in the duodenum, which is considered to be an important antibody isoform involved in mucosal surface protection responses [49]. In turn, the integrity of the duodenal mucosa acts as a balance between endogenous or exogenous aggressive factors and some protective mechanisms [50]. Therefore, the mixed silage may have some protective effect on the duodenal mucosa and improve the immune performance of the organism.
5. Conclusions
The levels of pesticide residues, heavy metals, and nitrites were generally lower than mill feed in line with Chinese feed safety production standards. In addition, the VC and VB contents was higher. Feeding experiment have found that the mixed silage do not adversely affect the growth performance and nutrient digestibility of Hu sheep and are beneficial to the development of rumen epithelial cells and improve the immune performance of the body. In conclusion, Chinese cabbage waste as a mixed silage ingredient does not have any impact on feed hygiene and safety and can have a beneficial effect on the healthy development of Hu sheep (Figure 4).
Figure 4.
Safety performance evaluation and effects on growth and health performance of the mixed silage.
Author Contributions
Conceptualization, M.W., J.X., Z.L. and C.L.; methodology, J.X., Z.L., Z.Z., Y.L. and L.H.; software, Z.L., Z.Z., C.X. and C.L.; validation, Z.L., C.L., L.H., Z.Z., Y.L., C.X., R.Q. and M.H.Z.; formal analysis, Z.L., Z.Z., C.X. and L.H.; investigation, Z.L., C.L., Y.L. and M.H.Z.; resources, Z.L., R.Q. and L.H.; data curation, Z.L., C.L., R.Q. and M.H.Z.; writing—original draft preparation, Z.L., J.X. and C.L.; writing—review and editing, Z.L., J.X. and M.W.; visualization, J.X. and M.W.; supervision, J.X. and M.W.; project administration, M.W.; funding acquisition, M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research is funded by the national corps of major scientific and technological “unveiling the list of commanding project” (2023AB078), the key program of state key Laboratory of Sheep Genetic Improvement and Healthy Production (NCG202232), the High End Foreign Expert Project of the Ministry of Science and Technology (G2023014066L), and the Priority Academic Program Development of Jiangsu Higher Education Institution (PAPD), P. R. China.
Institutional Review Board Statement
The animal study was reviewed and approved by the Animal Welfare Committee of Yangzhou Veterinarians of the Agriculture Ministry of China (Yangzhou, China, license no. syxk (Su)2019-0029). Written informed consent was obtained from the owners for the participation of their animals in this study.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The author hereby expresses his gratitude to the Siyang County Huifeng Goat Breeding Professional Cooperative (Suqian, Jiangsu) for the Hu sheep and breeding sites provided.
Conflicts of Interest
The authors declare that this study was conducted without any business or financial relationships that could be considered a potential conflict of interest. Lu Zhiqi now works for Ningxia Dairy Science and Innovation Center of Guangming Animal Husbandry Co., Ltd.
References
- Binod, P.; Sindhu, R.; Singhania, R.R.; Vikram, S.; Devi, L.; Nagalakshmi, S.; Kurien, N.; Sukumaran, R.K.; Pandey, A. Bioethanol production from rice straw: An overview. Bioresour. Technol. 2010, 101, 4767–4774. [Google Scholar] [CrossRef] [PubMed]
- Maiorella, B.L. Ethanol Fermentation. In Comprehensive Biotechnology; Moo-Young, M., Ed.; Pergamon Press: Oxford, UK, 1985; Volume III, pp. 861–914. [Google Scholar]
- Wang, X.; Song, J.; Liu, Z.; Zhang, G.; Zhang, Y. Fermentation Quality and Microbial Community of Corn Stover or Rice Straw Silage Mixed with Soybean Curd Residue. Animals 2022, 12, 919. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.B.; Tiwari, A.K.; Mohammad, A.; Prasad, N.; Srivastava, N.; Srivastava, K.; Singh, R.; Yoon, T.; Syed, A.; Bahkali, A.H. Enhanced Biogas Production Potential Analysis of Rice Straw: Biomass characterization, Kinetics and Anaerobic Co-Digestion investigations. Bioresour. Technol. 2022, 358, 127391. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Mathew, A.K.; Sindhu, R.; Pandey, A.; Binod, P. Potential of rice straw for bio-refining: An overview. Bioresour. Technol. 2016, 215, 29–36. [Google Scholar] [CrossRef]
- Ma, Y.; Yin, Y.; Liu, Y. A holistic approach for food waste management towards zero-solid disposal and energy/resource recovery. Bioresour. Technol. 2017, 228, 56–61. [Google Scholar] [CrossRef]
- Singh, R.; Patel, M. Effective utilization of rice straw in value-added by-products: A systematic review of state of art and future perspectives. Biomass Bioenergy 2022, 159, 106411. [Google Scholar] [CrossRef]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef]
- Idan, F.; Adogla-Bessa, T.; Amaning-Kwarteng, K. Preference, voluntary feed intake, and digestibility of sheep fed untreated rice straw and supplemented with sole or combined fodder tree leaves. Eur. J. Agric. Food Sci. 2020, 2, 1–8. [Google Scholar] [CrossRef]
- Tejaswini, G.S.; Mahadevakumar, S.; Sowmya, R.; Deepika, Y.S.; Meghavarshinigowda, B.R.; Nuthan, B.R.; Sharvani, K.A.; Amruthesh, K.N.; Sridhar, K.R. Molecular detection and pathological investigations on southern blight disease caused by Sclerotium rolfsii on cabbage (Brassica oleracea var. capitata): A new record in India. J. Phytopathol. 2022, 170, 363–372. [Google Scholar] [CrossRef]
- Cherdthong, A.; Suntara, C.; Khota, W. Lactobacillus casei TH14 and additives could modulate the quality, gas kinetics and the in vitro digestibility of ensilaged rice straw. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1690–1703. [Google Scholar] [CrossRef]
- Partovi, E.; Rouzbehan, Y.; Fazaeli, H.; Rezaei, J. Broccoli byproduct-wheat straw silage as a feed resource for fattening lambs. Transl. Anim. Sci. 2020, 4, txaa078. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.W.; Wang, C.; Fan, W.G.; Zhang, B.Y.; Li, Z.Z.; Li, D. Effects of Formic or Acetic Acid on the Storage Quality of Mixed Air-Dried Corn Stover and Cabbage Waste, and Microbial Community Analysis. Food Technol. Biotechnol. 2018, 56, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Hillion, M.L.; Moscoviz, R.; Trably, E.; Leblanc, Y.; Bernet, N.; Torrijos, M.; Escudie, R. Co-ensiling as a new technique for long-term storage of agro-industrial waste with low sugar content prior to anaerobic digestion. Waste Manag. 2018, 71, 147–155. [Google Scholar] [CrossRef] [PubMed]
- D’mello, J.; Placinta, C.; Macdonald, A. Fusarium mycotoxins: A review of global implications for animal health, welfare and productivity. Anim. Feed Sci. Tech. 1999, 80, 183–205. [Google Scholar] [CrossRef]
- Bueno, D.; Istamboulie, G.; Muñoz, R.; Marty, J.L. Determination of mycotoxins in food: A review of bioanalytical to analytical methods. Appl. Spectrosc. Rev. 2015, 50, 728–774. [Google Scholar] [CrossRef]
- Umapathi, R.; Ghoreishian, S.M.; Sonwal, S.; Rani, G.M.; Huh, Y.S. Portable electrochemical sensing methodologies for on-site detection of pesticide residues in fruits and vegetables. Coord. Chem. Rev. 2022, 453, 214305. [Google Scholar] [CrossRef]
- Sandeep, G.; Vijayalatha, K.; Anitha, T. Heavy metals and its impact in vegetable crops. Int. J. Chem. Stud. 2019, 7, 1612–1621. [Google Scholar]
- National Research Council (NRC). Nutrient Requirements of Small Ruminants, Sheep, Goats, Cervids, and New World Camelids; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Li, C.; Chen, N.; Zhang, X.; Shahzad, K.; Qi, R.; Zhang, Z.; Lu, Z.; Lu, Y.; Yu, X.; Zafar, M.H. Mixed silage with Chinese cabbage waste enhances antioxidant ability by increasing ascorbate and aldarate metabolism through rumen Prevotellaceae UCG-004 in Hu sheep. Front. Microbiol. 2022, 13, 978940. [Google Scholar] [CrossRef]
- Christensen, C.M. Storage of Cereal Grains and Their Products; American Association of Cereal Chemists: Saint Paul, MN, USA, 1974. [Google Scholar]
- Sun, X.; Tiffany, D.G.; Urriola, P.E.; Shurson, G.G.; Hu, B. Nutrition upgrading of corn-ethanol co-product by fungal fermentation: Amino acids enrichment and anti-nutritional factors degradation. Food Bioprod. Process. 2021, 130, 1–13. [Google Scholar] [CrossRef]
- Rusinamhodzi, L. The Role of Ecosystem Services in Sustainable Food Systems; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Yu, Z.; Fan, X.; Bai, C.; Tian, J.; Tharangani, R.; Bu, D.; Jia, T. Assessment of Forage Safety and Quality. In Research Progress on Forage Production, Processing and Utilization in China; Springer: Berlin/Heidelberg, Germany, 2022; pp. 145–181. [Google Scholar]
- Chattopadhyay, A. Pre-and post-harvest losses in vegetables IVI. In Advances in Postharvest Technologies of Vegetable Crops; Apple Academic Press: Cambridge, MA, USA, 2018; pp. 25–87. [Google Scholar]
- Storm, I.; Sørensen, J.L.; Rasmussen, R.R.; Nielsen, K.F.; Thrane, U. Mycotoxins in silage. Stewart Postharvest Rev. 2008, 4, 1–12. [Google Scholar]
- Storm, I.M.L.D.; Kristensen, N.; Raun, B.; Smedsgaard, J.; Thrane, U. Dynamics in the microbiology of maize silage during whole-season storage. J. Appl. Microbiol. 2010, 109, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Vandicke, J.; De Visschere, K.; Ameye, M.; Croubels, S.; De Saeger, S.; Audenaert, K.; Haesaert, G. Multi-mycotoxin contamination of maize silages in Flanders, Belgium: Monitoring mycotoxin levels from seed to feed. Toxins 2021, 13, 202. [Google Scholar] [CrossRef] [PubMed]
- Moreb, N.; Murphy, A.; Jaiswal, S.; Jaiswal, A.K. Cabbage; Elsevier: Amsterdam, The Netherlands, 2020; pp. 33–54. [Google Scholar]
- Mosha, T.; Gaga, H. Nutritive value and effect of blanching on the trypsin and chymotrypsin inhibitor activities of selected leafy vegetables. Plant Foods Hum. Nutr. 1999, 54, 271–283. [Google Scholar] [CrossRef] [PubMed]
- Tilley, J.; Terry, D.R. A two-stage technique for the in vitro digestion of forage crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Li, R.; Zheng, M.; Jiang, D.; Tian, P.; Zheng, M.; Xu, C. Replacing alfalfa with paper mulberry in total mixed ration silages: Effects on ensiling characteristics, protein degradation, and in vitro digestibility. Animals 2021, 11, 1273. [Google Scholar] [CrossRef]
- Vi, R.B.; McLeod, K.; Klotz, J.; Heitmann, R. Rumen development, intestinal growth and hepatic metabolism in the pre-and postweaning ruminant. J. Dairy Sci. 2004, 87, E55–E65. [Google Scholar]
- Gäbel, G.; Aschenbach, J.; Müller, F. Transfer of energy substrates across the ruminal epithelium: Implications and limitations. Anim. Health Res. Rev. 2002, 3, 15–30. [Google Scholar] [CrossRef]
- Steele, M.A.; Croom, J.; Kahler, M.; AlZahal, O.; Hook, S.E.; Plaizier, K.; McBride, B.W. Bovine rumen epithelium undergoes rapid structural adaptations during grain-induced subacute ruminal acidosis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2011, 300, R1515–R1523. [Google Scholar] [CrossRef]
- Leighton, B.; Nicholas, A.R.; Pogson, C.I. The pathway of ketogenesis in rumen epithelium of the sheep. Biochem. J. 1983, 216, 769–772. [Google Scholar] [CrossRef]
- VI, R.B. Use of isolated ruminal epithelial cells in the study of rumen metabolism. J. Nutr. 1998, 128, 293S–296S. [Google Scholar]
- Gaebel, G.; Martens, H.; Bell, M. The effect of low mucosal pH on sodium and chloride movement across the isolated rumen mucosa of sheep. Q. J. Exp. Physiol. Transl. Integr. 1989, 74, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.; Simmons, N.L. Functional organization of the bovine rumen epithelium. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 288, R173–R181. [Google Scholar] [CrossRef] [PubMed]
- Górka, P.; Kowalski, Z.; Zabielski, R.; Guilloteau, P. Invited review: Use of butyrate to promote gastrointestinal tract development in calves. J. Dairy Sci. 2018, 101, 4785–4800. [Google Scholar] [CrossRef] [PubMed]
- Arkhipov, V.I.; Pershina, E.V.; Levin, S.G. The role of anti-inflammatory cytokines in memory processing in a healthy brain. Behav. Brain Res. 2019, 367, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683. [Google Scholar] [CrossRef] [PubMed]
- Choy, E. Understanding the dynamics: Pathways involved in the pathogenesis of rheumatoid arthritis. Rheumatology 2012, 51, v3–v11. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef]
- Trakatellis, A.; Dimitriadou, A.; Exindari, M.; Scountzou, J.; Koliakos, G.; Christodoulou, D.; Malissiovas, N.; Antoniadis, A.; Polyzoni, T. Effect of pyridoxine deficiency on immunological phenomena. Postgrad. Med. J. 1992, 68, S70–S77. [Google Scholar]
- Wang, H.; Liu, Z.; Huang, M.; Wang, S.; Cui, D.; Dong, S.; Li, S.; Qi, Z.; Liu, Y. Effects of long-term mineral block supplementation on antioxidants, immunity, and health of Tibetan sheep. Biol. Trace Elem. Res. 2016, 172, 326–335. [Google Scholar] [CrossRef]
- Gonzalez-Quintela, A.; Alende, R.; Gude, F.; Campos, J.; Rey, J.; Meijide, L.; Fernandez-Merino, C.; Vidal, C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin. Exp. Immunol. 2008, 151, 42–50. [Google Scholar] [CrossRef]
- Greene, D.M.; Bondy, G.S.; Azcona-Olivera, J.I.; Pestka, J.J. Role of gender and strain in vomitoxin-induced dysregulation of IgA production and IgA nephropathy in the mouse. J. Toxicol. Environ. Health Part A Curr. Issues 1994, 43, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A. Canine IgA and IgA deficiency: Implications for immunization against respiratory pathogens. Can. Vet. J. 2019, 60, 1305. [Google Scholar] [PubMed]
- Abdel-Salam, O.M.; Czimmer, J.; Debreceni, A.; Szolcsányi, J.; Mózsik, G. Gastric mucosal integrity: Gastric mucosal blood flow and microcirculation. An overview. J. Physiol. 2001, 95, 105–127. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).