Experimental Study of Collateral Patency following Overlapped Multilayer Flow Modulators Deployment
Abstract
:1. Introduction
2. Materials and Methods
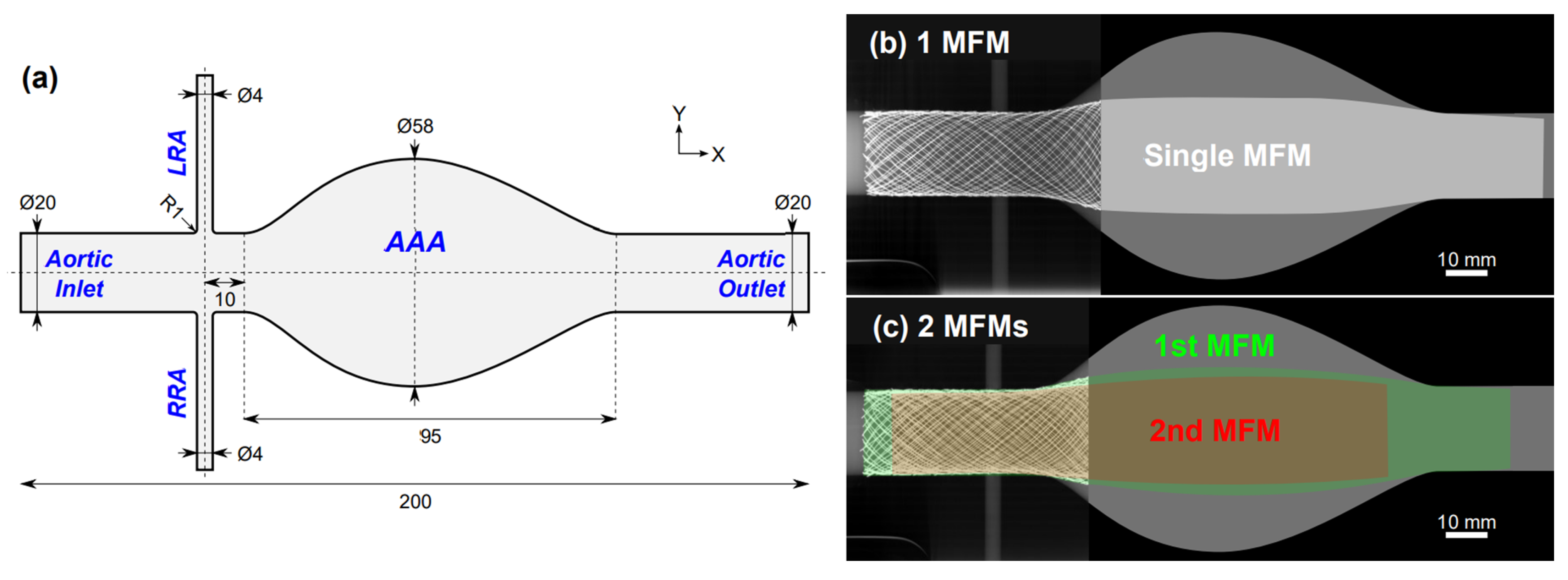
2.1. Phantom Geometry
2.2. MFM Devices
2.3. Micro-CT Imaging and Microstructure Evaluation
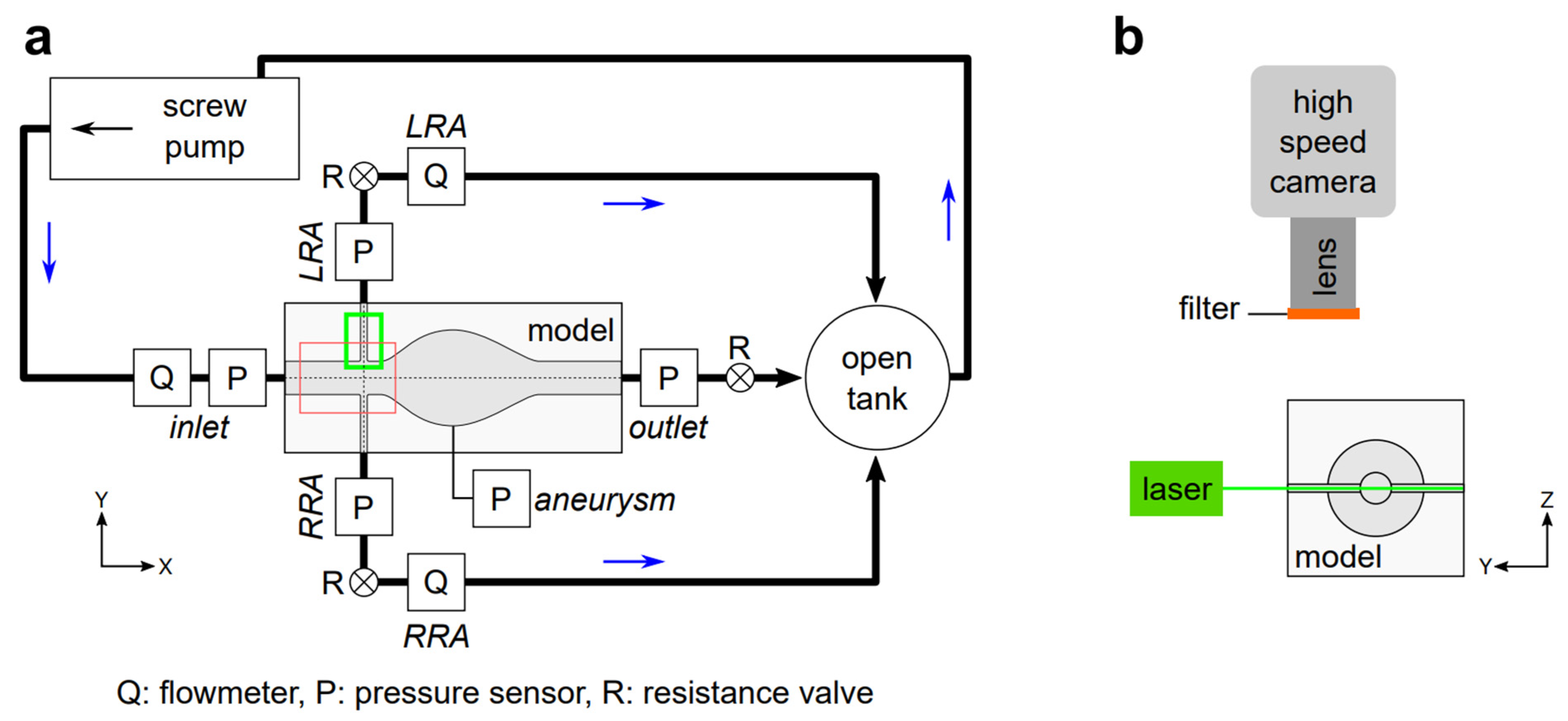
2.4. Circulation System
2.5. PIV Experiments
2.6. Flow Rate & Pressure Evaluation
- Acquire sensors values for 10 s without flow (zero initialization);
- Start the pump;
- Wait 30 s for the flow to stabilize;
- Start the acquisition of PIV images and sensors values for 30 s.
3. Results
3.1. Microstructure Analysis of the MFM Devices in 2 and 3 Dimensions
3.2. Flow Rate & Pressure Evaluation Reveal Branch Perfusion Preservation
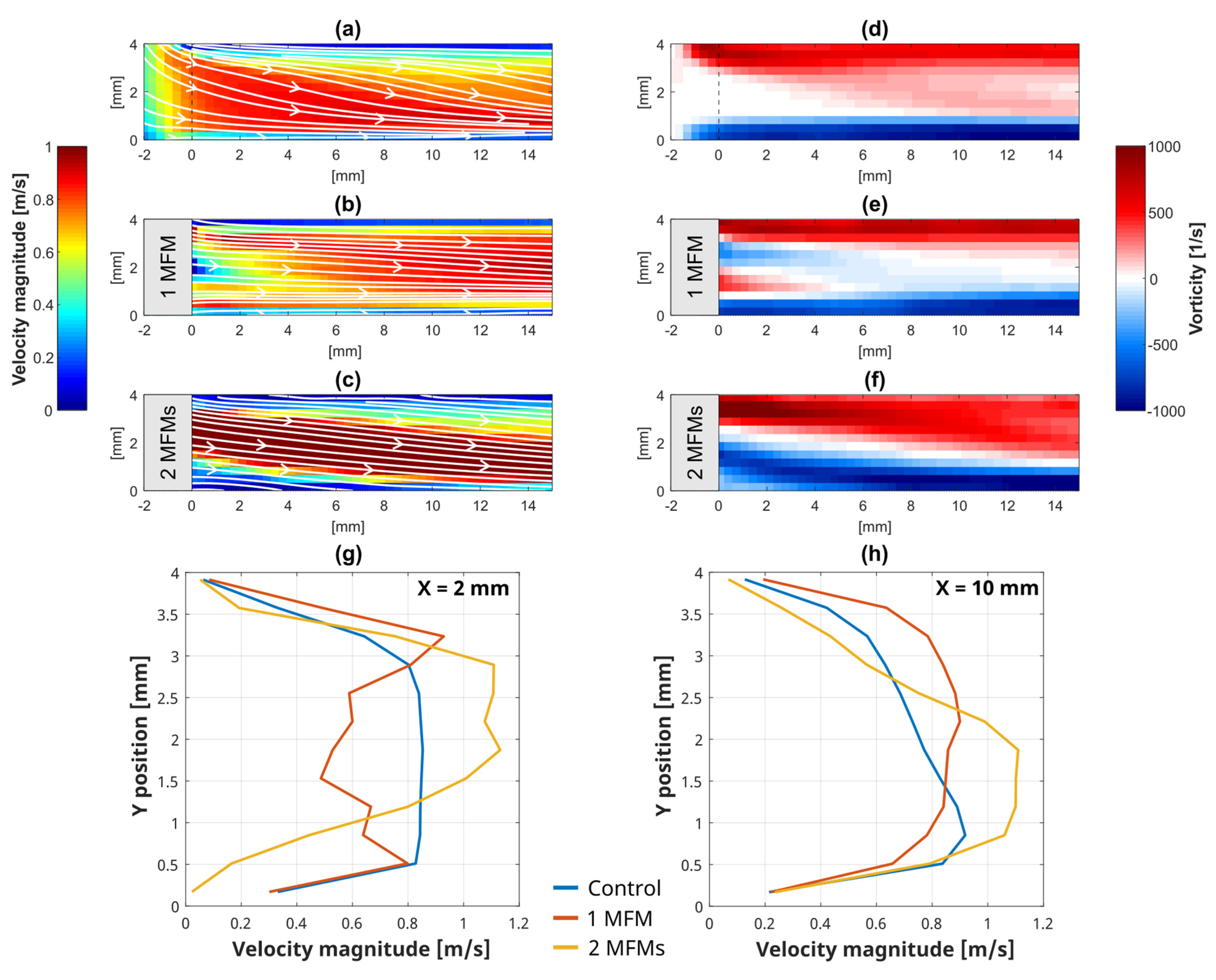
3.3. Local Flow Analysis Evaluated by PIV at the LRA Bifurcation
3.4. Study Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buck, D.B.; van Herwaarden, J.A.; Schermerhorn, M.L.; Moll, F.L. Endovascular Treatment of Abdominal Aortic Aneurysms. Nat. Rev. Cardiol. 2014, 11, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, M.; Donas, K.P.; Stavroulakis, K.; Stachmann, A.; Torsello, G.; Bisdas, T. Anatomical Suitability of the Zenith Off-the-Shelf (p-Branch) Endograft in Juxtarenal Aortic Aneurysms Previously Treated Using the Chimney Technique. J. Endovasc. Ther. 2017, 24, 223–229. [Google Scholar] [CrossRef] [PubMed]
- O’Mara, J.E.; Bersin, R.M. Endovascular Management of Abdominal Aortic Aneurysms: The Year in Review. Curr. Treat. Options Cardiovasc. Med. 2016, 18, 54. [Google Scholar] [CrossRef]
- Wanhainen, A.; Verzini, F.; van Herzeele, I.; Allaire, E.; Bown, M.; Cohnert, T.; Dick, F.; van Herwaarden, J.; Karkos, C.; Koelemay, M.; et al. Editor’s Choice–European Society for Vascular Surgery (ESVS) 2019 Clinical Practice Guidelines on the Management of Abdominal Aorto-Iliac Artery Aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019, 57, 8–93. [Google Scholar] [CrossRef] [Green Version]
- Saratzis, A.; Melas, N.; Mahmood, A.; Sarafidis, P. Incidence of Acute Kidney Injury (AKI) after Endovascular Abdominal Aortic Aneurysm Repair (EVAR) and Impact on Outcome. Eur. J. Vasc. Endovasc. Surg. 2015, 49, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Boyle, J.R. Poor Cardiac Function Is Associated With Renal Injury Following EVAR. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 725. [Google Scholar] [CrossRef] [Green Version]
- De Souza, L.R.; Oderich, G.S. Renal Function Deterioration in Complex Aortic Repair. In Endovascular Aortic Repair; Springer International Publishing: Cham, Switzerland, 2017; pp. 721–731. [Google Scholar]
- Ou, J.; Chan, Y.-C.; Chan, C.Y.-T.; Cheng, S.W.K. Geometric Alteration of Renal Arteries After Fenestrated Grafting and the Impact on Renal Function. Ann. Vasc. Surg. 2017, 41, 89–95. [Google Scholar] [CrossRef]
- Sultan, S.; Hynes, N.; Kavanagh, E.P.; Diethrich, E.B. How Does the Multilayer Flow Modulator Work? The Science Behind the Technical Innovation. J. Endovasc. Ther. 2014, 21, 814–821. [Google Scholar] [CrossRef] [Green Version]
- Tolva, V.S.; Bianchi, P.G.; Cireni, L.V.; Lombardo, A.; Keller, G.C.; Parati, G.; Casana, R.M. Multiple Multilayer Stents for Thoracoabdominal Aortic Aneurysm: A Possible New Tool for Aortic Endovascular Surgery. Int. J. Gen. Med. 2012, 5, 629–632. [Google Scholar] [CrossRef] [Green Version]
- Sultan, S.; Kavanagh, E.P.; Bonneau, M.; Kang, C.; Hynes, N. Abdominal Aortic Aneurysm Repair Using the Multilayer Flow Modulator in Porcine Animal Models. Univers. J. Med. Sci. 2015, 3, 1–10. [Google Scholar] [CrossRef]
- Benjelloun, A.; Henry, M.; Taberkant, M.; Berrado, A.; Houati, R.E.; Semlali, A. Multilayer Flow Modulator Treatment of Abdominal and Thoracoabdominal Aortic Aneurysms With Side Branch Coverage: Outcomes From a Prospective Single-Center Moroccan Registry. J. Endovasc. Ther. 2016, 23, 773–782. [Google Scholar] [CrossRef]
- Debing, E.; Aerden, D.; Gallala, S.; Vandenbroucke, F.; van den Brande, P. Stenting Complex Aorta Aneurysms with the Cardiatis Multilayer Flow Modulator: First Impressions. Eur. J. Vasc. Endovasc. Surg. 2014, 47, 604–608. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Chaichana, T. Investigation of the Hemodynamic Effect of Stent Wires on Renal Arteries in Patients with Abdominal Aortic Aneurysms Treated with Suprarenal Stent-Grafts. CardioVascular Interv. Radiol. 2009, 32, 647–657. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Chaichana, T. Fenestrated Stent Graft Repair of Abdominal Aortic Aneurysm: Hemodynamic Analysis of the Effect of Fenestrated Stents on the Renal Arteries. Korean J. Radiol. 2010, 11, 95. [Google Scholar] [CrossRef]
- Boersen, J.T.; Groot Jebbink, E.; Versluis, M.; Slump, C.H.; Ku, D.N.; de Vries, J.-P.P.M.; Reijnen, M.M.P.J. Flow and Wall Shear Stress Characterization after Endovascular Aneurysm Repair and Endovascular Aneurysm Sealing in an Infrarenal Aneurysm Model. J. Vasc. Surg. 2017, 66, 1844–1853. [Google Scholar] [CrossRef] [Green Version]
- Tupin, S.; Takase, K.; Ohta, M. Experimental Analysis of Pressure and Flow Alterations During and After Insertion of a Multilayer Flow Modulator into an AAA Model with Incorporated Branch. CardioVascular Interv. Radiol. 2021, 44, 1251–1259. [Google Scholar] [CrossRef]
- Zhang, M.; Tupin, S.; Li, Y.; Ohta, M. Association Between Aneurysmal Haemodynamics and Device Microstructural Characteristics After Flow-Diversion Treatments With Dual Stents of Different Sizes: A Numerical Study. Front. Physiol. 2021, 12, 663668. [Google Scholar] [CrossRef]
- International Organization for Standardization. Cardiovascular Implants and Extracorporeal Systems-Vascular Prostheses-Tubular Vascular Grafts and Vascular Patches; ISO 7198:2016; International Organization for Standardization: Geneva, Switzerland, 2016. [Google Scholar]
- Shida, S.; Kosukegawa, H.; Ohta, M. Development of a Methodology for Adaptation of Refractive Index Under Controlling Kinematic Viscosity for PIV. In Proceedings of the Volume 2: Biomedical and Biotechnology Engineering; Nanoengineering for Medicine and Biology, Denver, CO, USA, 11–17 November 2011; pp. 313–321. [Google Scholar] [CrossRef]
- Les, A.S.; Yeung, J.J.; Schultz, G.M.; Herfkens, R.J.; Dalman, R.L.; Taylor, C.A. Supraceliac and Infrarenal Aortic Flow in Patients with Abdominal Aortic Aneurysms: Mean Flows, Waveforms, and Allometric Scaling Relationships. Cardiovasc. Eng. Technol. 2010, 1, 39–51. [Google Scholar] [CrossRef]
- Bouillot, P.; Brina, O.; Ouared, R.; Yilmaz, H.; Farhat, M.; Erceg, G.; Lovblad, K.O.; Vargas, M.I.; Kulcsar, Z.; Pereira, V.M. Geometrical Deployment for Braided Stent. Med. Image Anal. 2016, 30, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Tupin, S.; Ohta, M. Assessing Porous Media Permeability in Non-Darcy Flow: A Re-Evaluation Based on the Forchheimer Equation. Materials 2020, 13, 2535. [Google Scholar] [CrossRef]
- Li, S.; Latt, J.; Chopard, B. Model for Pressure Drop and Flow Deflection in the Numerical Simulation of Stents in Aneurysms. Int. J. Numer. Methods Biomed. Eng. 2018, 34, e2949. [Google Scholar] [CrossRef]
- Tupin, S.; Saqr, K.M.; Ohta, M. Effects of Wall Compliance on Multiharmonic Pulsatile Flow in Idealized Cerebral Aneurysm Models: Comparative PIV Experiments. Exp. Fluids 2020, 61, 164. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Mashima, T.; Amagai, H.; Fujii, H.; Hayase, T.; Tanishita, K. Variation of Wall Shear Stress and Periodic Oscillations Induced in the Right-Angle Branch During Laminar Steady Flow. J. Fluids Eng. 2005, 127, 1013–1020. [Google Scholar] [CrossRef]


| Model | 1 MFM | 2 MFMs | ||||||
|---|---|---|---|---|---|---|---|---|
| Location | LRA | RRA | LRA | RRA | ||||
| Sample | – | – | outer | inner | both | outer | inner | both |
| 2D Porosity [%] | 61.3 | 66.4 | 64.2 | 64.9 | 39.0 | 63.6 | 67.0 | 38.0 |
| 3D Porosity [%] | 89.1 | 90.6 | 90.0 | 90.1 | 90.1 | 89.7 | 90.9 | 90.3 |
| Specific surface area [mm−1] | 2.54 | 2.21 | 2.38 | 2.32 | 2.35 | 2.39 | 2.10 | 2.25 |
| Pore density [mm−2] | 1.83 | 1.59 | 1.51 | 1.51 | 3.66 | 1.75 | 1.51 | 4.86 |
| Pore size [mm2] | 0.71 ± 0.41 | 0.93 ± 0.45 | 1.08 ± 0.62 | 0.74 ± 0.41 | 0.22 ± 0.17 | 0.83 ± 0.50 | 0.68 ± 0.29 | 0.21 ± 0.16 |
| Maximum pore size [mm2] | 1.31 | 1.50 | 1.90 | 1.33 | 0.60 | 1.59 | 1.05 | 0.55 |
| Model | Flow rate [L/min] | Pressure [mmHg] | ||||||
|---|---|---|---|---|---|---|---|---|
| Inlet | LRA | RRA | Inlet | LRA | RRA | Aneurysm | Outlet | |
| Control | 2.9 ± 0.1 | 0.498 ± 0.007 | 0.515 ± 0.007 | 83 ± 2 (0) | 69 ± 2 (14) | 69 ± 2 (14) | 82 ± 2 (1) | 81 ± 2 (2) |
| 1 MFM | 2.9 ± 0.1 | 0.492 ± 0.007 | 0.510 ± 0.007 | 82 ± 2 (0) | 69 ± 2 (13) | 68 ± 2 (14) | 81 ± 2 (1) | 80 ± 2 (2) |
| 2 MFMs | 2.9 ± 0.1 | 0.493 ± 0.007 | 0.506 ± 0.007 | 86 ± 2 (0) | 69 ± 2 (17) | 68 ± 2 (18) | 86 ± 2 (0) | 84 ± 2 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tupin, S.; Takase, K.; Ohta, M. Experimental Study of Collateral Patency following Overlapped Multilayer Flow Modulators Deployment. Fluids 2022, 7, 220. https://doi.org/10.3390/fluids7070220
Tupin S, Takase K, Ohta M. Experimental Study of Collateral Patency following Overlapped Multilayer Flow Modulators Deployment. Fluids. 2022; 7(7):220. https://doi.org/10.3390/fluids7070220
Chicago/Turabian StyleTupin, Simon, Kei Takase, and Makoto Ohta. 2022. "Experimental Study of Collateral Patency following Overlapped Multilayer Flow Modulators Deployment" Fluids 7, no. 7: 220. https://doi.org/10.3390/fluids7070220
APA StyleTupin, S., Takase, K., & Ohta, M. (2022). Experimental Study of Collateral Patency following Overlapped Multilayer Flow Modulators Deployment. Fluids, 7(7), 220. https://doi.org/10.3390/fluids7070220







