Flaxseed Fiber-Structured Nanoemulgels for Salad Dressing Applications: Processing and Stability
Abstract
1. Introduction
2. Results and Discussion
2.1. The Impact of the Number of Passes Through the Microfluidizer
2.1.1. Particle Size Distribution
2.1.2. Stress Sweep
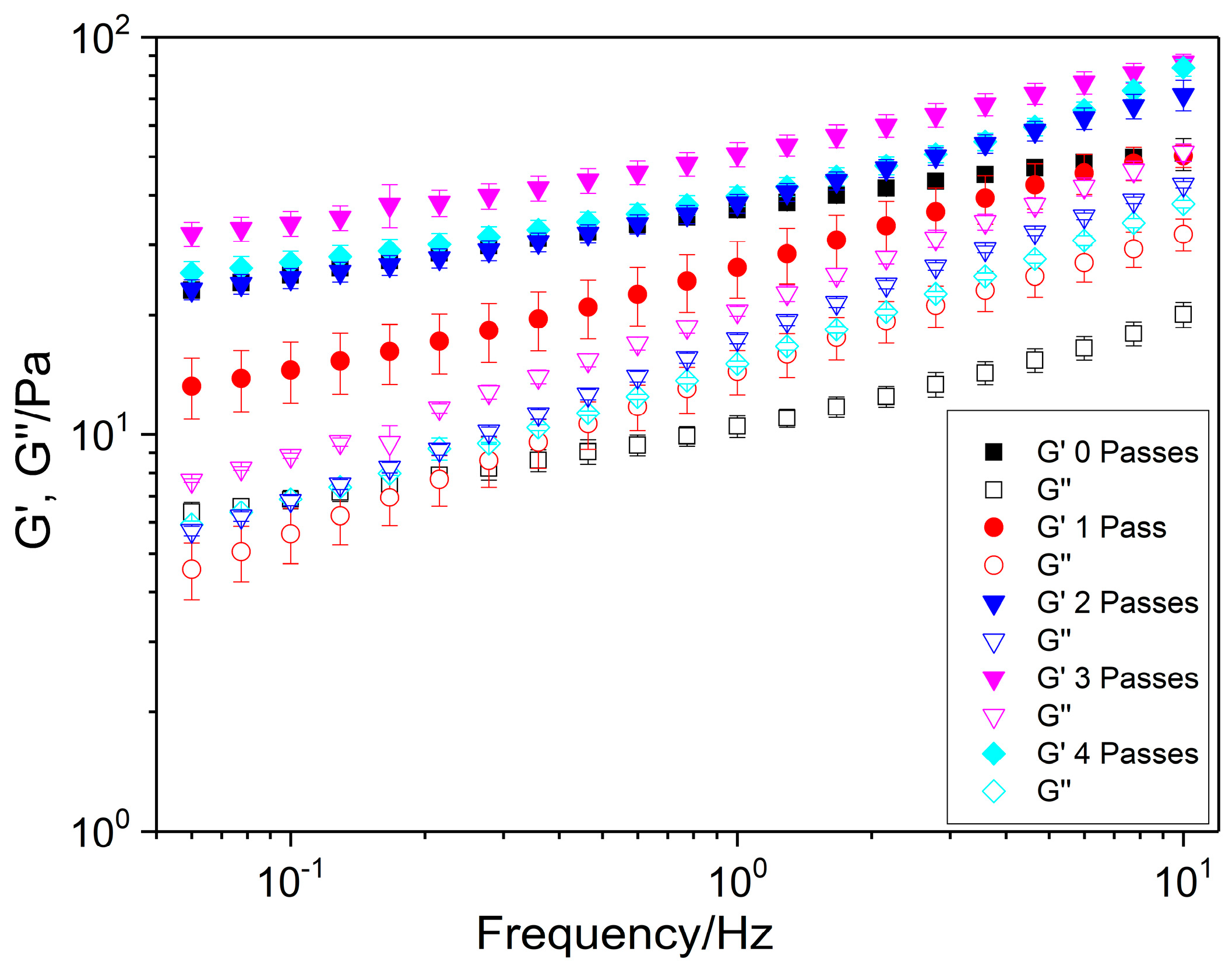
2.1.3. Frequency Sweep
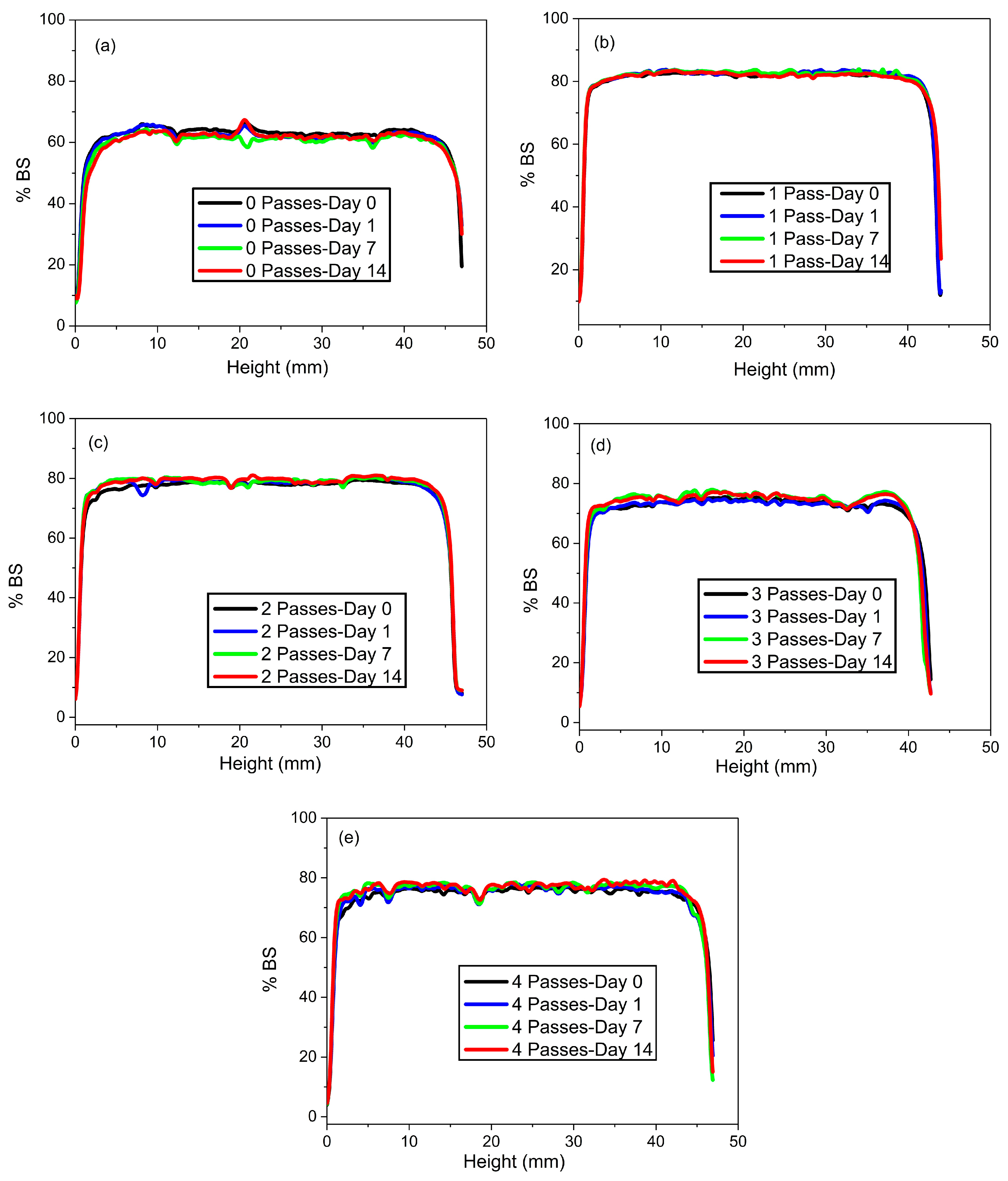
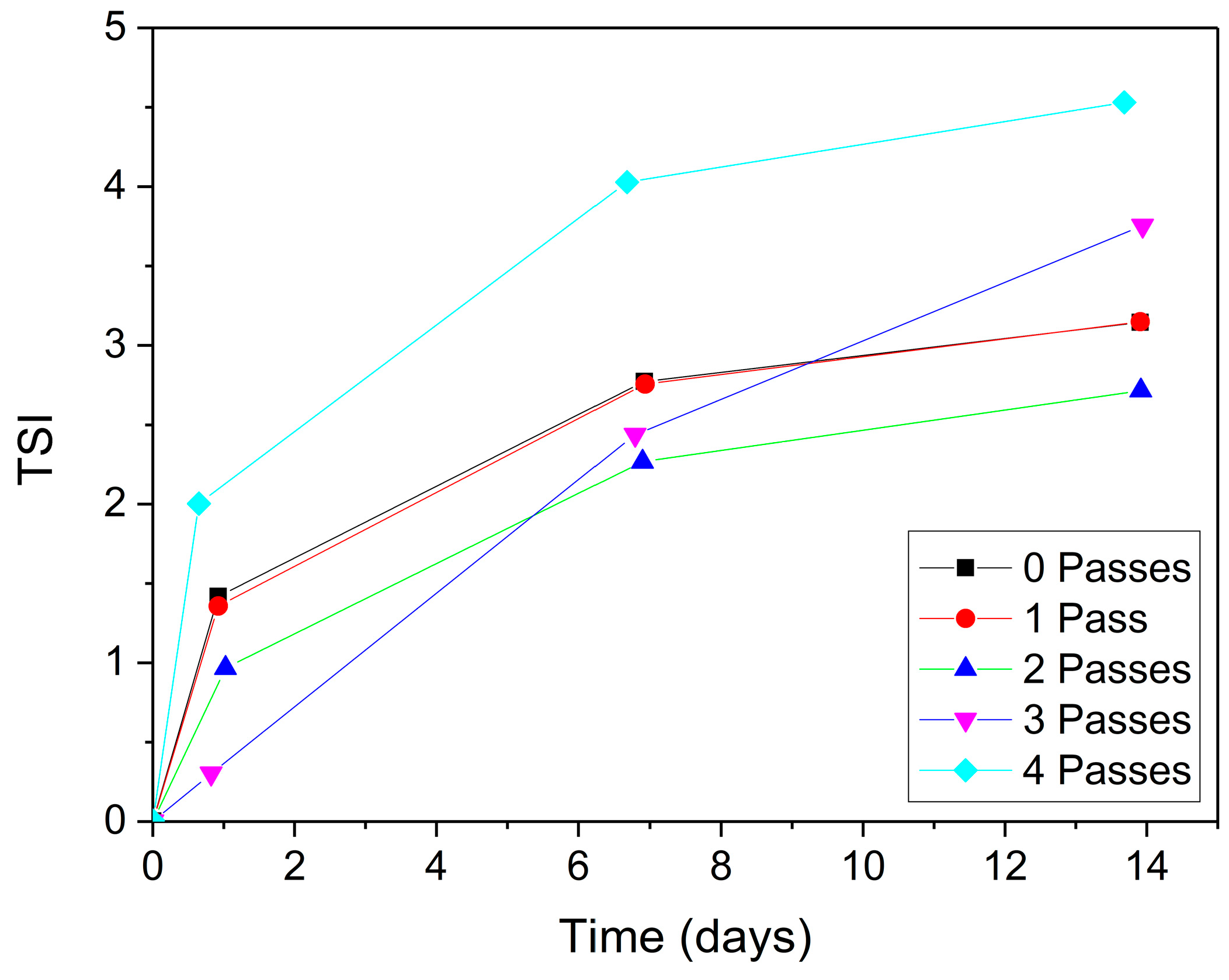
2.1.4. Physical Stability
2.2. Influence of Temperature
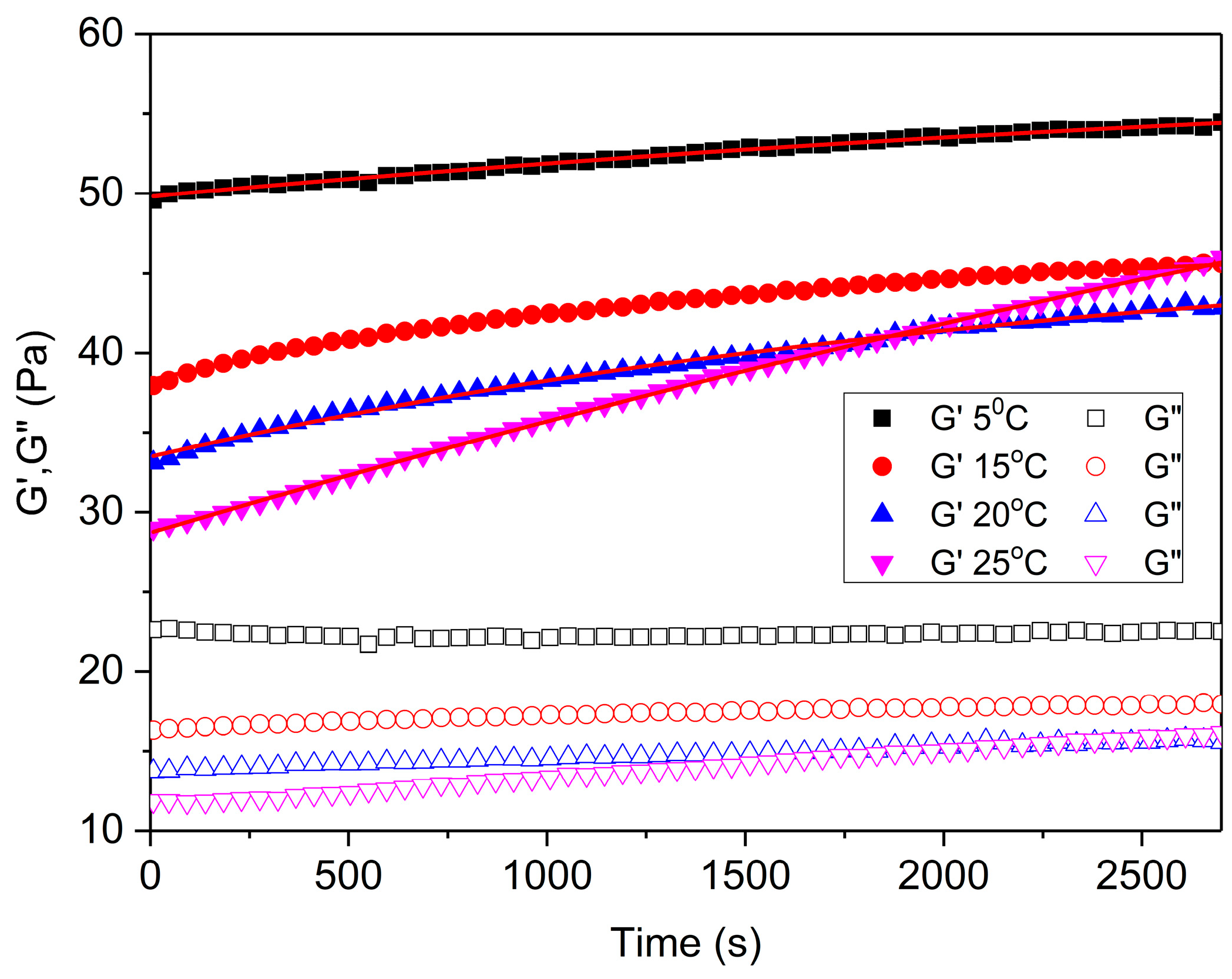
2.2.1. Time Sweep
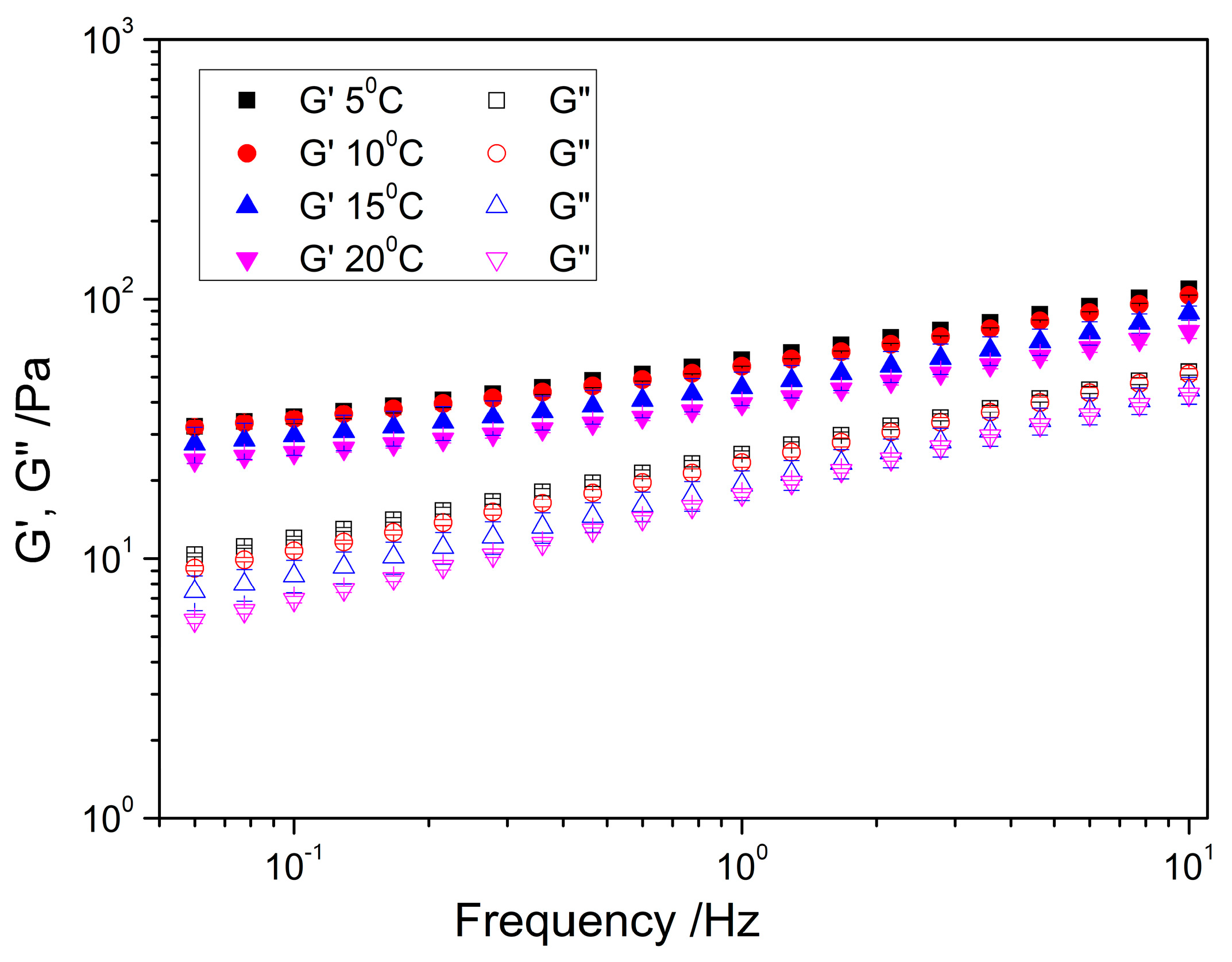
2.2.2. Frequency Sweep
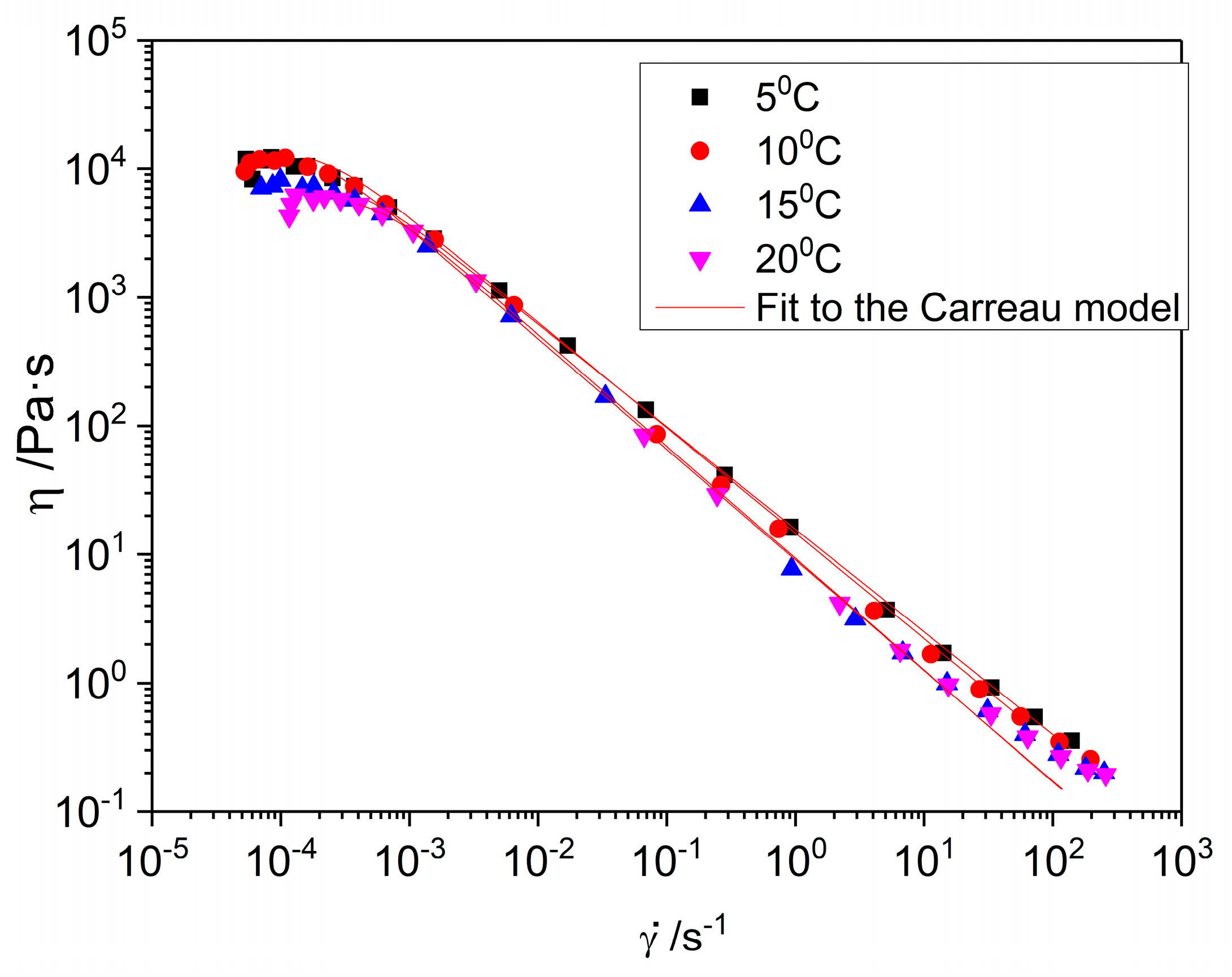
2.2.3. Flow Curves
3. Conclusions
4. Materials and Methods
4.1. Materials and Sample Preparation
4.2. Characterization Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alfaro-Rodríguez, M.C.; Prieto, P.; García, M.C.; Martín-Piñero, M.J.; Muñoz, J. Influence of Nanoemulsion/Gum Ratio on Droplet Size Distribution, Rheology and Physical Stability of Nanoemulgels Containing Inulin and Omega-3 Fatty Acids. J. Sci. Food Agric. 2022, 102, 6397–6403. [Google Scholar] [CrossRef]
- Khanna, S.; Singh, P.; Singh Chauhan, E. Characterization and Application of Nanoemulsion in Food Industry: A Review. Int. J. Sci. Res. Sci. Technol. 2024, 11, 672–679. [Google Scholar] [CrossRef]
- Aswathanarayan, J.B.; Vittal, R.R. Nanoemulsions and Their Potential Applications in Food Industry. Front. Sustain. Food Syst. 2019, 3, 95. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, H.; Chen, H.; Lin, J.; Wang, Q. Food-Grade Nanoemulsions: Preparation, Stability and Application in Encapsulation of Bioactive Compounds. Molecules 2019, 24, 4242. [Google Scholar] [CrossRef]
- Gengatharan, A.; Mohamad, N.V.; Zahari, C.N.M.C.; Vijayakumar, R. Oleogels: Innovative Formulations as Fat Substitutes and Bioactive Delivery Systems in Food and Beyond. Food Struct. 2023, 38, 100356. [Google Scholar] [CrossRef]
- Syan, V.; Kaur, J.; Sharma, K.; Patni, M.; Rasane, P.; Singh, J.; Bhadariya, V. An Overview on the Types, Applications and Health Implications of Fat Replacers. J. Food Sci. Technol. 2024, 61, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.M.; El-Gawad, M.A.M.A.; Kassem, J.M.; Salama, M. Application of Fat Replacers in Dairy Products: A Review. Foods Raw Mater. 2024, 12, 319–333. [Google Scholar] [CrossRef]
- McClements, D.J.; Bai, L.; Chung, C. Recent Advances in the Utilization of Natural Emulsifiers to Form and Stabilize Emulsions. Annu. Rev. Food Sci. Technol. 2017, 8, 205–236. [Google Scholar] [CrossRef]
- Dammak, I.; Sobral, P.J.D.A.; Aquino, A.; das Neves, M.A.; Conte-Junior, C.A. Nanoemulsions: Using Emulsifiers from Natural Sources Replacing Synthetic Ones—A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2721–2746. [Google Scholar] [CrossRef]
- Fedeniuk, R.W.; Biliaderis, C.G. Composition and Physicochemical Properties of Linseed (Linum usitatissimum L.) Mucilage. J. Agric. Food Chem. 1994, 42, 240–247. [Google Scholar] [CrossRef]
- Pramanik, J.; Kumar, A.; Prajapati, B. A Review on Flaxseeds: Nutritional Profile, Health Benefits, Value Added Products, and Toxicity. eFood 2023, 4, e114. [Google Scholar] [CrossRef]
- Hu, Y.; Shim, Y.Y.; Reaney, M.J.T. Flaxseed Gum Solution Functional Properties. Foods 2020, 9, 681. [Google Scholar] [CrossRef]
- Rashid, F.; Ahmed, Z.; Hussain, S.; Huang, J.Y.; Ahmad, A. Linum usitatissimum L. Seeds: Flax Gum Extraction, Physicochemical and Functional Characterization. Carbohydr. Polym. 2019, 215, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Oomah, B.D.; Kenaschuk, E.O.; Cui, W.; Mazza, G. Variation in the composition of water-soluble polysaccharides in flaxseed. J. Agric. Food Chem. 1995, 43, 1484–1488. [Google Scholar] [CrossRef]
- Wannerberger, K.; Nyman, M. Rheological and Chemical Properties of Mucilage in Different Varieties from Linseed (Linum usitatissimum). Acta Agric. Scand. 1991, 41, 311–319. [Google Scholar] [CrossRef]
- Alfaro-Rodríguez, M.C.; García, M.C.; Prieto-Vargas, P.; Muñoz, J. Rheological Properties and Physical Stability of Aqueous Dispersions of Flaxseed Fibers. Gels 2024, 10, 787. [Google Scholar] [CrossRef]
- Lorenc, F.; Jarošová, M.; Bedrníček, J.; Smetana, P.; Bárta, J. Structural Characterization and Functional Properties of Flaxseed Hydrocolloids and Their Application. Foods 2022, 11, 2304. [Google Scholar] [CrossRef]
- Kaur, M.; Kaur, R.; Punia, S. Characterization of Mucilages Extracted from Different Flaxseed (Linum usitatissiumum L.) Cultivars: A Heteropolysaccharide with Desirable Functional and Rheological Properties. Int. J. Biol. Macromol. 2018, 117, 919–927. [Google Scholar] [CrossRef]
- Lorenc, F.; Jarošová, M.; Bedrníček, J.; Smetana, P.; Bárta, J. Recent trends in food and dietary applications of flaxseed mucilage: A mini review. Int. J. Food Sci. Technol. 2024, 59, 2111–2121. [Google Scholar] [CrossRef]
- Stewart, S.; Mazza, G. Effect of flaxseed gum on quality and stability of a model salad dressing 1. J. Food Qual. 2000, 23, 373–390. [Google Scholar] [CrossRef]
- Mueed, A.; Shibli, S.; Korma, S.A.; Madjirebaye, P.; Esatbeyoglu, T.; Deng, Z. Flaxseed bioactive compounds: Chemical composition, functional properties, food applications and health benefits-related gut microbes. Foods 2022, 11, 3307. [Google Scholar] [CrossRef] [PubMed]
- Kajla, P.; Sharma, A.; Sood, D.R. Flaxseed—A Potential Functional Food Source. J. Food Sci. Technol. 2015, 52, 1857–1871. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.; Prasad Professor, R.; Higginbottom, S.; Gupta Assistant Professor, A.; Reema Verma, C.; Prasad, R.; Gupta, A. Functional Properties and Health Benefits in Flaxseed Fiber and Oil (Linum usitatissimum L.). Int. J. Home Sci. 2017, 3, 368–369. [Google Scholar]
- Milutinov, J.; Krstonošić, V.; Ćirin, D.; Pavlović, N. Emulgels: Promising Carrier Systems for Food Ingredients and Drugs. Polymers 2023, 15, 2302. [Google Scholar] [CrossRef]
- Santos, J.; Trujillo-Cayado, L.A.; Alcaide, M.D.Á.; Alfaro, M.D.C. Impact of Microfluidization on the Emulsifying Properties of Zein-Based Emulsions: Influence of Diutan Gum Concentration. Materials 2021, 14, 3695. [Google Scholar] [CrossRef]
- Delmas, T.; Piraux, H.; Couffin, A.-C.; Texier, I.; Vinet, F.; Poulin, P.; Cates, M.E.; Bibette, J. How to Prepare and Stabilize Very Small Nanoemulsions. Langmuir 2011, 27, 1683–1692. [Google Scholar] [CrossRef]
- Llinares, R.; Ramírez, P.; Carmona, J.A.; Trujillo-Cayado, L.A.; Muñoz, J. Assessment of Fennel Oil Microfluidized Nanoemulsions Stabilization by Advanced Performance Xanthan Gum. Foods 2021, 10, 693. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook; Vincentz Network GmbH & Co. KG: Hannover, Germany, 2006. [Google Scholar]
- García González, C.; Del Socorro Cely García, M.; García, J.M.; Alfaro-Rodriguez, M.C. A Comparison of the Effect of Temperature on the Rheological Properties of Diutan and Rhamsan Gum Aqueous Solutions. Fluids 2019, 4, 22. [Google Scholar] [CrossRef]
- Rao, M.A. Flow and Functional Models for Rheological Properties of Fluid Foods. In Rheology of Fluid and Semisolid Foods: Principles and Applications; Rao, M.A., Ed.; Aspen Publishers: Gaithersburg, MD, USA, 1999. [Google Scholar]
- Augusto, P.E.; Cristianini, M.; Ibarz, A. Effect of temperature on dynamic and steady-state shear rheological properties of siriguela (Spondias purpurea L.) pulp. J. Food Eng. 2012, 108, 283–289. [Google Scholar] [CrossRef]
- Martin-Piñero, M.J.; García, M.C.; Muñoz, J.; Alfaro-Rodriguez, M.C. Influence of the Welan Gum Biopolymer Concentration on the Rheological Properties, Droplet Size Distribution and Physical Stability of Thyme Oil/W Emulsions. Int. J. Biol. Macromol. 2019, 133, 270–277. [Google Scholar] [CrossRef]
- Steffe, J.F. Rheological Methods in Food Process Engineering; Freeman Press: Dallas, TX, USA, 1996. [Google Scholar]
- Patel, A.R.; Cludts, N.; Bin Sintang, M.D.; Lesaffer, A.; Dewettinck, K. Edible Oleogels Based on Water Soluble Food Polymers: Preparation, Characterization and Potential Application. Food Funct. 2014, 5, 2833–2841. [Google Scholar] [CrossRef]
- Yang, M.; Liu, F.; Tang, C.H. Properties and Microstructure of Transglutaminase-Set Soy Protein-Stabilized Emulsion Gels. Food Res. Int. 2013, 52, 409–418. [Google Scholar] [CrossRef]
- Muñoz, J.; Alfaro-Rodríguez, M.C.; Prieto-Vargas, P.; Lobo, C.; Garcia, M.C. Preparation of Nanoemulgels containing lemon essential oil and pectin: Physical stability and rheological properties. Appl. Sci. 2023, 13, 12662. [Google Scholar] [CrossRef]
- Kasprzak, M.M.; Berski, W.; Krystyjan, M.; Jamróz, E.; Florczuk, A.; Tkaczewska, J.; Zając, M.; Domagała, J.; Lett, A.M.; Ptasznik, S. Effects of Fibre Addition and Processing on the Stability, Rheology and in Vitro Gastric Digestion of Whey Protein-Xanthan Gum Stabilised Emulsions with High Oil Phase. LWT 2023, 178, 114465. [Google Scholar] [CrossRef]
- Scarpelli, F.; Ionescu, A.; Aiello, I.; La Deda, M.; Crispini, A.; Ghedini, M.; Brunelli, E.; Sest, S.; Godbert, N. High Order in a Self-Assembled Iridium (III) Complex Gelator Towards Nanostructured IrO2 Thin Films. Chem. Asian J. 2017, 12, 2703–2710. [Google Scholar] [CrossRef]
- Makeiff, D.A.; Cho, J.Y.; Godbert, N.; Smith, B.; Azyat, K.; Wagner AKulka, M.; Carlini, R. Supramolecular gels from alkylated benzimidazolone derivatives. J. Mol. Liq. 2021, 339, 116723. [Google Scholar] [CrossRef]
- McCarthy, N.A.; Kennedy, D.; Hogan, S.A.; Kelly, P.M.; Thapa, K.; Murphy, K.M.; Fenelon, M.A. Emulsification Properties of Pea Protein Isolate Using Homogenization, Microfluidization and Ultrasonication. Food Res. Int. 2016, 89, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zheng, Q.; Jin, M.; Chen, Y.; Guo, L.; Lin, J.; Zou, Y. Fabrication of Gel-like Emulsions with γ-Zein Particles Using Microfluidization: Structure Formation and Rheological Properties. Food Res. Int. 2022, 158, 111514. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Cayado, L.A.; Santos, J.; Calero, N.; Del Carmen Alfaro, M.; Muñoz, J. Influence of the Homogenization Pressure on the Rheology of Biopolymer-Stabilized Emulsions Formulated with Thyme Oil. Fluids 2019, 4, 29. [Google Scholar] [CrossRef]
- Mobuchon, C.; Carreau, P.; Heuzey, M.C. Effect of flow history on the structure of a non-polar polymer/clay nanocomposite model system. Rheol. Acta 2007, 46, 1045–1056. [Google Scholar] [CrossRef]
- Mobuchon, C.; Carreau, P.J.; Heuzey, M.-C.; Reddy, N.K.; Vermant, J. Anisotropy of Nonaqueous Layered Silicate Suspensions Subjected to Shear Flow. J. Rheol. 2009, 53, 517–538. [Google Scholar] [CrossRef]
- Moreira, R.; Chenlo, F.; Torres, M.D.; Prieto, D.M. Influence of the particle size on the rheological behaviour of chestnut flour doughs. J. Food Eng. 2010, 100, 270–277. [Google Scholar] [CrossRef]
- Niknam, R.; Ghanbarzadeh, B.; Ayaseh, A.; Rezagholi, F. The Effects of Plantago Major Seed Gum on Steady and Dynamic Oscillatory Shear Rheology of Sunflower Oil-in-Water Emulsions. J. Texture Stud. 2018, 49, 536–547. [Google Scholar] [CrossRef]
- Carreau, P.J. Rheological equations from molecular network theories. J. Rheol. 1972, 16, 99–127. [Google Scholar] [CrossRef]
- Lopes da Silva, J.A.; Gonçalves, M.P.; Rao, M.A. Influence of Temperature on the Dynamic and Steady-Shear Rheology of Pectin Dispersions. Carbohydr. Polym. 1994, 23, 77–87. [Google Scholar] [CrossRef]
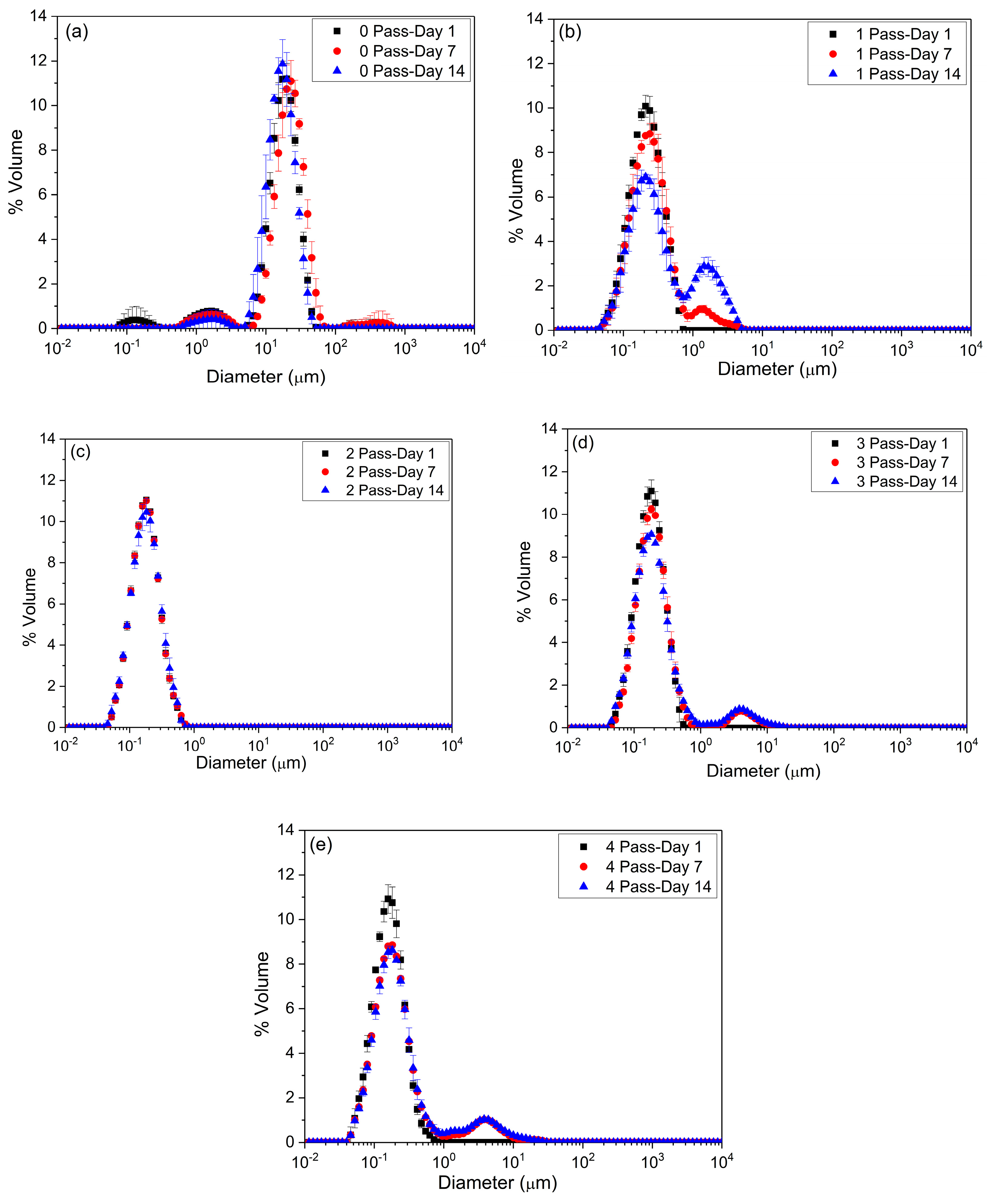

| Day 1 | |||
| D[3,2] ± SD (μm) | D[4,3] ± SD (μm) | span ± SD | |
| 0 Passes | 6.25 ± 3.83 a,* | 21.23 ± 7.57 α,* | 1.45 ± 0.25 v,* |
| 1 Pass | 0.17 ± 0.01 c,* | 0.22 ± 0.01 β,* | 1.43 ± 0.09 v,* |
| 2 Passes | 0.15 ± 0.01 c,* | 0.18 ± 0.01 β,* | 1.35 ± 0.01 v,* |
| 3 Passes | 0.14 ± 0.01 c,* | 0.17 ± 0.01 β,* | 1.30 ± 0.01 v,* |
| 4 Passes | 0.13 ± 0.01 c,* | 0.17 ± 0.01 β,* | 1.33 ± 0.01 v,* |
| Day 7 | |||
| 0 Passes | 9.91 ± 0.16 b,* | 28.23 ± 13.58 α,* | 1.45 ± 0.35 v,* |
| 1 Pass | 0.19 ± 0.02 c,* | 0.35 ± 0.02 β,* | 2.06 ± 0.22 w,* |
| 2 Passes | 0.15 ± 0.01 c,* | 0.19 ± 0.01 β,* | 1.37 ± 0.01 v,* |
| 3 Passes | 0.16 ± 0.01 c,* | 0.47 ± 0.01 β,* | 1.75 ± 0.03 v,* |
| 4 Passes | 0.15 ± 0.01 c,* | 0.69 ± 0.02 β,* | 7.08 ± 0.47 x,* |
| Day 14 | |||
| 0 Passes | 10.85 ± 2.76 b,* | 16.28 ± 0.29 α,* | 1.22 ± 0.07 v,* |
| 1 Pass | 0.22 ± 0.03 c,* | 0.62 ± 0.06 β,* | 6.57 ± 0.49 y,* |
| 2 Passes | 0.15 ± 0.01 c,* | 0.19 ± 0.01 β,* | 1.43 ± 0.09 v,* |
| 3 Passes | 0.15 ± 0.01 c,* | 0.55 ± 0.01 β,* | 2.64 ± 0.16 w,* |
| 4 Passes | 0.16 ± 0.01 c,* | 0.73 ± 0.06 β,* | 9.50 ± 0.61 z,* |
| Temperature | (Pa) ± SD | () (Pa) ± SD | k ± SD | R2 |
|---|---|---|---|---|
| G′/5 °C | 49.8 ± 0.03 a,* | 9.5 ± 0.09 c,* | 2.5×10−4 ± 0.08 e,* | 0.99 |
| G′/10 °C | 38.3 ± 0.05 a,* | 8.8 ± 0.11 c,* | 6.4×10−4 ± 2.02·10−5 e,* | 0.99 |
| G′/15 °C | 33.5 ± 0.07 a,* | 14.2 ± 0.45 c,* | 6.4×10−4 ± 2.40·10−5 e,* | 0.99 |
| G′/20 °C | 28.7 ± 0.04 b,* | 56.5 ± 2.69 d,* | 1.3×10−4 ± 7.59·10−6 e,* | 0.99 |
| Temperature | K ± SD (Pa) | n ± SD | R2 |
|---|---|---|---|
| G′/5 °C | 1.76 ± 4.89×10−4 x,* | 0.27 ± 6.76×10−4 α,* | 0.99 |
| G′/10 °C | 1.73 ± 8.57×10−4 x,* | 0.27 ± 1.00×10−3 α,* | 0.99 |
| G′/15 °C | 1.64 ± 1.00×10−3 x,* | 0.29 ± 2.00×10−3 α,* | 0.99 |
| G′/20 °C | 1.59 ± 4.99×10−4 x,* | 0.28 ± 8.05×10−4 α,* | 0.99 |
| Temperature | η0 ± SD (Pa·s) | ± SD (s−1) | n | R2 |
|---|---|---|---|---|
| 5 °C | 11,103.7 ± 286.02 a,* | 4.01×10−4 ± 5.29×10−6 α,* | 0.21 x,* | 0.99 |
| 10 °C | 11,760.33 ± 1508.68 a,* | 2.98×10−4 ± 3.25×10−5 α,* | 0.16 x,* | 0.99 |
| 15 °C | 11,494.9 ± 302.53 a,* | 1.46×10−4 ± 4.55×10−5 α,* | 0.15 x,* | 0.99 |
| 20 °C | 12,659.97 ± 340.03 a,* | 2.66×10−4 ± 4.10×10−5 α,* | 0.13 x,* | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfaro-Rodríguez, M.-C.; Vela, F.; García-González, M.-C.; Muñoz, J. Flaxseed Fiber-Structured Nanoemulgels for Salad Dressing Applications: Processing and Stability. Gels 2025, 11, 678. https://doi.org/10.3390/gels11090678
Alfaro-Rodríguez M-C, Vela F, García-González M-C, Muñoz J. Flaxseed Fiber-Structured Nanoemulgels for Salad Dressing Applications: Processing and Stability. Gels. 2025; 11(9):678. https://doi.org/10.3390/gels11090678
Chicago/Turabian StyleAlfaro-Rodríguez, María-Carmen, Fátima Vela, María-Carmen García-González, and José Muñoz. 2025. "Flaxseed Fiber-Structured Nanoemulgels for Salad Dressing Applications: Processing and Stability" Gels 11, no. 9: 678. https://doi.org/10.3390/gels11090678
APA StyleAlfaro-Rodríguez, M.-C., Vela, F., García-González, M.-C., & Muñoz, J. (2025). Flaxseed Fiber-Structured Nanoemulgels for Salad Dressing Applications: Processing and Stability. Gels, 11(9), 678. https://doi.org/10.3390/gels11090678







