Rheological and Physical Properties of Mucilage Hydrogels from Cladodes of Opuntia ficus-indica: Comparative Study with Pectin
Abstract
1. Introduction
2. Results and Discussion
2.1. Flow Curve Analysis
2.2. Determination of Dynamic Rheological Properties
2.3. ζ-Potential and Particle Size Distribution and Poly Dispersibility Index (PDI)
2.4. Released Liquid Mass and Water Activity
2.5. Fourier Transform–Infrared Spectroscopic Analysis (FT-IR)
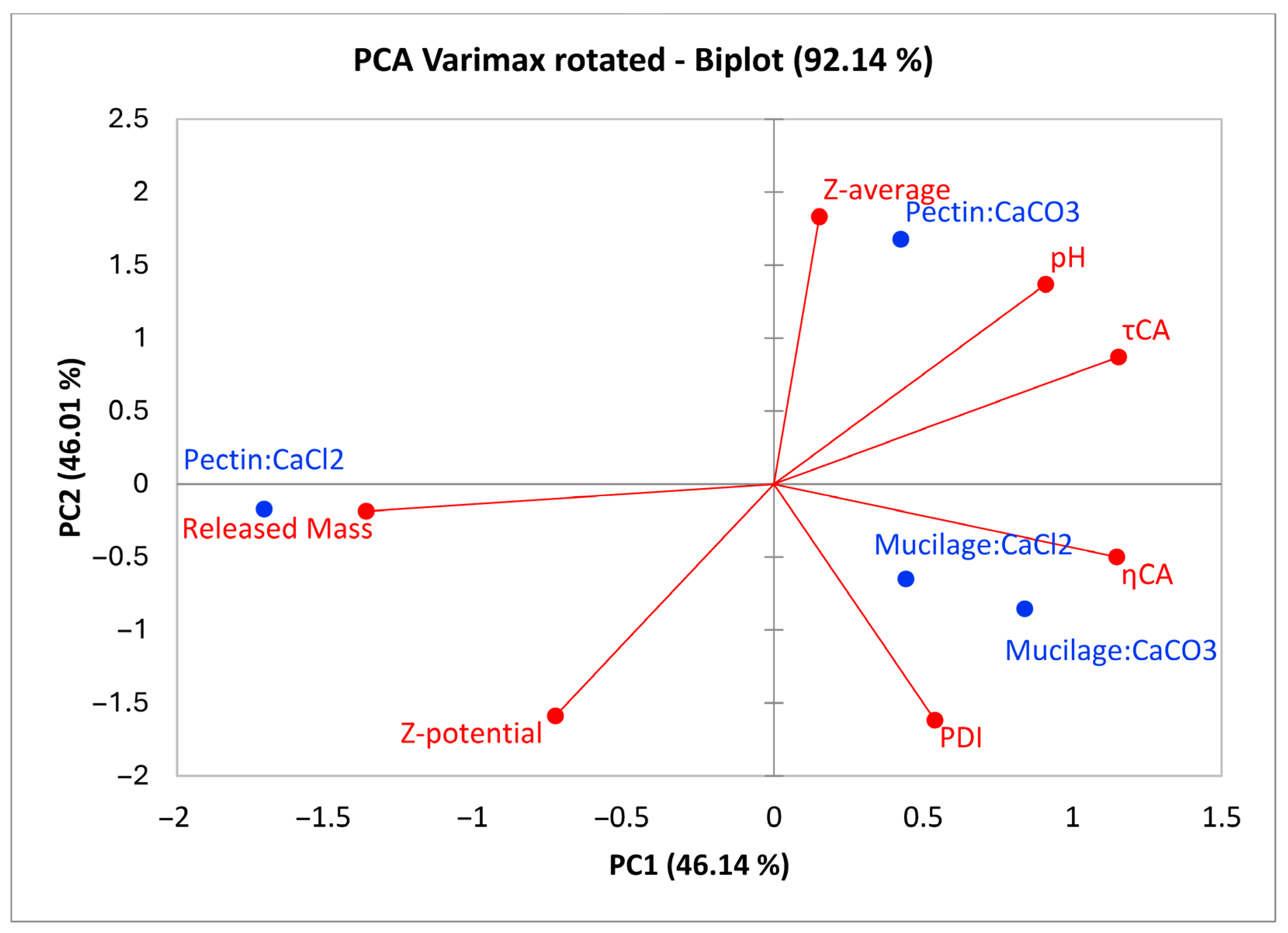
2.6. Multivariate Evaluation of Gels Physical-Chemical Properties
3. Conclusions
4. Materials and Methods
4.1. Extraction and Freeze-Drying of Cactus Pear Mucilage
4.2. Mucilage and Pectin Hydrogels Preparation
4.3. Rheological Measurements
4.3.1. Flow Curve Analysis
4.3.2. Viscoelastic Behavior
4.4. ζ-Potential, Particle Size Distribution, and Polydispersity Index
4.5. Released Liquid Mass (RM), Water Activity (aW), and pH
4.6. Fourier Transform–Infrared Spectroscopic Analysis (FT-IR)
4.7. Statistical Analysis
4.8. Multivariate Evaluation of Gel Physical–Chemical Properties
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roman-Benn, A.; Contador, C.A.; Li, M.W.; Lam, H.M.; Ah-Hen, K.; Ulloa, P.E.; Ravanal, M.C. Pectin: An overview of sources, extraction and applications in food products, biomedical, pharmaceutical and environmental issues. Food Chem. Adv. 2023, 2, 100192. [Google Scholar] [CrossRef]
- Said, N.S.; Olawuyi, I.F.; Lee, W.Y. Pectin Hydrogels: Gel-Forming Behaviors, Mechanisms, and Food Applications. Gels 2023, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, A.T.; Thibault, J.F.; Ralet, M.C. Citrus pectin: Structure and application in acid dairy drinks. J. Tree. For. Sci. Biotech. 2008, 2, 60–70. [Google Scholar]
- Srivastava, P.; Malviya, R. Sources of pectin, extraction and its applications in pharmaceutical industry—An overview. Indian. J. Nat. Prod. Resour. 2011, 2, 10–18. [Google Scholar]
- Zhao, X.; Ye, F.; Wu, Z.; Zhou, Y.; Lei, L.; Zhou, S.; Zhao, G. Sucrose and Ca2+ synergistically regulate the rheological properties of apple high-methoxyl pectin. Int. J. Biol. Macromol. 2024, 271 Pt 1, 132397. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25. [Google Scholar] [CrossRef]
- Sáenz, C.; Sepúlveda, E.; Matsuhiro, B. Opuntia spp. mucilage’s: A functional component with industrial perspectives. J. Arid. Environ. 2004, 57, 275–290. [Google Scholar] [CrossRef]
- Kalegowda, P.; Singh Chauhan, A.; Mysore Nanjaraj Urs, S. Opuntia dillenii (Ker-gawl) haw fruit peel pectin: Physicochemical, rheological, and functional behavior. J. Food Process. Preserv. 2017, 41, 1057–1064. [Google Scholar] [CrossRef]
- Liguori, G.; Gaglio, R.; Greco, G.; Gentile, C.; Settanni, L.; Inglese, P. Effect of Opuntia ficus-indica Mucilage Edible Coating on Processed Cactus Pear Fruits. Agronomy 2021, 11, 1963. [Google Scholar] [CrossRef]
- Quinzio, C.; Ayunta, C.; Alancay, M.; de Mishima, B.L.; Iturriaga, L. Physicochemical and rheological properties of mucilage extracted from Opuntia ficus indica (L. Miller). Comparative study with guar gum and xanthan gum. J. Food Meas. Charact. 2018, 12, 459–470. [Google Scholar] [CrossRef]
- Fraeye, I.; Colle, I.; Vandevenne, E.; Duvetter, T.; Van Buggenhout, S.; Moldenaers, P.; Van Loey, A.; Hendrickx, M. Influence of pectin structure on texture of pectin-calcium gels. Innov. Food Sci. Emerg. Technol. 2010, 11, 401–409. [Google Scholar] [CrossRef]
- Capitani, M.I.; Corzo-Rios, L.J.; Chel-Guerrero, L.A.; Betancur-Ancona, D.A.; Nolasco, S.M.; Tomás, M.C. Rheological properties of aqueous dispersions of chia (Salvia hispanica L.) mucilage. J. Food Eng. 2015, 149, 70–77. [Google Scholar] [CrossRef]
- Enoch, K.; Somasundaram, A.A. Rheological insights on Carboxymethyl cellulose hydrogels. Int. J. Biol. Macromol. 2023, 253, 127481. [Google Scholar] [CrossRef] [PubMed]
- Cofelice, M.; Messia, M.C.; Marconi, E.; Cuomo, F.; Lopez, F. Effect of the xanthan gum on the rheological properties of alginate hydrogels. Food Hydrocoll. 2023, 142, 108768. [Google Scholar] [CrossRef]
- Acar, H.; Kurt, A. Purified salep glucomannan synergistically interacted with xanthan gum: Rheological and textural studies on a novel pH-/thermo-sensitive hydrogel. Food Hydrocoll. 2020, 101, 105463. [Google Scholar] [CrossRef]
- Sandolo, C.; Matricardi, P.; Alhaique, F.; Coviello, T. Dynamo-mechanical and rheological characterization of guar gum hydrogels. Eur. Polym. J. 2007, 43, 3355–3367. [Google Scholar] [CrossRef]
- Martin, J.; Adolf, D. The sol-gel transition in chemical gels. Annu. Rev. Phys. Chem. 1991, 42, 311–339. [Google Scholar] [CrossRef]
- Muñoz-Almagro, N.; Vendrell-Calatayud, M.; Méndez-Albiñana, P.; Moreno, R.; Cano, M.P.; Villamiel, M. Extraction optimization and structural characterization of pectin from persimmon fruit (Diospyros kaki Thunb. var. Rojo brillante). Carbohydr. Polym. 2021, 272, 118411. [Google Scholar] [CrossRef] [PubMed]
- Kepekçi, R.A.; Yener İlçe, B.; Kanmazalp, S.D. Plant-derived biomaterials for wound healing. Stud. Nat. Prod. Chem. 2021, 70, 227–264. [Google Scholar]
- Pan, X.; Zhao, W.; Wang, Y.; Xu, Y.; Zhang, W.; Lao, F.; Liao, X.; Wu, J. Physicochemical and structural properties of three pectin fractions from muskmelon (Cucumis melo) and their correlation with juice cloud stability. Food Hydrocoll. 2022, 124 Pt B, 107313. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. The progress and application of vitamin E encapsulation—A review. Food Hydrocoll. 2021, 121, 106998. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Lara, C.R.; Cifuentes, G.R.; Gómez Castaño, J.A. Use of Opuntia ficus-indica Fruit Peel as a Novel Source of Mucilage with Coagulant Physicochemical/Molecular Characteristics. Polymers 2022, 14, 3832. [Google Scholar] [CrossRef] [PubMed]
- Pednekar, S.; Chun, J.; Morris, J.F. Bidisperse and polydisperse suspension rheology at large solid fraction. J. Rheol. 2018, 62, 513–526. [Google Scholar] [CrossRef]
- Wan, L.; Chen, Q.; Huang, M.; Liu, F.; Pan, S. Physiochemical, rheological and emulsifying properties of low methoxyl pectin prepared by high hydrostatic pressure-assisted enzymatic, conventional enzymatic, and alkaline de-esterification: A comparison study. Food Hydrocoll. 2019, 93, 146–155. [Google Scholar] [CrossRef]
- Liang, W.; Liao, J.; Qi, J.R.; Jiang, W.; Yang, X. Physicochemical characteristics and functional properties of high methoxyl pectin with different degree of esterification. Food Chem. 2022, 375, 131806. [Google Scholar] [CrossRef] [PubMed]
- Synytsya, A.; Čopíková, J.; Matějka, P.; Machovič, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Morales-Chávez, F.M.; Aguirre-Mancilla, C.L.; Medina-Torres, L.; Madera-Santana, T.J.; Grijalva-Verdugo, C.; Núñez-Colín, C.A.; Rodríguez-Núñez, J.R. Chemical, thermal, morphological, and rheological characterization of mucilage from different cactus pears cultivars (Opuntia spp.). J. Food Meas. Charact. 2024, 18, 7100–7111. [Google Scholar] [CrossRef]
- Du Toit, A.; De Wit, M.A. Process for Extracting Mucilage from Opuntia Ficus-Indica and Aloe Barbadensis. South Africa Patent No. PA153178/P, 12 May 2011. [Google Scholar]
- Voragen, F.; Schols, H.; Visser, R. Advances in Pectin and Pectinase Research. Ann. Bot. 2003, 94, 479–480. [Google Scholar]
- Richard, P.; Hilditch, S. D-Galacturonic acid catabolism in microorganisms and its biotechnological relevance. Appl. Microbiol. Biotechnol. 2009, 82, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Salehi, E.; Emam-djomeh, Z.; Fathi, M.; Askari, G. Opuntia ficus-indica Mucilage. Emerg. Nat. Hydrocoll. 2019, 425–449. [Google Scholar]
- Alvarez, M.D.; Canet, W. Dynamic Viscoelastic Behavior of Vegetable-Based Infant Purees. J. Texture Stud. 2013, 44, 205–224. [Google Scholar] [CrossRef]
- Ciron, C.I.E.; Gee, V.L.; Kelly, A.L.; Auty, M.A.E. Comparison of the effects of high-pressure microfluidization and conventional homogenization of milk on particle size, water retention and texture of non-fat and low-fat yoghurts. Int. Dairy. J. 2010, 20, 314–320. [Google Scholar] [CrossRef]
- ISO 18787:2017; (E) Foodstuffs—Determination of Water Activity. International Organization for Standardization: Geneva, Switzerland, 2017.
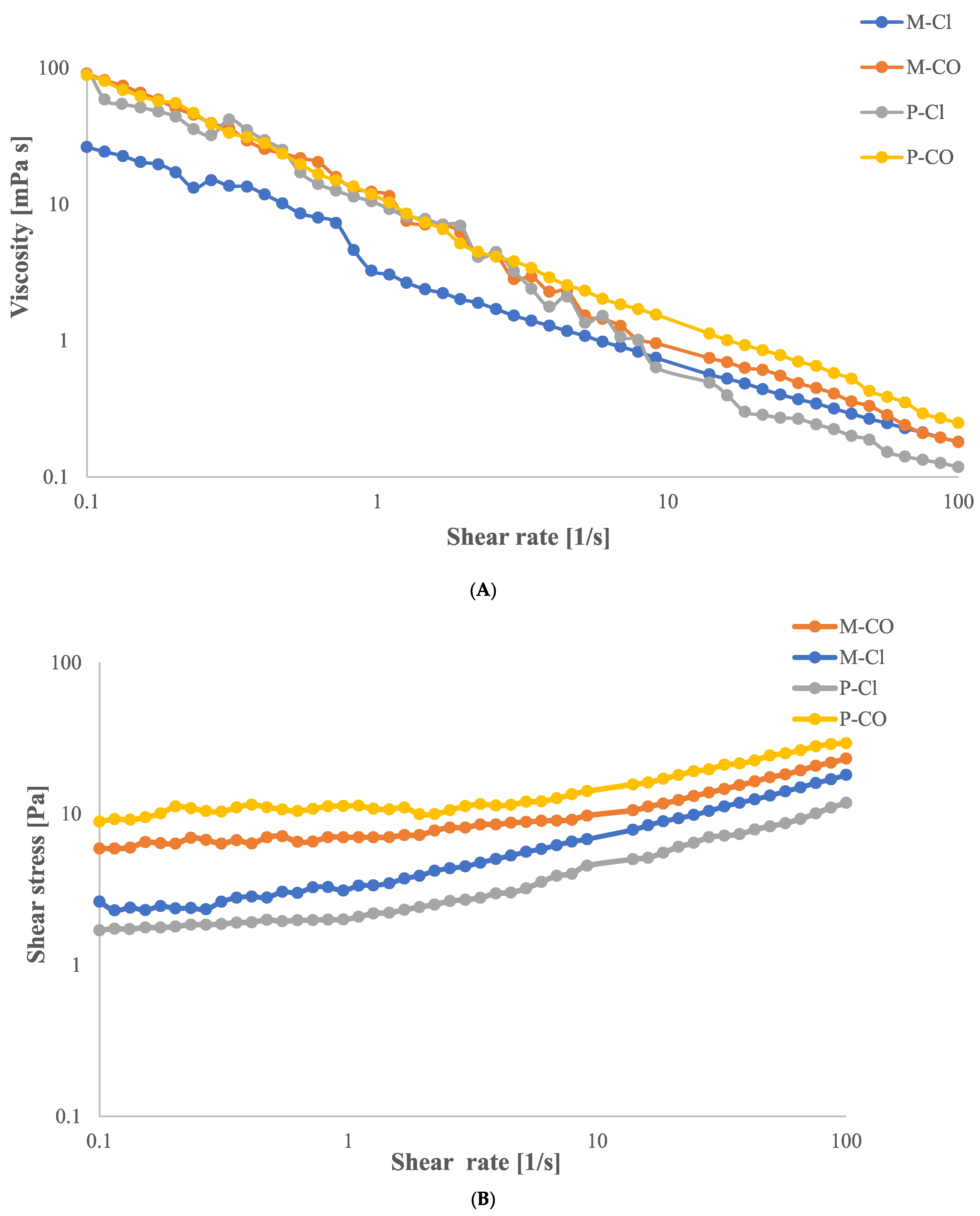
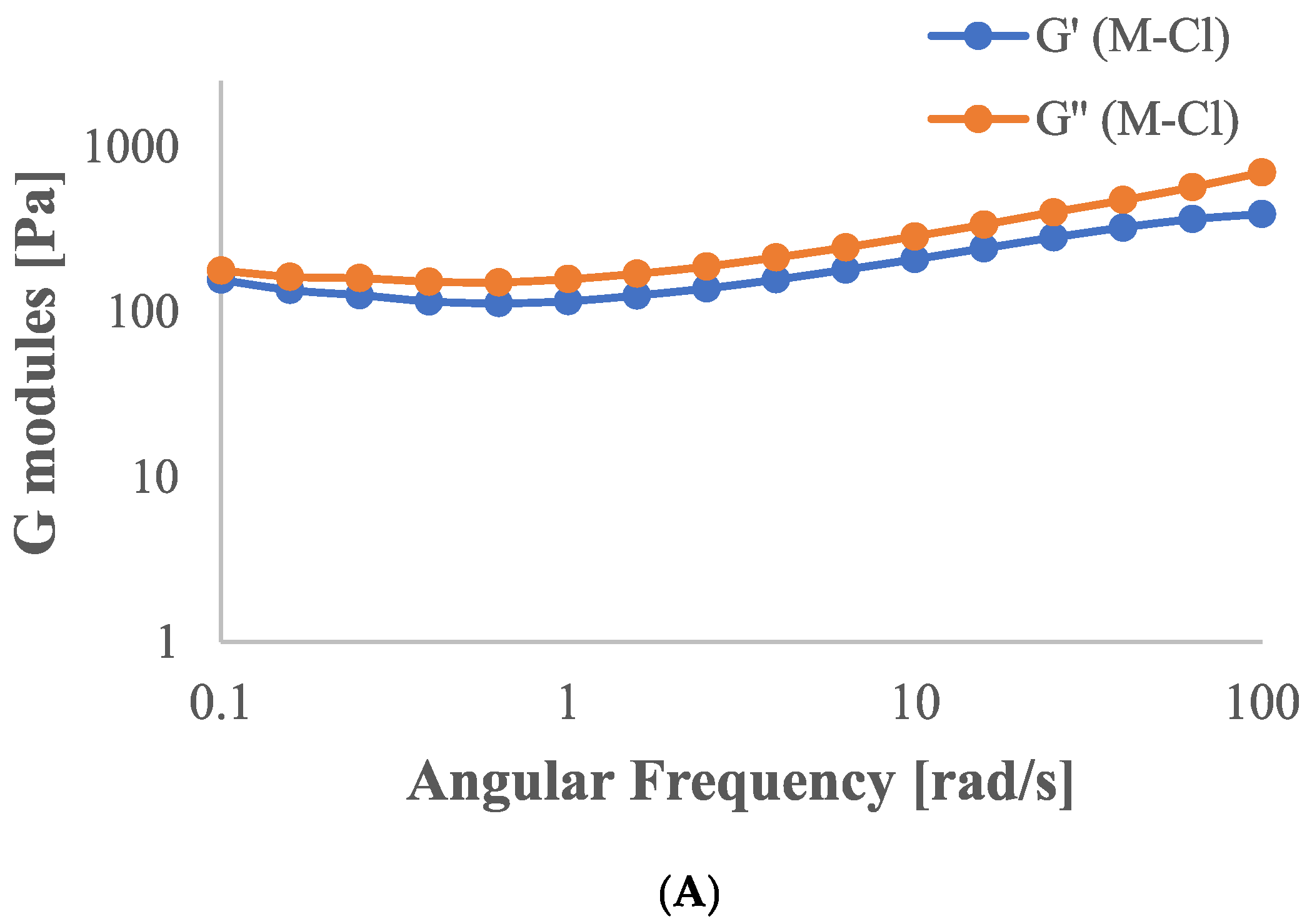
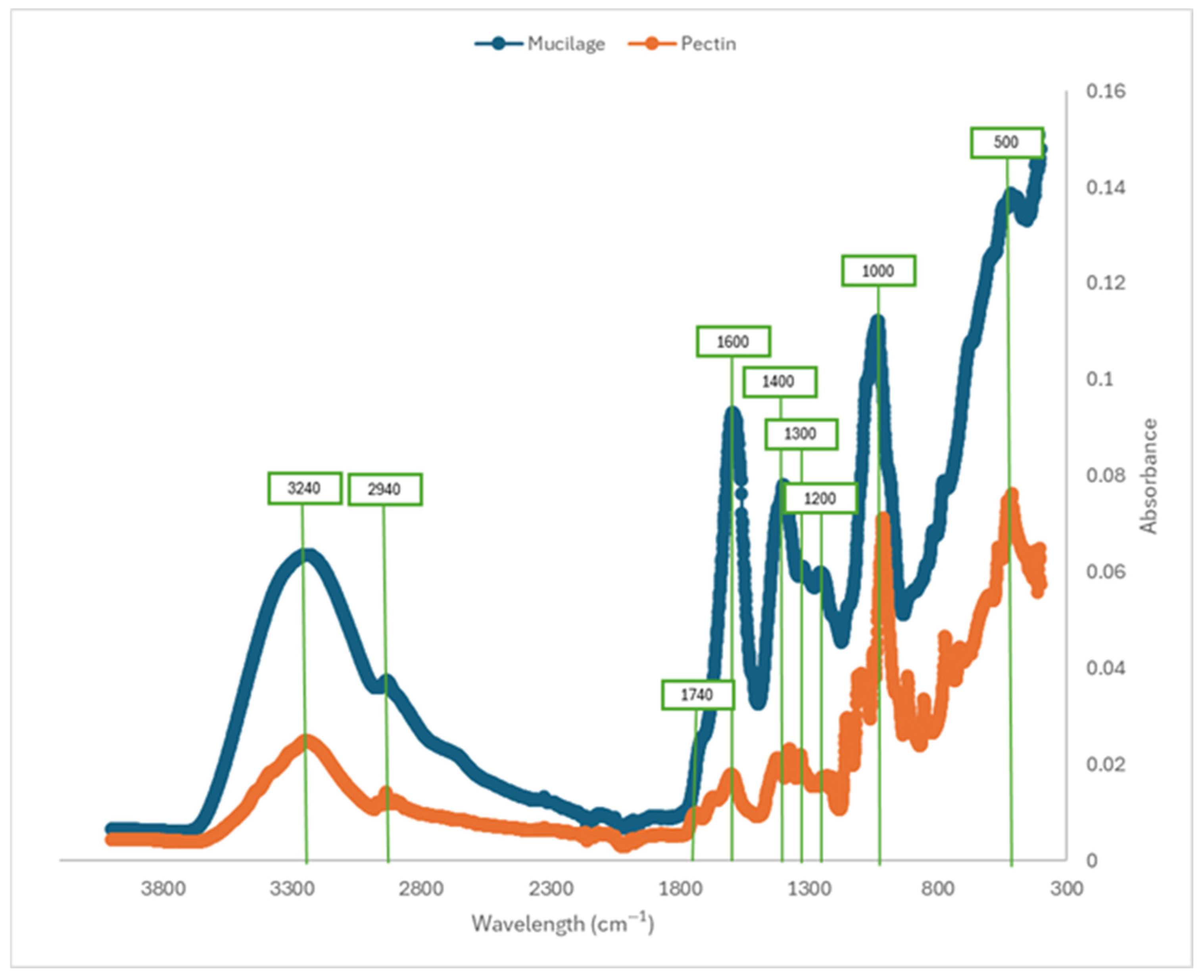
| Sample | Casson Viscosity ηCa [mPa·s] | Casson’s Yield Stress τCa [Pa] |
|---|---|---|
| M-Cl | 64.26 ± 5.2 | 4.01 ± 1.0 |
| M-CO | 52.62 ± 4.8 | 5.50 ± 0.8 |
| P-Cl | 24.40 ± 3.1 | 3.44 ± 1.0 |
| P-CO | 30.87 ± 2.9 | 11.48 ± 2.0 |
| Hydrogels | k′ | k″ | n′ | n″ | tanδ |
|---|---|---|---|---|---|
| M-Cl | 154.88 | 109.65 | 0.28 | 0.34 | 1.38 |
| M-CO | 371.53 | 295.12 | 0.31 | 0.33 | 1.30 |
| P-Cl | 346.74 | 208.93 | 0.18 | 0.26 | 1.89 |
| P-CO | 316.23 | 229.09 | 0.14 | 0.25 | 1.61 |
| Sample | ζ-Potential [mV] |
|---|---|
| Hydrocolloid | |
| Mucilage | −13 ± 2 |
| Pectin | −20 ± 16 |
| Sign. | *** |
| Salt | |
| CaCl2 | −8 ± 3 |
| CaCO3 | −25 ± 11 |
| Sign. | *** |
| Hydrogel samples | |
| M-Cl | −11.5 ± 0.6 b |
| M-CO | −14.8 ± 0.2 c |
| P-Cl | −5.4 ± 0.5 a |
| P-CO | −34.5 ± 0.5 d |
| Sign. | *** |
| Hydrocolloid in water | ζ-Potential [mV] |
| Mucilage | −7.2 ± 0.5 |
| Pectin | −9.9 ± 0.5 |
| Sign. | ** |
| Hydrogel Samples | Δζ-potential [mV] |
| M-Cl | −4.3 ± 0.09 |
| M-CO | −7.6 ± 0.68 |
| Sign. | ** |
| P-Cl | 4.6 ± 0.9 |
| P-CO | −24.6 ± 0.4 |
| Sign. | *** |
| Factor | pH |
|---|---|
| Hydrocolloid | |
| Mucilage | 4.8 ± 0.7 |
| Pectin | 5.4 ± 2.6 |
| Sign. | *** |
| Salt | |
| CaCl2 | 3.6 ± 0.7 |
| CaCO3 | 6.6 ± 1.3 |
| Sign. | *** |
| Hydrogel Samples | |
| M-Cl | 4.22 ± 0.02 c |
| M-CO | 5.4 ± 0.3 b |
| P-Cl | 2.95 ± 0.03 d |
| P-CO | 7.77 ± 0.01 a |
| Sign. | *** |
| Sample | Z-Average (d·nm) | PDI | Casson Viscosity [mPa·s] | Z-Potential |
|---|---|---|---|---|
| P-Cl | 517.5 | 0.595 | 65 ± 16 | −5.37 ± 0.53 |
| P-CO | 1243 | 0.427 | 49 ± 21 | −34.50 ± 0.50 |
| M-Cl | 563.7 | 0.732 | 20 ± 11 | −11.50 ± 0.56 |
| M-CO | 309.8 | 0.99 | 42 ± 12 | −14.83 ± 0.21 |
| Variable/Factor | aW | |
|---|---|---|
| Hydrocolloid | ||
| Mucilage | 0.93 ± 0.02 | |
| Pectin | 0.95 ± 0.01 | |
| Sign. | * | |
| Salt | ||
| CaCl2 | 0.94 ± 0.02 | |
| CaCO3 | 0.94 ± 0.02 | |
| Sign. | ns | |
| Hydrogel Samples | aW | Released Mass [% w/w] |
| P-Cl | 0.95 ± 0.01 | <LOQ b |
| P-CO | 0.95 ± 0.02 | <LOQ b |
| M-Cl | 0.92 ± 0.02 | 54 ± 3 a |
| M-CO | 0.93 ± 0.01 | <LOQ b |
| Sign. | ns | *** |
| Sample Coding | Water | Sucrose | Gelling Agent | Calcium Salt (1%) |
|---|---|---|---|---|
| (P-Cl) | 25 mL | 10 g | 1.0 g pectin | 0.25 g CaCl2 |
| (P-CO) | 25 mL | 10 g | 1.0 g pectin | 0.25 g CaCO3 |
| (M-Cl) | 25 mL | 10 g | 5.0 g mucilage | 0.25 g CaCl2 |
| (M-CO) | 25 mL | 10 g | 5.0 g mucilage | 0.25 g CaCO3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torregrossa, F.; Pollon, M.; Liguori, G.; Gargano, F.; Albanese, D.; Malvano, F.; Cinquanta, L. Rheological and Physical Properties of Mucilage Hydrogels from Cladodes of Opuntia ficus-indica: Comparative Study with Pectin. Gels 2025, 11, 556. https://doi.org/10.3390/gels11070556
Torregrossa F, Pollon M, Liguori G, Gargano F, Albanese D, Malvano F, Cinquanta L. Rheological and Physical Properties of Mucilage Hydrogels from Cladodes of Opuntia ficus-indica: Comparative Study with Pectin. Gels. 2025; 11(7):556. https://doi.org/10.3390/gels11070556
Chicago/Turabian StyleTorregrossa, Federica, Matteo Pollon, Giorgia Liguori, Francesco Gargano, Donatella Albanese, Francesca Malvano, and Luciano Cinquanta. 2025. "Rheological and Physical Properties of Mucilage Hydrogels from Cladodes of Opuntia ficus-indica: Comparative Study with Pectin" Gels 11, no. 7: 556. https://doi.org/10.3390/gels11070556
APA StyleTorregrossa, F., Pollon, M., Liguori, G., Gargano, F., Albanese, D., Malvano, F., & Cinquanta, L. (2025). Rheological and Physical Properties of Mucilage Hydrogels from Cladodes of Opuntia ficus-indica: Comparative Study with Pectin. Gels, 11(7), 556. https://doi.org/10.3390/gels11070556










