Radiation-Induced Synthesis of Polymer Networks Based on Thermoresponsive Ethylene Glycol Propylene Glycol Monomers
Abstract
1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation
4.3. UV-Vis Spectroscopy
4.4. Sol-Gel Conversion
4.5. FTIR Spectroscopy
4.6. Swelling Measurement
4.7. Calorimetric Measurement
4.8. Scanning Electron Microscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EG | Ethylene glycol |
| PG | Propylene glycol |
| EGPG | Ethylene glycol propylene glycol |
| PEG | Poly(ethylene glycol) |
| PPG | Poly(propylene glycol) |
| EG6MA | Ethylene glycol6 methacrylate |
| EG6PG3MA | (Ethylene glycol6-propylene glycol3) methacrylate |
| PG6EG3MA | (Propylene glycol6-ethylene glycol3) methacrylate |
| PG5MA | Propylene glycol5 methacrylate |
| OEGMA | Oligo(ethylene glycol) methacrylate |
| OPGMA | Oligo(propylene glycol) methacrylate |
| P(EGPG)MA | Poly(ethylene glycol-propylene glycol) methacrylate |
| PEG6MA | Poly(ethylene glycol)6 methacrylate |
| PPG5MA | Poly(propylene glycol)5 methacrylate |
| PEG6PG3MA | Poly((ethylene glycol)6-(propylene glycol)3) methacrylate |
| PPG6EG3MA | Poly((propylene glycol)6-(ethylene glycol)3) methacrylate |
| CP | Cloud point |
| LCST | Lower critical solution temperature |
| VPTT | Volume phase transition temperature |
| Qe | Swelling degree |
| SEM | Scanning electron microscopy |
| DSC | Differential Scanning Calorimetry |
| FTIR | Fourier Transform Infrared |
| HEMA | 2-Hydroxyethyl methacrylate |
| IA | Itaconic acid |
| EGDMA | Ethylene glycol dimethacrylate |
References
- Li, Z.; Zhou, Y.; Li, T.; Zhang, J.; Tian, H. Stimuli-responsive hydrogels: Fabrication and biomedical applications. View 2022, 3, 20200112. [Google Scholar] [CrossRef]
- Bakirov, A.; Kopishev, E.; Kadyrzhan, K.; Donbaeva, E.; Zhaxybayeva, A.; Duisembiyev, M.; Suyundikova, F.; Suleimenov, I. The method of direct and reverse phase portraits as a tool for systematizing the results of studies of phase transitions in solutions of thermosensitive polymers. Gels 2024, 10, 395. [Google Scholar] [CrossRef]
- Constantin, M.; Cristea, M.; Ascenzi, P.; Fundueanu, G. Lower critical solution temperature versus volume phase transition temperature in thermoresponsive drug delivery systems. Express Polym. Lett. 2011, 5, 839–848. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, T.; Guo, R.; Li, C.; Lu, C.; Yang, G.; Nie, J.; Wang, F.; Yang, X.; Chen, Z. Injectable and degradable PEG hydrogel with antibacterial performance for promoting wound healing. ACS Appl. Bio Mater. 2021, 4, 2769–2780. [Google Scholar] [CrossRef]
- Yesildag, C.; Ouyang, Z.; Zhang, Z.; Lensen, M.C.; Lensen, M.C. Micro-patterning of PEG-based hydrogels with gold nanoparticles using a reactive micro-contact-printing approach. Front. Chem. 2019, 6, 667. [Google Scholar] [CrossRef]
- Smeets, N.M.B.; Bakaic, E.; Patenaude, M.; Hoare, T. Injectable and tunable poly(ethylene glycol) analogue hydrogels based on poly(oligoethylene glycol methacrylate). Chem. Commun. 2014, 50, 3306–3309. [Google Scholar] [CrossRef] [PubMed]
- Smeets, N.M.B.; Bakaic, E.; Patenaude, M.; Hoare, T. Injectable poly(oligoethylene glycol methacrylate)-based hydrogels with tunable phase transition behaviours: Physicochemical and biological responses. Acta Biomater. 2014, 10, 4143–4155. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Nuttelman, C.R.; Anseth, K.S. The effects of crosslinking density on cartilage formation in photocrosslinkable hydrogels. Biomed. Sci. Instrum. 1999, 35, 309–314. [Google Scholar]
- Elisseeff, J.; McIntosh, W.; Anseth, K.; Riley, S.; Ragan, P.; Langer, R. Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks. J. Biomed. Mater. Res. A 2000, 51, 164–171. [Google Scholar] [CrossRef]
- Al-Gunaid, T.A.; Krupa, I.; Ouederni, M.; Krishnamoorthy, S.K.; Popelka, A. Enhancement of adhesion characteristics of low-density polyethylene using atmospheric plasma initiated-grafting of polyethylene glycol. Polymers 2021, 13, 1309. [Google Scholar] [CrossRef]
- Sun, S.T.; Wu, P.Y. On the thermally reversible dynamic hydration behavior of oligo(ethylene glycol) methacrylate-based polymers in water. Macromolecules 2013, 46, 236–246. [Google Scholar] [CrossRef]
- Cho, J.Y.; Lee, J.; Gutowska, A.; Tarasevich, B.J.; Sohn, Y.S.; Jeong, B. pH/temperature-sensitive poly(aspartic acid)-g-poly(propylene glycol). J. Drug Deliv. Sci. Tech. 2006, 16, 71–76. [Google Scholar] [CrossRef]
- Miladinovic, R.Z.; Micic, M.; Suljovrujic, E. Temperature/pH dual responsive OPGMA based copolymeric hydrogels prepared by gamma radiation: An optimisation study. J. Polym. Res. 2016, 23. [Google Scholar] [CrossRef]
- Barron, V.; Killion, J.A.; Pilkington, L.; Burke, G.; Geever, L.M.; Lyons, J.G.; McCullagh, E.; Higginbotham, C.L. Development of chemically cross-linked hydrophilic–hydrophobic hydrogels for drug delivery applications. Eur. Polym. J. 2016, 75, 25–35. [Google Scholar] [CrossRef]
- Burts, K.; Plisko, T.; Makarava, M.; Krasnova, M.; Penkova, A.; Ermakov, S.; Grigoryev, E.; Komolkin, A.; Bildyukevich, A. The effect of PEG-content and molecular weight of PEG-PPG-PEG block copolymers on the structure and performance of polyphenylsulfone ultra- and nanofiltration membranes. J. Membr. Sci. 2024, 704, 122869. [Google Scholar] [CrossRef]
- Suljovrujic, E.; Micic, M. Smart poly(oligo(propylene glycol) methacrylate) hydrogel prepared by gamma radiation. N. Instrum. Meth. B 2015, 342, 206–214. [Google Scholar] [CrossRef]
- Suljovrujic, E.; Krstic, M.; Rogic Miladinovic, Z.; Petrovic, S.; Leskovac, A.; Stamboliev, G. Optimization of thermoresponsive hydrogels based on oligomers with lower critical solution temperature (LCST) far below/above physiological temperatures for biomedical applications. React. Funct. Polym. 2023, 189, 105612. [Google Scholar] [CrossRef]
- Suljovrujic, E.; Rogic Miladinovic, Z.; Krstic, M. Swelling properties and drug release of new biocompatible POEGOPGMA hydrogels with VPTT near to the human body temperature. Polym. Bull. 2021, 78, 2405–2425. [Google Scholar] [CrossRef]
- Tomic, S.L.J.; Micic, M.M.; Filipovic, J.M.; Suljovrujic, E.H. Synthesis, characterization and controlled release of cephalexin drug from smart poly(2-hydroxyethyl methacrylate/poly(alkylene glycol)(meth)acrylates hydrogels. Chem. Eng. J. 2010, 160, 801–809. [Google Scholar] [CrossRef]
- Cao, H.; Guo, F.; Chen, Z.; Kong, X.Z. Preparation of thermoresponsive polymer nanogels of oligo(ethylene glycol) diacrylate-methacrylic acid and their property characterization. Nanoscale Res. Lett. 2018, 13, 209. [Google Scholar] [CrossRef]
- Dorey, S.; Pahl, I.; Uettwiller, I.; Priebe, P.; Hauk, A. Theoretical and practical considerations when selecting solvents for use in extractables studies of polymeric contact materials in single-use systems applied in the production of biopharmaceuticals. Ind. Eng. Chem. Res. 2018, 57, 7077–7089. [Google Scholar] [CrossRef]
- Nakamura, K.; Yano, K.; Ida, S.; Ida, D.; Ryoki, A.; Yamamoto, S.; Terashima, T.; Kanaoka, S. Solution properties of amphiphilic alternating copolymers of N-ethylmaleimide and 2-hydroxyethyl vinyl ether: Thermoresponsiveness in alcohols and cosolvency phenomenon in binary ethanol/water solutions. Macromolecules 2024, 57, 4439–4447. [Google Scholar] [CrossRef]
- Parke, S.A.; Birch, G.G. Solution properties of ethanol in water. Food Chem. 1999, 67, 241–246. [Google Scholar] [CrossRef]
- Kirschner, J.; Gomes, A.H.A.; Marinho, R.R.T.; Björneholm, O.; Ågren, H.; Carravetta, V.; Ottosson, N.; Brito, A.N.d.; Bakker, H.J. The molecular structure of the surface of water–ethanol mixtures. Phys. Chem. Chem. Phys. 2021, 23, 11568–11578. [Google Scholar] [CrossRef]
- Gereben, O.; Pusztai, L. Investigation of the structure of ethanol–water mixtures by molecular dynamics simulation I: Analyses concerning the hydrogen-bonded pairs. J. Phys. Chem. B 2015, 119, 3070–3084. [Google Scholar] [CrossRef] [PubMed]
- Dolenko, T.A.; Burikov, S.A.; Dolenko, S.A.; Efitorov, A.O.; Plastinin, I.V.; Yuzhakov, V.I.; Patsaeva, S.V. Raman spectroscopy of water−ethanol solutions: The estimation of hydrogen bonding energy and the appearance of clathrate-like structures in solutions. J. Phys. Chem. A 2015, 119, 10806–10815. [Google Scholar] [CrossRef]
- Koga, Y.; Nishikawa, K.; Westh, P. “Icebergs” or no “Icebergs” in aqueous alcohols?: Composition-dependent mixing schemes. J. Phys. Chem. A 2004, 108, 3873–3877. [Google Scholar] [CrossRef]
- Säckel, C.; von Klitzing, R.; Vogel, M. 2H and 17O NMR studies of solvent dynamics related to the cononsolvency of poly(N-isopropyl acrylamide) in ethanol–water mixtures. Soft Matter 2025, 21, 2738–2747. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, R.; Thijs, H.M.L.; Wouters, D.; Hoeppener, S.; Schubert, U.S. Tuning solution polymer properties by binary water–ethanol solvent mixtures. Soft Matter 2008, 4, 103–107. [Google Scholar] [CrossRef]
- Suljovrujic, E.; Rogic Miladinovic, Z.; Micic, M.; Suljovrujic, D.; Milicevic, D. The influence of monomer/solvent feed ratio on POEGDMA thermoresponsive hydrogels: Radiation-induced synthesis, swelling properties and VPTT. Radiat. Phys. Chem. 2019, 158, 37–45. [Google Scholar] [CrossRef]
- Jung, K.I.; Lee, D.G.; Bong, K.W.; Noh, S.M.; Um, M.S.; Choi, W.J.; Kim, B.; Jung, H.W. Effects of solvents on rheological and crosslinking properties of photo-polymerized poly(ethylene glycol) hydrogels. Korean J. Chem. Eng. 2017, 34, 1517–1523. [Google Scholar] [CrossRef]
- Ravi, N.; Mitra, A.; Hamilton, P.; Horkay, F. Characterization of the network properties of poly(ethylene glycol)–acrylate hydrogels prepared by variations in the ethanol–water solvent composition during crosslinking copolymerization. J. Polym. Sci. Part B Polym. 2002, 40, 2677–2684. [Google Scholar] [CrossRef]
- Amante, C.; Falcone, G.; Aquino, R.P.; Russo, P.; Nicolais, L.; Del Gaudio, P. In situ hydrogel formulation for advanced wound dressing: Influence of co-solvents and functional excipient on tailored alginate–pectin–chitosan blend gelation kinetics, adhesiveness, and performance. Gels 2024, 10, 3. [Google Scholar] [CrossRef]
- Zhao, Y.; Kremer, K. The effects of proline isomerization on the solvation behavior of elastin-like polypeptides in water–ethanol mixtures. Macromol. Rapid Commun. 2022, 43, 2100907. [Google Scholar] [CrossRef]
- Yang, J.; Rao, L.; Wang, Y.; Zhao, Y.; Liu, D.; Wang, Z.; Fu, L.; Wang, Y.; Yang, X.; Li, Y.; et al. Recent advances in smart hydrogels prepared by ionizing radiation technology for biomedical applications. Polymers 2022, 14, 4377. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Islam, M.; Hasan, M.K.; Nam, K.-W. A comprehensive review of radiation-induced hydrogels: Synthesis, properties, and multidimensional applications. Gels 2024, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.-O.; Park, J.-S.; Kim, Y.-A.; Yang, S.-J.; Jeong, S.-I.; Lee, J.-Y.; Lim, Y.-M. Gamma ray-induced polymerization and cross-linking for optimization of PPy/PVP hydrogel as biomaterial. Polymers 2020, 12, 111. [Google Scholar] [CrossRef]
- Ahmad, Z.; Salman, S.; Khan, S.A.; Amin, A.; Rahman, Z.U.; Al-Ghamdi, Y.O.; Akhtar, K.; Bakhsh, E.M.; Khan, S.B. Versatility of hydrogels: From synthetic strategies, classification, and properties to biomedical applications. Gels 2022, 8, 167. [Google Scholar] [CrossRef]
- Bebis, K.; Jones, M.W.; Haddleton, D.M.; Gibson, M.I. Thermoresponsive behaviour of poly[(oligo(ethyleneglycol methacrylate)]s and their protein conjugates: Importance of concentration and solvent system. Polym. Chem.-UK 2011, 2, 975–982. [Google Scholar] [CrossRef]
- Roufegari-Nejhad, E.; Sirousazar, M.; Abbasi-Chiyaneh, V.; Kheiri, F. Removal of methylene blue from aqueous solutions using poly(vinyl alcohol)/montmorillonite nanocomposite hydrogels: Taguchi optimization. J. Polym. Environ. 2019, 27, 2239–2249. [Google Scholar] [CrossRef]
- Kwon, K.-W.; Park, M.J.; Hwang, J.; Char, K. Effects of alcohol addition on gelation in aqueous solution of poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) triblock copolymer. Polym. J. 2001, 33, 404–410. [Google Scholar] [CrossRef]
- Chaibundit, C.; Ricardo, N.M.P.S.; Ricardo, N.M.P.S.; Muryn, C.A.; Madec, M.-B.; Yeates, S.G.; Booth, C. Effect of ethanol on the gelation of aqueous solutions of Pluronic F127. J. Colloid Interface Sci. 2010, 351, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Lutz, J.F. Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. Pol. Chem. 2008, 46, 3459–3470. [Google Scholar] [CrossRef]
- Ma, X.; Du, P.; Niu, B.; Zhang, J.; Yong, H.; He, X. LCST/UCST transition of acrylate copolymer with cosolvency behaviors in alcohol aqueous solutions. J. Mol. Liq. 2023, 375, 121305. [Google Scholar] [CrossRef]
- Bäckström, S.; Benavente, J.; Berg, R.; Stibius, K.; Larsen, M.; Bohr, H.; Helix-Nielsen, C. Tailoring properties of biocompatible PEG-DMA hydrogels with UV light. Mater. Sci. Appl. 2012, 03, 425–431. [Google Scholar] [CrossRef]
- Palencia, M. Functional transformation of Fourier-transform mid-infrared spectrum for improving spectral specificity by simple algorithm based on wavelet-like functions. J. Adv. Res. 2018, 14, 53–62. [Google Scholar] [CrossRef]
- Ciarleglio, G.; Toto, E.; Santonicola, M.G. Conductive and thermo-responsive composite hydrogels with poly(N-isopropylacrylamide) and carbon nanotubes fabricated by two-step photopolymerization. Polymers 2023, 15, 1022. [Google Scholar] [CrossRef]
- Radecki, M.; Spěváček, J.; Zhigunov, A.; Sedláková, Z.; Hanyková, L. Temperature-induced phase transition in hydrogels of interpenetrating networks of poly(N-isopropylacrylamide) and polyacrylamide. Eur. Polym. J. 2015, 68, 68–79. [Google Scholar] [CrossRef]
- Manek, E.; Tombácz, E.; Geissler, E.; László, K. Search for the origin of discrepancies in osmotic measurements of the PNIPAM—Water system. Period. Polytech. Chem. Eng. 2017, 61, 39–50. [Google Scholar] [CrossRef]
- Czakkel, O.; Berke, B.; László, K. Effect of graphene-derivatives on the responsivity of PNIPAM-based thermosensitive nanocomposites—A review. Eur. Polym. J. 2019, 116, 106–116. [Google Scholar] [CrossRef]
- Ghanbari, E.; Picken, S.J.; van Esch, J.H. Analysis of differential scanning calorimetry (DSC): Determining the transition temperatures, and enthalpy and heat capacity changes in multicomponent systems by analytical model fitting. J. Therm. Anal. Calorim. 2023, 148, 12393–12409. [Google Scholar] [CrossRef]
- Krstic, M.; Rogic Miladinovic, Z.; Barudzija, T.; Mladenovic, A.; Suljovrujic, E. Stimuli-responsive copolymeric hydrogels based on oligo(ethylene glycol) dimethacrylate for biomedical applications: An optimisation study of pH and thermoresponsive behaviour. React. Funct. Polym. 2022, 170, 105140. [Google Scholar] [CrossRef]
- Huaman, M.A.L.; Vega-Chacón, J.; Quispe, R.I.H.; Negrón, A.C.V. Synthesis and swelling behaviors of poly(2-hydroxyethyl methacrylate-co-itaconic acid) and poly(2-hydroxyethyl methacrylate-co-sodium itaconate) hydrogels as potential drug carriers. Results Chem. 2023, 5, 100917. [Google Scholar] [CrossRef]
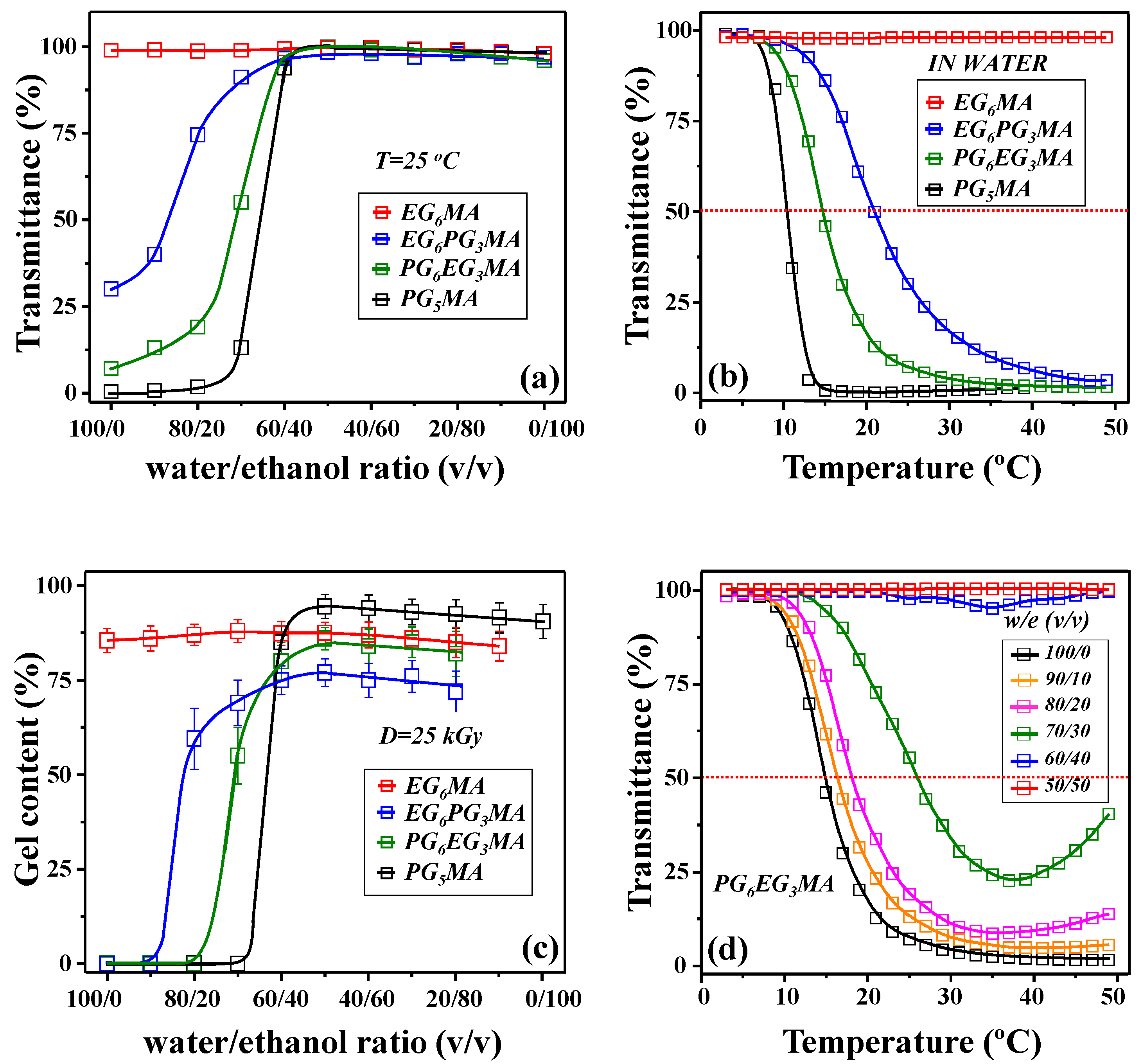
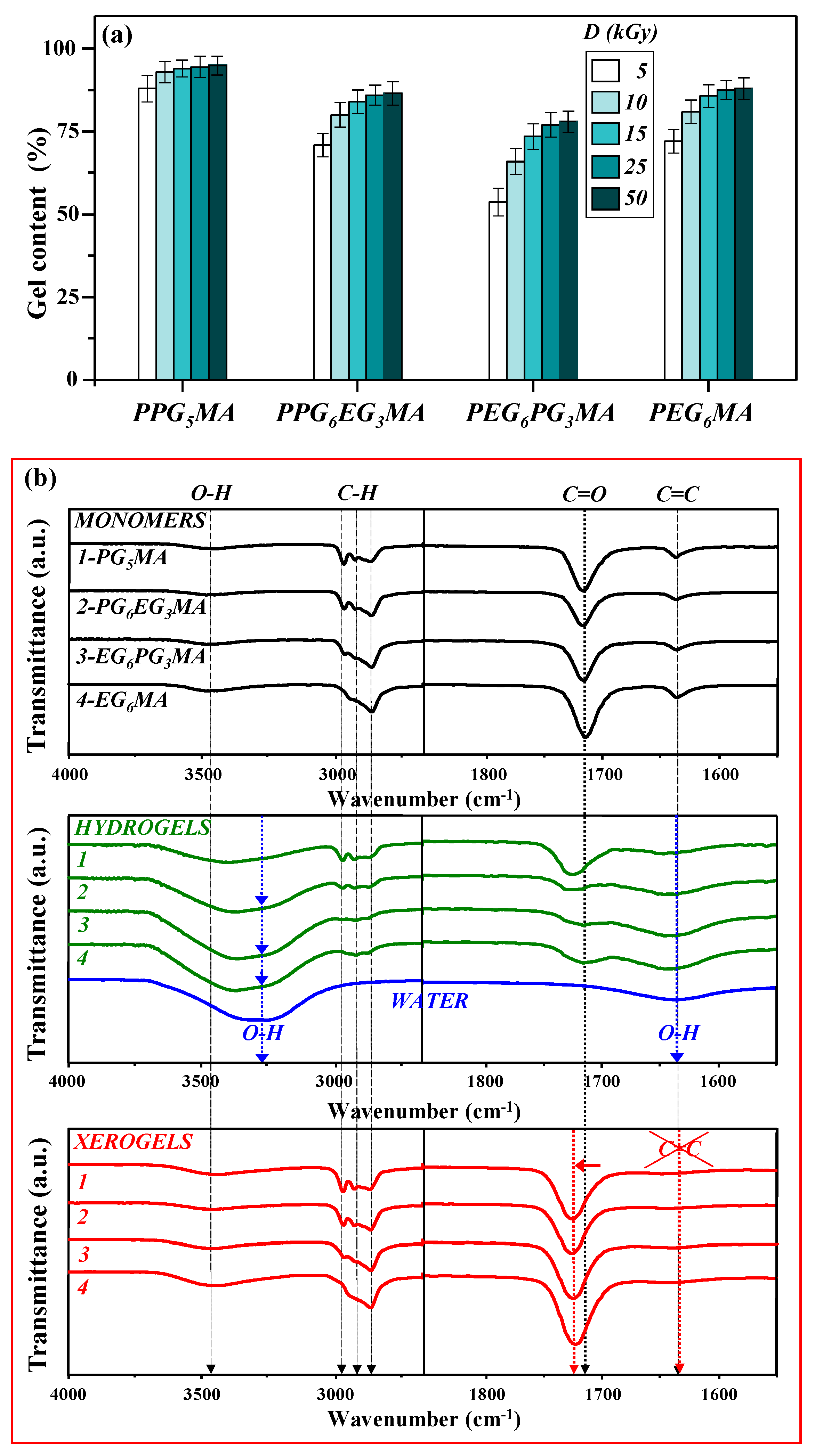
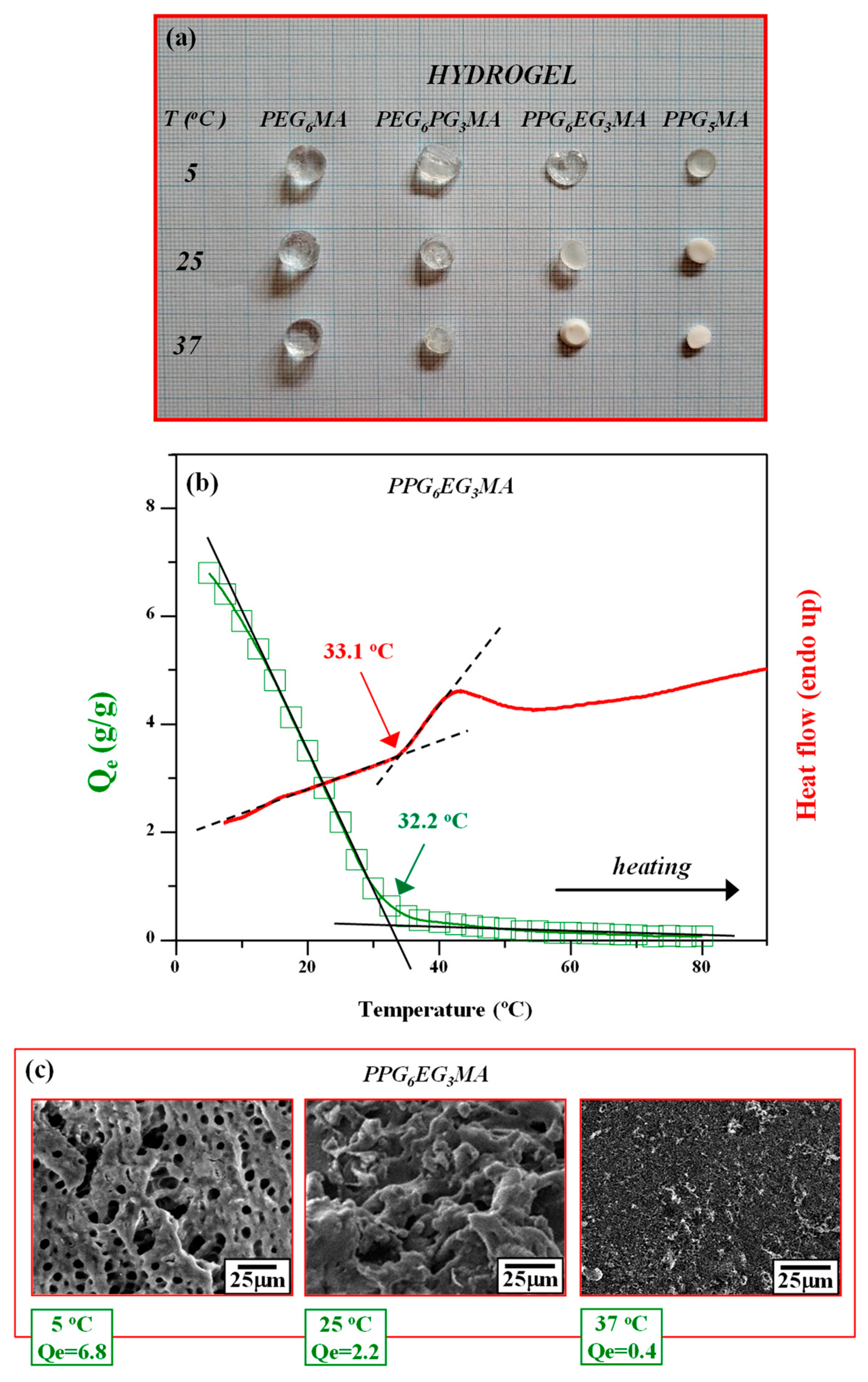
| Monomer/ Hydrogel | LCST (°C) | Qe (g/g) | VPTT (°C) | |||
|---|---|---|---|---|---|---|
| 5 °C | 25 °C | 37 °C | Swelling (Inf. Point) | Calorimetry (Onset Point) | ||
| (P)PG5MA | 10.4 | 2.7 ± 0.4 | 0.14 ± 0.04 | 0.1 ± 0.03 | 13.4 | 13.7 |
| (P)PG6EG3MA | 14.8 | 6.8 ± 0.6 | 2.2 ± 0.3 | 0.4 ± 0.1 | 32.2 | 33.1 |
| (P)EG6PG3MA | 21.1 | 10.3 ± 0.8 | 7.5 ± 0.6 | 4.0 ± 0.4 | 47.3 | 48.3 |
| (P)EG6MA | - | 9.3 ± 0.7 | 8 ± 0.6 | 7.1 ± 0.6 | 70.3 | 72.1 |
| Chemical Structure | Full Name | |
|---|---|---|
| Short Label | Mw (g/mol) | |
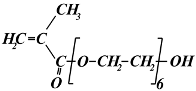 | (ethylene glycol)6 methacrylate | |
| EG6MA | 360 | |
 | (ethylene glycol)6-(propylene glycol)3 methacrylate | |
| EG6PG3MA | 524 | |
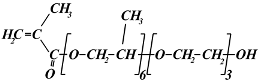 | (propylene glycol)6-(ethylene glycol)3 methacrylate | |
| PG6EG3MA | 566 | |
 | (propylene glycol)5 methacrylate | |
| PG5MA | 375 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stolic, A.; Rogic Miladinovic, Z.; Krstic, M.; Stamboliev, G.; Petrovic, V.; Suljovrujic, E. Radiation-Induced Synthesis of Polymer Networks Based on Thermoresponsive Ethylene Glycol Propylene Glycol Monomers. Gels 2025, 11, 488. https://doi.org/10.3390/gels11070488
Stolic A, Rogic Miladinovic Z, Krstic M, Stamboliev G, Petrovic V, Suljovrujic E. Radiation-Induced Synthesis of Polymer Networks Based on Thermoresponsive Ethylene Glycol Propylene Glycol Monomers. Gels. 2025; 11(7):488. https://doi.org/10.3390/gels11070488
Chicago/Turabian StyleStolic, Andjelka, Zorana Rogic Miladinovic, Maja Krstic, Georgi Stamboliev, Vladimir Petrovic, and Edin Suljovrujic. 2025. "Radiation-Induced Synthesis of Polymer Networks Based on Thermoresponsive Ethylene Glycol Propylene Glycol Monomers" Gels 11, no. 7: 488. https://doi.org/10.3390/gels11070488
APA StyleStolic, A., Rogic Miladinovic, Z., Krstic, M., Stamboliev, G., Petrovic, V., & Suljovrujic, E. (2025). Radiation-Induced Synthesis of Polymer Networks Based on Thermoresponsive Ethylene Glycol Propylene Glycol Monomers. Gels, 11(7), 488. https://doi.org/10.3390/gels11070488








