Gel Electrophoresis of an Oil Drop
Abstract
1. Introduction
2. Results and Discussion
2.1. Fundamental Electrokinetic Equations
2.2. Weak-Field Approximation
2.3. General Mobility Expression
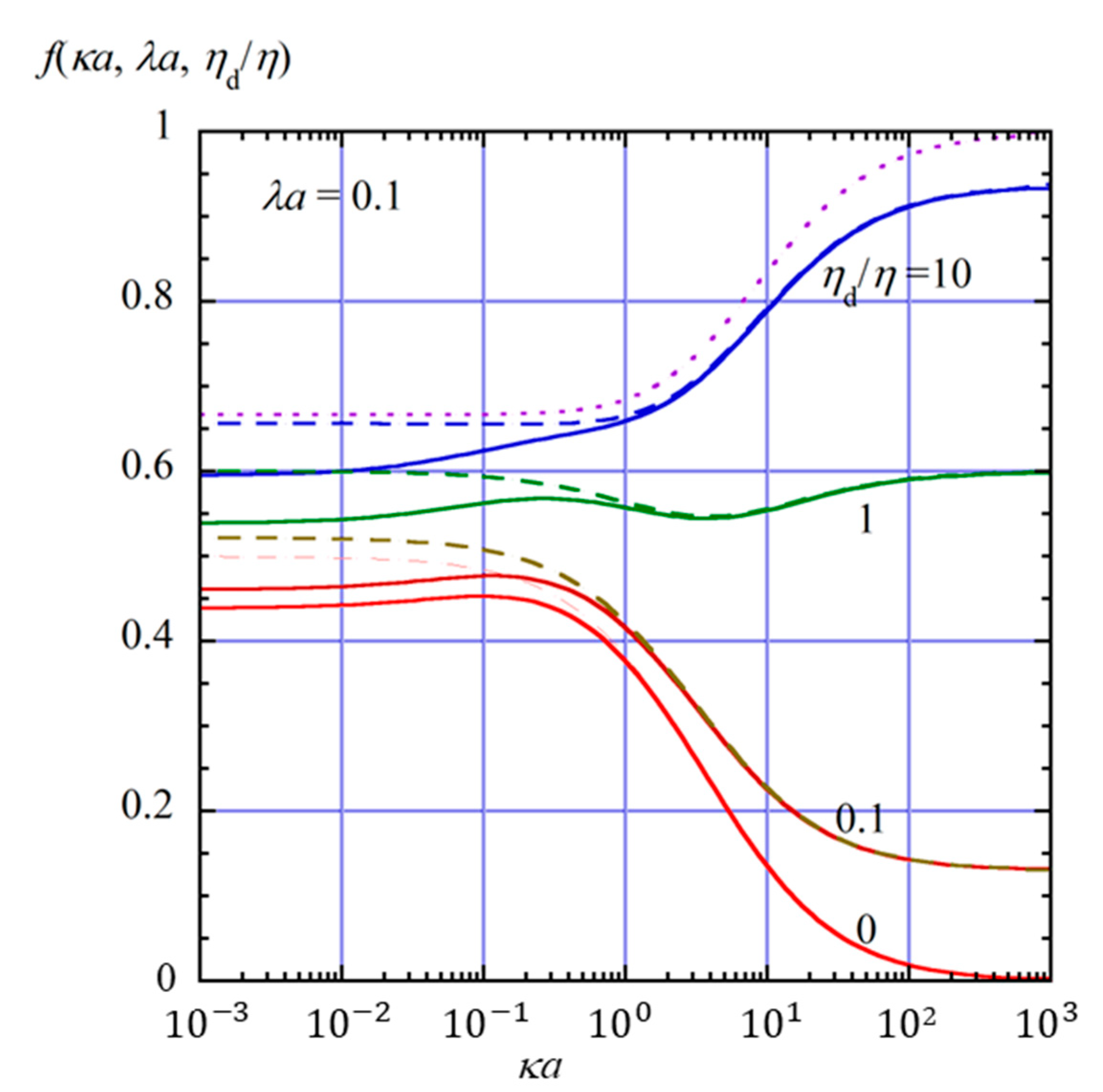
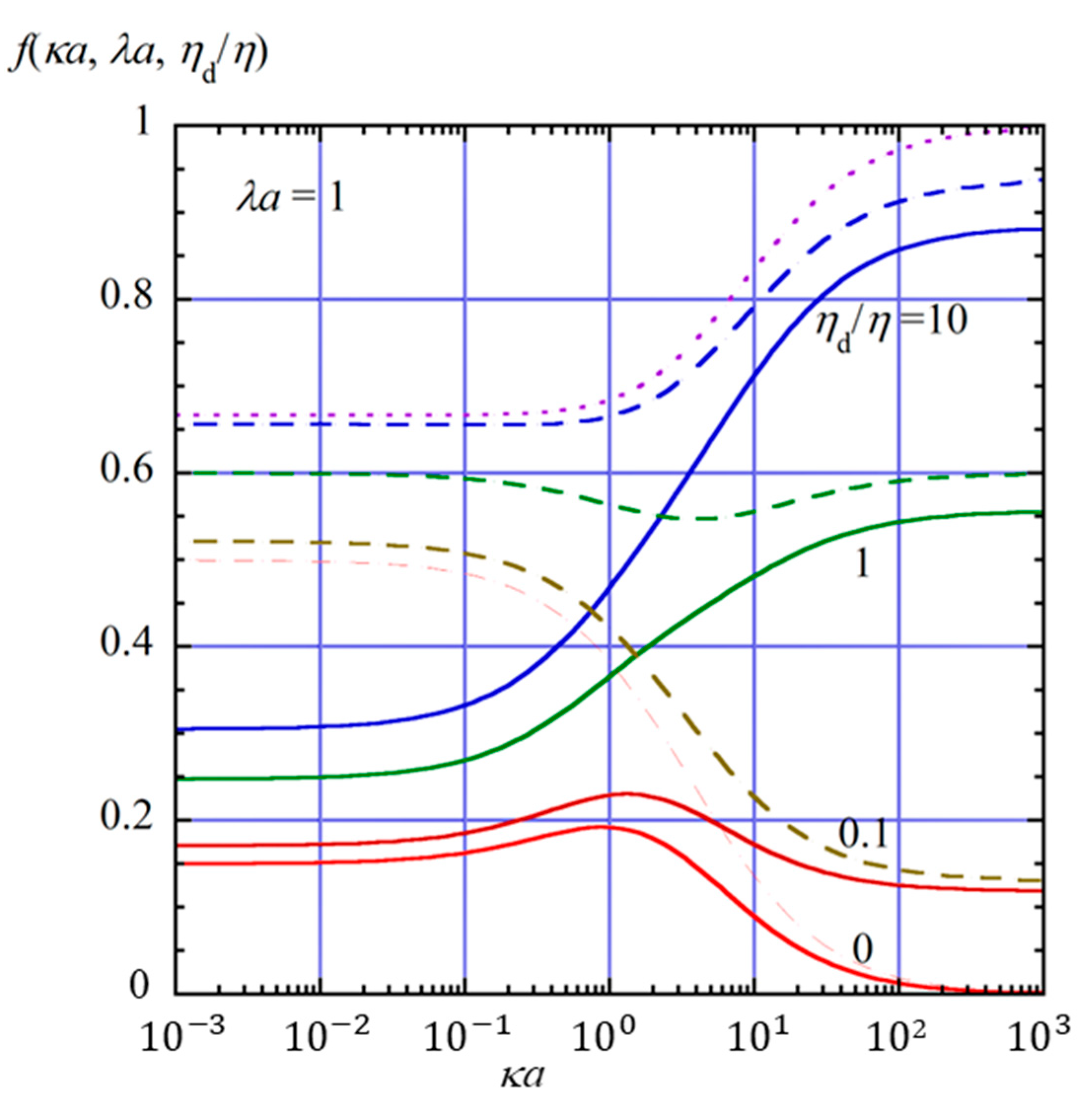
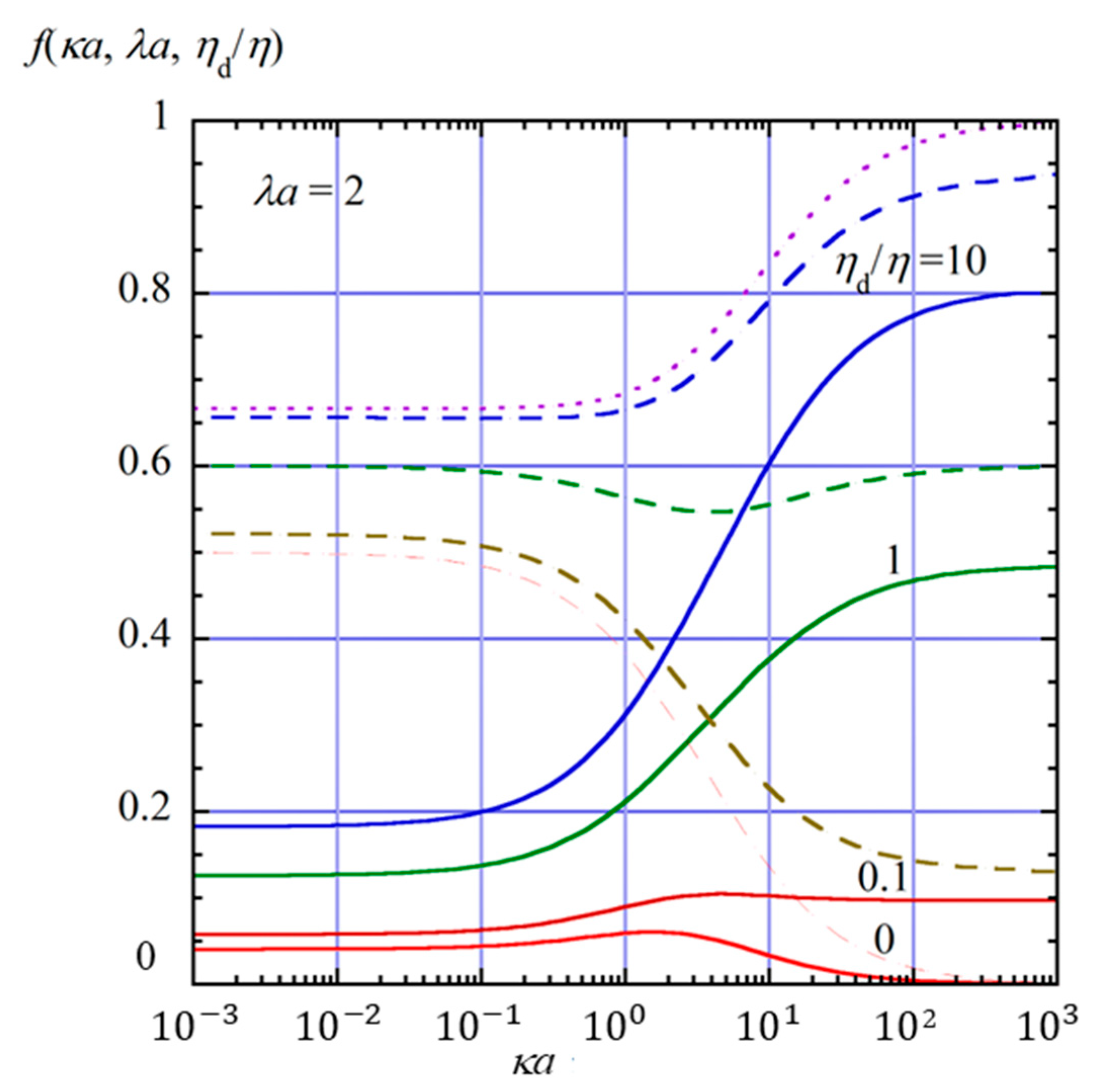
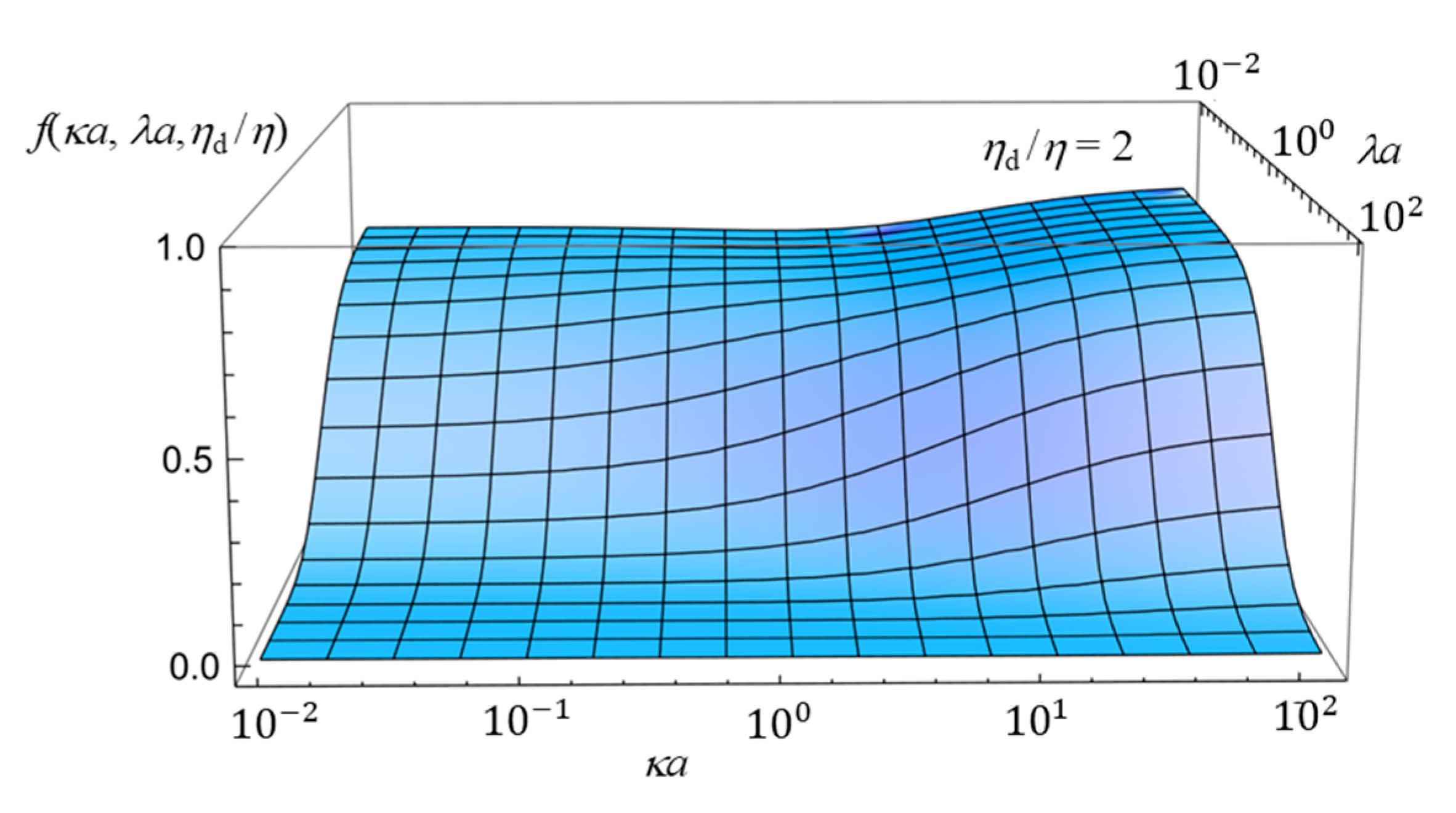
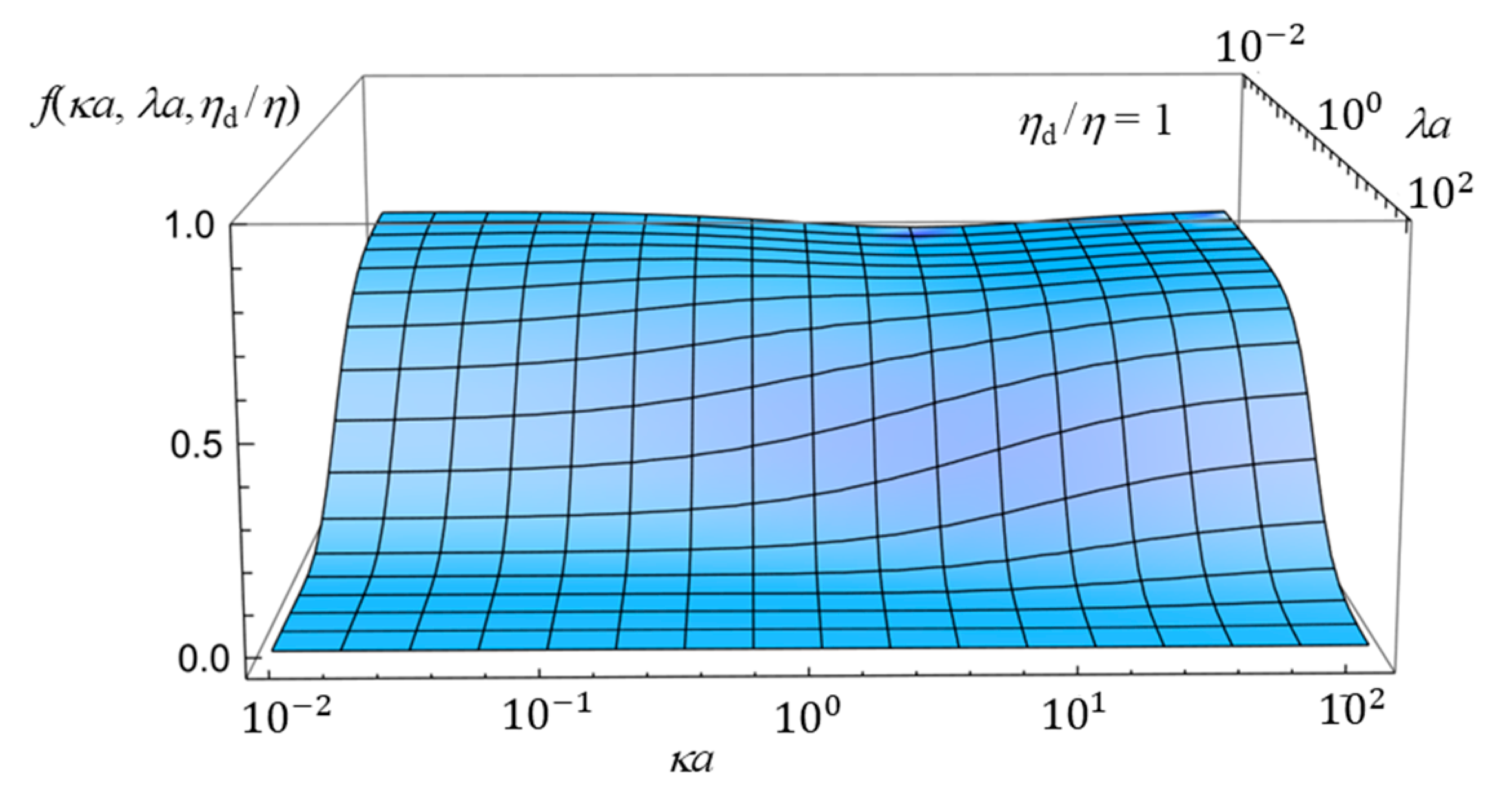
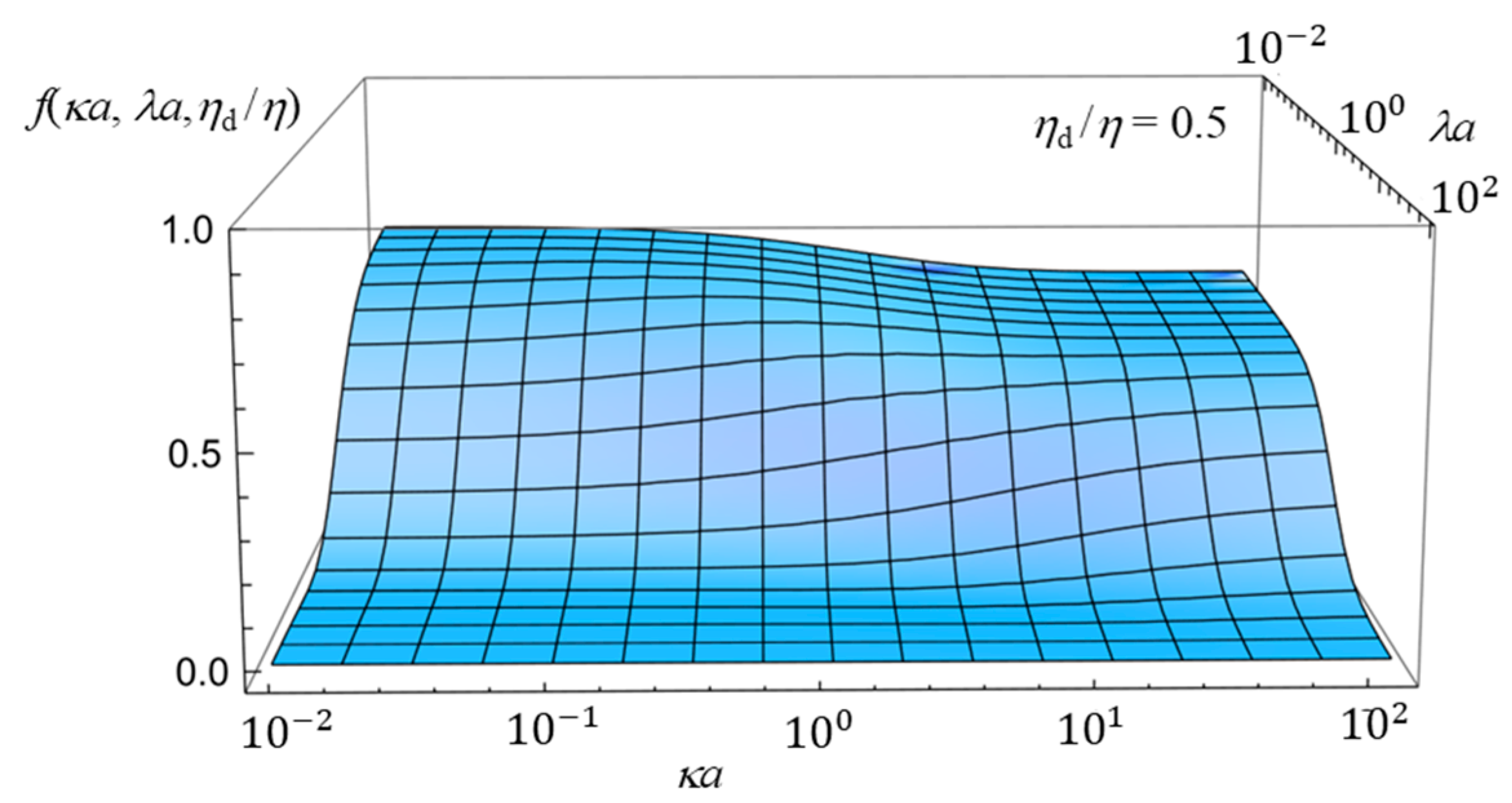
2.4. Numerical Results and Discussion
- (i)
- In the limit of λa → 0, Equation (61) reduces towhich agrees with the electrophoretic mobility μ for the free-solution electrophoresis of a weakly charged oil drop [52]. In the further limit of ηd/η → ∞, Equation (63) tends towhich agrees with Henry’s electrophoretic mobility of a rigid sphere in a free-electrolyte solution [30].
- (ii)
- In the limit of κa → ∞ (Smoluchowski limit), Equation (61) becomes
- (iii)
- In the limit of κa → 0 (Hückel limit), Equation (61) becomes
3. Conclusions
4. Materials and Methods
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allison, S.A.; Xin, Y.; Pei, H. Electrophoresis of spheres with uniform zeta potential in a gel modeled as an effective medium. J. Colloid Interface Sci. 2007, 313, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.A.; Pei, H.; Xin, Y. Review modeling the free solution and gel electrophoresis of biopolymers: The bead array-effective medium model. Biopolymers 2007, 87, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.J.; Ostoja-Starzewski, M. Electric-field-induced displacement of a charged spherical colloid embedded in an elastic Brinkman medium. Phys. Rev. E 2008, 77, 011404. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Hill, R.J. Steady electrical and micro-rheological response functions for uncharged colloidal inclusions in polyelectrolyte hydrogels. Proc. R. Soc. A 2010, 466, 213–235. [Google Scholar] [CrossRef]
- Tsai, P.; Lee, E. Gel electrophoresis in suspensions of charged spherical particles. Soft Matter 2011, 7, 5789–5798. [Google Scholar] [CrossRef]
- Tsai, P.; Huang, C.H.; Lee, E. Electrophoresis of a charged colloidal particle in porous media: Boundary effect of a solid plane. Langmuir 2011, 27, 13481–13488. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.P.; Huang, C.H.; Tseng, S. Gel electrophoresis: Importance of concentration-dependent permittivity and double-layer polarization. Chem. Eng. Sci. 2012, 84, 574–579. [Google Scholar] [CrossRef]
- Hsu, J.P.; Huang, C.H.; Tseng, S. Gel electrophoresis of a charge-regulated, bi-functional particle. Electrophoresis 2013, 34, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Hill, R.J. Nanoparticle gel electrophoresis: Bare charged spheres in polyelectrolyte hydrogels. J. Colloid Interface Sci. 2013, 394, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Allison, S.A.; Hill, R.J. Nanoparticle gel electrophoresis: Soft spheres in polyelectrolyte hydrogels under the Debye-Hückel approximation. J. Colloid Interface Sci. 2014, 423, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.A.; Li, F.; Hill, R.J. The electrophoretic mobility of a weakly charged “soft” sphere in a charged hydrogel: Application of the Lorentz reciprocal theorem. J. Phys. Chem. B 2014, 118, 8827–8838. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; De, S.; Gopmandal, P.P. Electrophoresis of a colloidal particle embedded in electrolyte saturated porous media. Chem. Eng. Sci. 2014, 118, 184–191. [Google Scholar] [CrossRef]
- Hill, R.J. Hydrogel charge regulation and electrolyte ion-concentration perturbations in nanoparticle gel electrophoresis. Proc. R. Soc. A 2015, 471, 20150523. [Google Scholar] [CrossRef]
- Allison, S.A.; Li, F.; Le, M. Electrophoretic mobility of a dilute, highly charged “soft” spherical particle in a charged hydrogel. J. Phys. Chem. B 2016, 120, 8071–8079. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.J. Electrokinetics of nanoparticle gel-electrophoresis. Soft Matter 2016, 12, 8030–8048. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; De, S. Gel electrophoresis and size selectivity of charged colloidal particles in a charged hydrogel medium. Chem. Eng. Sci. 2016, 141, 304–314. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; De, S. Nonlinear effects on electrophoresis of a charged dielectric nanoparticle in a charged hydrogel medium. Phys. Fluids 2016, 28, 092006. [Google Scholar] [CrossRef]
- Ohshima, H. Electrophoretic mobility of a charged spherical colloidal particle in an uncharged or charged polymer gel medium. Colloid Polym. Sci. 2019, 297, 719–728. [Google Scholar] [CrossRef]
- Ohshima, H. Gel electrophoresis of a soft particle. Adv. Colloid Interface Sci. 2019, 271, 101977. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H. Electrophoretic mobility of a soft particle in a polymer gel medium. Colloids Surf. A-Physicochem. Eng. Asp. 2021, 618, 126400. [Google Scholar] [CrossRef]
- Barman, S.S.; Bhattacharyya, S.; Dutta, P. Electrokinetic actuation of an uncharged polarizable dielectric droplet in charged hydrogel medium. Electrophoresis 2021, 42, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Bharti; Sarkar, S.; Ohshima, H.; Gopmandal, P.P. Gel electrophoresis of a hydrophobic liquid droplet with an equipotential slip surface. Langmuir 2022, 38, 8943–8953. [Google Scholar] [CrossRef] [PubMed]
- Bharti; Sarkar, S.; Ohshima, H.; Gopmandal, P.P. Electrophoresis of hydrophobic and polarizable liquid droplets in hydrogel medium. J. Mol. Liq. 2024, 395, 123810. [Google Scholar] [CrossRef]
- Saad, E.I.; Faltas, M.S. Time-dependent electrophoresis of a dielectric spherical particle embedded in Brinkman medium. Z. Angew. Math. Phys. 2018, 69, 43. [Google Scholar] [CrossRef]
- Saad, E.I. Start-up Brinkman electrophoresis of a dielectric sphere for Happel and Kuwabara models. Math. Meth. Appl. Sci. 2018, 41, 9578–9591. [Google Scholar] [CrossRef]
- Saad, E.I. Unsteady electrophoresis of a dielectric cylindrical particle suspended in porous medium. J. Mol. Liq. 2019, 289, 111050. [Google Scholar] [CrossRef]
- Sherief, H.H.; Faltas, M.S.; Ragab, K.E. Transient electrophoresis of a conducting spherical particle embedded in an electrolyte-saturated Brinkman medium. Electrophoresis 2021, 42, 1636–1647. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, H. Transient gel electrophoresis of a spherical colloidal particle. Gels 2023, 9, 356. [Google Scholar] [CrossRef] [PubMed]
- Ayman, M.; Saad, E.I.; Faltas, M.S. Transient electrophoresis of a conducting cylindrical colloidal particle suspended in a Brinkman medium. Z. Angew. Math. Phys. 2024, 75, 53. [Google Scholar] [CrossRef]
- Henry, D.C. The cataphoresis of suspended particles. Part I. The equation of cataphoresis. Proc. Roy. Soc. Lond. Ser. A. 1931, 133, 106–129. [Google Scholar] [CrossRef]
- O’Brien, R.W.; White, L.R. Electrophoretic mobility of a spherical colloidal particle. J. Chem. Soc. Faraday Trans. 2 1978, 74, 1607–1626. [Google Scholar] [CrossRef]
- Masliyah, J.H.; Bhattacharjee, S. Electrokinetic and Colloid Transport Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: New York, NY, USA, 2013. [Google Scholar]
- Lee, E. Theory of Electrophoresis and Diffusiophoresis of Highly Charged Colloidal Particles; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Ohshima, H. Fundamentals of Soft Interfaces in Colloid and Surface Chemistry; Elsevier: Amsterdam, The Netherlands, 2024. [Google Scholar]
- Brinkman, H.C. A calculation of the viscous force exerted by a flowing fluid on a dense swarm of particles. Appl. Sci. Res. 1949, 1, 27–34. [Google Scholar] [CrossRef]
- Debye, P.; Bueche, A.M. Intrinsic viscosity, diffusion, and sedimentation rate of polymers in solution. J. Chem. Phys. 1948, 16, 573–579. [Google Scholar] [CrossRef]
- Booth, F. The cataphoresis of spherical fluid droplets in electrolytes. J. Chem. Phys. 1951, 19, 1331–1336. [Google Scholar] [CrossRef]
- Ohshima, H.; Healy, T.W.; White, L.R. Electrokinetic phenomena in a dilute suspension of charged mercury drops. J. Chem. Soc. Faraday Trans 2 1984, 80, 1643–1667. [Google Scholar] [CrossRef]
- Baygents, J.C.; Saville, D.A. Electrophoresis of drops and bubbles. J. Chem. Soc. Faraday Trans. 1991, 87, 1883–1898. [Google Scholar] [CrossRef]
- Schnitzer, O.; Frankel, I.; Yariv, E. Electrokinetic flows about conducting drops. J. Fluid Mech. 2013, 722, 394–423. [Google Scholar] [CrossRef]
- Schnitzer, O.; Frankel, I.; Yariv, E. Electrophoresis of bubbles. J. Fluid Mech. 2014, 753, 49–79. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Majee, P.S. Nonlinear electrophoresis of a charged polarizable liquid droplet. Phys. Fluids 2018, 30, 082008. [Google Scholar] [CrossRef]
- Hill, R.J. Electrokinetic spectra of dilute surfactant-stabilized nano-emulsions. J. Fluid Mech. 2020, 902, A15. [Google Scholar] [CrossRef]
- Hill, R.J.; Afuwape, G. Dynamic mobility of surfactant-stabilized nano-drops: Unifying equilibrium thermodynamics, electro-kinetics and Marangoni effects. J. Fluid Mech. 2020, 895, A14. [Google Scholar] [CrossRef]
- Wu, Y.; Fan, L.; Jian, E.; Lee, E. Electrophoresis of a highly charged dielectric fluid droplet in electrolyte solutions. J. Colloid Interface Sci. 2021, 598, 358–368. [Google Scholar] [CrossRef]
- Rashidi, M.; Zargartalebi, M.; Benneker, A.M. Mechanistic studies of droplet electrophoresis: A review. Electrophoresis 2021, 42, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.Y.; Fan, L.; Tseng, J.; Lin, J.; Tseng, A.; Lee, E. Electrophoresis of a highly charged fluid droplet in dilute electrolyte solutions: Aanalytical Hückel-type solution. Electrophoresis 2022, 43, 1611–1616. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, P.; Ohshima, H.; Gopmandal, P.P. Electrophoresis of dielectric and hydrophobic spherical fluid droplets possessing uniform surface charge density. Langmuir 2022, 38, 11421–11431. [Google Scholar] [CrossRef] [PubMed]
- Tseng, J.; Su, J.; Chang, K.; Chang, A.; Chuang, L.; Lu, A.; Lee, R.; Lee, E. Electrophoresis of a dielectric droplet with constant surface charge density. Electrophoresis 2023, 44, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.J. Roles of interfacial-exchange kinetics and interfacial-charge mobility on fluid-sphere electrophoresis. J. Fluid Mech. 2025, 1005, A1. [Google Scholar] [CrossRef]
- Ohshima, H. Electrophoresis of a weakly charged oil drop in an electrolyte solution: Ion adsorption and Marangoni effects. Colloid Polym. Sci. 2025; in press. [Google Scholar] [CrossRef]
- Ashrafizadeh, S.N.; Seifollahi, Z.; Ganjizade, A.; Sadeghi, A. Electrophoresis of spherical soft particles in electrolyte solutions: A review. Electrophoresis 2020, 41, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, W.; Jia, M.; Liu, R.; Liu, Q.; Xiao, H.; Li, J.; Xue, Y.; Wang, Y.; Yan, C. Recent advances in microscale separation. Electrophoresis 2018, 39, 8–33. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohshima, H. Gel Electrophoresis of an Oil Drop. Gels 2025, 11, 555. https://doi.org/10.3390/gels11070555
Ohshima H. Gel Electrophoresis of an Oil Drop. Gels. 2025; 11(7):555. https://doi.org/10.3390/gels11070555
Chicago/Turabian StyleOhshima, Hiroyuki. 2025. "Gel Electrophoresis of an Oil Drop" Gels 11, no. 7: 555. https://doi.org/10.3390/gels11070555
APA StyleOhshima, H. (2025). Gel Electrophoresis of an Oil Drop. Gels, 11(7), 555. https://doi.org/10.3390/gels11070555





