Comparison of Ultrasound- and Microwave-Assisted Extraction Techniques on Chemical, Technological, Rheological, and Microstructural Properties of Starch from Mango Kernel
Abstract
1. Introduction
2. Results and Discussion
2.1. Amylose Content
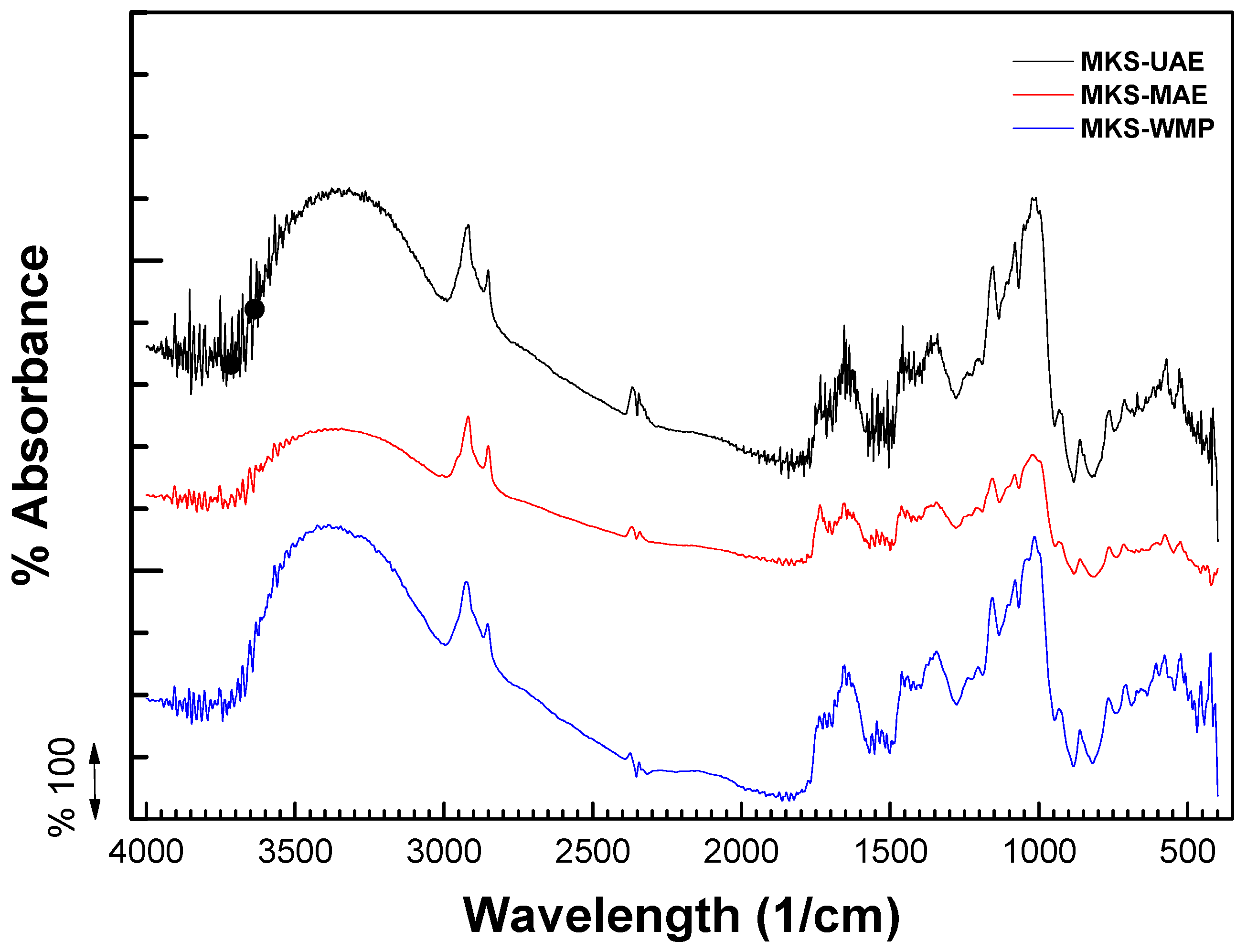
2.2. FTIR Analysis
2.3. Total Phenolic Compounds (TPC) and Antioxidant Capacity
2.4. Technological Properties
2.4.1. Water-Holding Capacity (WHC)
2.4.2. Oil-Holding Capacity (OHC)
2.4.3. Solubility and Swelling Power (SP)
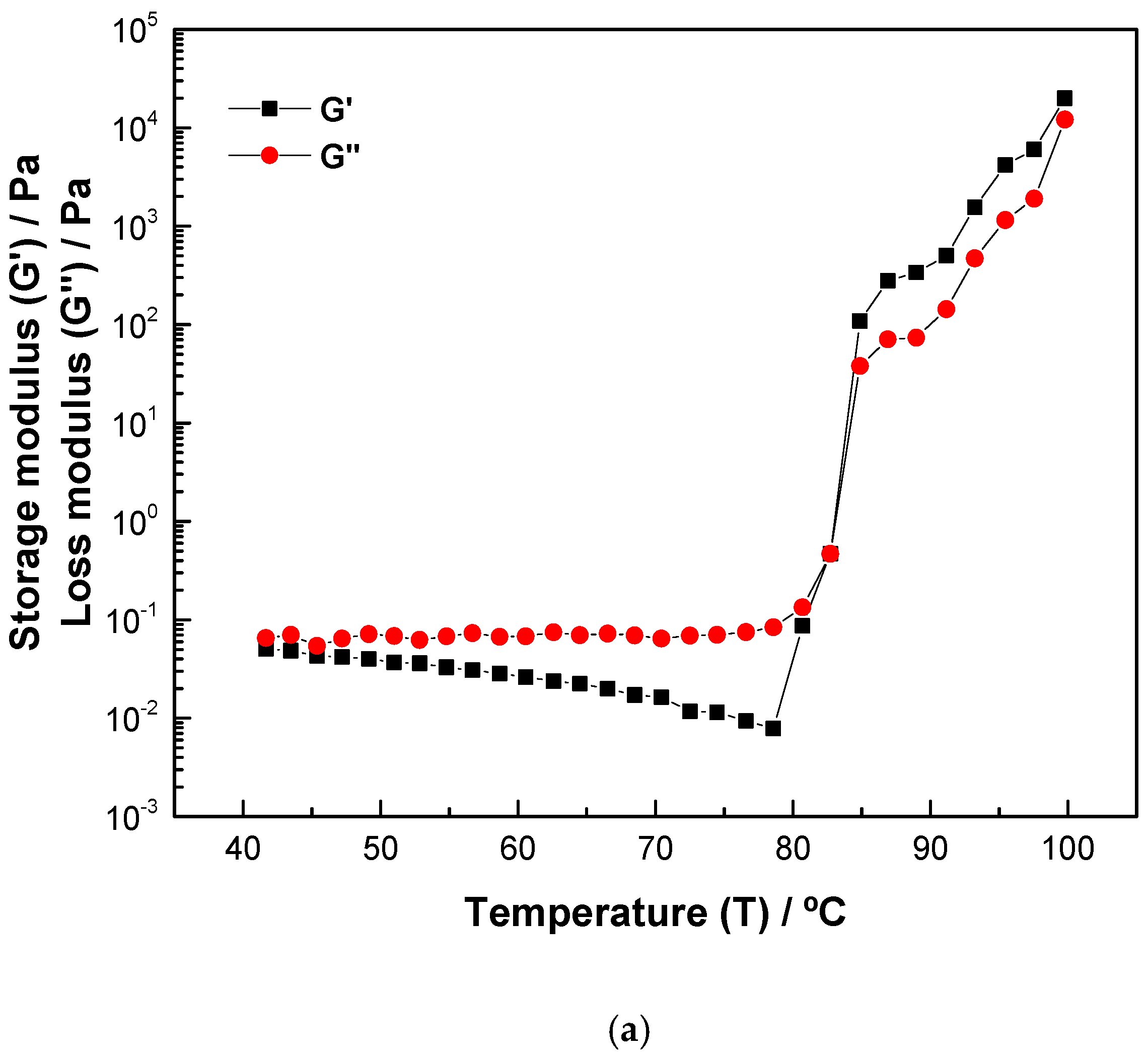
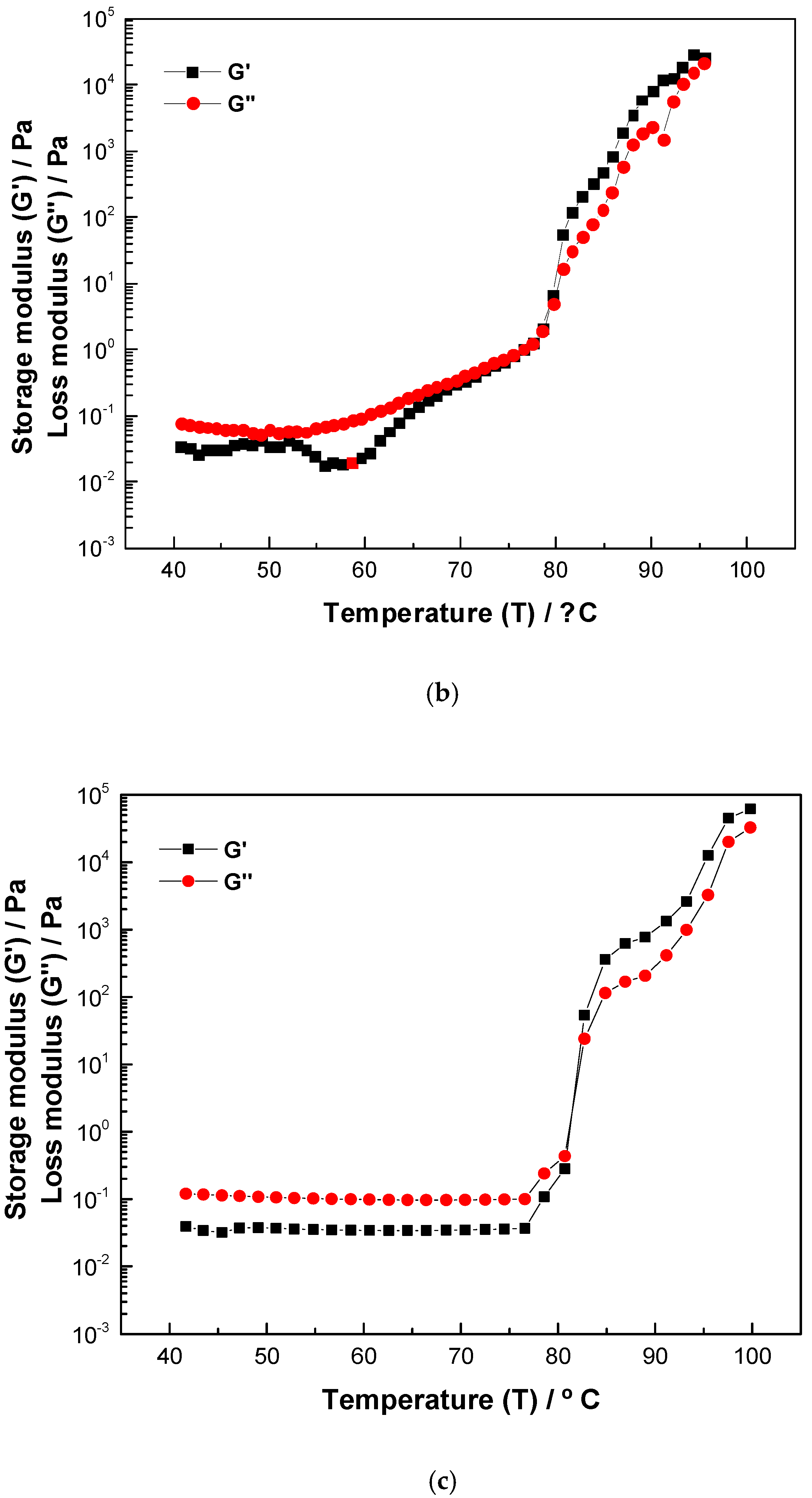
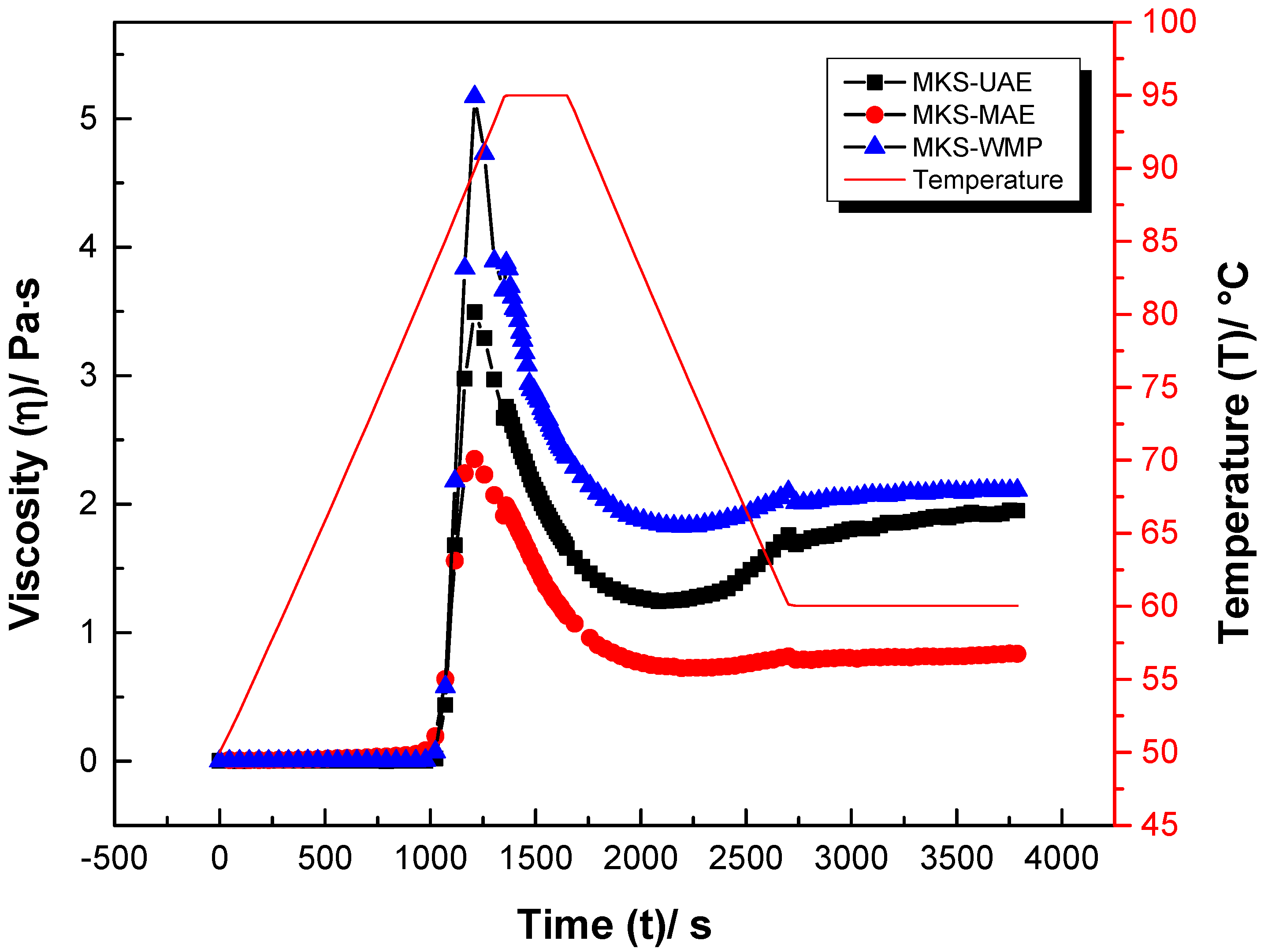
2.5. Rheological Properties
2.6. Pasting Properties
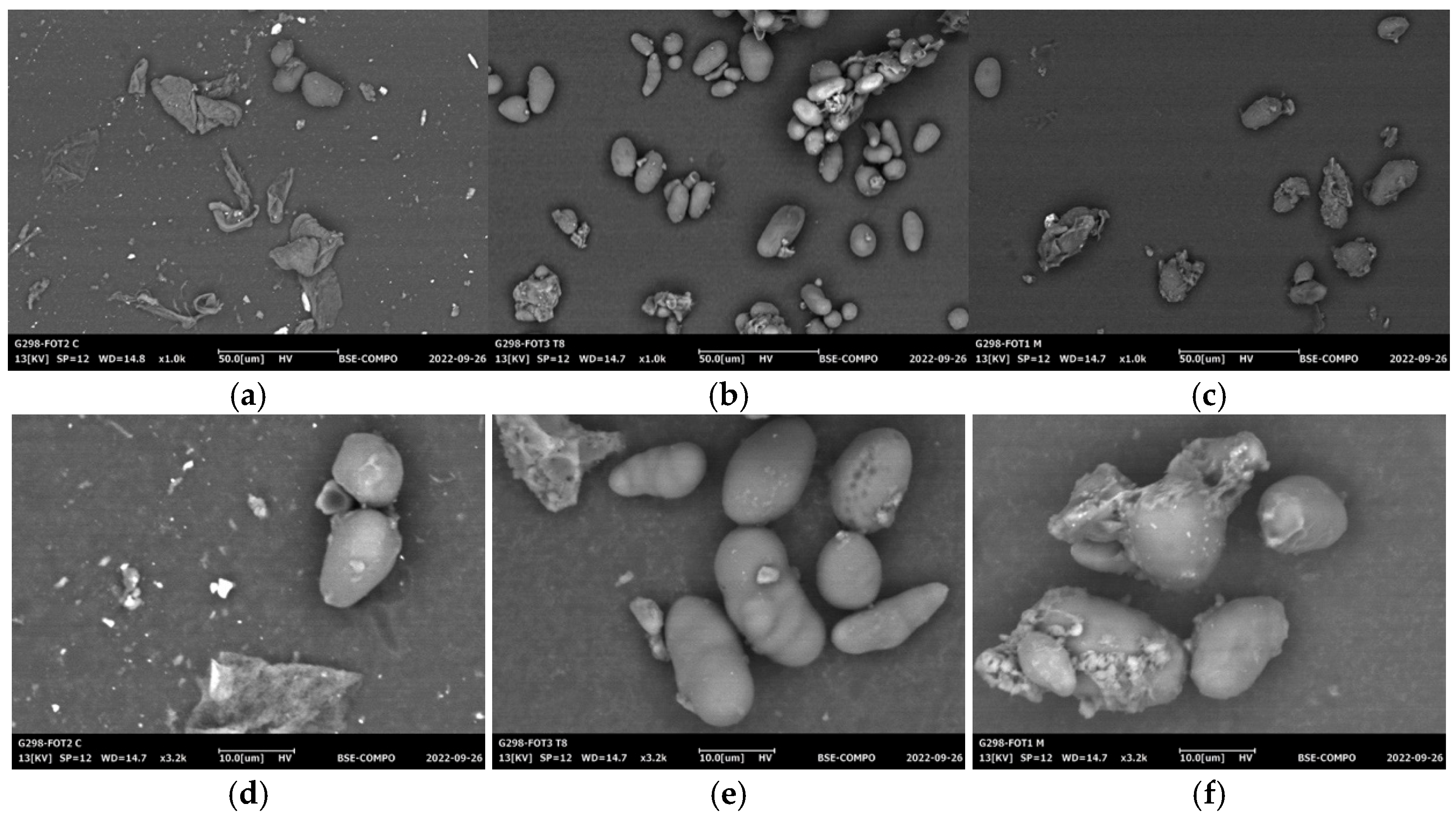
2.7. Scanning Electron Microscopy (SEM)
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Obtention of Mango Kernel Flour
4.3. Wet-Milling Process of Mango Kernel Starch
4.4. Ultrasound-Assisted Extraction (UAE) of Mango Kernel Starch
4.5. Microwave-Assisted Extraction (MAE) of Mango Kernel Starch
4.6. Determination of the Amylose and Amylopectin Content
4.7. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4.8. Determination of Total Phenolic Compounds (TPC) and Antioxidant Capacity
4.9. Technological Properties Analysis
4.10. Rheological Analysis
4.11. Microstructural Analysis
4.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Production of Mango, Mangosteens and Guava in India. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 27 March 2023).
- Torres-León, C.; Rojas, R.; Contreras-Esquivel, J.C.; Serna-Cock, L.; Belmares-Cerda, R.E.; Aguilar, C.N. Mango Seed: Functional and Nutritional Properties. Trends Food Sci. Technol. 2016, 55, 109–117. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Khalique, A. Promising Features of Mango (Mangifera indica L.) Kernel Oil: A Review. J. Food Sci. Technol. 2016, 53, 2185–2195. [Google Scholar] [CrossRef]
- Mwaurah, P.W.; Kumar, S.; Kumar, N.; Panghal, A.; Attkan, A.K.; Singh, V.K.; Garg, M.K. Physicochemical Characteristics, Bioactive Compounds and Industrial Applications of Mango Kernel and Its Products: A Review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2421–2446. [Google Scholar] [CrossRef] [PubMed]
- Punia Bangar, S.; Kumar, M.; Whiteside, W.S. Mango Seed Starch: A Sustainable and Eco-Friendly Alternative to Increasing Industrial Requirements. Int. J. Biol. Macromol. 2021, 183, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, T.A.S.; de Gusmão, R.P.; Moura, H.V.; Silva, H.A.; Cavalcanti-Mata, M.E.R.M.; Duarte, M.E.M. Production of Prebiotic Gluten-Free Bread with Red Rice Flour and Different Microbial Transglutaminase Concentrations: Modeling, Sensory and Multivariate Data Analysis. J. Food Sci. Technol. 2019, 56, 2949–2958. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Felisberto, M.H.F.; Sanches, E.A.; Campelo, P.H.; Clerici, M.T.P.S. Non-Conventional Starch Sources. Curr. Opin. Food Sci. 2021, 39, 93–102. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, N.; Sandhu, K.S.; Guraya, H.S. Physicochemical, Morphological, Thermal and Rheological Properties of Starches Separated from Kernels of Some Indian Mango Cultivars (Mangifera indica L.). Food Chem. 2004, 85, 131–140. [Google Scholar] [CrossRef]
- Nawab, A.; Alam, F.; Haq, M.A.; Hasnain, A. Effect of Guar and Xanthan Gums on Functional Properties of Mango (Mangifera Indica) Kernel Starch. Int. J. Biol. Macromol. 2016, 93, 630–635. [Google Scholar] [CrossRef]
- Chang, Y.; Yan, X.; Wang, Q.; Ren, L.; Tong, J.; Zhou, J. High Efficiency and Low Cost Preparation of Size Controlled Starch Nanoparticles through Ultrasonic Treatment and Precipitation. Food Chem. 2017, 227, 369–375. [Google Scholar] [CrossRef]
- Maniglia, B.C.; Castanha, N.; Rojas, M.L.; Augusto, P.E. Emerging Technologies to Enhance Starch Performance. Curr. Opin. Food Sci. 2021, 37, 26–36. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, W.; Pu, H.; Blennow, A.; Liu, X.; Guo, D. Short-Time Microwave Treatment Affects the Multi-Scale Structure and Digestive Properties of High-Amylose Maize Starch. Int. J. Biol. Macromol. 2019, 137, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Karaman, M.; Tuncel, N.B.; Yılmaz Tuncel, N. The Effect of Ultrasound-Assisted Extraction on Yield and Properties of Some Pulse Starches. Starch—Stärke 2017, 69, 1600307. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Wan, N.; Wang, X.; Yang, M. Extraction of High-Amylose Starch from Radix Puerariae Using High-Intensity Low-Frequency Ultrasound. Ultrason. Sonochem. 2019, 59, 104710. [Google Scholar] [CrossRef] [PubMed]
- González-Lemus, L.B.; Calderón-Domínguez, G.; Salgado-Cruz, M.d.l.P.; Díaz-Ramírez, M.; Ramírez-Miranda, M.; Chanona-Pérez, J.J.; Gϋemes-Vera, N.; Farrera-Rebollo, R.R. Ultrasound-Assisted Extraction of Starch from Frozen Jicama (P. erosus) Roots: Effect on Yield, Structural Characteristics and Thermal Properties. CyTA—J. Food 2018, 16, 738–746. [Google Scholar] [CrossRef]
- Bharti, I.; Singh, S.; Saxena, D.C. Exploring the Influence of Heat Moisture Treatment on Physicochemical, Pasting, Structural and Morphological Properties of Mango Kernel Starches from Indian Cultivars. LWT 2019, 110, 197–206. [Google Scholar] [CrossRef]
- Ferraz, C.A.; Fontes, R.L.S.; Fontes-Sant’Ana, G.C.; Calado, V.; López, E.O.; Rocha-Leão, M.H.M. Extraction, Modification, and Chemical, Thermal and Morphological Characterization of Starch From the Agro-Industrial Residue of Mango (Mangifera indica L) Var. Ubá. Starch—Stärke 2019, 71, 1800023. [Google Scholar] [CrossRef]
- Tesfaye, T.; Johakimu, J.K.; Chavan, R.B.; Sithole, B.; Ramjugernath, D. Valorisation of Mango Seed via Extraction of Starch: Preliminary Techno-Economic Analysis. Clean Technol. Environ. Policy 2018, 20, 81–94. [Google Scholar] [CrossRef]
- Dey, S.; Rathod, V.K. Ultrasound Assisted Extraction of β-Carotene from Spirulina Platensis. Ultrason. Sonochem. 2013, 20, 271–276. [Google Scholar] [CrossRef]
- Elakremi, M.; Sillero, L.; Ayed, L.; ben Mosbah, M.; Labidi, J.; ben Salem, R.; Moussaoui, Y.; Pistacia Vera, L. Leaves as a Renewable Source of Bioactive Compounds via Microwave Assisted Extraction. Sustain. Chem. Pharm. 2022, 29, 100815. [Google Scholar] [CrossRef]
- Shen, S.; Zhou, C.; Zeng, Y.; Zhang, H.; Hossen, M.A.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Structures, Physicochemical and Bioactive Properties of Polysaccharides Extracted from Panax Notoginseng Using Ultrasonic/Microwave-Assisted Extraction. LWT 2022, 154, 112446. [Google Scholar] [CrossRef]
- Sharma, M.; Dash, K.K. Microwave and Ultrasound Assisted Extraction of Phytocompounds from Black Jamun Pulp: Kinetic and Thermodynamics Characteristics. Innov. Food Sci. Emerg. Technol. 2022, 75, 102913. [Google Scholar] [CrossRef]
- Li, C.; Liu, W.; Gu, Z.; Fang, D.; Hong, Y.; Cheng, L.; Li, Z. Ultrasonic Pretreatment Improves the High-Temperature Liquefaction of Corn Starch at High Concentrations. Starch—Stärke 2017, 69, 1600002. [Google Scholar] [CrossRef]
- Han, L.; Cao, S.; Yu, Y.; Xu, X.; Cao, X.; Chen, W. Modification in Physicochemical, Structural and Digestive Properties of Pea Starch during Heat-Moisture Process Assisted by Pre- and Post-Treatment of Ultrasound. Food Chem. 2021, 360, 129929. [Google Scholar] [CrossRef] [PubMed]
- Seung, D. Amylose in Starch: Towards an Understanding of Biosynthesis, Structure and Function. New Phytol. 2020, 228, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, G.; Li, J.; Wang, W.; Hu, A.; Zheng, J. Structural and Physicochemical Properties of Resistant Starch under Combined Treatments of Ultrasound, Microwave, and Enzyme. Int. J. Biol. Macromol. 2023, 232, 123331. [Google Scholar] [CrossRef]
- Sun, H.; Fan, J.; Tian, Z.; Ma, L.; Meng, Y.; Yang, Z.; Zeng, X.; Liu, X.; Kang, L.; Nan, X. Effects of Treatment Methods on the Formation of Resistant Starch in Purple Sweet Potato. Food Chem. 2022, 367, 130580. [Google Scholar] [CrossRef]
- Li, Y.; Hu, A.; Zheng, J.; Wang, X. Comparative Studies on Structure and Physiochemical Changes of Millet Starch under Microwave and Ultrasound at the Same Power. Int. J. Biol. Macromol. 2019, 141, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Singh, L.; Sharanagat, V.S.; Patel, A.; Kumar, K. Effect of Microwave Treatment (Low Power and Varying Time) on Potato Starch: Microstructure, Thermo-Functional, Pasting and Rheological Properties. Int. J. Biol. Macromol. 2020, 155, 27–35. [Google Scholar] [CrossRef]
- Zhou, D.; Ma, Z.; Yin, X.; Hu, X.; Boye, J.I. Structural Characteristics and Physicochemical Properties of Field Pea Starch Modified by Physical, Enzymatic, and Acid Treatments. Food Hydrocoll. 2019, 93, 386–394. [Google Scholar] [CrossRef]
- Morales-Trejo, F.; Trujillo-Ramírez, D.; Aguirre-Mandujano, E.; Lobato-Calleros, C.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. Ultrasound-Assisted Extraction of Lychee (Litchi chinensis Sonn.) Seed Starch: Physicochemical and Functional Properties. Starch—Stärke 2022, 74, 2100092. [Google Scholar] [CrossRef]
- Sivasankar, T.; Paunikar, A.W.; Moholkar, V.S. Mechanistic Approach to Enhancement of the Yield of a Sonochemical Reaction. AIChE J. 2007, 53, 1132–1143. [Google Scholar] [CrossRef]
- Raza, H.; Ameer, K.; Ma, H.; Liang, Q.; Ren, X. Structural and Physicochemical Characterization of Modified Starch from Arrowhead Tuber (Sagittaria sagittifolia L.) Using Tri-Frequency Power Ultrasound. Ultrason. Sonochem. 2021, 80, 105826. [Google Scholar] [CrossRef]
- Falsafi, S.R.; Maghsoudlou, Y.; Rostamabadi, H.; Rostamabadi, M.M.; Hamedi, H.; Hosseini, S.M.H. Preparation of Physically Modified Oat Starch with Different Sonication Treatments. Food Hydrocoll. 2019, 89, 311–320. [Google Scholar] [CrossRef]
- Gani, A.; Gazanfar, T.; Jan, R.; Wani, S.M.; Masoodi, F.A. Effect of Gamma Irradiation on the Physicochemical and Morphological Properties of Starch Extracted from Lotus Stem Harvested from Dal Lake of Jammu and Kashmir, India. J. Saudi Soc. Agric. Sci. 2013, 12, 109–115. [Google Scholar] [CrossRef]
- Nadir, A.S.; Helmy, I.; Nahed, M.; Abdelmaguid; Waafa, M.; Abozeid, M.; Ramadan, M.T. Modification of Potato Starch by Some Different Physical Methods and Utilization in Cookies Production. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 556–569. [Google Scholar]
- Singh, R.; Sharanagat, V.S. Physico-Functional and Structural Characterization of Ultrasonic-Assisted Chemically Modified Elephant Foot Yam Starch. Int. J. Biol. Macromol. 2020, 164, 1061–1069. [Google Scholar] [CrossRef]
- Wang, H.; Xu, K.; Ma, Y.; Liang, Y.; Zhang, H.; Chen, L. Impact of Ultrasonication on the Aggregation Structure and Physicochemical Characteristics of Sweet Potato Starch. Ultrason. Sonochem. 2020, 63, 104868. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Aggarwal, M. Physicochemical, Thermal and Functional Properties of Gamma Irradiated Chickpea Starch. Int. J. Biol. Macromol. 2017, 97, 426–433. [Google Scholar] [CrossRef]
- Majeed, T.; Wani, I.A.; Hussain, P.R. Effect of Dual Modification of Sonication and γ-Irradiation on Physicochemical and Functional Properties of Lentil (Lens culinaris L.) Starch. Int. J. Biol. Macromol. 2017, 101, 358–365. [Google Scholar] [CrossRef]
- Palav, T. Impact of Microwave Heating on the Physico-Chemical Properties of a Starch-Water Model System. Carbohydr. Polym. 2007, 67, 596–604. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Saleem, S.; Khan, M.U.A.; Nishan, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. Characteristics of Starch Isolated from Microwave Heat Treated Lotus (Nelumbo nucifera) Seed Flour. Int. J. Biol. Macromol. 2018, 113, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Chen, B.; Zeng, H.; Guo, Z.; Lu, X.; Zhang, Y.; Zheng, B. Effect of Microwave Irradiation on the Physicochemical and Digestive Properties of Lotus Seed Starch. J. Agric. Food Chem. 2016, 64, 2442–2449. [Google Scholar] [CrossRef] [PubMed]
- Dundar, A.N.; Gocmen, D. Effects of Autoclaving Temperature and Storing Time on Resistant Starch Formation and Its Functional and Physicochemical Properties. Carbohydr. Polym. 2013, 97, 764–771. [Google Scholar] [CrossRef]
- Tung, C.-Y.M.; Dynes, P.J. Relationship between Viscoelastic Properties and Gelation in Thermosetting Systems. J. Appl. Polym. Sci. 1982, 27, 569–574. [Google Scholar] [CrossRef]
- Rocha, T.S.; Demiate, I.M.; Franco, C.M.L. Características estruturais e físico-químicas de amidos de mandioquinha-salsa (Arracacia xanthorrhiza). Food Sci. Technol. 2008, 28, 620–628. [Google Scholar] [CrossRef]
- Albano, K.M.; Franco, C.M.L.; Telis, V.R.N. Rheological Behavior of Peruvian Carrot Starch Gels as Affected by Temperature and Concentration. Food Hydrocoll. 2014, 40, 30–43. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Y.; Xu, Z.; Zhang, C.; Liu, X.; Sui, Z.; Corke, H. Microwave Irradiation Alters the Rheological Properties and Molecular Structure of Hull-Less Barley Starch. Food Hydrocoll. 2021, 120, 106821. [Google Scholar] [CrossRef]
- Zhong, Y.; Tian, Y.; Liu, X.; Ding, L.; Kirkensgaard, J.J.K.; Hebelstrup, K.; Putaux, J.L.; Blennow, A. Influence of Microwave Treatment on the Structure and Functionality of Pure Amylose and Amylopectin Systems. Food Hydrocoll. 2021, 119, 106856. [Google Scholar] [CrossRef]
- Wang, S.; Hu, X.; Wang, Z.; Bao, Q.; Zhou, B.; Li, T.; Li, S. Preparation and Characterization of Highly Lipophilic Modified Potato Starch by Ultrasound and Freeze-Thaw Treatments. Ultrason. Sonochem. 2020, 64, 105054. [Google Scholar] [CrossRef]
- Czechowska-Biskup, R.; Rokita, B.; Lotfy, S.; Ulanski, P.; Rosiak, J.M. Degradation of Chitosan and Starch by 360-KHz Ultrasound. Carbohydr. Polym. 2005, 60, 175–184. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Y.; Liu, Y.; Ouyang, J. Effect of Ultrasonic and Microwave Dual-Treatment on the Physicochemical Properties of Chestnut Starch. Polymers 2020, 12, 1718. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, S.; Lin, H.; Chen, L.; Qin, S.; Wu, W.; Zheng, B.; Guo, Z. Physicochemical Properties and Digestion of the Lotus Seed Starch-Green Tea Polyphenol Complex under Ultrasound-Microwave Synergistic Interaction. Ultrason. Sonochem. 2019, 52, 50–61. [Google Scholar] [CrossRef]
- Luo, Z.; Fu, X.; He, X.; Luo, F.; Gao, Q.; Yu, S. Effect of Ultrasonic Treatment on the Physicochemical Properties of Maize Starches Differing in Amylose Content. Starch—Stärke 2008, 60, 646–653. [Google Scholar] [CrossRef]
- Yılmaz, A.; Tugrul, N. Effect of Ultrasound-Microwave and Microwave-Ultrasound Treatment on Physicochemical Properties of Corn Starch. Ultrason. Sonochem. 2023, 98, 106516. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, M.; Li, W.; Liu, G.; Wang, W.; Zhi, W.; Wang, M.; Wang, R.; Hu, A.; Zheng, J. Effects of Dual Modification of Lysine and Microwave on Corn Starch: In Vitro Digestibility and Physicochemical Properties. Int. J. Biol. Macromol. 2022, 220, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Mieles-Gómez, L.; Quintana, S.E.; García-Zapateiro, L.A. Ultrasound-Assisted Extraction of Mango (Mangifera indica) Kernel Starch: Chemical, Techno-Functional, and Pasting Properties. Gels 2023, 9, 136. [Google Scholar] [CrossRef]
- Ramírez-Brewer, D.; Quintana, S.E.; García-Zapateiro, L.A. Effect of Microwave Treatment on Technological, Physicochemical, Rheological and Microstructural Properties of Mango (Mangifera indica) Kernel Starch Variety Tommy and Sugar. LWT 2023, 187, 115311. [Google Scholar] [CrossRef]
- Morrison, W.R.; Laignelet, B. An Improved Colorimetric Procedure for Determining Apparent and Total Amylose in Cereal and Other Starches. J. Cereal Sci. 1983, 1, 9–20. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M.B.T. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. ISBN 0076-6879. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, W.T. An Alkaline Water Retention Capacity Test for the Evaluation of Cookie Baking Potentialities of Soft Winter Wheat Flours. Cereal Chem. 1953, 30, 242–246. [Google Scholar]
- Medcalf, D.G. Wheat Starches I. Comparison of Physicochemical Properties. Cereal Chem. 1965, 42, 558–568. [Google Scholar]
- Jiang, Q.; Gao, W.; Li, X.; Man, S.; Shi, Y.; Yang, Y.; Huang, L.; Liu, C. Comparative Susceptibilities to Alkali-Treatment of A-, B- and C-Type Starches of Dioscorea Zingiberensis, Dioscorea Persimilis and Dioscorea Opposita. Food Hydrocoll. 2014, 39, 286–294. [Google Scholar] [CrossRef]
- López-Barraza, D.; Ortega-Ramos, A.; Torregroza-Fuentes, E.; Quintana, S.E.; García-Zapateiro, L.A. Rheological and Functional Properties of Hydrocolloids from Pereskia Bleo Leaves. Fluids 2021, 6, 349. [Google Scholar] [CrossRef]
| Sample Code | Yield % | Amylose Content g/100 g | TPC mg GAE/g | AA µMol Trolox/g |
|---|---|---|---|---|
| MKS-WMP | 42.05 ± 2.58 b | 28.46 ± 0.93 b | 84.89 ± 1.55 a | 18.15 ± 1.10 b |
| MKS-UAE | 50.40 ± 2.26 a | 33.84 ± 1.47 a | 90.85 ± 3.08 a | 16.12 ± 1.50 a |
| MKS-MAE | 48.43 ± 1.41 a | 32.21 ± 1.58 a | 89.60 ± 4.11 a | 15.24 ± 0.56 a |
| Sample Code | WHC g/100 g | OHC g/100 g | Solubility | Swelling Power | ||||
|---|---|---|---|---|---|---|---|---|
| 25 °C | 65 °C | 90 °C | 25 °C | 65 °C | 90 °C | |||
| MKS-WMP | 80.48 ± 2.41 b | 76.43 ± 1.63 b | 1.10 ± 0.07 c | 6.20 ± 0.15 c | 15.00 ± 0.58 b | 1.97 ± 0.09 b | 6.17 ± 0.04 a | 14.88 ± 0.47 b |
| MKS-UAE | 87.76 ± 2.01 a | 83.23 ± 1.83 a | 1.52 ± 0.04 a | 6.86 ± 0.08 a | 16.75 ± 0.30 a | 2.74 ± 0.32 a | 6.32 ± 1.02 a | 12.89 ± 0.85 a |
| MKS-MAE | 90.05 ± 2.54 a | 78.56 ± 1.32 a | 1.34 ± 0.05 b | 6.46 ± 0.12 b | 16.88 ± 0.38 a | 2.49 ± 0.46 ab | 6.45 ± 0.74 a | 15.72 ± 1.10 b |
| Sample Code | PT °C | PV Pa·s | FV Pa·s | TV Pa·s | BV Pa·s | SB Pa·s |
|---|---|---|---|---|---|---|
| MKS-WMP | 83.80 ± 1.67 b | 5.17 ± 0.25 c | 2.11 ± 0.10 c | 1.83 ± 0.09 c | 3.34 ± 0.16 c | 3.06 ± 0.15 b |
| MKS-UAE | 82.84 ± 1.47 ab | 3.49 ± 0.19 a | 1.94 ± 0.07 a | 1.23 ± 0.08 a | 2.26 ± 0.15 a | 1.54 ± 0.05 a |
| MKS-MAE | 79.85 ± 1.41 a | 2.35 ± 0.12 b | 0.83 ± 0.05 b | 0.72 ± 0.09 b | 1.63 ± 0.11 b | 1.52 ± 0.10 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mieles-Gómez, L.; Quintana, S.E.; García-Zapateiro, L.A. Comparison of Ultrasound- and Microwave-Assisted Extraction Techniques on Chemical, Technological, Rheological, and Microstructural Properties of Starch from Mango Kernel. Gels 2025, 11, 330. https://doi.org/10.3390/gels11050330
Mieles-Gómez L, Quintana SE, García-Zapateiro LA. Comparison of Ultrasound- and Microwave-Assisted Extraction Techniques on Chemical, Technological, Rheological, and Microstructural Properties of Starch from Mango Kernel. Gels. 2025; 11(5):330. https://doi.org/10.3390/gels11050330
Chicago/Turabian StyleMieles-Gómez, Luis, Somaris E. Quintana, and Luis A. García-Zapateiro. 2025. "Comparison of Ultrasound- and Microwave-Assisted Extraction Techniques on Chemical, Technological, Rheological, and Microstructural Properties of Starch from Mango Kernel" Gels 11, no. 5: 330. https://doi.org/10.3390/gels11050330
APA StyleMieles-Gómez, L., Quintana, S. E., & García-Zapateiro, L. A. (2025). Comparison of Ultrasound- and Microwave-Assisted Extraction Techniques on Chemical, Technological, Rheological, and Microstructural Properties of Starch from Mango Kernel. Gels, 11(5), 330. https://doi.org/10.3390/gels11050330









