Fullerene-Functionalized Cellulosic Hydrogel Biosensor with Bacterial Turn-on Fluorescence Response Derived from Carboxymethyl Cellulose for Intelligent Food Packaging with DFT Calculations and Molecular Docking
Abstract
1. Introduction
2. Results and Discussion
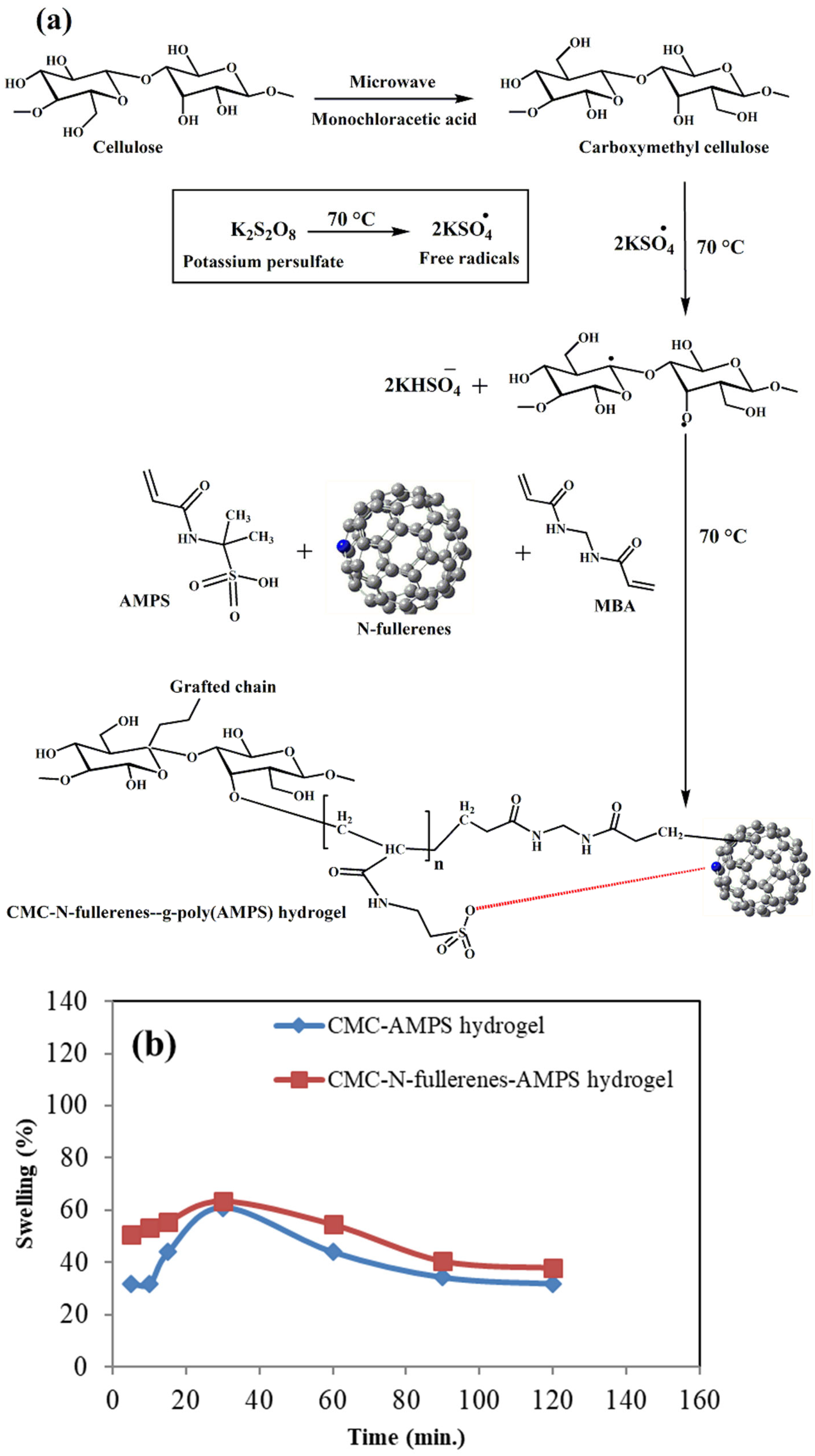
2.1. Proposed Mechanism of Carboxymethyl Cellulose–N-fullerene–g-poly(co-acrylamido-2-methyl-1-propane Sulfonic Acid) Hydrogel Formation and the Swelling Study
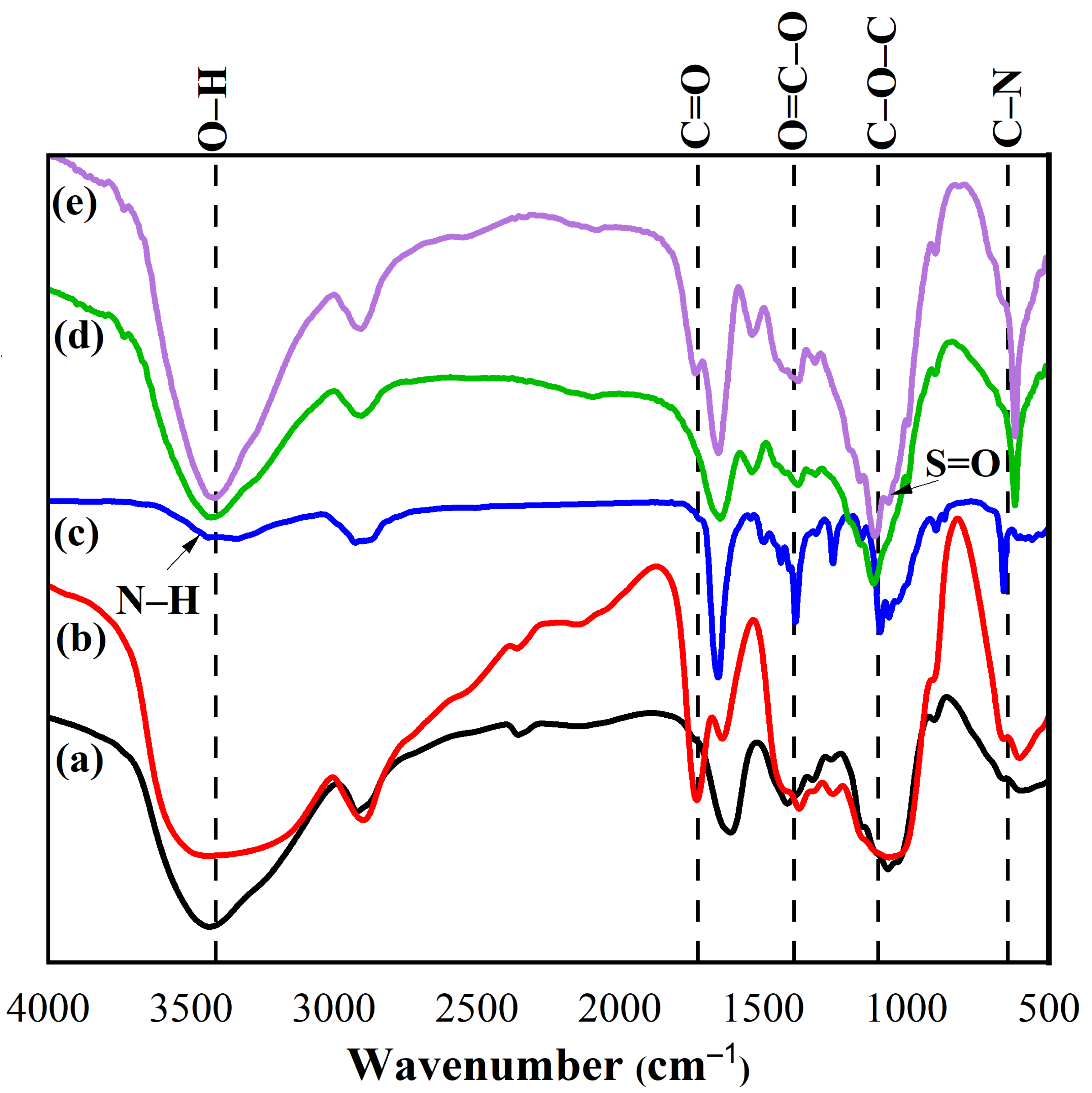
2.2. Fourier Transform Infrared Spectroscopy (FTIR) Spectra
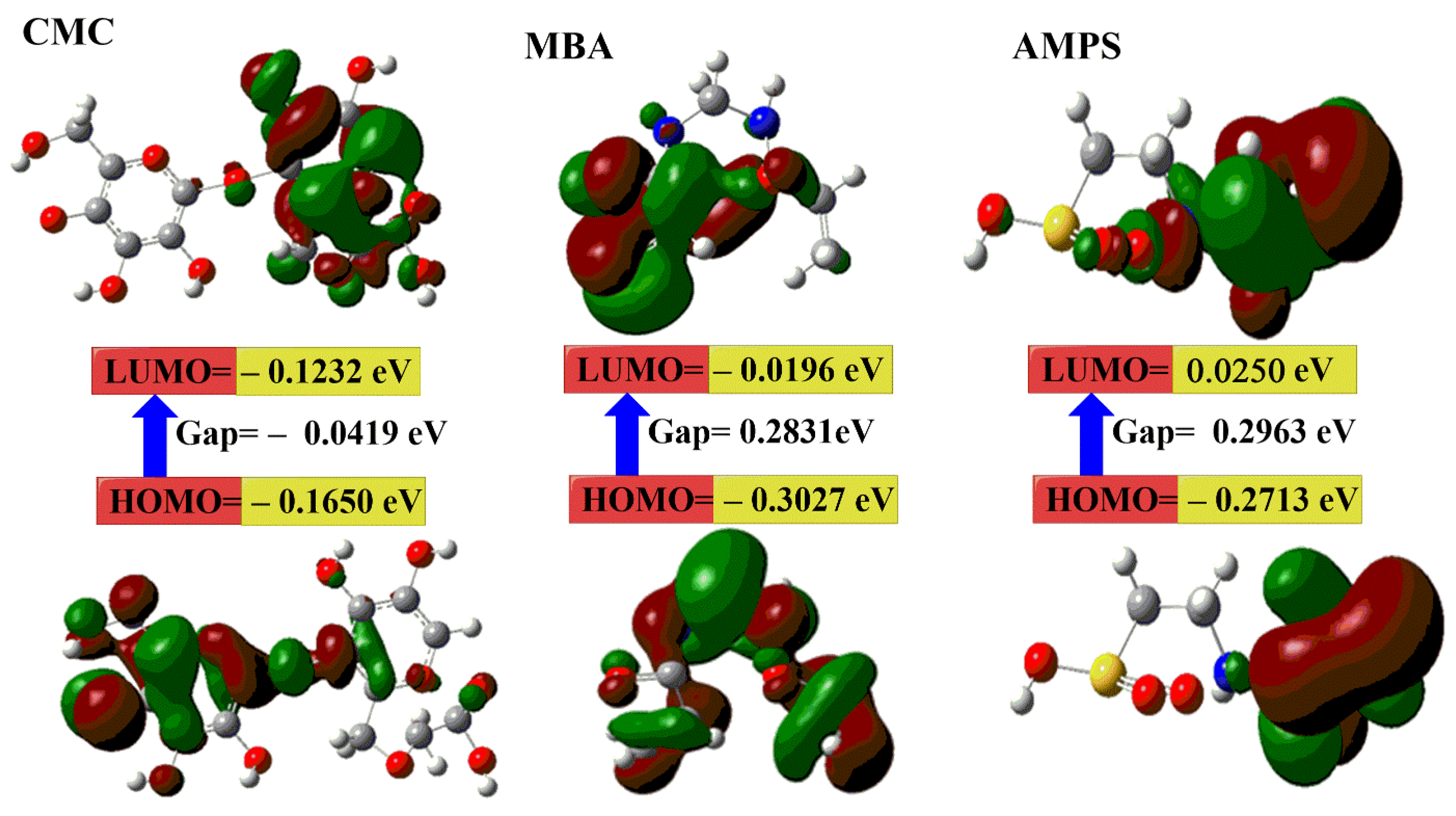
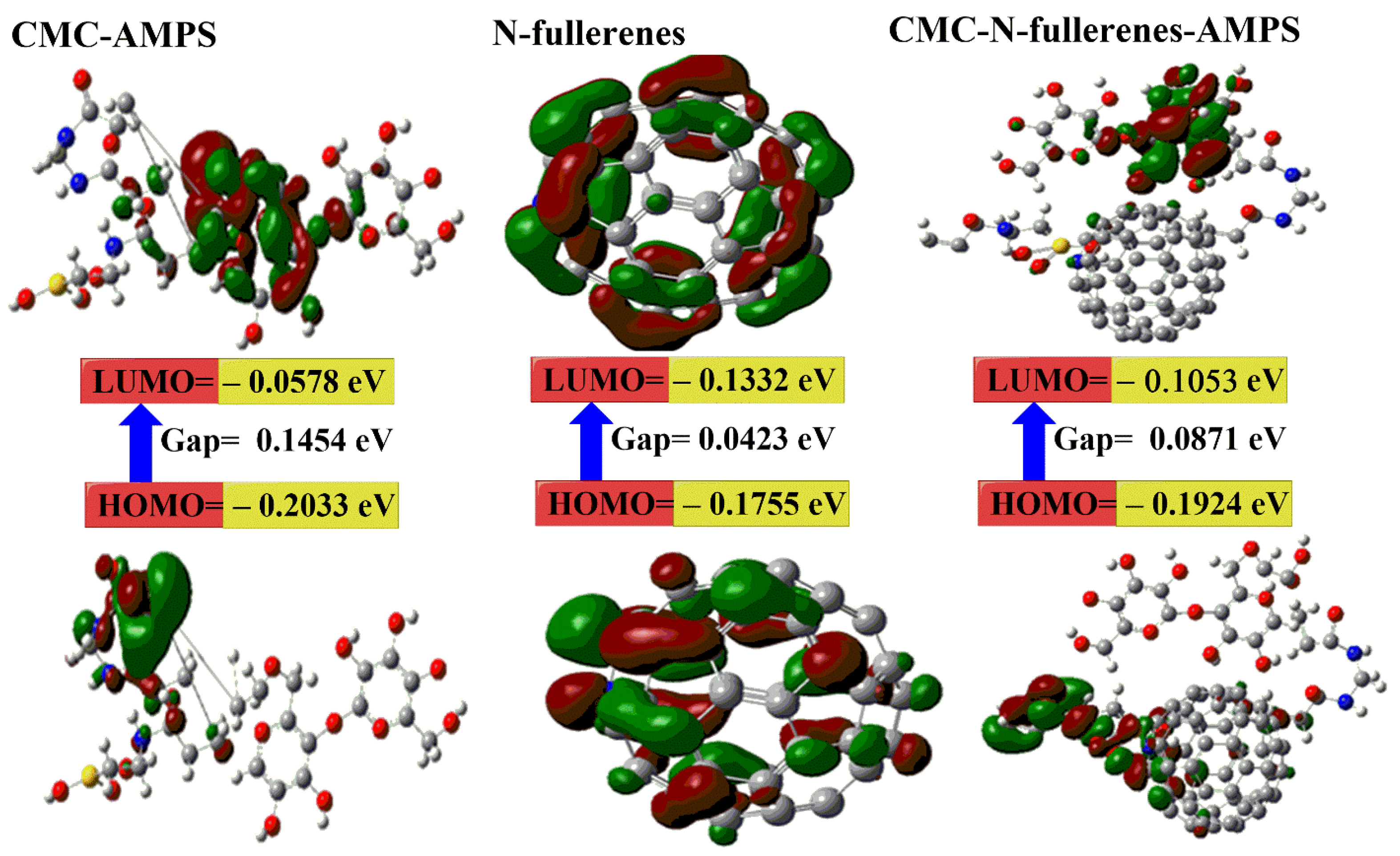
2.3. DFT Calculations
- a.
- b.
- The calculated Eg for the CMC–N-fullerene–AMPS hydrogel is the lowest (i.e., 0.0871 eV) compared to the CMC–AMPS hydrogel (i.e., 0.1454 eV), which in turn proves the strong chemical reaction between CMC, MBA, AMPS, and N-fullerenes in the CMC–N-fullerene–AMPS hydrogel compared to the CMC–AMPS hydrogel due to the presence of N-fullerenes [75,76].
- c.
- d.
- The CMC–N-fullerene–AMPS hydrogel is much softer than the CMC–AMPS hydrogel, which is a good approximation of the strong energy changes between the donor (HOMO) and acceptor (LUMO) in the CMC–N-fullerene–AMPS hydrogel [75].
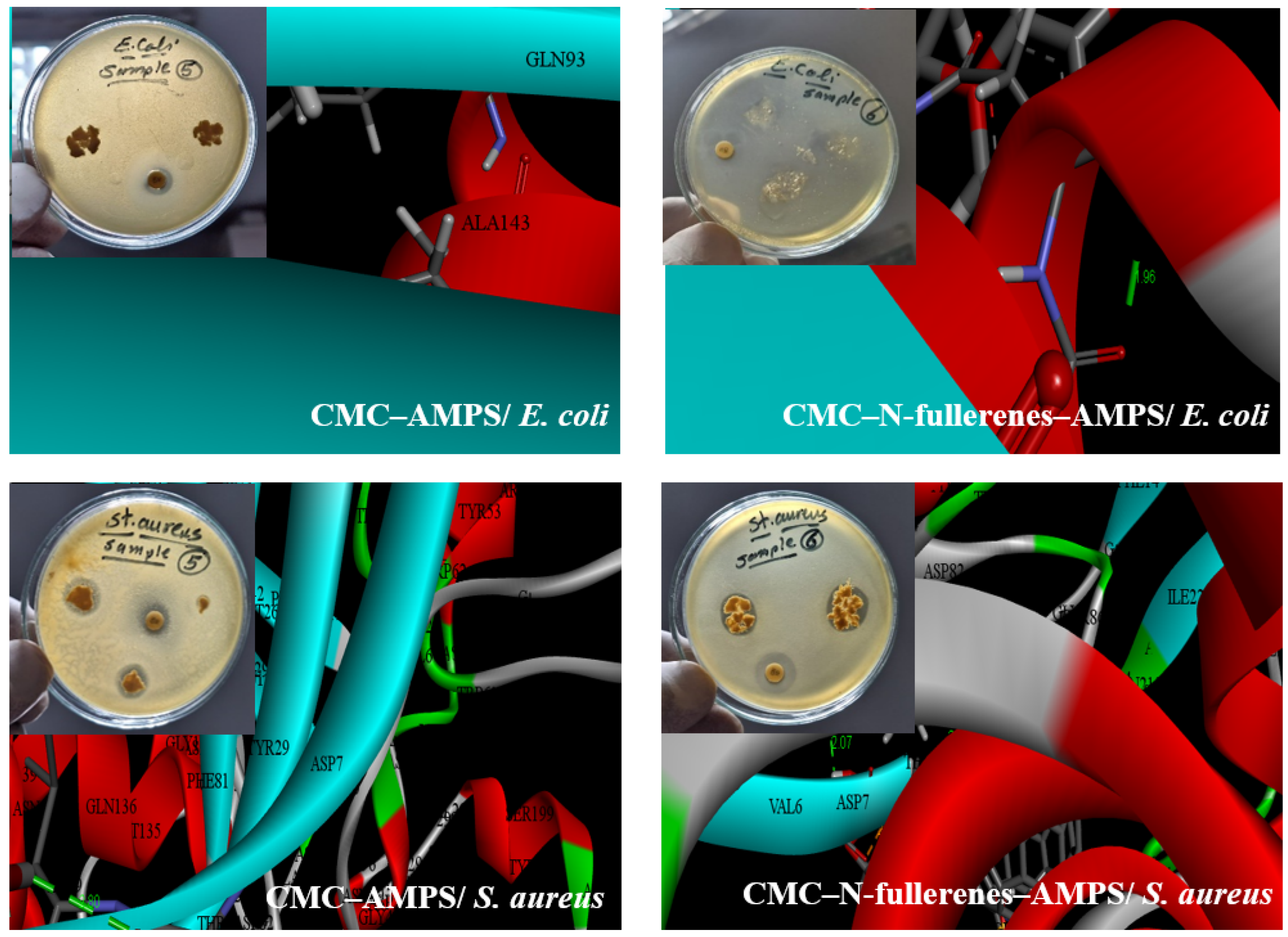
2.4. Antibacterial Activity and Molecular Docking Study
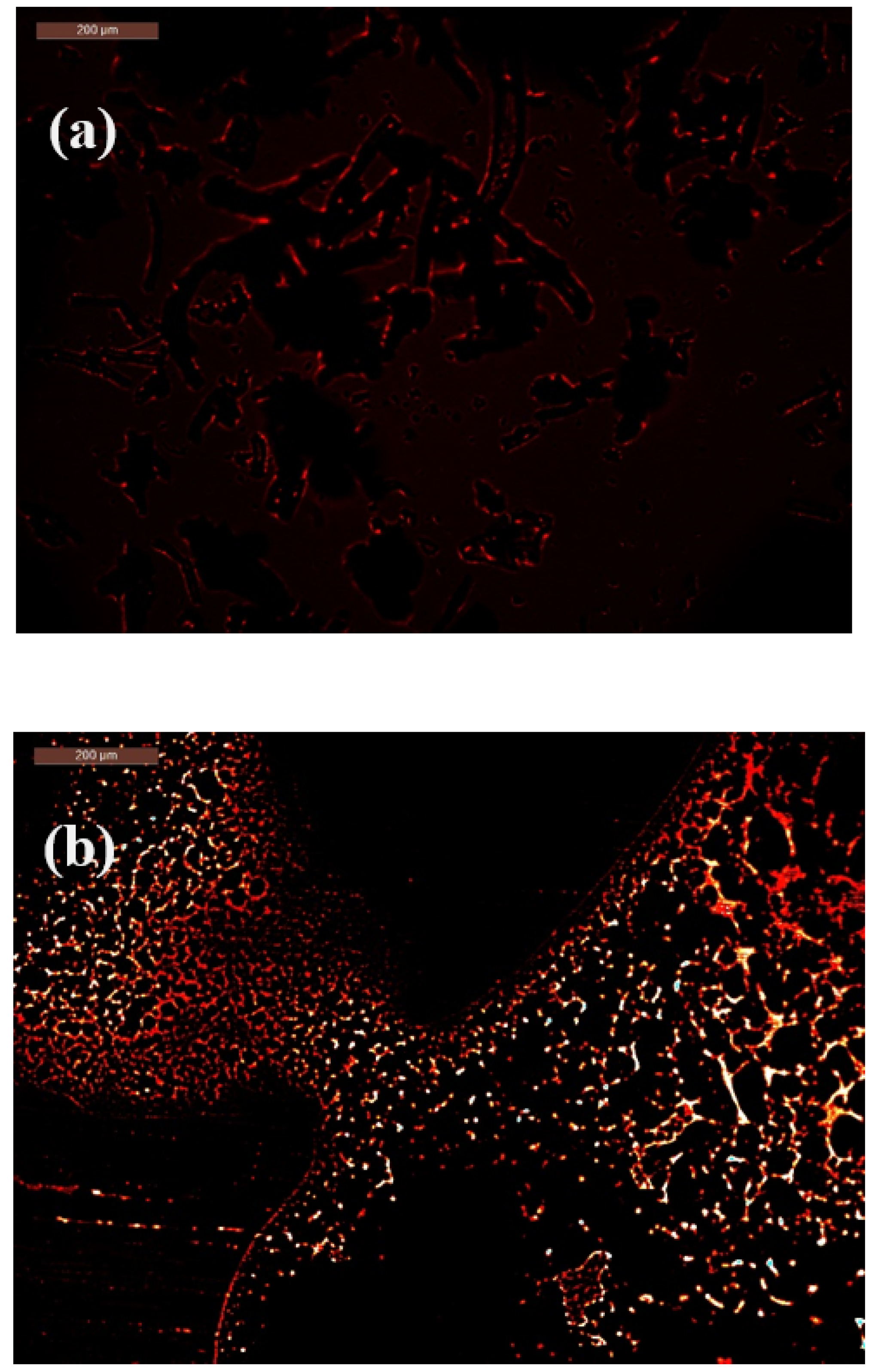
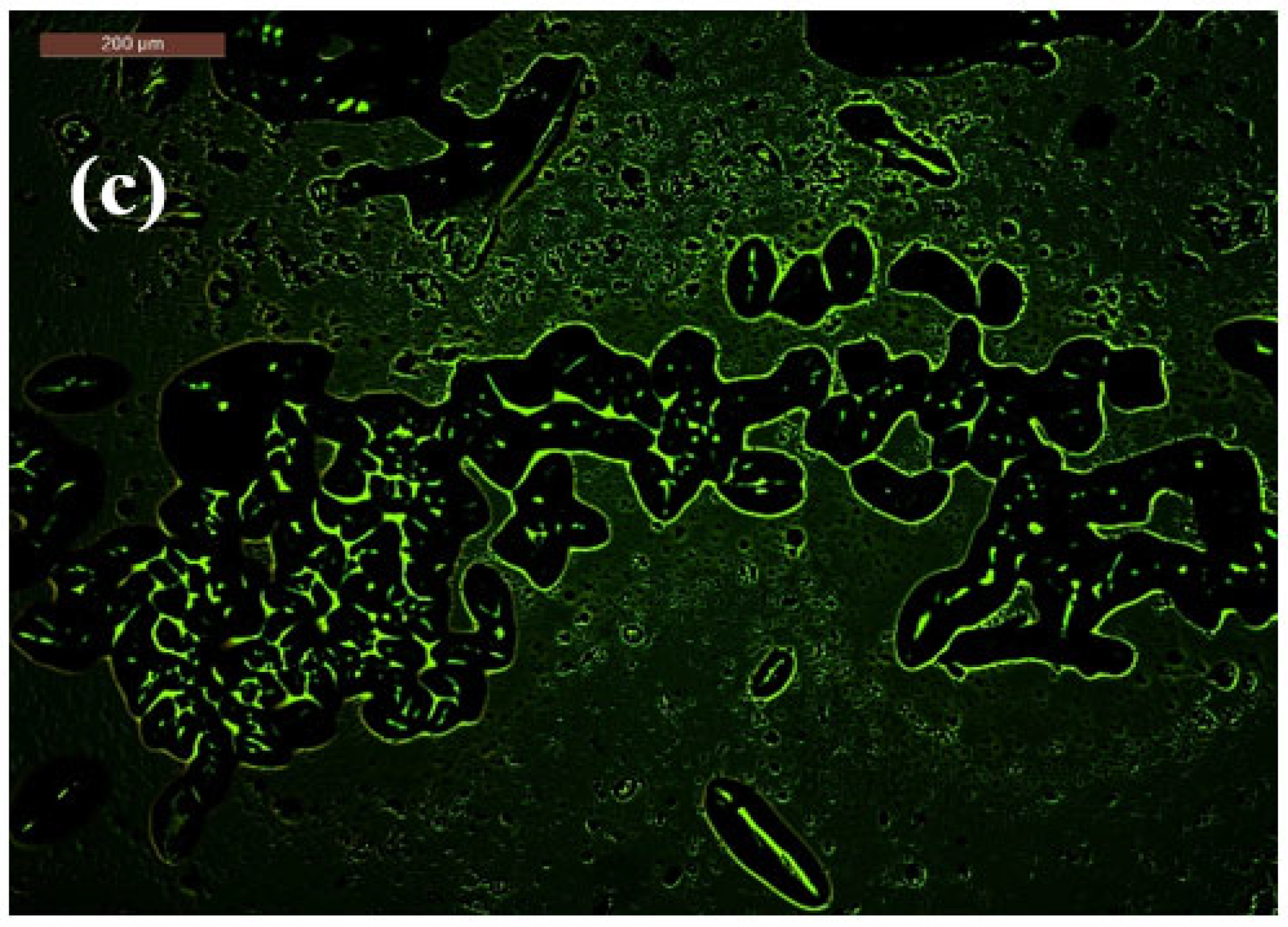
2.5. Mechanism of Bacterial-Induced Fluorescence Enhancement After Bacterial Contact
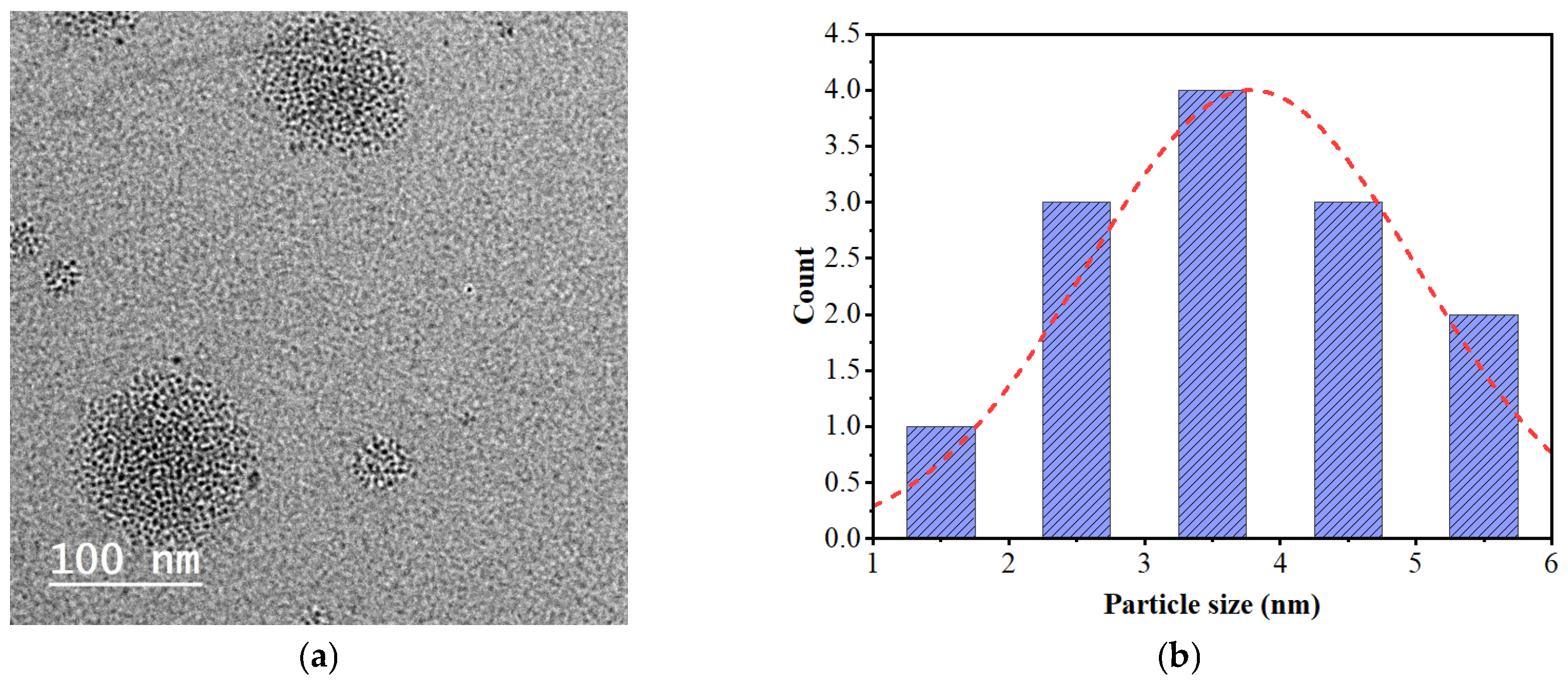
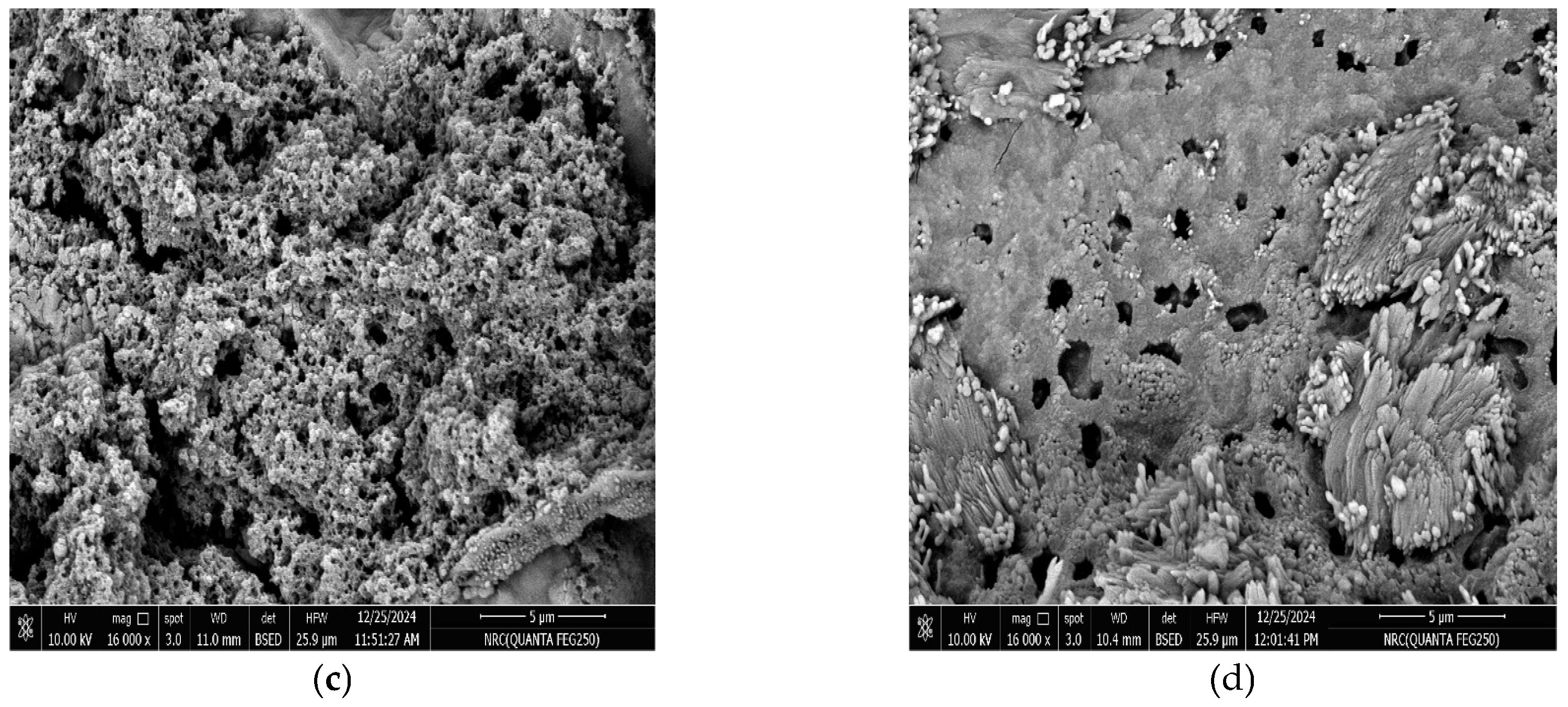
2.6. Morphological Observations
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Preparation of Cellulose
4.3. Microwave-Assisted Synthesis of Carboxymethyl Cellulose
4.4. Preparation of Nitrogen-Doped Fullerenes (N-fullerenes)
4.5. Preparation of Carboxymethyl cellulose–N-fullerene–g-poly(co-acrylamido-2-methyl-1-propane Sulfonic Acid)
4.6. Characterization
4.6.1. Morphological Observations
4.6.2. Fluorescence Microscope
4.6.3. Fourier-Transform Infrared (FTIR) Spectra
4.6.4. DFT Calculations
4.6.5. Swelling Behavior
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Squires, V.R.; Gaur, M.K. Food Security and Land Use Change Under Conditions of Climatic Variability: A Multidimensional Perspective; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Martindale, W. Global Food Security and Supply; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Aslam, J.; Parray, H.A.; Aslam, A.; Aslam, R. Food Waste Environmental Impact Assessment. In Sustainable Food Waste Management: Anti-corrosion Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 87–105. [Google Scholar]
- Singh, A.; Tyagi, P.K.; Garg, A. Sustainable Disposal Methods of Food Wastes in Hospitality Operations; IGI Global: Hershey, PA, USA, 2024. [Google Scholar]
- Wong, M.H.; Purchase, D.; Dickinson, N. Impacts, Management, and Recycling of Food Waste: Global Emerging Issues. In Food Waste Valorisation: Food, Feed, Fertiliser, Fuel and Value-Added Products; World Scientific: Singapore, 2023; pp. 3–31. [Google Scholar]
- Chapagain, A.; James, K. Accounting for the impact of food waste on water resources and climate change. In Food Industry Wastes; Academic Press: Cambridge, MA, USA, 2013; pp. 217–236. [Google Scholar] [CrossRef]
- Chai, X.; Tonjes, D.J.; Mahajan, D. Methane emissions as energy reservoir: Context, scope, causes and mitigation strategies. Prog. Energy Combust. Sci. 2016, 56, 33–70. [Google Scholar] [CrossRef]
- Zuberi, M.J.S.; Ali, S.F. Greenhouse effect reduction by recovering energy from waste landfills in Pakistan. Renew. Sustain. Energy Rev. 2015, 44, 117–131. [Google Scholar] [CrossRef]
- Ishangulyyev, R.; Kim, S.; Lee, S.H. Understanding food loss and waste—Why are we losing and wasting food? Foods 2019, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Thyberg, K.L.; Tonjes, D.J. Drivers of food waste and their implications for sustainable policy development. Resour. Conserv. Recycl. 2016, 106, 110–123. [Google Scholar] [CrossRef]
- Mosomi, E.K.; Olanrewaju, O.A.; Adeosun, S.O. Pivotal role of polylactide in carbon emission reduction: A comprehensive review. Eng. Rep. 2024, 6, e12909. [Google Scholar] [CrossRef]
- Soo, X.Y.D.; Muiruri, J.K.; Wu, W.Y.; Yeo, J.C.C.; Wang, S.; Tomczak, N.; Thitsartarn, W.; Tan, B.H.; Wang, P.; Wei, F. Bio-Polyethylene and Polyethylene Biocomposites: An Alternative toward a Sustainable Future. Macromol. Rapid Commun. 2024, 45, 2400064. [Google Scholar] [CrossRef]
- Macheca, A.D.; Mutuma, B.; Adalima, J.L.; Midheme, E.; Lúcas, L.H.; Ochanda, V.K.; Mhlanga, S.D. Perspectives on plastic waste management: Challenges and possible solutions to ensure its sustainable use. Recycling 2024, 9, 77. [Google Scholar] [CrossRef]
- Jiao, H.; Ali, S.S.; Alsharbaty, M.H.M.; Elsamahy, T.; Abdelkarim, E.; Schagerl, M.; Al-Tohamy, R.; Sun, J. A critical review on plastic waste life cycle assessment and management: Challenges, research gaps, and future perspectives. Ecotoxicol. Environ. Saf. 2024, 271, 115942. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S. Cellulosic schiff base hydrogel biosensor for bacterial detection with pH/thermo-responsitivity: DFT calculations and molecular docking. Int. J. Biol. Macromol. 2024, 283, 137389. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S. Novel, Speedy, and Eco-Friendly Carboxymethyl Cellulose-Nitrogen Doped Carbon Dots Biosensors with DFT Calculations, Molecular Docking, and Experimental Validation. Gels 2024, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Afolabi, O.A.; Turup Pandurangan, M.; Kanny, K. Types of Biobased Nanomaterials. In Biobased Nanomaterials: Applications in Biomedicine, Food Industry, Agriculture, and Environmental Sustainability; Springer: Berlin/Heidelberg, Germany, 2024; pp. 17–43. [Google Scholar]
- Gaur, M.; Misra, C.; Yadav, A.B.; Swaroop, S.; Maolmhuaidh, F.Ó.; Bechelany, M.; Barhoum, A. Biomedical applications of carbon nanomaterials: Fullerenes, quantum dots, nanotubes, nanofibers, and graphene. Materials 2021, 14, 5978. [Google Scholar] [CrossRef]
- Khan, T.; Singh, B.; Manikandan, M. Synthesis, Characteristics, and Applications of Nanomaterials. In Nanomaterials: The Building Blocks of Modern Technology: Synthesis, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2023; pp. 11–26. [Google Scholar]
- Prasad, R.D.; Sahoo, A.; Shrivastav, O.P.; Charmode, N.; Prasad, S.R.; Kamat, R.; Kajave, N.; Chauhan, J.; Banga, S.; Tamboli, U. A review on aspects of nanotechnology in food science and animal nutrition. ES Food Agrofor. 2022, 8, 12–46. [Google Scholar]
- Markovic, Z.; Trajkovic, V. Biomedical potential of the reactive oxygen species generation and quenching by fullerenes (C60). Biomaterials 2008, 29, 3561–3573. [Google Scholar] [CrossRef] [PubMed]
- Pantarotto, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Synthesis and biological properties of fullerene-containing amino acids and peptides. Mini Rev. Med. Chem. 2004, 4, 805–814. [Google Scholar] [CrossRef]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in food science: Applications, recent trends, and future perspectives. Nano-Micro Lett. 2020, 12, 1–34. [Google Scholar] [CrossRef]
- Papadochristopoulos, A.; Kerry, J.P.; Fegan, N.; Burgess, C.M.; Duffy, G. Natural anti-microbials for enhanced microbial safety and shelf-life of processed packaged meat. Foods 2021, 10, 1598. [Google Scholar] [CrossRef]
- Balasundaram, R.P.; Gunasekaran, P.; Ramaraj, A.; Jothiprakash, G.; Jaganathan, D. Role of Nanomaterials in Food Packaging Applications. In Introduction to Functional Nanomaterials; CRC Press: Boca Raton, FL, USA, 2024; pp. 290–301. [Google Scholar]
- Khan, A.; Ezati, P.; Tammina, S.K.; Riahi, Z. Packaging and Preservation. In Functional Nanomaterials and Nanocomposites for Biodegradable Food Packaging; Springer: Berlin/Heidelberg, Germany, 2025; p. 109. [Google Scholar]
- Sinha, S.; Sanfo, K.; Dallas, P.; Kumar, S.; Porfyrakis, K. Process parameter optimisation for endohedral metallofullerene synthesis via the arc-discharge method. Inorganics 2024, 12, 38. [Google Scholar] [CrossRef]
- Gupta, P.K. Mechanism of Nanotoxicity. In Nanotoxicology in Nanobiomedicine; Springer International Publishing: Cham, Switzerland; Springer: Berlin/Heidelberg, Germany, 2023; pp. 37–53. [Google Scholar]
- Gaganpreet; Pathania, Y. Carbon-Based Nanomaterials for Pollutants’ Treatment. In Carbon-Based Nanomaterials for Green Applications; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 355–381. [Google Scholar]
- Bharti, R.; Kaushik, P.; Naik, S.; Thakur, A.; Verma, M.; Sharma, R. Low Temperature Synthesis of Carbon Nanostructures. In Handbook of Functionalized Carbon Nanostructures: From Synthesis Methods to Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–45. [Google Scholar]
- Sharma, G. Biogenic carbon nanostructured materials for detection of cancer and medical applications: A mini review. Hybrid Adv. 2024, 5, 100166. [Google Scholar] [CrossRef]
- Danial, W.H. Synthesis of Carbon Nanostructures from Waste Materials. In Handbook of Functionalized Carbon Nanostructures: From Synthesis Methods to Applications; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–38. [Google Scholar]
- Tohamy, H.-A.S.; El-Sakhawy, M.; Kamel, S. A Greener Future: Carbon Nanomaterials from Lignocellulose. J. Renew. Mater. 2024, 13, 21–47. [Google Scholar]
- Levchenko, I.; Mandhakini, M.; Prasad, K.; Bazaka, O.; Ivanova, E.P.; Jacob, M.V.; Baranov, O.; Riccardi, C.; Roman, H.E.; Xu, S.; et al. Functional Nanomaterials from Waste and Low-Value Natural Products: A Technological Approach Level. Adv. Mater. Technol. 2022, 7, 2101471. [Google Scholar] [CrossRef]
- Kumar, K.; Kumar, R.; Kaushal, S.; Thakur, N.; Umar, A.; Akbar, S.; Ibrahim, A.A.; Baskoutas, S. Biomass waste-derived carbon materials for sustainable remediation of polluted environments: A comprehensive review. Chemosphere 2023, 345, 140419. [Google Scholar] [CrossRef] [PubMed]
- Poonia, K.; Singh, P.; Ahamad, T.; Van Le, Q.; Quang, H.H.P.; Thakur, S.; Mishra, A.K.; Selvasembian, R.; Hussain, C.M.; Nguyen, V.-H.; et al. Sustainability, performance, and production perspectives of waste-derived functional carbon nanomaterials towards a sustainable environment: A review. Chemosphere 2024, 352, 141419. [Google Scholar] [CrossRef]
- Salehi, B.; Wang, L. Critical review on nanomaterials for enhancing bioconversion and bioremediation of agricultural wastes and wastewater. Energies 2022, 15, 5387. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S.; El-Sakhawy, M.; Kamel, S. Fullerenes and tree-shaped/fingerprinted carbon quantum dots for chromium adsorption via microwave-assisted synthesis. RSC Adv. 2024, 14, 25785–25792. [Google Scholar] [CrossRef]
- Sabui, P.; Mallick, S.; Singh, K.R.; Natarajan, A.; Verma, R.; Singh, J.; Singh, R.P. Potentialities of fluorescent carbon nanomaterials as sensor for food analysis. Luminescence 2023, 38, 1047–1063. [Google Scholar] [CrossRef]
- Chen, D.; Gong, J.; Grüne, J.; Matulaitis, T.; Gillett, A.J.; Zhang, X.H.; Samuel, I.D.; Turnbull, G.A.; Zysman-Colman, E. Tetra-Donor Pyrazine Based Thermally Activated Delayed Fluorescence Emitters for Electroluminescence and Amplified Spontaneous Emission. Adv. Funct. Mater. 2024, 34, 2409592. [Google Scholar] [CrossRef]
- Hasan, M.; Shukla, A.; Mamada, M.; Adachi, C.; Lo, S.-C.; Namdas, E.B. Correlating exciton dynamics of thermally activated delayed-fluorescence emitters to efficiency roll-off in OLEDs. Phys. Rev. Appl. 2022, 18, 054082. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, G.; Chen, F.; Chen, J.; Zhang, Y.; Di, Y.; Chen, Y.; Zhu, Y.; Lin, M.; Chen, H. Thermally Activated Delayed Fluorescent Ag (I) Complexes for Highly Efficient Scintillation and High-Resolution X-Ray Imaging. Adv. Funct. Mater. 2024, 34, 2400436. [Google Scholar] [CrossRef]
- Feng, Q.; Zhu, S.; Wang, B.; Yu, F.; Li, H.; Yu, M.; Xu, M.; Xie, L. Thermally Activated Delayed Fluorescence Macrocycles for Organic Light-Emitting Diodes. Adv. Funct. Mater. 2024, 34, 2312622. [Google Scholar] [CrossRef]
- Baleizão, C.; Berberan-Santos, M.N. Thermally activated delayed fluorescence in fullerenes. Ann. N. Y. Acad. Sci. 2008, 1130, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Yuan, K.; Chen, T.; Xu, P.; Li, H.; Chen, R.; Zheng, C.; Zhang, L.; Huang, W. Thermally activated delayed fluorescence materials towards the breakthrough of organoelectronics. Adv. Mater. 2014, 26, 7931–7958. [Google Scholar] [CrossRef] [PubMed]
- Lichota, A.; Szabelski, M.; Krokosz, A. Quenching of protein fluorescence by fullerenol C60(OH)36 nanoparticles. Int. J. Mol. Sci. 2022, 23, 12382. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Bai, Y.; Mu, Y.; Pan, B.; Xing, B.; Lin, Y. Fluorescence quenching of fulvic acids by fullerene in water. Environ. Pollut. 2013, 172, 100–107. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, L.; Hu, Y.; Zhou, C.; Lan, W.; Fu, H.; She, Y. Nanomaterials as optical sensors for application in rapid detection of food contaminants, quality and authenticity. Sens. Actuators B Chem. 2021, 329, 129135. [Google Scholar] [CrossRef]
- Das, J.; Mishra, H.N. Recent advances in sensors for detecting food pathogens, contaminants, and toxins: A review. Eur. Food Res. Technol. 2022, 248, 1125–1148. [Google Scholar] [CrossRef]
- Bulgakov, R.; Galimov, D. Fullerene C 60 as a superefficient quencher of singlet exited states of polycyclic aromatic hydrocarbons. Russ. Chem. Bull. 2007, 56, 446–451. [Google Scholar] [CrossRef]
- Liu, S.; Ji, X.-X.; Zhu, J.-F. Recent progress in the synthesis and biomedical properties of natural biopolymer composites. Curr. Med. Chem. 2021, 28, 8243–8266. [Google Scholar] [CrossRef]
- Dobrzyńska-Mizera, M.; Dodda, J.M.; Liu, X.; Knitter, M.; Oosterbeek, R.N.; Salinas, P.; Pozo, E.; Ferreira, A.M.; Sadiku, E.R. Engineering of Bioresorbable Polymers for Tissue Engineering and Drug Delivery Applications. Adv. Healthc. Mater. 2024, 13, 2401674. [Google Scholar] [CrossRef]
- Dispenza, C.; Giacomazza, D.; Jonsson, M. Micro-to Nanoscale Bio-Hybrid Hydrogels Engineered by Ionizing Radiation. Biomolecules 2021, 11, 47. [Google Scholar] [CrossRef]
- Gurusamy, L.C. AMPs Based Rheology Modifier for Personal Care Industry. Ph.D. Thesis, Lehigh University, Bethlehem, PA, USA, 2019. [Google Scholar]
- Karchoubi, F.; Ghotli, R.A.; Pahlevani, H.; Salehi, M.B. New insights into nanocomposite hydrogels; A review on recent advances in characteristics and applications. Adv. Ind. Eng. Polym. Res. 2024, 7, 54–78. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S. Fluorescence ‘Turn-on’ Probe for Chromium Reduction, Adsorption and Detection Based on Cellulosic Nitrogen-Doped Carbon Quantum Dots Hydrogels. Gels 2024, 10, 296. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, H.; Pervaiz, F.; Rehman, S.; Noreen, S. Development of β-cyclodextrin/chitosan-co-poly (2-acrylamide-2-methylpropane sulphonic acid) cross-linked hybrid IPN-nanogels to enhance the solubility of rosuvastatin: An in vitro and in vivo attributes. J. Drug Deliv. Sci. Technol. 2022, 75, 103696. [Google Scholar] [CrossRef]
- Singha, N.R.; Dutta, A.; Mahapatra, M.; Roy, J.S.D.; Mitra, M.; Deb, M.; Chattopadhyay, P.K. In situ attachment of acrylamido sulfonic acid-based monomer in terpolymer hydrogel optimized by response surface methodology for individual and/or simultaneous removal (s) of M (III) and cationic dyes. ACS Omega 2019, 4, 1763–1780. [Google Scholar] [CrossRef]
- Mondal, S.; Das, S.; Nandi, A.K. A review on recent advances in polymer and peptide hydrogels. Soft Matter 2020, 16, 1404–1454. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S.; Mohamed, F.E.-Z.S.; El-Sakhawy, M. Novel microwave assisted carboxymethyl-graphene oxide and its hepatoprotective activity. BMC Pharmacol. Toxicol. 2024, 25, 50. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Liu, D.; Yi, M.; Chang, F.; Li, H.; Du, Y. A review of sulfate radical-based and singlet oxygen-based advanced oxidation technologies: Recent advances and prospects. Catalysts 2022, 12, 1092. [Google Scholar] [CrossRef]
- Seo, K.; Chung, I.-J. Reaction analysis of 3, 4-ethylenedioxythiophene with potassium persulfate in aqueous solution by using a calorimeter. Polymer 2000, 41, 4491–4499. [Google Scholar] [CrossRef]
- Hsu, S.-C.; Don, T.-M.; Chiu, W.-Y. Free radical degradation of chitosan with potassium persulfate. Polym. Degrad. Stab. 2002, 75, 73–83. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Bao, Y. Advances in cellulose-based superabsorbent hydrogels. RSC Adv. 2015, 5, 59745–59757. [Google Scholar] [CrossRef]
- El-Sakhawy, M.; Tohamy, H.-A.S.; AbdelMohsen, M.M.; El-Missiry, M. Biodegradable carboxymethyl cellulose based material for sustainable/active food packaging application. J. Thermoplast. Compos. Mater. 2024, 37, 2035–2050. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S.; El-Sakhawy, M.; Abdel-Halim, S.A.; El-Masry, H.M.; AbdelMohsen, M.M. Antimicrobial Plectranthus amboinicus emulsions prepared with amphiphilic cellulose stearate. In Euro-Mediterranean Journal for Environmental Integration; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–12. [Google Scholar]
- Cuba-Chiem, L.T.; Huynh, L.; Ralston, J.; Beattie, D.A. In situ particle film ATR FTIR spectroscopy of carboxymethyl cellulose adsorption on talc: Binding mechanism, pH effects, and adsorption kinetics. Langmuir 2008, 24, 8036–8044. [Google Scholar] [CrossRef] [PubMed]
- Cukrowicz, S.; Grabowski, B.; Kaczmarska, K.; Bobrowski, A.; Sitarz, M.; Tyliszczak, B. Structural studies (FTIR, XRD) of sodium carboxymethyl cellulose modified bentonite. Arch. Foundry Eng. 2020, 20, 119–125. [Google Scholar] [CrossRef]
- Jahit, I.; Nazmi, N.; Isa, M.; Sarbon, N. Preparation and physical properties of gelatin/CMC/chitosan composite films as affected by drying temperature. Int. Food Res. J. 2015, 23, 1068–1074. [Google Scholar]
- Mottola, S.; Viscusi, G.; Tohamy, H.-A.S.; El-Sakhawy, M.; Gorrasi, G.; De Marco, I. Application of electrospun N-doped carbon dots loaded cellulose acetate membranes as cationic dyes adsorbent. J. Environ. Manag. 2024, 370, 122714. [Google Scholar] [CrossRef] [PubMed]
- Mohkami, M.; Talaeipour, M. Investigation of the chemical structure of carboxylated and carboxymethylated fibres from waste paper via XRD and FTIR analysis. Bioresources 2011, 6, 1988–2003. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S. Speedy synthesis of magnetite/carbon dots for efficient chromium removal and reduction: A combined experimental and DFT approach. In Emergent Materials; Springer: Berlin/Heidelberg, Germany, 2024; pp. 1–13. [Google Scholar]
- Garcia-Borras, M.; Osuna, S.; Luis, J.M.; Swart, M.; Sola, M. The role of aromaticity in determining the molecular structure and reactivity of (endohedral metallo) fullerenes. Chem. Soc. Rev. 2014, 43, 5089–5105. [Google Scholar] [CrossRef]
- Nattagh, F.; Hosseini, S.; Esrafili, M.D. Effects of B and N doping/codoping on the adsorption behavior of C60 fullerene towards aspirin: A DFT investigation. J. Mol. Liq. 2021, 342, 117459. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S. Greener, Safer Packaging: Carbon Nanotubes/Gelatin-Enhanced Recycled Paper for Fire Retardation with DFT Calculations. J. Renew. Mater. 2024, 12, 1963–1983. [Google Scholar] [CrossRef]
- Tohamy, H.-A.S. Carboxymethyl hemicellulose hydrogel as a fluorescent biosensor for bacterial and fungal detection with DFT and molecular docking studies. Sci. Rep. 2025, 15, 741. [Google Scholar] [CrossRef]
- Varghese, M.; Balachandran, M. Antibacterial efficiency of carbon dots against Gram-positive and Gram-negative bacteria: A review. J. Environ. Chem. Eng. 2021, 9, 106821. [Google Scholar] [CrossRef]
- Niranjan, R.; Zafar, S.; Lochab, B.; Priyadarshini, R. Synthesis and characterization of sulfur and sulfur-selenium nanoparticles loaded on reduced graphene oxide and their antibacterial activity against gram-positive pathogens. Nanomaterials 2022, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Travlou, N.A.; Giannakoudakis, D.A.; Algarra, M.; Labella, A.M.; Rodríguez-Castellón, E.; Bandosz, T.J. S-and N-doped carbon quantum dots: Surface chemistry dependent antibacterial activity. Carbon 2018, 135, 104–111. [Google Scholar] [CrossRef]
- Ruan, Z.; Xu, Z.; Liu, T.; Chen, L.; Liu, X.; Chen, K.; Zhao, C. Multifunctional nitrogen-sulfur codoped carbon quantum dots: Determining reduced glutathione, broad-spectrum antibacterial activity, and cell imaging. Heliyon 2024, 10, e38177. [Google Scholar] [CrossRef]
- Saravanan, A.; Das, P.; Maruthapandi, M.; Aryal, S.; Michaeli, S.; Mastai, Y.; Luong, J.H.; Gedanken, A. Heteroatom co-doping (N, NS, NB) on carbon dots and their antibacterial and antioxidant properties. Surf. Interfaces 2024, 46, 103857. [Google Scholar] [CrossRef]
- Li, Y.; Han, Y.; Li, H.; Niu, X.; Zhang, D.; Wang, K. Antimicrobial hydrogels: Potential materials for medical application. Small 2024, 20, 2304047. [Google Scholar] [CrossRef]
- Fantini, J.; Yahi, N. Brain Lipids in Synaptic Function and Neurological Disease: Clues to Innovative Therapeutic Strategies for Brain Disorders; Academic Press: Cambridge, MA, USA, 2015. [Google Scholar]
- Baskar, A.V.; Benzigar, M.R.; Talapaneni, S.N.; Singh, G.; Karakoti, A.S.; Yi, J.; Al-Muhtaseb, A.a.H.; Ariga, K.; Ajayan, P.M.; Vinu, A. Self-assembled fullerene nanostructures: Synthesis and applications. Adv. Funct. Mater. 2022, 32, 2106924. [Google Scholar] [CrossRef]
- Lee, C.; Seo, Y.; Han, J.; Hwang, J.; Jeon, I. Perspectives on critical properties of fullerene derivatives for rechargeable battery applications. Carbon 2023, 210, 118041. [Google Scholar] [CrossRef]
- Omar Khudhur, Z.; HMaad, A.; AGhanimi, H.; Abdolmaleki, A. Fullerene nanoparticle as new therapeutic agent for the nervous system disorders. Nanomed. J. (NMJ) 2024, 11, 342–359. [Google Scholar]
- Hildebrandt, N.; Spillmann, C.M.; Algar, W.R.; Pons, T.; Stewart, M.H.; Oh, E.; Susumu, K.; Diaz, S.A.; Delehanty, J.B.; Medintz, I.L. Energy transfer with semiconductor quantum dot bioconjugates: A versatile platform for biosensing, energy harvesting, and other developing applications. Chem. Rev. 2017, 117, 536–711. [Google Scholar] [CrossRef]
- Szöllosi, J.; Damjanovich, S.; Mátyus, L. Application of fluorescence resonance energy transfer in the clinical laboratory: Routine and research. Cytom. J. Int. Soc. Anal. Cytol. 1998, 34, 159–179. [Google Scholar]
- Bhupathi, P.; Elhassan A-Elgadir, T.M.; Mohammed Ali, R.H.; Sanaan Jabbar, H.; Gulnoza, D.; Joshi, S.; Kadhem Abid, M.; Ahmed Said, E.; Alawadi, A.; Alsaalamy, A. Fluorescence Resonance Energy Transfer (FRET)-Based Sensor for Detection of Foodborne Pathogenic Bacteria: A Review. Crit. Rev. Anal. Chem. 2023, 1–18. [Google Scholar] [CrossRef] [PubMed]
- De, A.; Arora, R.; Jasani, A. Engineering aspects of bioluminescence resonance energy transfer systems. In Engineering in Translational Medicine; Springer: London, UK, 2013; pp. 257–300. [Google Scholar]
- Lone, M.S.; Bhat, P.A.; Afzal, S.; Chat, O.A.; Dar, A.A. Energy transduction through FRET in self-assembled soft nanostructures based on surfactants/polymers: Current scenario and prospects. Soft Matter 2021, 17, 425–446. [Google Scholar] [CrossRef]
- Gottschalk, G. Regulation of Bacterial Metabolism; Springer: Berlin/Heidelberg, Germany, 1986; pp. 178–207. [Google Scholar] [CrossRef]
- Somerville, G.A.; Proctor, R.A. At the crossroads of bacterial metabolism and virulence factor synthesis in Staphylococci. Microbiol. Mol. Biol. Rev. 2009, 73, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Hassanein, H.D.; Tohamy, H.A.S.; AbdelMohsen, M.M.; El-Masry, H.M.; El-Sakhawy, M. Preparation and characterization of cellulose acetate/corn silk extract films for potential antimicrobial application. Egypt. J. Chem. 2024, 68, 311–318. [Google Scholar] [CrossRef]
| DFT B3LYP/6–31G (d) | CMC | MBA | AMPS | CMC–AMPS | N-Fullerenes | CMC–N-fullerene–AMPS |
|---|---|---|---|---|---|---|
| ELUMO (eV) | −0.1232 | −0.0196 | 0.0250 | −0.0578 | −0.1332 | −0.1053 |
| EHOMO (eV) | −0.1650 | −0.3027 | −0.2713 | −0.2033 | −0.1755 | −0.1924 |
| Eg (eV) | 0.0419 | 0.2831 | 0.2963 | 0.1454 | 0.0423 | 0.0871 |
| ET (au) | 1434.557 | −525.486 | −939.989 | −2741.402 | −2290.040 | −5158.364 |
| μ (Debye) | 11.141 | 3.781 | 9.219 | 24.309 | 1.334 | 41.321 |
| ʷ (eV) | 0.0209 | 0.1415 | 0.1481 | 0.0727 | 0.0211 | 0.0435 |
| σ (eV) | 47.732 | 7.0646 | 6.748 | 13.750 | 47.281 | 22.962 |
| S (eV) | 23.866 | 3.5323 | 3.374 | 6.875 | 23.640 | 11.481 |
| ω (eV) | 0.4955 | 0.0917 | 0.0511 | 0.1172 | 0.5632 | 0.2543 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tohamy, H.-A.S. Fullerene-Functionalized Cellulosic Hydrogel Biosensor with Bacterial Turn-on Fluorescence Response Derived from Carboxymethyl Cellulose for Intelligent Food Packaging with DFT Calculations and Molecular Docking. Gels 2025, 11, 329. https://doi.org/10.3390/gels11050329
Tohamy H-AS. Fullerene-Functionalized Cellulosic Hydrogel Biosensor with Bacterial Turn-on Fluorescence Response Derived from Carboxymethyl Cellulose for Intelligent Food Packaging with DFT Calculations and Molecular Docking. Gels. 2025; 11(5):329. https://doi.org/10.3390/gels11050329
Chicago/Turabian StyleTohamy, Hebat-Allah S. 2025. "Fullerene-Functionalized Cellulosic Hydrogel Biosensor with Bacterial Turn-on Fluorescence Response Derived from Carboxymethyl Cellulose for Intelligent Food Packaging with DFT Calculations and Molecular Docking" Gels 11, no. 5: 329. https://doi.org/10.3390/gels11050329
APA StyleTohamy, H.-A. S. (2025). Fullerene-Functionalized Cellulosic Hydrogel Biosensor with Bacterial Turn-on Fluorescence Response Derived from Carboxymethyl Cellulose for Intelligent Food Packaging with DFT Calculations and Molecular Docking. Gels, 11(5), 329. https://doi.org/10.3390/gels11050329









