Botryosphaeriaceae Species Causing Stem Blight and Dieback of Blueberries in Serbia
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Isolations
2.2. Morphological and Ecological Characterization
2.3. DNA Amplification and Sequencing
2.4. Sequence and Phylogenetic Analyses
2.5. Pathogenicity Testing
2.6. Cultivar Susceptibility Testing
3. Results
3.1. Disease Symptoms and Isolates
3.2. Fungal Morphology
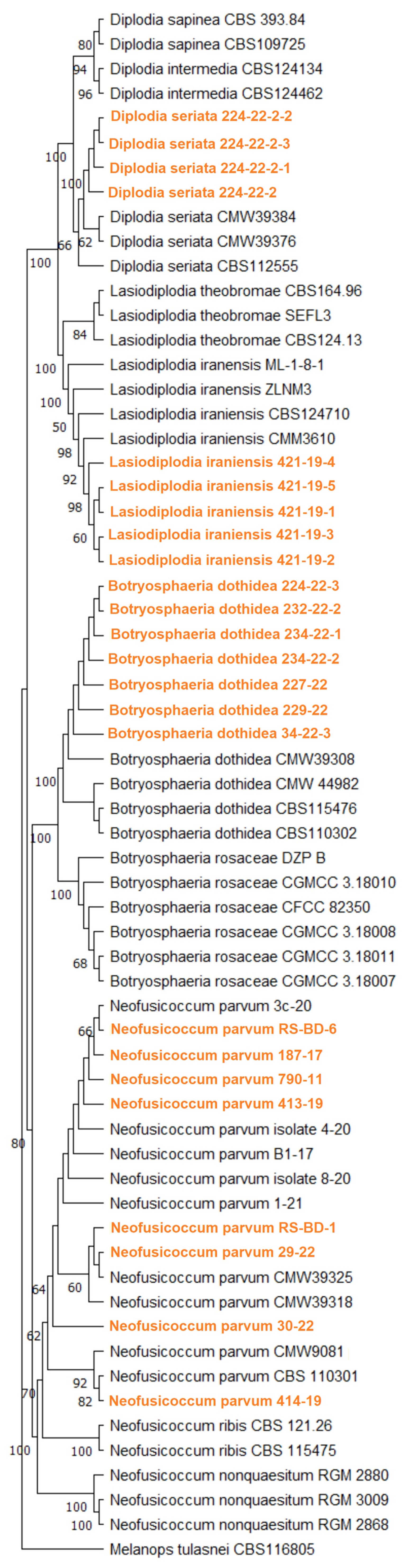
3.3. Molecular Identification and Phylogenetic Analyses
3.4. Pathogenicity
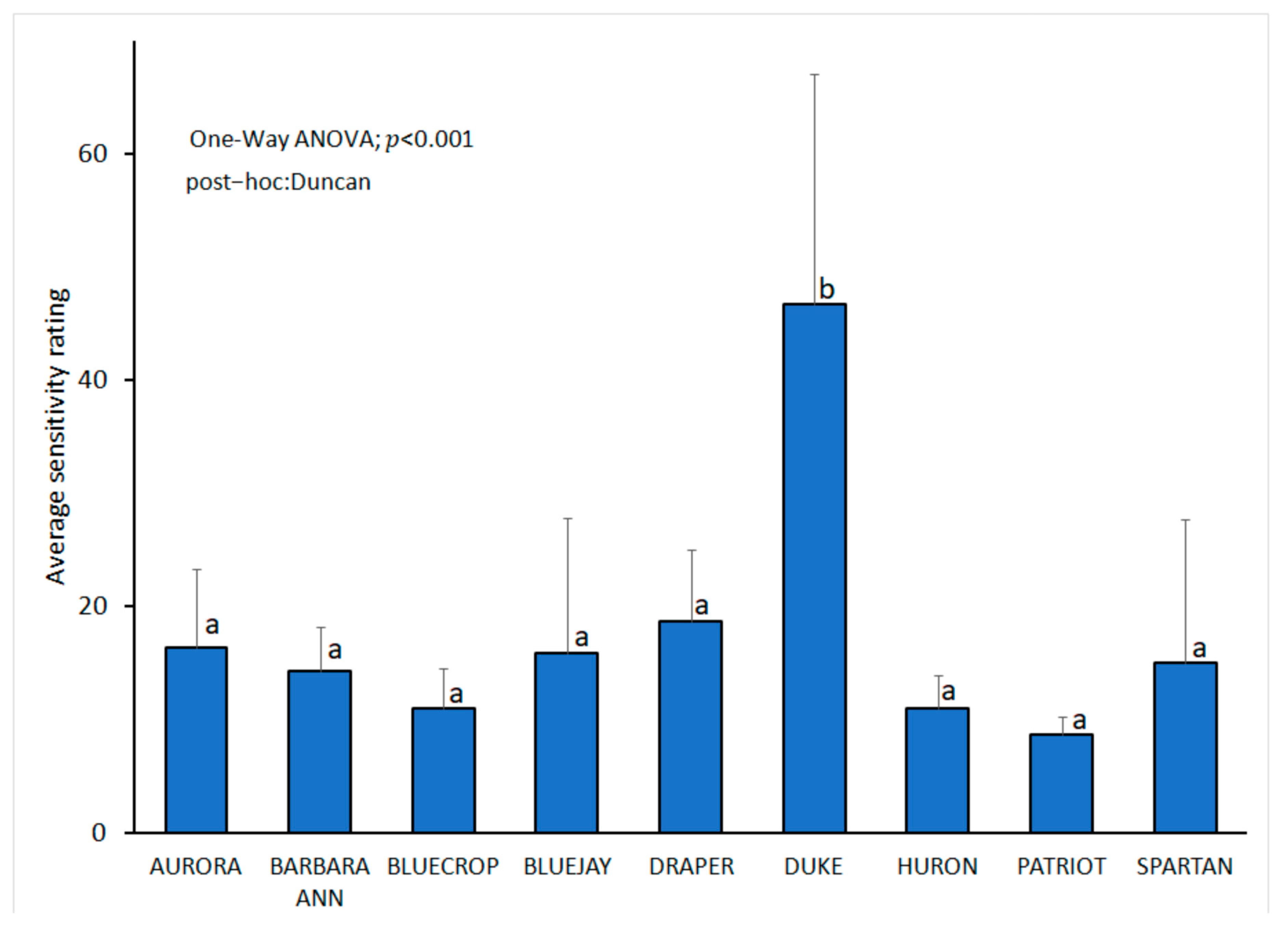
3.5. Cultivar Susceptibility
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PDA | Potato Dextrose Agar |
| PNA | Pine Needle Agar |
| PDB | Potato Dextrose Broth |
| ITS | Internal Transcribed Spacer |
| TEF1-α | Translation Elongation Factor 1α |
| TUB2 | Beta Tubulin |
| DNA | Deoxyribonucleic Acid |
| Nt | Nucleotide |
| Bp | Base Pair |
| Dpi | Days Per Inoculation |
| Cv | Cultivar |
| PCR | Polymerase Chain Reaction |
References
- Retamales, J.B.; Hancock, J.F. Blueberries, 2nd ed.; Bell and Bain Ltd.: Glasgow, UK, 2018; pp. 1–323. [Google Scholar]
- Kalt, W.; Dufour, D. Health functionality of blueberries. HortTechnology 1997, 7, 216–221. [Google Scholar] [CrossRef]
- Leposavić, A.; Jevremović, D. Blueberry-Technology of Growing, Protection and Processing; Scientific Pomological Society of Serbia: Belgrade, Serbia, 2020. [Google Scholar]
- Milivojević, J. Jagodaste Voćke; Univerzitet u Beogradu, Poljoprivredni Fakultet: Beograd-Zemun, Serbia, 2022; pp. 339–429. [Google Scholar]
- Milholland, R.D.; Galletta, G.J. Pathogenic variation among isolates of Botryosphaeria corticis on blueberry. Phytopathology 1969, 59, 1540–1543. [Google Scholar]
- Sammonds, J.; Billones, R.; Rocchetti, M.; Ridgway, H.J.; Walter, M.; Jaspers, M.V. Survey of blueberry farms for Botryosphaeria dieback and crown rot pathogens. N. Z. Plant Prot. 2009, 62, 238–242. [Google Scholar] [CrossRef]
- Wright, A.F.; Harmon, P.F. First report of Lasiodiplodia theobromae associated with stem blight of southern highbush blueberries in Florida. Plant Dis. 2009, 93, 962. [Google Scholar] [CrossRef]
- Xu, C.; Zhang, H.; Zhou, Z.; Hu, T.; Wang, S.; Wang, Y.; Cao, K. Identification and distribution of Botryosphaeriaceae species associated with blueberry stem blight in China. Eur. J. Plant Pathol. 2015, 143, 737–752. [Google Scholar] [CrossRef]
- Aviles, M.; De los Santos, B.; Borrero, C. Increase of canker disease severity in blueberries caused by Neofusicoccum parvum or Lasiodiplodia theobromae due to interaction with Macrophomina phaseolina root infection. Eur. J. Plant Pathol. 2021, 159, 655–663. [Google Scholar] [CrossRef]
- Tennakoon, K.M.S.; Ridgway, H.J.; Jaspers, M.V.; Jones, E.E. Botryosphaeriaceae species associated with blueberry dieback and sources of primary inoculum in propagation nurseries in New Zealand. Eur. J. Plant Pathol. 2017, 150, 363–374. [Google Scholar] [CrossRef]
- Ru, S.; Ding, S.; Oliver, J.E.; Amodu, A. A review of Botryosphaeria stem blight disease of blueberry from the perspective of plant breeding. Agriculture 2022, 13, 100. [Google Scholar] [CrossRef]
- Garcia, J.F.; Lawrence, D.P.; Morales-Cruz, A.; Travadon, R.; Minio, A.; Hernandez-Martinez, R.; Rolshausen, P.E.; Baumgartner, K.; Cantu, D. Phylogenomics of plant-associated Botryosphaeriaceae species. Front. Microbiol. 2021, 12, 652802. [Google Scholar] [CrossRef]
- Denman, S.; Crous, P.W.; Taylor, J.E.; Kang, J.C.; Pascoe, I.; Wingfield, M.J. An overview of the taxonomic history of Botryosphaeria, and a re-evaluation of its anamorphs based on morphology and ITS rDNA phylogeny. Stud. Mycol. 2000, 45, 129–140. [Google Scholar]
- Phillips, A.J.L.; Alves, A.; Abdollahzadeh, J.; Slippers, B.; Wingfield, M.J.; Groenewald, J.Z.; Crous, P.W. The Botryosphaeriaceae: Genera and species known from culture. Stud. Mycol. 2013, 76, 51–67. [Google Scholar] [CrossRef]
- Slippers, B.; Crous, P.W.; Jami, F.; Groenewald, J.Z.; Wingfield, M.J. Diversity in the Botryosphaeriales: Looking back, looking forward. Fungal Biol. 2017, 121, 307–321. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; He, W.; Zhang, Y. Stem blight of blueberry caused by Lasiodiplodia vaccinii sp. nov. in China. Plant Dis. 2019, 103, 2041–2050. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Bhoyroo, V.; Rampadarath, S.; Jeewon, R. Multigene phylogenetics and morphology reveal five novel Lasiodiplodia species associated with blueberries. Life 2021, 11, 657. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Plant health horizon scanning newsletter. EFSA Support. Publ. 2023, 20, 7813E. [Google Scholar] [CrossRef]
- Jibrin, M.O.; Liu, Q.; Huang, Y.; Urbina, H.; Gazis, R.; Zhang, S. Lasiodiplodia iraniensis, a new causal agent of tuber rot on yam (Dioscorea species) imported into the United States and implication for quarantine decisions. Plant Dis. 2022, 106, 3027–3032. [Google Scholar] [CrossRef]
- Abdollahzadeh, J.; Javadi, A.; Mohammadi Goltapeh, E.; Zare, R.; Phillips, A.J.L. Phylogeny and morphology of four new species of Lasiodiplodia from Iran. Persoonia 2010, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cruywagen, E.M.; Slippers, B.; Roux, J.; Wingfield, M.J. Phylogenetic species recognition and hybridization in Lasiodiplodia: A case study on species from baobabs. Fungal Biol. 2017, 121, 420–436. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Galvez, E.; Guerrero, P.; Barradas, C.; Crous, P.W.; Alves, A. Phylogeny and pathogenicity of Lasiodiplodia species associated with dieback of mango in Peru. Fungal Biol. 2017, 121, 452–465. [Google Scholar] [CrossRef]
- Bautista-Cruz, M.A.; Almaguer-Vergas, G.; Leyva-Mir, S.G.; Colinas-Leon, M.T.; Correia, K.C.; Camacho-Tapia, M.; Robles-Yerena, L.; Michereff, S.J.; Tovar-Pedraza, J.M. Phylogeny, distribution, and pathogenicity of Lasiodiplodia species associated with cankers and dieback symptoms of Persian lime in Mexico. Plant Dis. 2019, 103, 1156–1165. [Google Scholar] [CrossRef]
- Sakalidis, M.L.; Ray, J.D.; Lanoiselet, V.; Hardy, G.E.S.J.; Burgess, T.I. Pathogenic Botryosphaeriaceae associated with Mangifera indica in the Kimberley Region of Western Australia. Eur. J. Plant Pathol. 2011, 130, 379–391. [Google Scholar] [CrossRef]
- Al-Sadi, A.M.; Al-Wehaibi, A.N.; Al-Shariqi, R.M.; Al-Hammadi, M.S.; Al-Hosni, I.A.; Al-Mahmooli, I.H.; Al-Ghaithi, A.G. Population genetic analysis reveals diversity in Lasiodiplodia species infecting date palm, Citrus, and mango in Oman and the UAE. Plant Dis. 2013, 97, 1363–1369. [Google Scholar] [CrossRef]
- Marques, M.W.; Lima, N.B.; Morals, M.A., Jr.; Barbosa, M.A.G.; Souza, B.O.; Michereff, S.; Phillips, J.A.L.; Câmara, M.P.S. Species of Lasiodiplodia associated with mango in Brazil. Fungal Divers. 2013, 61, 181–193. [Google Scholar] [CrossRef]
- Li, G.Q.; Arnold, R.J.; Liu, F.F.; Li, J.Q.; Chen, S.F. Identification and pathogenicity of Lasiodiplodia species from Eucalyptus urophylla × grandis, Polyscias balfouriana and Bougainvillea spectabilis in Southern China. J. Phytopathol. 2015, 163, 956–967. [Google Scholar] [CrossRef]
- Netto, M.S.B.; Lima, W.G.; Correia, K.C.; Da Silva, C.F.B.; Thon, M.; Marting, R.B.; Miller, R.N.G.; Michereff, S.J.; Câmara, M.P.S. Analysis of phylogeny, distribution, and pathogenicity of Botryosphaeriaceae species associated with gummosis of Anacardium in Brazil, with a new species of Lasiodiplodia. Fungal Biol. 2017, 121, 437–451. [Google Scholar] [CrossRef]
- El-Ganainy, S.M.; Ismail, A.M.; Iqbal, Z.; Elshewy, E.S.; Alhudaib, K.A.; Almaghasla, M.I.; Magista, D. Diversity among Lasiodiplodia species causing dieback, root rot and leaf spot on fruit trees in Egypt, and a description of Lasiodiplodia newvalleyensis sp. nov. J. Fungi 2022, 8, 1203. [Google Scholar] [CrossRef]
- Negi, N.; Krishna, R.; Meena, R.K.; Pandey, A.; Bhandari, M.S.; Pandey, S. First report of Lasiodiplodia iraniensis causing leaf spot disease of Eucalyptus in India. Physiol. Mol. Plant Pathol. 2023, 127, 102113. [Google Scholar] [CrossRef]
- Silva, D.E.M.; Vieira, R.F.B.S.; Inokuti, E.M.; Almeida, M.M.M.; Cordeiro, M.V.M.; Lima, C.S.; Oster, A.H.; Silva, C.F.B. First report of Lasiodiplodia iraniensis causing crown rot on banana fruits in Brazil. Plant Dis. 2023, 107, 3315. [Google Scholar] [CrossRef]
- Piattino, V.; Aiello, D.; Dardani, G.; Martino, I.; Flores, M.; Aćimović, S.G.; Spadaro, D.; Polizzi, G.; Guarnaccia, V. Lasiodiplodia iraniensis and Diaporthe spp. are associated with twig dieback and fruit stem-end rot of sweet orange, Citrus sinensis, in Florida. Horticulturae 2024, 10, 406. [Google Scholar] [CrossRef]
- Popović, T.; Blagojević, J.; Aleksić, G.; Jelušić, A.; Krnjajić, S.; Milovanović, P. A blight disease on highbush blueberry associated with Macrophomina phaseolina in Serbia. Can. J. Plant Pathol. 2018, 40, 121–127. [Google Scholar] [CrossRef]
- Ristić, D.; Vučurović, I.; Živković, S.; Starović, M.; Delibašić, G.; Tanović, B.; Aleksić, G. Fusarium sporotrichioides—Novi patogen borovnice u Srbiji. In Proceedings of the XVI Simpozijum o Zaštiti Bilja u Bosni i Hercegovini, Sarajevo, Bosnia and Herzegovina, 5–7 November 2019; pp. 42–43. [Google Scholar]
- Jevremović, D.; Vasić, T.; Živković, S.; Vasilijević, B.; Marić, M.; Vojvodić, M.; Bulajić, A. Neopestalotiopsis clavispora: A causal agent of twig dieback on highbush blueberries in Serbia. J. Plant Dis. Prot. 2022, 129, 1277–1283. [Google Scholar] [CrossRef]
- Blagojević, J.; Aleksić, G.; Vučurović, I.; Starović, M.; Ristić, D. Exploring the phylogenetic diversity of Botryosphaeriaceae and Diaporthe species causing dieback and shoot blight of blueberry in Serbia. Phytopathology 2024, 114, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, O.; Sinclair, J. Basic Plant Pathology Methods, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar] [CrossRef]
- Espinoza, J.G.; Briceño, E.X.; Chávez, E.R.; Úrbez-Torres, J.R.; Latorre, B.A. Neofusicoccum spp. associated with stem canker and dieback of blueberry in Chile. Plant Dis. 2009, 93, 1187–1194. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes—Application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Glass, N.L.; Donaldson, G.C. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microbiol. 1995, 61, 1323–1330. [Google Scholar] [CrossRef]
- Carbone, I.; Kohn, L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 1999, 91, 553–556. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Zlatković, M.; Keča, N.; Wingfield, M.J.; Jami, F.; Slippers, B. Shot hole disease on Prunus laurocerasus caused by Neofusicoccum parvum. Antonie Leeuwenhoek 2016, 109, 543–564. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.P.; Zhang, M.; Dou, Z.P.; Zhang, Y. Botryosphaeria rosaceae sp. nov. and B. ramosa, new botryosphaeriaceous taxa from China. Mycosphere 2017, 8, 162–171. [Google Scholar] [CrossRef]
- Vučković, N.; Duduk, N.; Rekanović, E.; Duduk, B.; Vico, I. First report of Botryosphaeria dothidea causing root rot of sugar beet in Serbia. Plant Dis. 2024, 108, 3658. [Google Scholar] [CrossRef]
- Bezerra, J.D.P.; Crous, P.W.; Aiello, D.; Gullino, M.L.; Polizzi, G.; Guarnaccia, V. Genetic diversity and pathogenicity of Botryosphaeriaceae species associated with symptomatic citrus plants in Europe. Plants 2021, 10, 492. [Google Scholar] [CrossRef]
- de Silva, N.I.; Phillips, A.J.L.; Liu, J.K.; Lumyong, S.; Hyde, K.D. Phylogeny and morphology of Lasiodiplodia species associated with Magnolia forest plants. Sci. Rep. 2019, 9, 14355. Available online: https://www.nature.com/articles/s41598-019-50804-x (accessed on 23 September 2024). [CrossRef]
- Rodriguez-Galvez, E.; Hilário, S.; Lopes, A.; Alves, A. Diversity and pathogenicity of Lasiodiplodia and Neopestalotiopsis species associated with stem blight and dieback of blueberry plants in Peru. Eur. J. Plant Pathol. 2020, 157, 89–102. [Google Scholar] [CrossRef]
- Tennakoon, D.S.; Kuo, C.H.; Maharachchikumbura, S.S.N.; Thambugala, K.M.; Gentekaki, E.; Phillips, A.J.L.; Bhat, D.J.; Wanasinghe, D.N.; De Silva, N.I.; Promputtha, I.; et al. Taxonomic and phylogenetic contributions to Celtis formosana, Ficus ampelas, F. septica, Macaranga tanarius and Morus australis leaf litter inhabiting microfungi. Fungal Divers. 2021, 108, 1–215. [Google Scholar] [CrossRef]
- Rathnayaka, A.R.; Chethana, K.W.T.; Manawasinghe, I.S.; Wijesinghe, S.N.; De Silva, N.I.; Tennakoon, D.S.; Phillips, A.J.L.; Liu, J.K.; Jones, E.B.G.; Wang, Y.; et al. Lasiodiplodia: Generic revision by providing molecular markers, geographical distribution and haplotype diversity. Mycosphere 2023, 14, 1254–1339. [Google Scholar] [CrossRef]
- Machado, A.; Pinho, D.; Pereira, O. Phylogeny, identification and pathogenicity of the Botryosphaeriaceae associated with collar and root rot of the biofuel plant Jatropha curcas in Brazil, with a description of new species of Lasiodiplodia. Fungal Divers. 2014, 67, 231–247. [Google Scholar] [CrossRef]
- Hilario, S.; Lopes, A.; Santos, L.; Alves, A. Botryosphaeriaceae species associated with blueberry stem blight and dieback in the Centre region of Portugal. Eur. J. Plant Pathol. 2020, 156, 31–44. [Google Scholar] [CrossRef]
- Zlatković, M.; Keča, N.; Wingfield, M.J.; Jami, F.; Slippers, B. Botryosphaeriaceae associated with the die-back of ornamental trees in Serbia. Forest Pathol. 2016, 46, 267–282. [Google Scholar] [CrossRef]
- Zlatković, M.; Wingfield, M.J.; Jami, F.; Slippers, B. Genetic uniformity characterizes the Invasive spread of Neofusicoccum parvum. Plant Pathol. 2019, 68, 1153–1163. [Google Scholar] [CrossRef]
- Karličić, V.; Jovičić-Petrović, J.; Marojević, V.; Zlatković, M.; Orlović, S.; Raičević, V. Potential of Trichoderma spp. and Pinus sylvestris bark extracts as biocontrol agents against fungal pathogens residing in the Botryosphaeriales. Environ. Sci. Proc. 2021, 3, 99. [Google Scholar] [CrossRef]
- Vasić, M.; Duduk, N.; Vico, I.; Ivanović, M.S. First report of Botryosphaeria dothidea causing white rot of apple fruit in Serbia. Plant Dis. 2013, 97, 1659. [Google Scholar] [CrossRef]
- Vučković, N.; Vico, I.; Duduk, B.; Duduk, N. Diversity of Botryosphaeriaceae and Diaporthe species associated with postharvest apple fruit decay in Serbia. Phytopathology 2022, 112, 929–943. [Google Scholar] [CrossRef]
- Vučković, N.; Vico, I.; Duduk, N. First report of Botryosphaeria dothidea causing postharvest rot of quince fruits in Serbia. J. Plant Pathol. 2023, 105, 605. [Google Scholar] [CrossRef]
- Vico, I.; Žebeljan, A.; Vučković, N.; Vasić, M.; Duduk, N. First report of Diplodia seriata causing postharvest rot of quince fruit in Serbia. Plant Dis. 2017, 101, 1823. [Google Scholar] [CrossRef]
- Flor, N.C.; Wright, A.F.; Huguet-Tapia, J.; Harmon, P.F.; Liberti, D. Identification of fungi in the Botryosphaeriaceae family associated with stem blight of Vaccinium spp. in the southeastern United States. Fungal Biol. 2022, 126, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.N.; Zhao, H.H.; Yu, Y.Y.; Li, X.D.; Liang, C.; Li, B.D. The pathogen causing Lasiodiplodia twig blight of blueberry. Mycosystema 2016, 35, 657–665. [Google Scholar] [CrossRef]
- Wiseman, M.S.; Serdani, M.; Putnam, M.L. A new cane dieback disease of northern highbush blueberry in the United States caused by Lasiodiplodia mediterranea. Plant Dis. 2017, 101, 1317. [Google Scholar] [CrossRef]
- Scarlett, K.A.; Shuttleworth, L.A.; Collins, D.; Rothwell, C.T.; Guest, D.I.; Daniel, R. Botryosphaeriales associated with stem blight and dieback of blueberry (Vaccinium spp.) in New South Wales and Western Australia. Australas. Plant Pathol. 2019, 48, 45–57. [Google Scholar] [CrossRef]
- Rebollar-Alviter, A.; Boyzo-Marín, J.; Silva-Rojas, H.V.; Ramírez, G. Fungi and oomycete pathogens causing stem blight and root rots on blueberry in Central Mexico. Phytopathology 2013, 103, 119–120. [Google Scholar]
- Borrero, C.; Perez, S.; Aviles, M. First report of canker disease caused by Lasiodiplodia theobromae on blueberry bushes in Spain. Plant Dis. 2019, 103, 10. [Google Scholar] [CrossRef]
- Latorre, B.A.; Díaz, G.A.; Reed, M.P. Effect of water activity on in vitro mycelial growth of Neofusicoccum spp. infecting blueberry. Cienc. Investig. Agrar. 2012, 9, 221–228. [Google Scholar] [CrossRef]
- Babiker, E.M.; Stringer, S.J.; Sakhanokho, H.F.; Smith, B.J. Characterization and pathogenicity of stem blight complex isolates associated with stem blight disease on Vaccinium species. HortScience 2019, 54, 1199–1203. [Google Scholar] [CrossRef]
- Choi, S.; Paul, N.C.; Lee, K.-H.; Kim, H.-J.; Sang, H.M. Morphology, molecular phylogeny, and pathogenicity of Neofusicoccum parvum, associated with leaf spot disease of a new host, the Japanese bay tree (Machilus thunbergii). Forests 2021, 12, 440. [Google Scholar] [CrossRef]
- Milas, P.; Barra-Bucarei, L.; Castro, J.F.; Carrasco-Fernández, J.; Chilian, J.; Tapia, E.; Santelices, C.; Cisterna-Oyarce, V.; Muñoz, V.; Ortiz-Campos, J.; et al. Identification and distribution of species of Neofusicoccum that cause blueberry stem blight in Chile. Mycologia 2023, 115, 326–339. [Google Scholar] [CrossRef] [PubMed]
- Petrović, E.; Vrandečić, K.; Belušić Vozila, A.; Ćosić, J.; Godena, S. Diversity and pathogenicity of Botryosphaeriaceae species isolated from olives in Istria, Croatia, and evaluation of varietal resistance. Plants 2024, 13, 1813. [Google Scholar] [CrossRef]
- Li, X.; Yan, J.; Kong, F.; Qiao, G.; Zhang, Z.; Wang, Z. Botryosphaeria dothidea causing canker of grapevine newly reported in China. Plant Pathol. 2010, 59, 1170. [Google Scholar] [CrossRef]
- Yu, L.; Rarisara, I.; Xu, S.G.; Wu, X.; Zhao, J.R. First report of stem blight of blueberry caused by Botryosphaeria dothidea in China. Plant Dis. 2012, 96, 1697. [Google Scholar] [CrossRef]
- Yan, J.; Xie, Y.; Yao, S.; Wang, Z.; Li, X. Characterization of Botryosphaeria dothidea, the causal agent of grapevine canker in China. Australas. Plant Pathol. 2012, 41, 351–357. [Google Scholar] [CrossRef]
- Marsberg, A.; Kemler, M.; Jami, F.; Nagel, J.H.; Postma-Smidt, A.; Naidoo, S.; Wingfield, M.J.; Crous, P.W.; Spatafora, J.W.; Hesse, C.N.; et al. Botryosphaeria dothidea: A latent pathogen of global importance to woody plant health. Mol. Plant Pathol. 2017, 18, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Kovač, M.; Diminić, D.; Orlović, S.; Zlatković, M. Botryosphaeria Dothidea and Neofusicoccum Yunnanense Causing Canker and Die-Back of Sequoiadendron Giganteum in Croatia. Forests 2021, 12, 695. [Google Scholar] [CrossRef]
- Phillips, A.J.L.; Crous, P.W.; Alves, A. Diplodia seriata, the anamorph of “Botryosphaeria” obtusa. Fungal Divers. 2007, 25, 141–155. [Google Scholar]
- Gonzalez-Dominguez, E.; Alves, A.; León, M.; Armengol, J. Characterization of Botryosphaeriaceae species associated with diseased loquat (Eriobotrya japonica) in Spain. Plant Pathol. 2017, 66, 77–89. [Google Scholar] [CrossRef]
- Arrigoni, E.; Oliveira Longa, C.M.; Angeli, D.; Soini, M.; Pertot, I.; Perazzolli, M. A fast and reliable method for Diplodia seriata inoculation of trunks and assessment of fungicide efficacy on potted apple plants under greenhouse conditions. Phytopathol. Mediterr. 2019, 58, 163–173. [Google Scholar] [CrossRef]
- Manetti, G.; Brunetti, A.; Sciarroni, L.; Lumia, V.; Bechini, S.; Marangi, P.; Reverberi, M.; Scortichini, M.; Pilotti, M. Diplodia seriata Isolated from Declining Olive Trees in Salento (Apulia, Italy): Pathogenicity Trials Give a Glimpse That It Is More Virulent to Drought-Stressed Olive Trees and in a Warmth-Conditioned Environment. Plants 2024, 13, 2245. [Google Scholar] [CrossRef] [PubMed]
- Baskarathevan, J.; Jaspers, M.V.; Jones, E.E.; Cruickshank, R.H.; Ridgway, H.J. Genetic and pathogenic diversity of Neofusicoccum parvum in New Zealand vineyards. Fungal Biol. 2012, 116, 276–288. [Google Scholar] [CrossRef]
- Trotel-Aziz, P.; Robert-Siegwald, G.; Fernandez, O.; Leal, C.; Villaume, S.; Guise, J.F.; Abou-Mansour, E.; Lebrun, M.H.; Fontaine, F. Diversity of Neofusicoccum parvum for the production of the phytotoxic metabolites (-)-Terremutin and (R)-Mellein. J. Fungi 2022, 8, 319. [Google Scholar] [CrossRef]
- Belair, M.; Picot, A.; Lepais, O.; Masson, C.; Hébrard, M.; Moronvalle, A.; Comont, G.; Gabri Martin, V.M.; Tréguer, S.; Laloum, Y.; et al. Genetic diversity and population structure of Botryosphaeria dothidea and Neofusicoccum parvum on English walnut (Juglans regia L.) in France. Sci. Rep. 2024, 14, 19817. [Google Scholar] [CrossRef] [PubMed]
- Elena, G.; Garcia-Figueres, F.; Reigada, S.; Luque, J. Intraspecific variation in Diplodia seriata isolates occurring on grapevines in Spain. Plant Pathol. 2015, 64, 680–689. [Google Scholar] [CrossRef]
- Bhat, A.H.; Shah, M.D.; Padder, B.A.; Shah, Z.A.; Dar, E.A.; Fayaz, U.; Nain, M.S.; Ali, M.A.; Al-Hemaid, F.M.; Stępień, P.; et al. Morphological, pathogenic and genetic diversity in Diplodia seriata associated with black rot canker of apple in India. Sci. Rep. 2023, 13, 15682. [Google Scholar] [CrossRef]
- Trakunyingcharoen, T.; Lombard, L.; Groenewald, J.Z.; Cheewangkoon, R.; To-anun, C.; Crous, P.W. Caulicolous Botryosphaeriales from Thailand. Persoonia 2015, 34, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008, 28, 1–13. [Google Scholar]
- Dou, Z.P.; He, W.; Zhang, Y. Does morphology matter in taxonomy of Lasiodiplodia? An answer from Lasiodiplodia hyalina sp. nov. Mycosphere 2017, 8, 1014–1027. [Google Scholar] [CrossRef]
- Gallardo, R.K.; Zhang, Q.; Dossett, M.; Polashock, J.J.; Rodriguez-Saona, C.; Vorsa, N.; Edger, P.P.; Ashrafi, H.; Babiker, E.; Finn, C.E.; et al. Breeding trait priorities of the blueberry industry in the United States and Canada. HortScience 2018, 53, 1021–1028. [Google Scholar] [CrossRef]
- Sakalidis, M.L.; Hardy, G.E.S.J.; Burgess, T.I. Endophytes as potential pathogens of the baobab species Adansonia gregorii: A focus on the Botryosphaeriaceae. Fungal Ecol. 2011, 4, 1–14. [Google Scholar] [CrossRef]



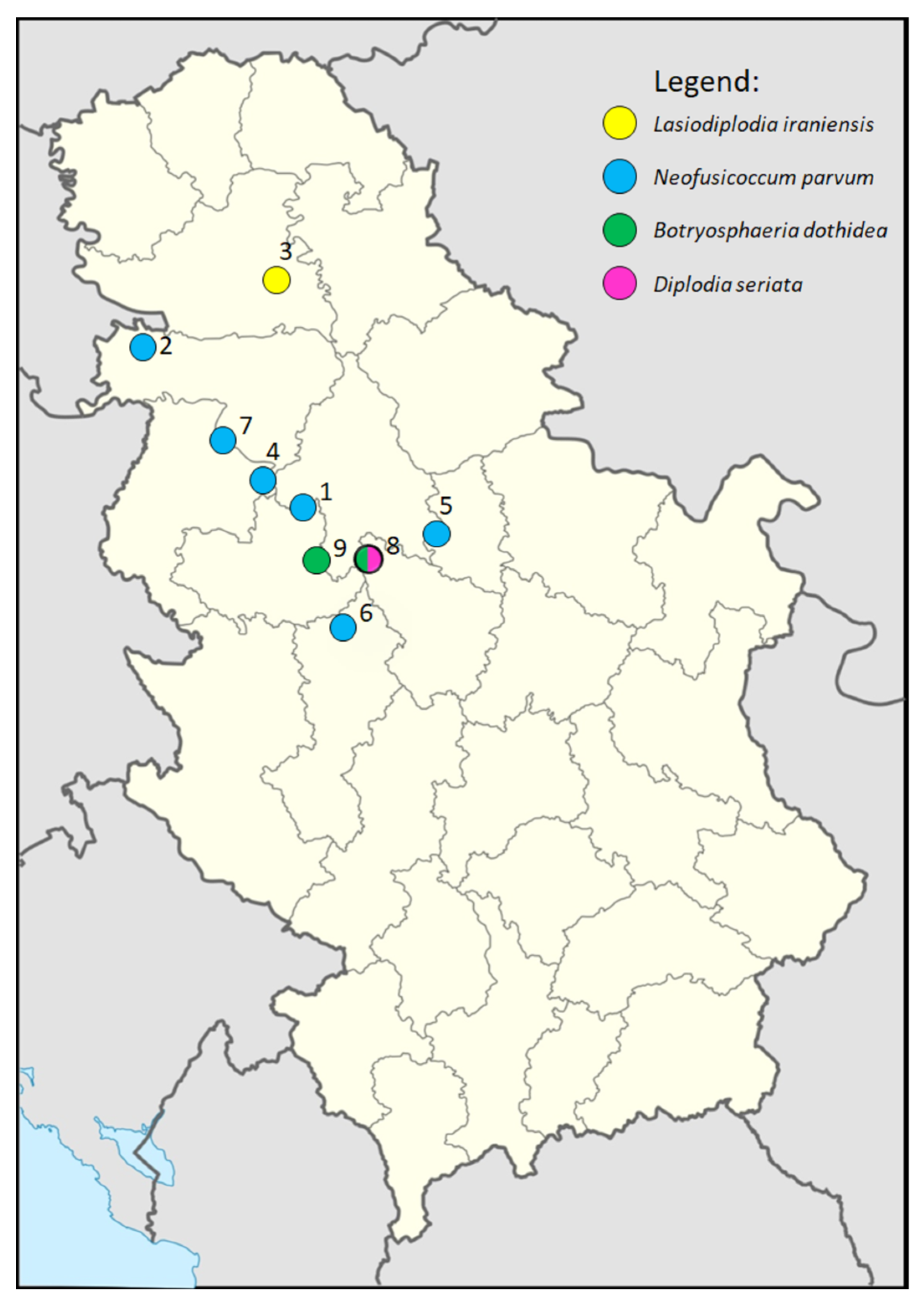
| No. * | Year | District | Locality | Blueberry Cultivar | Estimated Disease Incidence (%) | No. of Collected Samples | Fungal Species Detected (Positive Samples) | Isolates | |
|---|---|---|---|---|---|---|---|---|---|
| Culture | ITS Seq | ||||||||
| 1 | 2011 | Kolubara | Belanovica | Duke | 20 | 20 | Neofusicoccum parvum (16) Neopestalotiopsis spp. (11) Botrytis spp. (5) Epicoccum spp. (7) | 8 | 1 |
| 4 | 1 | ||||||||
| 3 | 1 | ||||||||
| 1 | 1 | ||||||||
| 2 | 2017 | Srem | Šid | Duke | 20 | 20 | Neofusicoccum parvum (18) Neopestalotiopsis spp. (14) | 14 11 | 2 1 |
| 3 | 2019 | Srem | Irig | Duke | 15 | 5 | Lasiodiplodia iraniensis (5) | 5 | 5 |
| 4 | Kolubara | Ub | Duke | 20 | 25 | Neofusicoccum parvum (18) | 6 | 2 | |
| 5 | 2020 | Belgrade | Sopot | Duke | 10 | 8 | Neofusicoccum parvum (7) Alternaria spp. (4) | 4 | 1 |
| 6 | Moravica | Gornji Milanovac | Duke | 30 | 7 | Neofusicoccum parvum (5) | 4 | 1 | |
| 7 | 2022 | Belgrade | Slatina | Duke | 15 | 20 | Neofusicoccum parvum (18) Diaporthe spp. (12) Peroneutypa spp. (7) Fusarium spp. (10) | 4 8 2 2 | 1 1 1 2 |
| 8 | Kolubara | Jajčić | Duke | 30 | 25 | Botryosphaeria dothidea (18) Diplodia seriata (8) Diaporthe spp. (5) Neopestalotiopsis spp. (16) | 12 5 1 4 | 4 4 1 3 | |
| 9 | Kolubara | Slavkovica | Duke | 25 | 23 | Botryosphaeria dothidea (15) Diaporthe spp. (17) | 8 8 | 3 2 | |
| GenBank Accessions | ||||||
|---|---|---|---|---|---|---|
| Species | Strain/Isolate | Host | Country | ITS | TEF1-α | β-Tubulin |
| Botryosphaeria dothidea | CBS115476 | Prunus sp. | Switzerland | AY236949 | AY236898 | AY236927 |
| Botryosphaeria dothidea | CBS110302 | Vitis vinifera | Portugal | AY259092 | AY573218 | EU673106 |
| Botryosphaeria dothidea | CMW44982 | Sequoiadendron giganteum | Serbia | KF575008 | KF575040 | KF575104 |
| Botryosphaeria dothidea | CMW39308 | Sequoiadendron giganteum | Serbia | KF575008 | KF575040 | KF575104 |
| Botryosphaeria dothidea | 34-22-3 | Vaccinium corymbosum | Serbia | PV235336 | PV296171 | PV278143 |
| Botryosphaeria dothidea | 234-22-1 | Vaccinium corymbosum | Serbia | PV268085 | PX056801 | PX056807 |
| Botryosphaeria dothidea | 227-22 | Vaccinium corymbosum | Serbia | PV263064 | PX056800 | PX056806 |
| Botryosphaeria dothidea | 229-22 | Vaccinium corymbosum | Serbia | PV268086 | PX056799 | PX056805 |
| Botryosphaeria dothidea | 224-22-3 | Vaccinium corymbosum | Serbia | PV263065 | PX056804 | PX056810 |
| Botryosphaeria dothidea | 234-22-2 | Vaccinium corymbosum | Serbia | PV263170 | PX056802 | PX056808 |
| Botryosphaeria dothidea | 232-22-2 | Vaccinium corymbosum | Serbia | PX048943 | PX056803 | PX056809 |
| Botryosphaeria rosaceae | CBSCGMCC 3.18007 | Malus sp. | China | KX197074 | KX197094 | KX197101 |
| Botryosphaeria rosaceae | CBSCGMCC 3.18008 | Amygdalus sp. | China | KX197075 | KX197095 | KX197102 |
| Botryosphaeria rosaceae | CFCC 82350 | Malus sp. | China | KX197079 | KX197097 | KX197106 |
| Botryosphaeria rosaceae | CGMCC3.18009 | Malus sp. | China | KX197076 | KX197096 | KX197103 |
| Botryosphaeria rosaceae | CBSCGMCC 3.18010 | Pyrus sp. | China | KX197077 | - | KX197104 |
| Botryosphaeria rosaceae | CBSCGMCC 3.18011 | Pyrus sp. | China | KX197078 | - | KX197105 |
| Diplodia intemerdia | CBS124134 | Cydonia sp. | Portugal | HM036528 | GQ923851 | KX464798 |
| Diplodia intermedia | CBS124462 | Malus sylvestris | Portugal | GQ923858 | GQ923826 | - |
| Diplodia sapinea | CBS393.84 | Pinus nigra | Netherlands | DQ458895 | DQ458880 | DQ458863 |
| Diplodia sapinea | CBS109725 | Pinus patula | South Africa | DQ458896 | DQ458881 | DQ458864 |
| Diplodia seriata | CMW39384 | Thuja occidentalis | Serbia | DQ458896 | DQ458881 | DQ458864 |
| Diplodia seriata | CMW39376 | Chamaecyparis pisifera | Serbia | KF574996 | KF575027 | KF575092 |
| Diplodia seriata | CBS112555 | Vitis vinifera | Portugal | AY259094 | AY573220 | DQ458856 |
| Diplodia seriata | 224-22-2 | Vaccinium corymbosum | Serbia | PV263172 | PV296172 | PV278144 |
| Diplodia seriata | 224-22-2-1 | Vaccinium corymbosum | Serbia | PX023087 | PX056793 | PX056796 |
| Diplodia seriata | 224-22-2-2 | Vaccinium corymbosum | Serbia | PX022810 | PX056794 | PX056797 |
| Diplodia seriata | 224-22-2-3 | Vaccinium corymbosum | Serbia | PX022815 | PX056795 | PX056798 |
| Dothiorella viticola | CBS 117009 | Vitis vinifera | Spain | AY905554 | AY905559 | EU673104 |
| Lasiodiplodia americana | CERC 1961 | Pistacia vera | USA: Arizona | KP217059 | KP217067 | KP217075 |
| L. avicenniae | CMW 41467 | Avicennia marina | South Africa | KP860835 | KP860680 | KP860758 |
| L. brasiliense | CMM 4015 | Mangifera indica | Brazil | JX464063 | JX464049 | - |
| L. bruguierae | CMW 41470 | Bruguiera gymnorrhiza | South Africa | NR_147358 | KP860678 | KP860756 |
| L. citricola | CBS 124707 | Citrus sp. | Iran | GU945354 | GU945340 | KP872405 |
| L. crassispora | CBS 118741 | Santalum album | Australia (WA) | DQ103550 | EU673303 | EU673133 |
| L. crassispora | CBS 121770 | Acacia mellifera | Namibia | EU101307 | EU101352 | - |
| L. endophytica | MFLUCC 18-1 | Magnolia candolii | China | MK501838 | MK584572 | MK550606 |
| L. egyptiacae | CBS 130992 | Mangifera indica | Egypt | JN814397 | JN814424 | - |
| L. euphorbicola | CMM 3609 | Jatropha curcas | Brazil | KF234543 | KF226689 | KF254926 |
| L. fujianensis | CGMCC: 3.19593 | Vaccinium corymbosum | China | MK802164 | OM144905 | MK816337 |
| L. gilanensis | CBS 124704 | Citrus sp. | Iran | GU945351 | GU945342 | KP872411 |
| L. gilanensis | CBS 128311 | Vitis vinifera | USA: Missouri | HQ288225 | HQ288267 | - |
| L. gonubiensis | CBS 115812 | Syzygium cordatum | South Africa | AY639595 | DQ103566 | DQ458860 |
| L. gravistriata | CMM 4564 | Anacardium humile | Brazil | KT250949 | KT250950 | - |
| L. hormozganensis | CBS 124709 | Olea sp. | Iran | GU945355 | GU945343 | KP872413 |
| L. iraniensis | ZLNM3 | Mangifera indica | Taiwan | OR534158 | OR552386 | OR551924 |
| L. iraniensis | ML-1-8-1 | Mangifera indica | Taiwan | OR534131 | OR552266 | OR551897 |
| L. iraniensis | CBS 124710 | Salvadora persica | Iran | GU945346 | GU945334 | KP872415 |
| L. iraniensis | CMM 3610 | Jatropha curcas | Brazil | KF234544 | KF226690 | KF254927 |
| L. iraniensis | 421-19-5 | Vaccinium corymbosum | Serbia | OR856066 | PP238619 | PP238615 |
| L. iraniensis | 421-19-4 | Vaccinium corymbosum | Serbia | OR878143 | PP372561 | PP238614 |
| L. iraniensis | 421-19-3 | Vaccinium corymbosum | Serbia | OR856065 | PP238618 | PP238613 |
| L. iraniensis | 421-19-2 | Vaccinium corymbosum | Serbia | OR856064 | PP238617 | PP238612 |
| L. iraniensis | 421-19 | Vaccinium corymbosum | Serbia | OR727299 | PP238616 | PP238611 |
| L. laeliocattleyae | CBS 167.28 | Laeliocattleya sp. | Italy | KU507487 | KU507454 | … |
| L. lignicola | MFLUCC 11-0435 | On dead wood | Thailand | JX646797 | KU887003 | JX646845 |
| L. lignicola | CBS 342.78 | Sterculia oblonga | Germany | KX464140 | KX464634 | KX464908 |
| L. macrospora | CMM 3833 | Jatropha curcas | Brazil | KF234557 | KF226718 | KF254941 |
| L. magnoliae | MFLUCC 18-0948 | Magnolia candolii | China | MK499387 | MK568537 | MK521587 |
| L. mahajangana | CBS 124927 | Terminalia catappa | Madagascar | FJ900595 | FJ900641 | FJ900630 |
| L. mahajangana | CMM 1325 | Citrus sinensis | Brazil | KT154760 | KT008006 | KT154767 |
| L. mahajangana | CBS 137785 | Retama raetam | Tunisia | KJ638317 | KJ638336 | - |
| L. margaritacea | CBS 122519 | Adansonia gibbosa | Australia (WA) | EU144050 | EU144065 | KX464903 |
| L. mediterranea | CBS 137783 | Quercus ilex | Italy | KJ638312 | KJ638331 | - |
| L. mitidjana | MUM 19.90 | Citrus sinensis | Algeria: Mitidja | MN104115 | MN159114 | - |
| L. parva | CBS 456.78 | Manihot esculenta | Colombia | EF622083 | EF622063 | KP872419 |
| L. plurivora | CBS 120832 | Prunus salicina | South Africa | EF445362 | EF445395 | KP872421 |
| L. pontae | CMM 1277 | Spondias purpurea | Brazil | KT151794 | KT151791 | KT151797 |
| L. pseudotheobromae | CBS 116459 | Gmelina arborea | Costa Rica | EF622077 | EF622057 | EU673111 |
| L. pseudotheobromae | SEGA21 | Vaccinium corymbosum | USA | JN607093 | JN607116 | JN607140 |
| L. pseudotheobromae | SEGA70 | Vaccinium corymbosum | USA | JN607095 | JN607118 | JN607142 |
| L. rubropurpurea | CBS 118740 | Eucalyptus grandis | Australia | DQ103553 | EU673304 | EU673136 |
| L. subglobosa | CMM 3872 | Jatropha curcas | Brazil | KF234558 | KF226721 | KF254942 |
| L. thailandica | CBS 138760 | Mangifera indica | Thailand | KJ193637 | KJ193681 | - |
| L. theobromae | CBS 111530 | Leucospermum sp. | USA: Hawaii | EF622074 | EF622054 | - |
| L. theobromae | CBS 124.13 | - | USA | DQ458890 | DQ458875 | DQ458858 |
| L. theobromae | CBS 164.96 | Fruit along coral reef coast | Papua New Guinea | AY640255 | AY640258 | EU673110 |
| L. theobromae | SEFL3 | Vaccinium corymbosum | USA | JN607091 | JN607114 | JN607138 |
| L. theobromae | SEFL28b | Vaccinium corymbosum | USA | JN607092 | JN607115 | JN607139 |
| L. vaccinii | CGMCC 3.19248 | Vaccinium corymbosum | China | MK157131 | MK157158 | MK157149 |
| L. venezuelensis | CBS 118739 | Acacia mangium | Venezuela | DQ103547 | EU673305 | EU673129 |
| L. viticola | CBS 128313 | Vitis vinifera | USA: Arkansas | HQ288227 | HQ288269 | HQ288306 |
| L. vitis | CBS 124060 | Vitis vinifera | - | KX464148 | KX464642 | KX464917 |
| Melanops tulasnei | CBS116805 | Quercus robur | Germany | FJ824769 | KF766423 | FJ824780 |
| Neofusicoccum nonquaesitum | RGM2880 | Vaccinium corymbosum | Chile | MT790243 | MT845319 | MT832803 |
| Neofusicoccum nonquaesitum | RGM3009 | Vaccinium corymbosum | Chile | MT790223 | MT845299 | MT832783 |
| Neofusicoccum nonquaesitum | RGM2868 | Vaccinium corymbosum | Chile | MT790266 | MT845342 | MT832826 |
| Neofusicoccum parvum | 8-20 | Vaccinium corymbosum | Serbia | OQ31660 | OQ342772 | OQ473020 |
| Neofusicoccum parvum | 3c-20 | Vaccinium corymbosum | Serbia | OQ316605 | OQ473018 | OQ342770 |
| Neofusicoccum parvum | B1-17 | Vaccinium corymbosum | Serbia | OQ316604 | OQ342769 | OQ473017 |
| Neofusicoccum parvum | 4-20 | Vaccinium corymbosum | Serbia | OQ316606 | OQ342771 | OQ473019 |
| Neofusicoccum parvum | 1-21 | Vaccinium corymbosum | Serbia | OQ316608 | OQ342773 | OQ473021 |
| Neofusicoccum parvum | CMW39325 | Aesculus hippocastanum | Serbia | KF575021 | KF575045 | KF575117 |
| Neofusicoccum parvum | CMW39318 | Chamaecyparis lawsoniana | Serbia | KF575022 | KF575046 | KF575118 |
| Neofusicoccum parvum | CBS110301 | Vitis vinifera | Portugal | AY259098 | AY573221 | EU673095 |
| Neofusicoccum parvum | ATCC58191 (CMW9081) | Populus nigra | New Zealand | AY236943 | AY236888 | AY236917 |
| Neofusicoccum parvum | 413-19 | Vaccinium corymbosum | Serbia | MW624690 | OL456720 | OL456719 |
| Neofusicoccum parvum | 414-19 | Vaccinium corymbosum | Serbia | MW624691 | OL456721 | OL415487 |
| Neofusicoccum parvum | 790-11 | Vaccinium corymbosum | Serbia | PV235269 | PV278148 | PV278140 |
| Neofusicoccum parvum | 187-17 | Vaccinium corymbosum | Serbia | PV226107 | PV278145 | PV278139 |
| Neofusicoccum parvum | 29-22 | Vaccinium corymbosum | Serbia | PV235282 | PV278146 | PV278137 |
| Neofusicoccum parvum | 30-22 | Vaccinium corymbosum | Serbia | PV235306 | PV278147 | PV278138 |
| Neofusicoccum parvum | RS-BD-1 | Vaccinium corymbosum | Serbia | PV235313 | PV278149 | PV278141 |
| Neofusicoccum parvum | RS-BD-6 | Vaccinium corymbosum | Serbia | PV235322 | PV278150 | PV278142 |
| Neofusicoccum ribis | CBS121.26 | Ribes sp. | USA | AF241177 | AY236879 | AY236908 |
| Neofusicoccum ribis | CBS115475 | Ribes sp. | USA | AY236935 | AY236877 | AY236906 |
| Lasiodiplodia spp. and Accession Number of the Isolate | Nucleotide Alignment Using L. endophytica MK584572 as Representative | |||||||
|---|---|---|---|---|---|---|---|---|
| 14 | 16 | 55 | 60–67 | 68 | 128 | 137 | 159 | |
| L.endophytica MK584572 [49] | C | C | G | - | T | C | C | G |
| L. thailandica MW183805 [51] | A | C | A | insertion | T | T | C | G |
| L. thailandica OQ509100 [52] | A | C | A | insertion | T | C | C | G |
| L. thailandica KJ93681 [52] | A | C | A | insertion | T | T | C | G |
| L. fujianensis MK887178 [17] | C | C | G | - | T | C | C | C |
| L. iraniensis GU945334 [20] | C | A | G | - | A | C | T | C |
| L. iraniensis GU945336 [20] | C | A | G | - | A | C | T | C |
| L. iraniensis GU945337 [20] | C | A | G | - | A | C | T | C |
| L. iraniensis ON975017 [31] | C | A | G | - | A | C | T | C |
| L. iraniensis OR114284 [30] | C | A | G | - | A | C | T | C |
| L. iraniensis PP389268 [32] | - | - | G | - | A | C | C | C |
| L. iraniensis PP389275 [32] | - | - | G | - | A | C | C | C |
| L. iraniensis PP389256 [32] | - | - | G | - | A | C | C | C |
| L. iraniensis (syn. L. jatrophicola) KF226690 [53] | C | A | G | - | A | C | C | C |
| L. iraniensis PP238619 (this study) | C | A | G | - | A | C | C | C |
| L. iraniensis PP372561 (this study) | C | A | G | - | A | C | C | C |
| L. iraniensis PP238618 (this study) | C | A | G | - | A | C | C | C |
| L. iraniensis PP238617 (this study) | C | A | G | - | A | C | C | C |
| L. iraniensis PP238616 (this study) | C | A | G | - | A | C | C | C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marić, M.; Vojvodić, M.; Jevremović, D.; Vasilijević, B.; Vasić, T.; Grkinić, M.; Bulajić, A. Botryosphaeriaceae Species Causing Stem Blight and Dieback of Blueberries in Serbia. J. Fungi 2025, 11, 686. https://doi.org/10.3390/jof11090686
Marić M, Vojvodić M, Jevremović D, Vasilijević B, Vasić T, Grkinić M, Bulajić A. Botryosphaeriaceae Species Causing Stem Blight and Dieback of Blueberries in Serbia. Journal of Fungi. 2025; 11(9):686. https://doi.org/10.3390/jof11090686
Chicago/Turabian StyleMarić, Miloš, Mira Vojvodić, Darko Jevremović, Bojana Vasilijević, Tanja Vasić, Miljan Grkinić, and Aleksandra Bulajić. 2025. "Botryosphaeriaceae Species Causing Stem Blight and Dieback of Blueberries in Serbia" Journal of Fungi 11, no. 9: 686. https://doi.org/10.3390/jof11090686
APA StyleMarić, M., Vojvodić, M., Jevremović, D., Vasilijević, B., Vasić, T., Grkinić, M., & Bulajić, A. (2025). Botryosphaeriaceae Species Causing Stem Blight and Dieback of Blueberries in Serbia. Journal of Fungi, 11(9), 686. https://doi.org/10.3390/jof11090686







