Phenotypic Impact and Multivariable Assessment of Antifungal Susceptibility in Candida auris Survival Using a Galleria mellonella Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Strains
2.1.1. Blood Culture Processing and Strain Identification
2.1.2. Phenotypic Classification
2.1.3. Antifungal Susceptibility Testing
2.2. Survival Assays in Galleria mellonella
2.2.1. Larvae Handling
2.2.2. Survival Assay Procedures
2.3. Statistical Analysis
3. Results
3.1. Correlation Analysis of Antifungal MICs Against C. auris: Exploring Associations and Managing Collinearity
3.2. Survival Assays in G. mellonella: Cox Regression and Elastic Net Analysis of Survival Predictors in a Control Model of C. auris Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Satoh, K.; Makimura, K.; Hasumi, Y.; Nishiyama, Y.; Uchida, K.; Yamaguchi, H. Candida auris sp. nov., a Novel Ascomycetous Yeast Isolated from the External Ear Canal of an Inpatient in a Japanese Hospital. Microbiol. Immunol. 2009, 53, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaitán, A.; Martínez, H.; Moret, A.M.; Calabuig, E.; Tasias, M.; Alastruey-Izquierdo, A.; Zaragoza, Ó.; Mollar, J.; Frasquet, J.; Salavert-Lletí, M.; et al. Detection and Treatment of Candida auris in an Outbreak Situation: Risk Factors for Developing Colonization and Candidemia by This New Species in Critically Ill Patients. Expert Rev. Anti-Infect. Ther. 2019, 17, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gaitán, A.; Moret, A.M.; Tasias-Pitarch, M.; Aleixandre-López, A.I.; Martínez-Morel, H.; Calabuig, E.; Salavert-Lletí, M.; Ramírez, P.; López-Hontangas, J.L.; Hagen, F.; et al. An Outbreak Due to Candida auris with Prolonged Colonisation and Candidaemia in a Tertiary Care European Hospital. Mycoses 2018, 61, 498–505. [Google Scholar] [CrossRef]
- Eyre, D.W.; Sheppard, A.E.; Madder, H.; Moir, I.; Moroney, R.; Quan, T.P.; Griffiths, D.; George, S.; Butcher, L.; Morgan, M.; et al. A Candida auris Outbreak and Its Control in an Intensive Care Setting. N. Engl. J. Med. 2018, 379, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, Biology, Antifungal Resistance, and Virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef] [PubMed]
- Chow, N.A.; Muñoz, J.F.; Gade, L.; Berkow, E.L.; Li, X.; Welsh, R.M.; Forsberg, K.; Lockhart, S.R.; Adam, R.; Alanio, A.; et al. Tracing the Evolutionary History and Global Expansion of Candida auris Using Population Genomic Analyses. mBio 2020, 11, e03364-19. [Google Scholar] [CrossRef]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.A.; Desjardins, C.A.; et al. Simultaneous Emergence of Multidrug-Resistant Candida auris on 3 Continents Confirmed by Whole-Genome Sequencing and Epidemiological Analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef]
- Chowdhary, A.; Sharma, C.; Meis, J.F. Candida auris: A Rapidly Emerging Cause of Hospital-Acquired Multidrug-Resistant Fungal Infections Globally. PLoS Pathog. 2017, 13, e1006290. [Google Scholar] [CrossRef]
- Iguchi, S.; Itakura, Y.; Yoshida, A.; Kamada, K.; Mizushima, R.; Arai, Y.; Uzawa, Y.; Kikuchi, K. Candida auris: A Pathogen Difficult to Identify, Treat, and Eradicate and Its Characteristics in Japanese Strains. J. Infect. Chemother. 2019, 25, 743–749. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019; Centers for Disease Control and Prevention (U.S.): Atlanta, GA, USA, 2019.
- Kadri, S.S. Key Takeaways from the U.S. CDC’s 2019 Antibiotic Resistance Threats Report for Frontline Providers. Crit. Care Med. 2020, 48, 939–945. [Google Scholar] [CrossRef]
- Suphavilai, C.; Ko, K.K.K.; Lim, K.M.; Tan, M.G.; Boonsimma, P.; Chu, J.J.K.; Goh, S.S.; Rajandran, P.; Lee, L.C.; Tan, K.Y.; et al. Detection and Characterisation of a Sixth Candida auris Clade in Singapore: A Genomic and Phenotypic Study. Lancet Microbe 2024, 5, 100878. [Google Scholar] [CrossRef] [PubMed]
- Fayed, B.; Lazreg, I.K.; AlHumaidi, R.B.; Qasem, M.A.A.A.; Alajmy, B.M.G.N.; Bojbarah, F.M.A.M.; Senok, A.; Husseiny, M.I.; Soliman, S.S.M. Intra-Clade Heterogeneity in Candida auris: Risk of Management. Curr. Microbiol. 2023, 80, 295. [Google Scholar] [CrossRef] [PubMed]
- Phan-Canh, T.; Kuchler, K. Do Morphogenetic Switching and Intraspecies Variation Enhance Virulence of Candida auris? PLoS Pathog. 2024, 20, e1012559. [Google Scholar] [CrossRef]
- Burrack, L.S.; Todd, R.T.; Soisangwan, N.; Wiederhold, N.P.; Selmecki, A. Genomic Diversity across Candida auris Clinical Isolates Shapes Rapid Development of Antifungal Resistance In Vitro and In Vivo. mBio 2022, 13, e00842-22. [Google Scholar] [CrossRef]
- Garcia-Bustos, V.; Pemán, J.; Ruiz-Gaitán, A.; Cabañero-Navalon, M.D.; Cabanilles-Boronat, A.; Fernández-Calduch, M.; Marcilla-Barreda, L.; Sigona-Giangreco, I.A.; Salavert, M.; Tormo-Mas, M.Á.; et al. Host–Pathogen Interactions upon Candida auris Infection: Fungal Behaviour and Immune Response in Galleria mellonella. Emerg. Microbes Infect. 2022, 11, 136–146. [Google Scholar] [CrossRef]
- Borman, A.M.; Szekely, A.; Johnson, E.M. Comparative Pathogenicity of United Kingdom Isolates of the Emerging Pathogen Candida auris and Other Key Pathogenic Candida Species. mSphere 2016, 1, e00189-16. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bustos, V.; Ruiz-Saurí, A.; Ruiz-Gaitán, A.; Sigona-Giangreco, I.A.; Cabañero-Navalon, M.D.; Sabalza-Baztán, O.; Salavert-Lletí, M.; Tormo, M.Á.; Pemán, J. Characterization of the Differential Pathogenicity of Candida auris in a Galleria mellonella Infection Model. Microbiol. Spectr. 2021, 9, e00013-21. [Google Scholar] [CrossRef]
- Papp, C.; Kocsis, K.; Tóth, R.; Bodai, L.; Willis, J.R.; Ksiezopolska, E.; Lozoya-Pérez, N.E.; Vágvölgyi, C.; Mora Montes, H.; Gabaldón, T.; et al. Echinocandin-Induced Microevolution of Candida parapsilosis Influences Virulence and Abiotic Stress Tolerance. mSphere 2018, 3, e00547-18. [Google Scholar] [CrossRef]
- Ben-Ami, R.; Garcia-Effron, G.; Lewis, R.E.; Gamarra, S.; Leventakos, K.; Perlin, D.S.; Kontoyiannis, D.P. Fitness and Virulence Costs of Candida albicans FKS1 Hot Spot Mutations Associated with Echinocandin Resistance. J. Infect. Dis. 2011, 204, 626–635. [Google Scholar] [CrossRef]
- Vincent, B.M.; Lancaster, A.K.; Scherz-Shouval, R.; Whitesell, L.; Lindquist, S. Fitness Trade-Offs Restrict the Evolution of Resistance to Amphotericin B. PLoS Biol. 2013, 11, e1001692. [Google Scholar] [CrossRef]
- Beyda, N.D.; Lewis, R.E.; Garey, K.W. Echinocandin Resistance in Candida Species: Mechanisms of Reduced Susceptibility and Therapeutic Approaches. Ann. Pharmacother. 2012, 46, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Sherry, L.; Ramage, G.; Kean, R.; Borman, A.; Johnson, E.M.; Richardson, M.D.; Rautemaa-Richardson, R. Biofilm-Forming Capability of Highly Virulent, Multidrug-Resistant Candida auris. Emerg. Infect. Dis. 2017, 23, 328–331. [Google Scholar] [CrossRef]
- Hernando-Ortiz, A.; Mateo, E.; Perez-Rodriguez, A.; De Groot, P.W.J.; Quindós, G.; Eraso, E. Virulence of Candida auris from Different Clinical Origins in Caenorhabditis elegans and Galleria mellonella Host Models. Virulence 2021, 12, 1063–1075. [Google Scholar] [CrossRef]
- EUCAST E.Def 7.4 EUCAST Method for Susceptibility Testing of Yeasts (v. 7.4 Valid from 13 October, 2023). European Committee on Antimicrobial Susceptibility Testing. 2023. Available online: https://www.eucast.org/astoffungi/methodsinantifungalsusceptibilitytesting/susceptibility_testing_of_yeasts (accessed on 8 May 2025).
- Pelletier, C.; Shaw, S.; Alsayegh, S.; Brown, A.J.P.; Lorenz, A. Candida auris Undergoes Adhesin-Dependent and -Independent Cellular Aggregation. PLoS Pathog. 2024, 20, e1012076. [Google Scholar] [CrossRef] [PubMed]
- Bing, J.; Guan, Z.; Zheng, T.; Zhang, Z.; Fan, S.; Ennis, C.L.; Nobile, C.J.; Huang, G. Clinical Isolates of Candida auris with Enhanced Adherence and Biofilm Formation Due to Genomic Amplification of ALS4. PLoS Pathog. 2023, 19, e1011239. [Google Scholar] [CrossRef]
- Louvet, M.; Li, J.; Brandalise, D.; Bachmann, D.; Sala De Oyanguren, F.; Labes, D.; Jacquier, N.; Genoud, C.; Mucciolo, A.; Coste, A.T.; et al. Ume6-Dependent Pathways of Morphogenesis and Biofilm Formation in Candida auris. Microbiol. Spectr. 2024, 12, e01531-24. [Google Scholar] [CrossRef]
- Forgács, L.; Borman, A.M.; Prépost, E.; Tóth, Z.; Kardos, G.; Kovács, R.; Szekely, A.; Nagy, F.; Kovacs, I.; Majoros, L. Comparison of in Vivo Pathogenicity of Four Candida auris Clades in a Neutropenic Bloodstream Infection Murine Model. Emerg. Microbes Infect. 2020, 9, 1160–1169. [Google Scholar] [CrossRef]
- Bing, J.; Guan, Z.; Zheng, T.; Ennis, C.L.; Nobile, C.J.; Chen, C.; Chu, H.; Huang, G. Rapid Evolution of an Adaptive Multicellular Morphology of Candida auris during Systemic Infection. Nat. Commun. 2024, 15, 2381. [Google Scholar] [CrossRef]
- Singh, R.; Kaur, M.; Chakrabarti, A.; Shankarnarayan, S.A.; Rudramurthy, S.M. Biofilm Formation by Candida auris Isolated from Colonising Sites and Candidemia Cases. Mycoses 2019, 62, 706–709. [Google Scholar] [CrossRef]
- Short, B.; Brown, J.; Delaney, C.; Sherry, L.; Williams, C.; Ramage, G.; Kean, R. Candida auris Exhibits Resilient Biofilm Characteristics In Vitro: Implications for Environmental Persistence. J. Hosp. Infect. 2019, 103, 92–96. [Google Scholar] [CrossRef]
- Brown, J.L.; Delaney, C.; Short, B.; Butcher, M.C.; McKloud, E.; Williams, C.; Kean, R.; Ramage, G. Candida auris Phenotypic Heterogeneity Determines Pathogenicity In Vitro. mSphere 2020, 5, e00371-20. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Ortiz, A.; Eraso, E.; Jauregizar, N.; De Groot, P.W.J.; Quindós, G.; Mateo, E. Efficacy of the Combination of Amphotericin B and Echinocandins against Candida auris In Vitro and in the Caenorhabditis elegans Host Model. Microbiol. Spectr. 2024, 12, e02086-23. [Google Scholar] [CrossRef]
- Das, S.; Singh, S.; Tawde, Y.; Dutta, T.K.; Rudramurthy, S.M.; Kaur, H.; Shaw, T.; Ghosh, A. Comparative Fitness Trade-Offs Associated with Azole Resistance in Candida auris Clinical Isolates. Heliyon 2024, 10, e32386. [Google Scholar] [CrossRef] [PubMed]
- Szekely, A.; Borman, A.M.; Johnson, E.M. Candida auris Isolates of the Southern Asian and South African Lineages Exhibit Different Phenotypic and Antifungal Susceptibility Profiles In Vitro. J. Clin. Microbiol. 2019, 57, e02055-18. [Google Scholar] [CrossRef]
- Zamith-Miranda, D.; Amatuzzi, R.F.; Munhoz Da Rocha, I.F.; Martins, S.T.; Lucena, A.C.R.; Vieira, A.Z.; Trentin, G.; Almeida, F.; Rodrigues, M.L.; Nakayasu, E.S.; et al. Transcriptional and Translational Landscape of Candida auris in Response to Caspofungin. Comput. Struct. Biotechnol. J. 2021, 19, 5264–5277. [Google Scholar] [CrossRef]
- Fayed, B.; Jayakumar, M.N.; Soliman, S.S.M. Caspofungin-Resistance in Candida auris Is Cell Wall-Dependent Phenotype and Potential Prevention by Zinc Oxide Nanoparticles. Med. Mycol. 2021, 59, 1243–1256. [Google Scholar] [CrossRef]
- Feng, W.; Yang, J.; Pan, Y.; Xi, Z.; Qiao, Z.; Ma, Y. The Correlation of Virulence, Pathogenicity, and Itraconazole Resistance with SAP Activity in Candida albicans Strains. Can. J. Microbiol. 2016, 62, 173–178. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, F.; Li, D.; Wang, S.; Pang, Z.; Chen, L.; Li, R.; Shi, D. Correlation Between Drug Resistance and Virulence of Candida Isolates from Patients with Candidiasis. Infect. Drug Resist. 2022, 15, 7459–7473. [Google Scholar] [CrossRef]
- Vale-Silva, L.A.; Sanglard, D. Tipping the Balance Both Ways: Drug Resistance and Virulence in Candida glabrata. FEMS Yeast Res. 2015, 15, fov025. [Google Scholar] [CrossRef]
- Mohammadi, F.; Ghasemi, Z.; Familsatarian, B.; Salehi, E.; Sharifynia, S.; Barikani, A.; Mirzadeh, M.; Hosseini, M.A. Relationship between Antifungal Susceptibility Profile and Virulence Factors in Candida albicans Isolated from Nail Specimens. Rev. Soc. Bras. Med. Trop. 2020, 53, e20190214. [Google Scholar] [CrossRef]
- Carolus, H.; Sofras, D.; Boccarella, G.; Sephton-Clark, P.; Biriukov, V.; Cauldron, N.C.; Lobo Romero, C.; Vergauwen, R.; Yazdani, S.; Pierson, S.; et al. Acquired Amphotericin B Resistance Leads to Fitness Trade-Offs That Can Be Mitigated by Compensatory Evolution in Candida auris. Nat. Microbiol. 2024, 9, 3304–3320. [Google Scholar] [CrossRef] [PubMed]
- Jenull, S.; Shivarathri, R.; Tsymala, I.; Penninger, P.; Trinh, P.-C.; Nogueira, F.; Chauhan, M.; Singh, A.; Petryshyn, A.; Stoiber, A.; et al. Transcriptomics and Phenotyping Define Genetic Signatures Associated with Echinocandin Resistance in Candida auris. mBio 2022, 13, e00799-22. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Niu, Y.; Tan, J.; Liu, W.; Sun, M.; Yang, E.; Wang, Q.; Li, R.; Wang, Y.; Liu, W. Global Screening of Genomic and Transcriptomic Factors Associated with Phenotype Differences between Multidrug-Resistant and -Susceptible Candida haemulonii Strains. mSystems 2019, 4, e00459-19. [Google Scholar] [CrossRef]
- Bohner, F.; Papp, C.; Takacs, T.; Varga, M.; Szekeres, A.; Nosanchuk, J.D.; Vágvölgyi, C.; Tóth, R.; Gacser, A. Acquired Triazole Resistance Alters Pathogenicity-Associated Features in Candida auris in an Isolate-Dependent Manner. J. Fungi 2023, 9, 1148. [Google Scholar] [CrossRef]
- Lee, Y.; Puumala, E.; Robbins, N.; Cowen, L.E. Antifungal Drug Resistance: Molecular Mechanisms in Candida albicans and Beyond. Chem. Rev. 2021, 121, 3390–3411. [Google Scholar] [CrossRef]
- Ramos, L.S.; Barbosa, P.F.; Lorentino, C.M.A.; Lima, J.C.; Braga, A.L.; Lima, R.V.; Giovanini, L.; Casemiro, A.L.; Siqueira, N.L.M.; Costa, S.C.; et al. The Multidrug-Resistant Candida auris, Candida haemulonii Complex and Phylogenetic Related Species: Insights into Antifungal Resistance Mechanisms. Curr. Res. Microb. Sci. 2025, 8, 100354. [Google Scholar] [CrossRef]
- Forastiero, A.; Mesa-Arango, A.C.; Alastruey-Izquierdo, A.; Alcazar-Fuoli, L.; Bernal-Martinez, L.; Pelaez, T.; Lopez, J.F.; Grimalt, J.O.; Gomez-Lopez, A.; Cuesta, I.; et al. Candida Tropicalis Antifungal Cross-Resistance Is Related to Different Azole Target (Erg11p) Modifications. Antimicrob. Agents Chemother. 2013, 57, 4769–4781. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Muñoz, J.F.; Subotic, A.; Cruz, R.B.; Cuomo, C.A.; Dijck, P.V. Genome-Wide Analysis of Experimentally Evolved Candida auris Reveals Multiple Novel Mechanisms of Multidrug Resistance. mBio 2021, 12, e03333-20. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Jacobs, J.L.; Dennis, E.K.; Taimur, S.; Rana, M.; Patel, D.; Gitman, M.; Patel, G.; Schaefer, S.; Iyer, K.; et al. Candida auris Pan-Drug-Resistant to Four Classes of Antifungal Agents. Antimicrob. Agents Chemother. 2022, 66, e00053-22. [Google Scholar] [CrossRef]
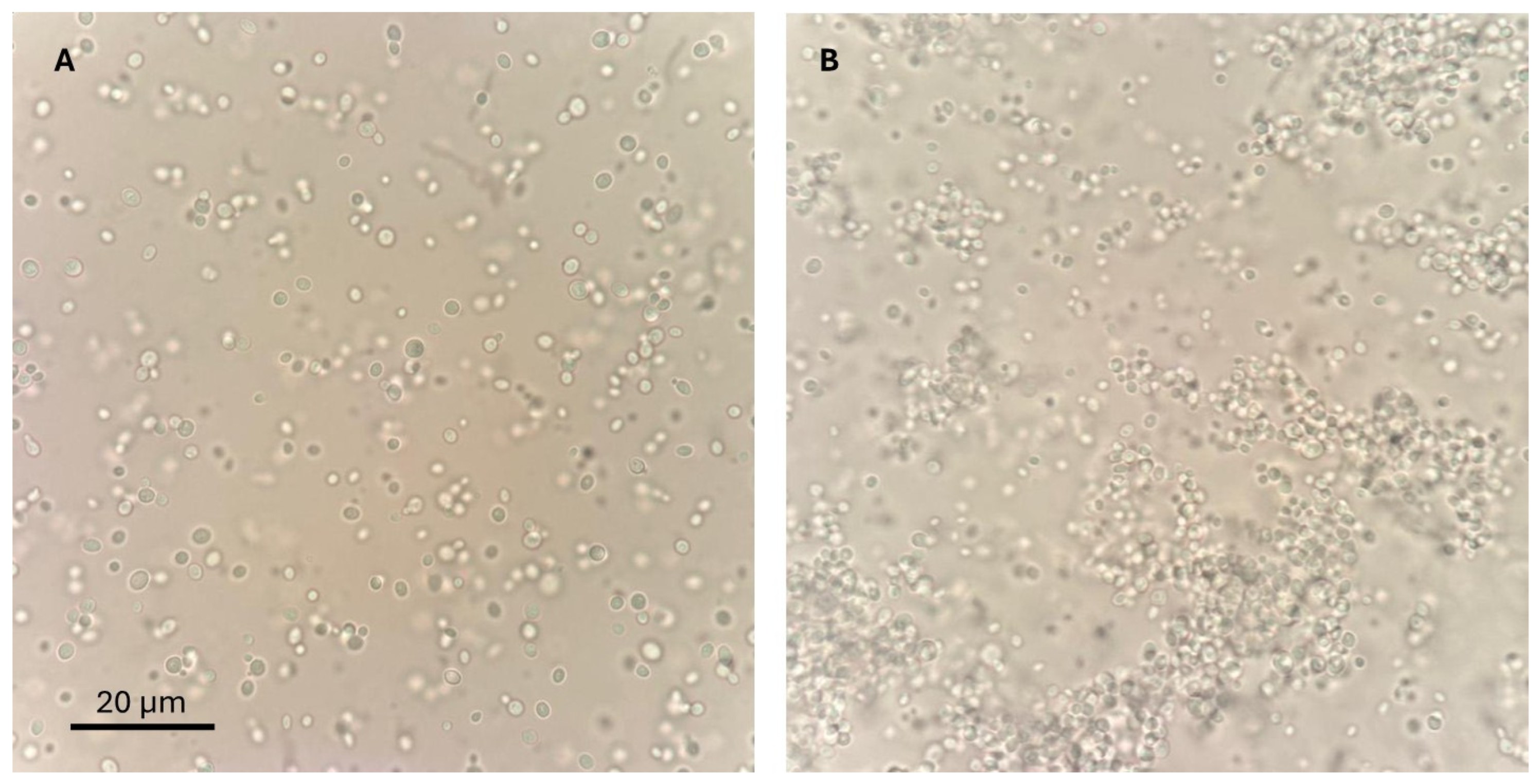
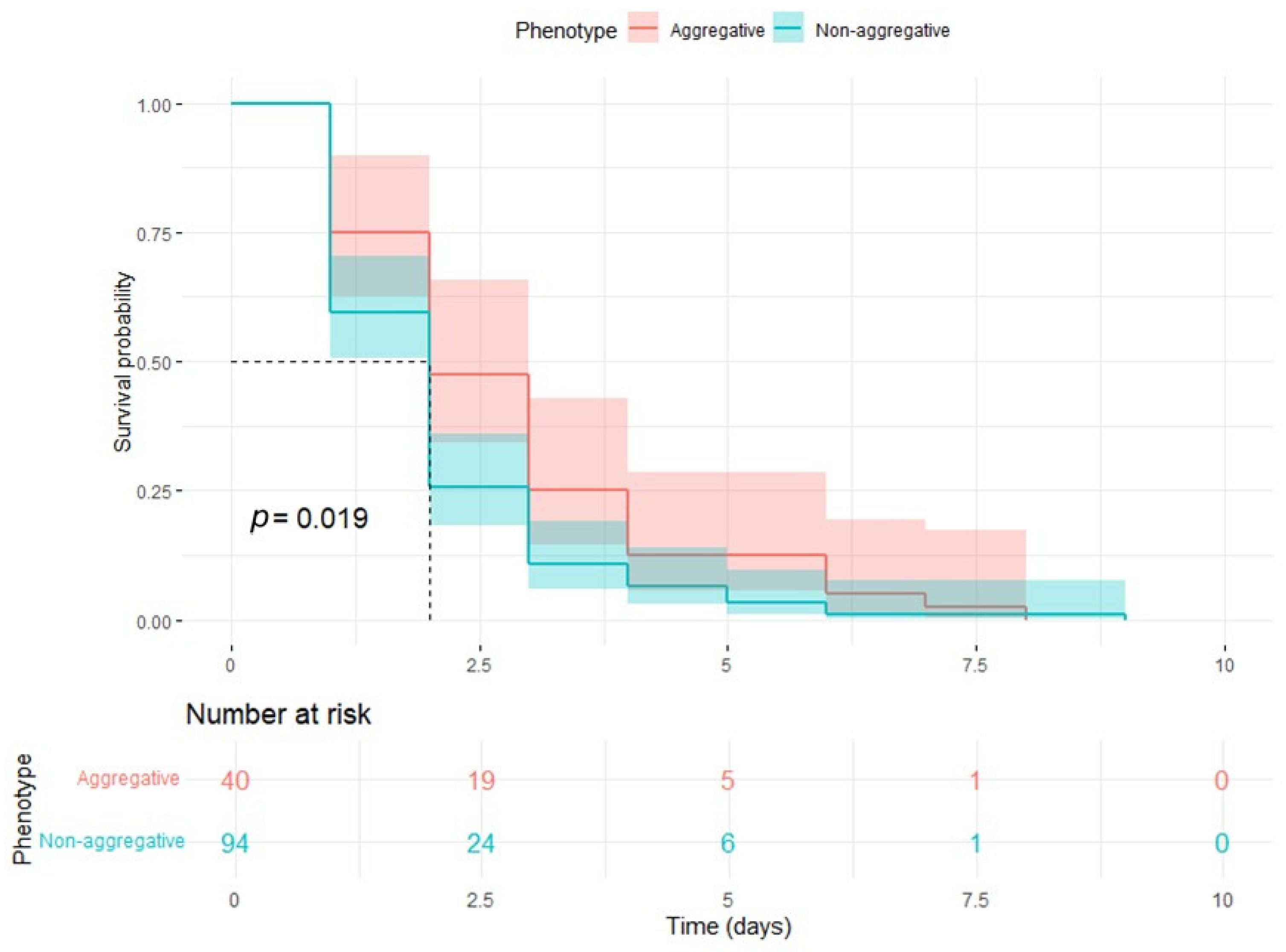
| Time | Number at Risk | Number of Events | Survival Probability | Standard Error |
|---|---|---|---|---|
| Invasive samples | ||||
| CJ104 | Blood | Polytraumatized | Surgical ICU | Aggregative |
| CJ173 | Blood | Polytraumatized | Surgical ICU | Aggregative |
| Cj198 | Blood | Pneumonia | Medical ICU | Aggregative |
| CJ98 | Blood | Polytraumatized | Surgical ICU | Non-aggregative |
| CJ175 | Blood | Status epilepticus | Medical ICU | Non-aggregative |
| CJ197 | Blood | Febrile neutropenia | Hematology | Non-aggregative |
| 312775 | Blood | Endocarditis | Medical ICU | Non-aggregative |
| Non-invasive epidemiological surveillance samples | ||||
| 124819 | Rectal | Extracorporeal mechanical oxygenation | Medical ICU | Non-aggregative |
| 182482 | Inguinal | Liver transplantation | Medical ICU | Non-aggregative |
| 253107 | Pharyngeal | Multiple myeloma | Medical ICU | Non-aggregative |
| MIC (mg/L) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strain | Phenotype | AMB MIC | AMB | 5FC MIC | 5FC | FLU MIC | FLU | ITR MIC | ITR | VOR MIC | VOR | POS MIC | POS | CAS MIC | CAS | ANI MIC | ANI | MCF MIC | MCF |
| 124819 | Non-aggregative | 0.5 | S | <0.06 | S | >256 | R | 0.06 | IE | 0.03 | IE | 0.015 | IE | 0.03 | S | 0.06 | S | 0.03 | S |
| 182482 | Non-aggregative | 0.5 | S | <0.06 | S | >256 | R | 0.06 | IE | 0.03 | IE | 0.015 | IE | 0.03 | S | 0.03 | S | 0.03 | S |
| 253107 | Non-aggregative | 0.5 | S | <0.06 | S | >256 | R | 0.06 | IE | 0.03 | IE | 0.015 | IE | 0.03 | S | 0.125 | S | 0.03 | S |
| CJ98 | Non-aggregative | 0.25 | S | 0.12 | S | >256 | R | 0.25 | IE | 2 | IE | 0.03 | IE | 0.5 | S | 0.06 | S | 0.06 | S |
| CJ175 | Non-aggregative | 0.5 | S | 0.06 | S | >256 | R | 0.125 | IE | 2 | IE | 0.06 | IE | 0.03 | S | 0.125 | S | 0.06 | S |
| CJ197 | Non-aggregative | 2 | R | 0.25 | S | >256 | R | 0.25 | IE | 4 | IE | 0.06 | IE | 0.5 | S | 0.5 | S | 0.25 | S |
| 312775 | Non-aggregative | 0.5 | S | 0.06 | S | >256 | R | 0.125 | IE | 8 | IE | 0.06 | IE | 0.03 | S | 0.125 | S | 0.06 | S |
| CJ104 | Aggregative | 0.5 | S | 0.06 | S | >256 | R | 0.125 | IE | 2 | IE | 0.06 | IE | 0.03 | S | 0.125 | S | 0.06 | S |
| CJ173 | Aggregative | 0.5 | S | <0.06 | S | >256 | R | 0.06 | IE | 2 | IE | 0.03 | IE | 0.06 | S | 0.06 | S | 0.06 | S |
| CJ198 | Aggregative | 0.25 | S | 0.06 | S | >256 | R | 0.25 | IE | 1 | IE | 0.03 | IE | 0.06 | S | 0.125 | S | 0.06 | S |
| Amphotericin B MIC | Flucytosine MIC | Itraconazole MIC | Voriconazole MIC | Caspofungin MIC | Anidulafungin MIC | Micafungin MIC | Posaconazole MIC | |
|---|---|---|---|---|---|---|---|---|
| Amphotericin B MIC | 1.000 | 0.659 * | 0.191 | 0.297 * | 0.346 * | 0.912 * | 0.372 * | 0.938 * |
| Flucytosine MIC | 0.659 * | 1.000 | 0.829 * | 0.490 * | 0.820 * | 0.882 * | 0.558 * | 0.815 * |
| Itraconazole MIC | 0.191 | 0.829 * | 1.000 | 0.340 * | 0.789 * | 0.551 * | 0.357 * | 0.458 * |
| Voriconazole MIC | 0.297 * | 0.490 * | 0.340 * | 1.000 | 0.226 | 0.445 * | 0.712 * | 0.428 * |
| Caspofungin MIC | 0.346 * | 0.820 * | 0.789 * | 0.226 | 1.000 | 0.587 * | 0.108 * | 0.427 |
| Anidulafungin MIC | 0.912 * | 0.882 * | 0.551 * | 0.445 * | 0.587 * | 1.000 | 0.526 * | 0.973 * |
| Micafungin MIC | 0.372 * | 0.558 * | 0.357 * | 0.712 * | 0.108 * | 0.526 * | 1.000 | 0.560 * |
| Posaconazole MIC | 0.938 * | 0.815 * | 0.458 * | 0.428 * | 0.427 | 0.973 * | 0.560 * | 1.000 |
| Phenotype | 0.116 | 0.009 | −0.101 | −0.167 | −0.020 | −0.326 | −0.339 | −0.363 |
| Variable | Coefficient | Hazard Ratio | Lower 95% Confidence Interval | Upper 95% Confidence Interval | Z-Score | p-Value |
|---|---|---|---|---|---|---|
| Non-aggregative phenotype | 0.883 | 2.418 | 1.190 | 4.913 | 2.441 | 0.015 * |
| Invasive origin | 1.078 | 2.939 | 0.830 | 10.41 | 1.671 | 0.095 |
| Amphotericin B MIC | 1.038 | 2.823 | 0.1661 | 47.96 | 0.718 | 0.473 |
| Flucytosine MIC | −0.991 | 0.371 | 3.7 × 10−6 | 3.72 × 104 | −0.169 | 0.866 |
| Voriconazole MIC | −0.116 | 0.891 | 0.771 | 1.029 | −1.571 | 0.116 |
| Micafungin MIC | −9.872 | 5.16 × 10−5 | 1 × 10−18 | 2.6 × 109 | −0.613 | 0.540 |
| Time | Number at Risk | Number of Events | Survival Probability | Standard Error | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|
| 1 | 40 | 10 | 0.75 | 0.0685 | 0.62713 | 0.897 |
| 2 | 30 | 11 | 0.475 | 0.079 | 0.34293 | 0.658 |
| 3 | 19 | 9 | 0.25 | 0.0685 | 0.14616 | 0.428 |
| 4 | 10 | 5 | 0.125 | 0.0523 | 0.05506 | 0.284 |
| 6 | 5 | 3 | 0.05 | 0.0345 | 0.01295 | 0.193 |
| 7 | 2 | 1 | 0.025 | 0.0247 | 0.00361 | 0.173 |
| 8 | 1 | 1 | 0.0 | NA | NA | NA |
| Time | Number at Risk | Number of Events | Survival Probability | Standard Error | Lower 95% CI | Upper 95% CI |
|---|---|---|---|---|---|---|
| 1 | 94 | 38 | 0.5957 | 0.0506 | 0.50436 | 0.7037 |
| 2 | 56 | 32 | 0.2553 | 0.045 | 0.18078 | 0.3606 |
| 3 | 24 | 14 | 0.1064 | 0.0318 | 0.05921 | 0.1911 |
| 4 | 10 | 4 | 0.0638 | 0.0252 | 0.02943 | 0.1384 |
| 5 | 6 | 3 | 0.0319 | 0.0181 | 0.01048 | 0.0972 |
| 6 | 3 | 2 | 0.0106 | 0.0106 | 0.00151 | 0.0747 |
| 9 | 1 | 1 | 0.0 | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarruiz, J.; Ruiz-Gaitán, A.C.; Cabanero-Navalon, M.D.; Pemán, J.; Blanes-Hernández, R.; de Cossio, S.; Garcia-Bustos, V. Phenotypic Impact and Multivariable Assessment of Antifungal Susceptibility in Candida auris Survival Using a Galleria mellonella Model. J. Fungi 2025, 11, 406. https://doi.org/10.3390/jof11060406
Alvarruiz J, Ruiz-Gaitán AC, Cabanero-Navalon MD, Pemán J, Blanes-Hernández R, de Cossio S, Garcia-Bustos V. Phenotypic Impact and Multivariable Assessment of Antifungal Susceptibility in Candida auris Survival Using a Galleria mellonella Model. Journal of Fungi. 2025; 11(6):406. https://doi.org/10.3390/jof11060406
Chicago/Turabian StyleAlvarruiz, Jorge, Alba Cecilia Ruiz-Gaitán, Marta Dafne Cabanero-Navalon, Javier Pemán, Rosa Blanes-Hernández, Santiago de Cossio, and Victor Garcia-Bustos. 2025. "Phenotypic Impact and Multivariable Assessment of Antifungal Susceptibility in Candida auris Survival Using a Galleria mellonella Model" Journal of Fungi 11, no. 6: 406. https://doi.org/10.3390/jof11060406
APA StyleAlvarruiz, J., Ruiz-Gaitán, A. C., Cabanero-Navalon, M. D., Pemán, J., Blanes-Hernández, R., de Cossio, S., & Garcia-Bustos, V. (2025). Phenotypic Impact and Multivariable Assessment of Antifungal Susceptibility in Candida auris Survival Using a Galleria mellonella Model. Journal of Fungi, 11(6), 406. https://doi.org/10.3390/jof11060406







