Mycobiota and Antifungal Antibodies as Emerging Targets for the Diagnosis and Prognosis of Human Diseases
Abstract
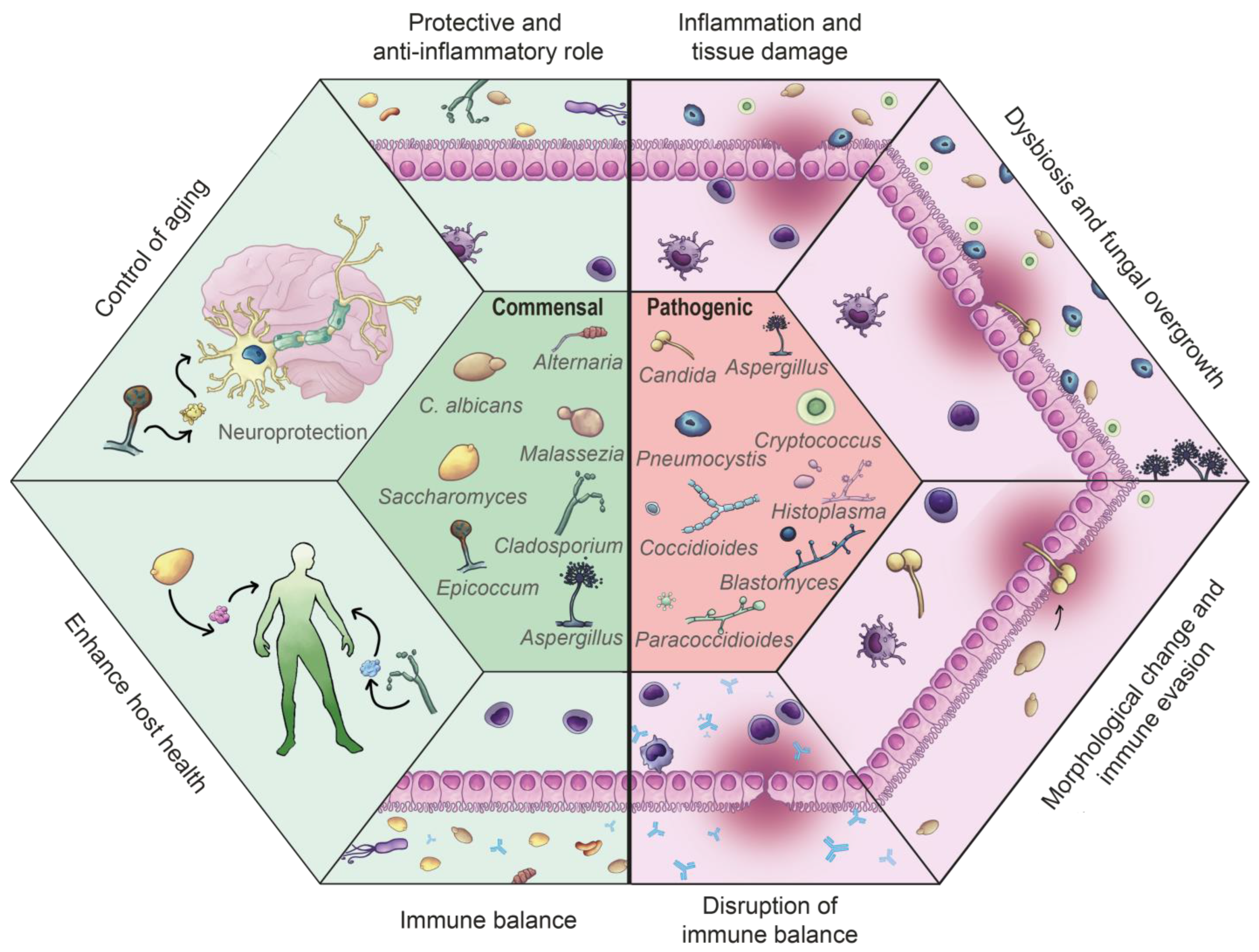
1. Fungal Microorganisms: Their Place in the Human Body
2. Fungal Communities in the Human Organism
2.1. The Process of Fungal Commensalisms
2.2. The Process of Fungal Infection
3. The Interplay Between the Immune System and Fungi
3.1. Activation of Adaptive Immunity
3.1.1. Adaptive Humoral Immunity Against Fungal Infections
3.1.2. Humoral Immunity and Commensal Fungi
3.2. Antibodies as Biomarkers
4. Antifungal Antibodies as Tools for Diagnosis and Predicting Chronic Immune-Mediated Inflammatory Non-Communicable Disease
4.1. Current Applications
4.2. Future Potential
| Type of Illnesses | Disease, Disorder or Condition | Immune and Mycobiota Imbalance | Reference |
|---|---|---|---|
| Gut disease | Crohn’s disease | Increase in ASCA, ALCA, ACCA, AMCA antibodies, often associated with severe or complicated disease Pathogenic antibodies to mannose glycan associated with IgG glycosylation signature Candida spp. overgrowth | [90,92,100,101,102,103] |
| Colorectal cancer | Increased Candida spp., decreased Saccharomyces spp. | [115,116] | |
| Cognitive disorders | Autism spectrum disorder | C. albicans overgrowth and increase in IgG antibodies | [90,104,111] |
| Bipolar disorder | Increased antibodies against S. cerevisiae Elevated ASCA markers | [105,107] | |
| Schizophrenia | Increased antibodies against C. albicans: diagnostic marker in males, prognostic marker for cognitive decline in females. Elevated ASCA markers (especially in antipsychotic-naive individuals Higher Candida spp., C. dubliensis | [105,106] | |
| Alzheimer’s disease | Elevated antibodies against Candida spp. in some patients Prevalence of Alternaria spp., Botrytis spp., Candida spp., and Malassezia spp. | [108,112] | |
| Parkinson’s disease | Increased ASCA antibodies Presence of Malassezia | [109,113] | |
| Multiple sclerosis | Presence of Trichosporon mucoides and Candida deformans | [114,119] |
5. Challenges and Limitations
6. Future Perspective
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACCAs | Anti-chitobioside antibodies |
| AD | Alzheimer’s disease |
| AILD | Autoimmune liver disease |
| ALCAs | anti-laminaribioside antibodies |
| AMCA | anti-mannobioside IgG |
| AMPs | Antimicrobial peptides |
| APCs | Antigen-presenting cells |
| ASCAs | Anti-Saccharomyces cerevisiae antibodies |
| ASD | Autism spectrum disorder |
| ASF mice | Altered Schaedler flora mice |
| BD | Behçet’s disease |
| BD | Bipolar disorder |
| CD | Crohn’s disease |
| CRC | Colorectal cancer |
| DCs | Dendritic cells |
| DIA | Dot immunobinding assay |
| ELISA | Enzyme-linked immune sorbent assay |
| FMT | Fecal microbiota transplant |
| GC-B | Germinal center B |
| IBD | Inflammatory bowel disease |
| IECs | Intestinal epithelial cells |
| Igs | Immunoglobulins |
| IL | Interleukin |
| IMID | Immune-mediated inflammatory disease |
| MAC | Membrane attack complex |
| MALDI-TOF MS | Matrix Assisted Laser Desorption Ionisation—Time of Flight Mass Spectrometry |
| MHC | Major histocompatibility complex |
| MS | Multiple sclerosis |
| nAbs | Natural antibodies |
| NGS | Next-generation sequencing |
| PAMPs | Pathogen-associated molecular patterns |
| PBC | Primary biliary cirrhosis |
| PD | Parkinson’s disease |
| PMNs | Polymorphonuclear leukocytes |
| PRRs | Pattern recognition receptors |
| PSC | Primary sclerosing cholangitis |
| ROS | Reactive oxygen species |
| SCZ | Schizophrenia |
| SD | Seborrheic dermatitis |
| SHM | Somatic hypermutation |
| SWOT | Strengths, weaknesses, opportunities, and threats analysis |
| Tc cells | Cytotoxic T cells |
| TCRs | T cell receptors |
| Tfh cells | Follicular helper T cells |
| Th cells | Helper T cells |
| Treg cells | Regulatory T cells |
| UC | Ulcerative Colitis |
| WB | Western blotting |
References
- Diez-Martin, E.; Hernandez-Suarez, L.; Muñoz-Villafranca, C.; Martin-Souto, L.; Astigarraga, E.; Ramirez-Garcia, A.; Barreda-Gómez, G. Inflammatory Bowel Disease: A Comprehensive Analysis of Molecular Bases, Predictive Biomarkers, Diagnostic Methods, and Therapeutic Options. Int. J. Mol. Sci. 2024, 25, 7062. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Sig. Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Limon, J.J.; Skalski, J.H.; Underhill, D.M. Commensal Fungi in Health and Disease. Cell Host Microbe 2017, 22, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Round, J.L. Defining Dysbiosis and Its Influence on Host Immunity and Disease. Cell Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Underhill, D.M.; Iliev, I.D. The Mycobiota: Interactions between Commensal Fungi and the Host Immune System. Nat. Rev. Immunol. 2014, 14, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Vaishnava, S. Microbial Underdogs: Exploring the Significance of Low-Abundance Commensals in Host-Microbe Interactions. Exp. Mol. Med. 2023, 55, 2498–2507. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Li, H.; Wu, A.; He, J.; Mao, X.; Dai, Z.; Tian, G.; Cai, J.; Tang, J.; Luo, Y. Composition, Influencing Factors, and Effects on Host Nutrient Metabolism of Fungi in Gastrointestinal Tract of Monogastric Animals. Animals 2025, 15, 710. [Google Scholar] [CrossRef]
- Commensalism—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/biochemistry-genetics-and-molecular-biology/commensalism (accessed on 23 March 2025).
- Rogalski, M.A.; Stewart Merrill, T.; Gowler, C.D.; Cáceres, C.E.; Duffy, M.A. Context-Dependent Host-Symbiont Interactions: Shifts along the Parasitism-Mutualism Continuum. Am. Nat. 2021, 198, 563–575. [Google Scholar] [CrossRef]
- Santiago, M.F.M.; King, K.C.; Drew, G.C. Interactions between Insect Vectors and Plant Pathogens Span the Parasitism–Mutualism Continuum. Biol. Lett. 2023, 19, 20220453. [Google Scholar] [CrossRef]
- Herrera, P.; Schuster, L.; Wentrup, C.; König, L.; Kempinger, T.; Na, H.; Schwarz, J.; Köstlbacher, S.; Wascher, F.; Zojer, M.; et al. Molecular Causes of an Evolutionary Shift along the Parasitism-Mutualism Continuum in a Bacterial Symbiont. Proc. Natl. Acad. Sci. USA 2020, 117, 21658–21666. [Google Scholar] [CrossRef]
- Shevchuk, Y.; Kuypers, K.; Janssens, G.E. Fungi as a Source of Bioactive Molecules for the Development of Longevity Medicines. Ageing Res. Rev. 2023, 87, 101929. [Google Scholar] [CrossRef] [PubMed]
- Li, X.V.; Leonardi, I.; Iliev, I.D. Gut Mycobiota in Immunity and Inflammatory Disease. Immunity 2019, 50, 1365–1379. [Google Scholar] [CrossRef]
- Wu, X.; Xia, Y.; He, F.; Zhu, C.; Ren, W. Intestinal Mycobiota in Health and Diseases: From a Disrupted Equilibrium to Clinical Opportunities. Microbiome 2021, 9, 60. [Google Scholar] [CrossRef]
- Yang, J.; Yang, H.; Li, Y. The Triple Interactions between Gut Microbiota, Mycobiota and Host Immunity. Crit. Rev. Food Sci. Nutr. 2023, 63, 11604–11624. [Google Scholar] [CrossRef] [PubMed]
- Santos-Fernandez, E.; Martin-Souto, L.; Antoran, A.; Areitio, M.; Aparicio-Fernandez, L.; Bouchara, J.-P.; Schwarz, C.; Rementeria, A.; Buldain, I.; Ramirez-Garcia, A. Microbiota and Fungal-Bacterial Interactions in the Cystic Fibrosis Lung. FEMS Microbiol. Rev. 2023, 47, fuad029. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, Y.; Shen, S.; Hou, Y.; Chen, Y.; Wang, T. The Mycobiota of the Human Body: A Spark Can Start a Prairie Fire. Gut Microbes 2020, 11, 655. [Google Scholar] [CrossRef]
- Leonardi, I.; Paramsothy, S.; Doron, I.; Semon, A.; Kaakoush, N.O.; Clemente, J.C.; Faith, J.J.; Borody, T.J.; Mitchell, H.M.; Colombel, J.-F.; et al. Fungal Trans-Kingdom Dynamics Linked to Responsiveness to Fecal Microbiota Transplantation (FMT) Therapy in Ulcerative Colitis. Cell Host Microbe 2020, 27, 823–829.e3. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, C.; Liu, X.; Jiang, S.; Huo, D.; Cai, K.; Zhang, J. Biodiversity Responses of Gut Mycobiota and Bacteriophages Induced by Probiotic Consumption. J. Funct. Foods 2023, 106, 105615. [Google Scholar] [CrossRef]
- Zhang, F.; Aschenbrenner, D.; Yoo, J.Y.; Zuo, T. The Gut Mycobiome in Health, Disease, and Clinical Applications in Association with the Gut Bacterial Microbiome Assembly. Lancet Microbe 2022, 3, e969–e983. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, A.L.; Underhill, D.M. The Mycobiome of the Human Urinary Tract: Potential Roles for Fungi in Urology. Ann. Transl. Med. 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- d’Enfert, C.; Kaune, A.-K.; Alaban, L.-R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Fróis-Martins, R.; Morelli, M.; et al. The Impact of the Fungus-Host-Microbiota Interplay upon Candida albicans Infections: Current Knowledge and New Perspectives. FEMS Microbiol. Rev. 2020, 45, fuaa060. [Google Scholar] [CrossRef]
- Mahalingam, S.S.; Jayaraman, S.; Pandiyan, P. Fungal Colonization and Infections-Interactions with Other Human Diseases. Pathogens 2022, 11, 212. [Google Scholar] [CrossRef]
- Turunen, J.; Paalanne, N.; Reunanen, J.; Tapiainen, T.; Tejesvi, M.V. Development of Gut Mycobiome in Infants and Young Children: A Prospective Cohort Study. Pediatr. Res. 2023, 94, 486–494. [Google Scholar] [CrossRef]
- Iliev, I.D.; Leonardi, I. Fungal Dysbiosis: Immunity and Interactions at Mucosal Barriers. Nat. Rev. Immunol. 2017, 17, 635–646. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Tennakoon, D.S.; Jayatunga, D.P.W.; Hongsanan, S.; Xie, N. Humans vs. Fungi: An Overview of Fungal Pathogens against Humans. Pathogens 2024, 13, 426. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Mudalagiriyappa, S.; Nanjappa, S.G. T Cell Responses to Control Fungal Infection in an Immunological Memory Lens. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A. Immunity to Invasive Fungal Diseases. Annu. Rev. Immunol. 2022, 40, 121–141. [Google Scholar] [CrossRef]
- Cramer, R.A.; Perfect, J.R. CHAPTER 2—Recent Advances in Understanding Human Opportunistic Fungal Pathogenesis Mechanisms. In Clinical Mycology, 2nd ed.; Anaissie, E.J., McGinnis, M.R., Pfaller, M.A., Eds.; Churchill Livingstone: Edinburgh, Scotland, 2009; pp. 15–31. ISBN 978-1-4160-5680-5. [Google Scholar]
- Casadevall, A. Fungi and the Rise of Mammals. PLoS Pathog. 2012, 8, e1002808. [Google Scholar] [CrossRef]
- Muñoz, J.F.; McEwen, J.G.; Clay, O.K.; Cuomo, C.A. Genome Analysis Reveals Evolutionary Mechanisms of Adaptation in Systemic Dimorphic Fungi. Sci. Rep. 2018, 8, 4473. [Google Scholar] [CrossRef] [PubMed]
- Olivier, F.A.B.; Hilsenstein, V.; Weerasinghe, H.; Weir, A.; Hughes, S.; Crawford, S.; Vince, J.E.; Hickey, M.J.; Traven, A. The Escape of Candida albicans from Macrophages Is Enabled by the Fungal Toxin Candidalysin and Two Host Cell Death Pathways. Cell Rep. 2022, 40, 111374. [Google Scholar] [CrossRef]
- Singh, D.K.; Tóth, R.; Gácser, A. Mechanisms of Pathogenic Candida Species to Evade the Host Complement Attack. Front. Cell Infect. Microbiol. 2020, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Chávez, M.J.; Pérez-García, L.A.; Niño-Vega, G.A.; Mora-Montes, H.M. Fungal Strategies to Evade the Host Immune Recognition. J. Fungi 2017, 3, 51. [Google Scholar] [CrossRef] [PubMed]
- Rai, L.S.; Wijlick, L.V.; Bougnoux, M.-E.; Bachellier-Bassi, S.; d’Enfert, C. Regulators of Commensal and Pathogenic Life-Styles of an Opportunistic Fungus—Candida albicans. Yeast 2021, 38, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Niemiec, M.J.; Kapitan, M.; Polke, M.; Jacobsen, I.D. Commensal to Pathogen Transition of Candida albicans. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Academic Press: Oxford, UK, 2017; pp. 696–713. ISBN 978-0-12-811737-8. [Google Scholar]
- Witchley, J.N.; Penumetcha, P.; Abon, N.V.; Woolford, C.A.; Mitchell, A.P.; Noble, S.M. Candida albicans Morphogenesis Programs Control the Balance between Gut Commensalism and Invasive Infection. Cell Host Microbe 2019, 25, 432–443.e6. [Google Scholar] [CrossRef]
- Esher, S.K.; Zaragoza, O.; Alspaugh, J.A. Cryptococcal Pathogenic Mechanisms: A Dangerous Trip from the Environment to the Brain. Mem. Inst. Oswaldo Cruz 2018, 113, e180057. [Google Scholar] [CrossRef] [PubMed]
- Nikou, S.-A.; Zhou, C.; Griffiths, J.S.; Kotowicz, N.K.; Coleman, B.M.; Green, M.J.; Moyes, D.L.; Gaffen, S.L.; Naglik, J.R.; Parker, P.J. The Candida albicans Toxin Candidalysin Mediates Distinct Epithelial Inflammatory Responses through P38 and EGFR-ERK Pathways. Sci. Signal 2022, 15, eabj6915. [Google Scholar] [CrossRef]
- Bacher, P.; Hohnstein, T.; Beerbaum, E.; Röcker, M.; Blango, M.G.; Kaufmann, S.; Röhmel, J.; Eschenhagen, P.; Grehn, C.; Seidel, K.; et al. Human Anti-Fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell 2019, 176, 1340–1355.e15. [Google Scholar] [CrossRef] [PubMed]
- Pathakumari, B.; Liang, G.; Liu, W. Immune Defence to Invasive Fungal Infections: A Comprehensive Review. Biomed. Pharmacother. 2020, 130, 110550. [Google Scholar] [CrossRef]
- McComb, S.; Thiriot, A.; Akache, B.; Krishnan, L.; Stark, F. Introduction to the Immune System. In Immunoproteomics: Methods and Protocols; Fulton, K.M., Twine, S.M., Eds.; Springer: New York, NY, USA, 2019; pp. 1–24. ISBN 978-1-4939-9597-4. [Google Scholar]
- Su, B.; Ng, L.G. Immunological Modulation in Health and Disease. Cell Mol. Immunol. 2023, 20, 981–982. [Google Scholar] [CrossRef]
- Dellière, S.; Aimanianda, V. Humoral Immunity Against Aspergillus fumigatus. Mycopathol. 2023, 188, 603–621. [Google Scholar] [CrossRef]
- Burgess, T.B.; Condliffe, A.M.; Elks, P.M. A Fun-Guide to Innate Immune Responses to Fungal Infections. J. Fungi 2022, 8, 805. [Google Scholar] [CrossRef]
- Ward, R.A.; Vyas, J.M. The First Line of Defense: Effector Pathways of Anti-Fungal Innate Immunity. Curr. Opin. Microbiol. 2020, 58, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, T.N.; Fierer, J. Innate Immune Receptors and Defense Against Primary Pathogenic Fungi. Vaccines 2020, 8, 303. [Google Scholar] [CrossRef] [PubMed]
- Sauls, R.S.; McCausland, C.; Taylor, B.N. Histology, T-Cell Lymphocyte. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Crameri, R.; Blaser, K. Allergy and Immunity to Fungal Infections and Colonization. Eur. Respir. J. 2002, 19, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Casadevall, A. Antibody Immunity and Invasive Fungal Infections. Infect. Immun. 1995, 63, 4211–4218. [Google Scholar] [CrossRef] [PubMed]
- Shukla, M.; Chandley, P.; Rohatgi, S. The Role of B-Cells and Antibodies against Candida Vaccine Antigens in Invasive Candidiasis. Vaccines 2021, 9, 1159. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Rezk, A.; Li, H.; Gommerman, J.L.; Prat, A.; Bar-Or, A.; On behalf of the Canadian B Cells in MS Team. Antibody-Independent Function of Human B Cells Contributes to Antifungal T Cell Responses. J. Immunol. 2017, 198, 3245–3254. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, A.; Sil, A.; Goel, S.; Vignesh, P.; Rawat, A.; Jindal, A.K. Disseminated Aspergillosis in X-Linked Agammaglobulinemia: Beyond the Norm. J. Clin. Immunol. 2024, 45, 24. [Google Scholar] [CrossRef]
- Doron, I.; Leonardi, I.; Li, X.V.; Fiers, W.D.; Semon, A.; Bialt-DeCelie, M.; Migaud, M.; Gao, I.H.; Lin, W.-Y.; Kusakabe, T.; et al. Human Gut Mycobiota Tune Immunity via CARD9-Dependent Induction of Anti-Fungal IgG Antibodies. Cell 2021, 184, 1017–1031.e14. [Google Scholar] [CrossRef]
- Verma, A.; Wüthrich, M.; Deepe, G.; Klein, B. Adaptive Immunity to Fungi. Cold Spring Harb. Perspect. Med. 2015, 5, a019612. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Alfaraj, A.H.; Alshengeti, A.; Alawfi, A.; Alwarthan, S.; Alhajri, M.; Al-Najjar, A.H.; Al Fares, M.A.; Najim, M.A.; Almuthree, S.A.; et al. Antibodies to Combat Fungal Infections: Development Strategies and Progress. Microorganisms 2023, 11, 671. [Google Scholar] [CrossRef] [PubMed]
- Etxebarria, A.; Díez-Martín, E.; Astigarraga, E.; Barreda-Gómez, G. Role of the Immune System in Renal Transplantation, Types of Response, Technical Approaches and Current Challenges. Immuno 2022, 2, 548–570. [Google Scholar] [CrossRef]
- IQWiG. In Brief: What Are the Organs of the Immune System? In InformedHealth.org; Institute for Quality and Efficiency in Health Care (IQWiG): Cologne, Germany, 2023. [Google Scholar]
- Cano, R.L.E.; Lopera, H.D.E. Introduction to T and B Lymphocytes. In Autoimmunity: From Bench to Bedside; El Rosario University Press: Bogota, Colombia, 2013. [Google Scholar]
- Elluru, S.R.; Kaveri, S.V.; Bayry, J. The Protective Role of Immunoglobulins in Fungal Infections and Inflammation. Semin. Immunopathol. 2015, 37, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Chear, C.T.; Nallusamy, R.; Chan, K.C.; Mohd Tap, R.; Baharin, M.F.; Syed Yahya, S.N.H.; Krishnan, P.B.; Mohamad, S.B.; Ripen, A.M. Atypical Presentation of Severe Fungal Necrotizing Fasciitis in a Patient with X-Linked Agammaglobulinemia. J. Clin. Immunol. 2021, 41, 1178–1186. [Google Scholar] [CrossRef]
- Rapaka, R.R.; Ricks, D.M.; Alcorn, J.F.; Chen, K.; Khader, S.A.; Zheng, M.; Plevy, S.; Bengtén, E.; Kolls, J.K. Conserved Natural IgM Antibodies Mediate Innate and Adaptive Immunity against the Opportunistic Fungus Pneumocystis murina. J. Exp. Med. 2010, 207, 2907–2919. [Google Scholar] [CrossRef]
- Reyneveld, G.I.; Savelkoul, H.F.J.; Parmentier, H.K. Current Understanding of Natural Antibodies and Exploring the Possibilities of Modulation Using Veterinary Models. A Review. Front. Immunol. 2020, 11, 2139. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sabater, A.; Autaa, G.; Sterlin, D.; Jerbi, A.; Villette, R.; Holm, J.B.; Parizot, C.; Selim, S.; Senghor, Y.; Ghillani-Dalbin, P.; et al. Systemic Anti-Commensal Response to Fungi Analyzed by Flow Cytometry Is Related to Gut Mycobiome Ecology. Microbiome 2020, 8, 159. [Google Scholar] [CrossRef]
- Wheeler, M.L.; Limon, J.J.; Underhill, D.M. Immunity to Commensal Fungi: Detente and Disease. Annu. Rev. Pathol. 2017, 12, 359–385. [Google Scholar] [CrossRef] [PubMed]
- Gouba, N.; Hien, Y.E.; Guissou, M.L.; Fonkou, M.D.M.; Traoré, Y.; Tarnagda, Z. Digestive Tract Mycobiota and Microbiota and the Effects on the Immune System. Human. Microbiome J. 2019, 12, 100056. [Google Scholar] [CrossRef]
- Swidergall, M.; LeibundGut-Landmann, S. Immunosurveillance of Candida albicans Commensalism by the Adaptive Immune System. Mucosal Immunol. 2022, 15, 829–836. [Google Scholar] [CrossRef]
- Gutierrez, M.W.; Arrieta, M.-C. The Intestinal Mycobiome as a Determinant of Host Immune and Metabolic Health. Curr. Opin. Microbiol. 2021, 62, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Larrea, A.; Elexpe, A.; Díez-Martín, E.; Torrecilla, M.; Astigarraga, E.; Barreda-Gómez, G. Neuroinflammation in the Evolution of Motor Function in Stroke and Trauma Patients: Treatment and Potential Biomarkers. Curr. Issues Mol. Biol. 2023, 45, 8552–8585. [Google Scholar] [CrossRef] [PubMed]
- Egiguren-Ortiz, J.; Domínguez-Fernández, C.; Razquin, J.; Heras-García, L.D.l.; Astigarraga, E.; Miguelez, C.; Barreda-Gómez, G. Reactive Antibodies against Brain Antigens as Serological Biomarkers of Neurodegenerative Diseases. Adv. Neurol. 2024, 3, 2058. [Google Scholar] [CrossRef]
- Vignoli, A.; Tenori, L.; Morsiani, C.; Turano, P.; Capri, M.; Luchinat, C. Serum or Plasma (and Which Plasma), That Is the Question. J. Proteome Res. 2022, 21, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Plebani, M.; Banfi, G.; Bernardini, S.; Bondanini, F.; Conti, L.; Dorizzi, R.; Ferrara, F.E.; Mancini, R.; Trenti, T. Serum or Plasma? An Old Question Looking for New Answers. Clin. Chem. Lab. Med. (CCLM) 2020, 58, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Solberg, H.; Bartholdy, C.; Landsy, L.H.; Andresen, L.O. Stability of Anti-Drug Antibodies in Human Serum Samples. J. Immunol. Methods 2024, 525, 113616. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Ó’Fágáin, C.; O’Kennedy, R. Antibody Stability: A Key to Performance—Analysis, Influences and Improvement. Biochimie 2020, 177, 213–225. [Google Scholar] [CrossRef]
- Oosterwegel, M.J.; Ibi, D.; Portengen, L.; Probst-Hensch, N.; Tarallo, S.; Naccarati, A.; Imboden, M.; Jeong, A.; Robinot, N.; Scalbert, A.; et al. Variability of the Human Serum Metabolome over 3 Months in the EXPOsOMICS Personal Exposure Monitoring Study. Environ. Sci. Technol. 2023, 57, 12752–12759. [Google Scholar] [CrossRef]
- Richardson, M.; Page, I. Role of Serological Tests in the Diagnosis of Mold Infections. Curr. Fungal Infect. Rep. 2018, 12, 127–136. [Google Scholar] [CrossRef]
- Shinfuku, K.; Suzuki, J.; Takeda, K.; Kawashima, M.; Morio, Y.; Sasaki, Y.; Nagai, H.; Watanabe, A.; Matsui, H.; Kamei, K. Validity of Platelia Aspergillus IgG and Aspergillus Precipitin Test To Distinguish Pulmonary Aspergillosis from Colonization. Microbiol. Spectr. 2023, 11, e0343522. [Google Scholar] [CrossRef]
- Lackner, M.; Lass-Flörl, C. Commercial Molecular Tests for Fungal Diagnosis from a Practical Point of View. Methods Mol. Biol. 2017, 1508, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; He, Z.; Guo, Y.; Ran, X.; Cheng, Y.; He, Z. A Novel Quantitative Double Antigen Sandwich ELISA for Detecting Total Antibodies against Candida albicans Enolase 1. Eur. J. Clin. Microbiol. Infect. Dis. 2024, 43, 1815–1823. [Google Scholar] [CrossRef]
- Martin-Souto, L.; Antoran, A.; Areitio, M.; Aparicio-Fernandez, L.; Martín-Gómez, M.T.; Fernandez, R.; Astigarraga, E.; Barreda-Gómez, G.; Schwarz, C.; Rickerts, V.; et al. Dot Immunobinding Assay for the Rapid Serodetection of Scedosporium/Lomentospora in Cystic Fibrosis Patients. J. Fungi 2023, 9, 158. [Google Scholar] [CrossRef] [PubMed]
- Taborda, C.P.; Camargo, Z.P. Diagnosis of Paracoccidioidomycosis by Dot Immunobinding Assay for Antibody Detection Using the Purified and Specific Antigen Gp43. J. Clin. Microbiol. 1994, 32, 554–556. [Google Scholar] [CrossRef]
- Reboli, A.C. Diagnosis of Invasive Candidiasis by a Dot Immunobinding Assay for Candida Antigen. Detection. J. Clin. Microbiol. 1993, 31, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Flori, P.; Hennequin, C.; Dubus, J.-C.; Reynaud-Gaubert, M.; Charpin, D.; Vergnon, J.M.; Gay, P.; Colly, A.; Piarroux, R.; et al. Evaluation of the Aspergillus Western Blot IgG Kit for Diagnosis of Chronic Aspergillosis. J. Clin. Microbiol. 2015, 53, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Piarroux, R.P.; Romain, T.; Martin, A.; Vainqueur, D.; Vitte, J.; Lachaud, L.; Gangneux, J.-P.; Gabriel, F.; Fillaux, J.; Ranque, S. Multicenter Evaluation of a Novel Immunochromatographic Test for Anti-Aspergillus IgG Detection. Front. Cell Infect. Microbiol. 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Etienne, K.A.; Kano, R.; Balajee, S.A. Development and Validation of a Microsphere-Based Luminex Assay for Rapid Identification of Clinically Relevant Aspergilli. J. Clin. Microbiol. 2009, 47, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, G.; Moscardelli, A.; Colella, A.; Marafini, I.; Salvatori, S. Immune-Mediated Inflammatory Diseases: Common and Different Pathogenic and Clinical Features. Autoimmun. Rev. 2023, 22, 103410. [Google Scholar] [CrossRef]
- Puig, L.; Ruiz de Morales, J.G.; Dauden, E.; Andreu, J.L.; Cervera, R.; Adán, A.; Marsal, S.; Escobar, C.; Hinojosa, J.; Palau, J.; et al. La Prevalencia de Diez Enfermedades Inflamatorias Inmunomediadas (IMID) En España. Rev. Esp. Salud Publica 2020, 93, e201903013. [Google Scholar]
- Tarn, J.; Lendrem, D.; Barnes, M.; Casement, J.; Ng, W.-F. Comorbidities in the UK Primary Sjögren’s Syndrome Registry. Front. Immunol. 2022, 13, 864448. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Li, L.; Liu, C.; Yan, S.; Li, Y. Meta-Analysis of Anti-Saccharomyces cerevisiae Antibodies as Diagnostic Markers of Behçet’s Disease with Gastrointestinal Involvement. BMJ Open 2020, 10, e033880. [Google Scholar] [CrossRef] [PubMed]
- Sendid, B.; Cornu, M.; Cordier, C.; Bouckaert, J.; Colombel, J.F.; Poulain, D. From ASCA Breakthrough in Crohn’s Disease and Candida albicans Research to Thirty Years of Investigations about Their Meaning in Human Health. Autoimmun. Rev. 2024, 23, 103486. [Google Scholar] [CrossRef]
- Doron, I.; Kusakabe, T.; Iliev, I.D. Immunoglobulins at the Interface of the Gut Mycobiota and Anti-Fungal Immunity. Semin. Immunol. 2023, 67, 101757. [Google Scholar] [CrossRef]
- Chandrakumar, A.; Georgy, M.; Agarwal, P.; ’t Jong, G.W.; El-Matary, W. Anti-Saccharomyces cerevisiae Antibodies as a Prognostic Biomarker in Children With Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2019, 69, 82–87. [Google Scholar] [CrossRef]
- Muratori, P.; Muratori, L.; Guidi, M.; Maccariello, S.; Pappas, G.; Ferrari, R.; Gionchetti, P.; Campieri, M.; Bianchi, F.B. Anti-Saccharomyces cerevisiae Antibodies (ASCA) and Autoimmune Liver Diseases. Clin. Exp. Immunol. 2003, 132, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Ghozzi, M.; Mankai, A.; Mechi, F.; Ben Chedly, Z.; Kallala, O.; Melayah, S.; Trabelsi, A.; Ghedira, I. High Frequency of Anti-Saccharomyces cerevisiae Antibodies in Chronic Hepatitis C. Arab. J. Gastroenterol. 2024, 25, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, T.; Vaziri, H.; Wu, G.Y. Primary Sclerosing Cholangitis and Inflammatory Bowel Disease: A Review. J. Clin. Transl. Hepatol. 2022, 10, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Sakly, W.; Jeddi, M.; Ghedira, I. Anti-Saccharomyces cerevisiae Antibodies in Primary Biliary Cirrhosis. Dig. Dis. Sci. 2008, 53, 1983–1987. [Google Scholar] [CrossRef]
- Fresko, I.; Ugurlu, S.; Ozbakir, F.; Celik, A.; Yurdakul, S.; Hamuryudan, V.; Yazici, H. Anti-Saccharomyces cerevisiae Antibodies (ASCA) in Behçet’s Syndrome. Clin. Exp. Rheumatol. 2005, 23, S67–S70. [Google Scholar] [PubMed]
- Dickerson, F.; Adamos, M.; Katsafanas, E.; Khushalani, S.; Origoni, A.; Savage, C.; Schweinfurth, L.; Stallings, C.; Sweeney, K.; Alaedini, A.; et al. The Association between Immune Markers and Recent Suicide Attempts in Patients with Serious Mental Illness: A Pilot Study. Psychiatry Res. 2017, 255, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Gaifem, J.; Rodrigues, C.S.; Petralia, F.; Alves, I.; Leite-Gomes, E.; Cavadas, B.; Dias, A.M.; Moreira-Barbosa, C.; Revés, J.; Laird, R.M.; et al. A Unique Serum IgG Glycosylation Signature Predicts Development of Crohn’s Disease and Is Associated with Pathogenic Antibodies to Mannose Glycan. Nat. Immunol. 2024, 25, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Dotan, I.; Fishman, S.; Dgani, Y.; Schwartz, M.; Karban, A.; Lerner, A.; Weishauss, O.; Spector, L.; Shtevi, A.; Altstock, R.T.; et al. Antibodies Against Laminaribioside and Chitobioside Are Novel Serologic Markers in Crohn’s Disease. Gastroenterology 2006, 131, 366–378. [Google Scholar] [CrossRef]
- Yorulmaz, E.; Adalı, G.; Yorulmaz, H.; Taşan, G.; Gürses, S.; Ayaş, M.R.; Tuncer, İ. The Correlation between New Serological Markers and Disease Phenotype and Activation in Inflammatory Bowel Disease. Middle East. J. Dig. Dis. 2022, 14, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Smids, C.; Horjus Talabur Horje, C.S.; Groenen, M.J.M.; van Koolwijk, E.H.M.; Wahab, P.J.; van Lochem, E.G. The Value of Serum Antibodies in Differentiating Inflammatory Bowel Disease, Predicting Disease Activity and Disease Course in the Newly Diagnosed Patient. Scand. J. Gastroenterol. 2017, 52, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Boschetti, G.; Rinaudo-Gaujous, M.; Moreau, A.; Del Tedesco, E.; Bonneau, J.; Presles, E.; Mounsef, F.; Clavel, L.; Genin, C.; et al. Association of Anti-Glycan Antibodies and Inflammatory Bowel Disease Course. J. Crohn’s Colitis 2015, 9, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Hughes, H.K.; Ashwood, P. Anti-Candida albicans IgG Antibodies in Children With Autism Spectrum Disorders. Front. Psychiatry 2018, 9, 627. [Google Scholar] [CrossRef]
- Severance, E.G.; Gressitt, K.L.; Stallings, C.R.; Katsafanas, E.; Schweinfurth, L.A.; Savage, C.L.; Adamos, M.B.; Sweeney, K.M.; Origoni, A.E.; Khushalani, S.; et al. Candida albicans Exposures, Sex Specificity and Cognitive Deficits in Schizophrenia and Bipolar Disorder. Npj Schizophr. 2016, 2, 16018. [Google Scholar] [CrossRef]
- Severance, E.G.; Alaedini, A.; Yang, S.; Halling, M.; Gressitt, K.L.; Stallings, C.R.; Origoni, A.E.; Vaughan, C.; Khushalani, S.; Leweke, F.M.; et al. Gastrointestinal Inflammation and Associated Immune Activation in Schizophrenia. Schizophr. Res. 2012, 138, 48–53. [Google Scholar] [CrossRef]
- Severance, E.G.; Gressitt, K.L.; Yang, S.; Stallings, C.R.; Origoni, A.E.; Vaughan, C.; Khushalani, S.; Alaedini, A.; Dickerson, F.B.; Yolken, R.H. Seroreactive Marker for Inflammatory Bowel Disease and Associations with Antibodies to Dietary Proteins in Bipolar Disorder. Bipolar Disord. 2014, 16, 230–240. [Google Scholar] [CrossRef]
- Alonso, R.; Pisa, D.; Rábano, A.; Carrasco, L. Alzheimer’s Disease and Disseminated Mycoses. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.-Y.; Fang, Y.; Li, C.; Xia, D.-D.; Zhang, G.; Wen, Y.; Zhang, S.-Z.; Hu, L.; Gu, L.-Y.; et al. Elevated Serum Anti-Saccharomyces cerevisiae Antibody Accompanied by Gut Mycobiota Dysbiosis as a Biomarker of Diagnosis in Patients with de Novo Parkinson Disease. Eur. J. Neurol. 2023, 30, 3462–3470. [Google Scholar] [CrossRef] [PubMed]
- Dorko, E.; Jenca, A.; Pilipcinec, E.; Tkáciková, L. Detection of Anti-Candida Antibodies by the Indirect Immunofluorescence Assay in Patients with Cancer in the Orofacial Region. Folia Microbiol. 2002, 47, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Strati, F.; Cavalieri, D.; Albanese, D.; De Felice, C.; Donati, C.; Hayek, J.; Jousson, O.; Leoncini, S.; Renzi, D.; Calabrò, A.; et al. New Evidences on the Altered Gut Microbiota in Autism Spectrum Disorders. Microbiome 2017, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Pisa, D.; Fernández-Fernández, A.M.; Carrasco, L. Infection of Fungi and Bacteria in Brain Tissue From Elderly Persons and Patients With Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 159. [Google Scholar] [CrossRef]
- Laurence, M.; Benito-León, J.; Calon, F. Malassezia and Parkinson’s Disease. Front. Neurol. 2019, 10, 758. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Jiang, M.-L.; Jiang, R.; Pang, T.; Zhang, C.-J. The Roles of Fungus in CNS Autoimmune and Neurodegeneration Disorders. Front. Immunol. 2023, 13, 1077335. [Google Scholar] [CrossRef]
- Gamal, A.; Elshaer, M.; Alabdely, M.; Kadry, A.; McCormick, T.S.; Ghannoum, M. The Mycobiome: Cancer Pathogenesis, Diagnosis, and Therapy. Cancers 2022, 14, 2875. [Google Scholar] [CrossRef] [PubMed]
- Pandey, H.; Tang, D.W.T.; Wong, S.H.; Lal, D. Gut Microbiota in Colorectal Cancer: Biological Role and Therapeutic Opportunities. Cancers 2023, 15, 866. [Google Scholar] [CrossRef]
- Ramirez-Garcia, A.; Arteta, B.; Abad-Diaz-de-Cerio, A.; Pellon, A.; Antoran, A.; Marquez, J.; Rementeria, A.; Hernando, F.L. Candida albicans Increases Tumor Cell Adhesion to Endothelial Cells in Vitro: Intraspecific Differences and Importance of the Mannose Receptor. PLoS ONE 2013, 8, e53584. [Google Scholar] [CrossRef]
- Gagnière, J.; Raisch, J.; Veziant, J.; Barnich, N.; Bonnet, R.; Buc, E.; Bringer, M.-A.; Pezet, D.; Bonnet, M. Gut Microbiota Imbalance and Colorectal Cancer. World J. Gastroenterol. 2016, 22, 501–518. [Google Scholar] [CrossRef]
- Alonso, R.; Fernández-Fernández, A.M.; Pisa, D.; Carrasco, L. Multiple Sclerosis and Mixed Microbial Infections. Direct Identification of Fungi and Bacteria in Nervous Tissue. Neurobiol. Dis. 2018, 117, 42–61. [Google Scholar] [CrossRef] [PubMed]
- Iliev, I.D.; Brown, G.D.; Bacher, P.; Gaffen, S.L.; Heitman, J.; Klein, B.S.; Lionakis, M.S. Focus on Fungi. Cell 2024, 187, 5121–5127. [Google Scholar] [CrossRef]
- Maas, E.; Penders, J.; Venema, K. Fungal-Bacterial Interactions in the Human Gut of Healthy Individuals. J. Fungi 2023, 9, 139. [Google Scholar] [CrossRef]
- Genome. Available online: https://www.ncbi.nlm.nih.gov/datasets/genome/ (accessed on 5 February 2025).
- Wang, Y.; He, F.; Liu, B.; Wu, X.; Han, Z.; Wang, X.; Liao, Y.; Duan, J.; Ren, W. Interaction between Intestinal Mycobiota and Microbiota Shapes Lung Inflammation. Imeta 2024, 3, e241. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.-Y.; Chi, Y.-Y.; Han, J.-X.; Kong, P.; Liang, Z.; Wang, D.; Xiang, H.; Xie, Q. Litchi Chinensis Seed Prevents Obesity and Modulates the Gut Microbiota and Mycobiota Compositions in High-Fat Diet-Induced Obese Zebrafish. Food Funct. 2022, 13, 2832–2845. [Google Scholar] [CrossRef] [PubMed]
- Ruchti, F.; Zwicky, P.; Becher, B.; Dubrac, S.; LeibundGut-Landmann, S. Epidermal Barrier Impairment Predisposes for Excessive Growth of the Allergy-Associated Yeast Malassezia on Murine Skin. Allergy 2024, 79, 1531–1547. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, I.; Gao, I.H.; Lin, W.-Y.; Allen, M.; Li, X.V.; Fiers, W.D.; De Celie, M.B.; Putzel, G.G.; Yantiss, R.K.; Johncilla, M.; et al. Mucosal Fungi Promote Gut Barrier Function and Social Behavior via Type 17 Immunity. Cell 2022, 185, 831–846.e14. [Google Scholar] [CrossRef] [PubMed]
- Diez-Martin, E.; Astigarraga, E.; Barreda-Gómez, G. Personalized On-Chip Sample Evaluation Devices for Biomedical Applications: Advantages, Challenges, and Opportunities. In Biosensors for Personalized Healthcare; Mahato, K., Chandra, P., Eds.; Springer Nature: Singapore, 2024; pp. 225–252. ISBN 978-981-9754-73-1. [Google Scholar]
- Filiz, Y.; Esposito, A.; De Maria, C.; Vozzi, G.; Yesil-Celiktas, O. A Comprehensive Review on Organ-on-Chips as Powerful Preclinical Models to Study Tissue Barriers. Prog. Biomed. Eng. 2024, 6. [Google Scholar] [CrossRef] [PubMed]
- García-Gamboa, R.; Díaz-Torres, O.; Senés-Guerrero, C.; Gradilla-Hernández, M.S.; Moya, A.; Pérez-Brocal, V.; Garcia-Gonzalez, A.; González-Avila, M. Associations between Bacterial and Fungal Communities in the Human Gut Microbiota and Their Implications for Nutritional Status and Body Weight. Sci. Rep. 2024, 14, 5703. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L. Immunoglobulins in Defense, Pathogenesis and Therapy of Fungal Diseases. Cell Host Microbe 2012, 11, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Krylov, V.B.; Kuznetsov, A.N.; Polyanskaya, A.V.; Tsarapaev, P.V.; Yashunsky, D.V.; Kushlinskii, N.E.; Nifantiev, N.E. ASCA-Related Antibodies in the Blood Sera of Healthy Donors and Patients with Colorectal Cancer: Characterization with Oligosaccharides Related to Saccharomyces cerevisiae Mannan. Front. Mol. Biosci. 2023, 10, 1296828. [Google Scholar] [CrossRef] [PubMed]
- Elizalde, J.; Mayora, K.; Rivas, L.A.; Sanz, A.J.; Tolentino-Cortez, T.; Barreda-Gómez, G.; Tijero, M. Multi-Parametric Point of Care Device for Allergen-Specific IgE Detection in Veterinary Applications. Procedia Eng. 2016, 168, 1410–1413. [Google Scholar] [CrossRef]
- Hernandez-Suarez, L.; Diez-Martin, E.; Egiguren-Ortiz, J.; Fernandez, R.; Etxebarria, A.; Astigarraga, E.; Miguelez, C.; Ramirez-Garcia, A.; Barreda-Gómez, G. Serological Antibodies against Kidney, Liver, and Spleen Membrane Antigens as Potential Biomarkers in Patients with Immune Disorders. Int. J. Mol. Sci. 2024, 25, 2025. [Google Scholar] [CrossRef]

| Strengths | Weaknesses |
|---|---|
| Emerging interest in the mycobiota Technological advancement in sequencing and bioinformatics Potential of antifungal antibodies as biomarkers Opportunities for personalized medicine | Limited mycobiota research and data Insufficient fungal databases Cross-reactivity in antibody detection Animal model limitations |
| Opportunities | Threats |
| Expansion of fungal databases Multiplex detection for better diagnosis Early disease screening using antibodies Targeted therapies for fungal dysbiosis | Lack of large-scale validation studies Bacterial dominance masking fungal effects Regulatory hurdles for clinical implementation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diez-Martin, E.; Hernandez-Suarez, L.; Astigarraga, E.; Ramirez-Garcia, A.; Barreda-Gómez, G. Mycobiota and Antifungal Antibodies as Emerging Targets for the Diagnosis and Prognosis of Human Diseases. J. Fungi 2025, 11, 296. https://doi.org/10.3390/jof11040296
Diez-Martin E, Hernandez-Suarez L, Astigarraga E, Ramirez-Garcia A, Barreda-Gómez G. Mycobiota and Antifungal Antibodies as Emerging Targets for the Diagnosis and Prognosis of Human Diseases. Journal of Fungi. 2025; 11(4):296. https://doi.org/10.3390/jof11040296
Chicago/Turabian StyleDiez-Martin, Eguzkiñe, Leidi Hernandez-Suarez, Egoitz Astigarraga, Andoni Ramirez-Garcia, and Gabriel Barreda-Gómez. 2025. "Mycobiota and Antifungal Antibodies as Emerging Targets for the Diagnosis and Prognosis of Human Diseases" Journal of Fungi 11, no. 4: 296. https://doi.org/10.3390/jof11040296
APA StyleDiez-Martin, E., Hernandez-Suarez, L., Astigarraga, E., Ramirez-Garcia, A., & Barreda-Gómez, G. (2025). Mycobiota and Antifungal Antibodies as Emerging Targets for the Diagnosis and Prognosis of Human Diseases. Journal of Fungi, 11(4), 296. https://doi.org/10.3390/jof11040296







