Ozone Treatment Inhibited the Blue Mold Development and Maintained the Main Active Ingredient Content in Radix astragali Infected by Penicillium polonicum Through Activating Reactive Oxygen Species Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Spore Suspension
2.2. Preparation of Ozone Gas and Treatment of Radix astragali (RA)
2.3. Effect of Ozone Treatment on Weight Loss Rate of Fresh R. astragali
2.4. Effect of Ozone Treatment on Disease Incidence of Fresh R. astragali
2.5. Effect of Ozone Application on the Patulin Production in Fresh R. astragali
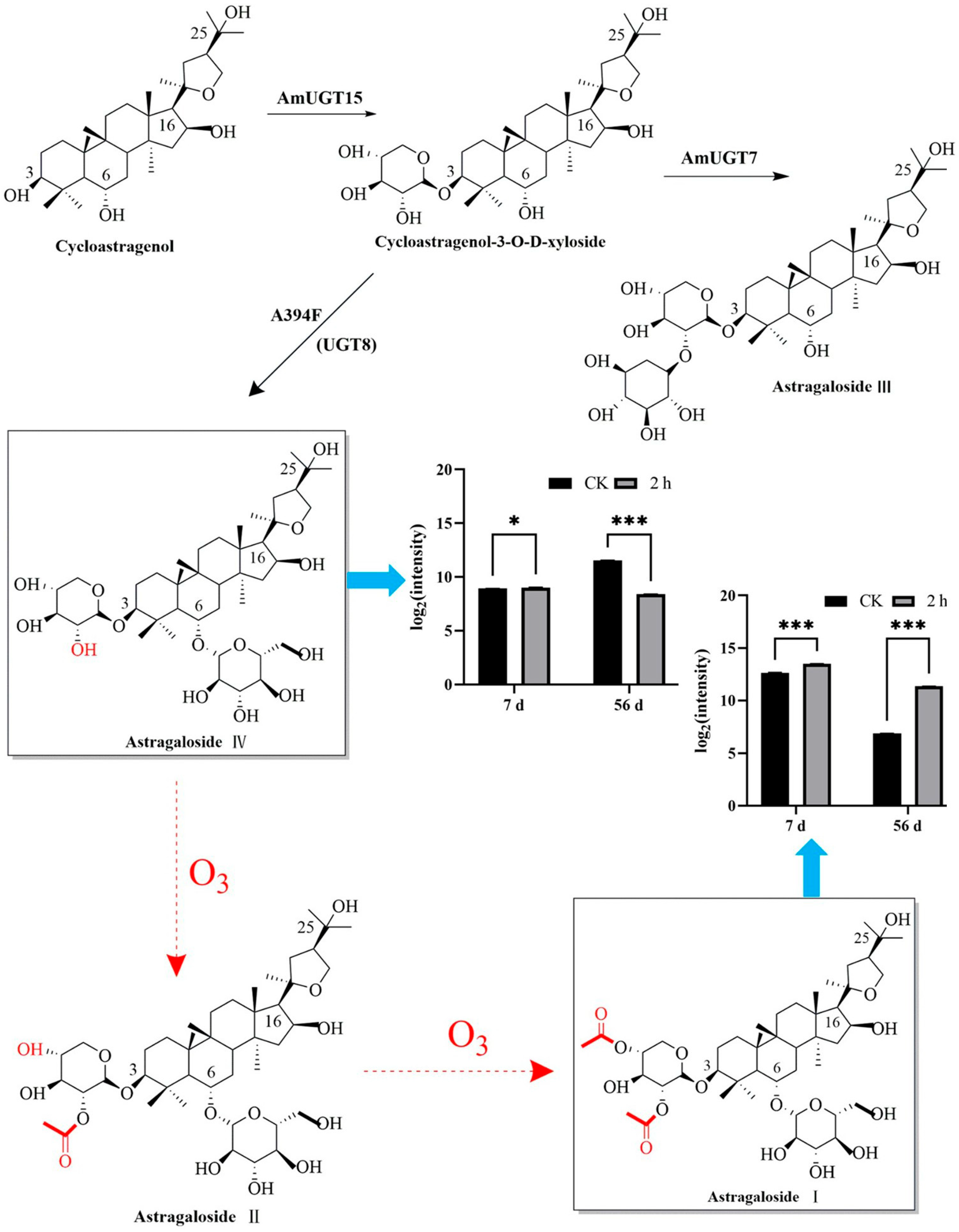
2.6. Effect of Ozone Treatment on the Main Active Ingredients of Fresh R. astragali
2.7. Malonaldehyde (MDA) Content Assay
2.8. The Production Rate of O2−· and H2O2 Content Assay
2.9. Enzymatic Activities Assay
2.9.1. Assay of the Enzymatic Activities Involved in ROS Production
2.9.2. Assay of the Enzymatic Activities Involved in ROS Depletion
2.9.3. Assay of the Key Enzymatic Activities Involved in AsA-GSH Cycle
2.10. Statistical Analysis
3. Results
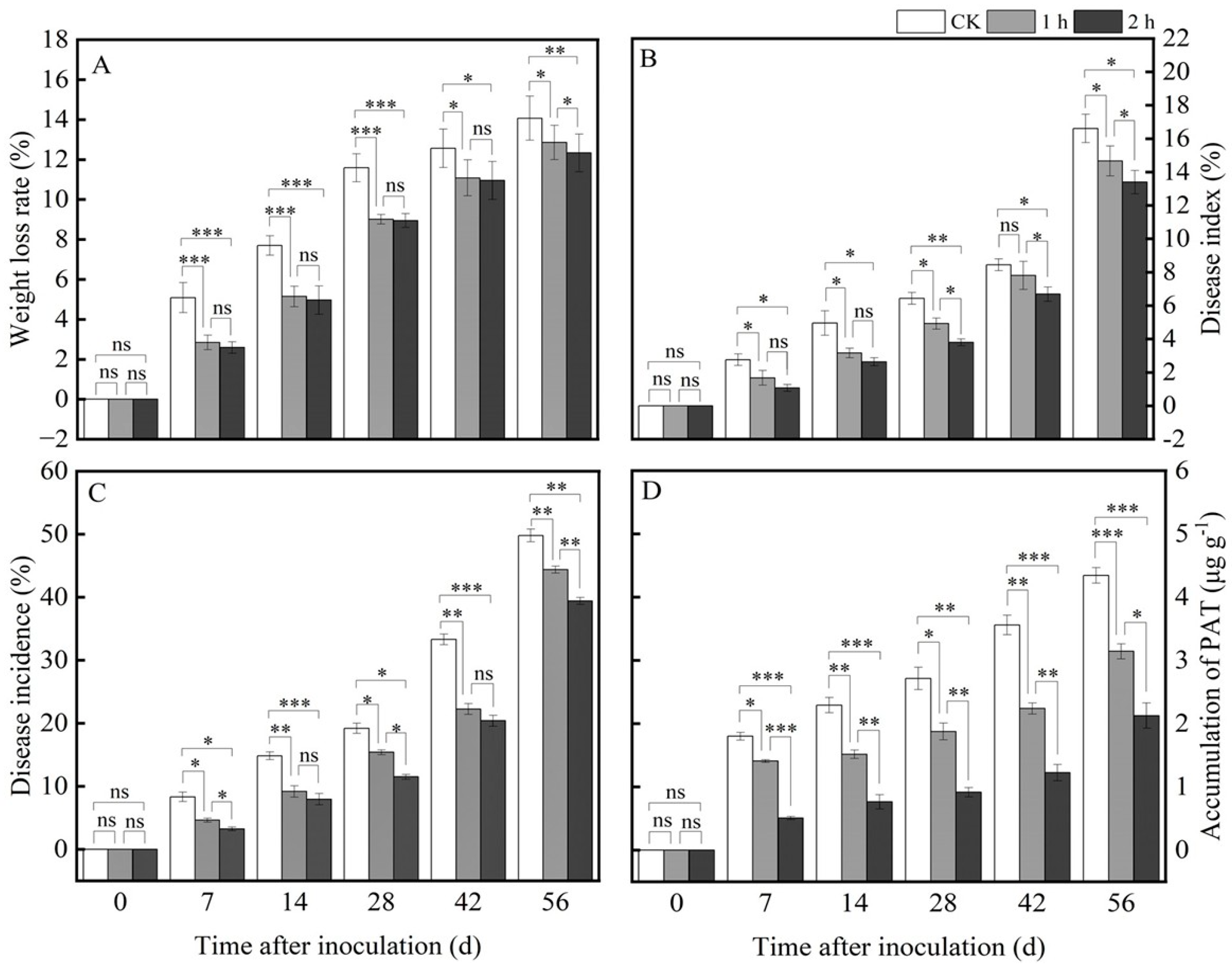
3.1. Ozone Treatment Delayed Water Loss in Fresh R. astragali
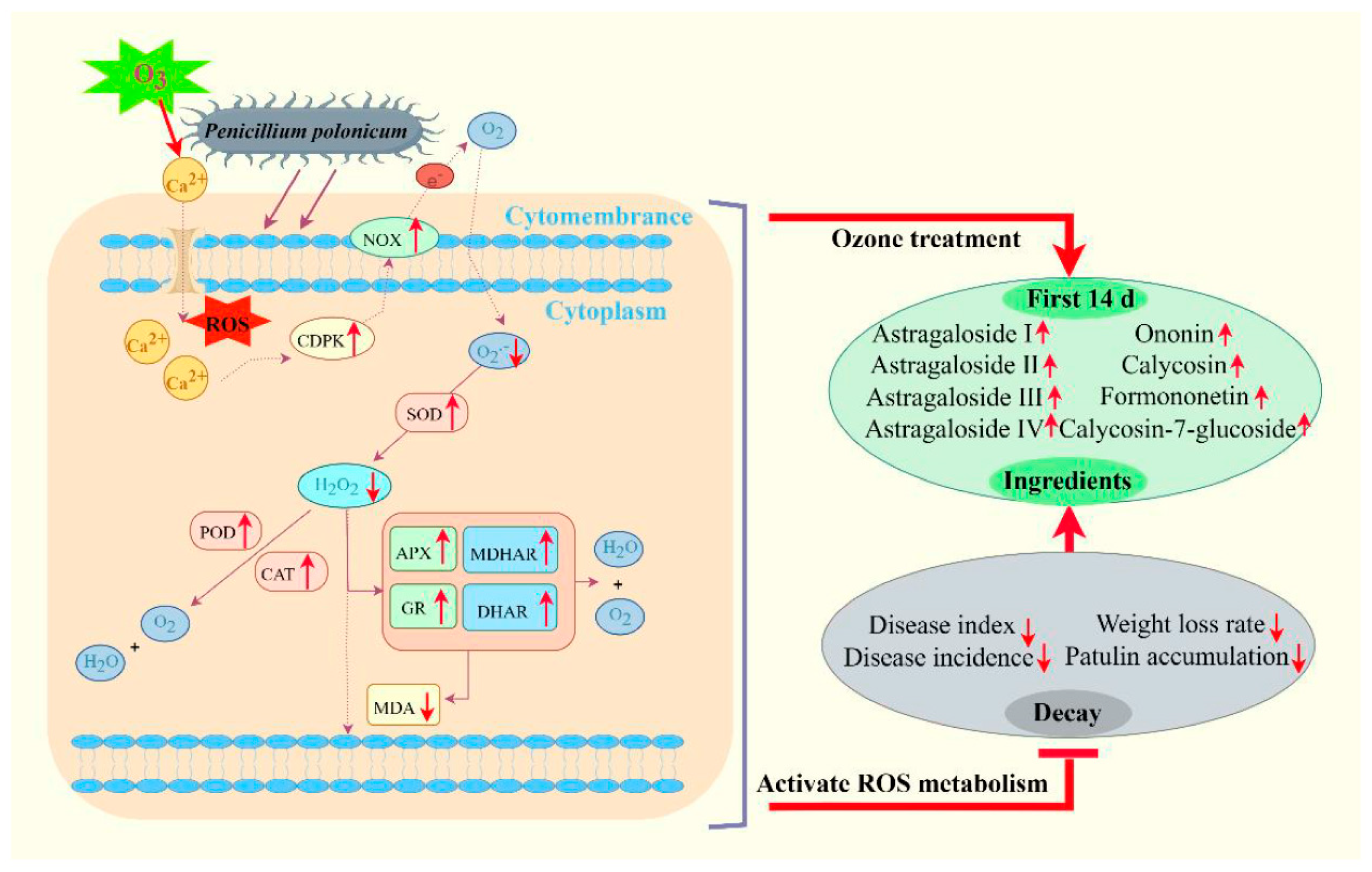
3.2. Ozone Treatment Inhibited the Blue Mold Development and Patulin Accumulation in Fresh R. astragali
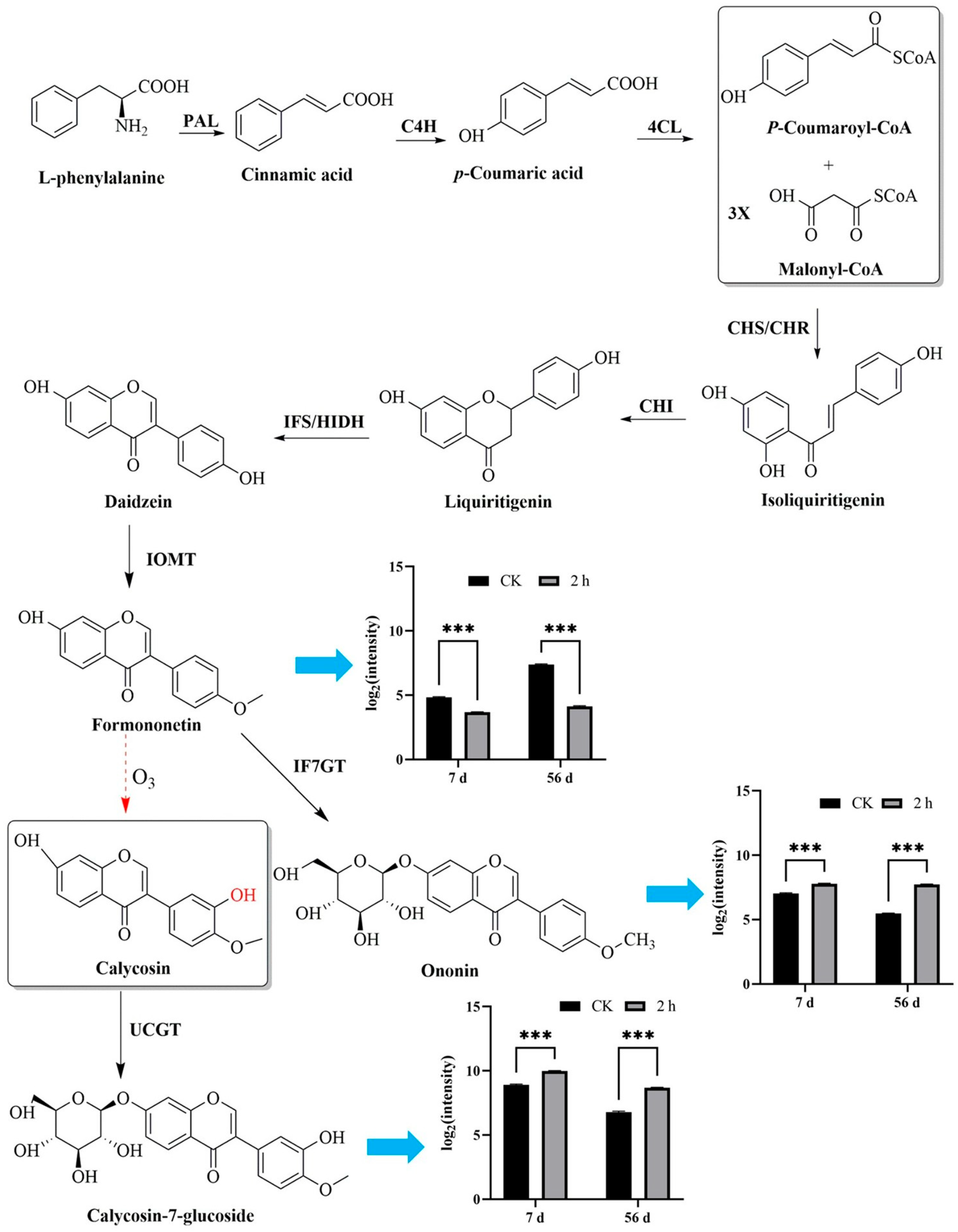
3.3. Ozone Treatment Maintained the Main Active Ingredient Contents of Fresh R. astragali
3.4. Ozone Treatment Activated ROS Metabolism and Kept Redox Homeostasis in Fresh R. astragali
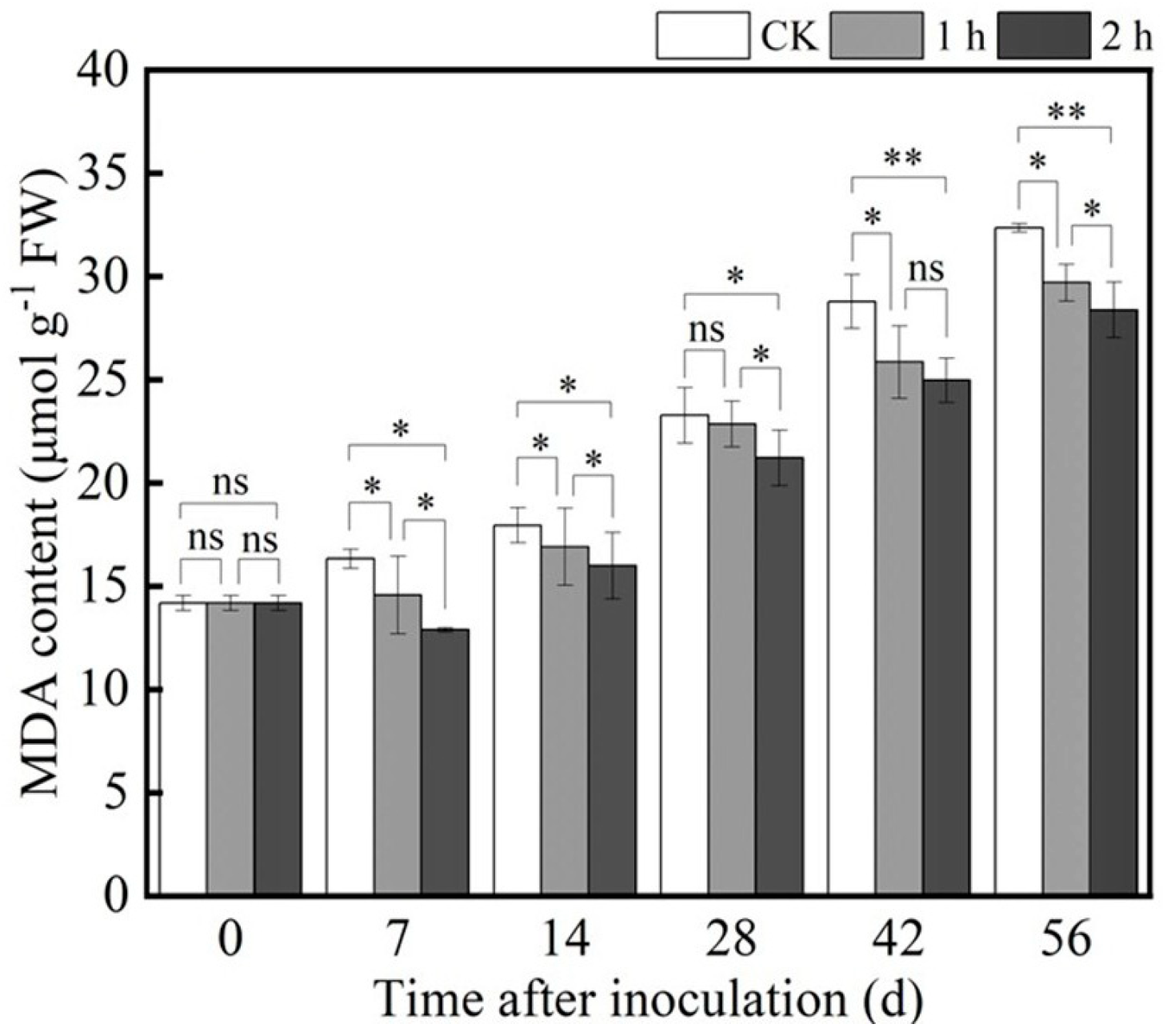
3.4.1. Ozone Treatment Decreased MDA Content in Fresh R. astragali
3.4.2. Ozone Treatment Decreased the O2−· Production Rate and H2O2 Content in Fresh R. astragali
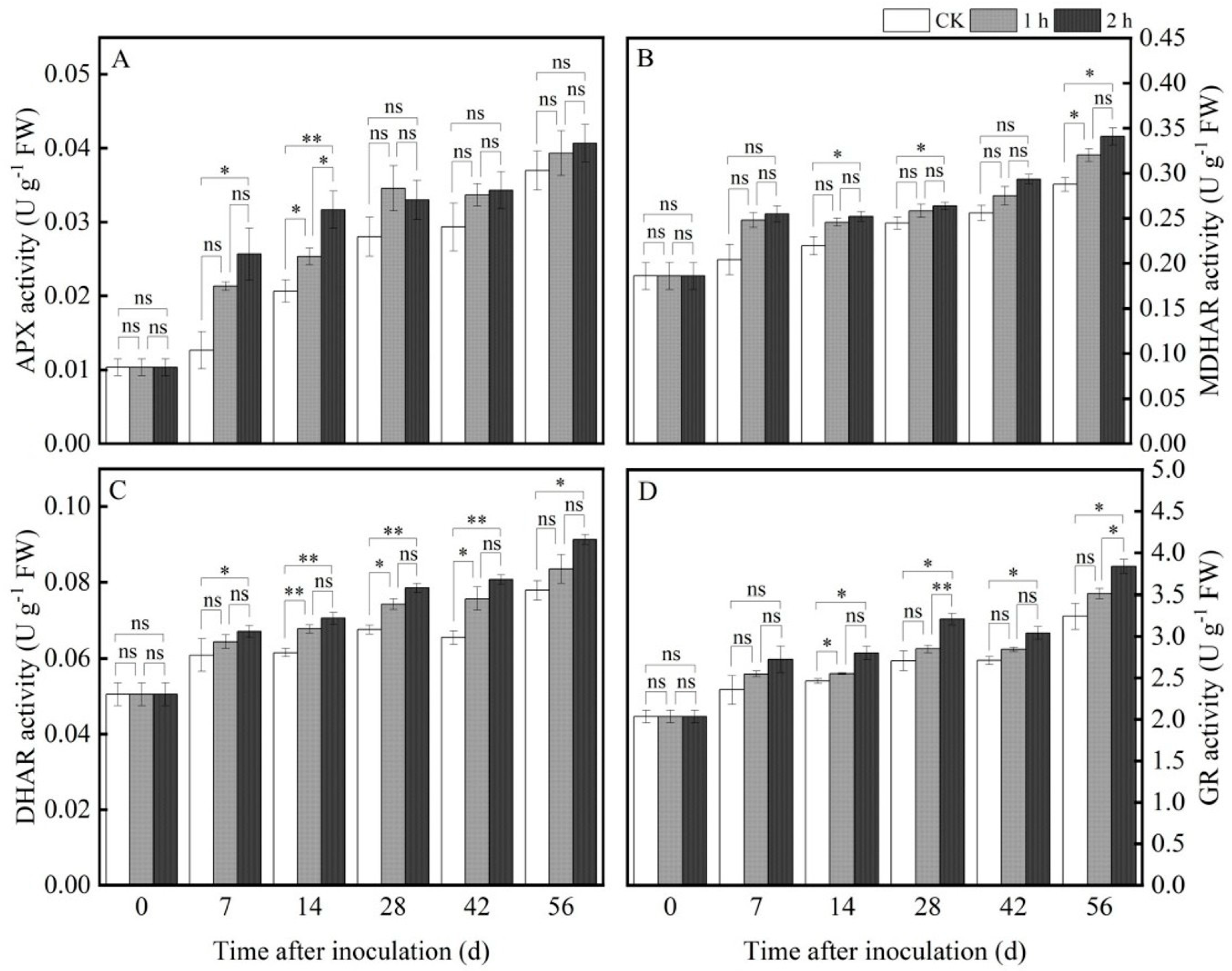
3.4.3. Ozone Treatment Increased the Activities of ROS Metabolism-Related Enzymes
3.4.4. Ozone Treatment Increased the Activities of the Enzymes Involved in AsA-GSH Cycle
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Pharmacopoeia of the People’s Republic of China: Volume I; Chinese Pharmacopoeia Commission: Beijing, China, 2020.
- Chang, X.; Chen, X.; Guo, Y.; Gong, P.; Pei, S.; Wang, D.; Wang, P.; Wang, M.; Chen, F. Advances in chemical composition, extraction techniques, analytical methods, and biological activity of Astragali Radix. Molecules 2022, 27, 1058. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xiao, B.; Sun, T. Antitumor and immunomodulatory activity of Astragalus membranaceus polysaccharides in H22 tumor-bearing mice. Int. J. Biol. Macromol. 2013, 62, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, H.; Luo, Y. Anti-Aging Implications of Astragalus Membranaceus (Huangqi): A Well-Known Chinese Tonic. Aging Dis. 2017, 8, 868. [Google Scholar] [CrossRef]
- Auyeung, K.K.; Han, Q.B.; Ko, J.K. Astragalus membranaceus: A Review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, X.F.; Liu, H.; Wang, W.; Liu, X.; Li, X.; Wu, X. Astragalus polysaccharides inhibit avian infectious bronchitis virus infection by regulating viral replication. Microb. Pathog. 2018, 114, 124–128. [Google Scholar] [CrossRef]
- Shahzad, M.; Shabbir, A.; Wojcikowski, K.; Wohlmuth, H.; Gobe, G.C. The antioxidant effects of Radix Astragali (Astragalus membranaceus and related species) in protecting tissues from injury and disease. Curr. Drug Targets 2016, 17, 1331–1340. [Google Scholar] [CrossRef]
- Zhang, A.; Zheng, Y.; Que, Z.; Zhang, L.; Lin, S.; Le, V.; Liu, J.; Tian, J. Astragaloside IV inhibits progression of lung cancer by mediating immune function of Tregs and CTLs by interfering with IDO. J. Cancer Res. Clin. 2014, 140, 1883–1890. [Google Scholar] [CrossRef]
- Zhang, L.; Qian, D.W.; Bu, F.S.; Huang, K.D.; Guo, S.; Yan, H.; Ouyang, Z.; Zhao, J.J.; Yu, J.Q.; Duan, J.A. Study on quality evaluation of Astragali Radix based on UPLC-MS. Chin. J. Pharm. Anal. 2020, 40, 722–732. [Google Scholar]
- Li, L.; Hou, X.J.; Xu, R.F. Research review on the pharmacological effects of astragaloside IV. Fundam. Clin. Pharmacol. 2017, 31, 17–36. [Google Scholar] [CrossRef]
- Li, L.L.; Huang, J.Z. Advances in pharmacological studies of calycosin-7-O-β-D-glucoside. J. Hainan Med. Univ. 2020, 26, 156–160. [Google Scholar]
- Wang, J.; Zhang, J.; Ma, J.; Liu, L.; Li, J.; Shen, T.; Tian, Y. The major component of cinnamon oil as a natural substitute against Fusarium solani on Astragalus membranaceus. J. Appl. Microbiol. 2022, 132, 3125–3141. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Yang, C.; Wei, L.; Cui, L.; Wang, Y.; Osei, R. First Report of Botrytis cinerea Causing Gray Mold on Astragalus membranaceus in China. Plant Dis. 2022, 106, 1520. [Google Scholar] [CrossRef]
- Liu, J.T.; Gao, Y.G.; Zang, P.; Xiao, R.X.; Zhao, Y.; He, Z.M.; Zhu, H.Y.; Zhang, L.X. First report of Penicillium oxalicum causing blue mold on Astragalus membranaceus in Jilin Province, China. Plant Dis. 2018, 102, 2637. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, Q.J.; Sun, K.; Guo, Z.X.; Chi, X.L.; Huang, L.Q. Population characteristics and threatened of wild Angelica sinensis in Gansu province. China J. Chin. Mater. Med. 2019, 44, 2987–2995. [Google Scholar]
- Zhang, Y.; Nan, M.N.; Xue, H.L. Isolating, identifying, and analyzing the biological characteristics of pathogens causing postharvest disease in fresh Radix astragali. Horticulturae 2023, 9, 1019. [Google Scholar] [CrossRef]
- Xi, J.H.; Yang, D.Y.; Xue, H.L.; Liu, Z.; Bi, Y.; Zhang, Y.; Yang, X.; Shang, S. Isolation of the main pathogens causing postharvest disease in fresh Angelica sinensis during different storage stages and impacts of ozone treatment on disease development and mycotoxin production. Toxins 2023, 15, 154. [Google Scholar] [CrossRef]
- Ma, B.; Kan, W.L.T.; Zhu, H.; Li, S.L.; Lin, G. Sulfur fumigation reducing systemic exposure of ginsenosides and weakening immunomodulatory activity of ginseng. J. Ethnopharmacol. 2017, 195, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Ong, M.K.; Forney, C.F. Effect of ozone pre-conditioning on quality and antioxidant capacity of papaya fruit during ambient storage. Food Chem. 2014, 142, 19–26. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Camele, I.; Sofo, A.; Mazzone, G.; Caivano, M.; Masi, S.; Caniani, D. Mycoremediation effect of Trichoderma harzianum strain T22 combined with ozonation in diesel-contaminated sand. Chemosphere 2020, 252, 126597. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Wu, S.; Qiu, T.; Blaženović, I.; Sun, J.; Zhang, Y.; Sun, X.; Ji, J. Evaluation of the toxicity and chemical alterations of deoxynivalenol degradation products under ozone treatment. Food Control 2021, 124, 107937. [Google Scholar] [CrossRef]
- Matłok, N.; Piechowiak, T.; Zardzewiały, M.; Balawejder, M. Effects of post-harvest ozone treatment on some molecular stability markers of amelanchier alnifolia nutt. fruit during cold storage. Int. J. Mol. Sci. 2022, 23, 11152. [Google Scholar] [CrossRef]
- Luo, A.; Bai, J.; Li, R.; Fang, Y.; Li, L.; Wang, D.; Zhang, L.; Liang, J.; Huang, T.; Kou, L. Effects of ozone treatment on the quality of kiwifruit during postharvest storage affected by Botrytis cinerea and Penicillium expansum. J. Phytopathol. 2019, 167, 470–478. [Google Scholar] [CrossRef]
- Li, L.; Xue, H.L.; Bi, Y.; Zhang, R.; Kouasseu, C.J.; Liu, Q.; Nan, M.; Pu, L.; Prusky, D. Ozone treatment inhibits dry rot development and diacetoxyscirpenol accumulation in inoculated potato tuber by influencing growth of Fusarium sulphureum and ergosterol biosynthesis. Postharvest Biol. Technol. 2022, 185, 111796. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, W. Effect of postmortem aging on tenderness and water-holding capacity of braised chicken. Food Ferment. Ind. 2019, 45, 105–110. [Google Scholar]
- Lv, B.Y.; Yang, X.; Xue, H.L.; Nan, M.; Zhang, Y.; Liu, Z.; Bi, Y.; Shang, S. Isolation of main pathogens causing postharvest disease in fresh codonopsis pilosula during different storage stages and ozone control against disease and mycotoxin accumulation. J. Fungi 2023, 9, 146. [Google Scholar] [CrossRef]
- Yang, L.; Xue, H.L.; Liu, Z.G.; Liu, Q.; Zhang, Q.; Nan, M. The effects of different ambient pH on the pathogenicity of Fusarium sulphureum and reactive oxygen species metabolism in F. sulphureum inoculation muskmelon fruits. Physiol. Mol. Plant Pathol. 2022, 122, 101893. [Google Scholar] [CrossRef]
- Bao, G.; Bi, Y.; Li, Y.; Kou, Z.; Hu, L.; Ge, Y.; Wang, Y.; Wang, D. Overproduction of reactive oxygen species involved in the pathogenicity of Fusarium in potato tubers. Physiol. Mol. Plant Pathol. 2014, 86, 35–42. [Google Scholar] [CrossRef]
- Ong, M.K.; Ali, A. Antifungal action of ozone against Colletotrichum gloeosporioides and control of papaya anthracnose. Postharvest Biol. Technol. 2015, 100, 113–119. [Google Scholar] [CrossRef]
- Vahisalu, T.; Puzõrjova, I.; Brosché, M.; Valk, E.; Lepiku, M.; Moldau, H.; Pechter, P.; Wang, Y.S.; Lindgren, O.; Salojärvi, J.; et al. Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1: Ozone-triggered stomatal responses. Plant J. 2010, 62, 442–453. [Google Scholar] [CrossRef]
- Piechowiak, T.; Antos, P.; Józefczyk, R.; Kosowski, P.; Skrobacz, K.; Balawejder, M. Impact of ozonation process on the microbiological contamination and antioxidant capacity of highbush blueberry (Vaccinum corymbosum L.) fruit during cold storage. Ozone Sci. Eng. 2019, 41, 376–385. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Zhang, Y. Effect of ozone treatment on active oxygen metabolism of postharvest apricot fruits. J. Chin. Inst. Food Sci. Technol. 2023, 23, 345–352. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Parvin, K.; Bhuiyan, T.F.; Anee, T.I.; Nahar, K.; Hossen, M.S.; Zulfiqar, F.; Alam, M.M.; Fujita, M. Regulation of ROS metabolism in plants under environmental stress: A Review of Recent Experimental Evidence. Int. J. Mol. Sci. 2020, 21, 8695. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.L.; Bi, Y.; Raza, H.; Wang, H.J.; Pu, L.M.; Nan, M.M.; Cheng, X.Y.; Wang, Y.; Li, Y.C. Detection of NEO in muskmelon fruits inoculated with Fusarium sulphureum and its control by postharvest ozone treatment. Food Chem. 2018, 254, 193–200. [Google Scholar]
- Li, C.; Tao, J.; Wu, Z. Gaseous ozone regulates reactive oxygen species metabolism and ascorbate–glutathione cycle to delay the senescence of fresh-cut red pitaya (Selenicereus undatus) fruit. Sci. Hortic. 2023, 312, 111839. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, H.; Zhang, N.; Dong, C.; Ji, H.; Yu, J.; Ban, Z.; Yan, R.; Zhang, T.; Chen, C.; et al. Effects of ozone treatment on gene profiling involved in ASA-GSH cycle in postharvest cantaloupe. Sci. Hortic. 2023, 312, 111843. [Google Scholar] [CrossRef]
- Kim, Y.B.; Thwe, A.A.; Li, X.; Tuan, P.A.; Zhao, S.; Park, C.G.; Lee, J.W.; Park, S.U. Accumulation of flavonoids and related gene expressions in different organs of Astragalus membranaceus Bge. Appl. Biochem. Biotech. 2014, 173, 2076–2085. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, J.; Liu, Q.; Zhang, Q.; Liu, Z.; Xue, H.; Feng, Y. Ozone Treatment Inhibited the Blue Mold Development and Maintained the Main Active Ingredient Content in Radix astragali Infected by Penicillium polonicum Through Activating Reactive Oxygen Species Metabolism. J. Fungi 2025, 11, 402. https://doi.org/10.3390/jof11060402
Xi J, Liu Q, Zhang Q, Liu Z, Xue H, Feng Y. Ozone Treatment Inhibited the Blue Mold Development and Maintained the Main Active Ingredient Content in Radix astragali Infected by Penicillium polonicum Through Activating Reactive Oxygen Species Metabolism. Journal of Fungi. 2025; 11(6):402. https://doi.org/10.3390/jof11060402
Chicago/Turabian StyleXi, Jihui, Qili Liu, Qingru Zhang, Zhiguang Liu, Huali Xue, and Yuqin Feng. 2025. "Ozone Treatment Inhibited the Blue Mold Development and Maintained the Main Active Ingredient Content in Radix astragali Infected by Penicillium polonicum Through Activating Reactive Oxygen Species Metabolism" Journal of Fungi 11, no. 6: 402. https://doi.org/10.3390/jof11060402
APA StyleXi, J., Liu, Q., Zhang, Q., Liu, Z., Xue, H., & Feng, Y. (2025). Ozone Treatment Inhibited the Blue Mold Development and Maintained the Main Active Ingredient Content in Radix astragali Infected by Penicillium polonicum Through Activating Reactive Oxygen Species Metabolism. Journal of Fungi, 11(6), 402. https://doi.org/10.3390/jof11060402





