A Comprehensive Review on Chemical Structures and Bioactivities of Ostropomycetidae Lichens
Abstract
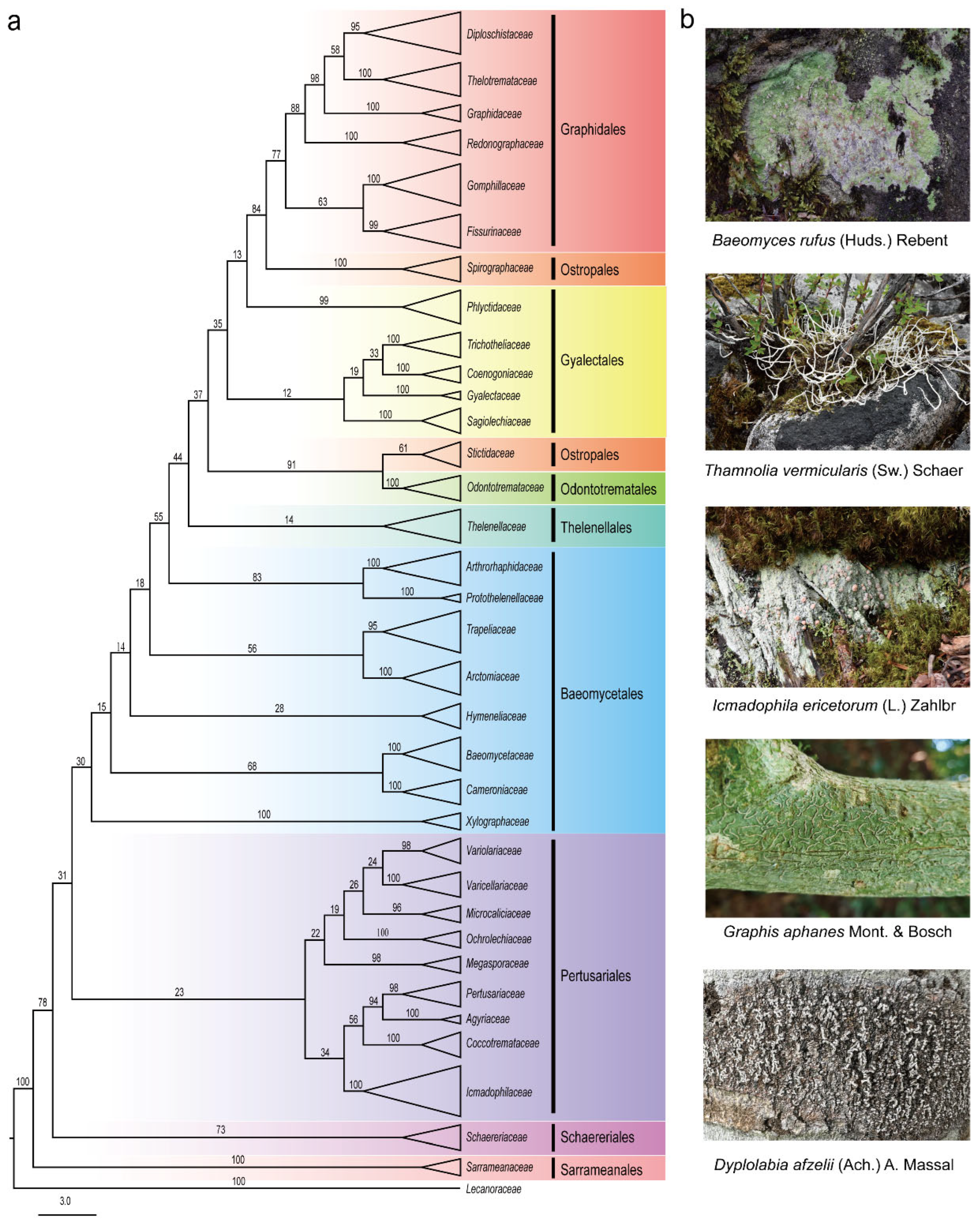
1. Introduction
2. Phenolic Compounds and Bioactivities
2.1. Phenols
2.1.1. Monophenol Derivatives
2.1.2. Polyhydroxylated Derivatives
2.2. Depsides and Their Derivatives
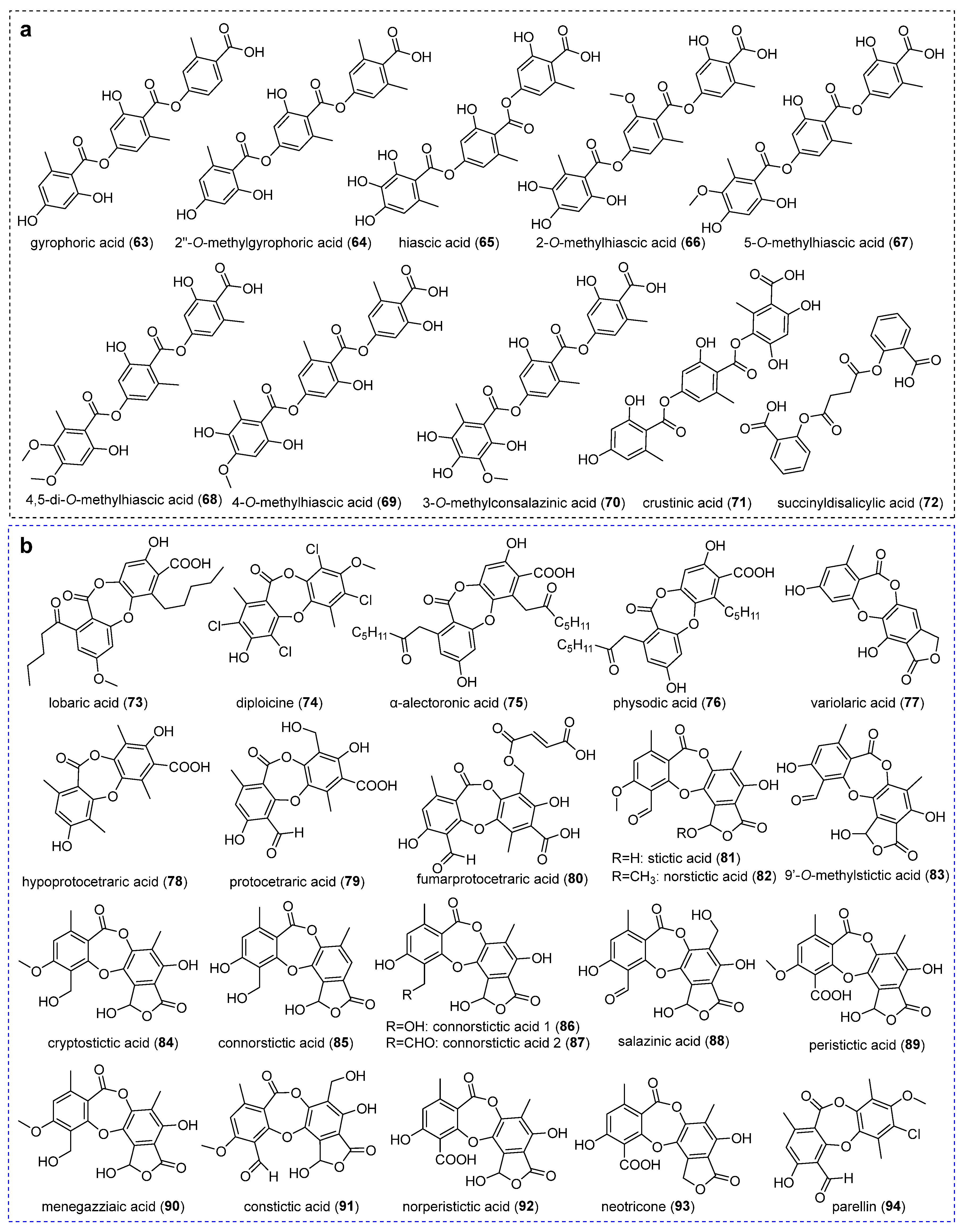
2.2.1. Orcinol-Type Depsides
2.2.2. β-Orcinol-Type Depsides
2.2.3. Hybrid-Type Depsides
2.2.4. Benzyldepsides
2.3. Tridepsides
2.4. Depsidones and Bioactivities
2.4.1. Orcinol-Type Depsidones
2.4.2. β-Orcinol-Type Depsidones
3. Aliphatic Acids and Bioactivities
4. Polyketides and Bioactivities
4.1. Lactone-Type Compounds
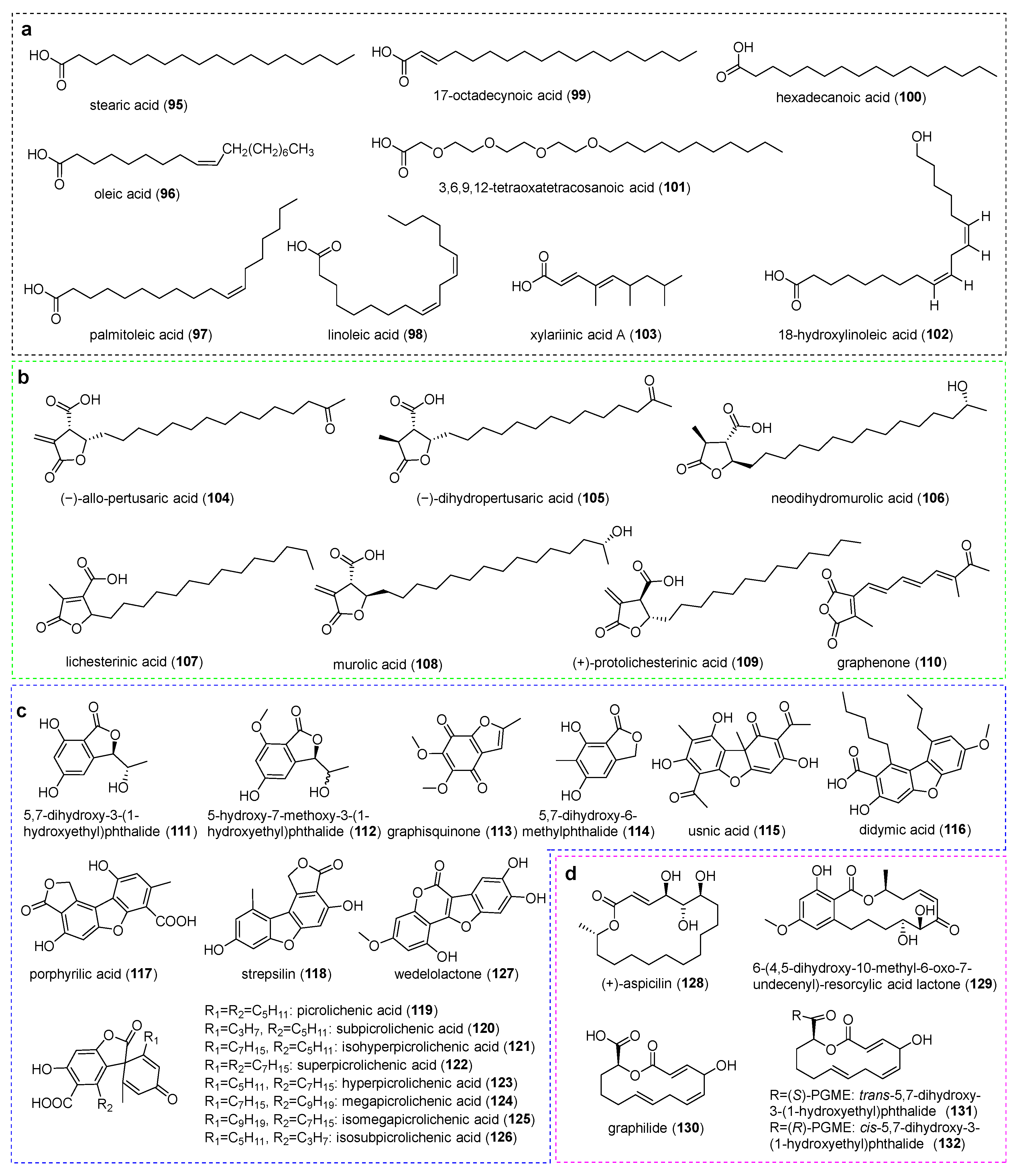
4.2. Phthalides and Dibenzofurans
4.3. Macrolides
4.4. Chromones
4.5. Xanthones
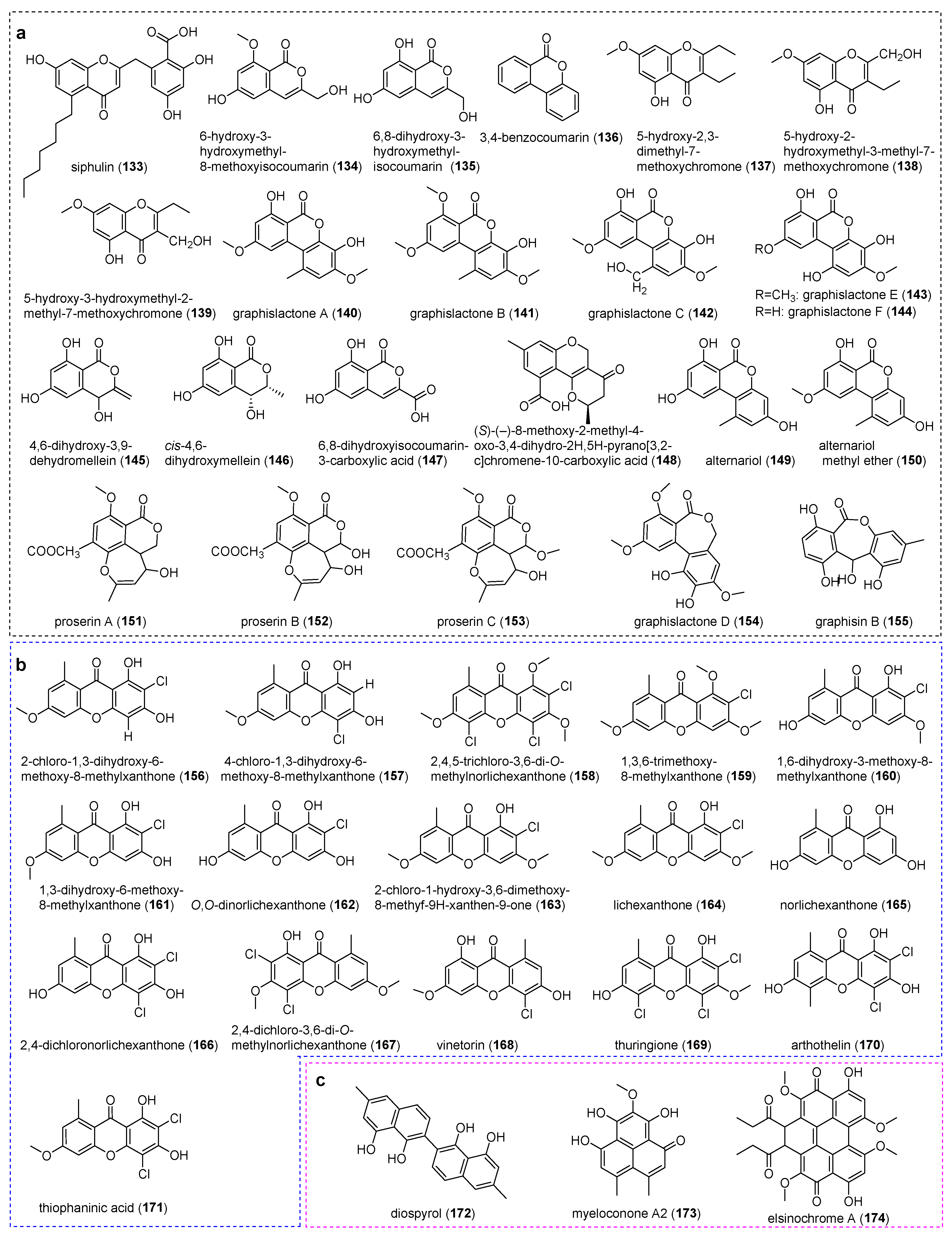
4.6. Other Polyketides
5. Terpenoids and Bioactivities
6. Steroids and Bioactivities

7. Non-Ribosomal Peptides and Bioactivities
8. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lücking, R.; Hodkinson, B.P.; Leavitt, S.D. Corrections and amendments to the 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota. Bryologist 2017, 120, 58–69. [Google Scholar] [CrossRef]
- Ranković, B. Lichen Secondary Metabolites: Bioactive Properties and Pharmaceutical Potential; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Pogam, P.L.; Herbette, G.; Boustie, J. Analysis of lichen metabolites, a variety of approaches. In Recent Advances in Lichenology: Modern Methods and Approaches in Biomonitoring and Bioprospection; Springer: New Delhi, India, 2015; Volume 1, p. 273. [Google Scholar]
- Nelsen, M.P.; Gargas, A. Phylogenetic distribution and evolution of secondary metabolites in the lichenized fungal genus Lepraria (Lecanorales: Stereocaulaceae). Nova Hedwig. 2008, 86, 115–131. [Google Scholar] [CrossRef]
- Ahmad, N.; Ritz, M.; Calchera, A.; Otte, J.; Schmitt, I.; Brueck, T.; Mehlmer, N. Biosynthetic potential of Hypogymnia holobionts: Insights into secondary metabolitepathways. J. Fungi. 2023, 9, 546. [Google Scholar] [CrossRef] [PubMed]
- Calcott, M.J.; Ackerley, D.F.; Knight, A.; Keyzers, R.A.; Owen, J.G. Secondary metabolism in the lichen symbiosis. Chem. Soc. Rev. 2018, 47, 1730–1760. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, L.; Liu, T. Concurrent production of carotenoids and lipid by a filamentous microalga Trentepohlia Arborum. Bioresour. Technol. 2016, 214, 567–573. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.D.; Wu, Y.C.; Cheng, J.; Jia, Z.F. Research progress of Nostoc commune in the age of omics. J. Liaocheng Univ. (Nat. Sci. Ed.) 2025, 38, 286–298. [Google Scholar] [CrossRef]
- Weiss, M.B.; Borges, R.M.; Sullivan, P.; Domingues, J.P.B.; da Silva, F.H.S.; Trindade, V.G.S.; Luo, S.; Orjala, J.; Crnkovic, C.M. Chemical diversity of cyanobacterial natural products. Nat. Prod. Rep. 2024, 42, 6–49. [Google Scholar] [CrossRef]
- Dar, T.U.H.; Dar, S.A.; Islam, S.U.; Mangral, Z.A.; Dar, R.; Singh, B.P.; Verma, P.; Haque, S. Lichens as a repository of bioactive compounds: An open window for green therapy against diverse cancers. Semin. Cancer. Biol. 2022, 86, 1120–1137. [Google Scholar] [CrossRef]
- Adenubi, O.T.; Famuyide, I.M.; McGaw, L.J.; Eloff, J.N. Lichens: An update on their ethnopharmacological uses and potential as sources of drug leads. J. Ethnopharmacol. 2022, 298, 115657. [Google Scholar] [CrossRef]
- Reeb, V.; Lutzoni, F.; Roux, C. Contribution of RPB2 to multilocus phylogenetic studies of the euascomycetes (Pezizomycotina, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol. Phylogenet Evol. 2004, 32, 1036–1060. [Google Scholar] [CrossRef]
- Jaklitsch, W.; Baral, H.O.; Lücking, R.; Lumbsch, H.T.; Frey, W. Syllabus of Plant Families—A. Engler’s Syllabus der Pflanzenfamilien Part 1/2: Ascomycota; Schweizerbart Science Publishers: Stuttgart, Germany, 2016; Volume 13. [Google Scholar]
- Wijayawardene, N.N.; Hyde, K.D.; Dai, D.Q.; Sánchez-García, M.; Goto, B.T.; Saxena, R.K.; Erdoğdu, M.; Selçuk, F.; Rajeshkumar, K.C.; Aptroot, A.; et al. Outline of Fungi and fungus-like taxa—2021. Mycosphere 2022, 13, 53–453. [Google Scholar] [CrossRef]
- Manojlovic, N.T.; Vasiljevic, P.J.; Nikolic, D.; Bogdanovic-Dusanovic, G.; Markovic, Z.S.; Najman, S. The isolation, analytical characterization by HPLC–UV and NMR spectroscopy, cytotoxic and antioxidant activities of baeomycesic acid from Thamnolia vermicularis var. subuliformis. Hem. Ind. 2011, 65, 591–598. [Google Scholar] [CrossRef]
- Christensen, S.N.; Alstrup, V. Chemical and morphological variation in Baeomyces rufus, including B. speciosus, (Baeomycetaceae, Lecanorales, Ascomycotina) with special reference to Denmark. Nova. Hedwig. 1990, 51, 469–474. [Google Scholar]
- Do, T.H.; Duong, T.H.; Ho, M.T.; Pham, D.D.; Nguyen, T.H.; Aonbangkhen, C.; Sichaem, J.; Nguyen, C.H.; Vo, T.P.; Nguyen, N.H.; et al. A new diphenyl ether from the cultured lichen mycobiont of Graphis cf. handelii. Nat. Prod. Res. 2024, 1–7, in press. [Google Scholar] [CrossRef]
- Guo, J.; Li, Z.L.; Wang, A.L.; Liu, X.Q.; Wang, J.; Guo, X.; Jing, Y.K.; Hua, H.M. Three new phenolic compounds from the lichen Thamnolia vermicularis and their antiproliferative effects in prostate cancer cells. Planta Med. 2011, 77, 2042–2046. [Google Scholar] [CrossRef]
- Dukic, V.; Usman, M.; Khalid, A.N.; Manojlovic, A.; Zaric, M.; Canovic, P.; Zivkovic-Zaric, R.; Manojlovic, N. Phytochemical composition and antitumor activity of a new arctic lichen Anamylopsora pakistanica. Nat. Prod. Res. 2024, in press. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Guo, Q.; Ding, H. Identification and antibacterial activity of Thamnolia vermicularis and Thamnolia subuliformis. J. Microbiol. Methods 2022, 203, 106628. [Google Scholar] [CrossRef]
- Shaheen, S.; Iqbal, Z.; Hussain, M. First report of dye yielding potential and compounds of lichens; a cultural heritage of Himalayan communities, Pakistan. Pak. J. Bot. 2019, 51, 341–360. [Google Scholar] [CrossRef]
- Osyczka, P.; Latkowska, E.; Rola, K. Metabolic processes involved with sugar alcohol and secondary metabolite production in the hyperaccumulator lichen Diploschistes muscorum reveal its complex adaptation strategy against heavy-metal stress. Fungal. Biol. 2021, 125, 999–1008. [Google Scholar] [CrossRef]
- Sedrpoushan, A.; Haghi, H.; Sohrabi, M. A new secondary metabolite profiling of the lichen Diploschistes diacapsis using liquid chromatography electrospray ionization tandem mass spectrometry. Inorg. Chem. Commun. 2022, 145, 110006. [Google Scholar] [CrossRef]
- Torres-Benitez, A.; Ortega-Valencia, J.E.; Jara-Pinuer, N.; Sanchez, M.; Vargas-Arana, G.; Gomez-Serranillos, M.P.; Simirgiotis, M.J. Antioxidant and antidiabetic activity and phytoconstituents of lichen extracts with temperate and polar distribution. Front. Pharmacol. 2023, 14, 1251856. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cai, Y.; Zhao, X.; Wu, C.; Liu, J.; Niu, T.; Shan, X.; Lu, Y.; Ruan, Y.; He, J. Investigation of the chemical structure of anti-amyloidogenic constituents extracted from Thamnolia vermicularis. J. Ethnopharmacol. 2022, 289, 115059. [Google Scholar] [CrossRef] [PubMed]
- Le, T.K.; Duong, T.H.; Nguyen, H.T.; Pham, N.K.; Vo, T.P.; Nguyen, N.H.; Niamnont, N.; Sichaem, J.; Tran, T.M. Antimicrobial sesquiterpenes from the cultured mycobiont Diorygma pruinosum against methicillin-resistant Staphylococcus aureus isolated from Vietnamese street foods. RSC Adv. 2024, 14, 4871–4879. [Google Scholar] [CrossRef] [PubMed]
- Goo, M.H.; Kim, J.H.; Park, H.; Lee, J.H.; Youn, U.J. Two new phenolic compounds from the Antarctic lichen Pertusaria dactylina. Chem. Nat. Compd. 2020, 56, 27–29. [Google Scholar] [CrossRef]
- Koo, M.H.; Kim, M.J.; Seo, J.E.; Kim, J.H.; Han, S.J.; Kim, I.C.; Lee, J.H.; Youn, U.J. A new chlorinated phenolic compound from the Antarctic lichen, Pertusaria dactylina. Nat. Prod. Commun. 2020, 15, 1–4. [Google Scholar] [CrossRef]
- Pittayakhajonwut, P.; Sri-Indrasutdhi, V.; Dramae, A.; Lapanun, S.; Suvannakad, R.; Tantichareon, M. Graphisins A and B from the lichen Graphis tetralocularis. Aust. J. Chem. 2009, 62, 389–391. [Google Scholar] [CrossRef]
- Takenaka, Y.; Taguchi, S.; Le, D.H.; Hamada, N.; Tanahashi, T. 3,4-dihydro-2,5-pyrano[3,2-]chromene and benzophenone derivatives from cultured lichen mycobionts of Vietnamese Graphis sp. Heterocycles 2013, 87, 2651–2657. [Google Scholar] [CrossRef]
- Upreti, D.K.; Divakar, P.K.; Shukla, V.; Bajpai, R. Recent Advances in Lichenology: Modern Methods and Approaches in Biomonitoring and Bioprospection; Springer: New Delhi, India, 2015; Volume 1. [Google Scholar]
- Urena-Vacas, I.; Gonzalez-Burgos, E.; Divakar, P.K.; Gomez-Serranillos, M.P. Lichen depsides and tridepsides: Progress in pharmacological approaches. J. Fungi. 2023, 9, 116. [Google Scholar] [CrossRef]
- Kjaer, A.; Kjaer, D. Synthesis of siphulin, a naturally-occurring homoflavone. Acta. Chem. Scand. B 1985, 39, 65–68. [Google Scholar] [CrossRef]
- Bonny, S.; Paquin, L.; Carrie, D.; Boustie, J.; Tomasi, S. Ionic liquids based microwave-assisted extraction of lichen compounds with quantitative spectrophoto densitometry analysis. Anal. Chim. Acta 2011, 707, 69–75. [Google Scholar] [CrossRef]
- Millot, M.; Tomasi, S.; Articus, K.; Rouaud, I.; Bernard, A.; Boustie, J. Metabolites from the lichen Ochrolechia parella growing under two different heliotropic conditions. J. Nat. Prod. 2007, 70, 316–318. [Google Scholar] [CrossRef] [PubMed]
- Rankovic, B.; Misic, M. The antimicrobial activity of the lichen substances of the lichens Cladonia furcata, Ochrolechia androgyna, Parmelia caperata and Parmelia conspresa. Biotechnol. Biotec. Eq. 2008, 22, 1013–1016. [Google Scholar] [CrossRef]
- Spribille, T.; Pérez Ortega, S.; Tonsberg, T.; Schirokauer, D. Lichens and lichenicolous fungi of the Klondike Gold Rush National Historic Park, Alaska, in a global biodiversity context. Bryologist 2010, 113, 439–515. [Google Scholar] [CrossRef]
- Yeash, E.A.; Letwin, L.; Malek, L.; Suntres, Z.; Knudsen, K.; Christopher, L.P. Biological activities of undescribed North American lichen species. J. Sci. Food. Agric. 2017, 97, 4721–4726. [Google Scholar] [CrossRef]
- Brodo, I.M. Studies in the lichen genus Ochrolechia. 2. Corticolous species of North America. Can. J. Bot. 1991, 69, 733–772. [Google Scholar] [CrossRef]
- Koparal, A.T.; Ulus, G.; Zeytinoglu, M.; Tay, T.; Turk, A.O. Angiogenesis inhibition by a lichen compound olivetoric acid. Phytother. Res. 2010, 24, 754–758. [Google Scholar] [CrossRef]
- Emsen, B.; Aslan, A.; Togar, B.; Turkez, H. In vitro antitumor activities of the lichen compounds olivetoric, physodic and psoromic acid in rat neuron and glioblastoma cells. Pharm. Biol. 2016, 54, 1748–1762. [Google Scholar] [CrossRef]
- Archer, A.W. A chemical and morphological arrangement of the lichen genus Pertusaria—Additional data and corrections. Mycotaxon 1995, 55, 385–389. [Google Scholar]
- Fernández-Pastor, I.; González-Menéndez, V.; Martínez Andrade, K.; Serrano, R.; Mackenzie, T.A.; Benítez, G.; Casares-Porcel, M.; Genilloud, O.; Reyes, F. Xerophytic lichens from Gypsiferous outcrops of arid areas of Andalusia as a source of anti-phytopathogenic depsides. J. Fungi 2023, 9, 887. [Google Scholar] [CrossRef]
- Halici, M.G.; Kocakaya, M.; Sweeney, K.; Fankhauser, J.D.; Schmitt, I. Pertusaria paramerae (Pertusariales, Ascomycota), a species with variable secondary chemistry, and a new lichen record for Turkey. Nova Hedwig. 2010, 91, 223–230. [Google Scholar] [CrossRef]
- Bendz, G.; Santesson, J.; Wachtmei, C. Studies on chemistry of lichens. 22. the chemistry of genus Siphula. I. Acta. Chem. Scand. 1965, 19, 1250–1252. [Google Scholar] [CrossRef]
- Omarsdottir, S.; Freysdottir, J.; Olafsdottir, E.S. Immunomodulating polysaccharides from the lichen Thamnolia vermicularis var. subuliformis. Phytomedicine 2007, 14, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W.J.; Wang, Q.Q.; Ma, L.; Hu, L.H. β-orcinol-type depsides from the lichen Thamnolia vermicularis. Nat. Prod. Res. 2013, 27, 804–808. [Google Scholar] [CrossRef]
- Santesson, J. Chemical studies on lichens.6. chemistry of genus Siphula 2. Acta Chem. Scand. 1967, 21, 1833–1837. [Google Scholar] [CrossRef]
- Ingolfsdottir, K.; Wiedemann, B.; Birgisdottir, M.; Nenninger, A.; Jonsdottir, S.; Wagner, H. Inhibitory effects of baeomycesic acid from the lichen Thamnolia subuliformis on 5-lipoxygenase in vitro. Phytomedicine 1997, 4, 125–128. [Google Scholar] [CrossRef]
- Bucar, F.; Schneider, I.; Ogmundsdottir, H.; Ingolfsdottir, K. Anti-proliferative lichen compounds with inhibitory activity on 12(S)-HETE production in human platelets. Phytomedicine 2004, 11, 602–606. [Google Scholar] [CrossRef]
- Liu, J.; Wu, R.; Yuan, S.; Kelleher, R.; Chen, S.; Chen, R.; Zhang, T.; Obaidi, I.; Sheridan, H. Pharmacogenomic analysis of combined therapies against Glioblastoma based on cell markers from single-cell sequencing. Pharmaceuticals 2023, 16, 1533. [Google Scholar] [CrossRef]
- Bjelland, T.; Grube, M.; Hoem, S.; Jorgensen, S.L.; Daae, F.L.; Thorseth, I.H.; Ovreas, L. Microbial metacommunities in the lichen-rock habitat. Environ. Microbiol. Rep. 2011, 3, 434–442. [Google Scholar] [CrossRef]
- Barbosa-Silva, A.M.; Silva, A.C.; Pereira, E.C.G.; Buail, M.L.L.; Silva, N.H.; Cáceaes, M.E.S.; Aptroot, A.; Bezerra-Gusmao, M.A. Richness of lichens consumed by in the semi-arid region of Brazil. Sociobiology 2019, 66, 154–160. [Google Scholar] [CrossRef]
- Elix, J.A.; Barclay, C.E.; Wardlaw, J.H.; Archer, A.W.; Yu, S.H.; Kantvilas, G. Four new β-orcinol meta-depsides from Pertusaria and Siphula lichens. Aust. J. Chem. 1999, 52, 837–840. [Google Scholar] [CrossRef]
- Yu, H.Y.; Shen, X.Q.; Liu, D.; Hong, M.H.; Lu, Y.H. The protective effects of β-sitosterol and vermicularin from Thamnolia vermicularis (Sw.) Ach. against skin aging in vitro. An. Acad. Bras. Cienc. 2019, 91, e20181088. [Google Scholar] [CrossRef]
- Harper, S.H.; Letcher, R.M. Chemistry of lichen constituents. Part III. Haemathamnolic acid: A new β-orcinol depside from Pertusaria rhodesiaca Vainio. J. Chem. Soc. C-Org. 1967, 17, 1603–1608. [Google Scholar] [CrossRef]
- Lawrey, J.D. Correlations between lichen secondary chemistry and grazing activity by Pallifera varia. Bryologist 1980, 83, 328–334. [Google Scholar] [CrossRef]
- Harikrishnan, A.; Veena, V.; Lakshmi, B.; Shanmugavalli, R.; Theres, S.; Prashantha, C.N.; Shah, T.; Oshin, K.; Togam, R.; Nandi, S. Atranorin, an antimicrobial metabolite from lichen Parmotrema rampoddense exhibited in vitro anti-breast cancer activity through interaction with Akt activity. J. Biomol. Struct. Dyn. 2021, 39, 1248–1258. [Google Scholar] [CrossRef]
- Mendili, M.; Khadhri, A.; Mediouni-Ben Jemaa, J.; Andolfi, A.; Tufano, I.; Aschi-Smiti, S.; DellaGreca, M. Anti-inflammatory potential of compounds isolated from Tunisian lichens species. Chem. Biodivers. 2022, 19, e202200134. [Google Scholar] [CrossRef]
- Bruun, T. Siphulin a chromenone lichen acid. Acta Chem. Scand. 1965, 19, 1677–1693. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S. Phytochemical investigation of the Australian lichens Ramalina glaucescens and Xanthoria parietina. Nat. Prod. Commun. 2009, 4, 959–964. [Google Scholar] [CrossRef]
- Thadhani, V.M.; Karunaratne, V. Potential of lichen compounds as antidiabetic agents with antioxidative properties: A review. Oxid. Med. Cell. Longev. 2017, 2017, 2079697. [Google Scholar] [CrossRef]
- Sisodia, R.; Geol, M.; Verma, S.; Rani, A.; Dureja, P. Antibacterial and antioxidant activity of lichen species Ramalina roesleri. Nat. Prod. Res. 2013, 27, 2235–2239. [Google Scholar] [CrossRef]
- Lai, D.; Odimegwu, D.C.; Esimone, C.; Grunwald, T.; Proksch, P. Phenolic compounds with in vitro activity against respiratory syncytial virus from the Nigerian lichen Ramalina farinacea. Planta Med. 2013, 79, 1440–1446. [Google Scholar] [CrossRef]
- Thuy Le, H.; Vu, Y.T.; Duong, G.H.; Le, T.K.; Dang, M.K.; Pham, D.D.; Pham, N.K.; Sichaem, J.; Nguyen, N.H.; Duong, T.H. Bio-guided isolation of alpha-glucosidase inhibitory compounds from Vietnamese lichen Roccella montagnei. Chem. Biodivers. 2024, 21, e202400438. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.M.; Aonbangkhen, C.; Duong, T.H.; Nguyen, T.H.; Ho, M.T.; Chavasiri, W.; Wongsuwan, S.; Chatwichien, J.; Giao Vo, T.P.; Nguyen, N.H.; et al. Diphenyl ethers from the cultured lichen mycobiont of Graphis handelii Zahlbr. Heliyon 2024, 10, e25763. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, H.; Sohrabi, M.; Yousefi, M.; Boustie, J. Tridepsides as potential bioactives: A review on their chemistry and the global distribution of their lichenic and non-lichenic natural sources. Front. Fungal Biol. 2023, 4, 1088966. [Google Scholar] [CrossRef] [PubMed]
- Kosanic, M.; Rankovic, B.; Stanojkovic, T.; Vasiljevic, P.; Manojlovic, N. Biological activities and chemical composition of lichens from Serbia. Excli. J. 2014, 13, 1226–1238. [Google Scholar]
- Cardile, V.; Graziano, A.C.E.; Avola, R.; Piovano, M.; Russo, A. Potential anticancer activity of lichen secondary metabolite physodic acid. Chem. Biol. Interact. 2017, 263, 36–45. [Google Scholar] [CrossRef]
- Goga, M.; Kello, M.; Vilkova, M.; Petrova, K.; Backor, M.; Adlassnig, W.; Lang, I. Oxidative stress mediated by gyrophoric acid from the lichen Umbilicaria hirsuta affected apoptosis and stress/survival pathways in HeLa cells. BMC Complem. Altern. Med. 2019, 19, 221. [Google Scholar] [CrossRef]
- Mohammadi, M.; Zambare, V.; Suntres, Z.; Christopher, L. Isolation, characterization, and breast cancer cytotoxic activity of gyrophoric acid from the lichen Umbilicaria muhlenbergii. Processes 2022, 10, 1361. [Google Scholar] [CrossRef]
- Mohammadi, M.; Bagheri, L.; Badreldin, A.; Fatehi, P.; Pakzad, L.; Suntres, Z.; van Wijnen, A.J. Biological effects of gyrophoric acid and other lichen derived metabolites, on cell proliferation, apoptosis and cell signaling pathways. Chem. Bio. Interact. 2022, 351, 109768. [Google Scholar] [CrossRef]
- Seo, C.; Choi, Y.H.; Ahn, J.S.; Yim, J.H.; Lee, H.K.; Oh, H. PTP1B inhibitory effects of tridepside and related metabolites isolated from the Antarctic lichen Umbilicaria antarctica. J. Enzym. Inhib. Med. Ch. 2009, 24, 1133–1137. [Google Scholar] [CrossRef][Green Version]
- Shim, J.H. Anti-aging effects of gyrophoric acid on UVA-irradiated normal human dermal fibroblasts. Nat. Prod. Commun. 2020, 15, 1–8. [Google Scholar] [CrossRef]
- Gupta, A.; Sahu, N.; Singh, A.P.; Singh, V.K.; Singh, S.C.; Upadhye, V.J.; Mathew, A.T.; Kumar, R.; Sinha, R.P. Exploration of novel lichen compounds as inhibitors of SARS-CoV-2 Mpro: Ligand-based design, molecular dynamics, and ADMET analyses. Appl. Biochem. Biotechnol. 2022, 194, 6386–6406. [Google Scholar] [CrossRef]
- Elix, J.A.; Barbero, M.; Giralt, M.; Lumbsch, H.T.; Mccaffery, L.F. 2″-O-methylgyrophoric acid, a new lichen tridepside. Aust. J. Chem. 1995, 48, 1761–1765. [Google Scholar] [CrossRef]
- Das, A.K.; Sharma, A.; Kathuria, D.; Ansari, M.J.; Bhardwaj, G. Chemistry, Biology and Pharmacology of Lichen; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2024; p. 331. [Google Scholar]
- Khayat, M.T.; Ghazawi, K.F.; Samman, W.A.; Alhaddad, A.A.; Mohamed, G.A.; Ibrahim, S.R. Recent advances on natural depsidones: Sources, biosynthesis, structure-activity relationship, and bioactivities. PeerJ 2023, 11, e15394. [Google Scholar] [CrossRef] [PubMed]
- Bonny, S.; Hitti, E.; Boustie, J.; Bernard, A.; Tomasi, S. Optimization of a microwave-assisted extraction of secondary metabolites from crustose lichens with quantitative spectrophoto densitometry analysis. J. Chromatogr. A 2009, 1216, 7651–7656. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Kim, J.; Yim, J.H.; Lee, H.K.; Pyo, S. Anti-inflammatory activity of lobaric acid via suppressing NF-kappaB/MAPK pathways or NLRP3 inflammasome activation. Planta Med. 2019, 85, 302–311. [Google Scholar] [CrossRef]
- Williams, D.E.; Loganzo, F.; Whitney, L.; Togias, J.; Harrison, R.; Singh, M.P.; McDonald, L.A.; Kathirgamanathar, S.; Karunaratne, V.; Andersen, R.J. Depsides isolated from the Sri Lankan lichen Parmotrema sp. exhibit selective Plk1 inhibitory activity. Pharm. Biol. 2011, 49, 296–301. [Google Scholar] [CrossRef][Green Version]
- Bauer, J.; Waltenberger, B.; Noha, S.M.; Schuster, D.; Rollinger, J.M.; Boustie, J.; Chollet, M.; Stuppner, H.; Werz, O. Discovery of depsides and depsidones from lichen as potent inhibitors of microsomal prostaglandin E2 synthase-1 using pharmacophore models. Chem. Med. Chem. 2012, 7, 2077–2081. [Google Scholar] [CrossRef]
- Stojanovic, I.Z.; Najman, S.; Jovanovic, O.; Petrovic, G.; Najdanovic, J.; Vasiljevic, P.; Smelcerovic, A. Effects of depsidones from Hypogymnia physodes on HeLa cell viability and growth. Folia. Biol. 2014, 60, 89–94. [Google Scholar] [CrossRef]
- Sahin, E.; Psav, S.D.; Avan, I.; Candan, M.; Sahinturk, V.; Koparal, A.T. Lichen-derived physodic acid exerts cytotoxic and anti-invasive effects in human lung cancer. Rend. Lincei-Sci. Fis. 2021, 32, 511–520. [Google Scholar] [CrossRef]
- Cardile, V.; Graziano, A.C.E.; Avola, R.; Madrid, A.; Russo, A. Physodic acid sensitizes LNCaP prostate cancer cells to TRAIL-induced apoptosis. Toxicol. Vitr. 2022, 84, 105432. [Google Scholar] [CrossRef]
- Elix, J.A.; Kalb, K.; Wardlaw, J.H. Neotricone and norperistictic acid, two new depsidones from the lichen genus Phaeographis. Aust. J. Chem. 2003, 56, 315–317. [Google Scholar] [CrossRef]
- Nishanth, K.S.; Sreerag, R.S.; Deepa, I.; Mohandas, C.; Nambisan, B. Protocetraric acid: An excellent broad spectrum compound from the lichen Usnea albopunctata against medically important microbes. Nat. Prod. Res. 2015, 29, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Honda, N.K.; Pavan, F.R.; Coelho, R.G.; de Andrade Leite, S.R.; Micheletti, A.C.; Lopes, T.I.; Misutsu, M.Y.; Beatriz, A.; Brum, R.L.; Leite, C.Q. Antimycobacterial activity of lichen substances. Phytomedicine 2010, 17, 328–332. [Google Scholar] [CrossRef]
- Manojlovic, N.; Rankovic, B.; Kosanic, M.; Vasiljevic, P.; Stanojkovic, T. Chemical composition of three Parmelia lichens and antioxidant, antimicrobial and cytotoxic activities of some their major metabolites. Phytomedicine 2012, 19, 1166–1172. [Google Scholar] [CrossRef]
- Huynh, B.L.C.; Pham, N.K.T.; Nguyen, T.P. Paresordin A, a new diphenyl cyclic peroxide from the lichen Parmotrema praesorediosum. J. Asian. Nat. Prod. Res. 2022, 24, 190–195. [Google Scholar] [CrossRef]
- de Barros Alves, G.M.; de Sousa Maia, M.B.; de Souza Franco, E.; Galvao, A.M.; da Silva, T.G.; Gomes, R.M.; Martins, M.B.; da Silva Falcao, E.P.; de Castro, C.M.; da Silva, N.H. Expectorant and antioxidant activities of purified fumarprotocetraric acid from Cladonia verticillaris lichen in mice. Pulm. Pharmacol. Ther. 2014, 27, 139–143. [Google Scholar] [CrossRef]
- Yanez, O.; Osorio, M.I.; Osorio, E.; Tiznado, W.; Ruiz, L.; Garcia, C.; Nagles, O.; Simirgiotis, M.J.; Castaneta, G.; Areche, C.; et al. Antioxidant activity and enzymatic of lichen substances: A study based on cyclic voltammetry and theoretical. Chem. Biol. Interact. 2023, 372, 110357. [Google Scholar] [CrossRef]
- Miyagawa, H.; Hamada, N.; Sato, M.; Ueno, T. Pigments from the cultured lichen mycobionts of Graphis scripta and G. desquamescens. Phytochemistry 1994, 36, 1319–1322. [Google Scholar] [CrossRef]
- Zhang, J.R.; Liu, L.; Xue, X.D.; Ren, Q. Pertusaria wui sp. nov. on bamboo from Yunnan, China. Mycotaxon 2021, 136, 719–723. [Google Scholar] [CrossRef]
- Paukov, A.G.; Teptina, A.Y.; Pushkarev, E.V. Heavy metal uptake by chemically distinct lichens from Aspicilia spp. growing on ultramafic rocks. Aust. J. Bot. 2015, 63, 111–118. [Google Scholar] [CrossRef]
- Pejin, B.; Iodice, C.; Bogdanovic, G.; Kojic, V.; Tesevic, V. Stictic acid inhibits cell growth of human colon adenocarcinoma HT-29 cells. Arab. J. Chem. 2017, 10, S1240–S1242. [Google Scholar] [CrossRef]
- Do, T.H.; Duong, T.H.; Vu, Y.T.; Tran, H.P.; Nguyen, T.T.; Sichaem, J.; Nguyen, N.H.; Nguyen, H.T.; Pham, D.D. Alpha-glucosidase inhibitory compounds from Vietnamese lichen Usnea baileyi: In vitro and in silico aspects. RSC Adv. 2024, 14, 32624–32636. [Google Scholar] [CrossRef] [PubMed]
- Odonovan, D.G.; Roberts, G.; Keogh, M.F. Structure of the β-orcinol depsidones, connorstictic and consalazinic acids. Phytochemistry 1980, 19, 2497–2499. [Google Scholar] [CrossRef]
- Correche, E.R.; Enriz, R.D.; Piovano, M.; Garbarino, J.; Gomez-Lechon, M.J. Cytotoxic and apoptotic effects on hepatocytes of secondary metabolites obtained from lichens. Atla-Altern. Lab. Anim. 2004, 32, 605–615. [Google Scholar] [CrossRef]
- Kumar, T.K.; Siva, B.; Kiranmai, B.; Alli, V.J.; Jadav, S.S.; Reddy, A.M.; Boustie, J.; Le Devehat, F.; Tiwari, A.K.; Suresh Babu, K. Salazinic acid and norlobaridone from the lichen Hypotrachyna cirrhata: Antioxidant activity, alpha-glucosidase inhibitory and molecular docking studies. Molecules 2023, 28, 7840. [Google Scholar] [CrossRef]
- Gaikwad, S.B.; Mapari, S.V.; Sutar, R.R.; Syed, M.; Khare, R.; Behera, B.C. In vitro and in silico studies of lichen compounds atranorin and salazinic acid as potential antioxidant, antibacterial and anticancer agents. Chem. Biodivers. 2023, 20, e202301229. [Google Scholar] [CrossRef]
- Esmaeili, A.; Rajaee, S. The preparation of hyaluronic acid nanoparticles from Aspicilia lichens using bifido bacteria for help in the treatment of diabetes in rats in vivo. Phytother. Res. 2017, 31, 1590–1599. [Google Scholar] [CrossRef]
- Shanmugam, K.; Srinivasan, M.; Neelakantan, H.G. Insights into in vitro phenotypic plasticity, growth and secondary metabolites of the mycobiont isolated from the lichen Platygramme caesiopruinosa. Arch. Microbiol. 2021, 204, 90. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, M.L.; Lin, L.; Ledesma-Amaro, R.; Wang, K.; Ji, X.J.; Huang, H. Dynamic regulation combined with systematic metabolic engineering for high-level palmitoleic acid accumulation in oleaginous yeast. Metab. Eng. 2025, 89, 33–46. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; He, H.M. The role of linoleic acid in skin and hair health: A review. Int. J. Mol. Sci. 2024, 26, 246. [Google Scholar] [CrossRef]
- Cartron, M.L.; England, S.R.; Chiriac, A.I.; Josten, M.; Turner, R.; Rauter, Y.; Hurd, A.; Sahl, H.G.; Jones, S.; Foster, S.J. Bactericidal activity of the human skin fatty acid cis-6-hexadecanoic acid on Staphylococcus aureus. Antimicrob. Agents Chemother. 2014, 58, 3599–3609. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.W.; Lee, I.K.; Kim, Y.S.; Lee, S.; Lee, H.J.; Yu, S.H.; Yun, B.S. Xylarinic acids A and B, new antifungal polypropionates from the fruiting body of Xylaria polymorpha. J. Antibiot. 2007, 60, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Huneck, S.; Tonsberg, T.; Bohlmann, F. Lichen substances. 144. (−)-allo-pertusaric acid and (−)-dihydropertusaric acid from the lichen Pertusaria albescens. Phytochemistry 1986, 25, 453–459. [Google Scholar] [CrossRef]
- Igoli, J.O.; Gray, A.I.; Clements, C.J.; Kantheti, P.; Singla, R.K. Antitrypanosomal activity & docking studies of isolated constituents from the lichen Cetraria islandica: Possibly multifunctional scaffolds. Curr. Top. Med. Chem. 2014, 14, 1014–1021. [Google Scholar] [CrossRef]
- Brisdelli, F.; Perilli, M.; Sellitri, D.; Piovano, M.; Garbarino, J.A.; Nicoletti, M.; Bozzi, A.; Amicosante, G.; Celenza, G. Cytotoxic activity and antioxidant capacity of purified lichen metabolites: An in vitro study. Phytother. Res. 2013, 27, 431–437. [Google Scholar] [CrossRef]
- Bessadottir, M.; Skuladottir, E.A.; Gowan, S.; Eccles, S.; Ogmundsdottir, S.; Ogmundsdottir, H.M. Effects of anti-proliferative lichen metabolite, protolichesterinic acid on fatty acid synthase, cell signalling and drug response in breast cancer cells. Phytomedicine 2014, 21, 1717–1724. [Google Scholar] [CrossRef]
- León, A.; Del-Ángel, M.; Ávila, J.L.; Delgado, G. Phthalides: Distribution in nature, chemical reactivity, synthesis, and biological activity. Prog. Chem. Org. Nat. Prod. 2017, 104, 252. [Google Scholar] [CrossRef]
- Wei, X.D.; Zeng, Y.P.; Sun, C.; Meng, F.C.; Wang, Y.B. Recent advances in natural phthalides: Distribution, chemistry, and biological activities. Fitoterapia 2022, 160, 105223. [Google Scholar] [CrossRef]
- Takenaka, Y.; Morimoto, N.; Hamada, N.; Tanahashi, T. Phenolic compounds from the cultured mycobionts of Graphis proserpens. Phytochemistry 2011, 72, 1431–1435. [Google Scholar] [CrossRef]
- Liang, X.; Chen, W.; Jiang, B.; Xiao, C.J. Dibenzofurans from nature: Biosynthesis, structural diversity, sources, and bioactivities. Bioorg. Chem. 2024, 144, 107107. [Google Scholar] [CrossRef]
- Millot, M.; Dieu, A.; Tomasi, S. Dibenzofurans and derivatives from lichens and ascomycetes. Nat. Prod. Rep. 2016, 33, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xuan, M.; Huang, C.; Wang, C. Advances in research on bioactivity, toxicity, metabolism, and pharmacokinetics of usnic acid in vitro and in vivo. Molecules 2022, 27, 7469. [Google Scholar] [CrossRef]
- Luo, H.; Ren, M.; Lim, K.M.; Koh, Y.J.; Wang, L.S.; Hur, J.S. Antioxidative activity of lichen Thamnolia vermicularis in vitro. Mycobiology 2006, 34, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Araujo, A.A.; de Melo, M.G.; Rabelo, T.K.; Nunes, P.S.; Santos, S.L.; Serafini, M.R.; Santos, M.R.; Quintans-Junior, L.J.; Gelain, D.P. Review of the biological properties and toxicity of usnic acid. Nat. Prod. Res. 2015, 29, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Dieu, A.; Millot, M.; Champavier, Y.; Mambu, L.; Chaleix, V.; Sol, V.; Gloaguen, V. Uncommon chlorinated xanthone and other antibacterial compounds from the lichen Cladonia incrassata. Planta Med. 2014, 80, 931–935. [Google Scholar] [CrossRef]
- Tatipamula, V.K.; Tatipamula, V.B. Dibenzofurans from Cladonia corniculata Ahti and Kashiw inhibit key enzymes involved in inflammation and gout: An in vitro approach. Indian. J. Chem. B 2021, 60, 715–719. [Google Scholar] [CrossRef]
- Elix, J.A.; Calanasan, C.A.; Archer, A.W. Subpicrolichenic acid and superpicrolichenic acid, 2 new depsones from Pertusaria lichens. Aust. J. Chem. 1991, 44, 1487–1493. [Google Scholar] [CrossRef]
- Elix, J.A.; Venables, D.A.; Archer, A.W. Further new depsones from the lichen Pertusaria truncata. Aust. J. Chem. 1994, 47, 1345–1353. [Google Scholar] [CrossRef]
- Cai, H.; Wu, Y.; Zhang, X. A comprehensive review on wedelolactone: Natural sources, total synthesis, and pharmacological activities. Chin. J. Nat. Med. 2025, 23, 169–181. [Google Scholar] [CrossRef]
- Yin, Y.; Mu, F.; Zhang, L.; Zhao, J.; Gong, R.; Yin, Y.; Zheng, L.; Du, Y.; Jin, F.; Wang, J. Wedelolactone activates the PI3K/AKT/NRF2 and SLC7A11/GPX4 signalling pathways to alleviate oxidative stress and ferroptosis and improve sepsis-induced liver injury. J. Ethnopharmacol. 2025, 344, 119557. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kinoshita, Y.; Kinoshita, K.; Koyama, K.; Takahashi, K. A zearalenone derivative from the liquid culture of the lichen, Baeomyces placophyllus. J. Hattori. Bot. Lab. 2002, 285–289. [Google Scholar] [CrossRef]
- Le, D.H.; Takenaka, Y.; Hamada, N.; Miyawaki, H.; Tanahashi, T. A 14-membered macrolide and isocoumarin derivatives from the cultured lichen mycobionts of Graphis vestitoides. Chem. Pharm. Bull. 2013, 61, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, U.; Verma, S.; Tonk, R.K. Chromenone: An emerging scaffold in anti-Alzheimer drug discovery. Bioorg. Med. Chem. Lett. 2024, 111, 129912. [Google Scholar] [CrossRef] [PubMed]
- Kornsakulkarn, J.; Thongpanchang, C.; Lapanun, S.; Srichomthong, K. Isocoumarin glucosides from the scale insect fungus Torrubiella tenuis BCC 12732. J. Nat. Prod. 2009, 72, 1341–1343. [Google Scholar] [CrossRef]
- Archer, A.W.; Elix, J.A. The lichen genus Pertusaria (lichenised Ascomycotina) in Papua New Guinea: Three new species and two new reports. Mycotaxon 1998, 69, 311–318. [Google Scholar]
- Takenaka, Y.; Tanahashi, T.; Nagakura, N.; Hamada, N. 2,3-Dialkylchromones from mycobiont cultures of the lichen Graphis scripta. Heterocycles 2000, 53, 1589–1593. [Google Scholar] [CrossRef]
- Tanahashi, T.; Takenaka, Y.; Nagakura, N.; Hamada, N. 6H-Dibenzo[b,d]pyran-6-one derivatives from the cultured lichen mycobionts of Graphis spp. and their biosynthetic origin. Phytochemistry 2003, 62, 71–75. [Google Scholar] [CrossRef]
- Hormazabal, E.; Schmeda-Hirschmann, G.; Astudillo, L.; Rodriguez, J.; Theoduloz, C. Metabolites from Microsphaeropsis olivacea, an endophytic fungus of Pilgerodendron uviferum. Z. Naturforsch. C J. Biosci. 2005, 60, 11–21. [Google Scholar] [CrossRef]
- Lee, Y.; Jang, H.R.; Lee, D.; Lee, J.; Jung, H.R.; Cho, S.Y.; Lee, H.Y. Graphislactone A, a fungal antioxidant metabolite, reduces lipogenesis and protects against diet-induced hepatic steatosis in mice. Int. J. Mol. Sci. 2024, 25, 1096. [Google Scholar] [CrossRef]
- Avantaggiato, G.; Solfrizzo, M.; Tosi, L.; Zazzerini, A.; Fanizzi, F.P.; Visconti, A. Isolation and characterization of phytotoxic compounds produced by Phomopsis helianthi. Nat. Toxins 1999, 7, 119–127. [Google Scholar] [CrossRef]
- Bacha, S.A.S.; Li, Y.; Nie, J.; Xu, G.; Han, L.; Farooq, S. Comprehensive review on patulin and Alternaria toxins in fruit and derived products. Front. Plant. Sci. 2023, 14, 1139757. [Google Scholar] [CrossRef] [PubMed]
- Solhaug, A.; Eriksen, G.S.; Holme, J.A. Mechanisms of action and toxicity of the mycotoxin alternariol: A review. Basic Clin. Pharmacol. Toxicol. 2016, 119, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Badiali, C.; Petruccelli, V.; Brasili, E.; Pasqua, G. Xanthones: Biosynthesis and trafficking in plants, fungi and lichens. Plants 2023, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Elix, J.A.; Musidlak, H.W.; Sala, T.; Sargent, M.V. Structure and synthesis of some lichen xanthones. Aust. J. Chem. 1978, 31, 145–155. [Google Scholar] [CrossRef]
- Kawakami, H.; Suzuki, C.; Yamaguchi, H.; Hara, K.; Komine, M.; Yamamoto, Y. Norlichexanthone produced by cultured endolichenic fungus induced from Pertusaria laeviganda and its antioxidant activity. Biosci. Biotechnol. Biochem. 2019, 83, 996–999. [Google Scholar] [CrossRef]
- Ebada, S.S.; Schulz, B.; Wray, V.; Totzke, F.; Kubbutat, M.H.; Muller, W.E.; Hamacher, A.; Kassack, M.U.; Lin, W.; Proksch, P. Arthrinins A-D: Novel diterpenoids and further constituents from the sponge derived fungus Arthrinium sp. Bioorg. Med. Chem. 2011, 19, 4644–4651. [Google Scholar] [CrossRef]
- Han, L.; Zheng, W.; He, Z.; Qian, S.; Ma, X.; Kang, J.C. Endophytic fungus Biscogniauxia petrensis produces antibacterial substances. PeerJ 2023, 11, e15461. [Google Scholar] [CrossRef]
- Huneck, S.; Hofle, G. Study on 13C-NMR and Structure of Chloroxanthones from Lichens. Tetrahedron 1978, 34, 2491–2502. [Google Scholar] [CrossRef]
- Ernst-Russell, M.A.; Chai, C.L.L.; Elix, J.A.; McCarthy, P.M. Myeloconone A2, a new phenalenone from the lichen Myeloconis erumpens. Aust. J. Chem. 2000, 53, 1011–1013. [Google Scholar] [CrossRef]
- Daub, M.E.; Herrero, S.; Chung, K.R. Reactive oxygen species in plant pathogenesis: The role of perylenequinone photosensitizers. Antioxid. Redox Signal. 2013, 19, 970–989. [Google Scholar] [CrossRef]
- Fazio, A.T.; Adler, M.T.; Parnmen, S.; Lücking, R.; Maier, M.S. Production of the bioactive pigment elsinochrome A by a cultured mycobiont strain of the lichen. Mycol. Prog. 2018, 17, 479–487. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.S.; Mahomoodally, M.F.; Rengasamy, K.R.R. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.H.; Chen, K.X.; Zhang, L.Q.; Li, Y.M. Advance in biological activities of natural guaiane-type sesquiterpenes. Med. Chem. Res. 2019, 28, 1339–1358. [Google Scholar] [CrossRef]
- Do, T.H.; Aree, T.; Nguyen, H.H.; Nguyen, H.C.; Vo, T.P.G.; Nguyen, N.H.; Duong, T.H. Two new guaiane-sesquiterpenes from the cultured lichen mycobiont of Diorygma pruinosum. Phytochem. Lett. 2022, 52, 59–62. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Duong, T.H.; Duong, G.H.; Nguyen, H.T.; Nguyen, T.H.T.; Phan, N.H.N.; Vo, T.P.G.; Tran, T.M.D.; Nguyen, T.P. Hydroxydiorygmone A, a new guaiane-sesquiterpene from the cultured lichen mycobiont of Diorygma sp. Nat. Prod. Commun. 2024, 19, 1–9. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Aree, T.; Nguyen, H.T.; Tran, T.M.; Nguyen, T.P.; Vo, T.G.; Nguyen, N.H.; Duong, T.H. Diorygmones A-B, two new guaiane-sesquiterpenes from the cultured lichen mycobiont of Diorygma sp. Nat. Prod. Res. 2024, 38, 2282–2287. [Google Scholar] [CrossRef]
- Tian, X.H.; Hong, L.L.; Jiao, W.H.; Lin, H.W. Natural sesquiterpene quinone/quinols: Chemistry, biological activity, and synthesis. Nat. Prod. Rep. 2023, 40, 718–749. [Google Scholar] [CrossRef]
- Flores-Bocanegra, L.; Augustinović, M.; Raja, H.A.; Kurina, S.J.; Maldonado, A.C.; Burdette, J.E.; Falkinham, J.O., 3rd; Pearce, C.J.; Oberlies, N.H. Cytotoxic and antimicrobial drimane meroterpenoids from a fungus of the Stictidaceae (Ostropales, Ascomycota). Tetrahedron Lett. 2021, 68, 152896. [Google Scholar] [CrossRef]
- Mierau, V.; Rojas de la Parra, V.; Sterner, O.; Anke, T. The dasyscyphins A-C and niveulone, new biologically active compounds from the ascomycete Dasyscyphus niveus. J. Antibiot. 2006, 59, 53–56. [Google Scholar] [CrossRef]
- Khanna, V.G.; Kannabiran, K.; Getti, G. Leishmanicidal activity of saponins isolated from the leaves of Eclipta prostrata and Gymnema sylvestre. Indian J. Pharmacol. 2009, 41, 32–35. [Google Scholar] [CrossRef]
- Yuyama, K.T.; Fortkamp, D.; Abraham, W.R. Eremophilane-type sesquiterpenes from fungi and their medicinal potential. Biol. Chem. 2017, 399, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Le, D.H.; Takenaka, Y.; Hamada, N.; Tanahashi, T. Eremophilane-type sesquiterpenes from cultured lichen mycobionts of Sarcographa tricosa. Phytochemistry 2013, 91, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Vo, V.G.; Le, H.D.; Tran, T.N.; Nguyen, N.H.; Vo, T.G.; Sichaem, J.; Nguyen, V.K.; Duong, T.H. A new eremophilane-sesquiterpene from the cultured lichen mycobiont of Graphis sp. Nat. Prod. Res. 2022, 36, 319–325. [Google Scholar] [CrossRef]
- Shiono, Y.; Muslihah, N.I.; Suzuki, T.; Ariefta, N.R.; Anwar, C.; Nurjanto, H.H.; Aboshi, T.; Murayama, T.; Tawaraya, K.; Koseki, T.; et al. New eremophilane and dichlororesorcinol derivatives produced by endophytes isolated from Ficus ampelas. J. Antibiot. 2017, 70, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Motohashi, K.; Hashimoto, J.; Inaba, S.; Khan, S.T.; Komaki, H.; Nagai, A.; Takagi, M.; Shin-ya, K. New sesquiterpenes, JBIR-27 and -28, isolated from a tunicate-derived fungus, Penicillium sp. SS080624SCf1. J. Antibiot. 2009, 62, 247–250. [Google Scholar] [CrossRef]
- Del Valle, P.; Figueroa, M.; Mata, R. Phytotoxic eremophilane sesquiterpenes from the coprophilous fungus Penicillium sp. G1-a14. J. Nat. Prod. 2015, 78, 339–342. [Google Scholar] [CrossRef]
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant sterols: Diversity, biosynthesis, and physiological functions. Biochemistry 2016, 81, 819–834. [Google Scholar] [CrossRef]
- Ndifor, A.R.; Dominique, N.; Claude, M.D.; Raoul, K.; Ebouel, F.L.E.; Dongmo, Y.K.M.; Pantaleon, A.; Henoumont, C.; Stanislaus, N.N.; Laurent, S.; et al. Constituents, nutrient content, and in vitro antioxidant and anti-inflammatory activity of Tricholomopsis aurea (Agaricomycetes) from cameroon. Int. J. Med. Mushrooms 2025, 27, 71–85. [Google Scholar] [CrossRef]
- Merdivan, S.; Lindequist, U. Ergosterol peroxide: A mushroom-derived compound with promising biological activities—A review. Int. J. Med. Mushrooms 2017, 19, 93–105. [Google Scholar] [CrossRef]
- Valitova, J.; Renkova, A.; Beckett, R.; Minibayeva, F. Stigmasterol: An enigmatic plant stress sterol with versatile functions. Int. J. Mol. Sci. 2024, 25, 8122. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Zhu, L.; Wang, X.; Meng, F.; Xia, L.; Zhang, H. Advances in stigmasterol on its anti-tumor effect and mechanism of action. Front. Oncol. 2022, 12, 1101289. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; Benkhaira, N.; Bourais, I.; Benali, T.; Lee, L.H.; El Omari, N.; Sheikh, R.A.; Goh, K.W.; Ming, L.C.; Bouyahya, A. Health benefits and pharmacological properties of stigmasterol. Antioxidants 2022, 11, 1912. [Google Scholar] [CrossRef] [PubMed]
- Uttu, A.J.; Sallau, M.S.; Iyun, O.R.A.; Ibrahim, H. In vitro antimicrobial studies of some major bioactive compounds isolated from Strychnos innocua (Delile) root bark. Steroids 2023, 195, 109241. [Google Scholar] [CrossRef] [PubMed]
- Jahn, L.; Storm-Johannsen, L.; Seidler, D.; Noack, J.; Gao, W.; Schafhauser, T.; Wohlleben, W.; van Berkel, W.J.H.; Jacques, P.; Kar, T.; et al. The endophytic fungus Cyanodermella asteris influences growth of the nonnatural host plant Arabidopsis thaliana. Mol. Plant Microbe Interact. 2022, 35, 49–63. [Google Scholar] [CrossRef]
- Vassaux, A.; Tarayre, C.; Arguëlles-Arias, A.; Compère, P.; Delvigne, F.; Fickers, P.; Jahn, L.; Lang, A.; Leclère, V.; Ludwig-Müller, J.; et al. Astin C production by the endophytic fungus Cyanodermella asteris in planktonic and immobilized culture conditions. Biotechnol. J. 2019, 14, e1800624. [Google Scholar] [CrossRef]
- Shen, Y.; Luo, Q.; Xu, H.; Gong, F.; Zhou, X.; Sun, Y.; Wu, X.; Liu, W.; Zeng, G.; Tan, N.; et al. Mitochondria-dependent apoptosis of activated T lymphocytes induced by astin C, a plant cyclopeptide, for preventing murine experimental colitis. Biochem. Pharmacol. 2011, 82, 260–268. [Google Scholar] [CrossRef]
- Venugopal, A.; Glaudline, S.; Raj, D.P.; Lakshiakanthan, E.; Vijayanand, S.; Sevanan, M. An overview of the therapeutic potential of understudied lichen species for novel drug discovery. J. Appl. Natl. Sci. 2024, 16, 1516–1529. [Google Scholar] [CrossRef]
- Ren, Q. Key to the Lichen genera of China. J. Liaocheng Univ. (Nat. Sci. Ed.) 2023, 36, 107–110. [Google Scholar] [CrossRef]
- Zhu, M.L.; Chen, L.W.; Zhao, Y.F.; Jia, Z.F. Echinoplaca infuscata sp. nov. and new records of the genus Echinoplaca from Yunnan, China. Mycotaxon 2023, 137, 715–723. [Google Scholar] [CrossRef]
- Cui, C.; Li, Y.J.; Xu, J.H.; Zhao, X.; Jia, Z.F. Diorygma tiantaiense sp. nov. and a checklist and key to Diorygma species from China. Diversity 2024, 16, 213. [Google Scholar] [CrossRef]
- Dou, M.Z.; Li, M.; Jia, Z.F. New species and records of Chapsa (Graphidaceae) in China. MycoKeys 2021, 85, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.H.; Hao, C.Y.; Zhao, X.; Jia, Z.F. New species and record in the genus Carbacanthographis (lichenized Ascomycota, Graphidaceae) from China. Phytotaxa 2024, 670, 285–292. [Google Scholar] [CrossRef]
- Shi, K.J.; Guo, T.L.; Jia, Z.F.; Zhao, X. Four new records of the lichen genus Fissurina from China. Herzogia 2023, 36, 488–496. [Google Scholar] [CrossRef]
- Liu, L.; Ren, Q. Aspicilia lixianensis, A. nivalis, and A. pycnocarpa spp. nov. from China. Mycotaxon 2021, 136, 739–748. [Google Scholar] [CrossRef]
- Ren, Q.; Alexander, G.P.; Sun, Z.S.; Yan, Q.D. Circinaria weii sp. nov., a new lichen species from China. Bryologist 2025, 128, 30–36. [Google Scholar] [CrossRef]
- Zhang, J.R.; Xue, X.D.; Liu, L.; Qiu, X.Y.; Ren, Q. Lepra yunlingensis and L. taiwanensis spp. nov. from China. Mycotaxon 2022, 136, 779–784. [Google Scholar] [CrossRef]
- Fan, J.; Wei, P.L.; Li, Y.; Zhang, S.; Ren, Z.; Li, W.; Yin, W.B. Developing filamentous fungal chassis for natural product production. Bioresour. Technol. 2025, 415, 131703. [Google Scholar] [CrossRef]
- Kalra, R.; Conlan, X.A.; Goel, M. Recent advances in research for potential utilization of unexplored lichen metabolites. Biotechnol. Adv. 2023, 62, 108072. [Google Scholar] [CrossRef]



| Bioactivity | Compounds | Lichen Species | References |
|---|---|---|---|
| Antitumor | Haematommic acid (4) | A. pakistanica | [19] |
| Olivetoric acid (30) | Ochrolechia spp. | [39,41] | |
| Baeomycesic acid (38) | Siphula spp. | [48] | |
| Thamnoliadepside A (42) | T. vermicularis | [18] | |
| Atranorin (55) | Siphula spp., Baeomyces spp. | [58] | |
| Sekikaic acid (57) | S. ceratites, L. alphoplaca | [61] | |
| Alectorialic acid (62) | A. pakistanica | [19] | |
| Gyrophoric acid (63) | P. mccroryae, O. androgyna, D. diacapsis, A. pakistanica | [69] | |
| α-alectoronic acid (75) | O. parella, A. radiosa | [35] | |
| Physodic acid (76) | D. diacapsis | [84,85] | |
| Variolaric acid (77) | O. parella, A. radiosa | [35] | |
| Protocetraric acid (79) | O. androgyna | [89] | |
| Stictic acid (81) | Baeomyces spp., Pertusaria spp. | [96] | |
| Salazinic acid (88) | P. pseudocorallina | [89] | |
| (+)-protolichesterinic acid (109) | Ochrolechia spp. | [110,111] | |
| 5,7-dihydroxy-6-methylphthalide (114) | A. pakistanica | [19] | |
| Usnic acid (115) | Baeomyces spp., Aspicilia spp. | [19] | |
| 6-(4,5-dihydroxy-10-methyl-6-oxo-7-undecenyl)-resorcylic acid lactone (129) | B. placophyllus | [126] | |
| Graphislactone A (140) | G. prunicola, G. cognata, G. scripta | [133] | |
| Norlichexanthone (165) | P. laeviganda | [141] | |
| Pruinosone (175) | D. pruinosum | [149] | |
| Diorygmones A–B (177–178) | Diorygma sp. | [151] | |
| Dasyscyphin F (185) | Stictidaceae | [153] | |
| Petasol (186) and sporogen AO-1 (189) | S. tricosa | [159] | |
| Graphilane (194) | Graphis sp. | [158] | |
| Ergosterol peroxide (196) | O. parella, S. tricosa | [164] | |
| Stigmasterol (197) | Aspicilia sp. | [167] | |
| Astin C (200) | C. asteris | [171] | |
| Anti-inflammatory | Barbatinic acid (47) | T. vermicularis | [47] |
| Atranorin (55) | Siphula spp., Aspicilia spp., Baeomyces spp. | [59] | |
| Physodic acid (76) | D. diacapsis | [82] | |
| Wedelolactone (127) | O. frigida | [125] | |
| Ergosterol peroxide (196) | O. parella, S. tricosa | [163] | |
| Stigmasterol (197) | Aspicilia sp. | [167] | |
| Astin C (200) | C. asteris | [170] | |
| Antibacterial | Squamatic acid (37) | Baeomyces spp., Thamnolia spp. | [20] |
| Baeomycesic acid (38) | Siphula spp. | [117] | |
| Gyrophoric acid (63) | Ochrolechia spp., P. mccroryae, | [67] | |
| Protocetraric acid (79) | O. androgyna | [36,87] | |
| Lobaric acid (73) | T. vermicularis, L. alphoplaca | [80] | |
| Fumarprotocetraric acid (80) | O. androgyna | [36] | |
| Stictic acid (81) | Baeomyces spp., Pertusaria spp., Diorygma sp. | [36] | |
| Hexadecanoic acid (100) | P. caesiopruinosa | [106] | |
| Usnic acid (115) | Baeomyces spp., Aspicilia spp. | [16,19] | |
| Didymic acid (116) | P. flavens | [120] | |
| Wedelolactone (127) | O. frigida | [124] | |
| 6,8-dihydroxy-3-hydroxymethylisocoumarin (135) | G. proserpens | [129] | |
| Vinetorin (168) | Pertusaria sp. | [142] | |
| Elsinochrome A (174) | G. elongata | [146] | |
| Diorygmone B (178) | Diorygma sp. | [26] | |
| Diorygmones C–D (180–181) | D. pruinosum | [26] | |
| Dasyscyphin F (185) | Stictidaceae | [155] | |
| Campesterol (198) | Aspicilia sp. | [168] | |
| β-sitosterol (199) | T. vermicularis, D. pruinosum | [168] | |
| Antifungal | Methyl-3-chloro-2-hydroxy-4-methoxy-6-pentylbenzoate (16) | P. dactylina | [27] |
| Lecanoric acid (29) | Siphula spp., Diploschistes spp. | [36] | |
| Divaricatic acid (31) | Pertusaria spp. | [43] | |
| Gyrophoric acid (63) | Ochrolechia spp., P. mccroryae, D. diacapsis, A. pakistanica | [67] | |
| Xylarinic acid A (103) | G. handelii | [107] | |
| Pruinosone (175) and hydroxypruinosone (176) | D. pruinosum | [149] | |
| Antiviral | Sekikaic acid (57) | S. ceratites, L. alphoplaca | [64] |
| Handelone (59) | G. handelii | [66] | |
| Variolaric acid (77) | O. parella, A. radiosa | [35] | |
| Usnic acid (115) | Baeomyces spp., Aspicilia spp. | [119] | |
| 6,8-dihydroxy-3-hydroxymethylisocoumarin (135) | G. proserpens | [129] | |
| Antioxidant | Orsellinic acid (6) | Diploschistes spp., O. frigida | [24] |
| Prephenic acid (8), hypoxyphenone (9), and tetrafucol A (28) | O. frigida | [24] | |
| Lecanoric acid (29) | Siphula spp., Diploschistes spp. | [24,38] | |
| Cyperine (36) | P. contortuplicata | [24] | |
| Atranorin (55) | Siphula spp., Baeomyces spp. | [38] | |
| Aekikaic acid (57) | S. ceratites, L. alphoplaca | [62] | |
| Gyrophoric acid (63) | Ochrolechia spp., P. mccroryae, D. diacapsis, A. pakistanica | [67] | |
| Lobaric acid (73) | T. vermicularis, L. alphoplaca | [80] | |
| Fumarprotocetraric acid (80) | O. androgyna | [91,92] | |
| Norstictic acid (82) | Siphula spp., Aspicilia spp., Graphis spp. | [79] | |
| 3,6,9,12-tetraoxapentacosanoic acid (101), 18-hydroxylinoleic acid (102), wedelolactone (127), and Diospyrol (173) | O. frigida | [24] | |
| Stigmasterol (197) | Aspicilia sp. | [167] | |
| Anti-angiogenic | Olivetoric acid (30) | Ochrolechia spp. | [40] |
| Anti-neurodegenerative diseases | Methylbenzoic acids (10,11,12) and vermicularin (41) | T. vermicularis | [25] |
| Baeomycesic acid (38) | Siphula spp. | [25] | |
| Antitubercular | Acremonidin E (25) | Graphis sp. | [29] |
| Protocetraric acid (79) | O. androgyna | [88] | |
| Anti-herbivore | Protocetraric acid (79) | O. androgyna | [57] |
| Stictic acid (81) | Baeomyces spp., Pertusaria spp., Diorygma sp. | [57] | |
| Norstictic acid (82) | Siphula spp., Aspicilia spp., Graphis spp. | [57] | |
| (+)-aspicilin (128) | Aspicilia spp. | [57] | |
| Antitrypanosomal | Lichesterinic acid (107) and (+)- protolichesterinic acid (109) | Ochrolechia spp. | [109] |
| Dasyscyphin C (183) | Stictidaceae | [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hao, C.; Jiang, S.; Ju, Y.; Li, W.; Jia, Z. A Comprehensive Review on Chemical Structures and Bioactivities of Ostropomycetidae Lichens. J. Fungi 2025, 11, 369. https://doi.org/10.3390/jof11050369
Wang Y, Hao C, Jiang S, Ju Y, Li W, Jia Z. A Comprehensive Review on Chemical Structures and Bioactivities of Ostropomycetidae Lichens. Journal of Fungi. 2025; 11(5):369. https://doi.org/10.3390/jof11050369
Chicago/Turabian StyleWang, Yunhui, Chengyue Hao, Shuhao Jiang, Yanhu Ju, Wei Li, and Zefeng Jia. 2025. "A Comprehensive Review on Chemical Structures and Bioactivities of Ostropomycetidae Lichens" Journal of Fungi 11, no. 5: 369. https://doi.org/10.3390/jof11050369
APA StyleWang, Y., Hao, C., Jiang, S., Ju, Y., Li, W., & Jia, Z. (2025). A Comprehensive Review on Chemical Structures and Bioactivities of Ostropomycetidae Lichens. Journal of Fungi, 11(5), 369. https://doi.org/10.3390/jof11050369






