Evaluation of the Synergistic Activity of Antimicrobial Peptidomimetics or Colistin Sulphate with Conventional Antifungals Against Yeasts of Medical Importance
Abstract
1. Introduction
2. Materials and Methods
2.1. Fungal Isolates and Media
2.2. Antimicrobial Agents
2.3. Inoculum Preparation
2.4. Microdilution Assay for Antifungal Susceptibility Testing
2.5. Checkerboard Assay to Test for Synergy
2.6. Time-Kill Assay
2.7. Ergosterol Binding and Sorbitol Protection Assays
2.8. Cellular Leakage Effect
2.9. Membrane-Perturbing Assay
2.10. Assessment of Mitochondrial Permeability Using Rhodamine 123
2.11. Activity on Germ Tube Formation in C. albicans
2.12. Statistical Analysis
3. Results
3.1. MIC of Antimicrobials
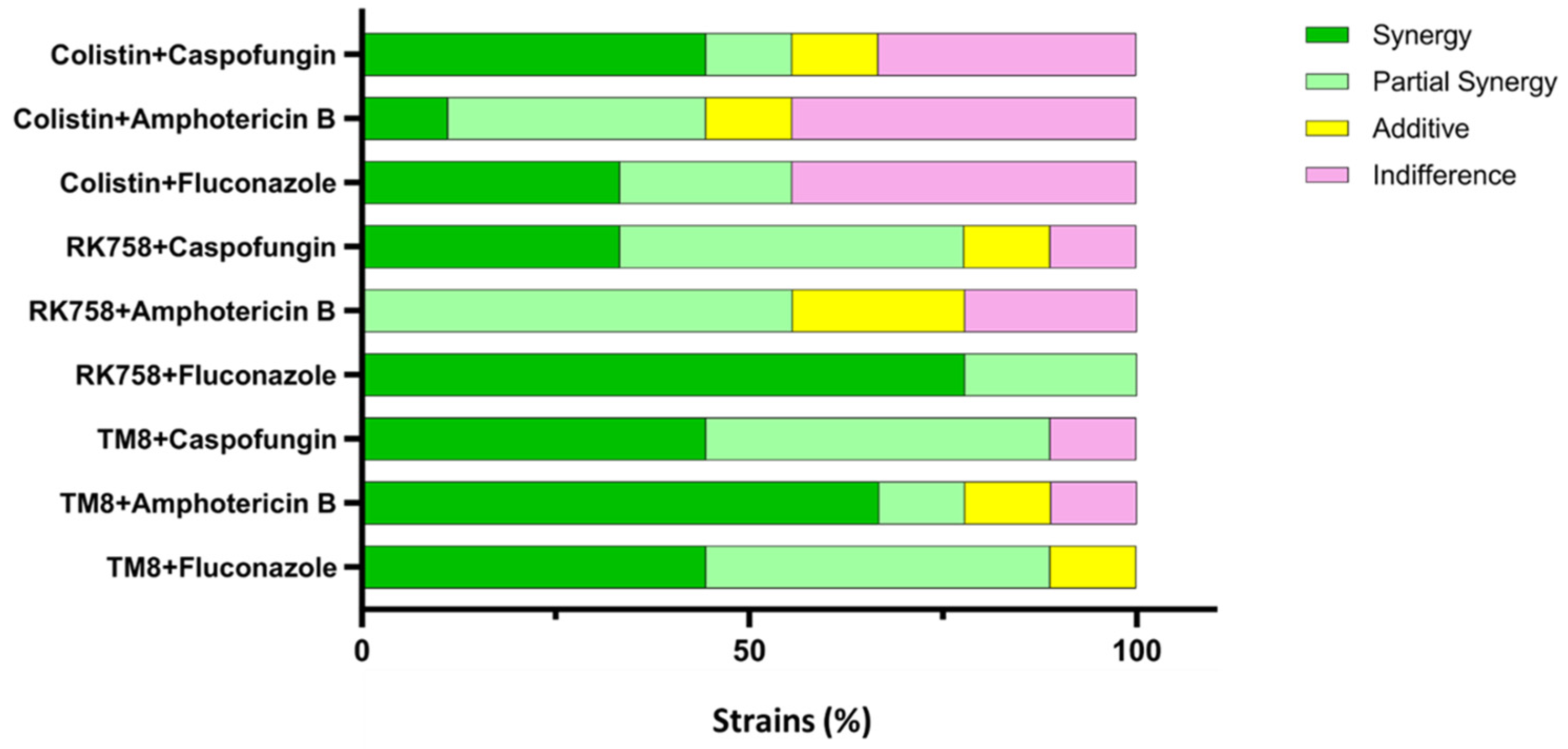
3.2. Checkerboard Assay
3.3. Time-Kill Assay
3.4. Ergosterol Binding and Sorbitol Protection Assays
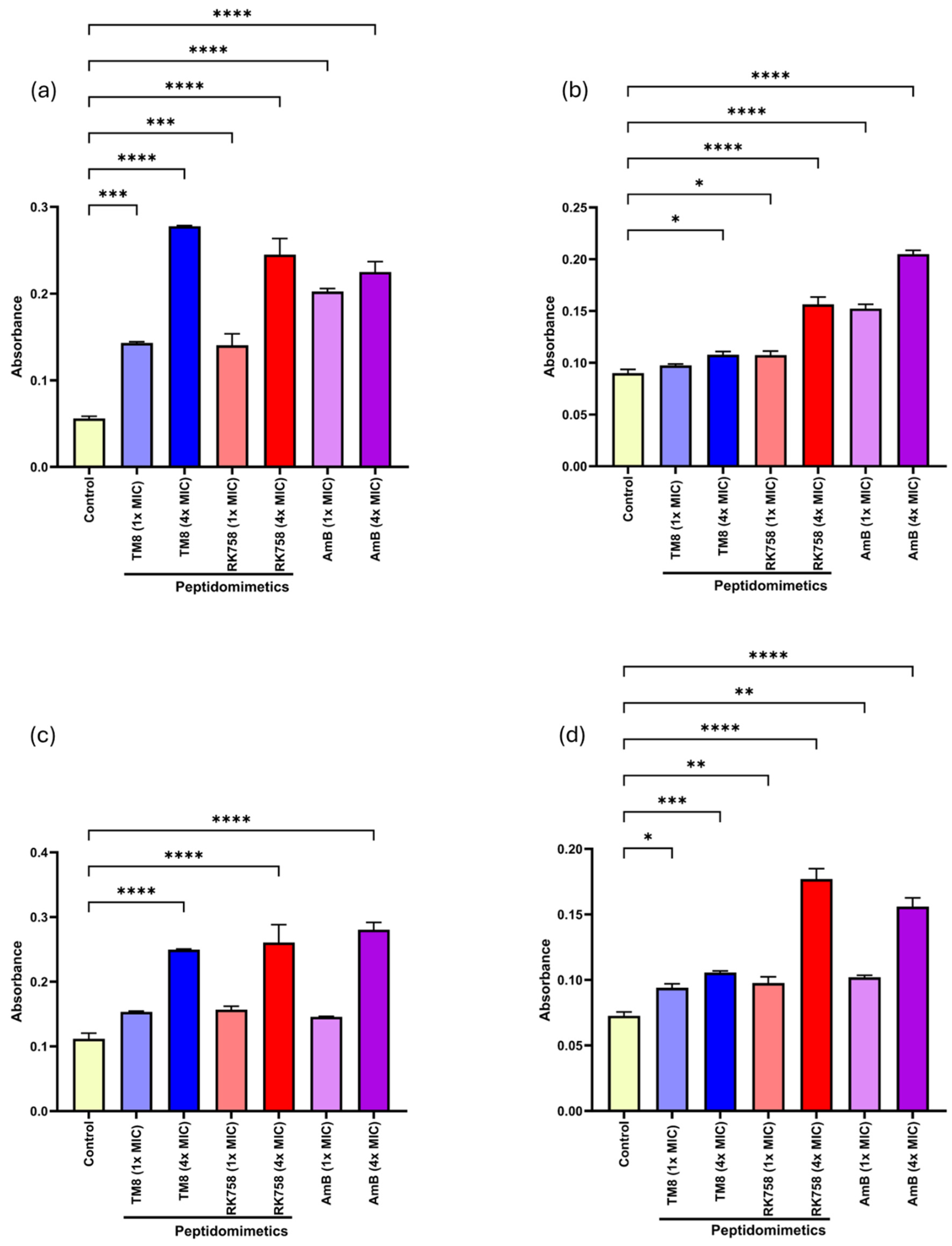
3.5. Cellular Leakage Assay
3.6. Membrane-Perturbating Assay
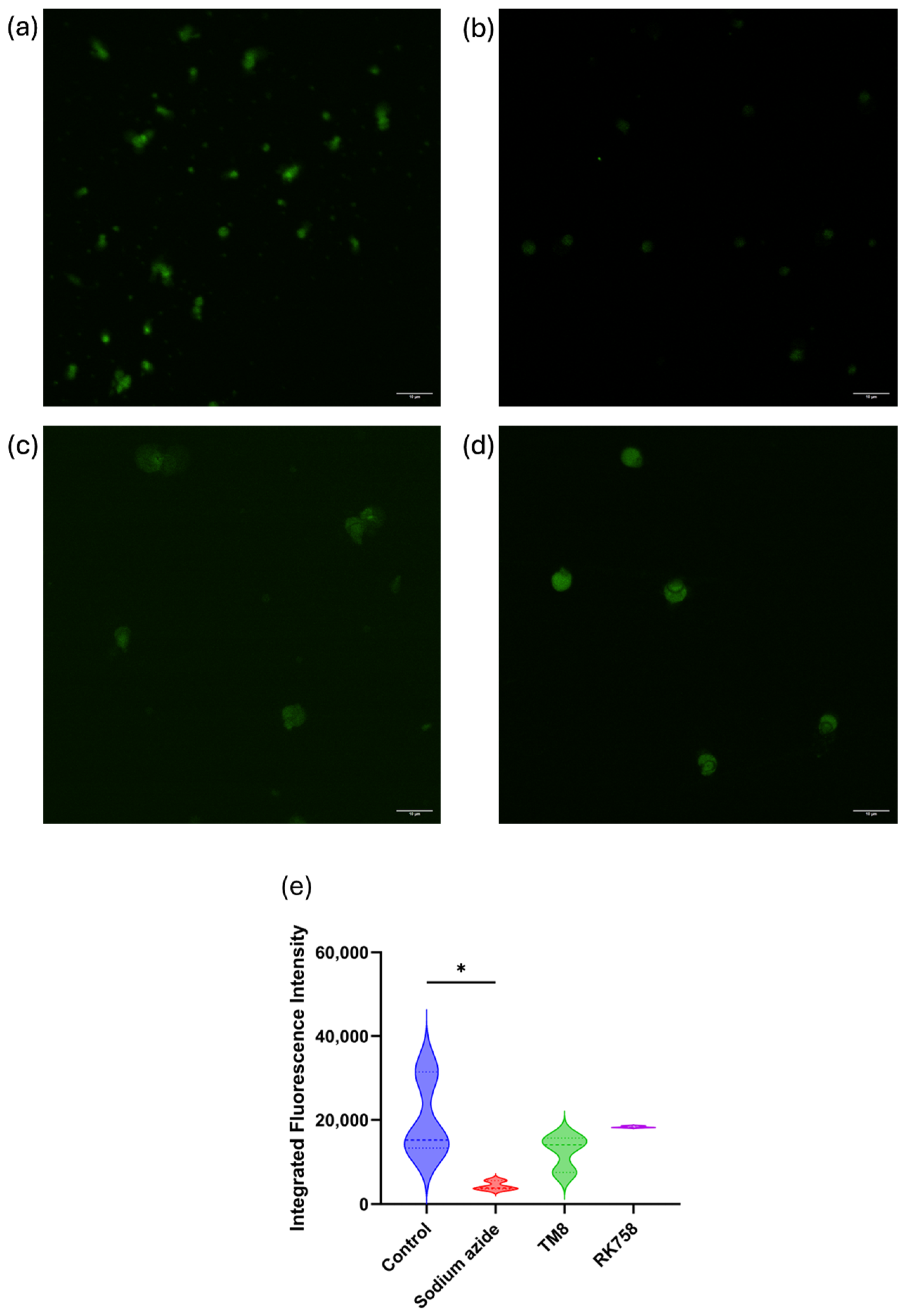
3.7. Mitochondrial Permeability Using Rhodamine 123
3.8. Germ Tube Formation Assay
4. Discussion
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mayer, F.L.; Wilson, D.; Hube, B. Candida albicans pathogenicity mechanisms. Virulence 2013, 4, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.A.; Butler, G. The Candida pathogenic species complex. Cold Spring Harb. Perspect. Med. 2014, 4, a019778. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Zeidler, U.; Bougnoux, M.-E.; Lupan, A.; Helynck, O.; Doyen, A.; Garcia, Z.; Sertour, N.; Clavaud, C.; Munier-Lehmann, H.; Saveanu, C. Synergy of the antibiotic colistin with echinocandin antifungals in Candida species. J. Antimicrob. Chemother. 2013, 68, 1285–1296. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024, 24, e428–e438. [Google Scholar] [CrossRef]
- Seyoum, E.; Bitew, A.; Mihret, A. Distribution of Candida albicans and non-albicans Candida species isolated in different clinical samples and their in vitro antifungal suscetibity profile in Ethiopia. BMC Infect. Dis. 2020, 20, 231. [Google Scholar] [CrossRef]
- Malinovská, Z.; Čonková, E.; Váczi, P. Biofilm formation in medically important Candida species. J. Fungi 2023, 9, 955. [Google Scholar] [CrossRef]
- Takashima, M.; Sugita, T. Taxonomy of Pathogenic Yeasts Candida, Cryptococcus, Malassezia, and Trichosporon Current Status, Future Perspectives, and Proposal for Transfer of Six Candida Species to the Genus Nakaseomyces. Med. Mycol. J. 2022, 63, 119–132. [Google Scholar] [CrossRef]
- Liu, F.; Hu, Z.D.; Zhao, X.M.; Zhao, W.N.; Feng, Z.X.; Yurkov, A.; Alwasel, S.; Boekhout, T.; Bensch, K.; Hui, F.L.; et al. Phylogenomic analysis of the Candida auris- Candida haemuli clade and related taxa in the Metschnikowiaceae, and proposal of thirteen new genera, fifty-five new combinations and nine new species. Persoonia—Mol. Phylogeny Evol. Fungi 2024, 52, 22–43. [Google Scholar] [CrossRef]
- Parambath, S.; Dao, A.; Kim, H.Y.; Zawahir, S.; Alastruey Izquierdo, A.; Tacconelli, E.; Govender, N.; Oladele, R.; Colombo, A.; Sorrell, T.; et al. Candida albicans—A systematic review to inform the World Health Organization Fungal Priority Pathogens List. Med. Mycol. 2024, 62, myae045. [Google Scholar] [CrossRef]
- Lass-Flörl, C.; Kanj, S.S.; Govender, N.P.; Thompson III, G.R.; Ostrosky-Zeichner, L.; Govrins, M.A. Invasive candidiasis. Nat. Rev. Dis. Primers 2024, 10, 20. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Kim, H.Y.; Stocker, S.; Kidd, S.; Alastruey-Izquierdo, A.; Dao, A.; Harrison, T.; Wahyuningsih, R.; Rickerts, V.; Perfect, J.; et al. Pichia kudriavzevii (Candida krusei): A systematic review to inform the World Health Organisation priority list of fungal pathogens. Med. Mycol. 2024, 62, myad132. [Google Scholar] [CrossRef]
- Mishra, S.K.; Yasir, M.; Willcox, M. Candida auris: An emerging antimicrobial-resistant organism with the highest level of concern. Lancet Microbe 2023, 4, e482–e483. [Google Scholar] [CrossRef]
- Kean, R.; Ramage, G. Combined antifungal resistance and biofilm tolerance: The global threat of Candida auris. mSphere 2019, 4, e00458-19. [Google Scholar] [CrossRef] [PubMed]
- Askari, F.; Kaur, R. Candida glabrata: A Tale of Stealth and Endurance. ACS Infect. Dis. 2024, 11, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Savini, V.; Catavitello, C.; Onofrillo, D.; Masciarelli, G.; Astolfi, D.; Balbinot, A.; Febbo, F.; D’Amario, C.; D’Antonio, D. What do we know about Candida guilliermondii? A voyage throughout past and current literature about this emerging yeast. Mycoses 2011, 54, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Carvalhaes, C.G.; DeVries, S.; Rhomberg, P.R.; Castanheira, M. Impact of COVID-19 on the antifungal susceptibility profiles of isolates collected in a global surveillance program that monitors invasive fungal infections. Med. Mycol. 2022, 60, myac028. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Fang, W.; Wu, J.; Cheng, M.; Zhu, X.; Du, M.; Chen, C.; Liao, W.; Zhi, K.; Pan, W. Diagnosis of invasive fungal infections: Challenges and recent developments. J. Biomed. Sci. 2023, 30, 42. [Google Scholar] [CrossRef]
- Cornely, O.A.; Sprute, R.; Bassetti, M.; Chen, S.C.A.; Groll, A.H.; Kurzai, O.; Lass-Flörl, C.; Ostrosky-Zeichner, L.; Rautemaa-Richardson, R.; Revathi, G.; et al. Global guideline for the diagnosis and management of candidiasis: An initiative of the ECMM in cooperation with ISHAM and ASM. Lancet Infect. Dis. 2025, 25, e280–e293. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and mechanisms of antifungal resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Mishra, S.K.; Akter, T.; Urmi, U.L.; Enninful, G.; Sara, M.; Shen, J.; Suresh, D.; Zheng, L.; Mekonen, E.S.; Rayamajhee, B.; et al. Harnessing Non-Antibiotic Strategies to Counter Multidrug-Resistant Clinical Pathogens with Special Reference to Antimicrobial Peptides and Their Coatings. Antibiotics 2025, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.E.; Larsen, C.J.; Franzyk, H.; Regenberg, B. Antifungal properties of peptidomimetics with an arginine-[β-(2, 5, 7-tri-tert-butylindol-3-yl) alanine]-arginine motif against Saccharomyces cerevisiae and Zygosaccharomyces bailii. FEMS Yeast Res. 2015, 15, fov011. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, R.; Willcox, M.; Black, D.S.; Kumar, N. Short cationic peptidomimetic antimicrobials. Antibiotics 2019, 8, 44. [Google Scholar] [CrossRef]
- Browne, K.; Kuppusamy, R.; Walsh, W.R.; Black, D.S.; Willcox, M.D.; Kumar, N.; Chen, R. Antimicrobial Peptidomimetics Prevent the Development of Resistance against Gentamicin and Ciprofloxacin in Staphylococcus and Pseudomonas Bacteria. Int. J. Mol. Sci. 2023, 24, 14966. [Google Scholar] [CrossRef]
- Kataria, A.; Sharma, R.; Sharma, S.; Singh, B.; Kaur, G.; Yakubu, C.M. Recent applications of bio-engineering principles to modulate the functionality of proteins in food systems. Trends Food Sci. Technol. 2021, 113, 54–65. [Google Scholar] [CrossRef]
- Méndez-Samperio, P. Peptidomimetics as a new generation of antimicrobial agents: Current progress. Infect. Drug Resist. 2014, 7, 229. [Google Scholar] [CrossRef]
- Urmi, U.L.; Vijay, A.K.; Willcox, M.D.; Attard, S.; Enninful, G.; Kumar, N.; Islam, S.; Kuppusamy, R. Exploring the efficacy of peptides and mimics against Influenza A Virus, Adenovirus, and murine norovirus. Int. J. Mol. Sci. 2024, 25, 7030. [Google Scholar] [CrossRef]
- Toepfer, S.; Keniya, M.V.; Lackner, M.; Monk, B.C. Azole Combinations and Multi-Targeting Drugs That Synergistically Inhibit Candidozyma auris. J. Fungi 2024, 10, 698. [Google Scholar] [CrossRef] [PubMed]
- Bibi, M.; Murphy, S.; Benhamou, R.I.; Rosenberg, A.; Ulman, A.; Bicanic, T.; Fridman, M.; Berman, J. Combining colistin and fluconazole synergistically increases fungal membrane permeability and antifungal cidality. ACS Infect. Dis. 2021, 7, 377–389. [Google Scholar] [CrossRef]
- Nielsen, J.E.; Alford, M.A.; Yung, D.B.Y.; Molchanova, N.; Fortkort, J.A.; Lin, J.S.; Diamond, G.; Hancock, R.E.; Jenssen, H.v.; Pletzer, D. Self-assembly of antimicrobial peptoids impacts their biological effects on ESKAPE bacterial pathogens. ACS Infect. Dis. 2022, 8, 533–545. [Google Scholar] [CrossRef]
- Jenssen, H. Self-assembling peptoid-based antimicrobial nanomaterials. Nanomedicine 2025, 20, 917–919. [Google Scholar] [CrossRef]
- Mishra, S.K.; Yasir, M.; Kuppusamy, R.; Wong, E.H.H.; Hui, A.; Sørensen, K.; Lin, J.S.; Jenssen, H.; Barron, A.E.; Willcox, M. Antimicrobial activity of peptoids against Metallo-β-lactamase-producing Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and other WHO priority pathogens, including Candida auris. J. Appl. Microbiol. 2025, 136, lxaf031. [Google Scholar] [CrossRef]
- Critchley, I.A.; Douglas, L.J. Role of glycosides as epithelial cell receptors for Candida albicans. Microbiology 1987, 133, 637–643. [Google Scholar] [CrossRef]
- Casalini, G.; Giacomelli, A.; Antinori, S. The WHO fungal priority pathogens list: A crucial reappraisal to review the prioritisation. Lancet Microbe 2024, 5, 717–724. [Google Scholar] [CrossRef]
- Willcox, M.; Webb, B.; Thakur, A.; Harty, D. Interactions between Candida species and platelets. J. Med. Microbiol. 1998, 47, 103–110. [Google Scholar] [CrossRef]
- Webb, B.; Willcox, M.; Thomas, C.; Harty, D.; Knox, K. The effect of sodium hypochlorite on potential pathogenic traits of Candida albicans and other Candida species. Oral Microbiol. Immunol. 1995, 10, 334–341. [Google Scholar] [CrossRef]
- Kurtzman, C.P.; Suzuki, M. Phylogenetic analysis of ascomycete yeasts that form coenzyme Q-9 and the proposal of the new genera Babjeviella, Meyerozyma, Millerozyma, Priceomyces, and Scheffersomyces. Mycoscience 2010, 51, 2–14. [Google Scholar] [CrossRef]
- Chatterjee, P.; Choi, H.; Ochoa, B.; Garmon, G.; Coppin, J.D.; Allton, Y.; Lukey, J.; Williams, M.D.; Navarathna, D.; Jinadatha, C. Clade-specific variation in susceptibility of Candida auris to broad-spectrum ultraviolet C light (UV-C). Infect. Control Hosp. Epidemiol. 2020, 41, 1384–1387. [Google Scholar] [CrossRef]
- Kuppusamy, R.; Yasir, M.; Yu, T.T.; Voli, F.; Vittorio, O.; Miller, M.J.; Lewis, P.; Black, D.S.; Willcox, M.; Kumar, N. Tuning the anthranilamide peptidomimetic design to selectively target planktonic bacteria and biofilm. Antibiotics 2023, 12, 585. [Google Scholar] [CrossRef]
- PubChem Compound Summary for CID 3365, Fluconazole. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Fluconazole (accessed on 4 May 2025).
- PubChem Compound Summary for CID 139586083. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Caspofungin (accessed on 4 May 2025).
- PubChem Compound Summary for CID 5280965, Amphotericin B. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Amphotericin-B (accessed on 4 May 2025).
- PubChem Compound Summary for CID 133109993. 2024. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Colistin (accessed on 4 May 2025).
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 3rd ed.; CLSI document M27-A3; CLSI: Malvern, PA, USA, 2008. [Google Scholar]
- CLSI. Epidemiological Cutoff Values for Antifungal Susceptibility Testing, 4th ed.; CLSI supplement M57S; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2022. [Google Scholar]
- Black, C.; Al Mahmud, H.; Howle, V.; Wilson, S.; Smith, A.C.; Wakeman, C.A. Development of a polymicrobial checkerboard assay as a tool for determining combinatorial antibiotic effectiveness in polymicrobial communities. Antibiotics 2023, 12, 1207. [Google Scholar] [CrossRef] [PubMed]
- Timurkaynak, F.; Can, F.; Azap, Ö.K.; Demirbilek, M.; Arslan, H.; Karaman, S.Ö. In vitro activities of non-traditional antimicrobials alone or in combination against multidrug-resistant strains of Pseudomonas aeruginosa and Acinetobacter baumannii isolated from intensive care units. Int. J. Antimicrob. Agents 2006, 27, 224–228. [Google Scholar] [CrossRef]
- Mun, S.-H.; Joung, D.-K.; Kim, Y.-S.; Kang, O.-H.; Kim, S.-B.; Seo, Y.-S.; Kim, Y.-C.; Lee, D.-S.; Shin, D.-W.; Kweon, K.-T. Synergistic antibacterial effect of curcumin against methicillin-resistant Staphylococcus aureus. Phytomedicine 2013, 20, 714–718. [Google Scholar] [CrossRef]
- Haji, S.H.; Ali, F.A.; Aka, S.T.H. Synergistic antibacterial activity of silver nanoparticles biosynthesized by carbapenem-resistant Gram-negative bacilli. Sci. Rep. 2022, 12, 15254. [Google Scholar] [CrossRef]
- Pankey, G.; Ashcraft, D.; Kahn, H.; Ismail, A. Time-kill assay and Etest evaluation for synergy with polymyxin B and fluconazole against Candida glabrata. Antimicrob. Agents Chemother. 2014, 58, 5795–5800. [Google Scholar] [CrossRef]
- Escalante, A.; Gattuso, M.; Pérez, P.; Zacchino, S. Evidence for the Mechanism of Action of the Antifungal Phytolaccoside B Isolated from Phytolacca tetramera Hauman. J. Nat. Prod. 2008, 71, 1720–1725. [Google Scholar] [CrossRef]
- Frost, D.J.; Brandt, K.D.; Cugier, D.; Goldman, R. A whole-cell Candida albicans assay for the detection of inhibitors towards fungal cell wall synthesis and assembly. J. Antibiot. 1995, 48, 306–310. [Google Scholar] [CrossRef]
- Zorić, N.; Kosalec, I.; Tomić, S.; Bobnjarić, I.; Jug, M.; Vlainić, T.; Vlainić, J. Membrane of Candida albicans as a target of berberine. BMC Complement. Altern. Med. 2017, 17, 268. [Google Scholar] [CrossRef]
- Merlino, F.; Carotenuto, A.; Casciaro, B.; Martora, F.; Loffredo, M.R.; Di Grazia, A.; Yousif, A.M.; Brancaccio, D.; Palomba, L.; Novellino, E. Glycine-replaced derivatives of [Pro3, DLeu9] TL, a temporin L analogue: Evaluation of antimicrobial, cytotoxic and hemolytic activities. Eur. J. Med. Chem. 2017, 139, 750–761. [Google Scholar] [CrossRef]
- Kondori, N.; Baltzer, L.; Dolphin, G.; Mattsby-Baltzer, I. Fungicidal activity of human lactoferrin-derived peptides based on the antimicrobial αβ region. Int. J. Antimicrob. Agents 2011, 37, 51–57. [Google Scholar] [CrossRef]
- Richardson, M.; Smith, H. Production of germ tubes by virulent and attenuated strains of Candida albicans. J. Infect. Dis. 1981, 144, 565–569. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 3rd ed.; CLSI supplement M27M44S; CLSI: Malvern, PA, USA, 2022. [Google Scholar]
- CDC. Antifungal Susceptibility Testing for C. auris. Available online: https://www.cdc.gov/candida-auris/hcp/laboratories/antifungal-susceptibility-testing.html (accessed on 3 December 2024).
- Harris, M.R.; Coote, P.J. Combination of caspofungin or anidulafungin with antimicrobial peptides results in potent synergistic killing of Candida albicans and Candida glabrata in vitro. Int. J. Antimicrob. Agents 2010, 35, 347–356. [Google Scholar] [CrossRef]
- Lu, H.; Shrivastava, M.; Whiteway, M.; Jiang, Y. Candida albicans targets that potentially synergize with fluconazole. Crit. Rev. Microbiol. 2021, 47, 323–337. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R. Fluconazole resistance in Candida species: A current perspective. Infect. Drug Resist. 2017, 10, 237–245. [Google Scholar] [CrossRef]
- Katsipoulaki, M.; Stappers, M.H.T.; Malavia-Jones, D.; Brunke, S.; Hube, B.; Gow, N.A.R. Candida albicans and Candida glabrata: Global priority pathogens. Microbiol. Mol. Biol. Rev. 2024, 88, e0002123. [Google Scholar] [CrossRef]
- Lindberg, E.; Hammarström, H.; Ataollahy, N.; Kondori, N. Species distribution and antifungal drug susceptibilities of yeasts isolated from the blood samples of patients with candidemia. Sci. Rep. 2019, 9, 3838. [Google Scholar] [CrossRef]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2020, 59, 14–30. [Google Scholar] [CrossRef]
- Ben-Ami, R. Treatment of invasive candidiasis: A narrative review. J. Fungi 2018, 4, 97. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Perlin, D.S. Echinocandin resistance in Candida. Clin. Infect. Dis. 2015, 61, S612–S617. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, H.; Li, J.; Zhang, Y.; Wang, Z.; Sun, S. Antifungal activity and potential mechanism of action of caspofungin in combination with ribavirin against Candida albicans. Int. J. Antimicrob. Agents 2023, 61, 106709. [Google Scholar] [CrossRef]
- Prayag, P.S.; Patwardhan, S.A.; Joshi, R.S.; Dhupad, S.; Rane, T.; Prayag, A.P. Comparative efficacies of the three echinocandins for Candida auris candidemia: Real world evidence from a tertiary centre in India. Med. Mycol. 2024, 62, myae065. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Srinivasan, A.; Uppuluri, P.; Ramasubramanian, A.K.; López-Ribot, J.L. Antifungal therapy with an emphasis on biofilms. Curr. Opin. Pharmacol. 2013, 13, 726–730. [Google Scholar] [CrossRef]
- Ahmady, L.; Gothwal, M.; Mukkoli, M.M.; Bari, V.K. Antifungal drug resistance in Candida: A special emphasis on amphotericin B. APMIS 2024, 132, 291–316. [Google Scholar] [CrossRef]
- Zhu, P.; Li, Y.; Guo, T.; Liu, S.; Tancer, R.J.; Hu, C.; Zhao, C.; Xue, C.; Liao, G. New antifungal strategies: Drug combination and co-delivery. Adv. Drug Deliv. Rev. 2023, 198, 114874. [Google Scholar] [CrossRef] [PubMed]
- Shaban, S.; Patel, M.; Ahmad, A. Fungicidal activity of human antimicrobial peptides and their synergistic interaction with common antifungals against multidrug-resistant Candida auris. Int. Microbiol. 2023, 26, 165–177. [Google Scholar] [CrossRef]
- Balkrishna, A.; Kharayat, B.; Rastogi, S.; Kabdwal, M.; Haldar, S.; Varshney, A. Withania somnifera seed oil exhibits antibiofilm properties against drug-resistant Candida auris clinical isolate through modulation in cell permeability. J. Appl. Microbiol. 2023, 134, lxad087. [Google Scholar] [CrossRef]
- Park, J.; Kim, H.; Kang, H.-K.; Choi, M.-C.; Park, Y. Lycosin-II exhibits antifungal activity and inhibits dual-species biofilm by Candida albicans and Staphylococcus aureus. J. Fungi 2022, 8, 901. [Google Scholar] [CrossRef]
- Kretschmar, M.; Hube, B.; Bertsch, T.; Sanglard, D.; Merker, R.; Schröder, M.; Hof, H.; Nichterlein, T. Germ tubes and proteinase activity contribute to virulence of Candida albicans in murine peritonitis. Infect. Immun. 1999, 67, 6637–6642. [Google Scholar] [CrossRef]
- Li, X.; Hu, Q.; Lin, Q.; Luo, J.; Xu, J.; Chen, L.; Xu, L.; Lin, X. Inhibition of Candida albicans in vivo and in vitro by antimicrobial peptides chromogranin A-N12 through microRNA-155/suppressor of cytokine signaling 1 axis. Bioengineered 2022, 13, 2513–2524. [Google Scholar] [CrossRef]
- D’Auria, F.D.; Casciaro, B.; De Angelis, M.; Marcocci, M.E.; Palamara, A.T.; Nencioni, L.; Mangoni, M.L. Antifungal activity of the frog skin peptide temporin G and its effect on Candida albicans virulence factors. Int. J. Mol. Sci. 2022, 23, 6345. [Google Scholar] [CrossRef] [PubMed]
- Cesare, G.B.D.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial Peptides: A New Frontier in Antifungal Therapy. mBio 2020, 11, e02123-20. [Google Scholar] [CrossRef]
- Sara, M.; Yasir, M.; Kalaiselvan, P.; Hui, A.; Kuppusamy, R.; Kumar, N.; Chakraborty, S.; Yu, T.T.; Wong, E.H.H.; Molchanova, N.; et al. The activity of antimicrobial peptoids against multidrug-resistant ocular pathogens. Cont. Lens Anterior Eye 2024, 47, 102124. [Google Scholar] [CrossRef] [PubMed]
- Bidaud, A.; Djenontin, E.; Botterel, F.; Chowdhary, A.; Dannaoui, E. Colistin interacts synergistically with echinocandins against Candida auris. Int. J. Antimicrob. Agents 2020, 55, 105901. [Google Scholar] [CrossRef] [PubMed]
- Yousfi, H.; Ranque, S.; Rolain, J.-M.; Bittar, F. In vitro polymyxin activity against clinical multidrug-resistant fungi. Antimicrob. Resist. Infect. Control. 2019, 8, 66. [Google Scholar] [CrossRef]
- Schwarz, P.; Nikolskiy, I.; Bidaud, A.-L.; Sommer, F.; Bange, G.; Dannaoui, E. In vitro activity of amphotericin B in combination with colistin against fungi responsible for invasive infections. J. Fungi 2022, 8, 115. [Google Scholar] [CrossRef]
- Carton, J.D.; de-la-Fuente, I.; Sevillano, E.; Jauregizar, N.; Quindós, G.; Eraso, E.; Guridi, A. In Vitro Assessment of Fluconazole and Cyclosporine A Antifungal Activities: A Promising Drug Combination Against Different Candida Species. J. Fungi 2025, 11, 133. [Google Scholar] [CrossRef]
- Cowen, L.E.; Singh, S.D.; Köhler, J.R.; Collins, C.; Zaas, A.K.; Schell, W.A.; Aziz, H.; Mylonakis, E.; Perfect, J.R.; Whitesell, L. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc. Natl. Acad. Sci. USA 2009, 106, 2818–2823. [Google Scholar] [CrossRef]



| Yeasts | Lab Designation | Strain ID | Nature of Strain | Isolation Source | Country of Isolation |
|---|---|---|---|---|---|
| Candida albicans | C. albicans 002 [34,35] | GRI 681 | WHO critical-priority; adherent strain | Cervix from an asymptomatic woman | UK |
| C. albicans 003 [35,36] | Y5499 | WHO critical-priority; strain showing resistance to complement attack but unable to grow in serum | Human saliva | Australia | |
| C. tropicalis | C. tropicalis 001 [35,36,37] | N/A | WHO high-priority; strain aggregating platelets within the shortest time among other non-albicans/auris Candida | Human saliva | Australia |
| C. parapsilosis complex | C. parapsilosis 001 [35,36,37] | Y316 | WHO high-priority; recently recognised as a complex of 4 species: C. parapsilosis, C. orthopsilosis, C. metapsilosis, Lodderomyces elongisporus | Human saliva | Australia |
| Meyerozyma guilliermondii | C. guillermondii 001 [16,36,37,38] | Y324 | This species is constitutively less susceptible to polyenes and echinocandins as compared to other yeast-like fungi | Human saliva | Australia |
| Nakaseomyces glabratus | C. glabrata 001 [8,35] | N/A | WHO high-priority | Human saliva | Australia |
| Pichia kudriavzevii | C. krusei 001 [35,36,37] | Y301 | WHO medium-priority; strong capacity for adhering to polystyrene and acrylic surfaces | Human saliva | Australia |
| Kluyveromyces marxianus | C. kefyr 001 [36,37] | Y83 | High capacity for adhering to buccal mucosa among non-albicans Candida | Human saliva | Australia |
| Candidozyma auris | C. auris 04 [9,35,39] | AR0384 | WHO critical-priority; aggregative strain | Blood | South Africa |
| Antifungal Class | Antifungal | Molecular Weight | Structure |
|---|---|---|---|
| Azoles | Fluconazole [41] | 306.27 g/mol | 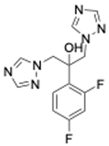 |
| Echinocandins | Caspofungin [42] | 1093.3 g/mol |  |
| Polyenes | Amphotericin B [43] | 924.1 g/mol | 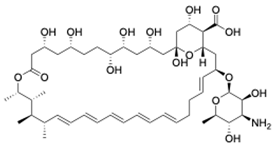 |
| Antibiotic Class | Antibiotic | Molecular Weight | Structure |
| Polymyxin | Colistin [44] | 1155.4 g/mol |  |
| Peptidomimetic | Molecular Weight | Structure | |
| RK758 [25] | 759.71 g/mol |  | |
| TM8 [31,33] | 1115.52 g/mol | 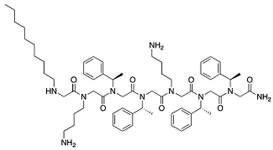 | |
| Candida Species | MIC (µg/mL) | |||||
|---|---|---|---|---|---|---|
| Fluconazole | AmB | Caspofungin | Colistin | TM8 | RK758 | |
| C. albicans 002 | 2 | 4 | 2 | 2000 | 15.6 | 12 |
| C. albicans 003 | 256 | 4 | 2 | 2000 | 15.6 | 24 |
| P. kudriavzevii 001 | 128 | 2 | 2 | 4000 | 7.8 | 12 |
| C. tropicalis 001 | 8 | 0.5 | 2 | 250 | 7.8 | 12 |
| N. glabratus 001 | 8 | 0.25 | 2 | 2000 | 15.6 | 12 |
| M. guilliermondii 001 | 4 | 0.125 | 4 | 2000 | 7.8 | 12 |
| C. parapsilosis 001 | 1 | 0.25 | 8 | 2000 | 7.8 | 48 |
| K. marxianus 001 | 1 | 0.25 | 2 | 2000 | 7.8 | 12 |
| C. auris 04 | 128 | 4 | 1 | 1000 | 31.2 | 12 |
| MIC50 | 8 | 0.5 | 2 | 2000 | 7.8 | 12 |
| MIC90 | 128 | 4 | 8 | 2000 | 31.2 | 24 |
| Geometric mean MIC of the antifungals against Candida spp. | 13.5 | 0.8 | 2.3 | 1587.4 | 11.5 | 15.1 |
| Strains | Antimicrobial Combinations | MIC (µg/mL) | FIC | FICI | Interpretation | ||||
|---|---|---|---|---|---|---|---|---|---|
| Antifungal | Antifungal in Combination with Peptidomimetic/Colistin | Peptidomimetic/Colistin | Peptidomimetic/Colistin in Combination with Antifungal | Antifungal | Peptidomimetic/Colistin | ||||
| C. albicans 002 | Fluco + TM8 | 2 | 0.25 | 15.6 | 3.9 | 0.125 | 0.25 | 0.38 | Synergy |
| Fluco + RK758 | 2 | 0.125 | 12 | 4 | 0.0625 | 0.25 | 0.31 | Synergy | |
| Fluco + Colistin | 2 | 0.125 | 2000 | 62.5 | 0.0625 | 0.031 | 0.094 | Synergy | |
| AmB + TM8 | 4 | 0.002 | 15.6 | 15.6 | 0.0005 | 1 | 1.0005 | Indifference | |
| AmB + RK758 | 4 | 0.125 | 12 | 6 | 0.031 | 0.5 | 0.53 | P. Synergy | |
| AmB + Colistin | 4 | 4 | 2000 | 2000 | 1 | 1 | 2 | Indifference | |
| Caspo + TM8 | 2 | 0.25 | 15.6 | 3.9 | 0.125 | 0.25 | 0.38 | Synergy | |
| Caspo + RK758 | 2 | 0.5 | 12 | 3 | 0.25 | 0.25 | 0.50 | Synergy | |
| Caspo + Colistin | 2 | 2 | 2000 | 250 | 1 | 0.125 | 1.13 | Indifference | |
| C. albicans 003 | Fluco + TM8 | 256 | 2 | 15.6 | 3.9 | 0.0078 | 0.25 | 0.26 | Synergy |
| Fluco + RK758 | 256 | 1 | 24 | 6 | 0.0039 | 0.25 | 0.25 | Synergy | |
| Fluco + Colistin | 256 | 256 | 2000 | 2000 | 1 | 1 | 2 | Indifference | |
| AmB + TM8 | 4 | 1 | 15.6 | 0.98 | 0.25 | 0.063 | 0.31 | Synergy | |
| AmB + RK758 | 4 | 2 | 24 | 6 | 0.5 | 0.25 | 0.75 | P. Synergy | |
| AmB + Colistin | 4 | 4 | 2000 | 2000 | 1 | 1 | 2 | Indifference | |
| Caspo + TM8 | 2 | 0.25 | 15.6 | 3.9 | 0.125 | 0.25 | 0.38 | Synergy | |
| Caspo + RK758 | 2 | 0.5 | 24 | 6 | 0.25 | 0.25 | 0.50 | Synergy | |
| Caspo + Colistin | 2 | 2 | 2000 | 1000 | 1 | 0.5 | 1.50 | Indifference | |
| P. kudriavzevii 001 | Fluco + TM8 | 128 | 2 | 7.8 | 3.9 | 0.016 | 0.5 | 0.52 | P. Synergy |
| Fluco + RK758 | 128 | 16 | 12 | 3 | 0.125 | 0.25 | 0.38 | Synergy | |
| Fluco + Colistin | 128 | 32 | 4000 | 1000 | 0.25 | 0.25 | 0.50 | Synergy | |
| AmB + TM8 | 2 | 0.25 | 7.8 | 1.95 | 0.125 | 0.25 | 0.38 | Synergy | |
| AmB + RK758 | 2 | 2 | 12 | 12 | 1 | 1 | 2 | Indifference | |
| AmB + Colistin | 2 | 1 | 4000 | 1000 | 0.5 | 0.25 | 0.75 | P. Synergy | |
| Caspo + TM8 | 2 | 1 | 7.8 | 1.95 | 0.5 | 0.25 | 0.75 | P. Synergy | |
| Caspo + RK758 | 2 | 1 | 12 | 6 | 0.5 | 0.5 | 1 | Additive | |
| Caspo + Colistin | 2 | 2 | 4000 | 4000 | 1 | 1 | 2 | Indifference | |
| C. tropicalis 001 | Fluco + TM8 | 8 | 0.5 | 7.8 | 3.9 | 0.063 | 0.5 | 0.56 | P. Synergy |
| Fluco + RK758 | 8 | 0.031 | 12 | 6 | 0.0039 | 0.5 | 0.504 | P. Synergy | |
| Fluco + Colistin | 8 | 0.015 | 250 | 62.5 | 0.0019 | 0.25 | 0.25 | Synergy | |
| AmB + TM8 | 0.5 | 0.125 | 7.8 | 1.95 | 0.25 | 0.25 | 0.50 | Synergy | |
| AmB + RK758 | 0.5 | 0.25 | 12 | 0.75 | 0.5 | 0.063 | 0.56 | P. Synergy | |
| AmB + Colistin | 0.5 | 0.25 | 250 | 62.5 | 0.5 | 0.25 | 0.75 | P. Synergy | |
| Caspo + TM8 | 2 | 0.5 | 7.8 | 1.95 | 0.25 | 0.25 | 0.5 | Synergy | |
| Caspo + RK758 | 2 | 1 | 12 | 3 | 0.5 | 0.25 | 0.75 | P. Synergy | |
| Caspo + Colistin | 2 | 0.06 | 250 | 7.8 | 0.03 | 0.031 | 0.061 | Synergy | |
| N. glabratus 001 | Fluco + TM8 | 8 | 4 | 15.6 | 7.8 | 0.5 | 0.5 | 1 | Additive |
| Fluco + RK758 | 8 | 2 | 12 | 6 | 0.25 | 0.5 | 0.75 | P. Synergy | |
| Fluco + Colistin | 8 | 8 | 2000 | 2000 | 1 | 1 | 2 | Indifference | |
| AmB + TM8 | 0.25 | 0.125 | 15.6 | 7.8 | 0.5 | 0.5 | 1 | Additive | |
| AmB + RK758 | 0.25 | 0.125 | 12 | 6 | 0.5 | 0.5 | 1 | Additive | |
| AmB + Colistin | 0.25 | 0.125 | 2000 | 2000 | 0.5 | 1 | 1.5 | Indifference | |
| Caspo + TM8 | 2 | 0.031 | 15.6 | 7.8 | 0.016 | 0.5 | 0.52 | P. Synergy | |
| Caspo + RK758 | 2 | 2 | 12 | 12 | 1 | 1 | 2 | Indifference | |
| Caspo + Colistin | 2 | 0.5 | 2000 | 31.25 | 0.25 | 0.016 | 0.27 | Synergy | |
| M. guilliermondii 001 | Fluco + TM8 | 4 | 1 | 7.8 | 1.95 | 0.25 | 0.25 | 0.5 | Synergy |
| Fluco + RK758 | 4 | 0.5 | 12 | 3 | 0.125 | 0.25 | 0.375 | Synergy | |
| Fluco + Colistin | 4 | 4 | 2000 | 2000 | 1 | 1 | 2 | Indifference | |
| AmB + TM8 | 0.125 | 0.03125 | 7.8 | 3.9 | 0.25 | 0.5 | 0.75 | P. Synergy | |
| AmB + RK758 | 0.125 | 0.0625 | 12 | 6 | 0.5 | 0.5 | 1 | Additive | |
| AmB + Colistin | 0.125 | 0.0625 | 2000 | 2000 | 0.5 | 0.5 | 1 | Additive | |
| Caspo + TM8 | 4 | 0.25 | 7.8 | 3.9 | 0.0625 | 0.5 | 0.56 | P. Synergy | |
| Caspo + RK758 | 4 | 0.25 | 12 | 6 | 0.0625 | 0.5 | 0.56 | P. Synergy | |
| Caspo + Colistin | 4 | 1 | 2000 | 250 | 0.25 | 0.125 | 0.38 | Synergy | |
| C. parapsilosis 001 | Fluco + TM8 | 1 | 0.016 | 7.8 | 3.9 | 0.016 | 0.5 | 0.52 | P. Synergy |
| Fluco + RK758 | 1 | 0.25 | 48 | 6 | 0.25 | 0.125 | 0.38 | Synergy | |
| Fluco + Colistin | 1 | 0.5 | 2000 | 250 | 0.5 | 0.125 | 0.63 | P. Synergy | |
| AmB + TM8 | 0.25 | 0.0625 | 7.8 | 1.95 | 0.25 | 0.25 | 0.5 | Synergy | |
| AmB + RK758 | 0.25 | 0.125 | 48 | 12 | 0.50 | 0.25 | 0.75 | P. Synergy | |
| AmB + Colistin | 0.25 | 0.0625 | 2000 | 500 | 0.25 | 0.25 | 0.5 | Synergy | |
| Caspo + TM8 | 8 | 0.0039 | 7.8 | 7.8 | 0.0005 | 1 | 1 | Indifference | |
| Caspo + RK758 | 8 | 4 | 48 | 12 | 0.5 | 0.25 | 0.75 | P. Synergy | |
| Caspo + Colistin | 8 | 0.125 | 2000 | 500 | 0.016 | 0.25 | 0.27 | Synergy | |
| K. marxianus 001 | Fluco + TM8 | 1 | 0.25 | 7.8 | 3.9 | 0.25 | 0.5 | 0.75 | P. Synergy |
| Fluco + RK758 | 1 | 0.25 | 12 | 3 | 0.25 | 0.25 | 0.5 | Synergy | |
| Fluco + Colistin | 1 | 1 | 4000 | 4000 | 1 | 1 | 2 | Indifference | |
| AmB + TM8 | 0.25 | 0.0625 | 7.8 | 1.95 | 0.25 | 0.25 | 0.5 | Synergy | |
| AmB + RK758 | 0.25 | 0.125 | 12 | 3 | 0.5 | 0.25 | 0.75 | P. Synergy | |
| AmB + Colistin | 0.25 | 0.0625 | 4000 | 2000 | 0.25 | 0.5 | 0.75 | P. Synergy | |
| Caspo + TM8 | 2 | 0.5 | 7.8 | 1.95 | 0.25 | 0.25 | 0.5 | Synergy | |
| Caspo + RK758 | 2 | 0.25 | 12 | 1.5 | 0.125 | 0.125 | 0.25 | Synergy | |
| Caspo + Colistin | 2 | 1 | 4000 | 2000 | 0.5 | 0.5 | 1 | P. Synergy | |
| C. auris 04 | Fluco + TM8 | 128 | 16 | 31.2 | 7.8 | 0.125 | 0.25 | 0.38 | Synergy |
| Fluco + RK758 | 128 | 32 | 12 | 3 | 0.25 | 0.25 | 0.5 | Synergy | |
| Fluco + Colistin | 128 | 64 | 4000 | 1000 | 0.5 | 0.25 | 0.75 | P. Synergy | |
| AmB + TM8 | 4 | 1 | 31.2 | 7.8 | 0.25 | 0.5 | 0.5 | Synergy | |
| AmB + RK758 | 4 | 0.25 | 12 | 12 | 0.0625 | 1 | 1.06 | Indifference | |
| AmB + Colistin | 4 | 4 | 4000 | 4000 | 1 | 1 | 2 | Indifference | |
| Caspo + TM8 | 1 | 0.5 | 31.2 | 7.8 | 0.5 | 0.25 | 0.75 | P. Synergy | |
| Caspo + RK758 | 1 | 0.25 | 12 | 6 | 0.25 | 0.5 | 0.75 | P. Synergy | |
| Caspo + Colistin | 1 | 0.5 | 4000 | 2000 | 0.5 | 0.5 | 1 | Additive | |
| Yeasts | Fold-Change in MIC (Increment) | |||
|---|---|---|---|---|
| TM8 | RK758 | |||
| Ergosterol (200 µg/mL) | Ergosterol (400 µg/mL) | Ergosterol (200 µg/mL) | Ergosterol (400 µg/mL) | |
| C. albicans 002 | 4 | 4 | 4 | 4 |
| P. kudriavzeviii 001 | 8 | 8 | 4 | 4 |
| C. tropicalis 001 | 8 | 4 | 4 | 4 |
| N. glabratus 001 | 4 | 4 | 8 | 4 |
| M. guilliermondii 001 | 8 | 8 | 4 | 4 |
| C. parapsilosis 001 | 4 | 4 | 4 | 4 |
| C. auris 04 | 4 | 4 | 4 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mishra, S.K.; Kuppusamy, R.; Nguyen, C.; Doeur, J.; Atwal, H.; Attard, S.; Sørensen, K.; Lin, J.S.; Wong, E.H.H.; Hui, A.; et al. Evaluation of the Synergistic Activity of Antimicrobial Peptidomimetics or Colistin Sulphate with Conventional Antifungals Against Yeasts of Medical Importance. J. Fungi 2025, 11, 370. https://doi.org/10.3390/jof11050370
Mishra SK, Kuppusamy R, Nguyen C, Doeur J, Atwal H, Attard S, Sørensen K, Lin JS, Wong EHH, Hui A, et al. Evaluation of the Synergistic Activity of Antimicrobial Peptidomimetics or Colistin Sulphate with Conventional Antifungals Against Yeasts of Medical Importance. Journal of Fungi. 2025; 11(5):370. https://doi.org/10.3390/jof11050370
Chicago/Turabian StyleMishra, Shyam Kumar, Rajesh Kuppusamy, Christina Nguyen, Jennifer Doeur, Harleen Atwal, Samuel Attard, Kristian Sørensen, Jennifer S. Lin, Edgar H. H. Wong, Alex Hui, and et al. 2025. "Evaluation of the Synergistic Activity of Antimicrobial Peptidomimetics or Colistin Sulphate with Conventional Antifungals Against Yeasts of Medical Importance" Journal of Fungi 11, no. 5: 370. https://doi.org/10.3390/jof11050370
APA StyleMishra, S. K., Kuppusamy, R., Nguyen, C., Doeur, J., Atwal, H., Attard, S., Sørensen, K., Lin, J. S., Wong, E. H. H., Hui, A., Barron, A. E., Kumar, N., & Willcox, M. (2025). Evaluation of the Synergistic Activity of Antimicrobial Peptidomimetics or Colistin Sulphate with Conventional Antifungals Against Yeasts of Medical Importance. Journal of Fungi, 11(5), 370. https://doi.org/10.3390/jof11050370









