Abstract
Russula, a genus of Basidiomycetes with considerable taxonomic diversity, holds significant potential in both traditional and modern medicinal practices. This comprehensive review explores the bioactive compounds identified in various Russula species, detailing their characterization, structural elucidation, and classification. The medicinal properties of these fungi are examined, with a focus on their antioxidant, anti-inflammatory, and immunomodulatory effects, supported by both historical usage and contemporary preclinical pharmacological research. The review also highlights emerging biotechnological applications including environmental remediation, antimicrobial agents, and functional food development. Safety and toxicological considerations are evaluated to provide a balanced perspective on the medicinal use of Russula. The review concludes by summarizing the key findings and emphasizing the importance of Russula in both traditional medicine and future clinically validated innovations.
1. Introduction
The family Russulaceae [] comprises a diverse group of fungi, including forms such as agaricoid, secotioid, pleurotoid, and gasteroid, which are well known for forming ectomycorrhizal relationships with a variety of host plants and have a worldwide distribution [,,,,,]. Molecular phylogenetic studies confirm that Russulaceae is a monophyletic group consisting of seven genera: Russula, Lactarius, Lactifluus, Boidinia, Multifurca Gloeopeniophorella, and Pseudoxenasma [,,]. Among these, Russula Pers. is the most dominant genus [,]. There are 3218 published species names under Russula (www.indexfungorum.org, accessed on 8 April 2025), reflecting the vast diversity and ongoing discovery of new species within this genus. This genus is characterized by its fleshy fruiting bodies, colorful and fragile caps, amyloid warty basidiospores, abundant sphaerocysts in the trama, and lack of latex and clamp connections in the hyphae [,,,,]. Russula species are commonly found in tropical and subtropical evergreen forests but have a broad distribution from Western Europe to North America, Africa, and various Asian countries, especially in China and India [,,,,,,,,,,,,,,,,,,,,,].
The genus Russula plays a significant ecological role as ectomycorrhizal fungi that form symbiotic relationships with trees and contribute to nutrient cycling in forest ecosystems [,,,,,,]. Traditional taxonomic classification has been based on morphological characteristics such as cap color, gill attachment, and spore print color, as well as the chemical reactions of different parts of the fruiting body, which are important taxonomic characters in Russula [,,,]. However, these features exhibit high variability and overlap between species, creating challenges for accurate identification [,]. Recent progress in molecular phylogenetics has provided deeper insights into the evolutionary relationships within Russula, leading to species reclassifications and the discovery of previously cryptic species [,,,]. The incorporation of molecular data has greatly improved the understanding of genetic diversity, evolutionary history, and ecological functions in this genus [,,,,,].
Many Russula species are highly valued for their edibility, with some, such as R. cyanoxantha and R. virescens, being globally renowned; in contrast, others, including R. griseocarnosa and R. adusta, hold regional commercial importance [,,,] (Figure 1, Table 1). Russula have long held historical and cultural significance, especially in Europe, Asia, and Eastern Europe, where they have been used in traditional cuisine and medicine for centuries [,,,,]. China has a long history of using various Russula species as traditional foods and medicines [,,,,]. Valued for their unique flavors, they are integral to regional dishes and are also employed in treating ailments such as indigestion and inflammation. In addition, they are nutritionally rich, providing carbohydrates, proteins, fiber, vitamins, and essential minerals, further contributing to their dietary and medicinal significance [,,,]. Extracts from these mushrooms have been shown to reduce inflammation and oxidative stress in various studies. These medicinal extracts are often utilized in various forms, including teas, tinctures, and capsules []. Beyond culinary and medicinal uses, these mushrooms have been symbolically significant in various cultures, representing fertility and prosperity, and have inspired artistic expressions and folklore due to their distinctive appearance []. Russula are processed into dietary supplements and nutraceuticals for their bioactive compounds, which offer immune-boosting and anti-aging benefits due to their rich content of antioxidants, vitamins, and minerals. In addition, Russula extracts are used in cosmetic products for their antioxidant properties, helping protect the skin from oxidative stress, improve hydration, reduce wrinkles, and enhance elasticity, making them valuable in anti-aging skincare [,].

Figure 1.
Russula species (a) Russula adusta (b) R. albonigra (c) R. alutacea (d) R. cyanoxantha (e) R. griseocarnosa (f) R. virescens (g) R. delica (h) R. foetens (https://www.inaturalist.org/; accessed on 10 March 2025, the images are used under the license Attribution Non-Commercial-No Derivs 4.0).

Table 1.
Edibility status of Russula species documented in this review.
The bioactive compounds in Russula demonstrate significant therapeutic potential due to their distinctive secondary metabolite profiles. These characteristic combinations of compounds—while sharing structural motifs with other fungi—exhibit marked antioxidant, anti-inflammatory, antimicrobial, and immunomodulatory activities, underscoring the pharmacological importance of this genus [,,,,]. As research continues, these compounds are being explored for their potential in drug discovery and natural product development, with advancements in biotechnological techniques further enhancing the ability to harness these properties for various applications. While Russula mushrooms are generally safe for consumption, it is important to consider potential toxicity, as some species contain harmful compounds [,]. Toxicological studies are essential to establish guidelines for safe use, especially as these mushrooms are increasingly integrated into modern medicine and biotechnology. Ongoing research into their medicinal potential, combined with a focus on safety, ensures that Russula will continue to be valuable both in traditional practices and in innovative scientific applications.
This comprehensive understanding of Russula, encompassing their taxonomical classification, ecological significance, and diverse applications, underscores their multifaceted value. Their integration into traditional and modern practices highlights both their cultural importance and potential in scientific research. As exploration into their bioactive compounds and medicinal properties continues, Russula stands as a vital resource for future biotechnological advancements and therapeutic developments.
2. Bioactive Compounds and Beneficial Medicinal Properties of Russula
Russula is enriched with several species like R. alboareolata, R. alutacea, R. helios, R. medullata, R. monspeliensis, and R. virescens, all of which are widely consumed as foods [,,]. In addition to their edibility, species of Russula are also used as traditional medicines for the treatment of various diseases like fever (R. cyanoxantha and R. nobilis), wound healing (R. luteotacta), treating gastritis and high blood pressure (e.g., R. delica and R. parazurea), and even in skin cancer (R. acrifolia) []. Moreover, some Russula species have also been traditionally used as tonics such as R. acrifolia, R. cyanoxantha, R. delica, R. luteotacta, R. nobilis, and R. parazurea []. In addition, R. luteotacta is also used as a sleep-promoting agent []. Apart from all this usefulness, there are reports about side effects and toxicities of some Russula species as well []. Russula densifolia, R. fragtissima, and R. rosacea can cause gastroenteritis, while R. olivacea can cause nausea, vomiting, and diarrhea []. Russula subnigricans can cause rhabdomyolysis, severe electrolyte disturbance (hypocalcemia), respiratory failure, acute renal failure, pulmonary edema, ventricular tachycardia, and circulatory shock []. Few species of Russula are poisonous, like R. emetica and R. nigricans []. Moreover, the consumption of R. risigallina, R. olivacea, and R. velenovskyi may poseharmful effects due to exceedingly elevated levels of Cr and Cd, as compared to reference safety limits []. In spite of the large number of taxa, the secondary metabolites of Russula have not been well researched. However, in the following part of this paper, we discuss various bioactive compounds (Figure 2 and Figure 3) produced by Russula species and their beneficial medicinal properties.
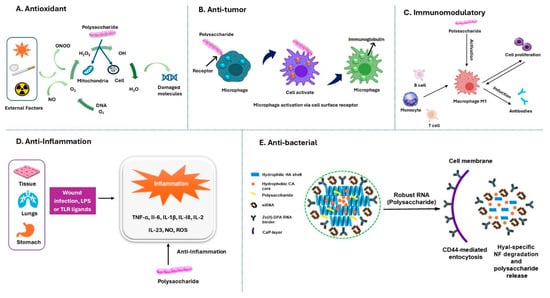
Figure 2.
Bioactive properties and mechanism of polysaccharides from Russula []. (A) Antioxidant: polysaccharides scavenge ROS, protecting cells from oxidative damage. (B) Anti-tumor: they activate macrophages, enhancing the immune response. (C) Immunomodulatory: they stimulate immune cells, boosting antibody production and cell proliferation. (D) Anti-inflammatory: they reduce cytokines (TNF-α, IL-6, and IL-1β) and oxidative stress. (E) Anti-bacterial: they facilitate bacterial RNA targeting and membrane degradation via CD44-mediated endocytosis.
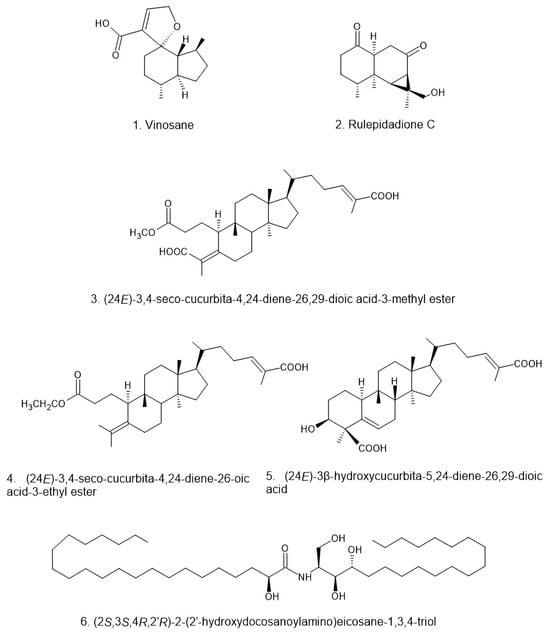
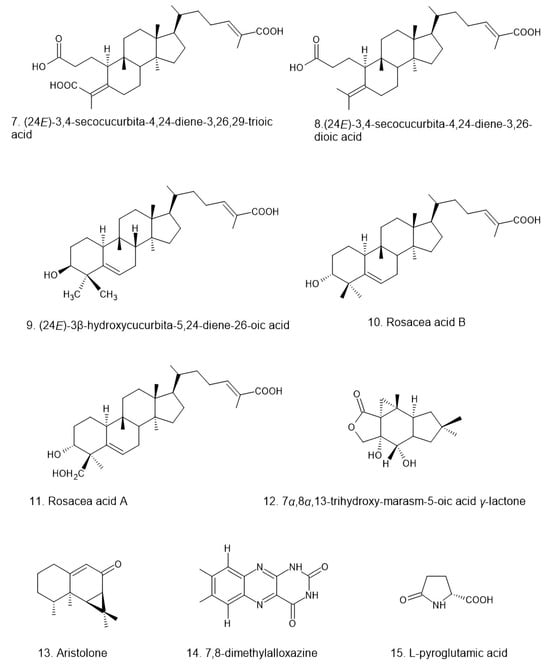
Figure 3.
Structural diversity of triterpenoids isolated from Russula, including seco-cucurbitane and lanostane derivatives []. These triterpenoids exhibit structural variations in oxidation patterns, side-chain modifications, and ring configurations, which may contribute to their diverse bioactivities.
2.1. Bioactive Compounds
2.1.1. Polysaccharides
Russula alatoreticula
A water-soluble, polysaccharide-rich extract (Rusalan) was isolated from the dried basidiocarps of R. alatoreticula, showing a triple helical conformation with glucose as the main monosaccharide. Rusalan exhibited strong antioxidant activity and effectively inhibited the growth of Staphylococcus aureus and Bacillus subtilis. In addition, it demonstrated immune-stimulatory effects in mouse macrophage cells []. The anti-oxidant activity of Rusalan has been demonstrated through its significant potential in scavenging hydroxyl, superoxide, and DPPH radicals, its metal ion chelating ability, and its capacity to donate electrons []. The extract also inhibits the growth of certain pathogenic bacteria []. Moreover, the crude polysaccharide showed high immune-modulatory activity without cytotoxicity []. Hence, Rusalan could be used as an ingredient for pharmaceutical use against free radicals, antibiotic-resistant pathogens, and hypo-immunity []. A β-glucan-enriched fraction, RualaCap, from the residue of R. alatoreticula after hot-water extraction, exhibited strong antioxidant and immune-enhancing properties. RualaCap demonstrated significant radical scavenging, chelating ability, and increased macrophage activity, along with the activation of key immune-related genes. These findings suggest its potential as a potent nutraceutical for immune stimulation []. Russula alatoreticula is rich in phenolic compounds and ascorbic acid, contributing to its strong antioxidant, antibacterial, and anticancer properties. The methanol extract demonstrated significant free radical quenching, Fe2+ ion chelation, and antibacterial activity, as well as apoptosis induction in Hep3B cells via the mitochondrial pathway. This suggests R. alatoreticula has substantial potential as a natural supplement for combating free radicals, pathogens, and hepatocellular carcinoma, with applications in food safety [].
Russula albonigra
The water-soluble polysaccharide fraction of R. albonigra was found to inhibit the replication of intracellular amastigotes in macrophages dose-dependently and revealed its anti-proliferating effect []. A water-soluble β-glucan was isolated from the alkaline extract of the ectomycorrhizal edible mushroom R. albonigra. This compound showed in vitro macrophage activation via NO production and splenocyte and thymocyte proliferation. Furthermore, it has exhibited potent antioxidant activities []. Moreover, a water-soluble glucan [] and a heteroglycan were isolated from the aqueous extract of R. albonigra []. These compounds showed in vitro macrophage activation via NO production, splenocytes, and thymocyte proliferation [].
Russula alutacea
Russula alutacea polysaccharides are alkali-soluble but water-insoluble, limiting their use. Acetylation improved their solubility and enhanced antioxidant activity. Both acetylated and water-soluble polysaccharides, along with vitamin C, showed strong superoxide anion scavenging, with acetylated polysaccharides having the best DPPH radical-scavenging ability []. Russula alutacea polysaccharides were chemically modified to enhance water solubility and biological activity. The sulfated polysaccharides showed the highest scavenging activity for hydroxyl radicals, while vitamin C was more effective against superoxide anions and DPPH radicals []. A purified polysaccharide isolated from R. alutacea significantly reduced cell morphological changes and nitric oxide (NO) production in LPS-induced RAW 264.7 cells, both extracellularly and intracellularly. It down-regulated NF-κB, iNOS, and COX-2 expression, and alleviated oxidative stress and mitochondrial dysfunction through MAPK signaling pathways. Thus, R. alutacea is potentially a resource for protecting against inflammatory and oxidative damage [].
Russula griseocarnosa
Polysaccharides from R. griseocarnosa demonstrate significant scavenging effects on superoxide anions and hydroxyl radicals, contributing to their strong antioxidative properties []. Alcohol extracts from R. griseocarnosa fruit bodies have been tested and shown to possess antibacterial properties against Escherichia coli and S. aureus []. RGP2, a polysaccharide from R. griseocarnosa, improved immune function in cyclophosphamide-induced immunosuppressed mice. With a molecular weight of 11.82 kDa, RGP2 enhanced spleen health, altered gut microbiota and serum metabolites, and boosted immune responses by affecting macrophages and T cells via the AKT/mTOR pathway []. Polysaccharides from R. griseocarnosa fruit bodies effectively neutralize hydroxyl and superoxide radicals. In vitro studies demonstrate that these polysaccharides significantly inhibit the proliferation of HeLa and SiHa cancer cells and enhance the phagocytic activity of peritoneal macrophages in mice, which, in turn, boosts the secretion of NO and cytokine IL-6, showcasing robust immunomodulatory properties []. Polysaccharide from R. griseocarnosa (PRG1-1) was found to enhance macrophage activation by increasing iNOS, COX-2, nitric oxide, and cytokine production. These effects are mediated through the NF-κB and MAPK signaling pathways, highlighting its potential as an immunomodulator [].
RGP1, a polysaccharide from R. griseocarnosa, was shown to improve hematopoietic function in K562 cells. In mice with cyclophosphamide-induced hematopoietic dysfunction, RGP1 reduced bone-marrow damage, increased long-term hematopoietic stem cells, and regulated myeloid cells in the blood. It promoted CD4+ T-cell differentiation without affecting other immune cells. RGP1’s benefits were linked to CD4+ T-cell activation and the Janus kinase/STAT3 pathway, supporting its potential for treating hematopoietic dysfunction []. RGP2, a polysaccharide from R. griseocarnosa, improved immune function in cyclophosphamide-induced immunosuppressed mice. With a molecular weight of 11.82 kDa, RGP2 enhanced spleen health, altered gut microbiota and serum metabolites, and boosted immune responses by affecting macrophages and T-cells via the AKT/mTOR pathway []. RGP1, a galactan from R. griseocarnosa, alleviated CTX-induced hematopoietic dysfunction by reducing bone marrow damage, increasing stem cell numbers, and promoting CD4+ T-cell differentiation via the JAK/STAT3 pathway. Its structure features a 1,6-α-D-Galp backbone with O-3 methylation and α-L-Fucp branching []. Polysaccharides from wild R. griseocarnosa (PRG) exhibited antioxidant activities evidenced by reducing power to scavenge the DPPH, ABTS, hydroxyl radical, and superoxide radical [,]. PRG showed the activity of anti-cervical carcinoma cells Hela and Siha. Hence, PRG has good antioxidant and inhibitory activities against cervical carcinoma cells, and PRG could be developed as a novel natural functional food []. A novel polysaccharide, PRG1-1, was obtained from R. griseocarnosa sporocarp and the cytotoxicity effects of PRG1-1 on human cervical carcinoma are associated with the apoptotic pathway. Hence, R. griseocarnosa showed a promising potential of bioactive PRG1-1 as a natural agent to inhibit tumor cell proliferation in the treatment of cervical carcinoma []. PRG1-1 also has the ability to activate macrophages through the NF-κB and MAPK pathways, demonstrating significant immunomodulatory potential [].
Russula pseudocyanoxantha
Russula pseudocyanoxantha, identified for its bioactive polysaccharides, was further explored by utilizing the solid remnants of conventional extraction processes, which contain therapeutic biopolymers. These were treated with cold alkali to yield a high-yield fraction (RP-CAP), characterized as having a β-glucan-rich carbohydrate backbone with a molecular weight of ~129.28 kDa. RP-CAP exhibited strong antioxidant and immune-boosting activities, promoting macrophage proliferation and inflammatory mediator synthesis through the TLR/NF-κB pathway. These findings suggest RP-CAP’s potential in functional foods and pharmaceuticals []. Russula pseudocyanoxantha has yielded a bioactive-rich fraction (RP-HAP) through extraction with a hot alkali solution. This β-glucan-enriched extract, with a molecular weight of approximately 111.25 kDa, demonstrated significant antioxidant activity and enhanced immune responses in RAW264.7 macrophage cells, indicating its potential for developing health-promoting pharmaceuticals [].
Russula virescens
Water-insoluble (1→3)-β-D-glucan was firstly isolated from the fresh fruiting bodies of Russula virescens and then sulfated using sulfur trioxide–pyridine complex. The native (1→3)-β-D-glucan did not show anti-tumor activity, while the sulfated derivatives exhibited enhanced anti-tumor activities against sarcoma 180 tumor cells []. Russula virescens was studied for its water-soluble polysaccharides, RVP-1 and RVP-2. These non-triple-helix hetero-polysaccharides, composed of galactose, glucose, mannose, and fructose, exhibited promising antidiabetic, anticancer, and immunological activities. RVP-1 and RVP-2 were found to inhibit α-glucosidase and α-amylase activities, suppress cancer cell proliferation, and activate immune responses, providing a scientific basis for their potential therapeutic use []. Sulfonic acid groups (–SO3H) can be incorporated into polysaccharide molecules by replacing hydroxyl groups (–OH) through a process called sulfonation. This modification improves the interaction between sulfated polysaccharides from R. virescens and bacterial receptors, enhancing recognition and binding by altering the polysaccharide’s spatial structure and conformation []. Sulfated derivatives of the water-soluble polysaccharide from R. virescens (RVP) were prepared with varying degrees of substitution, resulting in compounds with altered molecular weights and conformations. These sulfated polysaccharides showed enhanced antioxidant, anticoagulant, antibacterial, and anti-tumor activities compared to the non-sulfated RVP, with SRVP1–25 exhibiting the strongest scavenging and anticoagulant effects, and SRVP1–20 demonstrating the best antibacterial and anti-tumor properties []. RVP, a water-soluble galactoglucomannan from R. virescens, was extracted using an alkali method and adopts a semi-rigid triple-helix structure. It has a low protein content (0.95%) and a molecular weight of 8.91 × 105. RVP showed strong antioxidant activity by increasing cell viability, reducing malondialdehyde (MDA) levels, and enhancing antioxidant enzyme activity (SOD, CAT, and GSH-Px) in H2O2-induced oxidative stress models. These properties highlight its potential for health and wellness applications []. Furthermore, water-soluble polysaccharides from fruiting bodies of R. virescens could be developed as potential anti-oxidant, anti-coagulant, anti-bacterial, and anti-tumor agents for industrial and biomedical use [].
Russula vinosa
Polysaccharides derived from R. vinosa have been demonstrated to boost lymphocyte activity and suppress the growth of SiHa cancer cells []. Russula vinosa exhibited higher β-glucan levels when assessed using the Congo red method compared to many other wild and commercial mushrooms, highlighting its potential as a valuable source of β-glucans for use in the food industry and medicinal purposes []. The water-soluble and alkali-soluble polysaccharides from R. vinosa showed antioxidant and hepatoprotective effects in vitro []. Russula vinosa has been consumed as a food in South China for a long time. Furthermore, plant growth regulator compounds were also isolated from the fruiting bodies of R. vinosa []. This mushroom contains β-glucans, which show immunomodulatory, anticancer, and antioxidant activities []. The acid extracts of R. vinosa demonstrated the highest ABTS (+) scavenging activity []. Furthermore, R. vinosa extracts inhibited the proliferation of HeLa and HepG2 cells in a dose-dependent manner. These results indicate that R. vinosa polysaccharides have potential antioxidant activity []. Russula vinosa was investigated for its anti-inflammatory effects using two polysaccharides (CA-S and CA-L) extracted with citric acid. Both showed similar structures but differed in substitution. CA-S and CA-L reduced disease activity index (DAI) values in ulcerative colitis mice by 36.84% and 31.58%, respectively. CA-S, with a higher molecular weight and more hydroxyl groups, was more effective in reducing inflammation through reactive oxygen species scavenging and the modulation of the Nrf2 and NFκB pathways []. Russula vinosa Lindblad is a carbohydrate-rich edible fungus. Two polysaccharides, RP-1 and RP-5, were extracted using KOH-graded extraction, with RP-5 showing a stronger immunomodulatory effect. A structural analysis revealed β-d-glucopyranose linkages, with RP-5 containing an additional mannopyranose residue. RP-5 enhanced macrophage phagocytosis by 121.04%, compared to 42.15% for RP-1, and both activated the NF-κB pathway, highlighting their potential as bioactive compounds [].
Russula adusta, R. aurea, R. delica, R. emetica, and R. senecis
The polysaccharide named RAP was purified and characterized from R. adusta, showing a molecular weight of 5763 Da, with 80.03% total sugar, 0.17% protein, and 13.20% uronic acid. RAP primarily contained rhamnose, fucose, mannose, glucose, and galactose. RAP exhibited antioxidant activity, scavenging ·OH more effectively than O2−·, with activity increasing with concentration (0.25–8 mg/mL) []. Polysaccharides extracted from the mycelial culture of R. aurea inhibited the growth of sarcoma 180 and Ehrlich solid cancers by 70% and 60% in white mice, respectively []. Other research indicated that the water-insoluble (1–3)-β-D-glucan, isolated from the fresh fruiting bodies, did not show anti-tumor activity, whilst the sulfated derivatives exhibited enhanced anti-tumor properties []. The water-soluble polysaccharides also have antioxidant properties []. However, a study included R. aurea and R. sanguinea, but neither exhibited significant antioxidant, enzyme inhibitory, or antimutagenic activities [].
Leishmania donovani is the causative agent of visceral leishmaniasis or kala azar in the Indian subcontinent []. The water-soluble polysaccharide fraction of R. delica was found to inhibit the replication of intracellular amastigotes in macrophages dose-dependently, and it showed its anti-proliferating effect []. Hence, this finding could be used in further phytochemical and pharmacological investigations in search of novel anti-leishmanial leads []. Russula emetica polysaccharides extracted via the ultrasound-assisted extraction method showed high anti-diabetic and antihypertensive activities []. Bioactive polysaccharides from Rugibolutus extremiorientalis, R. emetica, and Phleobopus portentosus were extracted using refluxing and ultrasound-assisted methods. Russula extremiorientalis polysaccharides had the highest antioxidant activity, while R. emetica showed the strongest antidiabetic and antihypertensive effects. The polysaccharides mainly contained carbohydrates, proteins, and glucose, with R. extremiorientalis and R. emetica having β-glycosidic linkages and P. portentosus having both α- and β-glycosidic linkages []. The crude polysaccharide Rusenan was extracted from R. senecis, and it can be used as a potent free-radical scavenger and murine macrophage stimulator []. Moreover, as for antioxidant activity, the crude polysaccharide exhibited strong potential in scavenging superoxide radicals, inhibiting OH generation, stabilizing DPPH, quenching ABTS radicals, inhibiting β-carotene bleaching, and demonstrating reducing power and Fe2+chelating ability []. Rusenan also exhibited strong immune-stimulation activities at low concentrations and initiated innate immunity by promoting macrophage proliferation, phagocytosis, morphological changes, NO release, ROS production, and the transcription of TLR-4, TLR-2, NF-κB, COX-2, iNOS. Its effects were comparable to those of standard antioxidant drugs []. The administration of Russula powder and polysaccharides has been found to significantly lower blood glucose, total cholesterol, triglycerides, and low-density lipoprotein levels in hyperglycemic and hyperlipidemic mice, demonstrating a dose-dependent effect and highlighting Russula’s potent hypoglycemic and lipid-lowering properties. Similarly, polysaccharide injections from Russula in hyperlipidemic rats led to a 45.2% reduction in total cholesterol compared to the control group []. The phytochemical analysis of Cheimonophyllum candidissimus, Pleurotus sp., Russula sp., and Auricularia sp. revealed bioactive compounds such as alkaloids, tannins, phenols, saponins, and flavonoids. These mushrooms also contained significant levels of protein, fats, fiber, carbohydrates, and essential minerals. However, high concentrations of heavy metals like cadmium, zinc, lead, and copper were found, which could be harmful if consumed in large amounts []. Table 2 lists polysaccharides from various Russula species and their associated biological activities.

Table 2.
Polysaccharides from various Russula species and their associated biological activities.
2.1.2. Terpenes
Russula amarissima, R. brevipes, and R. cyanoxantha
Four aristolane sesquiterpenes were isolated from the fruiting bodies of R. amarissima and R. lepida []. Also, a seco-cucurbitane triterpene, 3,4-secocucurbita-4, 24E-diene-3-hydroxy-26-carboxylic acid, was isolated from both species []. Russula brevipes produces triterpenoid compounds such as Lactarorufin A and Russulactarorufin []. The sphingolipid components of several higher fungi were investigated, with three new phytosphingosine-derived ceramides identified, including russulamide from R. cyanoxantha [].
R. delica, R. foetens, and R. japonica
Protoilludane-type sesquiterpenoids have been isolated from R. delica []. Diethyl ether extract of the fruiting bodies of R. delica resulted in the isolation of a new norsesquiterpenoid, russulanorol, and eight known sesquiterpenoids, lactarorufin A, blennin C, furandiol, lactarorufin B, lactarolide A, 14-hydroxylactarolide A, 3-O-methyllactarolide B, and isolactarorufin [,]. Russula foetens is a poisonous mushroom that contains gastrointestinal irritants and several marasmane sesquiterpenes []. Methanol extract resulted in the isolation of a new marasmane sesquiterpene lactone named russulfoen, together with two known marasmane sesquiterpene lactones, 7α,8α,13-trihydroxy-marasm-5-oic acid γ-lactone [] and 8α,13-dihydroxy-marasm-5-oic acid γ-lactone [], one known ergosterol, (22E,24R)-5α,8α-epidioxyergosta-6,22-dien-3β-ol [], as well as (1R,2R)-1-phenylpropane-1,2-diol []. Russula foetens was shown to produce the marasmane sesquiterpenes Lactapiperanol A and Lactapiperanol E []. A cytotoxic marasmane sesquiterpene, Russulfoen, was produced by R. foetens []. Illudoid sesquiterpenes were obtained from the fruiting body of R. japonica with neurite outgrowth-promoting activity []. Russujaponols A–F, illudoid sesquiterpenes isolated from the fruiting body of Russula japonica, exhibit notable neurite outgrowth-promoting activity and possess potential anticancer properties [].
Russula lepida, R. nobilis, and R. queletii
Four aristolane sesquiterpenes were isolated from the fruiting bodies of R. lepida (=Russula rosea, www.indexfungorum.org, accessed on 26 March 2025) []. Three new triterpenoids and two new aristolane sesquiterpenoids were isolated from R. lepida. Notably, two of the compounds are the first naturally occurring seco-ring-A cucurbitane triterpenoids, while two others represent a rare type of aristolane sesquiterpenoids found among fungi [,]. From the extract of R. lepida fruiting bodies, which exhibit anti-tumor activity, four new cucurbitane-type triterpenoids were isolated: (24E)-3β-hydroxycucurbita-5,24-dien-26-oic acid, (24E)-3,4-seco-cucurbita-4,24-diene-3,26-dioic acid, (24E)-3,4-seco-cucurbita-4,24-diene-3,26,29-trioic acid, and lepidolide [,]. Fatty acid esters of velutinal, three new sesquiterpenoids, and Russulanobilines A–C, along with eight known compounds, were isolated from extracts of R. nobilis fruiting bodies []. These sesquiterpenes have unique structures for chemical defense machinery, which protects mushrooms against predators, parasites, and microorganisms []. Piperalol and piperdial, bioactive compounds isolated from R. queletii have shown potential in various biological activities, including antimicrobial and anticancer properties [].
Russula rosacea, R. sanguinaria, R. virescens, and R. vinosa
Two new triterpenes, identified as rosacea acids A and B, with a similar structure, were extracted from the fruiting bodies of R. rosacea (Bull) Gray em. Fr. (Russulaceae) []. From R. sanguinaria, several compounds, including 15-hydroxyblennin A, blennin A, C, and D, lactarorufin A, piperalol, and vellerolactone, were identified []. Three new lactarane-type sesquiterpenoids, sangusulactones A-C, and two known ones, blennin A and 15-hydroxyblennin A, were isolated from the methanol extract of the fruiting bodies of the inedible mushroom R. sanguinea []. The phytochemical and bioactive profile of R. virescens, identifying 633 phytochemicals, including fatty acids, amino acids, polyphenols, and terpenoids, highlights its nutritional and medicinal potential. Russula virescens phytochemicals were also linked to cancer treatment pathways, revealing potential targets like HSP90AA1 and AKT3. While the work demonstrates the efficacy of multiomics techniques for exploring mushroom bioactivity, limitations include the lack of quantitative analysis and reliance on reversed-phase chromatography []. Five compounds, including triterpenoids, alcohols, and phenol (1R,2S)-1-phenylpropane-1,2-diol, isolactarorufin, lactarorufin A, 8α,13-dihydroxy-marasm-5-oic acid γ-lactone, and 7α,8α,13-trihydroxy-marasm-5-oic acid γ-lactone, were extracted from the fruiting bodies of R. vinosa. In a bioassay that tested plant growth regulatory activity on lettuce, all of these compounds demonstrated growth-regulating effects []. Fifteen compounds, including six new ones, were isolated and purified from R. vinosa; triterpenoids and sesquiterpenoids were its main chemical constituents. They showed potential anti-inflammatory effects []. Table 3 summarizes the triterpenoids isolated from various Russula species and their bioactivities

Table 3.
Summary of triterpenoids isolated from various Russula species and their bioactivities.
2.2. Other Bioactive Compounds and Beneficial Medicinal Properties of Russula
2.2.1. Russula aeruginea, R. albonigra, R. alnetorum, R. brevipes, R. fragrantissima, R. nobilis, and R. ochroleuca
A nutrient analysis of the above species showed that the protein content varied between 28.12% and 42.86%, while the carbohydrate content ranged from 49.33% to 55% []. Russula aeruginea and R. brevipes exhibit significant antimicrobial and antioxidant activities, with R. brevipes showing strong antibacterial effects against B. subtilis and R. aeruginea demonstrating potent antifungal activity against Fusarium equiseti. The antioxidant potential of both species was confirmed through various assays, suggesting their potential as sources for developing new antimicrobial and antioxidant drugs []. An alcohol extract of R. albonigra showed protective effects against CCl4-induced liver damage in mice, normalizing liver enzymes and restoring antioxidant levels comparably to silymarin. These findings suggest its potential as a natural liver protectant [].
2.2.2. Russula alboareolata
The apoptotic activity of R. alboareolata ethanolic extract was tested on L929, HepG2, and HeLa cells. The extract induced significant apoptosis: 77.20% in L929 cells at 600 µg/mL, 73.69% in HeLa cells at 500 µg/mL, and 30.00% in HepG2 cells at 1000 µg/mL. Valinomycin was used as a positive control. The results suggest that the extract has potential apoptotic effects on both normal and cancer cells, indicating its possible application in dietary supplements or chemoprevention []. The ethanolic extract of R. alboareolata may be considered a natural supplement useful in the treatment of bacterial infections []. The anti-inflammatory effects of extracts from Russula species, including R. alboareolata, R. medullata, R. virescens, and R. helios, were studied. Russula alboareolata showed the strongest anti-inflammatory activity, inhibiting nitric oxide, prostaglandin E2, and COX-2, with minimal cytotoxicity, suggesting its potential as an effective anti-inflammatory agent [].
2.2.3. Russula alatoreticula
Russula alatoreticula methanol extract contains phenols, flavonoids, ascorbic acid, β-carotene, and lycopene []. Hence, it exhibited strong antioxidant activity through its ability to quench free radicals, chelate Fe2+ ions, and reduce components []. Furthermore, methanol extract showed effective antibacterial potential against six investigated microbes; B. subtilis, E. coli, Klebsiella pneumoniae, Listeria monocytogenes, S. aureus, and S. typhimurium []. The extract revealed promising anti-cancer properties as well []). Thus, R. alatoreticula can be utilized as a good source of natural supplement against free radicals, pathogenic bacteria, and hepatocellular carcinoma and, further, in the food safety industry []. Russula alatoreticula ethanolic extract, enriched with phenolics like pyrogallol and cinnamic acid, demonstrated strong antioxidant and antibacterial activities, particularly against Gram-positive bacteria. In addition, the extract showed potential in inhibiting Hep3B cell proliferation by inducing apoptosis through the intrinsic mitochondrial pathway [].
2.2.4. Russula alveolata, Russula aruea, Russula aurora, Russula alveolata, Russula cf. Compressa, Russula flavobrunnea var. aurantioflava, and Russula ochrocephala
The proximate compositions, minerals, and amino acid contents of R. alveolata, Russula cf. compressa, R. flavobrunnea var. aurantioflava, and R. ochrocephala from Burkina Faso were investigated. The analysis revealed the presence of various bioactive compounds, including volatile oil, sterols, triterpenes, carotenoids, and saponosides, along with essential amino acids such as phenylalanine, valine, threonine, isoleucine, methionine, leucine, and lysine []. A phytochemical analysis of the ethyl acetate fraction from R. aruea Pers fruiting bodies led to the identification of a new isolactarane, sesquiterpene, and 11 known compounds, including four sesquiterpenes, four sterols, one allitol, and two fatty acids. The sesquiterpenes in R. aruea may serve as chemotaxonomic markers []. In a study of phenolic acids in 26 mushroom species using HPLC–DAD, R. aurora was noted for its major phenolic compound, gallic acid. The method used also identified fumaric acid as the most abundant compound in many mushrooms and catechin hydrate in others. The study provided a standardized approach for phenolic acid profiling in mushrooms [].
2.2.5. Russula brevipes
The methanol extract of R. brevipes fruiting bodies and mycelia exhibited antioxidant activity with EC50 values of 0.89 mg/mL and 7.08 mg/mL, respectively, in the 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay []. In a separate study, the methanol and water extracts of R. brevipes exhibited reducing power with 3.60 mg and 0.95 mg of GAE/g, respectively. The Ferredoxin-reducing substance (FRS) assay recorded IC50 values of 1.60 mg/mL and 1.80 mg/mL, and the extracts achieved 271% and 705% inhibition of lipid peroxidation, respectively []. Russula brevipes was studied for its bioactive compounds, revealing that decoction and infusion methods, due to higher phenolic content, demonstrated superior radical scavenging and metal ion chelating activities. This highlights R. brevipes as a promising natural source for reducing oxidative stress [].
The 1H-NMR metabolomics profiling of six edible fungi, including R. delica and R. brevipes, confirmed the presence of essential amino acids, organic acids, nucleosides, and valuable nutraceuticals like betaine and carnitine. The analysis demonstrated how these Russula species, both phylogenetically related, could be grouped based on their chemical profiles, highlighting the nutritional and nutraceutical potential of these local foods [].
2.2.6. Russula chloroides, Russula cf. foetentoides, and Russula foetens
In R. foetens and R. a cf. foetentoides extracts, iron was the most abundant mineral in R. cf. foetentoides. Active secondary metabolites were identified, with gallic acid being the most concentrated phenolic compound. Russula foetens demonstrated the highest antibacterial and antifungal activities, particularly against S. aureus and F. equiseti. Both species exhibited significant antioxidant potential, with R. foetens showing the highest DPPH inhibition and reducing power, while R. cf. foetentoides had the highest ABTS inhibition, flavonoid, and total phenolic content []. Phenolic acids and flavonoids, like ferulic acid, gallic acid, and myricetin, found in R. chloroides, have been shown to enhance GST (glutathione S-transferase) activity [].
2.2.7. Russula cutefracta (=R. cyanoxantha) and Russula cyanoxantha
Russula cutefracta (=R. cyanoxantha, www.indexfungorum.org, accessed on 26 March 2025) inhibits degranulation in mast cells []. Russula cyanoxantha showed antifungal activity against Microsporum canis and antibacterial activity against Pseudomonas putida []. Ergosta-4, 6, 8(14), 22-tetraen-3-one (ergone), a bioactive steroid from R. cyanoxantha, has been demonstrated to possess cytotoxic and anti-proliferative activity towards HepG2 cells []. Lectins of this mushroom have significantly higher agglutination activity at 4 °C, as compared to room temperature []. A new phytosphingosine-type ceramide was isolated along with nine other compounds from extracts of the fruiting bodies of R. cyanoxanotha [].
2.2.8. Russula delica
The ethanolic extract of R. delica demonstrated antioxidant and antimicrobial activities, with inhibition values in the linoleic acid system increasing as the concentration increased. The extract contained 8.71 ± 0.56 μg mg−1 of total flavonoids and 47.01 ± 0.29 μg mg−1 of phenolic compounds, showing antibacterial but not anticandidal activity. These findings suggest that R. delica extracts could be valuable as antimicrobial and antioxidative agents in the food industry []. The methanolic extract of R. delica exhibited strong antioxidant activity, including reducing power and radical scavenging, surpassing some standard antioxidants like BHA, BHT, and α-tocopherol. The study also determined the total phenolic compounds, α-tocopherol, and β-carotene content in the extract, contributing to its potent antioxidant properties []. The ethanolic extract of R. delica shows antimicrobial activity against foodborne bacteria. The extract’s major phenolic component, catechin, was identified at 5.33 mg/L, and it demonstrated antioxidant properties, including 26% DPPH radical scavenging and 58% ferrous ion chelation. The extract also contained significant levels of total phenols, ascorbic acid, β-carotene, and lycopene []. Russula delica and R. vesca were among the 18 Portuguese wild mushrooms evaluated for their antioxidant properties. The study measured their radical-scavenging capacity, reducing power, and inhibition of lipid peroxidation, revealing that these mushrooms contain significant levels of antioxidants, including phenols and tocopherols. The research highlighted their potential for nutraceutical applications and emphasized the importance of managing and conserving these mushroom species [].
Russula delica demonstrated the highest antioxidant activity among the mushrooms studied. This species also showed significant potential for use in developing safe antioxidants []. Russula delica, R. lepida, and R. mustelina mushrooms exhibit high levels of protein (38.08–38.52%), crude fiber (9.59–19.78%), carbohydrates (39.29–41.64%), ash (12.7–13.80%), and fat (4.06–5.70%). They are rich in potassium, phosphorus, calcium, and magnesium, with R. delica having the highest calcium and phosphorus content. Containing 18 amino acids, with glutamic acid and valine as predominant, their essential amino acid to total amino acid ratios range from 0.40 to 0.45, highlighting their high biological protein value [].
The extract of R. delica was found to contain glycosaminoglycans, as identified using the dimethylmethylene blue (DMMB) dye-binding assay and UV-Vis spectrophotometry []. Fatty acids in Pleurotus ostreatus and R. delica were studied in total lipid, triacylglycerol, and phospholipid fractions, with palmitic, oleic, and linoleic acids as major components. Polyunsaturated fatty acids (PUFAs) were higher than monounsaturated (MUFAs) and saturated fatty acids (SFAs). Ethyl acetate extracts showed significant cytotoxicity against prostate carcinoma (PC-3) cells, with inhibition rates of 99.45–92.82% at 520–530 μg/mL and IC50 values of 274.53–297.77 μg/mL []. The 1H-NMR metabolomics profiling of six edible fungi, including R. delica and R. brevipes, confirmed the presence of essential amino acids, organic acids, nucleosides, and valuable nutraceuticals like betaine and carnitine. The analysis demonstrated how these Russula species, both phylogenetically related, could be grouped based on their chemical profiles, highlighting the nutritional and nutraceutical potential of these local foods [].
2.2.9. Russula densifolia, Russula emetica (M12), and Russula fellea
Extracts from R. densifolia, R. violeipes, and R. cyanoxantha showed strong antioxidant activities, including ABTS and DPPH radical scavenging, along with α-glucosidase and α-amylase inhibition. They also exhibited anti-inflammatory effects by inhibiting albumin denaturation and moderate antimicrobial activity. The ethanol extract of R. violeipes had notable cytotoxicity with an IC50 of 56.66 mg/mL, and the extracts contained significant levels of phenols and flavonoids []. Russula emetica (M12) showed multidrug resistance (MDR) reversal activity in paclitaxel-resistant P-glycoprotein (Pgp)-positive cancer cells, enhancing doxorubicin’s cytotoxicity in these cells. This suggests that compounds in R. emetica may be effective in reversing Pgp-associated drug resistance []. The anti-inflammatory and antimicrobial activities of 44 wild mushrooms from Rakuno Gakuen University in Japan were screened. Ten samples from five species, including Naematoloma fasciculare, Cortinarius balteatocumatilis, and R. rosacea, significantly reduced nitric oxide (NO) production, indicating strong anti-inflammatory effects [].
2.2.10. Russula fragilis, R. fragrantissima, and R. gnathangensis
The antimicrobial effects of protein extracts from rare mushrooms, including R. fragilis, Ganoderma resinaceum, and Inocybe grammata, were evaluated for their potential against common hospital pathogens. Mycena pura exhibited strong antagonism against E. coli. Unique protein patterns in exotic fungi further demonstrated significant inhibition of pathogens such as MRSA and salmonella, indicating that wild fungal peptides could have potential therapeutic applications []. Russula gnathangensis demonstrated strong antioxidant activities, suggesting its potential nutritional value for local communities [].
2.2.11. Russula griseocarnosa
Extracts from R. griseocarnosa fruit bodies have been shown to reduce oxidative damage caused by formaldehyde inhalation in mice. These bioactive-rich extracts are also used in dietary supplements and cosmetic products for their antioxidant, immune-enhancing, and anti-aging effects, supporting both overall health and skin care [].
Extracts from R. griseocarnosa have been found to increase glutathione and superoxide dismutase levels in mouse serum, enhancing the body’s ability to adapt to physical exercise, resist fatigue onset, and accelerate fatigue recovery [,].
Postharvest NO figuration stimulates phenolic and flavonoid accumulation and enhances the antioxidant activities in R. griseocarnosa. Thus, NO fumigation might have potential applications to enhance the bioactive compounds and improve the antioxidant activities of R. griseocarnosa []. This mushroom contained very useful phytochemicals such as caffeic acid, flavonoids, ergosterol, phenolics, protocatechuic acid, and β-carotene []. Moreover, the major component in R. griseocarnosa was quercetin. Bioactive substances, together with rich nutritional composition, lead to R. griseocarnosa as a potential nutritive source []. Research has demonstrated that fresh fruit bodies of R. griseocarnosa stimulate the activities of phenylalanine ammonia-lyase (PAL) and chalcone synthase, resulting in increased phenolic and flavonoid accumulation when treated with nitric oxide fumigation. This process enhances the bioactive compounds and improves the antioxidant properties of the mushrooms [].
2.2.12. Russula helios, Russula integra, Russula kivuensis, and Russula laurocerasi
The anti-inflammatory effects of extracts from Russula species, including R. alboareolata, R. medullata, R. virescens, and R. helios, were studied. R. alboareolata showed the strongest anti-inflammatory activity, inhibiting nitric oxide, prostaglandin E2, and COX-2, with minimal cytotoxicity, suggesting its potential as an effective anti-inflammatory agent []. A methanolic extract of R. integra showed a cytotoxic effect on non-small cell lung cancer cells (NCI-H460) []. The ethanolic extracts of five wild mushrooms from Tanzania’s Southern Highlands were analyzed using gas chromatography–mass spectrometry, revealing 75 chemical compounds, including fatty acids, carotenoids, alkaloids, phenols, terpenes, steroids, and amino acids. Key species studied included R. cellulata, R. kivuensis, Lactarius densifolius, L. gymnocarpoides, and Lactarius sp. []. The antioxidant properties of a phenolic extract from R. laurocerasi were evaluated using various in vitro assays. The extract showed strong antioxidant activity, particularly in hydroxyl radical scavenging, with a low EC50 value of 0.03 mg/mL. The antioxidant effects were correlated with the presence of total phenols and flavonoids, suggesting that these polyphenols are partly responsible for the observed activity. The findings indicate that R. laurocerasi could be a promising source of therapeutic antioxidants [].
2.2.13. Russula lepida
Russula lepida and P. ostreatus extracts from Himachal Pradesh, India were screened for phytochemicals and tested for antibacterial activity. Rich in bioactive compounds, the methanol extract was most effective, particularly against B. subtilis, highlighting its potential as a source of new antimicrobial agents and validating their traditional medicinal uses []. Thermostable lectins with Cu2+-induced enhancement, and potent antiproliferative and antitumor activities were isolated from R. lepida []. The R. lepida lectins have high antitumor activity, and therefore, they can be developed into agents for cancer therapy []. Russula lepida exhibited antiproliferative activity to hepatoma Hep G2 cells and human breast cancer MCF-7 cells [].
2.2.14. Russula luteotacta, Russula mairei (=R. nobilis), and Russula medullata
In a study, it was found that catechin was highest in R. luteotacta (2.09 mg/g dry weight) []. Ethanolic extracts prepared from R. mairei (=R. nobilis, https://www.indexfungorum.org/Names/Names.asp, accessed on 26 March 2025) have been shown selective anti-inflammatory activity by decreasing the production of NO and IL-6 but not TNF-α in LPS-stimulated RAW264.7 cells []. The anti-inflammatory effects of extracts from Russula species, including R. alboareolata, R. medullata, R. virescens, and R. helios, were studied. Russula alboareolata showed the strongest anti-inflammatory activity, inhibiting nitric oxide, prostaglandin E2, and COX-2, with minimal cytotoxicity, suggesting its potential as an effective anti-inflammatory agent [].
2.2.15. Russula mustelina
Russula mustelina, R. delica, and R. lepida were studied at three maturity stages—immature, mature, and post-mature, and it was revealed that protein, ash, crude fibers, lipids, and energy values increased with maturity, while carbohydrates were highest at the immature stage. Minerals like potassium, phosphorus, and calcium were most abundant at the mature stage. In addition, all essential amino acids were present at the immature stage, indicating that these mushrooms are valuable nutritional resources, especially at the mature and post-mature stages [].
2.2.16. Russula nigricans, Russula nobilis, Russula ochroleuca, Russula ochrocephala, and Russula paludosa
Nigricanin, which has interesting biological activities, has been isolated from the fruiting bodies of R. nigricans. This is the first ellagic acid derivative isolated from higher fungi []. Nigricanin P-hydroxybenzoic and cinnamic acids were identified in ethanolic extracts of R. nigricans, which showed antioxidant activity through the inhibition of thiobarbituric acid reactive substances formation and oxidative hemolysis []. The spirodioxolactone ochroleucin A1 is responsible for the red color produced when the stalk base of R. ochroleuca and R. viscida is treated with aqueous KOH, and it easily rearranges into the isomeric dilactone ochroleucin A2. Ochroleucin A1, along with the related hemiacetal ochroleucin B (5), is derived from the oxidative condensation of monomeric units, with their structures confirmed via MS, NMR, and quantum chemical calculations of CD spectra []. Water extract of R. paludosa showed an inhibitory effect on HIV-1 reverse transcriptase [].
2.2.17. Russula pseudocyanoxantha
Russula pseudocyanoxantha was found to be rich in phenolics, flavonoids, and antioxidants, with significant antibacterial properties, particularly against Gram-positive bacteria. The ethanol extract also exhibited a notable antiproliferative effect on Hep3B cells, suggesting its potential involvement in mitochondria-mediated pathways [].
Russula pseudocyanoxantha polysaccharide fraction was extracted using hot water, and the solid remnants of the extraction process, which still contained therapeutic biopolymers, were valorized. These remnants were treated with cold alkali, yielding a high-yield fraction (RP-CAP). Chemical analysis revealed the presence of various monomers, primarily β-glucan, with a homogeneous polymer of approximately 129.28 kDa. RP-CAP demonstrated potent antioxidant and immune-boosting activities, particularly at 100 μg/mL, through the TLR/NF-κB pathway. These findings suggest that RP-CAP has significant potential as a health-enhancing component in functional foods and pharmaceuticals [].
2.2.18. Russula rosea and Russula rosacea
A novel lectin with potent in vitro anti-tumor activity was isolated from Russula rosea, the first lectin reported from Russula []. Russula rosacea showed significant antitumor effects on sarcoma 180 in mice, likely due to its immunomodulating properties. Extracts from the mushroom, including those soluble in saline, hot water, and methanol, enhanced immune responses and prolonged survival in treated mice without showing cytotoxicity against cancer cell lines []. The fruiting bodies of R. rosacea were extracted using methanol and hot water. The extracts showed strong DPPH radical scavenging and chelating effects but a lower reducing power compared to BHT. With seven identified phenolic compounds, the extracts also demonstrated a moderate inhibition of acetylcholinesterase and butyrylcholinesterase and significant nitric oxide (NO) inhibition in LPS-induced cells, indicating their potential as natural sources of antioxidants and anti-inflammatory agents [].
2.2.19. Russula senecis
Russula senecis, a historically valued but neglected myco-resource, was explored for its health benefits. The ethanolic extract, rich in phenolics, flavonoids, ascorbic acid, and carotenoids, demonstrated strong antioxidant and antibiotic properties. It also showed selective anti-cancer activity against Hep3B cells, inducing apoptosis through the mitochondrial pathway. The study suggests that R. senecis has potential applications in the medicinal, pharmaceutical, and food industries []. The entire fruit bodies of R. senecis were used to isolate a β-glucan-enriched polysaccharide fraction (RuseHap) through consecutive hot water and cold alkaline extraction, reducing waste and fully utilizing the mushroom. The isolated RuseHap demonstrated strong antioxidant and immune-boosting properties, with potential pharmacotherapeutic applications, likely due to its interaction with Toll-like receptors (TLR2 and TLR4), leading to enhanced immune response and gene expression related to inflammation [].
2.2.20. Russula subnigricans
Five new chlorinated phenyl ethers, Russuphelins B, C, D, E, and F, have been isolated from R. subnigricans, and Russuphelins B, C and D exhibited cytotoxic activity in vitro against P388 leukemia cells []. A new cytotoxic substance, designated as russuphelin A, has been isolated from the mushroom R. subnigricans [].
2.2.21. Russula vesca
From R. vesca, two compounds, triyne acid, and triyinol, were isolated [].
Russula vesca was noted for its high carbohydrate content (71%) and significant magnesium levels (14 g/kg). This mushroom also exhibited lower moisture, lipid, sodium, and phosphorus contents compared to other species analyzed [].
Russula delica and R. vesca were among the 18 Portuguese wild mushrooms evaluated for their antioxidant properties. The study measured their radical-scavenging capacity, reducing power, and inhibition of lipid peroxidation, revealing that these mushrooms contain significant levels of antioxidants, including phenols and tocopherols. The research highlighted their potential for nutraceutical applications and emphasized the importance of managing and conserving these mushroom species []. Phenols, flavonoids, and antioxidant activity were assessed in aqueous and ethanolic extracts from Agaricus macrosporus and R. vesca. Aqueous extracts demonstrated higher antioxidant activity and phenol content than ethanolic extracts, with A. macrosporus showing more phenols and R. vesca exhibiting higher flavonoids. These results suggest that aqueous mushroom extracts could serve as effective substitutes for synthetic antioxidants in different industries [].
The antibacterial potential of aqueous and ethanolic extracts from A. macrosporus and R. vesca was investigated. Russula vesca extracts had higher total carbohydrate and protein content. Aqueous extracts showed superior antibacterial activity compared to ethanolic ones. Notably, the aqueous extract of R. vesca was more effective against Bacillus cereus (13.6 mm), Enterococcus faecalis (12.1 mm), E. coli (16.7 mm), and Pseudomonas aeruginosa (10.5 mm) than gentamicin or neomycin. These results highlight the potential of mushroom extracts for diverse industrial applications [].
2.2.22. Russula vinosa and Russula violeipes
Water-extracted polysaccharides from R. vinosa (WRP) were separated into three fractions: WRP-1, WRP-2, and WRP-3. WRP-1, a branched β-(1→3)-glucan with a rigid helical conformation, exhibited the strongest immunostimulatory activity. In contrast, WRP-2 and WRP-3, composed of galactoglucans with more flexible structures, showed lower immunostimulatory effects. All fractions promoted macrophage proliferation, phagocytosis, and the release of nitric oxide and cytokines, indicating their potential as natural immunostimulators in the food and pharmaceutical industries []. Extracts from R. densifolia, R. violeipes, and R. cyanoxantha showed strong antioxidant activities, including ABTS and DPPH radical scavenging, along with α-glucosidase and α-amylase inhibition. They also exhibited anti-inflammatory effects by inhibiting albumin denaturation and moderate antimicrobial activity. The ethanol extract of R. violeipes had notable cytotoxicity with an IC50 of 56.66 mg/mL, and the extracts contained significant levels of phenols and flavonoids [].
2.2.23. Russula virescens and Russula viscida
The chemical compositions of R. virescens were assessed among several wild edible mushroom species from Bukovina, Romania. The study measured water, crude protein, lipids, carbohydrates, and ash content. R. virescens had a carbohydrate content lower than Agaricus albolutescens, Boletus edulis, and Armillaria mellea. Its protein content varied between 10.12% and 15.15% dry weight. Notably, Russula virescens exhibited higher antiradical activity compared to other species, suggesting its potential benefits in antioxidant protection [].
Russula virescens exhibited an anti-inflammatory effect in the RAW 264.7 cell by suppressing the expression of STATs, a reduction in TNF-α, and NO production [].
A Chinese study suggests that R. virescens has beneficial effects on blood lipid regulation. Rats given high (600 mg/kg/day) and low (300 mg/kg/day) doses of R. virescens via stomach perfusion for 30 days had significantly (p < 0.05) lower levels of total cholesterol, total low-density lipoprotein cholesterol, and triglycerides than in the hyperlipidemia control group []. The Chinese study above also showed that rats given high and low doses of the mushroom had lower levels of serum and liver malondialdehyde (a biomarker used to measure levels of oxidative stress), and increased levels of the enzyme superoxide dismutase [].
The spirodioxolactone ochroleucin A1 is responsible for the red color produced when the stalk base of R. ochroleuca and R. viscida is treated with aqueous KOH, and it easily rearranges into the isomeric dilactone ochroleucin A2. Ochroleucin A1, along with the related hemiacetal ochroleucin B (5), is derived from the oxidative condensation of monomeric units, with their structures confirmed via MS, NMR, and quantum chemical calculations of CD spectra [].
2.2.24. Russula xerampelina
Russula xerampelina demonstrated antibacterial activity against Plasmodium falciparum []. The antibacterial potential of ethanolic extracts from R. xerampelina and Suillus granulatus mushrooms against P. aeruginosa was investigated, revealing an additive effect when combined. The extracts also displayed allelopathic effects, reducing the germination rates of Lactuca sativa (lettuce) and Solanum lycopersicum (tomato) seeds at higher concentrations. However, the seeds exhibited a positive response when analyzed for the allelopathic index, suggesting that these extracts could be effective in controlling Pseudomonas phytopathogens without causing significant phytotoxicity [].
Russula xerampelina, known as the “shrimp mushroom”, emits a strong shellfish-like odor. Analysis using SPME and GC–MS identified trimethylamine and trimethylamine N-oxide as the primary volatile compounds, with trimethylamine responsible for its fishy, cooked-seafood aroma []. Table 4 lists the bioactive properties of various Russula sp.

Table 4.
Bioactive properties of various Russula species.
3. Biotechnological Applications
The trace element levels in Russula species from the East Black Sea region were analyzed. Russula foetens had the lowest Hg level at 0.06 mg/kg, and R. cyanoxantha had the highest Cd level at 3.16 mg/kg. The study highlighted the metal bioaccumulation in these mushrooms, with specific focus on Cd and Hg levels []. Russula cyanoxantha from Dambovita County, Romania, showed iron concentrations four times higher than the average of the studied species. This indicates R. cyanoxantha’s strong ability to bioaccumulate iron while maintaining normal growth []. The antifungal effects of R. cyanoxantha were investigated against the plant pathogens Fusarium moniliforme and F. culmorum, which cause paleness sickness and root corrosion. Dried mushroom extracts, prepared using acetone and chloroform, exhibited significant antagonistic effects against both Fusarium species, with clear zones of inhibition observed, comparable to commercial antibiotics like amoxicillin and erythromycin []. Bacteria can colonize a wide variety of medical devices, leading to local and systemic infectious complications such as site infections, catheter-related bloodstream infections, and endocarditis []. Those bacteria are able to grow and adhere to almost every surface, forming architecturally complex communities termed biofilms []. Russula delica extract inhibits the biofilm production of E. coli, Proteus mirabilis, P. aeruginosa, and Acinetobacter baumannii []. Mercury (Hg) contamination in Russula ochroleuca was studied at ten unpolluted sites in northern Poland. Hg levels were 0.017 to 0.43 μg/g in caps and 0.011 to 0.24 μg/g in stipes. Caps had higher Hg concentrations than stipes, with bioconcentration factors of 0.57 to 5.6 for caps and 0.50 to 3.3 for stipes. Higher Hg levels were found in mushrooms from Trójmiejski Landscape Park. The study suggests Russula ochroleuca could be used as a bioindicator for environmental Hg pollution [].
Russula species such as R. atropurpurea [,,], R. bresadolae [,], R. ochroleuca [], and R. pumila [] can accumulate remarkably high concentrations of Zn and substantially contribute to the cycling and environmental sequestration of metal elements. Russula species can accumulate and translocate heavy metals under natural pH conditions. While the concentrations of iron (Fe), zinc (Zn), and copper (Cu) varied by species, these mushrooms exhibit a low capacity to accumulate these metals but demonstrate significant mobility within their fruiting bodies []. The production of vinegar from the wild edible mushroom R. delica using microwave-assisted enzymatic hydrolysis extraction resulted in a product with high nutritional value, significant antioxidant activity, and a unique aroma. The process yielded a vinegar with 10.95% alcohol content, 5.60% acetic acid, and notable levels of phenolic compounds. Thirteen volatile compounds were identified, contributing to its distinct aroma. This study represents the first analysis of vinegar derived from a mushroom, showcasing its potential for future commercial production [].
Russula vinosa Lindblad, a traditional food and medicinal resource rich in polyphenolic compounds, was mixed fermented with Saccharomyces boulardii and Lactobacillus lactis. The ethanol extract of the mixed bacterial fermentation product (EMFP) exhibited enhanced antioxidant activity and introduced 186 new compounds, including organic and phenolic acids. EMFP addition significantly improved bread quality by reducing hardness and chewiness while enhancing resilience and antioxidant properties. However, excessive EMFP negatively affected sensory attributes. A 0.5% EMFP addition was optimal for balancing quality, sensory evaluation, and antioxidant benefits, supporting its potential application in functional foods [].
A novel laccase from R. virescens was purified using chromatography and gel filtration. The 69 kDa monomeric enzyme has an N-terminal sequence AIGPTAELVV and optimal activity at pH 2.2 and 60 °C. It was inhibited by Cu2+ and other inhibitors. The laccase degrades phenolic compounds and decolorizes various laboratory and textile dyes, with a Km of 0.1 mM for specific substrates []. Halogen speciation analysis using HPLC-ICPMS/MS revealed the presence of dichloroacetic acid (DCAA) in R. nigricans at concentrations of 23–37 mg/kg. This compound was not detected in other Russula species or mushrooms from the same regions, suggesting that R. nigricans may biosynthesize DCAA. This finding challenges the traditional view of DCAA as merely a pollutant from water disinfection, highlighting its natural occurrence in living organisms []. Significant bioaccumulation of manganese and nickel of R. delica underscores the importance of understanding heavy metal accumulation. This knowledge is crucial for assessing potential health risks and ecological impacts, particularly in regions with varying soil metal concentrations. Monitoring and analyzing these metal levels can inform safer foraging practices and contribute to environmental health assessments []. Russula delica mushroom/bentonite clay (RDBNC) was tested as a low-cost bionanosorbent for removing methylene blue (MB) and malachite green (MG) dyes. Adsorption followed Freundlich isotherm and pseudo-second-order kinetics, driven by π–π interactions, hydrogen bonding, and electrostatic forces. RDBNC maintained efficiency after four cycles, and thermodynamic analysis confirmed spontaneous, exothermic adsorption. It also exhibited antibacterial effects against E. coli, making it a promising eco-friendly material for dye removal []. Table 5 presents the trace element levels and biotechnological applications of various Russula species.

Table 5.
Trace element levels and biotechnological applications of various Russula species.
4. Toxicity
Russula subnigricans is a toxic mushroom known for causing fatal poisoning when mistakenly ingested. The identification of its responsible toxin remained elusive for about 50 years due to the toxin’s instability and frequent misidentification of the mushroom. Recently, researchers isolated the unstable toxin and identified its structure. In addition, they discovered a unique chemical marker, cyclopropylacetyl-(R)-carnitine, which helps distinguish R. subnigricans from similar species []. A case involving seven family members poisoned by R. subnigricans revealed symptoms ranging from gastrointestinal issues to rhabdomyolysis, with one fatality. This case highlights that R. subnigricans can cause rhabdomyolysis and emphasizes the need for early recognition and intensive supportive care for affected individuals []. Rhabdomyolytic mushroom poisoning, a newly recognized syndrome, is on the rise globally, with R. subnigricans identified as a cause. This report details the first recorded case in Korea, involving a 51-year-old man who developed rhabdomyolysis, acute kidney injury, and severe complications, ultimately leading to death. The case underscores the importance of considering mushroom poisoning, particularly from R. subnigricans, in unexplained rhabdomyolysis cases []. Russula subnigricans poisoning, a newly identified syndrome, causes severe rhabdomyolysis, acute kidney injury, and cardiomyopathy. In a recent case series, two out of six patients died from severe complications, including metabolic acidosis and irreversible shock. This poisoning should be considered in cases of rhabdomyolysis with unknown origins [].
The essential and non-essential element contents of eight Russula species were evaluated, revealing high levels of potassium, magnesium, and calcium. Elevated metal concentrations were found in R. risigallina, R. olivacea, and R. velenovskyi, with chromium levels in R. risigallina and R. olivacea exceeding safe limits. Health risk indices suggested potential risks from chromium and cadmium in some species []. The potential myotoxicity of various edible mushrooms, including Russula spp., Cantharellus cibarius, Albatrellus ovinus, and Leccinum versipelle, was assessed after reports of Tricholoma flavovirens causing delayed rhabdomyolysis and fatalities. In a study involving 86 mice, the consumption of these mushrooms at high doses led to increased plasma creatine kinase activity, indicating potential muscle damage, though no histological changes were observed in muscle or liver tissues []. Myotoxic mushroom poisoning was studied in Thailand over a 5-year period, involving 41 patients. Symptoms included gastrointestinal issues and myalgia, with rhabdomyolysis developing 24–48 h after ingestion. Some mushrooms were identified as Russula species. Key issues were acute kidney injury (51.5%), hyperkalemia (33.3%), and a 26.8% mortality rate. Effective treatment required early detection and monitoring of serum potassium, creatinine, and CPK levels, with interventions including fluids, urine alkalinization, and dialysis []. A case series of five patients revealed a unique instance of hemolysis in a patient with glucose-6-phosphate dehydrogenase deficiency, suggesting that the mushroom’s toxin may trigger hemolysis in susceptible individuals. This finding underscores the need for further research on the toxic effects of R. subnigricans [].
Mushroom poisoning cases, including R. subnigricans, are on the rise, with increasing severity and fatality. A report described a family with R. subnigricans poisoning complicated by severe rhabdomyolysis. A 64-year-old man initially misdiagnosed with myocardial infarction, later found to have rhabdomyolysis from mushroom poisoning, was hospitalized alongside two other family members with similar symptoms. After intensive care and fluid resuscitation, all patients recovered without complications. Early identification and supportive care are crucial in managing mushroom poisoning cases []. Even 36 years after the Chernobyl disaster, consuming wild mushrooms in Ukraine’s Polissya remains risky due to high radionuclide contamination. Imleria badia and Tricholoma equestre showed the highest 137Cs levels, while R. emetica had the highest 90Sr levels. Annual effective doses from consuming these mushrooms ranged from 0.0014 to 8.71 mSv, depending on contamination levels []. Cadmium (Cd) and lead (Pb) levels in wild mushrooms from Poland’s “Green Lungs” region were assessed and compared to those in cultivated species. R. vinosa exhibited the highest Pb level among wild mushrooms at 2.61 μg/g, while R. heterophylla had a lower Cd concentration at 0.10 μg/g. Cultivated mushrooms generally showed lower levels of both metals. While Pb intake from wild mushrooms is considered safe, consuming Rozites caperatus and Boletus chrysenteron may exceed the provisional tolerable monthly intake (PTMI) for Cd, posing a potential risk of toxicity []. Russula virescens can accumulate heavy metals, posing health risks, with Cd, Pb, Cu, Zn, Co, Cr, Mn, Ni, and Fe analyzed in mushrooms and soil using the DGT technique. A correlation between R-values (metal resupply capacity) and bioaccumulation factors (BAFs) showed that faster resupply increases metal uptake. While soil contamination was below legal limits, high Cu levels in mushrooms may pose risks, especially for children [].
5. Cultivation Challenges of Russula: Current Knowledge and Limitations
The cultivation of Russula presents significant challenges due to its obligate ectomycorrhizal nature, requiring symbiotic relationships with specific host trees for successful growth and fruiting [,,,]. Unlike commercially cultivated saprotrophic mushrooms such as Agaricus bisporus or P. ostreatus, Russula species, including R. brevipes and R. griseocarnosa, depend entirely on living root systems of host trees for nutrient exchange and fruiting body formation [,,,]. This biological constraint has hindered artificial cultivation efforts, as these mushrooms typically fail to produce fruiting bodies in axenic culture or on standard artificial substrates [,,].
Limited success has been achieved through mycorrhizal synthesis approaches with particular Russula species []. For example, researchers have demonstrated the formation of functional ectomycorrhizae between R. brevipes and Pinus densiflora seedlings under controlled laboratory conditions, though reliable fruiting body production remains elusive []. Nevertheless, R. griseocarnosa, cannot be cultivated artificially and is solely harvested from natural habitats. Currently, there is limited understanding of controlled Russula cultivation and its associated microbial interactions [,]. Another Russula sp., has shown some promise in laboratory mycelial growth experiments but similarly fails to fruit without its natural tree partners (Wang & Guerin-Laguette, unpublished).
The cultivation barriers for Russula species are multifaceted. Each species exhibits specific host requirements, with R. cyanoxantha preferentially associating with Fagus trees in European forests, while R. emetica shows a stronger affinity for Betula species in northern ecosystems [,]. In addition, Russula mycelium grows significantly slower in culture compared to commercial mushroom species, and its complex nutrient requirements are difficult to replicate in artificial systems [,]. A patent proposes an innovative method for cultivating Russula species using sunflower byproducts as a substrate. The formula combines sunflower plates, stalks, seed shells, and cakes with bran, lime, and gypsum (63–65% moisture), reportedly achieving robust mycelial growth and impressive 130–142% biological efficiency. This approach not only offers a potential cultivation method but also provides sustainable utilization of agricultural waste material []. The fundamental biological understanding of the molecular dialogue between Russula fungi and their host plants remains incomplete, further complicating efforts to cultivate these fungi.
Current production, therefore, relies heavily on wild harvesting, raising concerns about sustainability and ecological impacts. Some researchers have proposed “wild-simulated” cultivation methods, where forest areas are intentionally inoculated with desired Russula species, as demonstrated with R. olivacea in managed oak forests [,,]. While not actual cultivation in the agricultural sense, this approach may help conserve natural populations while allowing for some level of production management. As research continues, particularly in understanding the molecular basis of the Russula–host interaction, opportunities may arise to develop more reliable cultivation methods for these ecologically and gastronomically important fungi.
6. Future Works and Drawbacks
Future research on Russula should focus on several key areas to fully unlock its medicinal potential. One important direction is the expanded phytochemical profiling of Russula species, using advanced analytical techniques like metabolomics and high-throughput screening. This would help comprehensively identify and characterize novel bioactive compounds with therapeutic potential. Special attention should be given to compounds whose production may depend on symbiotic interactions with host trees. In addition, there is a need for rigorous clinical trials and pharmacological studies to validate the efficacy and safety of these compounds in treating specific diseases. While in vitro and animal studies provide valuable insights, translating these findings into human health benefits requires well-designed clinical research.
Biotechnological applications also hold great promise for Russula. Future work should explore innovative approaches to cultivate these mushrooms sustainably, optimize yield, particularly by developing cultivation systems that maintain essential ectomycorrhizal relationships and develop efficient methods for extracting bioactive compounds. Advancing genomic and molecular studies on Russula could provide deeper insights into the biosynthetic pathways and host–symbiont signaling mechanisms responsible for producing these valuable compounds, potentially enabling the genetic engineering of Russula strains with enhanced therapeutic properties or facultative growth capabilities. Safety and toxicology research remains a critical area of focus. Although many Russula species are safe for consumption, the potential toxicity of some species necessitates comprehensive studies to establish safe consumption levels and identify any harmful effects. This is particularly important, as Russula species are increasingly considered for integration into modern medicinal practices.
However, there are several drawbacks that must be addressed. One significant challenge is the limited clinical evidence supporting the therapeutic efficacy of Russula bioactive compounds in humans. While existing studies offer promising results, most have been conducted in vitro or on animal models, which may not fully translate to human applications. The current inability to cultivate many Russula species without their natural host trees severely limits the standardized production of bioactive compounds for clinical research. In addition, the concentration of bioactive compounds in Russula species can vary widely, depending on factors such as geographical location, environmental conditions, host tree species, and cultivation practices. This variability complicates the standardization necessary for therapeutic use. Another concern is the potential toxicity of some Russula species. Despite their widespread use in traditional medicine and as food, not all species have been thoroughly studied for safety, which could pose health risks. The challenges of large-scale cultivation also present obstacles, as Russula mushrooms require specific growth conditions that are difficult to replicate artificially, and they are prone to contamination, potentially limiting the availability of Russula-derived products. Finally, integrating Russula bioactive compounds into pharmaceuticals or nutraceuticals may face regulatory hurdles, particularly for compounds derived from symbiotic cultivation systems, as obtaining approval from health authorities often requires extensive safety and efficacy data. These regulatory challenges could slow down the development and commercialization of Russula products despite their potential benefits. Addressing these drawbacks will be essential to realizing the full therapeutic potential of Russula in the future.
7. Conclusions
The current understanding of Russula species highlights their considerable pharmacological potential, supported by the presence of bioactive compounds with antioxidant, anti-inflammatory, antimicrobial, and immunomodulatory properties. However, translating this potential into practical applications requires addressing several critical research challenges.
A primary need lies in achieving comprehensive taxonomic clarification through integrated approaches that combine morphological and molecular techniques. This will help resolve existing ambiguities related to species complexes and cryptic diversity. In addition, detailed studies are necessary to elucidate the mechanisms of bioactive compounds, including their molecular targets, pharmacokinetics, and pharmacodynamics. Rigorous toxicological assessments must also be conducted to establish safety profiles for potential medicinal use.
Furthermore, the development of standardized protocols for cultivation and extraction is essential to ensure consistent and reproducible yields of bioactive compounds. Addressing these gaps through multidisciplinary research will not only validate the Russula species as promising candidates for pharmaceutical development but also enhance our broader understanding of fungal secondary metabolism and its ecological roles. Moving forward, prioritizing translational research will be key to bridging ethnomycological knowledge with clinically validated therapeutic applications.
Author Contributions
Conceptualization, K.H. and S.C.K.; investigation, S.C.K.; resources, S.C.K.; data curation, K.H.; writing—original draft preparation, J.Y. and K.H.; writing—review and editing, J.Y., S.C.K., K.H., N.P., E.T., D.L. and Y.Z.; visualization, K.H.; supervision, K.H.; project administration, J.Y.; funding acquisition, D.L. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Yunnan Fundamental Research Project (grant no. 202401CF070040) and the Yunnan Department of Education Scientific Research Fund Project (grant no. 2023J0993).
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were produced.
Acknowledgments
Samantha C. Karunarathna thanks the High-Level Talent Recruitment Plan of Yunnan Province (“High-End Foreign Experts” Program), the National Natural Science Foundation of China (Grant No. 32260004), and the Key Laboratory of Yunnan Provincial Department of Education of the Deep-Time Evolution on Biodiversity from the Origin of the Pearl River, Qujing Normal University, Qujing, Yunnan 655011, China, for their support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lotsy, J.P. Vorträge über botanische Stammesgeschichte; Gustav Fischer: Jena, Germany, 1907; Volume 708. [Google Scholar] [CrossRef][Green Version]
- Miller, S.L.; McClean, T.M.; Walker, J.F.; Buyck, B. A molecular phylogeny of the Russulales including agaricoid, gasteroid and pleurotoid taxa. Mycologia 2001, 93, 344–354. [Google Scholar] [CrossRef]
- Kirk, P.M.; Cannon, P.F.; Minter, D.W.; Stalpers, J.A. Dictionary of the Fungi, 10th ed.; CABI International: Oxon, UK, 2008; pp. 608–609. [Google Scholar]
- Buyck, B.; Zoller, S.; Hofstetter, V. Walking the thin line… ten years later: The dilemma of above- versus below-ground features to support phylogenies in the Russulaceae (Basidiomycota). Fungal Divers. 2018, 89, 267–292. [Google Scholar] [CrossRef]
- Buyck, B.; Horak, E.; Cooper, J.; Wang, X. Introducing Russula subgen. Cremeo-ochraceae, a new and very small lineage sharing with Multifurca (Russulaceae) an identical, largely circum-Pacific distribution pattern. Fungal Syst. Evol. 2024, 14, 109–126. [Google Scholar] [CrossRef]
- Roy, N.; Beypih, J.; Tanti, B.; Dutta, A.K. Russula brunneoaurantiaca, a novel taxon of Russula subg. Crassotunicata from West Bengal, India, with morpho-molecular analysis and scanning electron microscopy. Microsc. Res. Tech. 2024, 87, 740–746. [Google Scholar] [CrossRef]
- Niu, C.; Liu, T.; Zhao, S.; Ren, J.; Zhao, Y.; Kang, X.; Qin, W.; Xie, X.; Zhang, X.; Wei, T.; et al. Multi-gene analysis of the Russula crown clade (Russulales, Basidiomycota) revealed six new species and Alboflavinae subsect. nov. from Fagaceae forests in China. Front. Plant Sci. 2024, 15, 1454035. [Google Scholar] [CrossRef]
- Buyck, B.; Hofstetter, V.; Eberhardt, U.; Verbeken, A.; Kauff, F. Walking the thin line between Russula and Lactarius: The dilemma of Russula subsect. Ochricompactae. Fungal Divers. 2008, 28, 15–40. [Google Scholar]
- Looney, B.P.; Meidl, P.; Piatek, M.J.; Miettinen, O.; Martin, F.M.; Matheny, P.B.; Labbé, J.L. Russulaceae: A new genomic dataset to study ecosystem function and evolutionary diversification of ectomycorrhizal fungi with their tree associates. New Phytol. 2018, 218, 54–65. [Google Scholar] [CrossRef]
- Thachunglura, V.L.; Chawngthu, Z.; Zothanzama, J.; Lallawmkima, B.; Lalbiakmawia, B.; Khumlianlal, J.; Rai, P.K. Russulaceae of Ailawng forest with an emphasis on Russula purpureoverrucosa (Russulaceae): A first report for India. Sci. Vis. 2023, 23, 41–47. [Google Scholar]
- He, M.-Q.; Zhao, R.-L.; Hyde, K.D.; Begerow, D.; Kemler, M.; Yurkov, A.; McKenzie, E.H.C.; Raspé, O.; Kakishima, M.; Sánchez-Ramírez, S.; et al. Notes, outline and divergence times of Basidiomycota. Fungal Divers. 2019, 99, 105–367. [Google Scholar] [CrossRef]
- Chen, B.; Liang, J.; Yu, F. Morphological studies and phylogenetic analyses unveil two notable new species of Russula Subg. Heterophyllinae from China. Diversity 2024, 16, 727. [Google Scholar] [CrossRef]
- Li, G.J.; Zhao, Q.; Zhao, D.; Yue, S.F.; Li, S.F.; Wen, H.A.; Liu, X.Z. Russula atroaeruginea and R. sichuanensis spp. nov. from southwest China. Mycotaxon 2013, 124, 173–188. [Google Scholar] [CrossRef]
- Song, J.; Li, H.; Wu, S.; Chen, Q.; Yang, G.; Zhang, J.; Liang, J.; Chen, B. Morphological and Molecular Evidence for Two New Species within Russula Subgenus Brevipes from China. Diversity 2022, 14, 112. [Google Scholar] [CrossRef]
- Paloi, S.; Kumla, J.; Karunarathna, S.C.; Lumyong, S.; Suwannarach, N. Taxonomic and phylogenetic evidence reveal two new Russula species (Russulaceae, Russulales) from northern Thailand. Mycol. Prog. 2023, 22, 72. [Google Scholar] [CrossRef]
- Chuchała, P.; Mikołajczyk, A.; Mleczko, P.; Karpowicz, F. The genus Russula Pers. (Russulales) in the Pieniny Mts.: Diversity and distribution. Pienin. Przyr. I Człowiek 2024, 16, 49–93. [Google Scholar]
- Hessler, L.R. A study of Russula types. Mem. Torrey Bot. Club 1960, 21, 1–59. [Google Scholar] [CrossRef]
- Romagnesi, H. Les Russules d’Europe et d’Afrique du Nord; Bordas: Paris, France, 1967. [Google Scholar]
- Singer, R. The Agaricales in Modern Taxonomy, 4th ed.; Koeltz Scientific Books: Koenigstein, Germany, 1986. [Google Scholar]
- Härkönen, M.; Buyck, B.; Saarimäki, T.; Mwasumbi, L. Tanzanian mushrooms and their uses 1. Russula. Karstenia 1993, 33, 11–50. [Google Scholar] [CrossRef]
- Miller, S.L.; Buyck, B. Molecular phylogeny of the genus Russula in Europe with a comparison of modern infrageneric classifications. Mycol. Res. 2002, 106, 259–276. [Google Scholar] [CrossRef]
- Bau, T.; Li, Y.; Irina, A.G.; Eugenia, M.B.; Wasiliy, A.S. Common wild edible mushroom resource of Russia. Edible Fungi China 2008, 27, 9–13. [Google Scholar]
- Das, K.; Atri, N.; Buyck, B. Three new species of Russula (Russulales) from India. Mycosphere 2013, 4, 722–732. [Google Scholar] [CrossRef]
- Buyck, B.; Jančovičová, S.; Adamčík, S. The Study of Russula in the Western United States. Cryptogam. Mycol. 2015, 36, 193–211. [Google Scholar] [CrossRef]
- Buyck, B.; Henkel, T.; Manz, C.; Cao, S.; Amalfi, M.; Wang, X.H. A revision of the African Russula radicans and allies in subgen. Heterophyllidiae provides an example of a clade that exhibits recent diversification and extensive phenotypic plasticity. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wisitrassameewong, K.; Park, M.S.; Lee, H.; Ghosh, A.; Das, K.; Buyck, B.; Looney, B.P.; Caboň, M.; Adamčík, S.; Kim, C.; et al. Taxonomic revision of Russula subsection Amoeninae from South Korea. MycoKeys 2020, 75, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Wisitrassameewong, K.; Manz, C.; Hampe, F.; Looney, B.P.; Boonpratuang, T.; Verbeken, A.; Thummarukcharoen, T.; Apichitnaranon, T.; Pobkwamsuk, M.; Caboň, M.; et al. Two new Russula species (fungi) from dry dipterocarp forest in Thailand suggest niche specialization to this habitat type. Sci. Rep. 2022, 12, 2826. [Google Scholar] [CrossRef] [PubMed]
- Shimono, Y.; Kasuya, T.; Hosaka, K. Russula ryukokuensis sp. nov., an outstanding species of the genus Russula (Russulaceae) having minute basidiomata from Japan. Bull. Natl. Mus. Nat. Sci. Ser. B Bot. 2021, 47, 1–2. [Google Scholar]
- Vera, M.; Adamčík, S.; Adamčíková, K.; Hampe, F.; Caboň, M.; Manz, C.; Ovrebo, C.; Piepenbring, M.; Corrales, A. Morphological and genetic diversification of Russula floriformis, sp. nov., along the Isthmus of Panama. Mycologia 2021, 113, 807–827. [Google Scholar] [CrossRef]
- Bastos, C.; Liberal, Â.; Moldão, M.; Catarino, L.; Barros, L. Ethnomycological prospect of wild edible and medicinal mushrooms from Central and Southern Africa—A review. Food Front. 2023, 4, 549–575. [Google Scholar] [CrossRef]
- Ashfaq, A.; Razzaq, A.; Naseer, A.; Khalid, A.N. Morphological and molecular evidence for a new species of Russula subgen. Compactae from Pakistan. Nord. J. Bot. 2024, 2025, e04411. [Google Scholar] [CrossRef]
- Canales, M.P.; Velay, J.M. El género Russula en Valdeinferno: Base para la realización de un catálogo de especies. Almoraima: Rev. Estud. Campogibraltareños 2024, 61, 201–210. (In Spanish) [Google Scholar]
- Gupta, A.; Dimri, R.; Mishra, S.; Kumar, S. Russula rosea: A wild edible mushroom of India. Edible Med. Mushrooms India 2024, 1, 38–46. [Google Scholar]
- Mahadevakumar, S.; Santhosh, C.R.; Nuthan, B.R.; Sridhar, K.R.; Satish, S.; Amruthesh, K.N. Ethnomedicinal Applications of 100 Wild Mushrooms of the Indian Subcontinent. In Ethnic Knowledge and Perspectives of Medicinal Plants; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 545–576. [Google Scholar]
- Miller, S.L.; Aime, M.C.; Henkel, T.W. Russulaceae of the Pakaraima Mountains of Guyana 5. Two newly described diminutive species in a novel lineage of the crown clade of Russula (Russulaceae). Phytotaxa 2024, 668, 117–129. [Google Scholar] [CrossRef]
- Naveed, M.; Jabeen, S.; Ijaz, H.; Azeem, M.; Khan, M.; Ullah, S. Russula iqbalii sp. nov., Identified in R. Subsect. Maculatinae from Pakistan, Based on Morphology, Microscopy, and Phylogeny. Microsc. Res. Tech. 2024, 88, 986–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-H.; Li, G.-J.; Phurbu, D.; He, M.-Q.; Zhang, M.-Z.; Zhu, X.-Y.; Li, J.-X.; Zhao, R.-L.; Cao, B. Four new species of Russula from the Xizang Autonomous Region and other provinces of China. Mycology 2024, 15, 210–237. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R. Ectomycorrhizal Mushrooms: Their Diversity, Ecology and Practical Applications. In Mycorrhiza—Function, Diversity, State of the Art; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Yu, W.Y.; Peng, M.H.; Wang, J.J.; Ye, W.Y.; Wang, Z.H.; Lu, G.D.; Bao, J.D. Micro-community associated with ectomycorrhizal Russula symbiosis and sporocarp-producing Russula in Fagaceae dominant nature areas in southern China. bioRxiv 2020. [Google Scholar] [CrossRef]
- Manz, C.; Adamčík, S.; Looney, B.P.; Corrales, A.; Ovrebo, C.; Adamčíková, K.; Hofmann, T.A.; Hampe, F.; Piepenbring, M. Four new species of Russula subsection Roseinae from tropical montane forests in western Panama. PLoS ONE 2021, 16, e0257616. [Google Scholar] [CrossRef]
- Jiang, S.; Ma, J.; Han, Y.-X.; Xue, R.; Su, L.-J.; Yu, T.-J.; Tang, L.-P. Russula rubrosquamosa (Russulaceae, Russulales), a new species from southwestern China. Mycoscience 2024, 65, 162–172. [Google Scholar] [CrossRef]
- Laurent-Webb, L.; Rech, P.; Bourceret, A.; Chaumeton, C.; Deveau, A.; Genola, L.; Januario, M.; Petrolli, R.; Selosse, M.A. Endophytic and ectomycorrhizal, an overlooked dual ecological niche? Insights from natural environments and Russula species. bioRxiv 2024. [Google Scholar] [CrossRef]
- Hughes, K.W.; Franklin, J.A.; Schweitzer, J.; Kivlin, S.N.; Case, A.; Aldrovandi, M.; Matheny, P.B.; Miller, A.N. Post-fire Quercus mycorrhizal associations are dominated by Russulaceae, Thelephoraceae, and Laccaria in the southern Appalachian Mountains. Mycol. Prog. 2025, 24, 16. [Google Scholar] [CrossRef]
- Adamčík, S.; Carteret, X.; Buyck, B. Type Studies on Some Russula Species Described by CH Peck. Cryptogam. Mycol. 2013, 34, 367–391. [Google Scholar] [CrossRef]
- Looney, B.P. Molecular annotation of type specimens of Russula species described by W.A. Murrill from the southeast United States. Mycotaxon 2015, 129, 255–268. [Google Scholar] [CrossRef]
- Xie, X.-C.; Buyck, B.; Song, Y. Species of Russula subgenera Archaeae, Compactae and Brevipedum (Russulaceae, Basidiomycota) from Dinghushan Biosphere Reserve. Eur. J. Taxon. 2023, 864, 28–63. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Y.-Y.; Yu, J.-L.; Yuan, R.; Li, F. Phylogenetic and morphological evidence for four new species of Russula (Russulaceae, Basidiomycota) from northwestern China. Eur. J. Taxon. 2024, 958, 48–76. [Google Scholar] [CrossRef]
- Adamčík, S.; Looney, B.; Caboň, M.; Jančovičová, S.; Adamčíková, K.; Avis, P.G.; Barajas, M.; Bhatt, R.P.; Corrales, A.; Das, K.; et al. The quest for a globally comprehensible Russula language. Fungal Divers. 2019, 99, 369–449. [Google Scholar] [CrossRef]
- Zhou, H.; Cheng, G.-Q.; Wang, Q.-T.; Guo, M.-J.; Zhuo, L.; Yan, H.-F.; Li, G.-J.; Hou, C.-L. Morphological Characteristics and Phylogeny Reveal Six New Species in Russula Subgenus Russula (Russulaceae, Russulales) from Yanshan Mountains, North China. J. Fungi 2022, 8, 1283. [Google Scholar] [CrossRef] [PubMed]
- Melera, S.; Ostellari, C.; Roemer, N.; Avis, P.G.; Tonolla, M.; Barja, F.; Narduzzi-Wicht, B. Analysis of morphological, ecological and molecular characters of Russula pectinatoides Peck and Russula praetervisa Sarnari, with a description of the new taxon Russula recondita Melera & Ostellari. Mycol. Prog. 2017, 16, 117–134. [Google Scholar] [CrossRef]
- Roy, N.; Chattopadhyay, P.; Dutta, A.K. A new species of Russula subg. Brevipes (Russulaceae) from the lateritic regions of West Bengal, India. N. Z. J. Bot. 2024, 63, 331–341. [Google Scholar] [CrossRef]
- Buyck, B.; Horak, E.; Cooper, J.A.; Song, Y. Russula (Basidiomycota, Russulales, Russulaceae) Subsection Roseinae “Down Under”. Cryptogam. Mycol. 2024, 45, 101–126. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Y.; Mi, F.; Tang, X.; He, X.; Cao, Y.; Liu, C.; Yang, D.; Dong, J.; Zhang, K.; et al. Recent advances in population genetics of ectomycorrhizal mushrooms Russula spp. Mycology 2015, 6, 110–120. [Google Scholar] [CrossRef]
- Caboň, M.; Eberhardt, U.; Looney, B.; Hampe, F.; Kolařík, M.; Jančovičová, S.; Verbeken, A.; Adamčík, S. New insights in Russula subsect. Rubrinae: Phylogeny and the quest for synapomorphic characters. Mycol. Prog. 2017, 16, 877–892. [Google Scholar] [CrossRef]
- Elliott, T.; Trappe, J. A worldwide nomenclature revision of sequestrate Russula species. Fungal Syst. Evol. 2018, 1, 229–242. [Google Scholar] [CrossRef]
- De Lange, R.; Adamčík, S.; Adamčíkova, K.; Asselman, P.; Borovička, J.; Delgat, L.; Hampe, F.; Verbeken, A. Enlightening the black and white: Species delimitation and UNITE species hypothesis testing in the Russula albonigra species complex. IMA Fungus 2021, 12, 20. [Google Scholar] [CrossRef]
- Looney, B.P.; Manz, C.; Matheny, P.B.; Adamčík, S. Systematic revision of the Roseinae clade of Russula, with a focus on eastern North American taxa. Mycologia 2022, 114, 270–302. [Google Scholar] [CrossRef] [PubMed]
- Nurhayat, O.D.; Putra, I.P.; Riffiani, R.; Taridala, S.A.A.; Arif, Z. Scrutinize the Taxonomical Identity of Green Edible Russula from Sulawesi (Indonesia). HAYATI J. Biosci. 2025, 32, 436–444. [Google Scholar] [CrossRef]
- Wang, X.H.; Yang, Z.L.; Li, Y.C.; Knudsen, H.; Liu, P.G. Russula griseocarnosa sp. nov. (Russulaceae, Russulales), a commercially important edible mushroom in tropical China: Mycorrhiza, phylogenetic position, and taxonomy. Nova Hedwig. 2009, 88, 269–282. [Google Scholar] [CrossRef]
- Li, G.J.; Liu, T.Z.; Li, S.M.; Zhao, S.Y.; Niu, C.Y.; Liu, Z.Z.; Xie, X.J.; Zhang, X.; Shi, L.Y.; Guo, Y.B.; et al. Four new species of Russula subsection Sardoninae from China. J. Fungi 2023, 9, 199. [Google Scholar] [CrossRef]
- Anh, C.N.; Chi, N.M.; Dell, B. Nutritional value of edible Russula griseocarnosa in Vietnam. Asian J. Agric. Rural. Dev. 2024, 14, 87–94. [Google Scholar] [CrossRef]
- Anh, C.N.; Chi, N.M.; Kiet, T.T.; Ha, N.T.N.; Dell, B. Harvest and trade of wild edible Russula griseocarnosa in North Vietnam. Asian J. Agric. Rural. Dev. 2024, 14, 128–139. [Google Scholar] [CrossRef]
- Cao, B.; Li, G.; Zhao, R. Species diversity and geographic components of Russula from the Greater and Lesser Khinggan Mountains. Biodivers. Sci. 2019, 27, 854. [Google Scholar]
- Haro-Luna, M.X.; Ruan-Soto, F.; Guzmán-Dávalos, L. Traditional knowledge, uses, and perceptions of mushrooms among the Wixaritari and mestizos of Villa Guerrero, Jalisco, Mexico. IMA Fungus 2019, 10, 16. [Google Scholar] [CrossRef]
- Pérez-Moreno, J.; Guerin-Laguette, A.; Rinaldi, A.C.; Yu, F.; Verbeken, A.; Hernández-Santiago, F.; Martínez-Reyes, M. Edible mycorrhizal fungi of the world: What is their role in forest sustainability, food security, biocultural conservation and climate change? Plants People Planet 2021, 3, 471–490. [Google Scholar] [CrossRef]
- Elkhateeb, W.A.; Daba, G.M. Bioactive potential of some fascinating edible mushrooms Macrolepiota, Russula, Amanita, Vovariella and Grifola as a treasure of multipurpose therapeutic natural product. J. Mycol. 2022, 5, 1–8. [Google Scholar]
- On-Nom, N.; Suttisansanee, U.; Chathiran, W.; Charoenkiatkul, S.; Thiyajai, P.; Srichamnong, W. Nutritional Security: Carbohydrate Profile and Folk Remedies of Rare Edible Mushrooms to Diversifying Food and Diet: Thailand Case Study. Sustainability 2023, 15, 14034. [Google Scholar] [CrossRef]
- Li, G.J.; Li, S.F.; Wen, H.A. The Russula species resource and its economic values of China. Acta Edulis Fungi 2010, 17, 155–160. [Google Scholar]
- Li, F.; Deng, Q.-L. Three new species of Russula from South China. Mycol. Prog. 2018, 17, 1305–1321. [Google Scholar] [CrossRef]
- Kostić, M.; Ivanov, M.; Fernandes, Â.; Pinela, J.; Calhelha, R.C.; Glamočlija, J.; Barros, L.; Ferreira, I.C.F.R.; Soković, M.; Ćirić, A. Antioxidant Extracts of Three Russula Genus Species Express Diverse Biological Activity. Molecules 2020, 25, 4336. [Google Scholar] [CrossRef]
- Li, G.-J.; Li, S.-M.; Buyck, B.; Zhao, S.-Y.; Xie, X.-J.; Shi, L.-Y.; Deng, C.-Y.; Meng, Q.-F.; Sun, Q.-B.; Yan, J.-Q.; et al. Three new Russula species in sect. Ingratae (Russulales, Basidiomycota) from southern China. MycoKeys 2021, 84, 103–139. [Google Scholar] [CrossRef]
- Liu, S.; Zhu, M.; Keyhani, N.O.; Wu, Z.; Lv, H.; Heng, Z.; Chen, R.; Dang, Y.; Yang, C.; Chen, J.; et al. Three New Species of Russulaceae (Russulales, Basidiomycota) from Southern China. J. Fungi 2024, 10, 70. [Google Scholar] [CrossRef]
- Khatua, S.; Dutta, A.K.; Acharya, K. Prospecting Russula senecis: A delicacy among the tribes of West Bengal. PeerJ 2015, 3, e810. [Google Scholar] [CrossRef]
- Khatua, S.; Acharya, K. Crude polysaccharides from two Russuloid myco-food potentiates murine macrophage by tuning TLR/NF-κB pathway. In Biotechnology and Biological Sciences; CRC Press: Boca Raton, FL, USA, 2019; pp. 281–286. [Google Scholar]
- Atri, N.S.; Mridu, M. Mushrooms—Some ethnomycological and sociobiological aspects. Kavaka 2018, 51, 11–19. [Google Scholar]
- Renthlei, L.; Lalhlenmawia, H.; Mishra, B.P.; Zothanzama, J. Proximate composition and micro-nutritional value of three Russula species from Mizoram, India. Sci. Vis. 2024, 24, 24–30. [Google Scholar] [CrossRef]
- Balkrishna, A.A.; Sharma, N.; Srivastava, D.; Chaudhary, P.; Arya, V. Medicinal Marvels: A Comprehensive Study at the Nutritional and Therapeutic Potential of Russula Mushrooms. Curr. Res. Environ. Appl. Mycol. J. Fungal Biol. 2024, 14, 71–88. [Google Scholar] [CrossRef]
- Arya, A. Beauty, Diversity, and Utility of Mushrooms on Postage Stamps. In Biology, Cultivation and Applications of Mushrooms; Springer: Singapore, 2022; pp. 403–432. [Google Scholar]
- Cheng, Y.; Gan, J.; Yan, B.; Wang, P.; Wu, H.; Huang, C. Polysaccharides from Russula: A review on extraction, purification, and bioactivities. Front. Nutr. 2024, 11, 1406817. [Google Scholar] [CrossRef] [PubMed]
- Sanmeea, R.; Dellb, B.; Lumyongc, P.; Izumorid, K.; Lumyong, S. Nutritive value of popular wild edible mushrooms from northern Thailand. Food Chem. 2003, 82, 527–532. [Google Scholar] [CrossRef]
- Mallick, S.; Dutta, A.; Dey, S.; Ghosh, J.; Mukherjee, D.; Sultana, S.S.; Mandal, S.; Paloi, S.; Khatua, S.; Acharya, K.; et al. Selective inhibition of Leishmania donovani by active extracts of wild mushrooms used by the tribal population of India: An in vitro exploration for new leads against parasitic protozoans. Exp. Parasitol. 2014, 138, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Niazi, A.R.; Shafique, M.; Imran, M.; Khalid, A.N. Evaluation of Mycochemical Analysis and In Vitro Biological Activities of Some Russula Species (Agaricomycetes) from Pakistan. Int. J. Med. Mushrooms 2021, 23, 35–43. [Google Scholar] [CrossRef]
- Manassila, M.; Sooksa-Nguan, T.; Boonkerd, N.; Rodtong, S.; Teaumroong, N. Phylogenetic diversity of wild edible Russula from northeastern Thailand on the basis of internal transcribed spacer sequence. Sci. Asia 2005, 31, 323–328. [Google Scholar] [CrossRef]
- Quiñónez-Martínez, M.; Ruan-Soto, F.; Aguilar-Moreno, I.E.; Garza-Ocañas, F.; Lebgue-Keleng, T.; Lavín-Murcio, P.A.; Enríquez-Anchondo, I.D. Knowledge and use of edible mushrooms in two municipalities of the Sierra Tarahumara, Chihuahua, Mexico. J. Ethnobiol. Ethnomedicine 2014, 10, 67. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Chu, Q.; Jia, R.; Chen, W.; Wang, Y.; Yu, X.; Zheng, X. Russula alutacea Fr. polysaccharide ameliorates inflammation in both RAW264.7 and zebrafish (Danio rerio) larvae. Int. J. Biol. Macromol. 2020, 145, 740–749. [Google Scholar] [CrossRef]
- Khatua, S.; Sen Gupta, S.; Ghosh, M.; Tripathi, S.; Acharya, K. Exploration of nutritional, antioxidative, antibacterial and anticancer status of Russula alatoreticula: Towards valorization of a traditionally preferred unique myco-food. J. Food Sci. Technol. 2021, 58, 2133–2147. [Google Scholar] [CrossRef]
- Khatua, S.; Acharya, K. Alkali treated antioxidative crude polysaccharide from Russula alatoreticula potentiates murine macrophages by tunning TLR/NF-κB pathway. Sci. Rep. 2019, 9, 1713. [Google Scholar] [CrossRef]
- Nandi, A.K.; Samanta, S.; Sen, I.K.; Devi, K.S.P.; Maiti, T.K.; Acharya, K.; Islam, S.S. Structural elucidation of an immunoenhancing heteroglycan isolated from Russula albonigra (Krombh.) Fr. Carbohydr. Polym. 2013, 94, 918–926. [Google Scholar] [CrossRef]
- Nandi, A.K.; Samanta, S.; Maity, S.; Sen, I.K.; Khatua, S.; Devi, K.S.P.; Acharya, K.; Maiti, T.K.; Islam, S.S. Antioxidant and immunostimulant β-glucan from edible mushroom Russula albonigra (Krombh.) Fr. Carbohydr. Polym. 2013, 99, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Tapwal, A.; Pandey, S.; Rishi, R.; Mishra, G.; Giri, K. Six unrecorded species of Russula (Russulales) from Nagaland, India and their nutrient composition. Nusant. Biosci. 2014, 6, 33–38. [Google Scholar] [CrossRef]
- Clericuzio, M.; Cassino, C.; Corana, F.; Vidari, G. Terpenoids from Russula lepida and R. amarissima (Basidiomycota, Russulaceae). Phytochemistry 2012, 84, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; He, Y.; Liang, Z.; Zhou, W.; Niu, T. Sulfation of (1→3)-β-d-glucan from the fruiting bodies of Russula virescens and antitumor activities of the modifiers. Carbohydr. Polym. 2009, 77, 628–633. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Liu, J.-C.; Yang, X.-D.; Kennedy, J.F. Purification, structural analysis and hydroxyl radical-scavenging capacity of a polysaccharide from the fruiting bodies of Russula virescens. Process. Biochem. 2010, 45, 874–879. [Google Scholar] [CrossRef]
- Ohtsuka, S.; Ueno, S.; Yoshikumi, C.; Hirose, F.; Ohmura, Y.; Wada, T.; Fujii, T.; Takahashi, E. Polysaccharides Having an Anticarcinogenic Effect and a Method of Producing Them from Species of Basidiomycetes. UK Patent 1331513, 26 September 1973. [Google Scholar]
- Li, Y.-M.; Zhong, R.-F.; Chen, J.; Luo, Z.-G. Structural characterization, anticancer, hypoglycemia and immune activities of polysaccharides from Russula virescens. Int. J. Biol. Macromol. 2021, 184, 380–392. [Google Scholar] [CrossRef]
- Çayan, F.; Deveci, E.; Tel-Çayan, G.; Duru, M.E. Identification and quantification of phenolic acid compounds of twenty-six mushrooms by HPLC–DAD. J. Food Meas. Charact. 2020, 14, 1690–1698. [Google Scholar] [CrossRef]
- Suri, O.P.; Shah, R.; Satti, N.K.; Suri, K.A. Russulactarorufin, a lactarane skeleton sesquiterpene from Russula brevipes. Phytochemistry 1997, 45, 1453–1455. [Google Scholar] [CrossRef]
- Shomali, N.; Onar, O.; Alkan, T.; Demirtaş, N.; Akata, I.; Yildirim, Ö. Investigation of the polyphenol composition, biological activities, and detoxification properties of some medicinal mushrooms from Turkey. Turk. J. Pharm. Sci. 2019, 16, 155–160. [Google Scholar] [CrossRef]
- Sutachit, S.; Sutachit, M. Medicinal Mushrooms: Past, Presentand Future. Ukr. Bot. J 2002, 5, 499–524. [Google Scholar]
- Jaengklang, C.; Jarikasem, S.; Sithisarn, P.; Klungsupya, P. Determination on antioxidant capacity and TLC analysis of ten Thai Russula mushroom extracts. Isan J. Pharm. Sci. 2015, 10, 241–250. [Google Scholar]
- Lee, P.-T.; Wu, M.-L.; Tsai, W.-J.; Ger, J.; Deng, J.-F.; Chung, H.-M. Rhabdomyolysis: An unusual feature with mushroom poisoning. Am. J. Kidney Dis. 2001, 38, E17. [Google Scholar] [CrossRef] [PubMed]
- Sanon, E.; OuÃ, J.C.; Ilboudo, S.; Guissou, M.K.; Guissou, P.I.; Sankara, P. Phytochemical screening and amino acids analysis of mushrooms from Burkina Faso. Afr. J. Biotechnol. 2017, 16, 1338–1344. [Google Scholar]
- Niazi, A.R.; Shafique, M.; Imran, M.; Latif, S. Estimation of some trace metals, bioactive compounds, curative antimicrobial and antioxidant agents from Russula foetens and Russula cf. foetentoides. Pak. J. Pharm. Sci. 2022, 35, 1371–1378. [Google Scholar] [PubMed]
- Hearst, M.; Nelson, D.; McCollum, G.; Ballard, L.M.; Millar, B.C.; Moore, S.; McClean, S.; Moore, J.E.; Rao, J.R. Antimicrobial properties of protein extracts from wild mushroom fungi and native plant species against hospital pathogens. J. Pharmacogn. Phytother. 2010, 2, 103–107. [Google Scholar]
- Khatua, S.; Dutta, A.K.; Chandra, S.; Paloi, S.; Das, K.; Acharya, K. Introducing a novel mushroom from mycophagy community with emphasis on biomedical potency. PLoS ONE 2017, 12, e0178050. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Kaneko, A.; Matsumoto, Y.; Hama, H.; Arihara, S. Russujaponols A− F, illudoid sesquiterpenes from the fruiting body of Russula japonica. J. Nat. Prod. 2006, 69, 1267–1270. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Matsumoto, Y.; Hama, H.; Tanaka, M.; Zhai, H.; Fukuyama, Y.; Arihara, S.; Hashimoto, T. Russujaponols G-L, illudoid sesquiterpenes, and their neurite outgrowth promoting activity from the fruit body of Russula japonica. Chem. Pharm. Bull. 2009, 57, 311–314. [Google Scholar] [CrossRef]
- Chelela, B.L.; Chacha, M.; Matemu, A. Chemical composition of ethanolic extracts of some wild mushrooms from Tanzania and their medicinal potentials. Int. J. Med. Mushrooms 2016, 18, 457–464. [Google Scholar] [CrossRef]
- Khatua, S.; Roy, T.; Acharya, K. Antioxidant and free radical scavenging capacity of phenolic extract from Russula laurocerasi. Asian J. Pharm. Clin. Res. 2013, 6, 156–160. [Google Scholar]
- Tan, J.W.; Dong, Z.J.; Du, Z.H.; Liu, J.K. Lepidolide, a novel seco-ring-A cucurbitane triterpenoid from Russula lepida (Basidiomycetes). Z. Naturforsch. C 2000, 57, 963–965. [Google Scholar] [CrossRef]
- Tan, J.; Dong, Z.; Hu, L.; Liu, J. Lepidamine, the first aristolane-type sesquiterpene alkaloid from the basidiomycete Russula lepida. Helvetica Chim. Acta 2003, 86, 307–309. [Google Scholar] [CrossRef]
- Maarisit, W.; Yamazaki, H.; Kanno, S.-I.; Tomizawa, A.; Lee, J.-S.; Namikoshi, M. Protein tyrosine phosphatase 1B inhibitory properties of seco-cucurbitane triterpenes obtained from fruiting bodies of Russula lepida. J. Nat. Med. 2017, 71, 334–337. [Google Scholar] [CrossRef]
- Appolinaire, K.K.; Hubert, K.K.; Eugène, K.J.; Ahipo, D.E.; Lucien, K.P. Proximate composition, minerals and amino acids profiles of selected wild edible Russula species from Côte d’Ivoire. Turk. J. Agric.-Food Sci. Technol. 2016, 4, 882–886. [Google Scholar] [CrossRef][Green Version]
- Joshi, M.; Pathania, P.; Sagar, A. Phytochemical analysis and in vitro antibacterial activity of Russula lepida and Pleurotus ostreatus from North West Himalayas. India. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 1032–1034. [Google Scholar]
- Zhang, G.; Sun, J.; Wang, H.; Ng, T. First isolation and characterization of a novel lectin with potent antitumor activity from a Russula mushroom. Phytomedicine 2010, 17, 775–781. [Google Scholar] [CrossRef]
- Oscar, J.G.; Kouamé, A.K.; Kouassi, H.K.; Eugène, J.P. Proximate composition and nutritional value of three edible mushrooms ectomycorrhizal (Russula mustelina, Russula delica and Russula lepida) from Côte d’Ivoire according to the maturity stages. World J. Adv. Res. Rev. 2019, 2, 21–30. [Google Scholar]
- Sontag, B.; Rüth, M.; Spiteller, P.; Arnold, N.; Steglich, W.; Reichert, M.; Bringmann, G. Chromogenic meroterpenoids from the mushrooms Russula ochroleuca and R. viscida. Eur. J. Org. Chem. 2006, 2006, 1023–1033. [Google Scholar] [CrossRef]
- Drewnowska, M.; Sąpór, A.; Jarzyńska, G.; Nnorom, I.C.; Sajwan, K.S.; Falandysz, J. Mercury in Russula mushrooms: Bioconcentration by yellow-ocher brittle gills Russula ochroleuca. J. Environ. Sci. Health Part A 2012, 47, 1577–1591. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Ng, T. A peptide with HIV-1 reverse transcriptase inhibitory activity from the medicinal mushroom Russula paludosa. Peptides 2007, 28, 560–565. [Google Scholar] [CrossRef]
- Khatua, S.; Acharya, K. Cold alkali-extractable antioxidative polysaccharide from Russula pseudocyanoxantha (Agaricomycetes), a novel mushroom, stimulates immune responses in RAW264. 7 cells by regulating the TLR/NF-κB pathway. Int. J. Med. Mushrooms 2024, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Basak, G.; Acharya, K. Evaluation of an antioxidative polysaccharide fraction from Russula pseudocyanoxantha, a novel mushroom, as a strategy to enhance innate immunity. Lett. Appl. NanoBioSci. 2024, 13, 157. [Google Scholar] [CrossRef]
- Sterner, O.; Bergman, R.; Franzén, C.; Wickberg, B. New sesquiterpenes in a proposed Russulaceae chemical defense system. Tetrahedron Lett. 1985, 26, 3163–3166. [Google Scholar] [CrossRef]
- Yaoita, Y.; Hirao, M.; Kikuchi, M.; Machida, K. Three new lactarane sesquiterpenoids from the mushroom Russula sanguinea. Nat. Prod. Commun. 2012, 7, 1133–1135. [Google Scholar] [CrossRef]
- Khatua, S.; Acharya, K. Water soluble antioxidative crude polysaccharide from Russula senecis elicits TLR modulated NF-κB signaling pathway and pro-inflammatory response in murine macrophages. Front. Pharmacol. 2018, 9, 985. [Google Scholar] [CrossRef]
- Khatua, S.; Acharya, K. Antioxidative and antibacterial ethanol extract from a neglected indigenous myco-food suppresses Hep3B proliferation by regulating ROS-driven intrinsic mitochondrial pathway. Biointerface Res. Appl. Chem. 2020, 11, 11202–11220. [Google Scholar] [CrossRef]
- Khatua, S.; Acharya, K. Isolation of crude polysaccharides from Russula senecis (Agaricomycetes): Characterization, antioxidant activity, and immune-enhancing properties. Int. J. Med. Mushrooms 2021, 23, 47–57. [Google Scholar] [CrossRef]
- Trakulsrichai, S.; Jeeratheepatanont, P.; Sriapha, C.; Tongpoo, A.; Wananukul, W. Myotoxic Mushroom Poisoning in Thailand: Clinical Characteristics and Outcomes. Int. J. Gen. Med. 2020, 13, 1139–1146. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Sousa, M.J.; Martins, A.; Ferreira, I.C. Tocopherols composition of Portuguese wild mushrooms with antioxidant capacity. Food Chem. 2010, 119, 1443–1450. [Google Scholar] [CrossRef]
- Hearn, M.T.W.; Jones, E.R.H.; Pellatt, M.G.; Thaller, V.; Turner, J.L. Natural acetylenes. Part XLII. Novel C7, C8, C9, and C10 polyacetylenes from fungal cultures. J. Chem. Soc. Perkin Trans. 1973, 1, 2785–2788. [Google Scholar] [CrossRef]
- Stojanova, M.; Ðukić, D.; Stojanova, M.T.; Lalević, B.; Nazari, S.H.; Bogevska, Z. Determination of polysaccharide content of Agaricus macrosporus and Russula vesca mushroom extracts. In Proceedings of the 4th International Symposium: Modern Trends in Agricultural Production, Rural Development, Agro-economy, Cooperatives and Environmental Protection, Vrnjačka Banja, Serbia, 29–30 June 2022; pp. 477–482. [Google Scholar] [CrossRef]
- Chen, J.; Shen, C.; He, T.; Yan, S.F. Anticancer and immunoregulation activities of a polysaccharide from Russula vinosa. Mod. Food Sci. Technol. 2016, 32, 16–21. (In Chinese) [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M. Evaluation of Polish wild mushrooms as beta-glucan sources. Int. J. Environ. Res. Public Health 2020, 17, 7299. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Tian, G.; Yan, H.; Geng, X.; Cao, Q.; Wang, H.; Ng, T.B. Characterization of polysaccharides with antioxidant and hepatoprotective activities from the wild edible mushroom Russula vinosa Lindblad. J. Agric. Food Chem. 2014, 62, 8858–8866. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Kawagishi, H. Plant growth regulators from mushrooms. J. Antibiot. 2020, 73, 657–665. [Google Scholar] [CrossRef]
- Yan, B.; Wang, R.; Fu, C.; Huang, C.; Lai, C.; Yong, Q. Procuring the polysaccharides with anti-inflammatory bioactivity from Russula vinosa Lindblad by citric acid extraction. Food Biosci. 2024, 59, 104079. [Google Scholar] [CrossRef]
- Yan, B.; Wu, H.; Zeng, K.; Huang, C.; Lai, C.; Yong, Q. Structural characterization and immunomodulatory activities of polysaccharides from Russula vinosa Lindblad extracted using KOH. J. Bioresour. Bioprod. 2025, 10, 1–9. [Google Scholar] [CrossRef]
- Panda, M.K.; Das, S.K.; Mohapatra, S.; Debata, P.R.; Tayung, K.; Thatoi, H. Mycochemical composition, bioactivities, and phylogenetic placement of three wild edible Russula species from Northern Odisha, India. Plant Biosyst.-Int. J. Deal. All Asp. Plant Biol. 2021, 155, 1041–1055. [Google Scholar] [CrossRef]
- Lovy, A.; Knowles, B.; Labbe, R.; Nolan, L. Activity of edible mushrooms against the growth of human T4 leukemic cancer cells, HeLa cervical cancer cells, and Plasmodium falciparum. J. Herbs Spices Med. Plants 2000, 6, 49–57. [Google Scholar] [CrossRef]
- Volcão, L.M.; Fernandes, C.L.F.; Ribeiro, A.C.; Brum, R.d.L.; Eslabão, C.F.; Badiale-Furlong, E.; Ramos, D.F.; Bernardi, E.; Júnior, F.M.R.d.S. Bioactive extracts of Russula xerampelina and Suillus granulatus in the in vitro control of Pseudomonas aeruginosa phytopathogenic. South Afr. J. Bot. 2021, 140, 218–225. [Google Scholar] [CrossRef]
- Wood, W.F.; Largent, D.L.; DeShazer, D.A. The cooked shellfish-odour of the mushroom Russula xerampelina. Biosyst. Ecol. 2024, 3, 1–3. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory Effects of Edible and Medicinal Mushrooms and Their Bioactive Immunoregulatory Products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef] [PubMed]
- Khatua, S.; Acharya, K. Exploring the Chemical Composition and Bioactivity of Ethanol Extract from Russula pseudocyanoxantha (Agaricomycetes), a Novel Mushroom of Tribal Preference in India. Int. J. Med. Mushrooms 2022, 24, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, H.; She, Z.; Lu, H.; Wen, M.; Wang, X.; Wei, Z.; Yang, S.; Guan, X.; Tong, Y.; et al. Phytochemical and medicinal profiling of Russula vinosa Lindbl (RVL) using multiomics techniques. LWT 2024, 192, 115723. [Google Scholar] [CrossRef]
- Hu, X.; Xu, B. Chemical compositions and health promoting effects of edible mushrooms from genus Russula. Phytomedicine Plus 2024, 6, 100677. [Google Scholar] [CrossRef]
- Chun, M.S.; Min, M.K.; Ryu, J.H.; Lee, D.S.; Lee, M.J.; Hyun, T.; Shon, S.W. Mortality Cases of Mushroom Poisoning with Russula subnigricans. Wilderness Environ. Med. 2023, 34, 372–376. [Google Scholar] [CrossRef]
- Parnmen, S.; Nooron, N.; Pringsulaka, O.; Binchai, S.; Rangsiruji, A. Discrimination of lethal Russula subnigricans from wild edible and morphologically similar mushrooms in the genus Russula using SCAR markers. Food Control. 2024, 158, 110239. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Akata, I.; Tepe, B. Metal concentration and health risk assessment of eight Russula mushrooms collected from Kizilcahamam-Ankara, Turkey. Environ. Sci. Pollut. Res. 2021, 28, 15743–15754. [Google Scholar] [CrossRef]
- Zhang, G.; Geng, H.; Zhao, C.; Li, F.; Li, Z.-F.; Lun, B.; Wang, C.; Yu, H.; Bie, S.; Li, Z. Chemical Constituents with Inhibitory Activity of NO Production from a Wild Edible Mushroom, Russula vinosa Lindbl, May Be Its Nutritional Ingredients. Molecules 2019, 24, 1305. [Google Scholar] [CrossRef]
- Khatua, S.; Chandra, S.; Acharya, K. Hot alkali-extracted antioxidative crude polysaccharide from a novel mushroom enhances immune response via TLR-mediated NF-κB activation: A strategy for full utilization of a neglected tribal food. J. Food Biochem. 2021, 45, e13594. [Google Scholar] [CrossRef]
- Zhao, T.; Sun, M.; Kong, L.; Wang, R.; Njateng, G.S.S.; Cheng, G. Phytochemical investigation on the fruiting body of Russula aruea Pers. Biochem. Syst. Ecol. 2019, 86, 103912. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.N.; Chen, T.; Tang, Q.Y.; Duan, Q.P.; Wang, B.J.; Yang, Q.S. Research on acetylation and antioxidant activity of Russula alutacea Fr. water-soluble polysaccharides. Bulg. Chem. Commun. 2017, 49, 59–63. [Google Scholar]
- Wang, B.; Yang, Q.; Chen, T.; Qin, X.; Tang, Q.; Xiao, Y.; Yang, Y.; Zhao, Y. Preparation and in vitro antioxidant activity of sulfated water-insoluble polysaccharides from Russula alutacea Fr. Southwest China J. Agric. Sci. 2017, 30, 2673–2679. [Google Scholar]
- Liu, Y.Z.; Gan, Y.K.; Chen, X.J.; Ming, T.H.; Zeng, X.Y. Comparison of Bacteriostatic Effects between Extracts from Russula and Cyclobalanopsis glauca. Food Sci. 2011, 32, 36–38. (In Chinese) [Google Scholar]
- Chatterjee, S.; Datta, R.; Dey, A.; Pradhan, P.; Acharya, K. In vivo hepatoprotective activity of ethanolic extract of Russula albonigra against carbon tetrachloride-induced hepatotoxicity in mice. Res. J. Pharm. Technol. 2012, 5, 1034–1038. [Google Scholar]
- He, T. Study on Structure and Bioactivity of Polysaccarides from Russula griseocarnosa. Master’s Thesis, South China University of Technology, Guangzhou, China, 2015. (In Chinese). [Google Scholar]
- Chen, Q.; Qi, C.; Peng, G.; Liu, Y.; Zhang, X.; Meng, Z. Immune-enhancing effects of a polysaccharide PRG1-1 from Russula griseocarnosa on RAW264.7 macrophage cells via the MAPK and NF-κB signalling pathways. Food Agric. Immunol. 2018, 29, 833–844. [Google Scholar] [CrossRef]
- Liu, Y.; Yong, T.; Cai, M.; Wu, X.; Guo, H.; Xie, Y.; Hu, H.; Wu, Q. Exploring the Potential of Russula griseocarnosa: A Molecular Ecology Perspective. Agriculture 2024, 14, 879. [Google Scholar] [CrossRef]
- Liu, X.; Dong, M.; Li, Y.; Li, L.; Zhang, Y.; Wang, C.; Wang, N.; Wang, D. Structural properties of glucan from Russula griseocarnosa and its immunomodulatory activities mediated via T cell differentiation. Carbohydr. Polym. 2024, 339, 122214. [Google Scholar] [CrossRef]
- Liu, X.; Dong, M.; Li, Y.; Li, L.; Zhang, Y.; Zhou, A.; Wang, D. Structural characterization of Russula griseocarnosa polysaccharide and its improvement on hematopoietic function. Int. J. Biol. Macromol. 2024, 263, 130355. [Google Scholar] [CrossRef]
- Chen, X.H.; Xia, L.X.; Zhou, H.B.; Qiu, G.Z. Chemical Composition and Antioxidant Activities of Russula griseocarnosa sp. nov. J. Agric. Food Chem. 2010, 58, 6966–6971. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, Y.; Liu, M.; Chen, Q.; Jiao, Y.; Meng, Z. Optimization extraction and bioactivities of polysaccharide from wild Russula griseocarnosa. Saudi Pharm. J. 2017, 25, 523–530. [Google Scholar] [CrossRef]
- Sun, Y.; He, H.; Wang, Q.; Yang, X.; Jiang, S.; Wang, D. A Review of Development and Utilization for Edible Fungal Polysaccharides: Extraction, Chemical Characteristics, and Bioactivities. Polymers 2022, 14, 4454. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Meng, Z. Purification, characterization and anti-tumor activities of polysaccharides extracted from wild Russula griseocarnosa. Int. J. Biol. Macromol. 2018, 109, 1054–1060. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Xiong, Q.; Yu, Y.; Peng, L. Sulfated modification, characterization, and potential bioactivities of polysaccharide from the fruiting bodies of Russula virescens. Int. J. Biol. Macromol. 2020, 154, 1438–1447. [Google Scholar] [CrossRef]
- Yu, G.; Liu, Y.; Gao, Y.; Jia, X.; Ma, R.; Li, T.; Feng, W.; Xu, C. Structure Characterization and Antioxidant Properties of a Triple Helix Galactoglucomannan from the Fruiting Bodies of Russula virescens (Agaricomycetes). Int. J. Med. Mushrooms 2025, 27, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Z.; Wang, L.; Walid, E.; Zhang, H. In Vitro Antioxidant and Anti-Proliferation Activities of Polysaccharides from Various Extracts of Different Mushrooms. Int. J. Mol. Sci. 2012, 13, 5801–5817. [Google Scholar] [CrossRef]
- Yimeng, L.I.; Wenzhi, L.I.; Ruifang, Z.H.; Ruihai, L.I.; Jian, C.H. Study on extraction process optimization, physicochemical properties, and antioxidant activity of polysaccharide from Russula adusta (Pers.) Fr. J. Henan Univ. Technol. (Nat. Sci. Ed.) 2020, 41, 19–26. [Google Scholar]
- Alkan, S.; Uysal, A.; Kasik, G.; Vlaisavljevic, S.; Berežni, S.; Zengin, G. Chemical Characterization, Antioxidant, Enzyme Inhibition and Antimutagenic Properties of Eight Mushroom Species: A Comparative Study. J. Fungi 2020, 6, 166. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; den Boer, M. WHO Leishmaniasis Control Team. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Kaewnarin, K.; Suwannarach, N.; Kumla, J.; Choonpicharn, S.; Tanreuan, K.; Lumyong, S. Characterization of Polysaccharides from Wild Edible Mushrooms from Thailand and Their Antioxidant, Antidiabetic, and Antihypertensive Activities. Int. J. Med. Mushrooms 2020, 22, 221–233. [Google Scholar] [CrossRef]
- Okwulehie, I.C.; Ogoke, J.A. Bioactive, nutritional and heavy metal constituents of some edible mushrooms found in Abia State of Nigeria. Int. J. Appl. Microbiol. Biotechnol. Res. 2013, 1, 7–15. [Google Scholar]
- Gao, J.-M.; Dong, Z.-J.; Liu, J.-K. A new ceramide from the basidiomycete Russula cyanoxantha. Lipids 2001, 36, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Clericuzio, M.; Vizzini, A. Terpenoids of Russula (Basidiomycota), with Emphasis on Cucurbitane Triterpenes. In Advances in Macrofungi; CRC Press: Boca Raton, FL, USA, 2019; pp. 254–277. [Google Scholar]
- Yaoita, Y.; Ono, H.; Kikuchi, M. A New Norsesquiterpenoid from Russula delica F R. Chem. Pharm. Bull. 2003, 51, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Yaoita, Y.; Watanabe, N.; Takano, D.; Kikuchi, M. Sesquiterpenoids from the fruit bodies of Russula delica. Nat. Med. 2004, 58, 235. [Google Scholar]
- Wang, X.-N.; Shen, J.-H.; Du, J.-C.; Liu, J.-K. Marasmane Sesquiterpenes Isolated from Russula foetens. J. Antibiot. 2006, 59, 669–672. [Google Scholar] [CrossRef]
- Daniewski, W.M.; Gumułka, M.; Ptaszyńska, K.; Skibicki, P.; Krajewski, J.; Gluziński, P. Marasmane lactones from Lactarius vellereus. Phytochemistry 1992, 31, 913–915. [Google Scholar] [CrossRef]
- Trudeau, S.; Morgan, J.B.; Shrestha, M.; Morken, J.P. Rh-Catalyzed Enantioselective Diboration of Simple Alkenes: Reaction Development and Substrate Scope. J. Org. Chem. 2005, 70, 9538–9544. [Google Scholar] [CrossRef]
- Matsuura, M.; Saikawa, Y.; Inui, K.; Nakae, K.; Igarashi, M.; Hashimoto, K.; Nakata, M. Identification of the toxic trigger in mushroom poisoning. Nat. Chem. Biol. 2009, 5, 465–467. [Google Scholar] [CrossRef]
- Kim, K.H.; Noh, H.J.; Choi, S.U.; Park, K.M.; Seok, S.-J.; Lee, K.R. Russulfoen, a new cytotoxic marasmane sesquiterpene from Russula foetens. J. Antibiot. 2010, 63, 575–577. [Google Scholar] [CrossRef]
- Tan, J.-W.; Dong, Z.-J.; Liu, J.-K. New terpenoids from basidiomycetes Russula lepida. Helv. Chim. Acta 2000, 83, 3191–3197. [Google Scholar] [CrossRef]
- Tan, J.W.; Xu, J.B.; Dong, Z.J.; Luo, D.Q.; Liu, J.K. Nigricanin, the first ellagic acid derived metabolite from the basidiomycete Russula nigricans. Helv. Chim. Acta 2004, 87, 1025–1028. [Google Scholar] [CrossRef]
- Malagòn, O.; Porta, A.; Clericuzio, M.; Gilardoni, G.; Gozzini, D.; Vidari, G. Structures and biological significance of lactarane sesquiterpenes from the European mushroom Russula nobilis. Phytochemistry 2014, 107, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.B.; Yang, G.H.; Wu, S.H.; Wang, S.F.; Li, G.Y.; Xu, W.K.; Meng, L.S.; Li, Z.Y. Chemical studies on Russula rosacea. Acta Pharm. Sin. 1994, 29, 39–43. [Google Scholar]
- Gilardoni, G.; Malagòn, O.; Tosi, S.; Clericuzio, M.; Vidari, G. Lactarane sesquiterpenes from the European mushrooms Lactarius aurantiacus, L. subdulcis, and Russula sanguinaria. Nat. Prod. Commun. 2014, 9, 1934578X1400900309. [Google Scholar] [CrossRef]
- Matsuzaki, N.; Wu, J.; Kawaide, M.; Choi, J.-H.; Hirai, H.; Kawagishi, H. Plant growth regulatory compounds from the mushroom Russula vinosa. Mycoscience 2016, 57, 404–407. [Google Scholar] [CrossRef][Green Version]
- Xu, M.-L.; Choi, J.-Y.; Jeong, B.-S.; Li, G.; Lee, K.-R.; Lee, C.-S.; Woo, M.-H.; Lee, E.S.; Jahng, Y.; Chang, H.-W.; et al. Cytotoxic constituents isolated from the fruit bodies of Hypsizigus marmoreus. Arch. Pharmacal Res. 2007, 30, 28–33. [Google Scholar] [CrossRef]
- Klungsupya, P. Apoptotic activity of ethanolic extract of Thai indigenous mushroom Russula alboareolata against L929, HeLa and HepG2 cells by MMP assay. Thai J. Pharm. Sci. (TJPS) 2016, 40, 1–9. [Google Scholar]
- Klungsupya, P.; Muangman, T.; Taengphan, W.; Pradermwong, K. Biological activities and phytochemical constituent assessments of Thai Russula mushroom extracts. Thai J. Pharm. Sci. (TJPS) 2018, 42, 46–50. [Google Scholar]
- Taengphan, W.; Klungsupya, P.; Maungman, T.; Pethtubtim, I.; Pradermwong, K.; Jangklang, C. Anti-inflammation and active compounds of four indigenous Thai Russula mushrooms. Biol. Chem. Res. 2019, 6, 155–162. [Google Scholar]
- Khatua, S.; Sikder, R.; Acharya, K. Chemical and biological studies on a recently discovered edible mushroom: A report. FABAD J. Pharm. Sci. 2018, 43, 241–247. [Google Scholar]
- Khatua, S.; Chandra, S.; Acharya, K. Expanding knowledge on Russula alatoreticula, a novel mushroom from tribal cuisine, with chemical and pharmaceutical relevance. Cytotechnology 2019, 71, 245–259. [Google Scholar] [CrossRef]
- Prasad, R.; Varshney, V.K.; Harsh, N.S.K.; Kumar, M. Antioxidant Capacity and Total Phenolics Content of the Fruiting Bodies and Submerged Cultured Mycelia of Sixteen Higher Basidiomycetes Mushrooms from India. Int. J. Med. Mushrooms 2015, 17, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Puttaraju, N.G.; Venkateshaiah, S.U.; Dharmesh, S.M.; Urs, S.M.N.; Somasundaram, R. Antioxidant Activity of Indigenous Edible Mushrooms. J. Agric. Food Chem. 2006, 54, 9764–9772. [Google Scholar] [CrossRef] [PubMed]
- Sharma, Y.P.; Sharma, R.; Khatua, S.; Acharya, K. Morphotaxonomy and comparative mycochemical study and antioxidant activity of hydromethanol, infusion and decoction extracts from Russula brevipes Peck. Indian Phytopathol. 2019, 72, 445–452. [Google Scholar] [CrossRef]
- Pacheco-Hernández, Y.; Villa-Ruano, N.; Lozoya-Gloria, E.; de Jesús Terán-Sánchez, E.; Becerra-Martínez, E. Revealing the 1H-NMR Profiling of Six Edible Mushrooms Consumed in the Northeastern Highlands of Puebla, Mexico. Chem. Biodivers. 2024, 10, e202301851. [Google Scholar] [CrossRef]
- Ye, M.; Jeon, Y.; Jin, H.; Kim, Y.; Lim, B. Russula cutefracta inhibits antigen-induced degranulation and Syk and MAPK phosphorylation in rat basophilic leukaemia cells. Allergol. Immunopathol. 2012, 40, 261–263. [Google Scholar] [CrossRef]
- Khan, F.; Chandra, R. Bioprospecting of Wild Mushrooms from India with Respect to Their Medicinal Aspects. Int. J. Med. Mushrooms 2019, 21, 181–192. [Google Scholar] [CrossRef]
- Zhao, S.; Zhao, Y.; Li, S.; Zhang, G.; Wang, H.; Ng, T.B. An antiproliferative ribonuclease from fruiting bodies of the wild mushroom Russula delica. J. Microbiol. Biotechnol. 2010, 20, 693–699. [Google Scholar] [CrossRef]
- Panchak, L.V. Russulaceae family mushrooms lectins: Function, purification, structural features and possibilities of practical applications. Biotechnol. Acta 2019, 12, 29–38. [Google Scholar] [CrossRef]
- Türkoğlu, A.; Duru, M.E.; Mercan, N. Antioxidant and Antimicrobial Activity of Russula delica Fr: An Edidle Wild Mushroom. Eurasian J. Anal. Chem. 2007, 2, 54–67. [Google Scholar] [CrossRef]
- Elmastas, M.; Isildak, O.; Turkekul, I.; Temur, N. Determination of antioxidant activity and antioxidant compounds in wild edible mushrooms. J. Food Compos. Anal. 2007, 20, 337–345. [Google Scholar] [CrossRef]
- Yaltirak, T.; Aslim, B.; Ozturk, S.; Alli, H. Antimicrobial and antioxidant activities of Russula delica Fr. Food Chem. Toxicol. 2009, 47, 2052–2056. [Google Scholar] [CrossRef] [PubMed]
- Amabye, T.G. Antioxidant and Anti-inflammatory Properties of Cultivated Mushrooms Grown in Mekelle City Tigray Ethiopia. Int. J. Nutr. Food Sci. 2015, 4, 578–583. [Google Scholar] [CrossRef][Green Version]
- Choocheep, K.; Nathip, N. Detection of a Non-animal Source of Glycosaminoglycans from Edible Mushrooms in Northern Thailand. Chiang Mai Univ. J. Nat. Sci. 2018, 17, 213–218. [Google Scholar] [CrossRef]
- Acay, H.; Baran, M. Fatty acid compositions of total lipid, phospholipid and triacylglycerol fractions of the wild edible mushroom Pleurotus ostreatus and Russula delica with cytotoxic activities on prostate carcinoma cell lines. Medicine 2019, 8, 736–740. [Google Scholar] [CrossRef]
- Choi, C.W.; Yoon, J.W.; Yon, G.H.; Kim, Y.S.; Ryu, S.Y.; Seok, S.J.; Kang, S.; Kim, Y.H. Multidrug resistance reversal activity of methanol extracts from basidiomycete mushrooms in cancer cells. Nat. Prod. Sci. 2012, 18, 239–243. [Google Scholar]
- Yamada, S.; Tanaka, M.; Miura, R.; Takeuchi, C.; Tu, Z.; Hu, D.; Matsuoka, K.; Sugawara, R.; Hoshibaa, T.; Yamaguchi, A. Anti-Inflammatory and Antimicrobial Activities of Aqueous Extracts of Wild Mushrooms from Japan. Int. J. Med. Mushrooms 2019, 21, 469–486. [Google Scholar] [CrossRef]
- Paul, C.; Das, N. Comparative study of bio-chemicals and antioxidant activities of two wild edible mushrooms Russula gnathangensis and Ramaria thindii from Sikkim Himalayas, India. Mushroom Res. 2021, 30, 41. [Google Scholar] [CrossRef]
- Lou, X.-H.; Gan, Y.-K.; Wang, L.-M.; Yan, L.; Yang, X. Protective effect of extract from Russula sp. on oxidative damage caused by formaldehyde. J. Toxicol. 2007, 21, 225–226. (In Chinese) [Google Scholar]
- Gan, Y.-K.; Chen, X.-J.; Wei, Q.-C.; Peng, S.-N.; Chen, L. The Influence of Temperature upon the Growth of the Natural Red Mushroom Hypha. J. Yulin Teach. Coll. (Nat. Sci.) 2007, 28, 58–60+64. (In Chinese) [Google Scholar]
- Gan, Y.-K.; Chen, X.-J.; Su, L.; He, Y. Effects of Aquatic Extraction Substance from Tenebrio molitor Linnaeus Feces on Mycelium Growth of Five Edible Fungi. J. Anhui Agric. Sci. 2008, 36, 11295–11296+11320. [Google Scholar]
- Dong, J.; Zhang, M.; Lu, L.; Sun, L.; Xu, M. Nitric oxide fumigation stimulates flavonoid and phenolic accumulation and enhances antioxidant activity of mushroom. Food Chem. 2012, 135, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Y.; Shen, X.; Chao, X.; Ho, C.C.; Cheng, X.L.; Zhang, Y.; Lin, R.C.; Du, K.J.; Luo, W.J.; Chen, J.Y.; et al. Nitric oxide as a developmental and metabolic signal in filamentous fungi. Mol. Microbiol. 2020, 113, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Butkhup, L.; Samappito, W.; Jorjong, S. Evaluation of bio-activities and phenolic contents of wild edible mushrooms from northeastern Thailand. Food Sci. Biotechnol. 2018, 27, 193–202. [Google Scholar] [CrossRef]
- O’Callaghan, Y.C.; O’Brien, N.M.; Kenny, O.; Harrington, T.; Brunton, N.; Smyth, T.J. Anti-Inflammatory Effects of Wild Irish Mushroom Extracts in RAW264. 7 Mouse Macrophage Cells. J. Med. Food 2015, 18, 202–207. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, G.; Hur, H.; Lee, U.; Lee, T. Immuno-potentiating and Antitumor Effects against Mouse Sarcoma 180 by Crude Polysaccharides Extracted from Fruiting Body of Russula rosacea. Korean J. Mycol. 2008, 36, 84–92. [Google Scholar] [CrossRef][Green Version]
- Yoon, K.N.; Lee, T.S. In vitro antioxidant, anti-hyperglycemic, anti-cholinesterase, and inhibition of nitric oxide production activities of methanol and hot water extracts of Russula rosacea mushroom. J. Mushroom 2015, 13, 1–10. [Google Scholar] [CrossRef]
- Takahashi, A.; Agatsuma, T.; Ohta, T.; Nunozawa, T.; Endo, T.; Nozoe, S. Russuphelins B, C, D, E and F, New Cytotoxic Substances from the Mushroom Russula subnigricans Hongo. Chem. Pharm. Bull. 1993, 41, 1726–1729. [Google Scholar] [CrossRef]
- Takahashi, A.; Agatsuma, T.; Matsuda, M.; Ohta, T.; Nunozawa, T.; Endo, T.; Nozoe, S. Russuphelin A, a New Cytotoxic Substane from the Mushroom Russula subnigricans Hongo. Chem. Pharm. Bull. 1992, 40, 3185–3188. [Google Scholar] [CrossRef]
- Adejumo, T.O.; Awosanya, O.B. Proximate and mineral composition of four edible mushroom species from South Western Nigeria. Afr. J. Biotechnol. 2005, 4, 1084–1088. [Google Scholar]
- Stojanova, M.; Djukic, D.; Veskovic-Moracanin, S.; Stojanova, M.T.; Mladenoska, I.; Limani-Bektashi, N. Determination of antioxidant potential of Agaricus macrosporus and Russula vesca mushroom extracts. In Proceedings of the III International Agricultural, Biological & Life Science Conference, Istanbul, Turkey, 15–17 October 2021. [Google Scholar]
- Zhang, H.; Li, C.; Lai, P.F.; Chen, J.; Xie, F.; Xia, Y.; Ai, L. Fractionation, chemical characterization and immunostimulatory activity of β-glucan and galactoglucan from Russula vinosa Lindblad. Carbohydr. Polym. 2021, 256, 117559. [Google Scholar] [CrossRef]
- Leahu, A.; Damian, C.; Oroian, M.; Ropciuc, S. Establishing the antioxidant activity based on chemical composition of wild edible mushrooms. Food Environ. Saf. J. 2016, 14, 398–406. [Google Scholar]
- Hur, J.; Young Choi, S.; Ou Lim, B. In vitro anti–inflammatory activity of Russula virescens in the macrophage like cell line RAW 264.7 activated by lipopolysaccharide. Nutr. Food Sci. 2012, 2, 142. [Google Scholar] [CrossRef]
- Wang, X.H.; Liu, P.G.; Yu, F.Q. Color Atlas of Wild Commercial Mushrooms in Yunnan; Yunnan Science and Technology Press: Kunming, China, 2004. [Google Scholar]
- Demirbaş, A. Heavy metal bioaccumulation by mushrooms from artificially fortified soils. Food Chem. 2001, 74, 293–301. [Google Scholar] [CrossRef]
- Georgescu, A.A.; Busuioc, G. Determination of heavy metals in several species of wild mushrooms and their influence on peroxidase activity. Lucr. Ştiinţifice 2011, 54, 62–66. [Google Scholar]
- Güler, P.; Khatun, S.; Cakilcioglu, U.; Chatterjee, N.C. Antifungal Effects of Russula cyanoxantha Against the Plant Pathogen Fusarium moniliforme and Fusarium culmorum. In Proceedings of the 3rd International Biotechnology and Biodiversity Conference & Exhibition, Diyarbakır, Turkey, 22–26 October 2012; pp. 9–11. [Google Scholar]
- Bonkat, G.; Widmer, A.F.; Rieken, M.; van der Merwe, A.; Braissant, O.; Müller, G.; Wyler, S.; Frei, R.; Gasser, T.C.; Bachmann, A. Microbial biofilm formation and catheter-associated bacteriuria in patients with suprapubic catheterisation. World J. Urol. 2013, 31, 565–571. [Google Scholar] [CrossRef]
- Branda, S.S.; Vik Å Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.F.R.; Lourenço, I.; Costa, E.; Martins, A.; Pintado, M. Wild Mushroom Extracts as Inhibitors of Bacterial Biofilm Formation. Pathogens 2014, 3, 667–679. [Google Scholar] [CrossRef]
- Sácký, J.; Leonhardt, T.; Kotrba, P. Functional analysis of two genes coding for distinct cation diffusion facilitators of the ectomycorrhizal Zn-accumulating fungus Russula atropurpurea. BioMetals 2016, 29, 349–363. [Google Scholar] [CrossRef]
- Leonhardt, T.; Sácký, J.; Šimek, P.; Šantrůček, J.; Kotrba, P. Metallothionein-like peptides involved in sequestration of Zn in the Zn-accumulating ectomycorrhizal fungus Russula atropurpurea. Metallomics 2014, 6, 1693–1701. [Google Scholar] [CrossRef]
- Leonhardt, T.; Sácký, J.; Kotrba, P. Functional analysis RaZIP1 transporter of the ZIP family from the ectomycorrhizal Zn-accumulating Russula atropurpurea. BioMetals 2018, 31, 255–266. [Google Scholar] [CrossRef]
- Vetter, J.; Siller, I.; Horváth, Z. Zinc content of sporocarps of basidiomycetous fungi. Mycologia 1997, 89, 481–483. [Google Scholar] [CrossRef]
- Borovička, J.; Řanda, Z. Distribution of iron, cobalt, zinc and selenium in macrofungi. Mycol. Prog. 2007, 6, 249–259. [Google Scholar] [CrossRef]
- Leonhardt, T.; Borovička, J.; Sácký, J.; Šantrůček, J.; Kameník, J.; Kotrba, P. Zn over accumulating Russula species clade together and use the same mechanism for the detoxification of excess Zn. Chemosphere 2019, 225, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Busuioc, G.; Elekes, C.C.; Stihi, C.; Iordache, S.; Ciulei, S.C. The bioaccumulation and translocation of Fe, Zn, and Cu in species of mushrooms from Russula genus. Environ. Sci. Pollut. Res. 2011, 18, 890–896. [Google Scholar] [CrossRef]
- Saithong, P.; Permpool, J.; Rattanaloeadnusorn, S.; Poompurk, P.; Khunnamwong, P.; Lomthong, T. Nutritional compositions, microbial quality, bioactivities, and volatile compounds of a novel vinegar from wild edible mushroom, Russula delica Fr. Food Prod. Process. Nutr. 2024, 6, 50. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Lu, J.-K.; Liu, L.-Z.; Sui, Z.-N.; Peng, Y.-Y.; Zhang, Y.; Qu, H.; Yi, J.-J. Fortification Effect of Mixed Fermentation Product of Russula vinosa Lindblad Supplementation on Physicochemical, Sensory and Antioxidant Properties of Wheat Bread. Food Med. Homol. 2025, 4, 71. [Google Scholar] [CrossRef]
- Zhu, M.-J.; Du, F.; Zhang, G.-Q.; Wang, H.-X.; Ng, T.-B. Purification a laccase exhibiting dye decolorizing ability from an edible mushroom Russula virescens. Int. Biodeterior. Biodegradation 2013, 82, 33–39. [Google Scholar] [CrossRef]
- Lajin, B.; Braeuer, S.; Borovička, J.; Goessler, W. Is the water disinfection by-product dichloroacetic acid biosynthesized in the edible mushroom Russula nigricans? Chemosphere 2021, 281, 130819. [Google Scholar] [CrossRef]
- Semreen, M.H.; Aboul-Enein, H.Y. Determination of Heavy Metal Content in Wild-Edible Mushroom from Jordan. Anal. Lett. 2011, 44, 932–941. [Google Scholar] [CrossRef]
- Yildirim, A.; Acay, H. Methylene blue and malachite green dyes adsorption onto Russula delica/bentonite/tripolyphosphate. Heliyon 2025, 11, e41250. [Google Scholar] [CrossRef]
- Matsuura, M.; Kato, S.; Saikawa, Y.; Nakata, M.; Hashimoto, K. Identification of Cyclopropylacetyl-(R)-carnitine, a Unique Chemical Marker of the Fatally Toxic Mushroom Russula subnigricans. Chem. Pharm. Bull. 2016, 64, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Mu, M.; Yang, F.; Yang, C. Russula subnigricans Poisoning: From Gastrointestinal Symptoms to Rhabdomyolysis. Wilderness Environ. Med. 2015, 26, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.T.; Han, J.H. A case of mushroom poisoning with Russula subnigricans: Development of rhabdomyolysis.; acute kidney injury, cardiogenic shock, and death. J. Korean Med. Sci. 2016, 31, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, P.; Kirsi, M.; Mustonen, A.-M. Suspected Myotoxicity of Edible Wild Mushrooms. Exp. Biol. Med. 2006, 231, 221–228. [Google Scholar] [CrossRef]
- Pu, Y.; Wang, F.; Zhang, H.; Chai, X.; Xiao, B. Hemolysis associated with Russula subnigricans ingestion in a patient with glucose-6-phosphate dehydrogenase deficiency. Clin. Toxicol. 2023, 61, 473–475. [Google Scholar] [CrossRef]
- Min, M.K.; Lee, D.; Shon, S.W.; Ryu, J.H.; Wang, I.; Lee, M.J.; Chun, M.; Hyun, T. Russula subnigricans Poisoning Causes Severe Rhabdomyolysis That Could be Misdiagnosed as Non-ST Segment Elevation Myocardial Infarction. Wilderness Environ. Med. 2022, 33, 324–328. [Google Scholar] [CrossRef]
- Grodzinskaya, A.A.; Nebesnyi, V.B.; Landin, V.P.; Gabriel, J. Radioactive Contamination of Wild Mushrooms from Ukraine under Conditions of Contrasting Radiation Loads: 36 Years after the Chernobyl Nuclear Power Plant Catastrophe. Int. J. Med. Mushrooms 2022, 24, 25–40. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Socha, K.; Witkowska, A.M.; Zujko, M.E.; Borawska, M.H. Cadmium and Lead in Wild Edible Mushrooms from the Eastern Region of Poland’s ‘Green Lungs’. Pol. J. Environ. Stud. 2013, 22, 1759–1765. [Google Scholar]
- Senila, M.; Resz, M.-A.; Senila, L.; Torok, I. Application of Diffusive Gradients in Thin-films (DGT) for assessing the heavy metals mobility in soil and prediction of their transfer to Russula virescens. Sci. Total. Environ. 2024, 909, 168591. [Google Scholar] [CrossRef]
- Huang, G.W.; Zhang, P. Symbiosis Cultivation of Ectomycorrhizal Mycelium. Edible Fungi China 2005, 24, 16–17. (In Chinese) [Google Scholar]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Wand, R.; Liu, C.-G.; Xu, J. The Separation and Cultivation of Ectomycorrhizal Fungi. Edible Fungi China 2013, 32, 4–7. (In Chinese) [Google Scholar]
- Hall, I.R.; Yun, W.; Amicucci, A. Cultivation of edible ectomycorrhizal mushrooms. Trends Biotechnol. 2003, 21, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Giomaro, G.M.; Sisti, D.; Zambonelli, A. Cultivation of edible ectomycorrhizal fungi by in vitro mycorrhizal synthesis. In In Vitro Culture of Mycorrhizas; Soil Biology; Declerck, S., Fortin, J.A., Strullu, D.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 4, pp. 253–267. [Google Scholar] [CrossRef]
- Tedersoo, L.; May, T.W.; Smith, M.E. Ectomycorrhizal lifestyle in fungi: Global diversity, distribution, and evolution of phylogenetic lineages. Mycorrhiza 2010, 20, 217–263. [Google Scholar] [CrossRef] [PubMed]
- Luan, Q.-S.; Jin, R.-Z.; Yun, L.-L. Study on Isolation and Cultivation of Wild Macro Fungi in Forest. In Proceedings of the 11th Chapter of the 2004 China Association for Science and Technology Annual Conference, Qionghai, China, 20–21 November 2004; pp. 489–491. (In Chinese). [Google Scholar]
- Karwa, A.; Varma, A.; Rai, M. Edible Ectomycorrhizal Fungi: Cultivation, Conservation and Challenges. In Diversity and Biotechnology of Ectomycorrhizae; Soil Biology; Rai, M., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 25. [Google Scholar] [CrossRef]
- Chen, Y.H. The theories and application of th cultivation of Russula vinosa. In Proceedings of the First Cross-Strait Symposium on Edible (Medicinal) Fungi, Fuzhou, China, 1 November 2005; pp. 97–100. (In Chinese). [Google Scholar]
- Yamada, A.; Ogura, T.; Ohmasa, M. Cultivation of mushrooms of edible ectomycorrhizal fungi associated with Pinus densiflora by in vitro mycorrhizal synthesis. Mycorrhiza 2001, 11, 59–66. [Google Scholar] [CrossRef]
- Hintikka, V.; Niemi, K. Aseptic culture of slowly growing mycorrhical Russula and Cortinarius species. Karstenia 1999, 39, 39–41. [Google Scholar] [CrossRef]
- Wang, E.J.; Jeon, S.M.; Jang, Y.; Ka, K.H. Mycelial growth of edible ectomycorrhizal fungi according to nitrogen sources. Korean J. Mycol. 2016, 44, 166–170. [Google Scholar] [CrossRef]
- Wu, J. Method for Preparing Russula Cultivation Material by Utilizing Sunflower by-Products (CN103601596B); China National Intellectual Property Administration: Beijing, China, 2015. [Google Scholar]
- Hortal, S.; Pera, J.; Parladé, J. Tracking mycorrhizas and extraradical mycelium of the edible fungus Lactarius deliciosus under field competition with Rhizopogon spp. Mycorrhiza 2008, 18, 69–77. [Google Scholar] [CrossRef]
- De Miguel, A.M.; Águeda, B.; Sánchez, S.; Parladé, J. Ectomycorrhizal fungus diversity and community structure with natural and cultivated truffle hosts: Applying lessons learned to future truffle culture. Mycorrhiza 2014, 24, S5–S18. [Google Scholar] [CrossRef]
- Lian, C.L. Research Progress on Cultivating Russula in Harvested Forest Lands. Fujian For. 2023, 22–23. (In Chinese) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).