Analysis of Amino Acid and Derivative Diversity and Antioxidant Capacity in Ophiocordyceps sinensis and Its Substitutes
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Metabolite Extraction
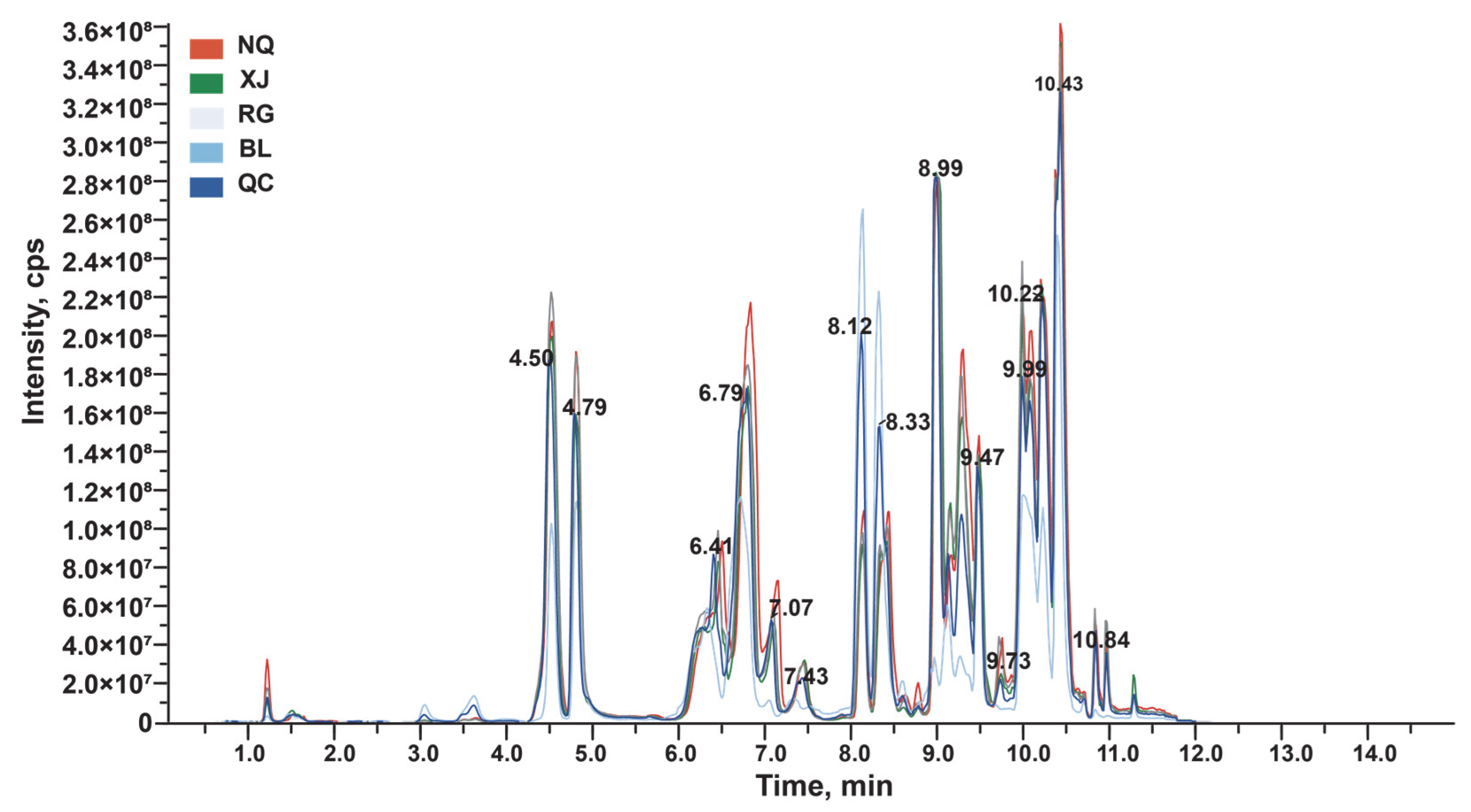
2.3. LC-MS/MS Analysis
2.4. Data Processing
2.5. Calibration Curves Quantification
2.6. Measurement of Physiological and Biochemical Parameters
3. Results
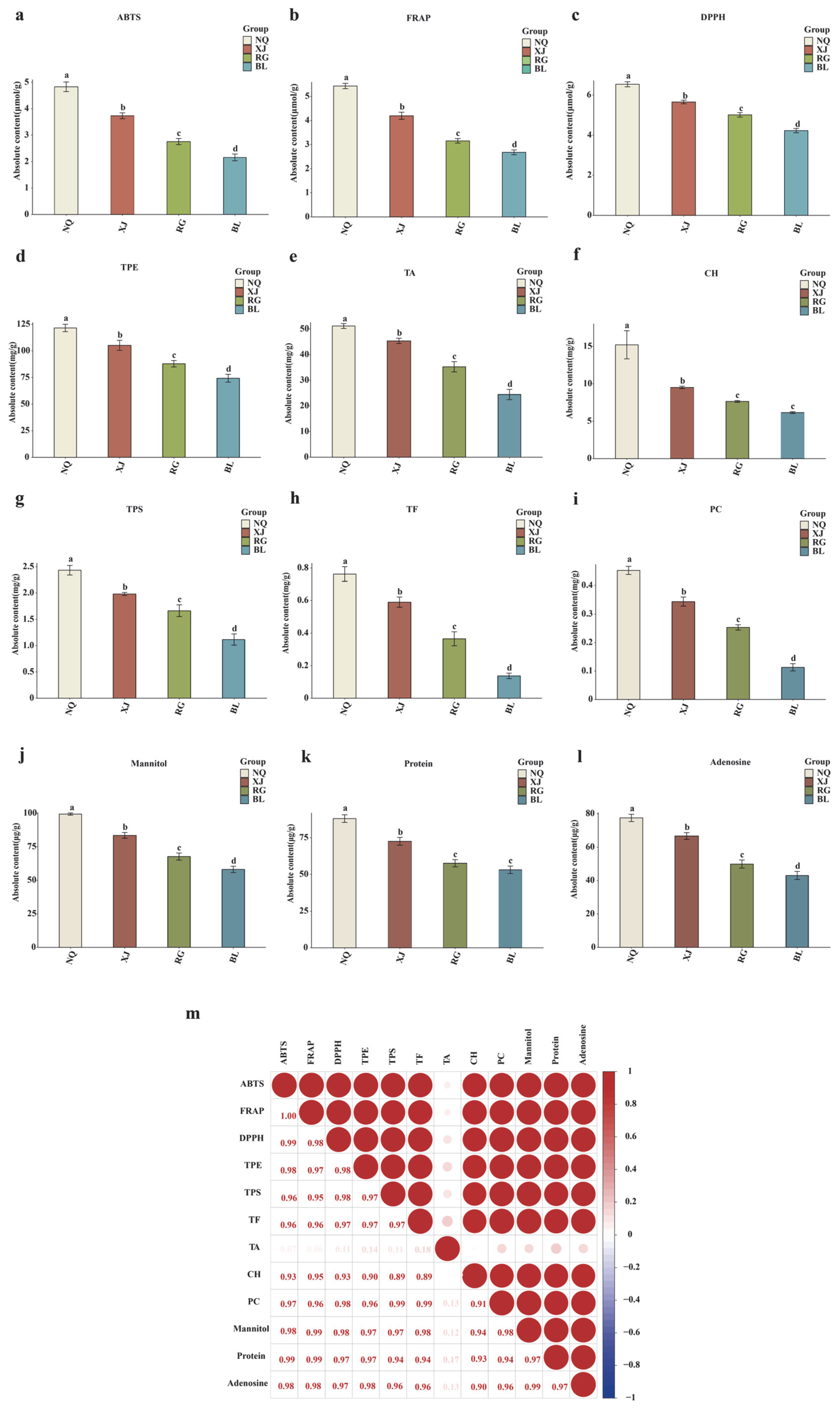
3.1. Physiological and Biochemical Characteristics of Four O. sinensis Samples
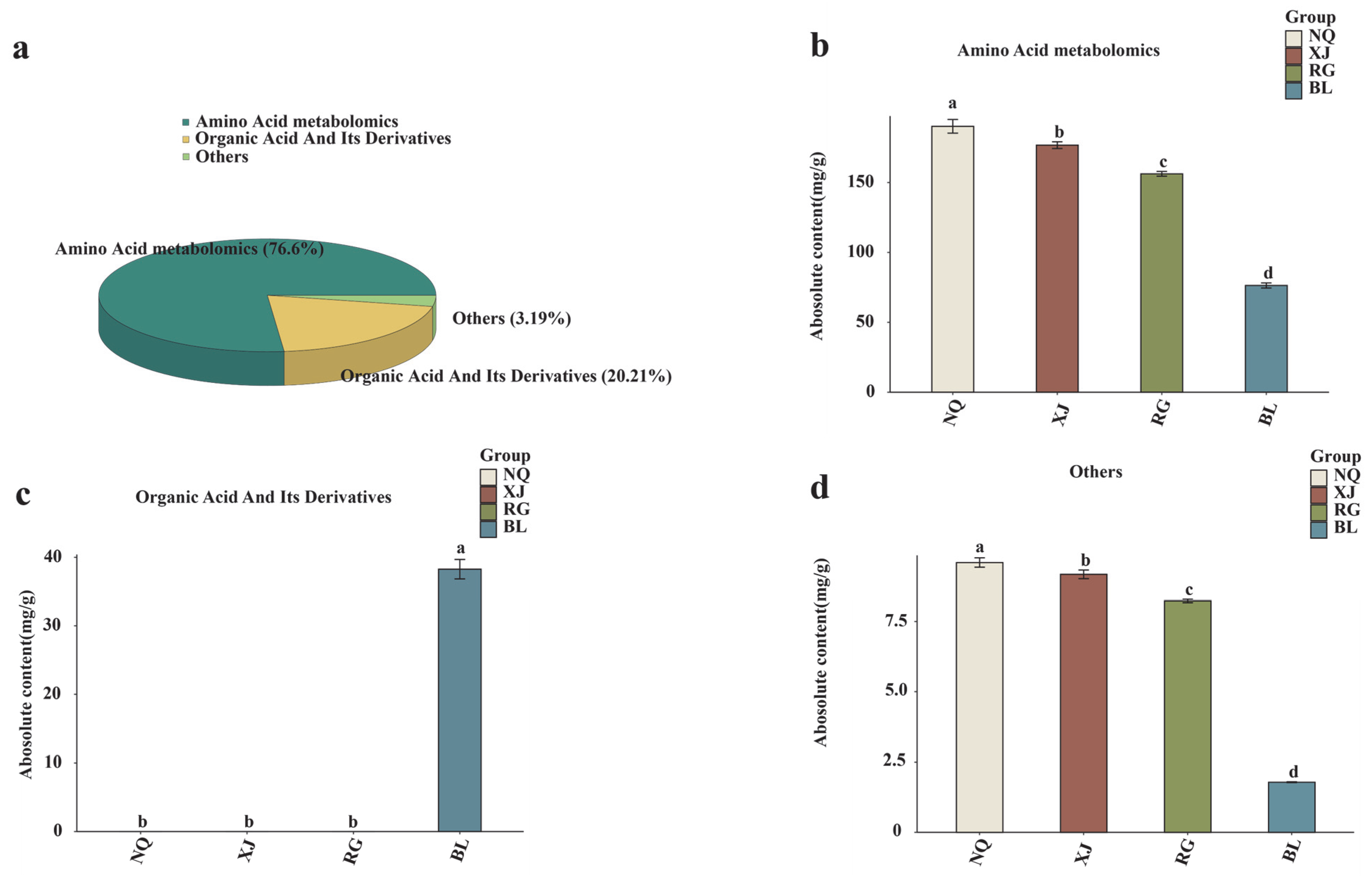
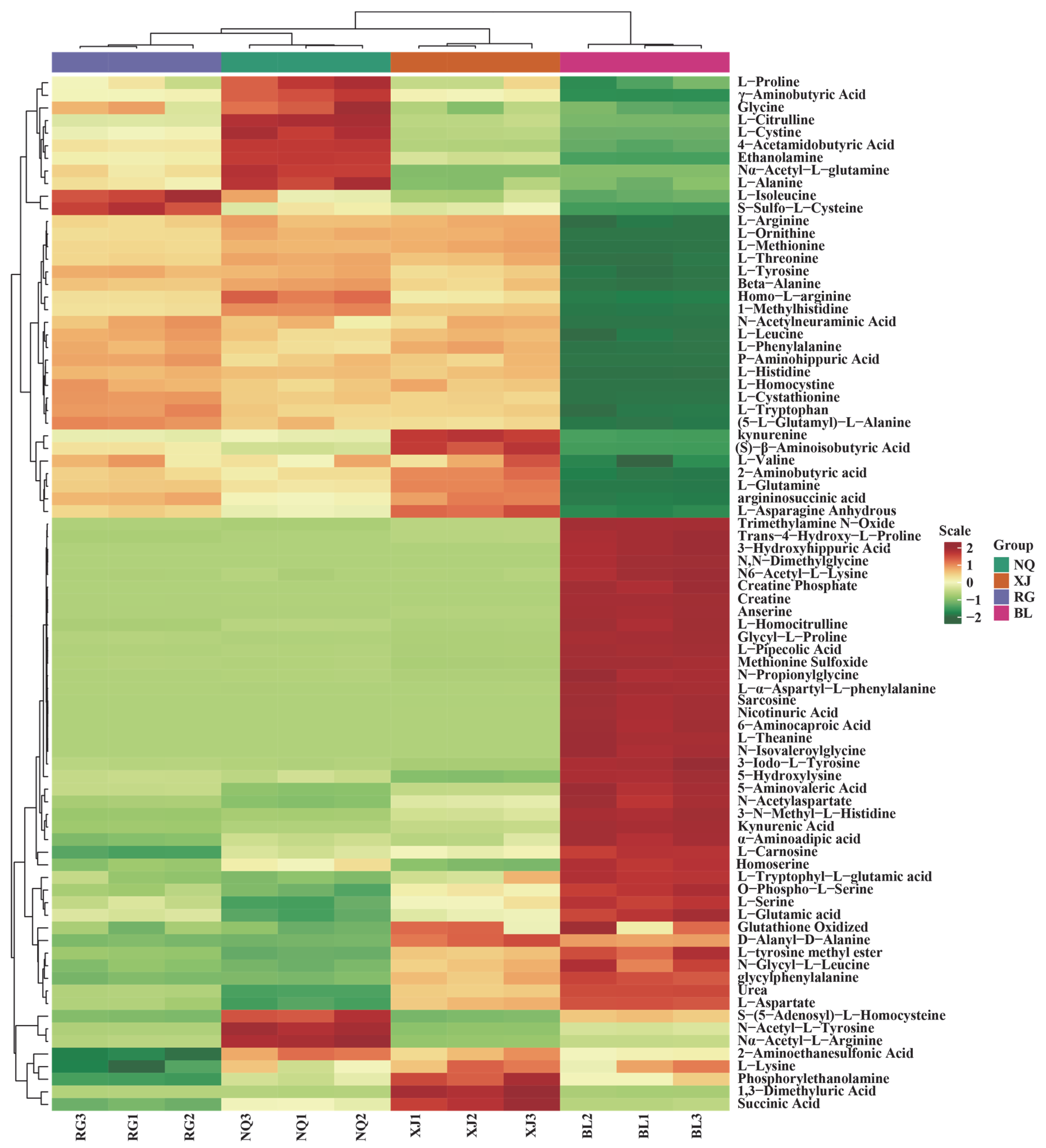
3.2. Amino Acid Metabolic Profiling
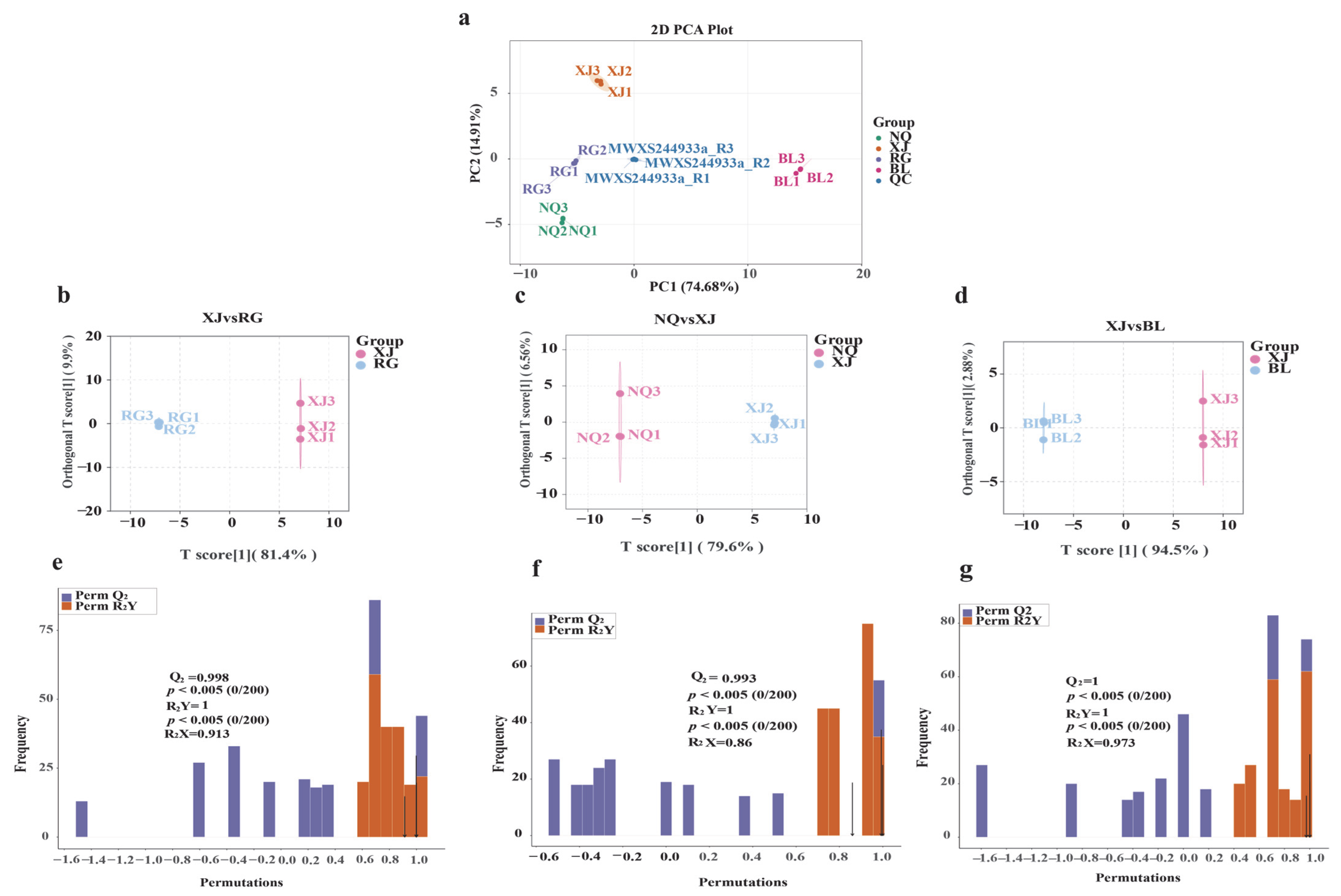
3.3. Multivariate Statistical Analysis of Amino Acid Profiles
3.4. Identification and Characterization of DAAMs
3.5. Correlation Between Amino Acids and Physiological-Biochemical Characteristics
3.6. KEGG Pathway Enrichment and Key Functional Annotations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhu, J.S.; Halpern, G.M.; Jones, K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis: Part II. J. Altern. Complement. Med. 1998, 4, 429–457. [Google Scholar] [CrossRef]
- Li, S.P.; Yang, F.Q.; Tsim, K.W. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J. Pharm. Biomed. Anal. 2006, 41, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Wang, T.; Tang, C.; He, M.; Li, Y.; Li, X. Effects of different drying methods on amino acid metabolite content and quality of Ophiocordyceps sinensis by LC-MS/MS combined with multivariate statistical methods. Metabolites 2024, 14, 459. [Google Scholar] [CrossRef]
- Liu, Q.B.; Lu, J.G.; Jiang, Z.H.; Zhang, W.; Li, W.J.; Qian, Z.M.; Bai, L.P. In situ chemical profiling and imaging of cultured and natural Cordyceps sinensis by TOF-SIMS. Front. Chem. 2022, 10, 862007. [Google Scholar] [CrossRef]
- Ng, T.B.; Wang, H.X. Pharmacological actions of Cordyceps, a prized folk medicine. J. Pharm. Pharmacol. 2005, 57, 1509–1519. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Q.; Weng, Q. Secondary metabolites (SMs) of Isaria cicadae and Isaria tenuipes. RSC Adv. 2018, 9, 172–184. [Google Scholar] [CrossRef]
- Nxumalo, W.; Elateeq, A.A.; Sun, Y. Can Cordyceps cicadae be used as an alternative to Cordyceps militaris and Cordyceps sinensis?—A review. J. Ethnopharmacol. 2020, 257, 112879. [Google Scholar] [CrossRef] [PubMed]
- Krishna, K.V.; Balasubramanian, B.; Park, S.; Bhattacharya, S.; Kadanthottu Sebastian, J.; Liu, W.C.; Pappuswamy, M.; Meyyazhagan, A.; Kamyab, H.; Chelliapan, S.; et al. Conservation of endangered Cordyceps sinensis through artificial cultivation strategies of C. militaris, an alternate. Mol. Biotechnol. 2025, 67, 1382–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Tang, C.; Chen, J.; Liang, J.; Li, Y.; Li, X. Accumulation characteristics of natural Ophiocordyceps sinensis metabolites driven by environmental factors. Metabolites 2024, 14, 414. [Google Scholar] [CrossRef]
- Wang, C.; Hou, X.X.; Rui, H.L.; Li, L.J.; Zhao, J.; Yang, M.; Sun, L.J.; Dong, H.R.; Cheng, H.; Chen, Y.P. Artificially cultivated Ophiocordyceps sinensis alleviates diabetic nephropathy and its podocyte injury via inhibiting P2X7R expression and NLRP3 inflammasome activation. J. Diabetes Res. 2018, 2018, 1390418. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Q.; Li, W.; Li, Q.; Qian, Z.; Liu, X.; Dong, C. A breakthrough in the artificial cultivation of Chinese cordyceps on a large-scale and its impact on science, the economy, and industry. Crit. Rev. Biotechnol. 2019, 39, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zou, G.; Liu, H.; Wei, Y. Application of CRISPR/Cas genome editing technology in the synthesis of secondary metabolites of filamentous fungi. Synth. Biol. J. 2023, 4, 738–755. [Google Scholar] [CrossRef]
- Kunhorm, P.; Chueaphromsri, P.; Chaicharoenaudomrung, N.; Noisa, P. Enhancement of cordycepin production from Cordyceps militaris culture by epigenetic modification. Biotechnol. Lett. 2022, 44, 581–593. [Google Scholar] [CrossRef]
- Zhong, X.; Gu, L.; Wang, H.; Lian, D.; Zheng, Y.; Zhou, S.; Zhou, W.; Gu, J.; Zhang, G.; Liu, X. Profile of Ophiocordyceps sinensis transcriptome and differentially expressed genes in three different mycelia, sclerotium and fruiting body developmental stages. Fungal Biol. 2018, 122, 943–951. [Google Scholar] [CrossRef]
- Gonda, I.; Bar, E.; Portnoy, V.; Lev, S.; Burger, J.; Schaffer, A.A.; Tadmor, Y.; Gepstein, S.; Giovannoni, J.J.; Katzir, N.; et al. Branched-chain and aromatic amino acid catabolism into aroma volatiles in Cucumis melo L. fruit. J. Exp. Bot. 2010, 61, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Nie, S.; Xie, M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020, 235, 115957. [Google Scholar] [CrossRef]
- Bastings, J.; van Eijk, H.M.; Olde Damink, S.W.; Rensen, S.S. d-amino acids in health and disease: A focus on cancer. Nutrients 2019, 11, 2205. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Rose, A.J.; Sijmonsma, T.P.; Bröer, A.; Pfenninger, A.; Herzig, S.; Schmoll, D.; Bröer, S. Mice lacking neutral amino acid transporter B0AT1 (Slc6a19) have elevated levels of FGF21 and GLP-1 and improved glycaemic control. Mol. Metab. 2015, 4, 406–417. [Google Scholar] [CrossRef]
- Hernandez, P.J.; Abel, T. The role of protein synthesis in memory consolidation: Progress amid decades of debate. Neurobiol. Learn. Mem. 2008, 89, 293–311. [Google Scholar] [CrossRef]
- Zhou, X.; Xiong, H.; Lu, Y.; Geng, M.; Lu, Y.; Zhang, L.; Zhu, X. PKD2 deficiency suppresses amino acid biosynthesis in ADPKD by impairing the PERK-TBL2-eIF2ɑ-ATF4 pathway. Biochem. Biophys. Res. Commun. 2021, 561, 73–79. [Google Scholar] [CrossRef]
- Çolakoğulları, M. Tirozin, Triiodotironin Ve Tiroksinin Antioksidan Etkinliğinin In Vitro Ve In Vivo Ortamlarda Araştırılması. Ph.D. Thesis, Bursa Uludag University, Bursa, Turkey, 2003. [Google Scholar]
- Kabil, O.; Vitvitsky, V.; Banerjee, R. Sulfur as a signaling nutrient through hydrogen sulfide. Annu. Rev. Nutr. 2014, 34, 171–205. [Google Scholar] [CrossRef]
- Stabler, S.P. Alterations in sulfur amino acids as biomarkers of disease. J. Nutr. 2020, 150, 2532s–2537s. [Google Scholar] [CrossRef]
- Yoder, J.A.; Benoit, J.B.; Denlinger, D.L.; Rivers, D.B. Stress-induced accumulation of glycerol in the flesh fly, Sarcophaga bullata: Evidence indicating anti-desiccant and cryoprotectant functions of this polyol and a role for the brain in coordinating the response. J. Insect Physiol. 2006, 52, 202–214. [Google Scholar] [CrossRef]
- Colovic, M.B.; Vasic, V.M.; Djuric, D.M.; Krstic, D.Z. Sulphur-containing Amino Acids: Protective Role Against Free Radicals and Heavy Metals. Curr. Med. Chem. 2018, 25, 324–335. [Google Scholar] [CrossRef]
- He, J.; Hewett, S.J. Nrf2 Regulates Basal Glutathione Production in Astrocytes. Int. J. Mol. Sci. 2025, 26, 687. [Google Scholar] [CrossRef]
- Shashidhar, M.G.; Giridhar, P.; Udaya Sankar, K.; Manohar, B. Bioactive principles from Cordyceps sinensis: A potent food supplement—A review. J. Funct. Foods 2013, 5, 1013–1030. [Google Scholar] [CrossRef]
- Pal, M.; Bhardwaj, A.; Manickam, M.; Tulsawani, R.; Srivastava, M.; Sugadev, R.; Misra, K. Protective Efficacy of the Caterpillar Mushroom, Ophiocordyceps sinensis (Ascomycetes), from India in Neuronal Hippocampal Cells against Hypoxia. Int. J. Med. Mushrooms 2015, 17, 829–840. [Google Scholar] [CrossRef]
- Chen, X.; Xie, N.; Feng, L.; Huang, Y.; Wu, Y.; Zhu, H.; Tang, J.; Zhang, Y. Oxidative stress in diabetes mellitus and its complications: From pathophysiology to therapeutic strategies. Chin. Med. J. 2025, 138, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Nikfar, S.; Larijani, B.; Abdollahi, M. A review on the role of antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005, 59, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Pachuta-Stec, A. Antioxidant Activity of 1,2,4-Triazole and its Derivatives: A Mini-Review. Mini Rev. Med. Chem. 2022, 22, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, A.V.; Gerasimova, E.L.; Brainina Kh, Z. Potentiometric study of antioxidant activity: Development and prospects. Crit. Rev. Anal. Chem. 2015, 45, 311–322. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, X.; Song, Q.; Lu, W.; Wu, T.; Zhang, Q.; Li, C. Determination and comparative analysis of 13 nucleosides and nucleobases in natural fruiting body of Ophiocordyceps sinensis and its substitutes. Mycology 2017, 8, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Fan, Y.; Wang, T.; Wang, J.; Xiao, M.; He, M.; Chang, X.; Li, Y.; Li, X. Metabolomic profiling of Floccularia luteovirens from different geographical regions proposes a novel perspective on their antioxidative activities. Antioxidants 2024, 13, 620. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Fan, Y.; Wang, T.; Wang, J.; Xiao, M.; He, M.; Chang, X.; Li, Y.; Li, X. Integrated amino acid profiling and 4D-DIA proteomics reveal protein quality divergence and metabolic adaptation in Cordyceps species. J. Fungi 2025, 11, 365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, S.; Yu, Q.; Shan, X.; Chen, L.; Deng, Y.; Hua, J.; Zhu, J.; Zhou, Q.; Jiang, Y.; et al. The influence of rolling pressure on the changes in non-volatile compounds and sensory quality of congou black tea: The combination of metabolomics, E-tongue, and chromatic differences analyses. Food Chem. X 2023, 20, 100989. [Google Scholar] [CrossRef]
- Paterson, R.R. Cordyceps: A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry 2008, 69, 1469–1495. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.X.; Wang, S.; Nie, S.; Marcone, M. Properties of Cordyceps Sinensis: A review. J. Funct. Foods 2013, 5, 550–569. [Google Scholar] [CrossRef]
- Hardy, L.M.; Frisdal, E.; Le Goff, W. Critical role of the human ATP-binding cassette G1 transporter in cardiometabolic diseases. Int. J. Mol. Sci. 2017, 18, 1892. [Google Scholar] [CrossRef]
- Stefková, J.; Poledne, R.; Hubácek, J.A. ATP-binding cassette (ABC) transporters in human metabolism and diseases. Physiol. Res. 2004, 53, 235–243. [Google Scholar] [CrossRef]
- Sun, S.; Wang, D.; Dong, D.; Xu, L.; Xie, M.; Wang, Y.; Ni, T.; Jiang, W.; Zhu, X.; Ning, N.; et al. Altered intestinal microbiome and metabolome correspond to the clinical outcome of sepsis. Crit. Care 2023, 27, 127. [Google Scholar] [CrossRef]
- Charvet, S.; Bock, N.A.; Kim, E.; Duhamel, S. Transcriptomics reveal a unique phago-mixotrophic response to low nutrient concentrations in the prasinophyte Pterosperma cristatum. ISME Commun. 2024, 4, ycae083. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Ali, S.; Karmoker, J.R.; Kadir, M.F.; Ahmed, M.U.; Nahar, Z.; Islam, S.M.A.; Islam, M.S.; Hasnat, A.; Islam, M.S. Evaluation of serum amino acids and non-enzymatic antioxidants in drug-naïve first-episode major depressive disorder. BMC Psychiatry 2020, 20, 333. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Jiang, Z.; Tao, M.; Wen, M.; Xiao, Z.; Zhang, L.; Zha, M.; Chen, J.; Liu, Z.; Zhang, L. Targeted and nontargeted metabolomics analysis for determining the effect of storage time on the metabolites and taste quality of keemun black tea. Food Chem. 2021, 359, 129950. [Google Scholar] [CrossRef]
- Wu, S.; Yu, Q.; Shen, S.; Shan, X.; Hua, J.; Zhu, J.; Qiu, J.; Deng, Y.; Zhou, Q.; Jiang, Y.; et al. Non-targeted metabolomics and electronic tongue analysis reveal the effect of rolling time on the sensory quality and nonvolatile metabolites of congou black tea. LWT 2022, 169, 113971. [Google Scholar] [CrossRef]
- Chen, X.; Yu, D. Metabolomics study of oral cancers. Metabolomics 2019, 15, 22. [Google Scholar] [CrossRef]
- Cui, L.; Lu, H.; Lee, Y.H. Challenges and emergent solutions for LC-MS/MS based untargeted metabolomics in diseases. Mass Spectrom. Rev. 2018, 37, 772–792. [Google Scholar] [CrossRef]
- Lin, Y.; Xiao, R.; Xia, B.H.; Zhang, Z.M.; Li, C.; Wu, P.; Liao, D.F.; Lin, L.M. Investigation of the idiosyncratic hepatotoxicity of Polygonum multiflorum Thunb. through metabolomics using GC-MS. BMC Complement. Med. Ther. 2021, 21, 120. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Evaluation of The Antioxidant Capacity of Food Products: Methods, Applications and Limitations. Processes 2022, 10, 2031. [Google Scholar] [CrossRef]
- Lyu, T.; Yang, X.; Zhao, C.; Wang, L.; Zhou, S.; Shi, L.; Dong, Y.; Dou, H.; Zhang, H. Comparative transcriptomics of high-altitude Vulpes and their low-altitude relatives. Front. Ecol. Evol. 2022, 10, 999411. [Google Scholar] [CrossRef]
- Du, Z.; Lin, W.; Yu, B.; Zhu, J.; Li, J. Integrated metabolomic and transcriptomic analysis of the flavonoid accumulation in the leaves of Cyclocarya paliurus at different altitudes. Front. Plant Sci. 2021, 12, 794137. [Google Scholar] [CrossRef]
- Tajalli, F.; Malekzadeh, K.; Soltanian, H.; Janpoor, J.; Rezaeian, S.; Pourianfar, H.R. Antioxidant capacity of several Iranian, wild and cultivated strains of the button mushroom. Braz. J. Microbiol. 2015, 46, 769–776. [Google Scholar] [CrossRef]
- Braga, P.C.; Antonacci, R.; Wang, Y.Y.; Lattuada, N.; Dal Sasso, M.; Marabini, L.; Fibiani, M.; Lo Scalzo, R. Comparative antioxidant activity of cultivated and wild Vaccinium species investigated by EPR, human neutrophil burst and COMET assay. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1987–1999. [Google Scholar]
- Li, M.; Meng, Q.; Zhang, H.; Shu, R.; Zhao, Y.; Wu, P.; Li, X.; Zhou, G.; Qin, Q.; Zhang, J. Changes in transcriptomic and metabolomic profiles of morphotypes of Ophiocordyceps sinensis within the hemocoel of its host larvae, Thitarodes xiaojinensis. BMC Genom. 2020, 21, 789. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Feng, Z.F.; Li, F.; Hou, Y.M. Host-Encoded Aminotransferase Import into the Endosymbiotic Bacteria Nardonella of Red Palm Weevil. Insects 2024, 15, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, Y.; Cui, J.L.; Ji, X.; Miao, S.M.; Zhang, G.; Li, Y.M. The composition characteristics of endophytic communities and their relationship with metabolites profile in Ephedra sinica under wild and cultivated conditions. Environ. Sci. Pollut. Res. Int. 2023, 30, 95648–95659. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, S.N.; Saikia, K.; Thakur, D. Characterization and selection of endophytic actinobacteria for growth and disease management of Tea (Camellia sinensis L.). Front. Plant Sci. 2022, 13, 989794. [Google Scholar] [CrossRef]
- Sun, T.; Jin, Y.; Rao, Z.; Liyan, W.; Tang, R.; Zaryab, K.M.; Li, M.; Li, Z.; Wang, Y.; Xu, J.; et al. Knockdown of Thitarodes host genes influences dimorphic transition of Ophiocordyceps sinensis in the host hemolymph. Front. Cell Infect. Microbiol. 2024, 14, 1451628. [Google Scholar] [CrossRef]
- Chen, B.; Sun, Y.; Luo, F.; Wang, C. Bioactive Metabolites and Potential Mycotoxins Produced by Cordyceps Fungi: A Review of Safety. Toxins 2020, 12, 410. [Google Scholar] [CrossRef]
- Bezerra, J.D.; Nascimento, C.C.; Barbosa Rdo, N.; da Silva, D.C.; Svedese, V.M.; Silva-Nogueira, E.B.; Gomes, B.S.; Paiva, L.M.; Souza-Motta, C.M. Endophytic fungi from medicinal plant Bauhinia forficata: Diversity and biotechnological potential. Braz. J. Microbiol. 2015, 46, 49–57. [Google Scholar] [CrossRef]
- Lin, S.; Liu, Z.Q.; Xue, Y.P.; Baker, P.J.; Wu, H.; Xu, F.; Teng, Y.; Brathwaite, M.E.; Zheng, Y.G. Biosynthetic pathway analysis for improving the cordycepin and cordycepic acid production in Hirsutella sinensis. Appl. Biochem. Biotechnol. 2016, 179, 633–649. [Google Scholar] [CrossRef]
- Guo, S.; Lin, M.; Xie, D.; Zhang, W.; Zhang, M.; Zhou, L.; Li, S.; Hu, H. Comparative metabolic profiling of wild Cordyceps species and their substituents by liquid chromatography-tandem mass spectrometry. Front. Pharmacol. 2022, 13, 1036589. [Google Scholar] [CrossRef]
- Shen, F.; Wang, T.; Zhang, R.; Zhong, B.; Wu, Z. Metabolism and release of characteristic components and their enzymatic mechanisms in Pericarpium Citri Reticulatae co-fermentation. Food Chem. 2024, 432, 137227. [Google Scholar] [CrossRef]
- Yang, H.; Lei, M.; Huang, L.; Wang, Y.; Sun, N.; Ban, L.; Wang, X.; Zhang, H. Study on the effects of environmental factors on enzyme activities during growth of Hypsizygus marmoreus. PLoS ONE 2022, 17, e0268107. [Google Scholar] [CrossRef]
- Zhang, W.; Alseekh, S.; Zhu, X.; Zhang, Q.; Fernie, A.R.; Kuang, H.; Wen, W. Dissection of the domestication-shaped genetic architecture of lettuce primary metabolism. Plant J. 2020, 104, 613–630. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, J.; Liu, X.; Wang, B.; Zhao, Y.; Liao, L.; Allan, A.C.; Sun, C.; Duan, Y.; Li, X.; et al. A metabolic perspective of selection for fruit quality related to apple domestication and improvement. Genome Biol. 2023, 24, 95. [Google Scholar] [CrossRef]
- Gibbons, J.G.; Salichos, L.; Slot, J.C.; Rinker, D.C.; McGary, K.L.; King, J.G.; Klich, M.A.; Tabb, D.L.; McDonald, W.H.; Rokas, A. The evolutionary imprint of domestication on genome variation and function of the filamentous fungus Aspergillus oryzae. Curr. Biol. 2012, 22, 1403–1409. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, Q.; Yao, Q.; Yang, Z.; Li, W.; Cheng, X.; Wen, Y.; Chen, R.; Xu, J.; Wang, X.; et al. Nonenzymatic lysine D-lactylation induced by glyoxalase II substrate SLG dampens inflammatory immune responses. Cell Res. 2025, 35, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Forero, I.; Rouzaut, A.; Palazon, A.; Dubrot, J.; Melero, I. Lysine 63 polyubiquitination in immunotherapy and in cancer-promoting inflammation. Clin. Cancer Res. 2009, 15, 6751–6757. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, S.M.; Gao, X.; Dai, Z.; Locasale, J.W. Methionine metabolism in health and cancer: A nexus of diet and precision medicine. Nat. Rev. Cancer 2019, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Lupoli, R.; Di Minno, A.; Spadarella, G.; Franchini, M.; Sorrentino, R.; Cirino, G.; Di Minno, G. Methylation reactions, the redox balance and atherothrombosis: The search for a link with hydrogen sulfide. Semin. Thromb. Hemost. 2015, 41, 423–432. [Google Scholar] [CrossRef]
- Mao, X.; Zeng, X.; Qiao, S.; Wu, G.; Li, D. Specific roles of threonine in intestinal mucosal integrity and barrier function. Front. Biosci. Elite Ed. 2011, 3, 1192–1200. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pu, J.; Jing, J.; Su, Z.; Cai, J.; Jia, G.; Zhao, H.; Tian, G. Threonine attenuates lipopolysaccharide-induced intestinal inflammatory responses in rabbits. Eur. J. Nutr. 2024, 64, 10. [Google Scholar] [CrossRef]
- Nishida, Y. Inhibition of lipid peroxidation by methylated analogues of uric acid. J. Pharm. Pharmacol. 1991, 43, 885–887. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. Dietary Glycine Is Rate-Limiting for Glutathione Synthesis and May Have Broad Potential for Health Protection. Ochsner J. 2018, 18, 81–87. [Google Scholar] [PubMed]
- Querio, G.; Antoniotti, S.; Levi, R.; Gallo, M.P. Trimethylamine N-Oxide Does Not Impact Viability, ROS Production, and Mitochondrial Membrane Potential of Adult Rat Cardiomyocytes. Int. J. Mol. Sci. 2019, 20, 3045. [Google Scholar] [CrossRef]
- Chomard, P.; Seguin, C.; Loireau, A.; Autissier, N.; Artur, Y. Effects of iodotyrosines, thyronines, iodothyroacetic acids and thyromimetic analogues on in vitro copper-induced oxidation of low-density lipoproteins. Biochem. Pharmacol. 1998, 55, 1591–1601. [Google Scholar] [CrossRef]
- Singh, M.; Tulsawani, R.; Koganti, P.; Chauhan, A.; Manickam, M.; Misra, K. Cordyceps sinensis increases hypoxia tolerance by inducing heme oxygenase-1 and metallothionein via Nrf2 activation in human lung epithelial cells. Biomed. Res. Int. 2013, 2013, 569206. [Google Scholar] [CrossRef]
- Alkhatib, A.; Feng, W.H.; Huang, Y.J.; Kuo, C.H.; Hou, C.W. Anserine Reverses Exercise-Induced Oxidative Stress and Preserves Cellular Homeostasis in Healthy Men. Nutrients 2020, 12, 1146. [Google Scholar] [CrossRef]
- Zhao, J.M.; Chen, X.; Cheng, K.; Shi, Q.; Peng, K. Anserine and glucosamine supplementation attenuates the levels of inflammatory markers in rats with rheumatoid arthritis. AMB Express 2020, 10, 57. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.; Jia, B.; Tang, C.; Feng, C.; Li, Y.; Li, X. Analysis of Amino Acid and Derivative Diversity and Antioxidant Capacity in Ophiocordyceps sinensis and Its Substitutes. J. Fungi 2025, 11, 711. https://doi.org/10.3390/jof11100711
Tang H, Jia B, Tang C, Feng C, Li Y, Li X. Analysis of Amino Acid and Derivative Diversity and Antioxidant Capacity in Ophiocordyceps sinensis and Its Substitutes. Journal of Fungi. 2025; 11(10):711. https://doi.org/10.3390/jof11100711
Chicago/Turabian StyleTang, Haoxu, Bing Jia, Chuyu Tang, Chao Feng, Yuling Li, and Xiuzhang Li. 2025. "Analysis of Amino Acid and Derivative Diversity and Antioxidant Capacity in Ophiocordyceps sinensis and Its Substitutes" Journal of Fungi 11, no. 10: 711. https://doi.org/10.3390/jof11100711
APA StyleTang, H., Jia, B., Tang, C., Feng, C., Li, Y., & Li, X. (2025). Analysis of Amino Acid and Derivative Diversity and Antioxidant Capacity in Ophiocordyceps sinensis and Its Substitutes. Journal of Fungi, 11(10), 711. https://doi.org/10.3390/jof11100711






