The Frailty, Fitness, and Psychophysical/Social Condition of Community-Dwelling Older Adults—Analysis of 5-Year Longitudinal Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
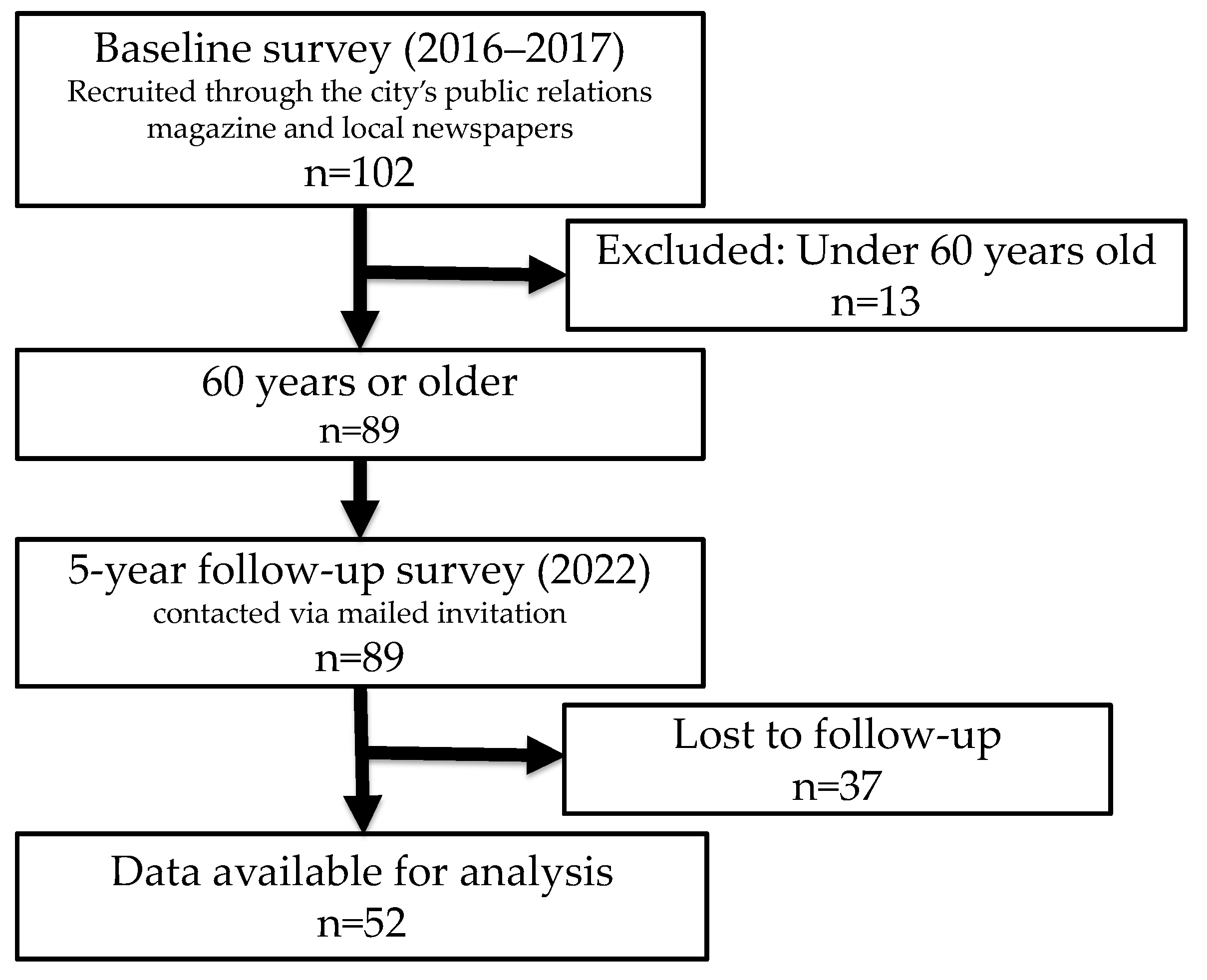
2.2. Participants
2.3. Measurements
2.3.1. Physique and Physical Fitness
2.3.2. Basic Characteristics, Health Perceptions, and Lifestyle Factors
2.3.3. Frailty
2.3.4. Depression
2.3.5. Social Isolation
2.3.6. The Kihon Checklist (KCL)
2.4. Data Analysis
3. Results
3.1. Characteristics of the Participants
3.2. Health Perception and Lifestyle Factors in the Robust and Frailty Risk Groups
3.3. Physique, Physical Fitness, and Psychosocial Conditions in the Robust and Frailty Risk Groups
3.4. Factors Associated with Frailty Risk After 5 Years
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Japan Geriatrics Society. Statement Regarding Frailty [in Japanese]. 2014. Available online: https://www.jpn-geriat-soc.or.jp/info/topics/pdf/20140513_01_01.pdf (accessed on 20 December 2023).
- Satake, S.; Shimada, H.; Yamada, M.; Kim, H.; Yoshida, H.; Gondo, Y.; Matsubayashi, K.; Matsushita, E.; Kuzuya, M.; Kozaki, K.; et al. Prevalence of frailty among community-dwellers and outpatients in Japan as defined by the Japanese version of the Cardiovascular Health Study criteria. Geriatr. Gerontol. Int. 2017, 17, 2629–2634. [Google Scholar] [CrossRef]
- Yamada, Y.; Nanri, H.; Watanabe, Y.; Yoshida, T.; Yokoyama, K.; Itoi, A.; Date, H.; Yamaguchi, M.; Miyake, M.; Yamagata, E.; et al. Prevalence of frailty assessed by fried and Kihon Checklist Indexes in a prospective cohort study: Design and demographics of the Kyoto-Kameoka longitudinal study. J. Am. Med. Dir. Assoc. 2017, 18, 733.e7–733.e15. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Sezgin, D.; O’Donovan, M.R.; Molloy, D.W.; Clegg, A.; Rockwood, K.; Liew, A. Prevalence of frailty in 62 countries across the world: A systematic review and meta-analysis of population-level studies. Age Ageing 2021, 50, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Pradana, A.A.; Chiu, H.L.; Lin, C.J.; Lee, S.C. Prevalence of frailty in Indonesia: A systematic review and meta-analysis. BMC Geriatr. 2023, 23, 778. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, Y.; Gao, Q.; Yuan, H.; Sun, L.; Xi, H.; Wu, W. Prevalence of frailty among Chinese community-dwelling older adults: A systematic review and meta-analysis. Int. J. Public Health 2023, 68, 1605964. [Google Scholar] [CrossRef]
- Melo, R.C.; Cipolli, G.C.; Buarque, G.L.A.; Yassuda, M.S.; Cesari, M.; Oude Voshaar, R.C.; Aprahamian, I. Prevalence of frailty in Brazilian older adults: A systematic review and meta-analysis. J. Nutr. Health Aging 2020, 24, 708–716. [Google Scholar] [CrossRef]
- Kojima, G.; Iliffe, S.; Taniguchi, Y.; Shimada, H.; Rakugi, H.; Walters, K. Prevalence of frailty in Japan: A systematic review and meta-analysis. J. Epidemiol. 2017, 27, 347–353. [Google Scholar] [CrossRef]
- Iwasaki, M.; Yoshihara, A.; Sato, N.; Sato, M.; Minagawa, K.; Shimada, M.; Nishimuta, M.; Ansai, T.; Yoshitake, Y.; Ono, T.; et al. A 5-year longitudinal study of association of maximum bite force with development of frailty in community-dwelling older adults. J. Oral Rehabil. 2018, 45, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Sawaya, Y.; Ishizaka, M.; Hashimoto, N.; Kubo, A.; Urano, T. Kihon Checklist items associated with the development of frailty and recovery to robust status during the COVID-19 pandemic. Geriatr. Gerontol. Int. 2022, 22, 745–752. [Google Scholar] [CrossRef]
- Ye, B.; Chen, H.; Huang, L.; Ruan, Y.; Qi, S.; Guo, Y.; Huang, Z.; Sun, S.; Chen, X.; Shi, Y.; et al. Changes in frailty among community-dwelling Chinese older adults and its predictors: Evidence from a two-year longitudinal study. BMC Geriatr. 2020, 20, 130. [Google Scholar] [CrossRef]
- Sheng, K.; Chen, H.; Qu, X. The effects of cognitive leisure activities on frailty transitions in older adults in China: A CHARLS-Based longitudinal study. BMC Public Health 2024, 24, 1405. [Google Scholar] [CrossRef]
- Wang, S.; Li, Q.; Wang, S.; Huang, C.; Xue, Q.L.; Szanton, S.L.; Liu, M. Sustained frailty remission and dementia risk in older adults: A longitudinal study. Alzheimers Dement 2024, 20, 6268–6277. [Google Scholar] [CrossRef] [PubMed]
- Oishi, R.; Segi, N.; Okazaki, M.; Ito, S.; Ouchida, J.; Yamauchi, I.; Seki, T.; Takegami, Y.; Ishizuka, S.; Hashizume, A.; et al. Longitudinal transitions in frailty and health-related quality of life investigated by a 5-year community study. Sci. Rep. 2025, 15, 15162. [Google Scholar] [CrossRef]
- Lee, Y.S.; Nishita, Y.; Tange, C.; Zhang, S.; Shimokata, H.; Lin, S.Y.; Chu, W.M.; Otsuka, R. Association between objective physical activity and frailty transition in community-dwelling prefrail Japanese older adults. J. Nutr. Health Aging 2025, 29, 100519. [Google Scholar] [CrossRef]
- Park, J.; Shin, H.E.; Kim, M.; Won, C.W.; Song, Y.M. Longitudinal association between eating alone and deterioration in frailty status: The Korean Frailty and Aging Cohort Study. Exp. Gerontol. 2023, 172, 112078. [Google Scholar] [CrossRef]
- Ministry of Internal Affairs and Communications. Statistical Topic No. 142: Our Country’s Elderly Population from the Perspective of Statistics—In Commemoration of “Respect for the Aged Day”. Available online: https://www.stat.go.jp/data/topics/pdf/topics142.pdf (accessed on 1 February 2025).
- Chan, R.; Leung, J.; Woo, J. Dietary patterns and risk of frailty in Chinese community-dwelling older people in Hong Kong: A prospective cohort study. Nutrients 2015, 7, 7070–7084. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, T.; Saida, K.; Tanaka, S.; Murayama, A.; Higuchi, D. Factors for the change in frailty status during the COVID-19 pandemic: A prospective cohort study over six- and 12-month periods in Japan. Geriatr. Nurs. 2022, 48, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Li, M.; Cao, J.; Zeng, L.; Jiang, C.; Lin, F. Association of metabolic syndrome severity with frailty progression among Chinese middle and old-aged adults: A longitudinal study. Cardiovasc. Diabetol. 2024, 23, 302. [Google Scholar] [CrossRef]
- Yuan, Y.; Peng, C.; Burr, J.A.; Lapane, K.L. Frailty, cognitive impairment, and depressive symptoms in Chinese older adults: An eight-year multi-trajectory analysis. BMC Geriatr. 2023, 23, 843. [Google Scholar] [CrossRef]
- Nerobkova, N.; Park, Y.S.; Park, E.C.; Shin, J. Frailty transition and depression among community-dwelling older adults: The Korean Longitudinal Study of Aging (2006–2020). BMC Geriatr. 2023, 23, 148. [Google Scholar] [CrossRef]
- Navarrete-Villanueva, D.; Gómez-Cabello, A.; Marín-Puyalto, J.; Moreno, L.A.; Vicente-Rodríguez, G.; Casajús, J.A. Frailty and physical fitness in elderly people: A systematic review and meta-analysis. Sports Med. 2021, 51, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Gobbens, R.J.; Luijkx, K.G.; Wijnen-Sponselee, M.T.; Schols, J.M. Towards an integral conceptual model of frailty. J. Nutr. Health Aging 2010, 14, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Mizuta, C.; Yamada, Y.; Okayama, Y.; Nakamura, E. Constructing an index of physical fitness age for Japanese elderly based on 7-year longitudinal data: Sex differences in estimated physical fitness age. Age 2012, 34, 203–214. [Google Scholar] [CrossRef]
- Yamagata, E.; Kimura, M.; Miyake, M.; Yamada, Y.; Masumoto, T.; Watanabe, Y.; Yoshida, T.; Yokoyama, K.; Yoshinaka, Y.; Sugihara, Y.; et al. Relationship between homebound status and physical fitness in the community-dwelling elderly population. Nihon Koshu Eisei Zasshi 2014, 61, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Yamada, Y.; Yoshida, T.; Yokoyama, K.; Miyake, M.; Yamagata, E.; Yamada, M.; Yoshinaka, Y.; Kimura, M.; for Kyoto-Kameoka Study Group. Comprehensive geriatric intervention in community-dwelling older adults: A cluster-randomized controlled trial. J. Cachexia Sarcopenia Muscle 2020, 11, 26–37. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yamagata, E.; Kimura, M. Comparison of lower-limb muscle quantity, quality, and physical functions between older adults with and without certified for long-term care and/or support. Appl. Gerontol. 2020, 14, 58–68. [Google Scholar] [CrossRef]
- Satake, S.; Arai, H. The revised Japanese version of the Cardiovascular Health Study criteria (revised J-CHS criteria). Geriatr. Gerontol. Int. 2020, 20, 992–993. [Google Scholar] [CrossRef]
- Sheikh, J.I.; Yesavage, J.A. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Yatomi, N. Examination of the factor structure and item characteristics of the shortened version of the Geriatric Depression Scale (GDS) for the elderly in Japan. Jpn. J. Gerontol. 1994, 16, 29–36. [Google Scholar]
- Kurimoto, A.; Awata, S.; Ohkubo, K.; Tsubota-Utsugi, M.; Asayama, K.; Takahashi, K.; Suenaga, K.; Satoh, H.; Imai, Y. Reliability and validity of the Japanese version of the abbreviated Lubben Social Network Scale. Nihon Ronen Igakkai Zasshi 2011, 48, 149–157. [Google Scholar] [CrossRef]
- Arai, H.; Satake, S. English translation of the Kihon Checklist. Geriatr. Gerontol. Int. 2015, 15, 518–519. [Google Scholar] [CrossRef] [PubMed]
- Sewo Sampaio, P.Y.; Sampaio, R.A.; Yamada, M.; Arai, H. Systematic review of the Kihon Checklist: Is it a reliable assessment of frailty? Geriatr. Gerontol. Int. 2016, 16, 893–902. [Google Scholar] [CrossRef]
- Nanri, H.; Yoshida, T.; Watanabe, Y.; Fujita, H.; Kimura, M.; Yamada, Y.; On Behalf Of The Kameoka Study Group. The association between habitual green tea consumption and comprehensive frailty as assessed by Kihon Checklist Indexes among an older Japanese population. Nutrients 2021, 13, 4149. [Google Scholar] [CrossRef]
- Koyama, S.; Otobe, Y.; Suzuki, M.; Kimura, Y.; Ishiyama, D.; Kojima, I.; Masuda, H.; Kusumi, H.; Yamada, M. Relationship between the Kihon Checklist and all-cause hospitalization among community-dwelling older adults. Geriatr. Gerontol. Int. 2022, 22, 132–137. [Google Scholar] [CrossRef]
- Kim, J.H. Multicollinearity and misleading statistical results. Korean J. Anesthesiol. 2019, 72, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Morita, A.; Takahashi, Y.; Takahashi, K.; Fujiwara, T. Depressive symptoms homophily among community-dwelling older adults in Japan: A social networks analysis. Front. Public Health 2022, 10, 965026. [Google Scholar] [CrossRef] [PubMed]
- Goto, T.; Fujiwara, S.; Koda, T.; Matsuda, T.; Kitamura, M.; Shirayama, Y.; Ichikawa, T. Biopsychosocial consideration of ikigai in older adults in Japan through a cross-sectional study. Geriatrics 2024, 9, 78. [Google Scholar] [CrossRef]
- Imaoka, M.; Nakao, H.; Nakamura, M.; Tazaki, F.; Hida, M.; Omizu, T.; Imai, R.; Takeda, M. Associations between depressive symptoms and geriatric syndromes in community-dwelling older adults in Japan: A cross-sectional study. Prev. Med. Rep. 2021, 22, 101353. [Google Scholar] [CrossRef]
- Oe, N.; Tadaka, E. Differences in loneliness and social isolation among community-dwelling older adults by household type: A nationwide survey in Japan. Healthcare 2023, 11, 1647. [Google Scholar] [CrossRef]
- Yokoro, M.; Otaki, N.; Imamura, T.; Tanino, N.; Fukuo, K. Association between social network and dietary variety among community-dwelling older adults. Public Health Nutr. 2023, 26, 2441–2449. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Rieker, J.A.; Gajewski, P.D.; Reales, J.M.; Ballesteros, S.; Golka, K.; Hengstler, J.G.; Wascher, E.; Getzmann, S. The impact of physical fitness, social life, and cognitive functions on work ability in middle-aged and older adults. Int. Arch. Occup. Environ. Health 2023, 96, 507–520. [Google Scholar] [CrossRef] [PubMed]
- Glasson, L.; Panter, J.; Ogilvie, D.; Patterson, R. The physical activity implications of retirement across occupational activity groups. Prev. Med. 2023, 173, 107570. [Google Scholar] [CrossRef] [PubMed]
- Nagai, K.; Tamaki, K.; Kusunoki, H.; Wada, Y.; Tsuji, S.; Itoh, M.; Sano, K.; Amano, M.; Hayashitani, S.; Yokoyama, R.; et al. Physical frailty predicts the development of social frailty: A prospective cohort study. BMC Geriatr. 2020, 20, 403. [Google Scholar] [CrossRef]
- Sato, S.; Yamaji, S. Koureisya no tairyokuhyouka no kanngaekata. Concept of physical fitness assessment in the elderly. In Koureisya no Tairyoku Oyobi Seikatsukatsudou no Sokutei to Hyouka [Measurement and Evaluation of Physical Fitness and Activities of Daily Living in the Elderly]; Demura, S., Miyaguchi, K., Sato, S., Sato, T., Ikemoto, Y., Eds.; Ichimura Shuppan: Tokyo, Japan, 2015; pp. 36–41. [Google Scholar]
- Tanaka, T.; Hirano, H.; Ikebe, K.; Ueda, T.; Iwasaki, M.; Shirobe, M.; Minakuchi, S.; Akishita, M.; Arai, H.; Iijima, K. Oral frailty five-item checklist to predict adverse health outcomes in community-dwelling older adults: A Kashiwa cohort study. Geriatr. Gerontol. Int. 2023, 23, 651–659. [Google Scholar] [CrossRef]
- Tanaka, T.; Takahashi, K.; Hirano, H.; Kikutani, T.; Watanabe, Y.; Ohara, Y.; Furuya, H.; Tetsuo, T.; Akishita, M.; Iijima, K. Oral frailty as a risk factor for physical frailty and mortality in community-dwelling elderly. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1661–1667. [Google Scholar] [CrossRef]
- Kusunoki, H.; Hasegawa, Y.; Nagasawa, Y.; Shojima, K.; Yamazaki, H.; Mori, T.; Tsuji, S.; Wada, Y.; Tamaki, K.; Nagai, K.; et al. Oral frailty and its relationship with physical frailty in older adults: A longitudinal study using the oral frailty five-item checklist. Nutrients 2024, 17, 17. [Google Scholar] [CrossRef]
- Funakubo, N.; Okazaki, K.; Hayashi, F.; Eguchi, E.; Nishimagi, M.; Nakano, H.; Nagao, M.; Yoshida, T.; Yokozuka, M.; Moriyama, N.; et al. Association of laughter and social communication with oral frailty among residents in Fukushima: A cross-sectional study. Sci. Rep. 2024, 14, 26818. [Google Scholar] [CrossRef]
| Measurement Item (Unit) | Purpose | Device Used | Procedure Summary | |
|---|---|---|---|---|
| 1 | Grip strength (kg) | Muscle strength | Digital grip strength meter (TKK5401: Takei Scientific Instruments, Niigata, Japan) | Each hand was tested twice with maximum effort. The higher value from each hand was averaged. |
| 2 | KES (kg) | Muscle strength | Leg strength measurement platform and tension meter (TKK5715, TKK5710e: Takei Scientific Instruments, Niigata, Japan) | Each leg was tested twice with maximum effort. The higher value from each leg was averaged. |
| 3 | 30 s chair standing test (numbers) | Lower limb muscular function | Standard chair | One trial: number of chair stands completed in 30 s |
| 4 | Vertical jump height (cm) | Muscle power | Jump meter (TKK5406: Takei Scientific Instruments, Niigata, Japan) | Two maximal jumps with arm swing; the highest value was used |
| 5 | Chair stepping (numbers) | Foot movement agility | Chair and floor markers (30 cm) | Open/close feet while seated between floor lines (30 cm apart); each performed one trial; 20-sec count |
| 6 | Single leg stance with eyes open (seconds) | Balance and stability | Stopwatch | Two trials with eyes open; the longest one-leg stance time was recorded |
| 7 | Sit-and-reach (cm) | Flexibility | Digital flexibility tester (TKK5112: Takei Scientific Instruments, Niigata, Japan) | Sit-and-reach test with straight legs; each performed two trials; the greater distance was used |
| 8 | FR (cm) | Balance | Digital mirror (PN-S3019030: Panasonic, Osaka, Japan) | Reach forward from standing posture; each performed two trials; the max distance was used |
| 9 | 10 m walking speed (m/s) | Gait speed | Stopwatch | 10 m walk at their usual pace; each performed two trials; the average time was used to calculate speed |
| 10 | TUG (seconds) | Functional mobility | Digital mirror (PN-S3019030: Panasonic, Osaka, Japan) | Each performed one trial: stand from chair, walk 3 m and back, sit down; the total time was used |
| Follow-Up Survey | ||||||||
|---|---|---|---|---|---|---|---|---|
| Robust Group | Frailty Risk Group | |||||||
| Robust | Pre-Frail | Frail | ||||||
| n | % | n | % | n | % | p | ||
| Baseline | Robust | 19 | 65.5% | 10 | 34.5% | 0 | 0.0% | 0.002 |
| Pre-frail | 4 | 17.4% | 18 | 78.3% | 1 | 4.3% | ||
| Frail | 0 | 0.0% | 0 | 0.0% | 0 | 0.0% | ||
| Robust Group | Frailty Risk Group | p | ||||
|---|---|---|---|---|---|---|
| Age (y) 1 | 77.4 ± 4.0 | 77.8 ± 5.3 | 0.745 | |||
| Sex 2 | Men | 11 | 55.0% | 9 | 45.0% | 0.260 |
| Women | 12 | 37.5% | 20 | 62.5% | ||
| Robust Group | Frailty Risk Group | ||||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | p | |||
| Baseline | Household composition | Living alone | 5 | 21.7% | 3 | 10.3% | 0.526 |
| Living with spouse only | 14 | 60.9% | 20 | 69.0% | |||
| Other | 4 | 17.4% | 6 | 20.7% | |||
| Perceived health | Healthy | 23 | 100.0% | 27 | 93.1% | 0.497 | |
| Unhealthy | 0 | 0.0% | 2 | 6.9% | |||
| Presence of hobbies | Yes | 23 | 100.0% | 26 | 89.7% | 0.245 | |
| No | 0 | 0.0% | 3 | 10.3% | |||
| Role in the household | Yes | 20 | 87.0% | 26 | 89.7% | 1.000 | |
| No | 3 | 13.0% | 3 | 10.3% | |||
| Work with income | Yes | 7 | 30.4% | 5 | 17.2% | 0.329 | |
| No | 16 | 69.6% | 24 | 82.8% | |||
| Participation in community activities | Yes | 6 | 26.1% | 6 | 20.7% | 0.746 | |
| No | 17 | 73.9% | 23 | 79.3% | |||
| Follow-up survey | Household composition | Living alone | 6 | 26.1% | 6 | 20.7% | 0.206 |
| Living with spouse only | 14 | 60.9% | 13 | 44.8% | |||
| Other | 3 | 13.0% | 10 | 34.5% | |||
| Perceived health | Healthy | 23 | 100.0% | 25 | 86.2% | 0.120 | |
| Unhealthy | 0 | 0.0% | 4 | 13.8% | |||
| Presence of hobbies | Yes | 22 | 95.7% | 29 | 100.0% | 0.442 | |
| No | 1 | 4.3% | 0 | 0.0% | |||
| Role in the household | Yes | 21 | 91.3% | 29 | 100.0% | 0.191 | |
| No | 2 | 8.7% | 0 | 0.0% | |||
| Work with income | Yes | 8 | 34.8% | 3 | 10.3% | 0.044 | |
| No | 15 | 65.2% | 26 | 89.7% | |||
| Participation in community activities | Yes | 8 | 34.8% | 6 | 20.7% | 0.348 | |
| No | 15 | 65.2% | 23 | 79.3% | |||
| Robust Group | Frailty Risk Group | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | Baseline | Follow-Up | Time Effect | Interaction | |||||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p | F | p | |||||
| Physique and physical fitness | ||||||||||||||||
| Height (cm) | 159.7 | ± | 8.7 | 159.1 | ± | 8.8 | 156.6 | ± | 6.8 | 155.6 | ± | 6.7 | 7.170 | 0.010 | 3.419 | 0.070 |
| Weight (kg) | 56.5 | ± | 10.7 | 57.0 | ± | 11.4 | 56.5 | ± | 8.1 | 56.0 | ± | 7.8 | 1.428 | 0.238 | 1.565 | 0.217 |
| BMI (kg/m2) | 22.0 | ± | 2.8 | 22.3 | ± | 3.0 | 23.0 | ± | 2.7 | 23.1 | ± | 3.1 | 0.429 | 0.515 | 0.511 | 0.478 |
| Grip strength (kg) | 30.6 | ± | 7.1 * | 29.0 | ± | 6.6 | 25.6 | ± | 6.7 | 23.6 | ± | 6.3 | 21.094 | <0.001 | 1.587 | 0.214 |
| KES (kg) | 35.2 | ± | 10.6 | 34.6 | ± | 8.7 | 28.8 | ± | 9.4 | 25.7 | ± | 10.4 | 5.224 | 0.027 | 3.056 | 0.087 |
| 30 s chair standing test (numbers) | 28.1 | ± | 6.0 * | 24.4 | ± | 5.5 | 24.4 | ± | 5.8 | 21.9 | ± | 4.6 | 2.632 | 0.111 | 0.527 | 0.472 |
| Vertical jump height (cm) | 30.1 | ± | 6.8 * | 25.5 | ± | 5.8 | 24.9 | ± | 6.5 | 20.2 | ± | 7.0 | 24.673 | <0.001 | 0.348 | 0.558 |
| Chair stepping test (numbers) | 31.9 | ± | 5.1 | 34.3 | ± | 5.5 | 31.0 | ± | 4.2 | 32.6 | ± | 5.9 | 1.655 | 0.204 | 0.183 | 0.671 |
| Single-leg stance with eyes open (s) | 54.9 | ± | 44.3 | 40.1 | ± | 30.9 | 35.8 | ± | 35.2 | 39.4 | ± | 39.3 | 0.314 | 0.578 | 3.167 | 0.081 |
| Sit-and-reach (cm) | 36.1 | ± | 13.9 | 32.6 | ± | 12.5 | 39.2 | ± | 8.6 | 33.9 | ± | 10.9 | 1.706 | 0.197 | 0.066 | 0.798 |
| FR (cm) | 34.0 | ± | 4.7 | 33.4 | ± | 5.3 | 32.7 | ± | 3.6 | 30.4 | ± | 6.6 | 4.865 | 0.032 | 2.191 | 0.145 |
| 10 m walking speed (m/s) | 1.43 | ± | 0.2 | 1.46 | ± | 0.2 | 1.37 | ± | 0.1 | 1.43 | ± | 0.2 | 0.000 | 0.991 | 0.194 | 0.661 |
| TUG (s) | 5.61 | ± | 0.9 | 5.74 | ± | 0.9 | 5.95 | ± | 0.9 | 6.64 | ± | 1.3 ††† | 3.466 | 0.069 | 6.926 | 0.011 |
| FAS | 0.25 | ± | 0.9 * | −0.10 | ± | 0.7 | −0.29 | ± | 0.6 | −0.62 | ± | 0.8 | 8.241 | 0.006 | 0.020 | 0.889 |
| Psychosocial conditions | ||||||||||||||||
| GDS | 2.5 | ± | 3.1 | 2.2 | ± | 3.1 | 3.0 | ± | 2.4 | 3.4 | ± | 2.6 | 0.764 | 0.386 | 1.412 | 0.241 |
| LSNS−6 | 18.3 | ± | 6.1 | 19.3 | ± | 7.0 | 16.6 | ± | 4.3 | 17.0 | ± | 4.5 | 0.116 | 0.735 | 0.320 | 0.575 |
| KCL total score | 2.78 | ± | 2.4 | 2.52 | ± | 1.8 | 3.97 | ± | 2.8 | 5.21 | ± | 3.1 | 0.034 | 0.855 | 4.045 | 0.050 |
| KCL IADL | 0.35 | ± | 0.7 | 0.17 | ± | 0.4 | 0.24 | ± | 0.5 | 0.28 | ± | 0.7 | 1.366 | 0.248 | 2.151 | 0.149 |
| KCL physical function | 0.96 | ± | 1.1 | 0.70 | ± | 0.8 | 1.00 | ± | 1.0 | 1.38 | ± | 0.9 | 0.790 | 0.378 | 4.005 | 0.051 |
| KCL nutrition | 0.22 | ± | 0.4 | 0.04 | ± | 0.2 | 0.38 | ± | 0.6 | 0.48 | ± | 0.6 | 1.613 | 0.210 | 3.605 | 0.063 |
| KCL oral function | 0.35 | ± | 0.5 ** | 0.52 | ± | 0.7 | 0.93 | ± | 0.8 | 0.72 | ± | 0.9 | 0.005 | 0.946 | 3.690 | 0.061 |
| KCL homebound | 0.04 | ± | 0.2 * | 0.26 | ± | 0.5 | 0.28 | ± | 0.5 | 0.52 | ± | 0.5 | 0.004 | 0.950 | 0.000 | 0.991 |
| KCL cognitive function | 0.64 | ± | 0.7 | 0.45 | ± | 0.7 | 0.38 | ± | 0.6 | 0.55 | ± | 0.8 | 1.407 | 0.241 | 1.483 | 0.229 |
| KCL depression | 0.26 | ± | 0.9 | 0.35 | ± | 0.8 | 0.76 | ± | 1.0 | 1.28 | ± | 1.1 | 0.343 | 0.561 | 1.329 | 0.254 |
| OR | 95% CI | p | |
|---|---|---|---|
| Sex 1 | 7.792 | 0.052–1163.22 | 0.421 |
| Grip Strength | 1.001 | 0.715–1.40 | 0.997 |
| 30 s chair standing test | 0.866 | 0.733–1.02 | 0.093 |
| Vertical jump height | 0.965 | 0.785–1.19 | 0.737 |
| TUG | 0.855 | 0.221–3.30 | 0.821 |
| FAS | 0.254 | 0.019–3.42 | 0.302 |
| KCL oral function | 5.331 | 1.593–17.84 | 0.007 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamagata, E.; Watanabe, Y.; Mitsuhashi, M.; Hashimoto, H.; Sugihara, Y.; Murata, N.; Komatsu, M.; Ebine, N.; Kimura, M. The Frailty, Fitness, and Psychophysical/Social Condition of Community-Dwelling Older Adults—Analysis of 5-Year Longitudinal Data. Geriatrics 2025, 10, 82. https://doi.org/10.3390/geriatrics10030082
Yamagata E, Watanabe Y, Mitsuhashi M, Hashimoto H, Sugihara Y, Murata N, Komatsu M, Ebine N, Kimura M. The Frailty, Fitness, and Psychophysical/Social Condition of Community-Dwelling Older Adults—Analysis of 5-Year Longitudinal Data. Geriatrics. 2025; 10(3):82. https://doi.org/10.3390/geriatrics10030082
Chicago/Turabian StyleYamagata, Emi, Yuya Watanabe, Miwa Mitsuhashi, Hidemi Hashimoto, Yuriko Sugihara, Naoko Murata, Mitsuyo Komatsu, Naoyuki Ebine, and Misaka Kimura. 2025. "The Frailty, Fitness, and Psychophysical/Social Condition of Community-Dwelling Older Adults—Analysis of 5-Year Longitudinal Data" Geriatrics 10, no. 3: 82. https://doi.org/10.3390/geriatrics10030082
APA StyleYamagata, E., Watanabe, Y., Mitsuhashi, M., Hashimoto, H., Sugihara, Y., Murata, N., Komatsu, M., Ebine, N., & Kimura, M. (2025). The Frailty, Fitness, and Psychophysical/Social Condition of Community-Dwelling Older Adults—Analysis of 5-Year Longitudinal Data. Geriatrics, 10(3), 82. https://doi.org/10.3390/geriatrics10030082






