In Vitro Anthelmintic Activity of Isatis tinctoria Extracts against Ewes’ Gastrointestinal Nematodes (GINs), a Possible Application for Animal Welfare
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction Procedure
2.2. Parasitological Study
2.2.1. Recovery of GIN Eggs
2.2.2. Coprocultures
2.2.3. Anthelmintic Efficacy
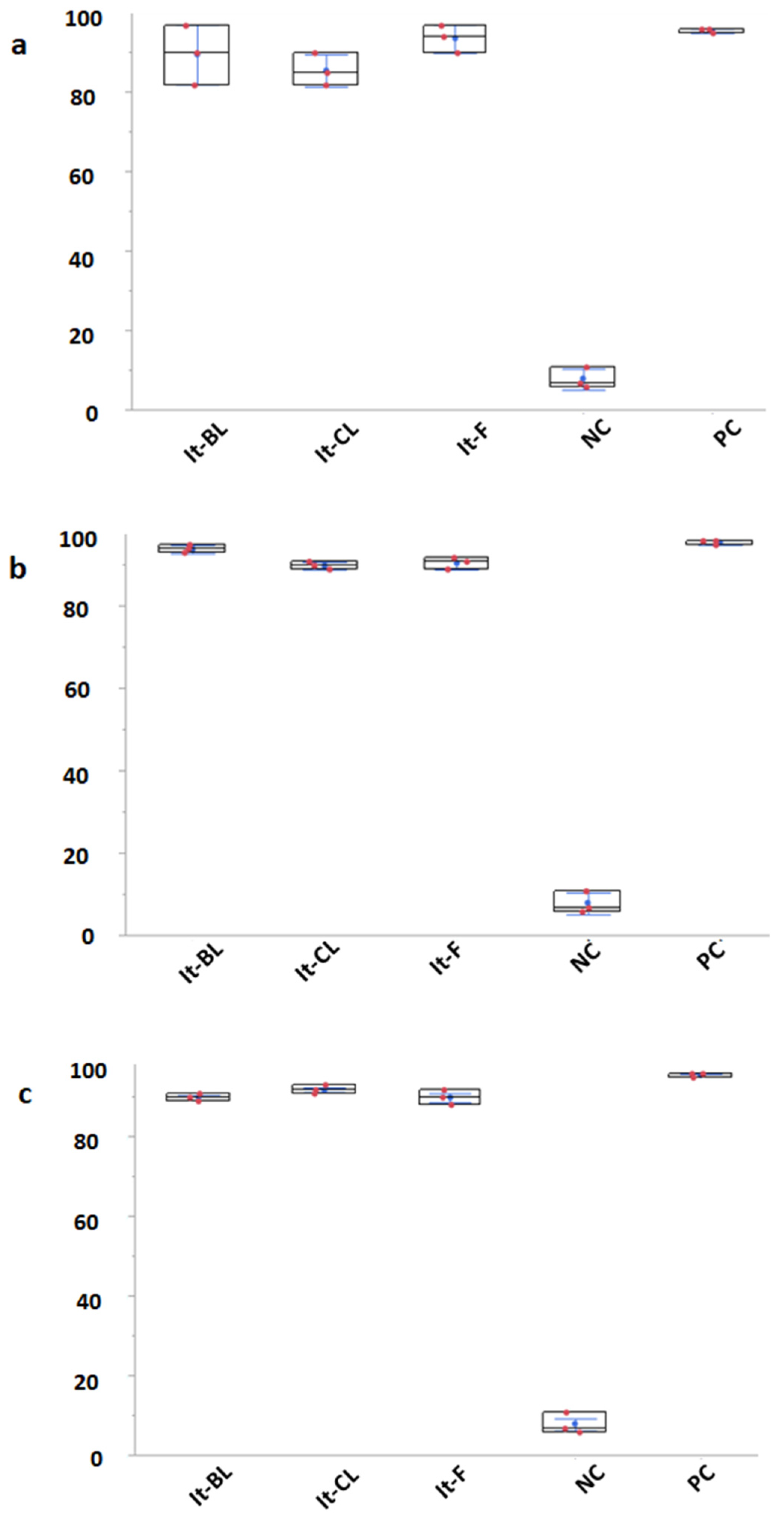
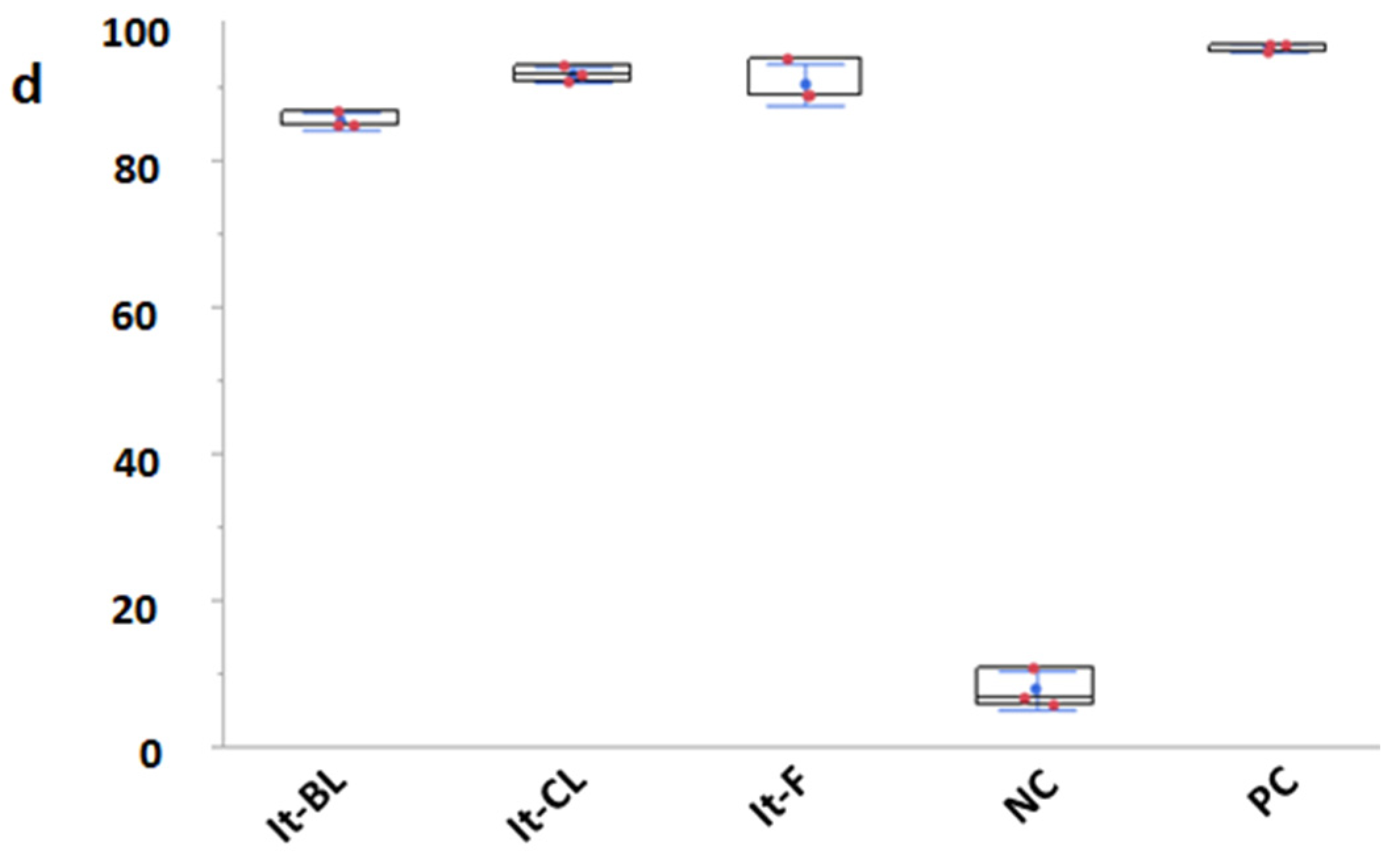
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Musella, V.; Catelan, D.; Rinaldi, L.; Lagazio, C.; Cringoli, G.; Biggeri, A. Covariate selection in multivariate spatial analysis of ovine parasitic infection. Prev. Vet. Med. 2011, 99, 69–77. [Google Scholar] [CrossRef]
- Mavrot, F.; Hertzberg, H.; Torgerson, P. Effect of gastro-intestinal nematode infection on sheep performance: A systematic review and meta-analysis. Parasites Vectors 2015, 8, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaplan, R.M.; Vidyashankar, A.N. An inconvenient truth: Global worming and anthelmintic resistance. Vet. Parasitol. 2012, 186, 70–78. [Google Scholar] [CrossRef]
- Rose, H.; Rinaldi, L.; Bosco, A.; Mavrot, F.; De Waal, T.; Skuce, P.; Charlier, J.; Torgerson, P.R.; Hertzberg, H.; Hendrickx, G.; et al. Widespread anthelmintic resistance in european farmed ruminants: A systematic review. Vet. Rec. 2015, 176, 546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosco, A.; Kießler, J.; Amadesi, A.; Varady, M.; Hinney, B.; Ianniello, D.; Maurelli, M.P.; Cringoli, G.; Rinaldi, L. The threat of reduced efficacy of anthelmintics against gastrointestinal nematodes in sheep from an area considered anthelmintic resistance-free. Parasites Vectors 2020, 13, 457. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The traditional medicine and modern medicine from natural products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrovska, B.B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bauri, R.K.; Tigga, M.N.; Kullu, S.S. A review on use of medicinal plants to control parasites. Indian J. Nat. Prod. Resour. 2015, 6, 268–277. [Google Scholar]
- Castagna, F.; Britti, D.; Oliverio, M.; Bosco, A.; Bonacci, S.; Iriti, G.; Ragusa, M.; Musolino, V.; Rinaldi, L.; Palma, E.; et al. In vitro anthelminthic efficacy of aqueous pomegranate (Punica granatum L.) extracts against gastrointestinal nematodes of sheep. Pathogens 2020, 9, 1063. [Google Scholar] [CrossRef]
- Cartea, M.E.; Velasco, P. Glucosinolates in Brassica foods: Bioavailability in food and significance for human health. Phytochem. Rev. 2008, 7, 213–229. [Google Scholar] [CrossRef]
- Lakshmanan, D.K.; Murugesan, S.; Rajendran, S.; Ravichandran, G.; Elangovan, A.; Raju, K.; Prathiviraj, R.; Pandiyan, R.; Thilagar, S. Brassica juncea (L.) Czern. leaves alleviate adjuvant-induced rheumatoid arthritis in rats via modulating the finest disease targets—IL2RA, IL18 and VEGFA. J. Biomol. Struct. Dyn. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.K.; Chatterjee, S.S.; Kumar, V. Antidepressant-like Effects of Brassica juncea L. leaves in diabetic rodents. Indian J. Exp. Biol. 2014, 52, 613. [Google Scholar] [PubMed]
- Kumar, S.; Andy, A. Minireview health promoting bioactive phytochemicals from Brassica. Int. Food Res. J. 2012, 19, 141–152. [Google Scholar]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Sobrinho Santos, E.M.; Almeida, A.C.; Santos, H.O.; Cangussu, A.R.; Costa, K.S.; Alves, J.N.; Bertucci Barbosa, L.C.; Souza Aguiar, R.W. Mechanism of Brassica oleracea performance in bovine infectious mastitis by bioinformatic analysis. Microb. Pathog. 2019, 129, 19–29. [Google Scholar] [CrossRef]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Speranza, J.; Miceli, N.; Taviano, M.F.; Ragusa, S.; Kwiecień, I.; Szopa, A.; Ekiert, H. Isatis tinctoria L. (Woad): A review of its botany, ethnobotanical uses, phytochemistry, biological activities, and biotechnological studies. Plants 2020, 9, 298. [Google Scholar] [CrossRef] [Green Version]
- Recio, M.C.; Cerdá-Nicolás, M.; Porterat, O.; Hamburger, M.; Ríos, J.L. Anti-inflammatory and antiallergic activity in vivo of lipophilic Isatis tinctoria extracts and tryptanthrin. Planta Med. 2006, 72, 539–546. [Google Scholar] [CrossRef] [Green Version]
- Condurso, C.; Verzera, A.; Romeo, V.; Ziino, M.; Trozzi, A.; Ragusa, S. The leaf volatile constituents of isatis tinctoria by solid-phase microextraction and gas chromatography/mass spectrometry. Planta Med. 2006, 72, 924–928. [Google Scholar] [CrossRef]
- Hamburger, M. Isatis tinctoria—From the rediscovery of an ancient medicinal plant towards a novel anti-inflammatory phytopharmaceutical. Phytochem. Rev. 2002, 1, 333–344. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines. Isatis root (Isatidis radix). In European Pharmacopoeia, 9th ed.; European Directorate for the Quality of Medicines: Strasburg, Germany, 2017; pp. 1399–1400. [Google Scholar]
- Sales, E.; Kanhonou, R.; Baixauli, C.; Giner, A.; Cooke, D.; Gilbert, K.; Arrillaga, I.; Segura, J.; Ros, R. Sowing date, transplanting, plant density and nitrogen fertilization affect indigo production from Isatis species in a Mediterranean region of Spain. Ind. Crops Prod. 2006, 23, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Spataro, G.; Negri, V. Adaptability and variation in Isatis tinctoria L.: A new crop for Europe. Euphytica 2008, 163, 89–102. [Google Scholar] [CrossRef]
- Miceli, N.; Cavò, E.; Ragusa, M.; Cacciola, F.; Mondello, L.; Dugo, L.; Acquaviva, R.; Malfa, G.A.; Marino, A.; D’Arrigo, M.; et al. Brassica incana Ten. (Brassicaceae): Phenolic constituents, antioxidant and cytotoxic properties of the leaf and flowering top extracts. Molecules 2020, 25, 1461. [Google Scholar] [CrossRef] [Green Version]
- Taviano, M.F.; Miceli, N.; Acquaviva, R.; Malfa, G.A.; Ragusa, S.; Giordano, D.; Cásedas, G.; Les, F.; López, V. Cytotoxic, antioxidant, and enzyme inhibitory properties of the traditional medicinal plant Matthiola incana (L.) R. Br. Biology 2020, 9, 163. [Google Scholar] [CrossRef]
- Taviano, M.F.; Filocamo, A.; Ragusa, S.; Cacciola, F.; Dugo, P.; Mondello, L.; Paterniti Mastrazzo, G.; De Rose, R.F.; Celano, M.; Lombardo, G.E.; et al. Phenolic profile, antioxidant and cytotoxic properties of polar extracts from leaves and flowers of Isatis tinctoria L. (Brassicaceae) growing in Sicily. Plant Biosyst. 2018, 152, 795–803. [Google Scholar] [CrossRef]
- Coles, G.C.; Bauer, C.; Borgsteede, F.H.M.; Geerts, S.; Klei, T.R.; Taylor, M.A.; Waller, P.J. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) Methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992, 44, 35–44. [Google Scholar] [CrossRef]
- van Wyk, J.A.; Mayhew, E. Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: A practical lab guide. Onderstepoort J. Vet. Res. 2013, 80, 1–14. [Google Scholar] [CrossRef]
- Great Britain. Ministry of Agriculture, Fisheries and Food. Manual of Veterinary Parasitological Laboratory Techniques; H.M. Stationery Office: London, UK, 1971; ISBN 9780112409182. [Google Scholar]
- Von Samson-Himmelstjerna, G.; Coles, G.C.; Jackson, F.; Bauer, C.; Borgsteede, F.; Cirak, V.Y.; Demeler, J.; Donnan, A.; Dorny, P.; Epe, C.; et al. Standardization of the egg hatch test for the detection of benzimidazole resistance in parasitic nematodes. Parasitol. Res. 2009, 105, 825–834. [Google Scholar] [CrossRef]
- Esteban-Ballesteros, M.; Sanchis, J.; Gutiérrez-Corbo, C.; Balaña-Fouce, R.; Rojo-Vázquez, F.A.; González-Lanza, C.; Martínez-Valladares, M. In vitro anthelmintic activity and safety of different plant species against the ovine gastrointestinal nematode Teladorsagia circumcincta. Res. Vet. Sci. 2019, 123, 153–158. [Google Scholar] [CrossRef]
- Mancilla-Montelongo, M.G.; Castañeda-Ramírez, G.S.; Gaudin-Barbier, E.; Canul-Velasco, M.L.; Chan-Pérez, J.I.; De la Cruz-Cortazar, Á.; Mathieu, C.; Fourquaux, I.; Sandoval-Castro, C.A.; Hoste, H.; et al. In vitro evaluation of the nutraceutical potential of Theobroma cacao pod husk and leaf extracts for small ruminants. Acta Parasitol. 2021, 66, 1122–1136. [Google Scholar] [CrossRef]
- Lima, C.S.; Pereira, M.H.; Gainza, Y.A.; Hoste, H.; Regasini, L.O.; de Souza Chagas, A.C. Anthelmintic effect of Pterogyne nitens (Fabaceae) on eggs and larvae of Haemonchus contortus: Analyses of structure-activity relationships based on phenolic compounds. Ind. Crops Prod. 2021, 164, 113348. [Google Scholar] [CrossRef]
- Mansfield, L.S.; Gamble, H.R.; Fetterer, R.H. Characterization of the eggshell of Haemonchus contortus—i. structural components. Comp. Biochem. Physiol. Part B Comp. Biochem. 1992, 103, 681–686. [Google Scholar] [CrossRef]
- Molan, A.-L.; Faraj, A.M. The effects of condensed tannins extracted from different plant species on egg hatching and larval development of Teladorsagia circumcincta (nematoda: Trichostrongylidae). Folia Parasitol. 2010, 57, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Cortes-Morales, J.A.; Olmedo-Juárez, A.; Trejo-Tapia, G.; González-Cortazar, M.; Domínguez-Mendoza, B.E.; Mendoza-de Gives, P.; Zamilpa, A. In vitro ovicidal activity of Baccharis conferta Kunth against Haemonchus contortus. Exp. Parasitol. 2019, 197, 20–28. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ragusa, M.; Miceli, N.; Piras, C.; Bosco, A.; Castagna, F.; Rinaldi, L.; Musella, V.; Taviano, M.F.; Britti, D. In Vitro Anthelmintic Activity of Isatis tinctoria Extracts against Ewes’ Gastrointestinal Nematodes (GINs), a Possible Application for Animal Welfare. Vet. Sci. 2022, 9, 129. https://doi.org/10.3390/vetsci9030129
Ragusa M, Miceli N, Piras C, Bosco A, Castagna F, Rinaldi L, Musella V, Taviano MF, Britti D. In Vitro Anthelmintic Activity of Isatis tinctoria Extracts against Ewes’ Gastrointestinal Nematodes (GINs), a Possible Application for Animal Welfare. Veterinary Sciences. 2022; 9(3):129. https://doi.org/10.3390/vetsci9030129
Chicago/Turabian StyleRagusa, Monica, Natalizia Miceli, Cristian Piras, Antonio Bosco, Fabio Castagna, Laura Rinaldi, Vincenzo Musella, Maria Fernanda Taviano, and Domenico Britti. 2022. "In Vitro Anthelmintic Activity of Isatis tinctoria Extracts against Ewes’ Gastrointestinal Nematodes (GINs), a Possible Application for Animal Welfare" Veterinary Sciences 9, no. 3: 129. https://doi.org/10.3390/vetsci9030129
APA StyleRagusa, M., Miceli, N., Piras, C., Bosco, A., Castagna, F., Rinaldi, L., Musella, V., Taviano, M. F., & Britti, D. (2022). In Vitro Anthelmintic Activity of Isatis tinctoria Extracts against Ewes’ Gastrointestinal Nematodes (GINs), a Possible Application for Animal Welfare. Veterinary Sciences, 9(3), 129. https://doi.org/10.3390/vetsci9030129










