Anthelmintic Properties of Essential Oils to Control Gastrointestinal Nematodes in Sheep—In Vitro and In Vivo Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oils and Chemical Analyses
2.2. Egg Hatch Test
2.3. Faecal Egg Count Reduction Test
2.4. Coproculture
2.5. Statistical Analyses
3. Results
3.1. GC-MS Analyses
3.2. Egg Hatch Test
3.3. Faecal Egg Count Reduction Test
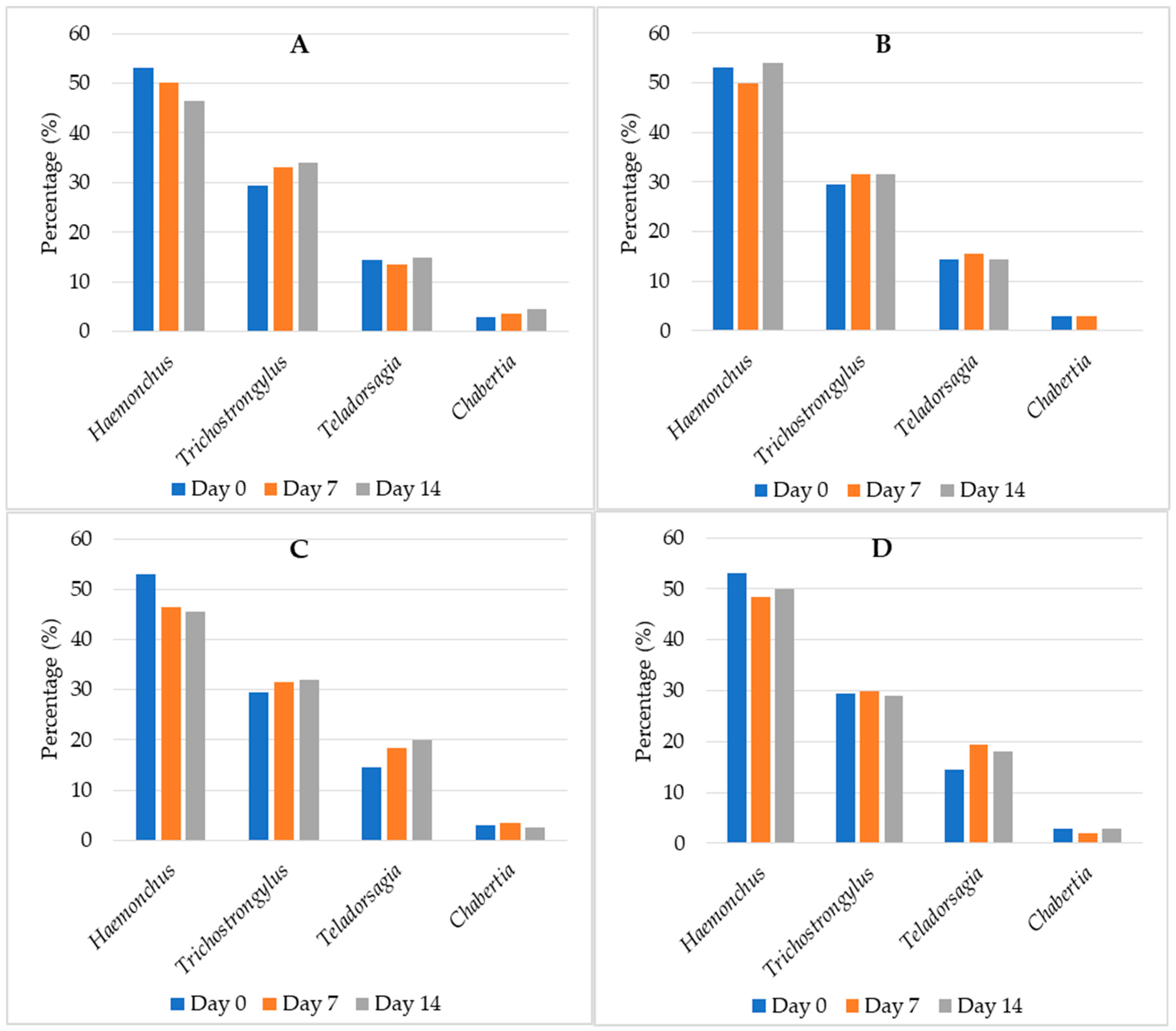
3.4. Coproculture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beleckė, A.; Kupčinskas, T.; Stadalienė, I.; Höglund, J.; Thamsborg, S.M.; Stuen, S.; Petkevičius, S. Anthelmintic resistance in small ruminants in the Nordic-Baltic region. Acta Vet. Scand. 2021, 63, 18. [Google Scholar] [CrossRef]
- Kulišić, Z.; Aleksić, N.; Đorđević, M.; Đorđević, M.M.; Gajić, B.; Tambur, Z.; Stevanović, J.; Stanimirović, Z. Prevalence and intensity of infection with gastrointestinal nematodes in sheep in eastern Serbia. Acta Vet.-Beograd. 2013, 63, 429–436. [Google Scholar] [CrossRef][Green Version]
- Roeber, F.; Jex, A.R.; Gasser, R.B. Impact of gastrointestinal parasitic nematodes of sheep, and the role of advanced molecular tools for exploring epidemiology and drug resistance—An Australian perspective. Parasit. Vectors 2013, 6, 153. [Google Scholar] [CrossRef]
- Bosco, A.; Kießler, J.; Amadesi, A.; Varady, M.; Hinney, B.; Ianniello, D.; Paola Maurelli, M.; Cringoli, G.; Rinaldi, L. The threat of reduced efficacy of anthelmintics against gastrointestinal nematodes in sheep from an area considered anthelmintic resistance-free. Parasit. Vectors 2020, 13, 457. [Google Scholar] [CrossRef]
- Zajac, A.M.; Garza, J. Biology, epidemiology, and control of gastrointestinal nematodes of small ruminants. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 73–87. [Google Scholar] [CrossRef]
- Chali, A.R.; Hunde, F.T. Study on prevalence of major gastrointestinal nematodes of sheep in Wayu Tuka and Diga District, Oromia Regional State. Vet. Med. Open J. 2021, 6, 13–21. [Google Scholar] [CrossRef]
- Abebe, T.; Yobsan, T.; Debela, A. Prevalence of major gastrointestinal nematode and degree of parasite infestation in sheep of Bako agricultural research center community based breeding program project small holder farms at Horro district. Dairy Vet. Sci. J. 2018, 8, 555740. [Google Scholar] [CrossRef]
- Abbas, R.Z.; Zaman, M.A.; Sindhu, Z.U.D.; Sharif, M.; Rafique, A.; Saeed, Z.; Rehman, T.U.; Siddique, F.; Zaheer, T.; Khan, M.K.; et al. Anthelmintic effects and toxicity analysis of herbal dewormer against the infection of Haemonchus contortus and Fasciola hepatica in goat. Pak. Vet. J. 2020, 40, 455–460. [Google Scholar] [CrossRef]
- Mavrot, F. Livestock Nematode Infection in a Changing World: Investigating the European Situation. Ph.D. Dissertation, University of Zürich, Zürich, Switzerland, 2016. [Google Scholar] [CrossRef]
- Kaplan, R.M. Biology, epidemiology, diagnosis and management of anthelmintic resistance in gastrointestinal nematodes of livestock. Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 17–30. [Google Scholar] [CrossRef]
- Pinto, N.B.; de Castro, L.M.; Azambuja, R.H.M.; Capella, G.D.A.; de Moura, M.Q.; Terto, W.D.; Freitag, R.A.; Jeske, S.T.; Villela, M.M.; Cleff, M.B.; et al. Ovicidal and larvicidal potential of Rosmarinus officinalis to control gastrointestinal nematodes of sheep. Rev. Bras. Parasitol. Vet. 2019, 28, 807–811. [Google Scholar] [CrossRef]
- Arsenopoulus, K.; Minoudi, S.; Symeonidou, I.; Triantafyllidis, A.; Katsafadou, A.I.; Lianou, D.T.; Fthenakis, G.C.; Papadopoulos, E. Frequency of resistance to benzimidazoles of Haemonchus contortus helminths from dairy sheep, goats, cattle and buffaloes in Greece. Pathogens 2020, 9, 347. [Google Scholar] [CrossRef]
- Zeineldin, M.; Abdelmegeid, M.; Barakat, R.; Ghanem, M. A review: Herbal medicine as an effective therapeutic approach for treating digestive disorders in small ruminants. Alex J. Vet. Sci. 2018, 56, 33–44. [Google Scholar] [CrossRef]
- Ferreira, L.E.; Benincasa, B.I.; Fachin, A.L.; França, S.C.; Contini, S.S.H.T.; Chagas, A.C.S.; Beleboni, R.O. Thymus vulgaris L. essential oil and its main component thymol: Anthelmintic effects against Haemonchus contortus from sheep. Vet. Parasitol. 2016, 228, 70–76. [Google Scholar] [CrossRef]
- Katiki, L.M.; Barbieri, A.M.E.; Araujo, R.C.; Veríssimo, C.J.; Louvandini, H.; Ferreira, J.F.S. Synergistic interaction of ten essential oils against Haemonchus contortus in vitro. Vet. Parasitol. 2017, 243, 47–51. [Google Scholar] [CrossRef]
- Macedo, I.T.F.; de Oliveira, L.M.B.; Andre, W.B.P.; Filho, J.V.A.; dos Santos, J.M.L.; Rondon, F.C.M.; Ribeiro, W.L.C.; Camurça-Vasconcelos, A.L.F.; de Oliveira, E.F.; de Paula, H.C.B.; et al. Anthelmintic effect of Cymbopogon citratus essential oil and its nanoemulsion on sheep gastrointestinal nematodes. Rev. Bras. Parasitol. Vet. 2019, 28, 522–527. [Google Scholar] [CrossRef]
- Zaman, M.A.; Qamar, W.; Mehreen, U.; Shahid, Z.; Khan, M.K.; Qamar, M.F.; Yousaf, S. In vitro experiments revealed the anthelmintic potential of herbal complex vagainst Haemonchus contortus. Pak. Vet. J. 2020, 40, 271–273. [Google Scholar] [CrossRef]
- André, W.P.P.; Ribeiro, W.L.C.; de Oliveira, L.M.B.; Macedo, I.T.F.; Rondon, F.C.R.; Bevilaqua, C.M.L. Essential oils and their bioactive compounds in the control of gastrointestinal nematodes of small ruminants. Acta Sci. Vet. 2018, 46, 1522. [Google Scholar] [CrossRef]
- Fayaz, M.R.; Abbas, R.Z.; Abbas, A.; Khan, M.K.; Raza, M.A.; Israr, M.; Khan, J.A.; Mahmood, M.S.; Saleemi, M.K.; Rehman, T.Z.; et al. Potential of botanical driven essential oils against Haemonchus contortus in small ruminants. Bol. Latinoam. Caribe Plant. Med. Aromát. 2019, 18, 533–543. [Google Scholar] [CrossRef]
- Nehme, R.; Andrés, S.; Pereira, R.B.; Jemaa, M.B.; Bouhallab, S.; Ceciliani, F.; López, S.; Rahali, F.Z.; Ksouri, R.; Pereira, D.M.; et al. Essential oils in livestock: From health to food quality. Antioxidants 2021, 10, 330. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Mancianti, F.; Ebani, V.V. Biological activity of essential oils. Molecules 2020, 25, 678. [Google Scholar] [CrossRef]
- Asrar, B.; Khan, I.U.; Homberg, J.R.; Haleem, D.J. Nigella sativa oil ameliorates chronic ethanol induced anxiety and impaired spatial memory by modulating noradrenaline levels. Pak. Vet. J. 2020, 40, 350–354. [Google Scholar] [CrossRef]
- Mucha, W.; Witkowska, D. The applicability of essential oils in different stages of production of animal-based foods. Molecules 2021, 26, 3798. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef]
- Yasmin, S.; Nawaz, M.; Anjum, A.A.; Ashraf, K.; Basra, M.A.R.; Mehmood, A.; Khan, I.; Malik, F. Phytochemical analysis and in vitro activity of essential oils of selected plants against Salmonella enteritidis and Salmonella gallinarum of poultry origin. Pak. Vet. J. 2020, 40, 139–144. [Google Scholar] [CrossRef]
- Masood, A.; Qureshi, A.S.; Shadid, R.U.; Jamil, H. Effects of oral administration of essential oil (Mix Oil®) on growth performance and intestinal morphometry of Japanese quails (Coturnix coturnix japonica). Pak. Vet. J. 2020, 40, 385–389. [Google Scholar] [CrossRef]
- Nardoni, S.; Pisteli, L.; Baronti, I.; Najar, B.; Pisseri, F.; Reidel, R.V.B.; Papini, R.; Perrucci, S.; Mancianti, F. Traditional Mediterranean plants: Characterization and use of an essential oils mixture to treat Malassezia otitis externa in atopic dogs. Nat. Prod. Res. 2017, 31, 1891–1894. [Google Scholar] [CrossRef]
- Yipel, F.A.; Acar, A.; Yipel, M. Effect of some essential oils (Allium sativum L., Origanum majorana L.) and ozonated olive oil on the treatment of ear mites (Otodectes cynotis) in cats. Turkish J. Vet. Anim. Sci. 2016, 40, 782–787. [Google Scholar] [CrossRef]
- Amer, A.M.; Amer, M.M. Efficacy and safety of essential oils mixture on tick infestation in dogs. Adv. Anim. Vet. Sci. 2020, 8, 398–407. [Google Scholar] [CrossRef]
- Monteiro, J.N.M.; Archanjo, A.B.; Passos, G.P.; Costa, A.V.; Porfirio, L.C.; Martins, I.V.F. Chenopodium ambrosioides L. essential oils and ethanol extract on control of canome Ancylostoma spp. Semin. Ciências Agrárias 2017, 38, 1947–1953. [Google Scholar] [CrossRef]
- Saha, S.; Lachance, S. Effect of essential oils on cattle gastrointestinal nematodes assessed by egg hatch, larval migration and mortality testing. J. Helminthol. 2019, 94, e111. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.E.; Benincasa, B.I.; Fachin, A.L.; Contini, S.H.T.; França, S.C.; Chagas, A.C.S.; Beleboni, R.O. Essential oils of Citrus aurantifola, Anthemis nobile and Lavandula officinalis: In vitro anthelmintic activities against Haemonchus contortus. Parasit. Vectors 2018, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Business Media: Carol Stream, IL, USA, 2012. [Google Scholar]
- Bosco, A.; Maurelli, M.P.; Ianniello, D.; Morgoglione, M.E.; Amadesi, A.; Coles, G.C.; Cringoli, G.; Rinaldi, L. The recovery of added nematode eggs from horse and sheep faeces by three methods. BMC Vet. Res. 2018, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Cringoli, G.; Maurelli, M.P.; Levecke, B.; Bosco, A.; Vercruysse, J.; Utzinger, J.; Rinaldi, L. The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infections in humans and animals. Nat. Protoc. 2017, 12, 1723–1732. [Google Scholar] [CrossRef]
- Ministry of Agriculture, Fisheries and Food (MAFF). Grande-Bretagne, Manual of Veterinary Parasitological Laboratory Techniques; HM Stationery Off: London, UK, 1986.
- van Wyk, J.A.; Mayhew, E. Morphological identification of parasitic nematode infective larvae of small ruminants and cattle: A practical lab guide. Onderstepoort J. Vet. Res. 2013, 80, 539. [Google Scholar] [CrossRef]
- Coles, G.C.; Bauer, C.; Borgsteede, F.H.; Geerts, S.; Klei, T.R.; Taylor, M.A.; Waller, P.J. World association for the advencement of veterinary parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet. Parasitol. 1992, 44, 35–44. [Google Scholar] [CrossRef]
- Macedo, I.T.F.; Bevilaqua, C.M.I.; de Oliveira, L.M.B.; Camurça-Vasconcelos, A.L.F.; Vieira, L.S.; Oliveira, F.R.; Queiroz-Junior, E.M.; Tome, A.R.; Nascimento, N.R.F. Anthelmintic effect of Eucalyptus staigeriana essential oil against goat gastrointestinal nematodes. Vet. Parasitol. 2010, 173, 93–98. [Google Scholar] [CrossRef]
- Fonseca, Z.A.A.S.; Coelho, W.A.C.; Andre, W.P.P.; Ribeiro, W.L.C.; Bessa, E.N.; Galindo, V.R.; Pereira, J.S.; Ahid, S.M.M. Use of herbal medicines in control of gastrointestinal nematodes of small ruminants: Efficacies and prospects. Rev. Bras. Hig. Sanid. Anim. 2013, 7, 233–249. [Google Scholar] [CrossRef]
- Levecke, B.; Dobson, R.J.; Speybroeck, N.; Vercruysse, J.; Charlier, J. Novel insights in the faecal egg count reduction test for monitoring drug efficacy against gastrointestinal nematodes of veterinary importance. Vet. Parasitol. 2012, 188, 391–396. [Google Scholar] [CrossRef]
- Soren, A.D.; Yadav, A.K. Evaluation of in vitro and in vivo anthelmintic efficacy of Cyperus compressus Linn., a traditionally used anthelmintic plant in parasite-animal models. Future J. Pharm. Sci. 2020, 6, 126. [Google Scholar] [CrossRef]
- Kenyon, F.; Greer, A.W.; Coles, G.C.; Cringoli, G.; Papadopoulus, E.; Cabaret, J.; Berrag, B.; Varady, M.; Van Wyk, J.A.; Thomas, E.; et al. The role of target selective treatments in the development of refugia-based approaches to the control of gastrointestinal nematodes of small ruminants. Vet. Parasitol. 2009, 164, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Maes, C.; Bouquillion, S.; Fauconnier, M.L. Encapsulation of essential oils for the development of biosourced pesticides with controlled release: A review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef] [PubMed]
- Hoste, H.; Torres-Acosta, J.F.; Alonso-Diaz, M.A.; Brunet, S.; Sandoval-Castro, C.; Adote, S.H. Identification and validation of bioactive plants for the control of gastrointestinal nematodes in small ruminants. Trop. Biomed. 2008, 25, 56–72. [Google Scholar] [PubMed]
- Radunz, M.; Helbig, E.; Borges, C.D.; Gandra, T.K.V.; Gandra, E.A. A mini-review on encapsulation of essential oils. J. Anal. Pharm. Res. 2018, 7, 00205. [Google Scholar] [CrossRef]
- Junkuszew, A.; Milerski, M.; Bojar, W.; Szczepaniak, K.; Le Scouarnec, J.; Tomczuk, K.; Dudko, P.; Studzinska, M.B.; Demkowska-Kutrzepa, M.; Bracik, K. Effect of various antiparasitic treatments on lamb growth and mortality. Small Rumin Res. 2015, 123, 306–313. [Google Scholar] [CrossRef]
- Katiki, L.M.; Chagas, A.C.S.; Bizzo, H.R.; Ferreira, J.F.S.; Amarante, A.F.T. Anthelmintic activity of Cymbopogon martini, Cymbopogon schoenanthus and Mentha piperita essential oils evaluated in four different in vitro tests. Vet. Parasitol. 2011, 183, 103–108. [Google Scholar] [CrossRef]
- Valente, A.H.; Roode, M.D.; Ernst, M.; Peña-Espinoza, M.; Bornancin, L.; Bonde, C.S.; Martínez-Valladares, M.; Ramünke, S.; Krücken, J.; Simonsen, H.T.; et al. Identification of compounds responsible for the anthelmintic effects of chicory (Cichorium intybus) by molecular networking and bio-guided fractionation. Int. J. Parasitol-Drug. 2021, 15, 105–114. [Google Scholar] [CrossRef]
- André, W.P.P.; Cavalcante, G.S.; Ribeiro, W.L.C.; dos Santos, J.M.L.; Macedo, I.T.F.; de Paula, H.C.B.; de Morais, S.M.; Bevilaqua, C.M.L. Anthelmintic effect of thymol and thymol acetate on sheep gastrointestinal nematodes and their toxicity in mice. Rev. Bras. Parasitol. Vet. 2017, 26, 323–330. [Google Scholar] [CrossRef]
- André, W.P.P.; Ribeiro, W.L.C.; Cavalcante, G.S.; dos Santos, J.M.L.; Macedo, I.T.F.; de Paula, H.C.B.; de Morais, S.M.; de Freitas, R.M.; Bevilaqua, C.M.L. Comparative efficacy and toxic effects of carvacryl acetate and carvacrol on sheep gastrointestinal nematodes and mice. Vet. Parasitol. 2016, 218, 52–58. [Google Scholar] [CrossRef]
- Camurça-Vasconcelos, A.L.F.; Bevilaqua, C.M.L.; Morais, S.M.; Maciel, M.V.; Costa, C.T.C.; Macedo, I.T.F.; Oliveira, L.M.B.; Braga, R.R.; Silva, R.A.; Vieira, L.S. Anthelmintic activity of Croton zehtneri and Lippia sidoides essential oils. Vet. Parasitol. 2007, 148, 288–294. [Google Scholar] [CrossRef]
- Grando, T.H.; Baldissera, M.D.; Gressler, L.T.; Sá, M.F.; Bortoluzzi, B.N.; Schafer, A.S.; Ebling, R.C.; Raffin, R.P.; Santos, R.C.V.; Stefani, L.M.; et al. Melaleuca alternifolia anthelmintic activity in gerbils experimentally infected by Haemonchus contortus. Exp. Parasitol. 2016, 170, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oils‘ chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1147–1154. [Google Scholar] [CrossRef]
- Fokou, J.B.H.; Dongmo, P.M.J.; Boyom, F.F. Essential oil’s chemical composition and pharmacological properties. In Essential Oils Oils of Nature; El-Shemy, H., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Andres, M.F.; González-Coloma, A.; Sanz, J.; Burrilo, J.; Sainz, P. Nematicidal activity of essential oils: A review. Phytochem. Rev. 2012, 11, 371–390. [Google Scholar] [CrossRef]
- Muthee, J.K. Anthelmintic efficacy of selected medicinal plants against gastrointestinal nematodes in naturally infected sheep in Kenya. J. Phytopharmacol. 2018, 7, 111–115. [Google Scholar] [CrossRef]
- Štrbac, F.; Bosco, A.; Amadesi, A.; Rinaldi, L.; Stojanović, D.; Simin, N.; Orčić, D.; Pušić, I.; Krnjajić, S.; Ratajac, R. Ovicidal potential of five different essential oils to control gastrointestinal nematodes of sheep. Pak. Vet. J. 2021, 41, 353–358. [Google Scholar] [CrossRef]
- Štrbac, F.; Bosco, A.; Amadesi, A.; Rinaldi, L.; Stojanović, D.; Simin, N.; Orčić, D.; Pušić, I.; Krnjajić, S.; Ratajac, R. In vitro ovicidal activity of two chemotypes of the yarrow (Achillea millefolium L.) essential oil against sheep gastrointestinal nematodes. Arch. Vet. Med. 2020, 13, 59–76. [Google Scholar] [CrossRef]
- Štrbac, F.; Bosco, A.; Amadesi, A.; Rinaldi, L.; Stojanović, D.; Simin, N.; Orčić, D.; Pušić, I.; Krnjajić, S.; Ratajac, R. In vitro ovicidal effect of common juniper (Juniperus communis L.) on sheep gastrointestinal nematodes. Vet. Rev. 2020, 1, 152–159. [Google Scholar]
| AI | Compound | % of Total Peak Area a | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TV1 | TV2 | AM1 | AM2 | SM | SH | MP | FV | HA | OV | JC | ||
| 925 | α-Thujene | - | 0.85 | - | 0.13 | 0.33 | 0.38 | - | - | - | - | 1.88 b |
| 932 | α-Pinene | 2.47 | 0.71 | 3.09 | 4.15 | 1.16 | 1.21 | 0.20 | 1.53 | 28.0 | 1.81 | 40.46 |
| 947 | Camphene | 0.62 | 0.42 | 1.37 | 0.26 | 0.44 | 0.46 | - | 0.06 | 0.17 | - | 0.33 |
| 972 | Sabinene | - | - | 1.87 | 5.48 | - | - | 1.28 | - | - | - | 14.04 |
| 976 | β-Pinene | 0.18 | 0.10 | 1.50 | 28.5 | 0.66 | - | 0.12 | 0.05 | 0.21 | 1.64 | 2.70 |
| 990 | β-Myrcene | 0.71 | 1.05 | - | - | 0.96 | 1.30 | 0.84 | 0.13 | - | 0.32 | 8.87 |
| 998 | δ-2-Carene | - | - | 1.40 | - | - | - | - | - | - | - | - |
| 1016 | α-Terpinene | - | 1.18 | 2.86 | 0.19 | 2.32 | 2.00 | - | - | 0.11 | 0.20 | 0.86 |
| 1024 | p-Cymene | 41.7 | 21.0 | 4.38 | 0.65 | 42.8 | 12.9 | 0.07 | - | - | 12.6 | 1.94 |
| 1026 | o-Cymene | - | 1.17 | - | - | - | 0.54 | - | - | - | - | - |
| 1027 | Limonene | 1.26 | - | - | 0.87 | 1.52 | - | 7.14 | 1.20 | 1.66 | - | 4.95 |
| 1030 | 1,8-Cineole | 0.66 | - | 41.69 | 11.7 | 0.73 | - | 2.64 | - | 0.57 | - | - |
| 1057 | γ-Terpinene | - | 8.11 | 1.13 | 0.40 | 14.6 | 29.7 | 4.46 | 0.29 | 0.31 | 2.63 | 1.42 |
| 1059 | Artemisia ketone | - | - | 4.31 | - | - | - | - | - | - | - | - |
| 1082 | Artemisia alcohol | - | - | 1.57 | - | - | - | - | - | - | - | - |
| 1088 | Fenchone | - | - | - | - | - | - | - | 19.5 | - | - | - |
| 1100 | Linalool | 4.37 | 2.77 | - | - | 1.20 | - | - | - | 0.30 | 1.43 | - |
| 1106 | cis-Thujone | - | - | 3.28 | - | - | - | - | - | - | - | - |
| 1115 | trans-Thujone | - | - | 2.13 | - | - | - | - | - | - | - | - |
| 1124 | Chrysanthenone | - | - | 2.55 | - | - | - | - | - | - | - | - |
| 1143 | Camphor | 0.22 | - | 8.37 | 1.57 | - | - | - | 0.15 | - | - | - |
| 1152 | Menthone | - | - | - | - | - | - | 3.33 | - | - | - | - |
| 1163 | Isomenthone | - | - | - | - | - | - | 6.04 | - | - | - | - |
| 1164 | Borneol | 0.69 | 1.24 | 3.57 | 0.47 | 1.27 | - | - | - | - | - | - |
| 1176 | Terpinen-4-ol | - | 0.62 | 3.37 | - | 0.78 | - | 7.88 | - | - | - | 2.85 |
| 1190 | α-Terpineol | 11.7 | 0.26 | 1.18 | 0.87 | - | - | 9.77 | - | 0.33 | - | - |
| 1198 | Estragole | - | - | - | - | - | - | 3.37 | - | - | - | |
| 1204 | trans-Dihydrocarvone | - | - | - | - | - | - | 14.6 | - | - | - | - |
| 1214 | Isodihydrocarveol | - | - | - | - | - | - | 6.25 | - | - | - | - |
| 1234 | trans-Chrysanthenyl acetate | - | - | 4.90 | - | - | - | - | - | - | - | - |
| 1253 | Piperitone | - | - | - | - | - | - | 25.4 | - | - | - | - |
| 1286 | Anethol | - | - | - | - | - | - | - | 73.4 | - | - | - |
| 1291 | Lavandulyl acetate | - | - | - | 1.30 | - | - | - | - | - | - | - |
| 1292 | Thymol | 31.6 | 54.5 | - | - | - | 0.36 | - | - | - | 0.97 | - |
| 1302 | Carvacrol | - | 3.95 | - | - | 28.1 | 49.5 | - | - | - | 76.2 | - |
| 1364 | Neryl acetate | - | - | - | - | - | - | - | - | 3.05 | - | - |
| 1375 | α-Copaene | - | - | - | - | - | - | - | - | 3.09 | - | 0.28 |
| 1384 | β-Bourbonene | - | - | - | 1.29 | - | - | 0.51 | - | - | - | - |
| 1391 | β-Elemene | - | - | - | - | - | - | - | - | - | - | 1.24 |
| 1402 | iso-Italicene | - | - | - | - | - | - | - | - | 3.20 | - | - |
| 1418 | β-caryophyllene | 0.82 | 1.69 | 0.59 | 18.7 | 2.46 | 1.25 | 1.69 | 6.36 | 2.23 | 1.71 | |
| 1442 | sesquiterpene | - | - | - | - | - | - | - | - | 2.09 | - | - |
| 1452 | α-Humulene | - | - | - | 4.08 | - | - | - | - | - | - | 1.31 |
| 1474 | sesquiterpene | - | - | - | - | - | - | - | - | 1.64 | - | - |
| 1479 | γ-Curcumene | - | - | - | - | - | - | - | - | 20.1 | - | - |
| 1480 | Germacrene D | - | - | 0.44 | 8.01 | - | - | - | - | - | - | 2.54 |
| 1482 | ar-Curcumene | - | - | - | - | - | - | - | - | 4.15 | - | - |
| 1485 | β-Selinene | - | - | - | - | - | - | - | - | 9.32 | - | - |
| 1493 | α-Selinene | - | - | - | - | - | - | - | - | 5.21 | - | - |
| 1499 | sesquiterpene | - | - | - | - | - | - | - | - | 1.33 | - | - |
| 1506 | sesquiterpene | - | - | - | - | - | - | - | - | 1.33 | - | - |
| 1512 | β-Curcumene | - | - | - | - | - | - | - | - | 1.89 | - | - |
| 1513 | γ-Cadinene | - | - | - | - | - | - | - | - | - | - | 1.09 |
| 1523 | δ-Cadinene | - | - | - | 0.90 | - | - | - | - | 1.53 | - | 2.46 |
| 1556 | Germacrene B | - | - | - | - | - | - | - | - | - | - | 2.32 |
| 1582 | Caryophyllene oxide | - | - | - | 2.49 | - | - | 2.14 | - | - | - | - |
| 1590 | Viridiflorol | - | - | - | 3.52 | - | - | - | - | - | - | - |
| Number of all identified compounds | 15 | 19 | 28 | 27 | 17 | 13 | 21 | 12 | 30 | 10 | 28 | |
| Concentration [mg/mL] | Thymus vulgaris 1 | Thymus vulgaris 2 | Achillea millefolium 1 | Achillea millefolium 2 | Satureja montana | Satureja hortensis |
|---|---|---|---|---|---|---|
| 50 | 100 ± 0 Aa | 100 ± 0 Aa | 99.5 ± 1.0 Aa | 97.3 ± 0.96 Aa | 100 ± 0 Aa | 100 ± 0 Aa |
| 12.5 | 100 ± 0 Aa | 99.5 ± 0.58 Aa | 98.0 ± 1.83 Aa | 90.0 ± 4.97 Ab | 100 ± 0 Aa | 100 ± 0 Aa |
| 3.125 | 95.3 ± 5.68 Aa | 100 ± 0 Aa | 95.3 ± 4.35 Aa | 73.0 ± 1.63 Bb | 100 ± 0 Aa | 100 ± 0 Aa |
| 0.781 | 97.5 ± 1.73 Aae | 100 ± 0 Aa | 87.5 ± 2.65 Bbe | 72.8 ± 8.42 Bc | 100 ± 0 Aa | 100 ± 0 Aa |
| 0.195 | 98.0 ± 1.83 Aa | 100 ± 0 Aa | 49.0 ± 1.63 Cb | 71.3 ± 3.3 Bc | 100 ± 0 Aa | 99.8 ± 0.5 Aa |
| 0.049 | 96.8 ± 2.22 Aa | 98.5 ± 0.58 Aa | 46.5 ± 3.0 Cb | 69.5 ± 3.7 Bc | 100 ± 0 Aa | 99.3 ± 0.96 Aa |
| Control (+) | 98.0 ± 0.82 A | 98.0 ± 0.82 A | 98.0 ± 0.82 A | 98.0 ± 0.82 A | 98.0 ± 0.82 A | 98.0 ± 0.82 A |
| Control (−) | 16.8 ± 5.56 B | 16.8 ± 5.56 B | 16.8 ± 5.56 D | 16.8 ± 5.56 C | 16.8 ± 5.56 B | 16.8 ± 5.56 B |
| Mentha x piperita | Foeniculum vulgare | Helichrysum arenarium | Origanum vulgare | Juniperus communis | Linalool: estragole | |
| 50 | 99.8 ± 0.5 Aa | 100 ± 0 Aa | 69.3 ± 2.22 Ab | 100 ± 0 Aa | 96.8 ± 1.71 Aa | 100 ± 0 Aa |
| 12.5 | 99.0 ± 0.82 Aa | 100 ± 0 Aa | 68.5 ± 2.89 Ac | 100 ± 0 Aa | 95.5 ± 1.73 ABab | 100 ± 0 Aa |
| 3.125 | 99.0 ± 0.82 Aa | 100 ± 0 Aa | 68.3 ± 3.59 Ab | 100 ± 0 Aa | 94.8 ± 0.96 ABa | 99.8 ± 0.5 Aa |
| 0.781 | 94.8 ± 1.71 Aae | 100 ± 0 Aa | 63.8 ± 1.26 ABd | 100 ± 0 Aa | 91.0 ± 1.63 Be | 47.0 ± 20. 5 Bf |
| 0.195 | 83.0 ± 1.63 Bd | 100 ± 0 Aa | 59.8 ± 2.22 Be | 100 ± 0 Aa | 85.5 ± 0.58 Cd | 29.5 ± 1.29 BCf |
| 0.049 | 72.5 ± 1.29 Cc | 100 ± 0 Aa | 59.8 ± 0.96 Bd | 100 ± 0 Aa | 81.0 ± 1.63 Ce | 29.5 ± 2.65 BCf |
| Control (+) | 98.0 ± 0.82 A | 98.0 ± 0.82 A | 98.0 ± 0.82 A | 98.0 ± 0.82 A | 98.0 ± 0.82 A | 98.0 ± 0.82 A |
| Control (−) | 16.8 ± 5.56 D | 16.8 ± 5.56 B | 16.8 ± 5.56 C | 16.8 ± 5.56 B | 16.8 ± 5.56 D | 16.8 ± 5.56 C |
| Tested Sample | Day 0 | Day 7 | Day 14 | |
|---|---|---|---|---|
| T. vulgaris type 1, 100 mg/kg | Mean EPG | 143.5 ± 170.6 Aa | 99.8 ± 123.1 ABb | 107.7 ± 115.1 Aab |
| Efficacy | / | 25.23% | 24.42% | |
| Linalool:estragole, 100 mg/kg | Mean EPG | 145.6 ± 198.2 Aa | 101.9 ± 148.2 ABb | 106.3 ± 124.8 Ab |
| Efficacy | / | 24.91% | 25.90% | |
| Fenbendazole, 5 mg/kg (C+) | Mean EPG | 221.4 ± 326.9 Aa | 37.0 ± 86.5 Ab | 24.8 ± 39.6 Bb |
| Efficacy | / | 82.74% | 88.93% | |
| Sunflower oil, 50 mL/animal (C−) | Mean EPG | 142.3 ± 149.1 Aa | 132.5 ± 119.4 Ba | 140 ± 100.1 Aa |
| Efficacy | / | / | / | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Štrbac, F.; Bosco, A.; Maurelli, M.P.; Ratajac, R.; Stojanović, D.; Simin, N.; Orčić, D.; Pušić, I.; Krnjajić, S.; Sotiraki, S.; et al. Anthelmintic Properties of Essential Oils to Control Gastrointestinal Nematodes in Sheep—In Vitro and In Vivo Studies. Vet. Sci. 2022, 9, 93. https://doi.org/10.3390/vetsci9020093
Štrbac F, Bosco A, Maurelli MP, Ratajac R, Stojanović D, Simin N, Orčić D, Pušić I, Krnjajić S, Sotiraki S, et al. Anthelmintic Properties of Essential Oils to Control Gastrointestinal Nematodes in Sheep—In Vitro and In Vivo Studies. Veterinary Sciences. 2022; 9(2):93. https://doi.org/10.3390/vetsci9020093
Chicago/Turabian StyleŠtrbac, Filip, Antonio Bosco, Maria Paola Maurelli, Radomir Ratajac, Dragica Stojanović, Nataša Simin, Dejan Orčić, Ivan Pušić, Slobodan Krnjajić, Smaragda Sotiraki, and et al. 2022. "Anthelmintic Properties of Essential Oils to Control Gastrointestinal Nematodes in Sheep—In Vitro and In Vivo Studies" Veterinary Sciences 9, no. 2: 93. https://doi.org/10.3390/vetsci9020093
APA StyleŠtrbac, F., Bosco, A., Maurelli, M. P., Ratajac, R., Stojanović, D., Simin, N., Orčić, D., Pušić, I., Krnjajić, S., Sotiraki, S., Saralli, G., Cringoli, G., & Rinaldi, L. (2022). Anthelmintic Properties of Essential Oils to Control Gastrointestinal Nematodes in Sheep—In Vitro and In Vivo Studies. Veterinary Sciences, 9(2), 93. https://doi.org/10.3390/vetsci9020093











